Published online Jun 19, 2022. doi: 10.5498/wjp.v12.i6.779
Peer-review started: January 6, 2022
First decision: March 13, 2022
Revised: April 12, 2022
Accepted: May 14, 2022
Article in press: May 14, 2022
Published online: June 19, 2022
Processing time: 158 Days and 23.7 Hours
As a common and serious psychiatric disorder, depression significantly affects psychosocial functioning and quality of life. However, the mechanism of depression is still enigmatic and perplexing, which limits its precise and effective therapeutic methods. Recent studies demonstrated that neuroinflammation activation plays an important role in the pathophysiology of depression. In this respect, high mobility group box 1 (HMGB1) may be a possible signaling inducer of neuroinflammation and can be a potential mechanistic and therapeutic target for depression. Herein, we review recent studies on the mechanistic and therapeutic targets of HMGB1 in depression and propose potential perspectives on this topic.
Core Tip: Limited reviews in the literature contributed to the high mobility group box 1 (HMGB1) in depression. This review provides a comprehensive mechanistic and therapeutic perspective on this topic and proposed that the future perspectives of HMGB1 in depression should be understanding the full signaling pathway of HMGB1 in depression, deeply investigating potential HMGB1 related therapeutic targets, and exploring the role of HMGB1 in depression and combined disease.
- Citation: Wang S, Guan YG, Zhu YH, Wang MZ. Role of high mobility group box protein 1 in depression: A mechanistic and therapeutic perspective. World J Psychiatry 2022; 12(6): 779-786
- URL: https://www.wjgnet.com/2220-3206/full/v12/i6/779.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i6.779
Depression is one of the most common, serious, and costly psychiatric disorders that affect psychosocial functioning and quality of life[1]. The aggregate point, one-year, and lifetime prevalence of depression in the community is about 12.9%, 7.2%, and 10.8%, respectively[2]. Crucially, the number of people suffering from depression worldwide has increased from 172 million in 1990 to 258 million in 2017, with an increase of 49.86%[3,4]. The stress of the outbreak of coronavirus disease 2019 and interpersonal isolation makes it even more people suffer depression recently[5-7]. However, the mechanism of depression is still enigmatic and perplexing, which limits its precise and effective therapeutic methods[8,9]. Recent studies demonstrated that the activation of neuroinflammation might play an important role in the pathophysiology of depression[10]. High mobility group box 1 (HMGB1), a chromosomal protein, has been found to perform an essential job in the neuroinflammation of several central nervous system diseases, which might also be a potential therapeutic target[11-15]. Rana et al[11] proposed that HMGB1-mediated neuroinflammation in depression could have insights into the pathogenesis understanding and therapeutic promise. Herein, with recent studies concerning this topic, we review the role of HMGB1 in depression and propose several potential key mechanistic and future therapeutic perspectives.
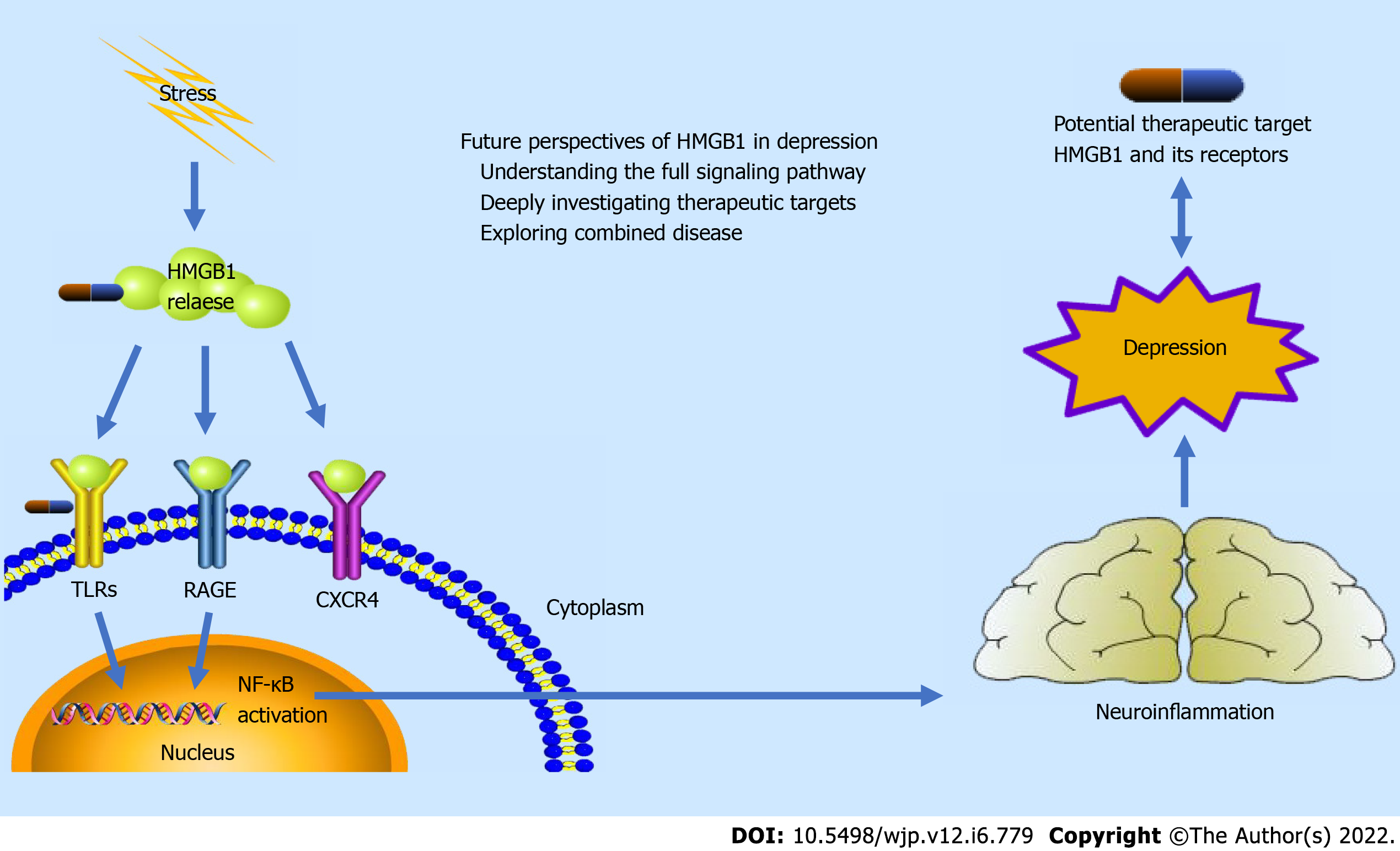
HMGB1 is the most researched protein in the HMGB family for inflammation as innate immune responses[16]. Expressed in nearly all eukaryotic cells, HMGB1 is a kind of chromatin-binding molecule to function in chromatin remodeling in the nucleus under normal physiological situations[13]. Whereas in stressful situations or pathological situations, caused by immune and other cells or cell injury and death, HMGB1 secretes or translates from nuclei to the cytoplasm and eventually excretes or releases to the extracellular milieu, acting as a mediator of inflammation extracellularly[17]. Placing on extracellular milieu, HMGB1 is acknowledged by plenty of binding receptors, mainly including Toll-like receptors (TLRs) and receptors for advanced glycation end products (RAGE), resulting in the expression of proinflammatory response elements and eventually in the inflammatory cascade[18]. The TLRs and RAGE are transmembrane proteins, which are located in the membrane of several cells such as monocytes, macrophages, dendritic cells, and neural cells[12]. For the HMGB1-TLRs pathway (mainly including TLR2 and TLR4), MyD88 dependent and independent pathways were activated, resulting in the simulation of NF-κB and induction of pro-inflammatory response[19]. For MyD88-dependent pathway, MyD88 serves as a domain-containing adaptor for the cytoplasmic Toll/ interleukin (IL)-1 receptor[20]. Stimulated by ligands, MyD88 recruits IL-1 receptor-associated kinase-4 (IRAK-4) to TLRs; and IRAK-1 is phosphorylated and then associates with TRAF6, thereby activating the IKK complex and leading to activation of MAP kinases (JNK, p38 MAPK) and NF-κB[21,22]. The MyD88-independent pathway also mediates the immune response via TRIF and TRAF3, leading to recruitment of IKKε/TBK1, phosphorylation of IRF3, and expression of interferon-β[23,24]. A recent study also indicated that the HMGB1-TLR4 pathway could activate Nod-like receptor protein 3 (NLRP3) inflammasome and then enhanced the production of IL-1β[25]. For the HMGB1-RAGE pathway, the downstream signaling is propagated by the Akt, MAPK, ERK, JAK-STAT1, and Rac pathways, ultimately promoting the activation of NF-κB and expression of the proinflammatory cytokines and chemokines, which contributes in the immune cells’ maturation and migration and surface receptors’ expression[26,27]. Fully reduced HMGB1 (fr-HMGB1), which is a kind of three redox states (fr-HMGB1, disulfide HMGB1, and sulfonyl HMGB1), can act as a chemoattractant through connections with RAGE[28]. Furthermore, binding with C-X-C motif chemokine receptor 4, promotes chemotactic activity (stimulates leukocyte recruitment)[29]. It should be noted that such inflammation activation can lead to inflammatory responses and cell injury and death, which promotes the further release of HMGB1 and upgrade of its receptors[30]. This may contribute to the aggravation and drug-resistance of HMGB1 related disease[15].
More recently, neuroinflammation has been proposed to play a significant role in several diseases including depression, epilepsy, stroke, traumatic brain injury, Parkinson's disease, and Alzheimer's disease[11-15]. HMGB1 is considered as an essential neuroinflammatory facilitator, which is released by glial cells and neurons upon inflammasome activation and acts as a pro-inflammatory cytokine[15]. Neurons are considered as a primary and necessary driver of neuroinflammation through release of HMGB1, with the subsequent amplification via recruitment of immunocompetent cells, including microglia and astrocytes[31]. HMGB1 has been proved that it releases from neurons in many central nervous system (CNS) diseases and then triggeres neuroinflammation as an upstream inflammatory mediator[15,32]. Activated by HMGB1, microglia functions as key contributor of the inflammatory processes sequentially influences neural cells, following by the activation of microglial NF-κB pathway and production of pro-inflammatory cytokines[33]. The study of Gao et al[34] using a Parkinson's disease model revealed that HMGB1 released from inflamed microglia and/or degenerating neurons, bound to microglial Mac1 and activated NF-κB pathway and nicotinamide adenine dinucleotide phosphate oxidase to stimulate production of multiple inflammatory and neurotoxic factors. Astrocytes are also a population of CNS cells with distinctive morphology and functions. Xiao et al[35] suggested that HMGB1 promoted the release of sonic hedgehog from astrocytes through signal pathway JNK, p38 and stat3 mediated by receptor RAGE in an animal model of multiple sclerosis, suggesting the important role of HMGB1-astrocytes medicated neuroinflammation. Also, some types of reactive astrocytes can also be induced by activated neuroinflammatory microglia and take parts in various human neurodegenerative diseases, formulating a complex immune network[36].
Depression is also found to closely link with neuroinflammation, which is mainly characterized by the increased mediators of inflammation and neurodegeneration[37]. Depressed patients have been found to have higher levels of proinflammatory cytokines, acute phase proteins, chemokines and cellular adhesion molecules, including IL-1β, IL-6, TNF-α and CRP[38,39]. Preclinical study based on animals also exhibited the activation of microglia together with enhanced inflammatory mediators. In a chronic mild stress (CMS) mouse model of depression, NLRP3-inflammasome/caspase-1/IL-1β axis microglia-mediated neuroinflammation was found being activated[40]. Another study suggested rats exposed to CMS exhibited a significant increase in inflammatory mediators, including TNF-α and IL-1β, activation of NF-κB signaling pathway in the hippocampus. Icariin, a flavonoid inhibiting neuroinflammation, could negatively regulated the activation of the NLRP3 inflammasome/caspase-1/IL-1β[41]. Chronic treatment with corticosterone and intraperitoneally administration of lipopolysaccharide depressed models also showed a higher expression level of pro-inflammatory phenotype characterized by IL-1β, IL-6, TNF-α and IκB-α[42,43]. These findings provide a powerful connection of neuroinflammation and depression.
Concerning HMGB1 medicates depression, recent studies suggested that HMGB1 might be a probable inducer of stress-mediated neuroinflammation in depression[11]. It has been proven that HMGB1 could activate neuroinflammatory reactions by inducing TNF-α to exhibit anhedonia-like behavior[44]. Based on the inescapable tail shock rats’ model, Weber et al[45] indicated that HMGB1 in the brain is a probable inducer of stress-mediated microglial priming by acting on the NLRP3 inflammasome and pro-inflammatory cytokines. Based on recent studies, stress-mediated depression-like behaviors were found to be induced by HMGB1 and glycogen synthase kinase-3 dependent TLR4 signaling, resulting in the activation of NF-κB and NLRP3 inflammasome; and the HMGB1 was additionally promoted in mice[46]. This stress-induced neuroinflammation can further make it more susceptible to depression[47]. Based on rats’ chronic unpredictable stress (CUS)-induced behavioral deficits, Franklin et al[48] discovered that CUS caused consistent upregulation of HMGB1 mRNA and RAGE mRNA in hippocampal microglia. They also found that HMGB1 infusion into the hippocampus caused anhedonic behavior and suggested that HMGB1-RAGE increased vulnerability to depressive-like behaviors long[10,48]. In addition, HMGB1 could also induce depressive behaviors by limiting the kynurenine pathway via suppression of activated enzymes[37]. Furthermore, based on preclinical studies, neuroinflammation induced by HMGB1 can mediate depressive behaviors such as reduction of locomotor activity and sucrose preference, which are analogs to the motivational deficits in depression[49,50].
Current studies proposed that interventions in the HMGB1 and related molecular in its neuroinflammation pathways have the potential to be a therapeutic target in several diseases like depression, epilepsy, cancers, stroke, and other local and systemic neuroinflammatory diseases[4,13,15]. The main potential therapeutic targets include anti-HMGB1 monoclonal antibody (mAb), HMGB1 inhibitors, and HMGB1 receptors and its related molecular in neuroinflammation pathway[15].
For depression, although several studies provided evidence on mediating HMGB1, this topic is still needing more effort. Traditionally commonly used anti-depression drugs based on the theory of serotonin-like selective serotonin reuptake inhibitors (SSRIs) as well as serotonin and norepinephrine reuptake inhibitors may be complicated to treat motivational deficits symptoms, suggesting additional neurotransmitters like dopamine dysfunction might be involved[51,52]. Also, recent studies suggested the anti-inflammatory and anti-oxidative effects may be one of the potential mechanisms of these anti-depression drugs[53]. Only limited studies are based on animal models concerning the therapeutic target of HMGB1 in anti-depression. Liu et al[50] and Fu et al[54] indicated that the anti-depressive-like behavior compound Hesperidin and Baicalin reduced the CUS-induced model by inhibition of neuroinflammatory actions via HMGB1-TLR4-NF-κB pathway or HMGB1-RAGE-NF-κB pathway. The anti-HMGB1 mAb is highly specific for HMGB1, which shows the value of target validation but also has potential for the treatment of neuroinflammation diseases, including depression[14]. Hisaoka-Nakashima et al[55] used the model of partial sciatic nerve ligation to introduce neuropathic pain and anxiodepressive-like behaviors in mice. They observed increased HMGB1 and microglia activation in the frontal cortex. Anti-HMGB1 mAb and glycyrrhizic acid (HMGB1 inhibitor) can reduce microglia activation and anxiodepressive-like behavior[55]. HMGB1 inhibitors can be another possible anti-HMGB1 strategy. Based on the CUS-induced model both in vivo and in vitro, glycyrrhizic acid, the inhibitor of HMGB1, may restrain HMGB1 thus improving depressive-like behaviors through regulating the kynurenine pathway[47,56]. Encouragingly, a recent clinical trial found that depressive symptoms are relieved more in SSRI+ glycyrrhizic acid than SSRI+ placebo, proving anti-inflammatory agents is effective in clinical use[57]. The HMGB1 receptors and their related molecular in neuroinflammation pathway can also be a therapeutic target. Cheng et al[46] based on an inescapable tail shock model suggested that TLR4 knockout mice were resistant to learned helplessness and GSK3 (a TLR4 signaling dependent kinase) inhibitor TDZD-8 reduced the stress-induced increases of hippocampal cytokines and chemokines.
Based on current studies, HMGB1 may be a possible signaling inducer of stress-mediated depression behaviors via HMGB1-TLRs signaling and HMGB1-RAGE signaling, followed by the activation of NF-κB and expression of the proinflammatory cytokines and chemokines, resulting in the neuroinflammation procedure[10]. This pathway suggests a possible mechanistic direction for depression. HMGB1 can also be a potential therapeutic target in depression by playing an important role in neurotransmitter-related anti-depression drugs, anti-HMGB1 mAb, HMGB1 inhibitors, and HMGB1 receptors[11]. However, there are still many challenges in further exploring HMGB1 as a potential mechanistic and therapeutic direction. The mechanistic illustration of HMGB1 in depression is provided as Figure 1.
As a multifunctional protein, HMGB1 has been extensively researched. Encouragingly, cell stresses and plenty of disease processes are found related to this important inflammation facilitator[58]. HMGB1 needs to translate from nuclei to the cytoplasm and eventually release to the extracellular milieu for its inflammatory function[16]. Thus, location and translocation are the keys to function[59]. The potential mechanism of HMGB1 translocation, however, is still not clear and requires further exploration in depression. Furthermore, most current studies only focus on the release of HMGB1 in neuroglial cells (especially neuroimmune cells), which requires more studies on the role of other neuronal cells[11]. Another potential perspective is about HMGB1 receptors. The different functions, distribution, and potential relationship among various HMGB1 receptors emerge as a research focus in inflammation[60,61]. For depression, the mechanism of neurotransmitters and neuroinflammation may make this question more interesting and meaningful[53,62]. It has to be admitted that only limited studies explored some parts of the HMGB1 as a mechanism in depression. There still is a long way to understand the full signaling pathway of HMGB1 and the complete mechanism of depression introduced by the neuroinflammation.
Although several studies indicated potential therapeutic targets as neurotransmitter-related anti-depression drugs, anti-HMGB1 mAb, HMGB1 inhibitors, and HMGB1 receptors, current researches are far from enough to provide evidence for potential therapy or clinical application[11]. The potential anti-inflammatory mechanisms and collaboration of neurotransmitter-related anti-depression drugs might be a clinically translational direction[53]. Furthermore, some anti-inflammatory and antiapoptotic compounds [such as (–)-Epigallocatechin-3-gallate and different microRNAs] can inhibit the HMGB1-NF-κB signaling pathway, which showed great potential in therapy of other HMGB1 related diseases[15]. These may also show effects in depression, which deserves further researches. The future need of the research is to deeply investigate potential HMGB1 related therapeutic targets using different animal models[63]. The ultimate goal is for contribution to human clinical applications, while the clinical study on glycyrrhizic acid (HMGB1 inhibitor) as an adjunctive treatment for depression is a meaningful attempt[57].
It is commonly observed in clinical practice that depression may combine with other diseases, such as epilepsy, stroke, and heart disease[64-66]. HMGB1 is found widely participating in different inflammation-related diseases on the nervous system, circulatory system, and others[13]. Thus, HMGB1 may be at the crossroads of depression and other combined diseases. These diseases may have consistent or similar pathogenesis as HMGB1 and response to the same therapeutic target on HMGB1. The researches and discussion of HMGB1 as a potential common mechanistic and therapeutic direction in depression and combined inflammation-related disease may be meaningful and beneficial. Figure 1 shows an illustration of mechanistic and therapeutic perspective of HMGB1 in depression.
Neuroinflammation activation plays an important role in the pathophysiology of depression. Playing an important role in neuroinflammation activation, HMGB1 may be a possible signaling inducer of depression. HMGB1 can also be a potential therapeutic target in depression by playing an important role in neurotransmitter-related anti-depression drugs, anti-HMGB1 mAb, HMGB1 inhibitors, and HMGB1 receptors. However, there are still many challenges in further exploring HMGB1 as a potential mechanistic and therapeutic direction. The future perspectives of HMGB1 in depression are understanding the full signaling pathway of HMGB1 in depression, deeply investigating potential HMGB1 related therapeutic targets, and exploring the role of HMGB1 in depression and combined disease.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Asmamaw M, Ethiopia; Ferreira LPS, Brazil A-Editor: Zhu JQ S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Tran BX, Ha GH, Nguyen DN, Nguyen TP, Do HT, Latkin CA, Ho CSH, Ho RCM. Global mapping of interventions to improve quality of life of patients with depression during 1990-2018. Qual Life Res. 2020;29:2333-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. Prevalence of Depression in the Community from 30 Countries between 1994 and 2014. Sci Rep. 2018;8:2861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1088] [Cited by in RCA: 928] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 3. | GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9354] [Cited by in RCA: 8390] [Article Influence: 1198.6] [Reference Citation Analysis (4)] |
| 4. | Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J Psychiatr Res. 2020;126:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 787] [Article Influence: 157.4] [Reference Citation Analysis (0)] |
| 5. | Wang S, Zhang Y, Ding W, Meng Y, Hu H, Liu Z, Zeng X, Wang M. Psychological distress and sleep problems when people are under interpersonal isolation during an epidemic: A nationwide multicenter cross-sectional study. Eur Psychiatry. 2020;63:e77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (4)] |
| 6. | Wang S, Zhang Y, Guan Y, Ding W, Meng Y, Hu H, Liu Z, Zeng X, Wang M. A nationwide evaluation of the prevalence of and risk factors associated with anxiety, depression and insomnia symptoms during the return-to-work period of coronavirus disease 2019 in China. Soc Psychiatry Psychiatr Epidemiol. 2021;56:2275-2286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Wang S, Ding W, Meng Y, Hu H, Liu Z, Zeng X, Guan Y, Wang M. Status and influential factors of anxiety depression and insomnia symptoms in the work resumption period of COVID-19 epidemic: A multicenter cross-sectional study. J Psychosom Res. 2020;138:110253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | do Prado-Lima PAS, Costa-Ferro ZSM, Souza BSF, da Cruz IBM, Lab B. Is there a place for cellular therapy in depression? World J Psychiatry. 2021;11:553-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Onaolapo AY, Onaolapo OJ. Glutamate and depression: Reflecting a deepening knowledge of the gut and brain effects of a ubiquitous molecule. World J Psychiatry. 2021;11:297-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (3)] |
| 10. | Franklin TC, Xu C, Duman RS. Depression and sterile inflammation: Essential role of danger associated molecular patterns. Brain Behav Immun. 2018;72:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 11. | Rana T, Behl T, Mehta V, Uddin MS, Bungau S. Molecular insights into the therapeutic promise of targeting HMGB1 in depression. Pharmacol Rep. 2021;73:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Paudel YN, Shaikh MF, Chakraborti A, Kumari Y, Aledo-Serrano Á, Aleksovska K, Alvim MKM, Othman I. HMGB1: A Common Biomarker and Potential Target for TBI, Neuroinflammation, Epilepsy, and Cognitive Dysfunction. Front Neurosci. 2018;12:628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 13. | Vijayakumar EC, Bhatt LK, Prabhavalkar KS. High Mobility Group Box-1 (HMGB1): A Potential Target in Therapeutics. Curr Drug Targets. 2019;20:1474-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Nishibori M, Mori S, Takahashi HK. Anti-HMGB1 monoclonal antibody therapy for a wide range of CNS and PNS diseases. J Pharmacol Sci. 2019;140:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Wang S, Guan Y, Li T. The Potential Therapeutic Role of the HMGB1-TLR Pathway in Epilepsy. Curr Drug Targets. 2021;22:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, Savitsky D, Ronfani L, Akira S, Bianchi ME, Honda K, Tamura T, Kodama T, Taniguchi T. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 546] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 17. | Andersson U, Yang H, Harris H. Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Expert Opin Ther Targets. 2018;22:263-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 226] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 18. | Andersson U, Yang H, Harris H. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Semin Immunol. 2018;38:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 224] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 19. | Zuo T, Yue Y, Wang X, Li H, Yan S. Luteolin Relieved DSS-Induced Colitis in Mice via HMGB1-TLR-NF-κB Signaling Pathway. Inflammation. 2021;44:570-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 565] [Cited by in RCA: 516] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 21. | Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5843] [Cited by in RCA: 6700] [Article Influence: 446.7] [Reference Citation Analysis (0)] |
| 22. | Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1230] [Article Influence: 175.7] [Reference Citation Analysis (0)] |
| 23. | Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2477] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 24. | Fitzgerald KA, Kagan JC. Toll-like Receptors and the Control of Immunity. Cell. 2020;180:1044-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 1380] [Article Influence: 276.0] [Reference Citation Analysis (0)] |
| 25. | Kim EJ, Park SY, Baek SE, Jang MA, Lee WS, Bae SS, Kim K, Kim CD. HMGB1 Increases IL-1β Production in Vascular Smooth Muscle Cells via NLRP3 Inflammasome. Front Physiol. 2018;9:313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Hudson BI, Lippman ME. Targeting RAGE Signaling in Inflammatory Disease. Annu Rev Med. 2018;69:349-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 365] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 27. | Massey N, Puttachary S, Bhat SM, Kanthasamy AG, Charavaryamath C. HMGB1-RAGE Signaling Plays a Role in Organic Dust-Induced Microglial Activation and Neuroinflammation. Toxicol Sci. 2019;169:579-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A, Raeli L, Shams SS, Yang H, Varani L, Andersson U, Tracey KJ, Bachi A, Uguccioni M, Bianchi ME. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 659] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 29. | Tirone M, Tran NL, Ceriotti C, Gorzanelli A, Canepari M, Bottinelli R, Raucci A, Di Maggio S, Santiago C, Mellado M, Saclier M, François S, Careccia G, He M, De Marchis F, Conti V, Ben Larbi S, Cuvellier S, Casalgrandi M, Preti A, Chazaud B, Al-Abed Y, Messina G, Sitia G, Brunelli S, Bianchi ME, Vénéreau E. High mobility group box 1 orchestrates tissue regeneration via CXCR4. J Exp Med. 2018;215:303-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 30. | Kim SW, Lee H, Lee HK, Kim ID, Lee JK. Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol Commun. 2019;7:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 31. | Yang H, Andersson U, Brines M. Neurons Are a Primary Driver of Inflammation via Release of HMGB1. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Sun Q, Wu W, Hu YC, Li H, Zhang D, Li S, Li W, Li WD, Ma B, Zhu JH, Zhou ML, Hang CH. Early release of high-mobility group box 1 (HMGB1) from neurons in experimental subarachnoid hemorrhage in vivo and in vitro. J Neuroinflammation. 2014;11:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 33. | Brevet M, Kojima H, Asakawa A, Atsuchi K, Ushikai M, Ataka K, Inui A, Kimura H, Sevestre H, Fujimiya M. Chronic foot-shock stress potentiates the influx of bone marrow-derived microglia into hippocampus. J Neurosci Res. 2010;88:1890-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci. 2011;31:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 282] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 35. | Xiao Y, Sun Y, Liu W, Zeng F, Shi J, Li J, Chen H, Tu C, Xu Y, Tan Z, Gong F, Shu X, Zheng F. HMGB1 Promotes the Release of Sonic Hedgehog From Astrocytes. Front Immunol. 2021;12:584097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3268] [Cited by in RCA: 5244] [Article Influence: 655.5] [Reference Citation Analysis (0)] |
| 37. | Beumer W, Gibney SM, Drexhage RC, Pont-Lezica L, Doorduin J, Klein HC, Steiner J, Connor TJ, Harkin A, Versnel MA, Drexhage HA. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. J Leukoc Biol. 2012;92:959-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 282] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 38. | Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1963] [Cited by in RCA: 2159] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 39. | Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, Ho RC. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer's disease: Systematic Review and Meta-Analysis. Sci Rep. 2018;8:12050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 407] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 40. | Lu M, Yang JZ, Geng F, Ding JH, Hu G. Iptakalim confers an antidepressant effect in a chronic mild stress model of depression through regulating neuro-inflammation and neurogenesis. Int J Neuropsychopharmacol. 2014;17:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Liu B, Xu C, Wu X, Liu F, Du Y, Sun J, Tao J, Dong J. Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience. 2015;294:193-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 42. | Chabry J, Nicolas S, Cazareth J, Murris E, Guyon A, Glaichenhaus N, Heurteaux C, Petit-Paitel A. Enriched environment decreases microglia and brain macrophages inflammatory phenotypes through adiponectin-dependent mechanisms: Relevance to depressive-like behavior. Brain Behav Immun. 2015;50:275-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 43. | Bay-Richter C, Janelidze S, Hallberg L, Brundin L. Changes in behaviour and cytokine expression upon a peripheral immune challenge. Behav Brain Res. 2011;222:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Wu TY, Liu L, Zhang W, Zhang Y, Liu YZ, Shen XL, Gong H, Yang YY, Bi XY, Jiang CL, Wang YX. High-mobility group box-1 was released actively and involved in LPS induced depressive-like behavior. J Psychiatr Res. 2015;64:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF. Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: a priming stimulus of microglia and the NLRP3 inflammasome. J Neurosci. 2015;35:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 46. | Cheng Y, Pardo M, Armini RS, Martinez A, Mouhsine H, Zagury JF, Jope RS, Beurel E. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav Immun. 2016;53:207-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 47. | Wang B, Lian YJ, Dong X, Peng W, Liu LL, Su WJ, Gong H, Zhang T, Jiang CL, Li JS, Wang YX. Glycyrrhizic acid ameliorates the kynurenine pathway in association with its antidepressant effect. Behav Brain Res. 2018;353:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Franklin TC, Wohleb ES, Zhang Y, Fogaça M, Hare B, Duman RS. Persistent Increase in Microglial RAGE Contributes to Chronic Stress-Induced Priming of Depressive-like Behavior. Biol Psychiatry. 2018;83:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 49. | Aucott H, Sowinska A, Harris HE, Lundback P. Ligation of free HMGB1 to TLR2 in the absence of ligand is negatively regulated by the C-terminal tail domain. Mol Med. 2018;24:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Liu L, Dong Y, Shan X, Li L, Xia B, Wang H. Anti-Depressive Effectiveness of Baicalin In Vitro and In Vivo. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 51. | Yohn SE, Errante EE, Rosenbloom-Snow A, Somerville M, Rowland M, Tokarski K, Zafar N, Correa M, Salamone JD. Blockade of uptake for dopamine, but not norepinephrine or 5-HT, increases selection of high effort instrumental activity: Implications for treatment of effort-related motivational symptoms in psychopathology. Neuropharmacology. 2016;109:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 52. | Randall PA, Lee CA, Podurgiel SJ, Hart E, Yohn SE, Jones M, Rowland M, López-Cruz L, Correa M, Salamone JD. Bupropion increases selection of high effort activity in rats tested on a progressive ratio/chow feeding choice procedure: implications for treatment of effort-related motivational symptoms. Int J Neuropsychopharmacol. 2014;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 53. | Gałecki P, Mossakowska-Wójcik J, Talarowska M. The anti-inflammatory mechanism of antidepressants - SSRIs, SNRIs. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 54. | Fu H, Liu L, Tong Y, Li Y, Zhang X, Gao X, Yong J, Zhao J, Xiao D, Wen K, Wang H. The antidepressant effects of hesperidin on chronic unpredictable mild stress-induced mice. Eur J Pharmacol. 2019;853:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 55. | Hisaoka-Nakashima K, Tomimura Y, Yoshii T, Ohata K, Takada N, Zhang FF, Nakamura Y, Liu K, Wake H, Nishibori M, Nakata Y, Morioka N. High-mobility group box 1-mediated microglial activation induces anxiodepressive-like behaviors in mice with neuropathic pain. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:347-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 56. | Wang B, Lian YJ, Su WJ, Peng W, Dong X, Liu LL, Gong H, Zhang T, Jiang CL, Wang YX. HMGB1 mediates depressive behavior induced by chronic stress through activating the kynurenine pathway. Brain Behav Immun. 2018;72:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 57. | Cao ZY, Liu YZ, Li JM, Ruan YM, Yan WJ, Zhong SY, Zhang T, Liu LL, Wu R, Wang B, Wang W, Bi XY, Wang YX, Su WJ, Jiang CL. Glycyrrhizic acid as an adjunctive treatment for depression through anti-inflammation: A randomized placebo-controlled clinical trial. J Affect Disord. 2020;265:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 58. | Yang H, Wang H, Andersson U. Targeting Inflammation Driven by HMGB1. Front Immunol. 2020;11:484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 409] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 59. | Deng M, Scott MJ, Fan J, Billiar TR. Location is the key to function: HMGB1 in sepsis and trauma-induced inflammation. J Leukoc Biol. 2019;106:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 60. | Nogueira-Machado JA, Volpe CM, Veloso CA, Chaves MM. HMGB1, TLR and RAGE: a functional tripod that leads to diabetic inflammation. Expert Opin Ther Targets. 2011;15:1023-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 61. | Gąsiorowski K, Brokos B, Echeverria V, Barreto GE, Leszek J. RAGE-TLR Crosstalk Sustains Chronic Inflammation in Neurodegeneration. Mol Neurobiol. 2018;55:1463-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 62. | Beurel E, Toups M, Nemeroff CB. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron. 2020;107:234-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 1217] [Article Influence: 243.4] [Reference Citation Analysis (0)] |
| 63. | Hao Y, Ge H, Sun M, Gao Y. Selecting an Appropriate Animal Model of Depression. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 64. | Tao K, Wang X. The comorbidity of epilepsy and depression: diagnosis and treatment. Expert Rev Neurother. 2016;16:1321-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Shao A, Lin D, Wang L, Tu S, Lenahan C, Zhang J. Oxidative Stress at the Crossroads of Aging, Stroke and Depression. Aging Dis. 2020;11:1537-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 66. | Carney RM, Freedland KE. Depression and coronary heart disease. Nat Rev Cardiol. 2017;14:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 424] [Article Influence: 47.1] [Reference Citation Analysis (0)] |