Published online Jul 19, 2022. doi: 10.5498/wjp.v12.i7.884
Peer-review started: January 31, 2022
First decision: April 18, 2022
Revised: April 29, 2022
Accepted: June 26, 2022
Article in press: June 26, 2022
Published online: July 19, 2022
Processing time: 168 Days and 11 Hours
Depression is a common, recurrent mental disorder and one of the leading causes of disability and global burden of disease worldwide. Up to 15%-40% of cases do not respond to diverse pharmacological treatments and, thus, can be defined as treatment-resistant depression (TRD). The development of biomarkers predictive of drug response could guide us towards personalized and earlier treatment. Growing evidence points to the involvement of the glutamatergic system in the pathogenesis of TRD. Specifically, the N-methyl-D-aspartic acid receptor (NMDAR) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), which are targeted by ketamine and esketamine, are proposed as promising pathways. A literature search was performed to identify studies on the genetics of the glutamatergic system in depression, focused on variables related to NMDARs and AMPARs. Our review highlights GRIN2B, which encodes the NR2B subunit of NMDAR, as a candidate gene in the pathogenesis of TRD. In addition, several studies have associated genes encoding AMPAR subunits with symptomatic severity and suicidal ideation. These genes encoding glutamatergic receptors could, therefore, be candidate genes for understanding the etiopathogenesis of TRD, as well as for understanding the pharmacodynamic mechanisms and response to ketamine and esketamine treatment.
Core Tip: Depression is a common mental disorder and one of the leading causes of disability worldwide. Up to 15%-40% of cases are considered treatment-resistant depression, which seems to be conditioned by environmental and genetic factors. The glutamatergic system, specifically N-methyl-D-aspartic acid receptor (NMDAR) dysfunction, has been proposed to be involved in the pathogenesis of treatment-resistant depression (TRD). A literature search was performed to identify studies on the genetics of the glutamatergic system in depression, focused on NMDAR and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor. Our review highlights GRIN2B, which encodes the NR2B subunit of NMDAR, as a candidate gene in the pathogenesis of TRD.
- Citation: Saez E, Erkoreka L, Moreno-Calle T, Berjano B, Gonzalez-Pinto A, Basterreche N, Arrue A. Genetic variables of the glutamatergic system associated with treatment-resistant depression: A review of the literature. World J Psychiatry 2022; 12(7): 884-896
- URL: https://www.wjgnet.com/2220-3206/full/v12/i7/884.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i7.884
Depression is characterized by sustained low mood, anhedonia, psychomotor inhibition and, frequently, somatic alterations that significantly affect an individual's functioning and, as such, poses a social and economic problem, as well as a health problem. It is, according to the World Health Organization, a common, recurrent mental disorder and one of the leading causes of disability and global burden of disease worldwide[1]. Thus, it has been highlighted as one of the priority conditions covered by the Mental Health Gap Action Programme. The 12-mo prevalence of major depressive disorder (MDD) is estimated to be approximately 6%[2], whereas the lifetime risk of depression is between 15% and 18%[3]. Thus, MDD is common, with almost one in five people experiencing one episode at some point in their lifetime. Between 2005 and 2015, the incidence of depression increased by 18.4% worldwide[4]. Regarding gender differences, the lifetime-incidence of a major depressive episode in females has been reported to be twice that of males[5].
Depression significantly affects family, social, and occupational functioning and is, therefore, a health, social and economic problem. A recent review calculated that the direct costs of depression, due to the higher use of healthcare services, may be up to 24,069€ per patient/year, depending on the jurisdiction wherein the analyses were performed[6]. Productivity losses, for their part, were estimated to be between 1963€ and 27364€ per person per year[6]. It is among the leading causes of loss of disability-adjusted life years, mainly in the age range between 10 and 49 years[7], and is the most frequently identified diagnosis in people who have died by suicide[8]. Thus, in recent years, depression has become a major target of public health policies[9,10] due to the consequences that both depression itself, as well as associated events such as suicide, have on society.
If depression is untreated or inadequately treated, it is associated with higher rates of medical morbidity, lower productivity, decreased life expectancy, higher rates of suicide, and higher rates of functional disability. However, sometimes, despite evidence-based treatment, the patient may not respond favorably to treatment. Even though we have a growing number of therapeutic alternatives available to treat depression, approximately half of patients do not respond, and up to two-thirds do not achieve remission after first-line treatment[11]. In this context, the development of biomarkers predictive of drug response, which could guide us towards personalized treatment for each patient, is a challenge for the future.
Although there is no consensus on the definition of treatment-resistant depression (TRD), it is a useful concept to characterize a group of patients with MDD that do not respond to traditional monoaminergic antidepressants. The European Medicines Agency considers TRD to be that which has not responded to two antidepressants of different classes, prescribed at adequate doses (within a therapeutic range), for the appropriate time (> 6 wk) and ensuring correct adherence to the protocol[12,13]. Some authors add that potentiation strategies, such as lithium, neuroleptic drugs, or electroconvulsive therapy, also need to have been used.
According to the well-known Sequenced Treatment Alternatives to Relieve Depression study, one-third of patients with depression could be classified as TRD, as they do not respond to two different antidepressant treatments[11]. Along the same lines, other works have described that 15%-40% of patients with MDD do not respond to multiple pharmacological treatments[14].
Patients with MDD are more likely to make attempts and/or complete suicide, as well as to experience more frequent relapses and hospitalizations, and to have a worse overall prognosis. In other words, they form a subgroup of depressive patients characterized by clinical severity and higher health and social costs[15].
Resistance to treatment seems to be conditioned by genetic and environmental factors[16]. The underlying genetic factors of individuals cannot be modified, but genetic information could be used to predict response and tailor treatments to the idiosyncrasies of each patient. Emerging evidence has shown that genetic variations associated with antidepressant responses appear to cluster in families, supporting the importance of these variations in the underlying mechanism of depression, especially in TRD[17].
Identifying biomarkers that can predict the antidepressant response could be helpful in designing the initial treatment, decreasing the need for trial-and-error testing, and also avoiding suffering and possible chronicity. Single nucleotide polymorphisms (SNPs) have been suggested to be a decisive factor in the antidepressant response; numerous genetic polymorphisms have been described as possible risk factors for MDD and TRD[18-21].
Albeit a clinically heterogeneous pathology, there is consistent evidence, based on twin and adoption studies, that there is a heritability of 29%-49% in MDD (reviewed in[22]). Research has also been performed to identify more genetically homogeneous groups of MDD, indicating that clinical severity, the need for certain therapeutic strategies, recurrent episodes, and postpartum depression show differences in heritability[23,24].
It is a polygenic disease caused by the combined effect of polymorphisms, common to the general population, in different genes[25]. The genetics of depression has been studied for years via a candidate gene approach, mainly focusing the study on genes involved in the serotonergic, noradrenergic, and dopaminergic pathways, targets of the usual treatments[26-28]. A recent literature review of 18 candidate genes showed that most of the studies performed lacked sufficient statistical power and, thus, questioned previous depression candidate gene findings[29]. More recent work has begun to focus on the glutamatergic pathway as a candidate in the study of genetic factors involved in depression[30].
In recent years, genome-wide association studies (GWAS) have proliferated in an attempt to identify genes involved in various pathologies, including depression. A recent GWAS identified 102 independent variants, 269 genes, and 15 gene-sets associated with depression, including both genes and gene pathways associated with synaptic structure and neurotransmission, providing further evidence of the importance of prefrontal brain regions. A previous GWAS implicated voltage-gated calcium channels, the D2 dopamine receptor and, interestingly, glutamate receptors[31]. The authors stated that all humans carry a lesser or greater number of genetic risk factors for MDD.
Along this line, many authors have investigated the interaction between genetics and environment in the pathogenesis of depression. Recent reviews concluded that various genetic polymorphisms in the serotonergic system moderate the association between adverse childhood experiences and depression[32], and that early-life stress produces transcriptomic changes that are moderated by the female sex[33].
Finally, postmortem studies have also been conducted to investigate differential gene expression in human brains. GluR gene expression in the dorsolateral prefrontal cortex has been studied in small postmortem cohorts of MDD subjects and controls, with inconclusive results to date[34,35]. Nonetheless, the data seemed to indicate a fundamental dysfunction of the glutamatergic system in the frontal cortex in MDD[36].
The neurotransmitter systems most studied in the etiopathogenesis of depression have been the serotonergic, noradrenergic, and dopaminergic systems, which are targeted by the most commonly used antidepressant drugs. However, another system involved is the glutamatergic system. Glutamate exerts its action via ionotropic receptors [N-methyl-D-aspartic acid receptor (NMDAR), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), and 2-carboxy-3-carboxymethyl-4-isopropenylpyrrolidine kainate receptors (KAR)] and metabotropic receptors. In the last two decades, the glutamatergic system, specifically NMDAR dysfunction, has been shown to be involved in the pathogenesis of TRD[37]. In particular, NMDAR antagonism has been highlighted, marking it a target of numerous drugs indicated for TRD[38], such as ketamine and esketamine[39,40].
Intravenous ketamine and intranasal esketamine, rather than inhibiting, activate glutamate release[41], resulting in a rapid antidepressant effect, a prompt disappearance of suicidal ideation[42,43], and a reduction of anhedonic symptoms[44]. This emerging hypothesis suggests that NMDAR antagonism in GABAergic interneurons (the mechanism of action of ketamine and esketamine) leads to glutamate release[45]. Regarding this gamma-amino butyric acid (GABA)-glutamate neurotransmitter system, animal and human studies have described that intravenous ketamine administration reduces GABA concentration in several brain areas, such as the frontal cortex[45-47].
Treatment with ketamine and esketamine has proven particularly useful in cases of TRD[48], thus their mechanism of action in glutamatergic pathways, being the major difference with respect to usual antidepressant treatments, is an interesting starting point for understanding the etiopathogenesis of TRD.
Central glutamatergic activity is measured at the peripheral level via plasma levels of glutamate (pGlu) and GABA (pGABA). pGlu and pGABA levels have been described to significantly correlate with cerebrospinal fluid glutamate levels[49,50], indicating that, although the plasma levels assessed derive from both the brain and the periphery, the plasma levels of these amino acids reflect brain concentrations[51]. Previous studies reported altered levels of pGlu in blood, cerebrospinal fluid, and prefrontal, frontal, and occipital cortical areas of patients with depression compared with healthy volunteers[52-55].
In relation to GABA, as a neurotransmitter system closely related to the glutamatergic, a recent meta-analysis indicated a decrease in pGABA levels in patients with depression compared with healthy controls, although the heterogeneity was significant[56].
All these findings indicate that alterations in the glutamatergic system may play a key role in the development of TRD. Therefore, it has been proposed that genes involved in glutamatergic transmission could be candidate genes to explain the neurobiological basis of TRD, i.e., genetic risk factors for the development of depression, especially TRD[55,57].
A literature search was performed to identify studies regarding the genetics of the glutamatergic system in depression. A total of 118 articles, published up to October 15, 2021, were retrieved from the PubMed and Reference Citation Analysis (https://www.referencecitationanalysis.com/) databases using broad search terms in order to identify as many potentially eligible studies as possible: [(NMDA receptor OR AMPA receptor) AND gene* AND depression]. An age filter was added: “Adults: 19+ years”. Studies were included according to three criteria: (1) They investigated the influence of genetics/epigenetics on glutamate receptors in depression; (2) They were systematic reviews, meta-analyses, narrative reviews, or original research studies; and (3) They were written in English or Spanish. The reference lists of the selected studies and reviews were also checked to identify additional relevant articles using a snowballing approach. Finally, 46 papers were included in the review.
This is not a systematic review, but a narrative one; it summarizes the findings described in the selected reports and, in this way, provides an overview of the subject. The main results are summarized in Table 1.
| Receptor | Gene | Marker | Ref. | Result |
| NMDA | GRIN2A | rs16966731 | Chen et al[79], 2021 | T allele associated with antidepressant effect of ketamine |
| GRIN2B | rs1805502 | Zhang et al[69], 2014 | G allele associated with TRD | |
| Arnold et al[70], 2009 | GT haplotype increased risk of TRD | |||
| rs890 | Zhang et al[69], 2014 | C allele associated with TRD | ||
| Arnold et al[70], 2009 | ||||
| rs2268115 | Sokolowski et al[72], 2013 | Associated with suicide attempts | ||
| rs220557 | Sokolowski et al[72], 2013 | Associated with suicide attempts | ||
| AMPA | GRIA2 | rs4302506 | Chiesa et al[91], 2012 | C allele associated with a lower age of onset in MDD |
| rs4400397 | Chiesa et al[91], 2012 | C allele associated with a lower age of onset in MDD | ||
| GRIA3 | rs4825476 | Laje and McMahon[17], 2007 | G allele associated with suicidal ideation | |
| Kainate | GRIK4 | rs1954787 | Horstmann et al[94], 2010 | CC haplotype associated with response to antidepressants |
| Serretti et al[95], 2012 | No significant associations | |||
| rs12800734 | Horstmann et al[94], 2010 | GG haplotype associated with response to antidepressants | ||
| Serretti et al[95], 2012 | No significant associations |
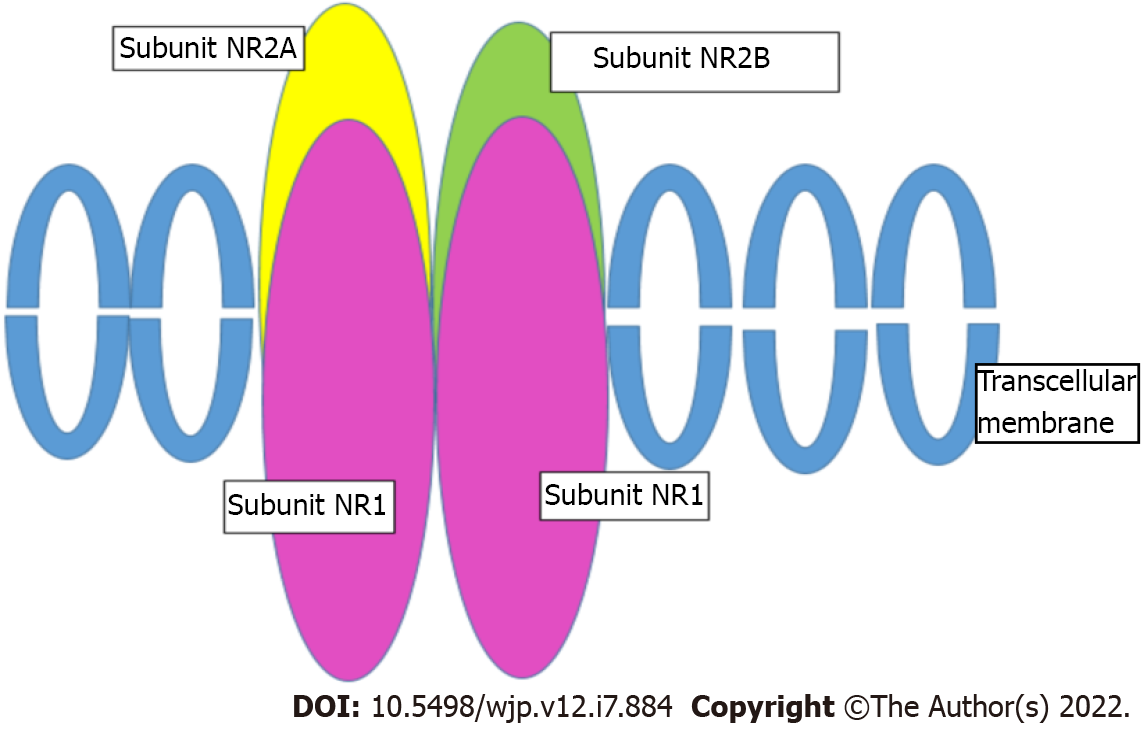
NMDAR, indicated as a therapeutic target in TRD[58], consists of four subunits (Figure 1). Two of them must be the NR1 subunit, mandatory for the receptor to be functional, while the other two subunits can be any of the four NR2 subunits (NR2A-D), or two NR3[59]. The NR2A-D subunits bind glutamate[60]. These subunits are encoded by the GRIN1, GRIN2A-D, and GRIN3 genes[61,62].
Associations between the functionality of these subunits and depression or response to antidepressant molecules were mostly found with the NR2B subunit. This is encoded by the GRIN2B gene, which is located on chromosome 12p12 and consists of 13 exons. Three potentially functional SNPs have been identified in this gene, all located in the 3'-untranslated region (UTR) that governs gene expression: rs1805502 (A to G), rs1806201 (T to C), and rs890 (A to C). They may contribute to the regulation of GRIN2B gene expression and influence glutamate release activity in the brain.
Ketamine users have also been reported to have a higher frequency of the rs1806201 TT genotype and a lower frequency of the CC genotype than controls, suggesting that this polymorphism may play a role in ketamine abuse[63]. Clinical trials report the superior therapeutic efficacy of NMDAR NR2B subunit antagonists over conventional antidepressants in patients with TRD[64,65].
Different GWAS have revealed a relationship between SNPs in the GRIN2B gene and depression[38,66,67]. In vivo studies evaluating glutamatergic activity at the brain level have shown that carriers of the rs1805502 G, rs1806201 T, or rs890 C allele have decreased glutamate concentrations in the anterior cingulate cortex. These alleles have been related to several psychiatric disorders[68-70], suggesting that they be a risk factor (genetic predictor) for TRD[69] in MDD patients. Recently, in a preclinical study in transgenic mice with selective mutations in the NR2B subunit in GABAergic interneurons, deletion of NR2B was found to block the antidepressant action of ketamine[71].
These GRIN2B gene polymorphisms have been described both as risk variables for MDD and predictors of TRD. An association was reported between the GT haplotype (rs1805502-rs890) and increased TRD risk compared with controls, as well as between the rs1805502 G allele and TRD (compared with non-resistant depression)[69].
A GWAS-based study reported a significant association between GRIN2B and suicide attempts, as well as a gene-environment relationship with a history of physical abuse in childhood and adolescence, which also increases the risk of suicide[72]. Indeed, they found that GRIN2B and ODC1 (encoding ornithine decarboxylase, a rate-limiting enzyme of the polyamine synthesis pathway) seem to be associated with severe suicide attempts, as well as with serious physical assault in childhood and adolescence[73,74], which in turn increases the risk of suicide attempts, thereby configuring a gene-by-environment interaction.
Finally, human postmortem studies found GRIN2B expression to be higher in suicidal MDD patients, compared with non-suicidal MDD patients[36], and in the locus coeruleus of depressed individuals[75]. It is therefore postulated that GRIN2B mRNA level may be a biomarker of suicide; indeed, GRIN2B genetic polymorphisms in MDD have been reported to predict treatment resistance, suicide attempts, and reasoning ability[72].
Based on the data described, GRIN2B is considered a promising candidate gene for MDD susceptibility, and more specifically for TRD, supporting the contention that TRD can be classified as a specific subtype of MDD[69].
Regarding other NMDAR subunits, postmortem studies in rodents using depression models have observed that chronic stress, besides increasing NR2B subunit mRNA, also increases NR1 and NR2A in several brain regions[76,77]. Postmortem studies in humans reported higher expression levels of GRIN1 and GRIN2A in the brains of depressed patients than in controls, and of GRIN2B in suicidal compared with non-suicidal MDD patients[36,78]. Likewise, the GRIN2A rs16966731 polymorphism (T to C, intron area) has been associated with the rapid and persistent antidepressant effect of ketamine[79]. Chandley et al[75] also reported altered expression of the GRIN2C gene at the locus ceruleus in depressed patients[75]. Finally, one paper reported that women with MDD had higher expression levels of all the NMDAR subunit genes; the only one not reaching statistical significance was GRIN3A[36].
From a gene-environment interaction perspective, an epigenetic study showed that GRIN1 methylation was a significant predictor of depression in a sample of abused children[80]. In one study, GRIN2A hypermethylation in the hippocampus and prefrontal cortex in postmortem studies was related to overexpression of the GluN2A subunit[81,82]. Interestingly, maternal separation increases the expression of this subunit in the hippocampus of adult rats, but not of subunit 2B. Numerous rat stress models have evaluated GRIN1A, GRIN2B, and GRIN2A with results similar to those described above[77,83,84].
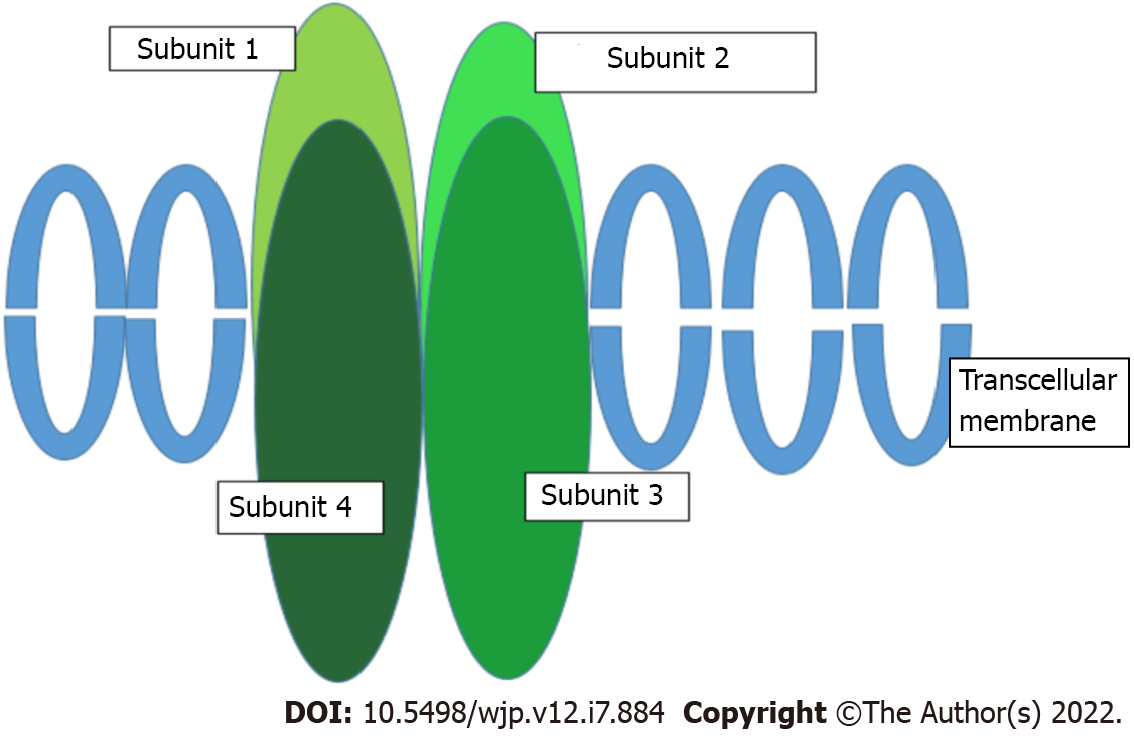
AMPARs are transmembrane ionotropic glutamatergic receptors and the main receptors mediating rapid synaptic neurotransmission in the brain. They consist of four subunits (GluR1-4) encoded by four genes (GRIA1-4)[85] (Figure 2). Evidence also suggests that the antidepressant mechanism of action of ketamine and esketamine involves the activation of AMPARs, with a subsequent increase in brain-derived neurotrophic factor levels (usually reduced in patients with depression)[45], as has been observed in rodent models[86]. Therefore, AMPARs have been proposed to play a key role in the antidepressant effect associated with ketamine[87].
Ketamine and its enantiomer, esketamine, lead to the disinhibition of glutamatergic neurons that modulate AMPARs by antagonizing the NMDAR of GABAergic interneurons[45,88]. In addition, ketamine metabolites such as hydroxynorketamine seem to exert their antidepressant effect via AMPAR activation[84,89].
In mouse models reproducing depression and stress, increased expression of AMPARs has been observed[76,90]. Postmortem studies described increased GRIA2-4 expression in the prefrontal cortex of MDD patients vs controls[36], and of GRIA3 in suicidal vs non-suicidal patients with MDD. As regards the GRIA2 gene, several authors have observed an association between carriers of the C allele (rs4302506; C to T, located in the coding exon) and carriers of the T allele (rs4400397; C to T, 3'-UTR), and a lower age of MDD onset[91]. Also, the G allele (rs4825476; G to A, intron 3) of the GRI3A gene has been associated with suicidal ideation in patients with major depression treated with monoaminergic antidepressants[17]. The AMPAR subunits GluA2-4 had significantly higher expression in female MDD patients[36].
Animal studies have suggested that ionotropic glutamate receptors play a role in the action of antidepressant drugs[92,93]. Another widely investigated gene is GRIK4, which encodes subunit 4 of the ionotropic glutamate KAR. Here, an association was observed between the rs1954787 polymorphism and antidepressant response[94]; however, the GRIK4 polymorphism with the highest predictive value for treatment outcome was rs12800734. Nonetheless, these findings have not been replicated in other studies, probably due to design differences[95]. Existing data also revealed increased expression of the KAR subunits, GluK1 and GluK2. In addition, the strongest predictor of suicide was GRIK3 (GluK3) expression in both sexes[36]. KARs appear to regulate L-glutamate release by functioning as facilitatory or inhibitory autoreceptors during repetitive synaptic activation. The KAR activity may contribute to excitotoxic cell death; however, the role of these receptors in the dorsolateral prefrontal cortex of MDD subjects remains to be elucidated. Genetic variation in GRIK3 has been associated with recurrent MDD[36].
In addition to ionotropic glutamatergic receptors, metabotropic receptors have also been involved in the genesis of MDD. Increased expression of GRIN1, GRIN2A-D, GRIA2-4, GRIK1-2, GRM1, GRM4, GRM5, and GRM7 has been observed in female MDD patients. In contrast, GRM5 expression was lower in male MDD patients relative to male controls. When suicidal MDD patients were compared with non-suicidal patients, GRIN2B, GRIK3, and GRM2 were expressed at higher levels in suicidal patients[36]. Recent studies showed that mGluR4 regulation is altered in male suicidal individuals, leading to higher expression of mGluR. Higher expression levels of the mGluR2 encoding gene, GRM2, were also detected; GRM2 has been proposed as a biomarker of suicide[36].
Repeated stress in male rats has been reported to be associated with lower expression of AMPARs and NMDARs, and, also, with a lower activity of these receptors. In contrast, in female rats exposed to stress, the expression of AMPARs and NMDARs was normalized via the activation of estrogen receptors, resulting in a neuroprotective and procognitive effect[96]. The authors proposed that, in female patients, estrogenic activity may lead to a differential response to ketamine; it should be noted that two-thirds of MDD patients are women.
Finally, there is downregulation of metabotropic receptors in mice reproducing models of depression, especially in the mGlu2 subunit, which is completely restored by ketamine administration[97].
The main limitation of this review is the scarcity and heterogeneity of the literature available on the topic. Few studies have employed similar methodology and, thus, there is limited replication of the described findings. Due to the small number of studies, all research conducted in humans and animals has been included in the review, although the extrapolation of the results, in this case, is limited. As we have noted, this is a narrative review, and limitations inherent to this type of review should also be mentioned: Study selection, data extraction, and synthesis were not protocol-based and, thus, could be prone to bias.
Nonetheless, it should also be noted that this is the first review, to our knowledge, of this specific topic, making it possible to summarize the current state of the art, highlighting the need to advance research in this field.
Patients with TRD often experience long periods of therapeutic trials with different antidepressant medications, resulting in a worse outcome, a delay in symptomatic remission, and an increased risk of fatal events, such as suicide. Therefore, the management of TRD with appropriate therapy could be facilitated by the identification of biological markers of TRD, which could guide treatment choice from the outset.
Although the serotonergic, noradrenergic, and dopaminergic pathways were those historically studied, more recent work indicates the involvement of the glutamatergic pathway. This proposal is consistent with new therapeutic strategies in TRD, such as ketamine and esketamine, which act mainly on glutamatergic receptors.
Our review highlights GRIN2B, which encodes the NR2B subunit of NMDAR, as a candidate gene in the pathogenesis of TRD. In addition, several studies have associated genes encoding AMPAR subunits with symptomatic severity and suicidal ideation. These genes encoding glutamatergic receptors could, therefore, be candidate genes for understanding the etiopathogenesis of TRD, as well as for understanding the pharmacodynamic mechanisms and response to ketamine and esketamine treatment. However, further empirical work is required to replicate the observed associations and to confirm the involvement of these genes in the pathogenesis of TRD.
We would like to thank Biocruces Bizkaia Health Research Institute.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Sociedad Española de Psiquiatría Biológica; Asociación Española de Neuropsiquiatría; Sociedad Española para el Estudio de los Trastornos de la Personalidad; International Society of Transference-focused Psychotherapy.
Specialty type: Psychiatry
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Arumugam VA, India; Ji Y, China; Wen XL, China S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | World Health Organization. Depression. [cited 10 January 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/depression. |
| 2. | Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1342] [Cited by in RCA: 1636] [Article Influence: 136.3] [Reference Citation Analysis (0)] |
| 3. | Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N, Karam AN, Kaur J, Kostyuchenko S, Lépine JP, Levinson D, Matschinger H, Mora ME, Browne MO, Posada-Villa J, Viana MC, Williams DR, Kessler RC. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1172] [Cited by in RCA: 1336] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 4. | GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545-1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5206] [Cited by in RCA: 4834] [Article Influence: 537.1] [Reference Citation Analysis (0)] |
| 5. | Gabilondo A, Rojas-Farreras S, Vilagut G, Haro JM, Fernández A, Pinto-Meza A, Alonso J. Epidemiology of major depressive episode in a southern European country: results from the ESEMeD-Spain project. J Affect Disord. 2010;120:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Coretti S, Rumi F, Cicchetti A. The Social Cost of Major Depression: A Systematic Review. Rev Eur Stud. 2019;11:73. |
| 7. | Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, Abdelalim A, Abdollahi M, Abdollahpour I, Abolhassani H, Aboyans V, Abrams EM, Abreu LG, Abrigo MRM, Abu-Raddad LJ, Abushouk AI, Acebedo A, Ackerman IN, Adabi M, Adamu AA, Adebayo OM, Adekanmbi V, Adelson JD, Adetokunboh OO, Adham D, Afshari M, Afshin A, Agardh EE, Agarwal G, Agesa KM, Aghaali M, Aghamir SMK, Agrawal A, Ahmad T, Ahmadi A, Ahmadi M, Ahmadieh H, Ahmadpour E, Akalu TY, Akinyemi RO, Akinyemiju T, Akombi B, Al-Aly Z, Alam K, Alam N, Alam S, Alam T, Alanzi TM, Albertson SB, Alcalde-Rabanal JE, Alema NM, Ali M, Ali S, Alicandro G, Alijanzadeh M, Alinia C, Alipour V, Aljunid SM, Alla F, Allebeck P, Almasi-Hashiani A, Alonso J, Al-Raddadi RM, Altirkawi KA, Alvis-Guzman N, Alvis-Zakzuk NJ, Amini S, Amini-Rarani M, Aminorroaya A, Amiri F, Amit AML, Amugsi DA, Amul GGH, Anderlini D, Andrei CL, Andrei T, Anjomshoa M, Ansari F, Ansari I, Ansari-Moghaddam A, Antonio CAT, Antony CM, Antriyandarti E, Anvari D, Anwer R, Arabloo J, Arab-Zozani M, Aravkin AY, Ariani F, Ärnlöv J, Aryal KK, Arzani A, Asadi-Aliabadi M, Asadi-Pooya AA, Asghari B, Ashbaugh C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204-1222. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11327] [Cited by in RCA: 9637] [Article Influence: 1927.4] [Reference Citation Analysis (35)] |
| 8. | Bachmann S. Epidemiology of Suicide and the Psychiatric Perspective. Int J Environ Res Public Health. 2018;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 642] [Cited by in RCA: 742] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 9. | Mendelson T, Eaton WW. Recent advances in the prevention of mental disorders. Soc Psychiatry Psychiatr Epidemiol. 2018;53:325-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | World Health Organization. Comprehensive Mental Health Action Plan 2013-2030 [cited 10 January 2022].. |
| 11. | Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2618] [Cited by in RCA: 3324] [Article Influence: 174.9] [Reference Citation Analysis (0)] |
| 12. | Culpepper L. Why do you need to move beyond first-line therapy for major depression? J Clin Psychiatry. 2010;71 Suppl 1:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Berlim MT, Fleck MP, Turecki G. Current trends in the assessment and somatic treatment of resistant/refractory major depression: an overview. Ann Med. 2008;40:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry. 2007;52:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 264] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Gibson TB, Jing Y, Smith Carls G, Kim E, Bagalman JE, Burton WN, Tran QV, Pikalov A, Goetzel RZ. Cost burden of treatment resistance in patients with depression. Am J Manag Care. 2010;16:370-377. [PubMed] |
| 16. | Kovacs D, Gonda X, Petschner P, Edes A, Eszlari N, Bagdy G, Juhasz G. Antidepressant treatment response is modulated by genetic and environmental factors and their interactions. Ann Gen Psychiatry. 2014;13:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Laje G, McMahon FJ. The pharmacogenetics of major depression: past, present, and future. Biol Psychiatry. 2007;62:1205-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Fabbri C, Di Girolamo G, Serretti A. Pharmacogenetics of antidepressant drugs: an update after almost 20 years of research. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:487-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Fabbri C, Drago A, Serretti A. Early antidepressant efficacy modulation by glutamatergic gene variants in the STAR*D. Eur Neuropsychopharmacol. 2013;23:612-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Myung W, Song J, Lim SW, Won HH, Kim S, Lee Y, Kang HS, Lee H, Kim JW, Carroll BJ, Kim DK. Genetic association study of individual symptoms in depression. Psychiatry Res. 2012;198:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Howard DM, Adams MJ, Shirali M, Clarke TK, Marioni RE, Davies G, Coleman JRI, Alloza C, Shen X, Barbu MC, Wigmore EM, Gibson J; 23andMe Research Team, Hagenaars SP, Lewis CM, Ward J, Smith DJ, Sullivan PF, Haley CS, Breen G, Deary IJ, McIntosh AM. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat Commun. 2018;9:1470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 367] [Cited by in RCA: 342] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 22. | Kendall KM, Van Assche E, Andlauer TFM, Choi KW, Luykx JJ, Schulte EC, Lu Y. The genetic basis of major depression. Psychol Med. 2021;51:2217-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 23. | Nguyen TD, Harder A, Xiong Y, Kowalec K, Hägg S, Cai N, Kuja-Halkola R, Dalman C, Sullivan PF, Lu Y. Genetic heterogeneity and subtypes of major depression. Mol Psychiatry. 2022;27:1667-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 24. | Kiewa J, Meltzer-Brody S, Milgrom J, Guintivano J, Hickie IB, Whiteman DC, Olsen CM, Colodro-Conde L, Medland SE, Martin NG, Wray NR, Byrne EM. Perinatal depression is associated with a higher polygenic risk for major depressive disorder than non-perinatal depression. Depress Anxiety. 2022;39:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Flint J, Kendler KS. The genetics of major depression. Neuron. 2014;81:484-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 406] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 26. | Chiesa A, Lia L, Lia C, Lee SJ, Han C, Patkar AA, Pae CU, Serretti A. Investigation of possible epistatic interactions between GRIA2 and GRIA4 variants on clinical outcomes in patients with major depressive disorder. J Int Med Res. 2013;41:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Phillips JL, Batten LA, Tremblay P, Aldosary F, Du L, Blier P. Impact of monoamine-related gene polymorphisms on hippocampal volume in treatment-resistant depression. Acta Neuropsychiatr. 2015;27:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | He M, He H, Yang L, Zhang J, Chen K, Duan Z. Functional tag SNPs inside the DRD2 gene as a genetic risk factor for major depressive disorder in the Chinese Han population. Int J Clin Exp Pathol. 2019;12:628-639. [PubMed] |
| 29. | Border R, Johnson EC, Evans LM, Smolen A, Berley N, Sullivan PF, Keller MC. No Support for Historical Candidate Gene or Candidate Gene-by-Interaction Hypotheses for Major Depression Across Multiple Large Samples. Am J Psychiatry. 2019;176:376-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 30. | Wang HQ, Wang ZZ, Chen NH. The receptor hypothesis and the pathogenesis of depression: Genetic bases and biological correlates. Pharmacol Res. 2021;167:105542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 31. | Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, Adams MJ, Agerbo E, Air TM, Andlauer TMF, Bacanu SA, Bækvad-Hansen M, Beekman AFT, Bigdeli TB, Binder EB, Blackwood DRH, Bryois J, Buttenschøn HN, Bybjerg-Grauholm J, Cai N, Castelao E, Christensen JH, Clarke TK, Coleman JIR, Colodro-Conde L, Couvy-Duchesne B, Craddock N, Crawford GE, Crowley CA, Dashti HS, Davies G, Deary IJ, Degenhardt F, Derks EM, Direk N, Dolan CV, Dunn EC, Eley TC, Eriksson N, Escott-Price V, Kiadeh FHF, Finucane HK, Forstner AJ, Frank J, Gaspar HA, Gill M, Giusti-Rodríguez P, Goes FS, Gordon SD, Grove J, Hall LS, Hannon E, Hansen CS, Hansen TF, Herms S, Hickie IB, Hoffmann P, Homuth G, Horn C, Hottenga JJ, Hougaard DM, Hu M, Hyde CL, Ising M, Jansen R, Jin F, Jorgenson E, Knowles JA, Kohane IS, Kraft J, Kretzschmar WW, Krogh J, Kutalik Z, Lane JM, Li Y, Lind PA, Liu X, Lu L, MacIntyre DJ, MacKinnon DF, Maier RM, Maier W, Marchini J, Mbarek H, McGrath P, McGuffin P, Medland SE, Mehta D, Middeldorp CM, Mihailov E, Milaneschi Y, Milani L, Mill J, Mondimore FM, Montgomery GW, Mostafavi S, Mullins N, Nauck M, Ng B, Nivard MG, Nyholt DR, O'Reilly PF, Oskarsson H, Owen MJ, Painter JN, Pedersen CB, Pedersen MG, Peterson RE, Pettersson E, Peyrot WJ, Pistis G, Posthuma D, Purcell SM, Quiroz JA, Qvist P, Rice JP, Riley BP, Rivera M, Saeed Mirza S, Saxena R, Schoevers R, Schulte EC, Shen L, Shi J, Shyn SI, Sigurdsson E, Sinnamon GBC, Smit JH, Smith DJ, Stefansson H, Steinberg S, Stockmeier CA, Streit F, Strohmaier J, Tansey KE, Teismann H, Teumer A, Thompson W, Thomson PA, Thorgeirsson TE, Tian C, Traylor M, Treutlein J, Trubetskoy V, Uitterlinden AG, Umbricht D, Van der Auwera S, van Hemert AM, Viktorin A, Visscher PM, Wang Y, Webb BT, Weinsheimer SM, Wellmann J, Willemsen G, Witt SH, Wu Y, Xi HS, Yang J, Zhang F; eQTLGen; 23andMe, Arolt V, Baune BT, Berger K, Boomsma DI, Cichon S, Dannlowski U, de Geus ECJ, DePaulo JR, Domenici E, Domschke K, Esko T, Grabe HJ, Hamilton SP, Hayward C, Heath AC, Hinds DA, Kendler KS, Kloiber S, Lewis G, Li QS, Lucae S, Madden PFA, Magnusson PK, Martin NG, McIntosh AM, Metspalu A, Mors O, Mortensen PB, Müller-Myhsok B, Nordentoft M, Nöthen MM, O'Donovan MC, Paciga SA, Pedersen NL, Penninx BWJH, Perlis RH, Porteous DJ, Potash JB, Preisig M, Rietschel M, Schaefer C, Schulze TG, Smoller JW, Stefansson K, Tiemeier H, Uher R, Völzke H, Weissman MM, Werge T, Winslow AR, Lewis CM, Levinson DF, Breen G, Børglum AD, Sullivan PF; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668-681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2291] [Cited by in RCA: 1940] [Article Influence: 277.1] [Reference Citation Analysis (0)] |
| 32. | Lipsky RK, McDonald CC, Souders MC, Carpio CC, Teitelman AM. Adverse childhood experiences, the serotonergic system, and depressive and anxiety disorders in adulthood: A systematic literature review. Neurosci Biobehav Rev. 2022;134:104495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Parel ST, Peña CJ. Genome-wide Signatures of Early-Life Stress: Influence of Sex. Biol Psychiatry. 2022;91:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 34. | Rodríguez-Muñoz M, Sánchez-Blázquez P, Callado LF, Meana JJ, Garzón-Niño J. Schizophrenia and depression, two poles of endocannabinoid system deregulation. Transl Psychiatry. 2017;7:1291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 36. | Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry. 2015;20:1057-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 37. | Meador-Woodruff JH, Hogg AJ Jr, Smith RE. Striatal ionotropic glutamate receptor expression in schizophrenia, bipolar disorder, and major depressive disorder. Brain Res Bull. 2001;55:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Fabbri C, Montgomery S, Lewis CM, Serretti A. Genetics and major depressive disorder: clinical implications for disease risk, prognosis and treatment. Int Clin Psychopharmacol. 2020;35:233-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Muthukumaraswamy SD, Shaw AD, Jackson LE, Hall J, Moran R, Saxena N. Evidence that Subanesthetic Doses of Ketamine Cause Sustained Disruptions of NMDA and AMPA-Mediated Frontoparietal Connectivity in Humans. J Neurosci. 2015;35:11694-11706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 40. | Kalmoe MC, Janski AM, Zorumski CF, Nagele P, Palanca BJ, Conway CR. Ketamine and nitrous oxide: The evolution of NMDA receptor antagonists as antidepressant agents. J Neurol Sci. 2020;412:116778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921-2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1233] [Cited by in RCA: 1291] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 42. | Andrade C. Ketamine for Depression, 6: Effects on Suicidal Ideation and Possible Use as Crisis Intervention in Patients at Suicide Risk. J Clin Psychiatry. 2018;79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Chen MH, Cheng CM, Gueorguieva R, Lin WC, Li CT, Hong CJ, Tu PC, Bai YM, Tsai SJ, Krystal JH, Su TP. Maintenance of antidepressant and antisuicidal effects by D-cycloserine among patients with treatment-resistant depression who responded to low-dose ketamine infusion: a double-blind randomized placebo-control study. Neuropsychopharmacology. 2019;44:2112-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 44. | Lally N, Nugent AC, Luckenbaugh DA, Niciu MJ, Roiser JP, Zarate CA Jr. Neural correlates of change in major depressive disorder anhedonia following open-label ketamine. J Psychopharmacol. 2015;29:596-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 45. | Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr, Gould TD. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev. 2018;70:621-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 757] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 46. | Boczek T, Lisek M, Ferenc B, Wiktorska M, Ivchevska I, Zylinska L. Region-specific effects of repeated ketamine administration on the presynaptic GABAergic neurochemistry in rat brain. Neurochem Int. 2015;91:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Silberbauer LR, Spurny B, Handschuh P, Klöbl M, Bednarik P, Reiter B, Ritter V, Trost P, Konadu ME, Windpassinger M, Stimpfl T, Bogner W, Lanzenberger R, Spies M. Effect of Ketamine on Limbic GABA and Glutamate: A Human In Vivo Multivoxel Magnetic Resonance Spectroscopy Study. Front Psychiatry. 2020;11:549903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | McIntyre RS, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, Brietzke E, Dodd S, Gorwood P, Ho R, Iosifescu DV, Lopez Jaramillo C, Kasper S, Kratiuk K, Lee JG, Lee Y, Lui LMW, Mansur RB, Papakostas GI, Subramaniapillai M, Thase M, Vieta E, Young AH, Zarate CA Jr, Stahl S. Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation. Am J Psychiatry. 2021;178:383-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 379] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 49. | Alfredsson G, Wiesel FA, Lindberg M. Glutamate and glutamine in cerebrospinal fluid and serum from healthy volunteers--analytical aspects. J Chromatogr. 1988;424:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Adinoff B, Kramer GL, Petty F. Levels of gamma-aminobutyric acid in cerebrospinal fluid and plasma during alcohol withdrawal. Psychiatry Res. 1995;59:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Petty F. Plasma concentrations of gamma-aminobutyric acid (GABA) and mood disorders: a blood test for manic depressive disease? Clin Chem. 1994;40:296-302. [PubMed] |
| 52. | Altamura C, Maes M, Dai J, Meltzer HY. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur Neuropsychopharmacol. 1995;5 Suppl:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Gao SF, Bao AM. Corticotropin-releasing hormone, glutamate, and γ-aminobutyric acid in depression. Neuroscientist. 2011;17:124-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 451] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 55. | Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 782] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 56. | Romeo B, Choucha W, Fossati P, Rotge JY. Meta-analysis of central and peripheral γ-aminobutyric acid levels in patients with unipolar and bipolar depression. J Psychiatry Neurosci. 2018;43:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 57. | Okada M, Kawano Y, Fukuyama K, Motomura E, Shiroyama T. Candidate Strategies for Development of a Rapid-Acting Antidepressant Class That Does Not Result in Neuropsychiatric Adverse Effects: Prevention of Ketamine-Induced Neuropsychiatric Adverse Reactions. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Park LT, Kadriu B, Gould TD, Zanos P, Greenstein D, Evans JW, Yuan P, Farmer CA, Oppenheimer M, George JM, Adeojo LW, Snodgrass HR, Smith MA, Henter ID, Machado-Vieira R, Mannes AJ, Zarate CA. A Randomized Trial of the N-Methyl-d-Aspartate Receptor Glycine Site Antagonist Prodrug 4-Chlorokynurenine in Treatment-Resistant Depression. Int J Neuropsychopharmacol. 2020;23:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 59. | Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, Lichnerova K, Cerny J, Krusek J, Dittert I, Horak M, Vyklicky L. Structure, function, and pharmacology of NMDA receptor channels. Physiol Res. 2014;63:S191-S203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 60. | Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2914] [Cited by in RCA: 2710] [Article Influence: 180.7] [Reference Citation Analysis (0)] |
| 61. | Mueller HT, Meador-Woodruff JH. NR3A NMDA receptor subunit mRNA expression in schizophrenia, depression and bipolar disorder. Schizophr Res. 2004;71:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Dean B, Gibbons AS, Boer S, Uezato A, Meador-Woodruff J, Scarr E, McCullumsmith RE. Changes in cortical N-methyl-D-aspartate receptors and post-synaptic density protein 95 in schizophrenia, mood disorders and suicide. Aust N Z J Psychiatry. 2016;50:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Fan N, An L, Zhang M, He H, Zhou Y, Ou Y. GRIN2B Gene Polymorphism in Chronic Ketamine Users. Am J Addict. 2020;29:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA Jr. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 240] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 65. | Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 414] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 66. | Aragam N, Wang KS, Anderson JL, Liu X. TMPRSS9 and GRIN2B are associated with neuroticism: a genome-wide association study in a European sample. J Mol Neurosci. 2013;50:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Myers SJ, Yuan H, Kang JQ, Tan FCK, Traynelis SF, Low CM. Distinct roles of GRIN2A and GRIN2B variants in neurological conditions. F1000Res. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 68. | Narita S, Onozawa Y, Yoshihara E, Nishizawa D, Numajiri M, Ikeda K, Iwahashi K. Association between N-methyl-D-aspartate Receptor Subunit 2B Gene Polymorphisms and Personality Traits in a Young Japanese Population. East Asian Arch Psychiatry. 2018;28:45-52. [PubMed] |
| 69. | Zhang C, Li Z, Wu Z, Chen J, Wang Z, Peng D, Hong W, Yuan C, Yu S, Xu Y, Xu L, Xiao Z, Fang Y. A study of N-methyl-D-aspartate receptor gene (GRIN2B) variants as predictors of treatment-resistant major depression. Psychopharmacology (Berl). 2014;231:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 70. | Arnold PD, Macmaster FP, Richter MA, Hanna GL, Sicard T, Burroughs E, Mirza Y, Easter PC, Rose M, Kennedy JL, Rosenberg DR. Glutamate receptor gene (GRIN2B) associated with reduced anterior cingulate glutamatergic concentration in pediatric obsessive-compulsive disorder. Psychiatry Res. 2009;172:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 71. | Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, Taylor SR, Duman CH, Delpire E, Picciotto M, Wohleb ES, Duman RS. GABA interneurons are the cellular trigger for ketamine's rapid antidepressant actions. J Clin Invest. 2020;130:1336-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 231] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 72. | Sokolowski M, Ben-Efraim YJ, Wasserman J, Wasserman D. Glutamatergic GRIN2B and polyaminergic ODC1 genes in suicide attempts: associations and gene-environment interactions with childhood/adolescent physical assault. Mol Psychiatry. 2013;18:985-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Engdahl E, Alavian-Ghavanini A, Forsell Y, Lavebratt C, Rüegg J. Childhood adversity increases methylation in the GRIN2B gene. J Psychiatr Res. 2021;132:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Buji RI, Abdul Murad NA, Chan LF, Maniam T, Mohd Shahrir MS, Rozita M, Shamsul AS, Mohamad Hussain R, Abdullah N, Jamal R, Nik Jaafar NR. Suicidal ideation in systemic lupus erythematosus: NR2A gene polymorphism, clinical and psychosocial factors. Lupus. 2018;27:744-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Chandley MJ, Szebeni A, Szebeni K, Crawford JD, Stockmeier CA, Turecki G, Kostrzewa RM, Ordway GA. Elevated gene expression of glutamate receptors in noradrenergic neurons from the locus coeruleus in major depression. Int J Neuropsychopharmacol. 2014;17:1569-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 76. | Pacheco A, Aguayo FI, Aliaga E, Muñoz M, García-Rojo G, Olave FA, Parra-Fiedler NA, García-Pérez A, Tejos-Bravo M, Rojas PS, Parra CS, Fiedler JL. Chronic Stress Triggers Expression of Immediate Early Genes and Differentially Affects the Expression of AMPA and NMDA Subunits in Dorsal and Ventral Hippocampus of Rats. Front Mol Neurosci. 2017;10:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 77. | Sathyanesan M, Haiar JM, Watt MJ, Newton SS. Restraint stress differentially regulates inflammation and glutamate receptor gene expression in the hippocampus of C57BL/6 and BALB/c mice. Stress. 2017;20:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 78. | Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1774-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 317] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 79. | Chen MH, Kao CF, Tsai SJ, Li CT, Lin WC, Hong CJ, Bai YM, Tu PC, Su TP. Treatment response to low-dose ketamine infusion for treatment-resistant depression: A gene-based genome-wide association study. Genomics. 2021;113:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Weder N, Zhang H, Jensen K, Yang BZ, Simen A, Jackowski A, Lipschitz D, Douglas-Palumberi H, Ge M, Perepletchikova F, O'Loughlin K, Hudziak JJ, Gelernter J, Kaufman J. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. J Am Acad Child Adolesc Psychiatry. 2014;53:417-24.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 81. | Kaut O, Schmitt I, Hofmann A, Hoffmann P, Schlaepfer TE, Wüllner U, Hurlemann R. Aberrant NMDA receptor DNA methylation detected by epigenome-wide analysis of hippocampus and prefrontal cortex in major depression. Eur Arch Psychiatry Clin Neurosci. 2015;265:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 82. | Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, Jurjus GJ, Dieter L, Duman RS. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol. 2013;16:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 83. | Adell A. Brain NMDA Receptors in Schizophrenia and Depression. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 84. | McKendrick G, Graziane NM. Drug-Induced Conditioned Place Preference and Its Practical Use in Substance Use Disorder Research. Front Behav Neurosci. 2020;14:582147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 85. | O'Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES. AMPA receptor potentiators for the treatment of CNS disorders. Curr Drug Targets CNS Neurol Disord. 2004;3:181-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 86. | Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator (LY392098). Neuropharmacology. 2001;40:1028-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 185] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 87. | Alshammari TK. The Ketamine Antidepressant Story: New Insights. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 88. | Widman AJ, McMahon LL. Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci U S A. 2018;115:E3007-E3016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 89. | Koike H, Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res. 2014;271:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 90. | Pandis C, Sotiriou E, Kouvaras E, Asprodini E, Papatheodoropoulos C, Angelatou F. Differential expression of NMDA and AMPA receptor subunits in rat dorsal and ventral hippocampus. Neuroscience. 2006;140:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 91. | Chiesa A, Crisafulli C, Porcelli S, Han C, Patkar AA, Lee SJ, Park MH, Jun TY, Serretti A, Pae CU. Influence of GRIA1, GRIA2 and GRIA4 polymorphisms on diagnosis and response to treatment in patients with major depressive disorder. Eur Arch Psychiatry Clin Neurosci. 2012;262:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | Pochwat B, Nowak G, Szewczyk B. An update on NMDA antagonists in depression. Expert Rev Neurother. 2019;19:1055-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 93. | Nowak G, Li Y, Paul IA. Adaptation of cortical but not hippocampal NMDA receptors after chronic citalopram treatment. Eur J Pharmacol. 1996;295:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 94. | Horstmann S, Lucae S, Menke A, Hennings JM, Ising M, Roeske D, Müller-Myhsok B, Holsboer F, Binder EB. Polymorphisms in GRIK4, HTR2A, and FKBP5 show interactive effects in predicting remission to antidepressant treatment. Neuropsychopharmacology. 2010;35:727-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 95. | Serretti A, Chiesa A, Crisafulli C, Massat I, Linotte S, Calati R, Kasper S, Bailer U, Lecrubier Y, Fink M, Antonijevic I, Forray C, Snyder L, Bollen J, Zohar J, De Ronchi D, Souery D, Mendlewicz J. Failure to replicate influence of GRIK4 and GNB3 polymorphisms on treatment outcome in major depression. Neuropsychobiology. 2012;65:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 96. | Ho MF, Correia C, Ingle JN, Kaddurah-Daouk R, Wang L, Kaufmann SH, Weinshilboum RM. Ketamine and ketamine metabolites as novel estrogen receptor ligands: Induction of cytochrome P450 and AMPA glutamate receptor gene expression. Biochem Pharmacol. 2018;152:279-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | Elhussiny MEA, Carini G, Mingardi J, Tornese P, Sala N, Bono F, Fiorentini C, La Via L, Popoli M, Musazzi L, Barbon A. Modulation by chronic stress and ketamine of ionotropic AMPA/NMDA and metabotropic glutamate receptors in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2021;104:110033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |