Published online Apr 19, 2022. doi: 10.5498/wjp.v12.i4.558
Peer-review started: March 18, 2021
First decision: July 15, 2021
Revised: August 20, 2021
Accepted: February 22, 2022
Article in press: February 22, 2022
Published online: April 19, 2022
Processing time: 391 Days and 0.8 Hours
Anorexia nervosa (AN) is a disabling, costly and potentially deadly illness. Treatment failure and relapse are common after completing treatment, and a substantial proportion of patients develop severe and enduring AN. The time from AN debut to the treatment initiation is normally unreasonably long. Over the past 20 years there has been empirical support for the efficacy of several treatments for AN. Moreover, outpatient treatment with family-based therapy or individual psychotherapy is associated with good outcomes for a substantial proportion of patients. Early intervention improves outcomes and should be a priority for all patients. Outpatient treatment is usually the best format for early intervention, and it has been demonstrated that even patients with severe or extreme AN can be treated as outpatients if they are medically stable. Inpatient care is more disruptive, more costly, and usually has a longer waiting list than does outpatient care. The decision as to whether to proceed with outpatient treatment or to transfer the patient for inpatient therapy may be difficult. The core aim of this opinion review is to provide the knowledge base needed for performing safe outpatient treatment of AN. The scientific essentials for outpatient treatment are described, including how to assess and manage the medical risks of AN and how to decide when transition to inpatient care is indicated. The following aspects are discussed: early intervention, outpatient treatment of AN, including outpatient psychotherapy for severe and extreme AN, how to determine when outpatient treatment is safe, and when transfer to inpatient healthcare is indicated. Emerging treatments, ethical issues and outstanding research questions are also addressed.
Core Tip: Outpatient psychotherapy is the mainstay of treatment of anorexia nervosa. Both early intervention and healthcare for severe and enduring anorexia nervosa are mainly performed in outpatient clinics. Even in severe and extreme anorexia nervosa outpatient psychotherapy is an alternative to inpatient treatment when the patient is medically stable. Medical management is essential for safe outpatient therapy. In this opinion review essentials in outpatient healthcare and medical management are discussed. Emerging therapies and outstanding research issues are addressed.
- Citation: Frostad S, Bentz M. Anorexia nervosa: Outpatient treatment and medical management. World J Psychiatry 2022; 12(4): 558-579
- URL: https://www.wjgnet.com/2220-3206/full/v12/i4/558.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i4.558
Anorexia nervosa (AN) is characterized by starvation, malnutrition, fear of weight gain and/or a disturbed body image, and severe dietary restriction or other weight-loss behaviors (e.g., purging, excessive physical activity). There are two subtypes of this condition: binge eating with purging (or only purging), and food restricting only[1,2]. Patients usually have a low body mass index (BMI), but some patients with rapid weight loss have a clinical picture of AN with a BMI within the normal range[3]. In addition, cognitive and emotional functioning are often markedly disturbed[4,5]. The prognosis is poor for a substantial proportion of patients[6], and mortality rates are high[7]. The comparative efficacy of available treatments is described in recent systematic reviews and meta-analyses[1,4]. The core aim of the present opinion review is to present an overview of the scientific essentials for AN outpatient treatment, including how to assess and manage the medical risks of AN, and how to decide when transition to inpatient care is indicated. Emerging therapies and outstanding research questions are addressed.
The diagnostic criteria for AN in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) are provided in Table 1[2]. Diagnosing AN is usually straightforward, although sometimes the use of additional informants such as parents is necessary[4].
| Diagnostic variable | |
| Diagnostic criteria | (1) Restriction of energy intake relative to requirements in anorexia nervosa leads to significantly low body weight for the patient´s age, sex, developmental trajectory and physical health. Significantly low weight is defined as a weight that is less than the minimal normal weight or (in children and adolescents) less than the minimum expected weight; (2) Intense fear of gaining weight or of becoming fat, or persistent behaviour that interferes with weight gain, even though the patient has a significantly low weight; and (3) Disturbance in the way in which one´s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or persistent lack of recognition of the seriousness of the current low body weight |
| Subtype designation | Restricting subtype: During the past 3 mo, the patient has not engaged in recurrent episodes of binge-eating or purging behaviour (i.e. self-induced vomiting or the misuse of laxatives, diuretics or enemas). Weight loss is primarily through dieting, fasting or excessive exercise, or all of these methods; Binge-eating/purging subtype: During the past 3 mo, the patient has engaged in recurrent episodes of binge-eating or purging behaviour (i.e., self-induced vomiting or the misuse of laxatives, diuretics or enemas) |
| Current severity | Mildly severe low body weight is defined as BMI > 17.00 kg/m2; Moderately severe low body weight is defined as a BMI of 16.00-16.99 kg/m2; Severe low body weight is defined as a BMI of 15.00-15.99 kg/m2; Extremely severe low body weight is defined as BMI < 15.00 kg/m2[1,2] |
Inflammatory bowel disease (Crohn’s disease or ulcerative colitis), malignancies, thyrotoxicosis and diabetes can present a clinical picture similar to AN. In rare cases AN can be mimicked by a cerebral tumor including pituitary adenoma[4]. Patients with severe depression can experience weight loss due to loss of appetite or a belief that they do not deserve food. A patient with schizophrenia might avoid food due to various delusions[4]. Avoidant/restrictive food-intake disorder (ARFID) was initially regarded as a disorder of childhood, but is now regarded as an age-neutral disorder[4]. Core symptoms are food avoidance or restriction (volume or variety), together with weight loss or faltering growth, nutritional deficiencies, dependence on nutritional supplements for sufficient intake, and/or psychosocial impairment[4]. Although patients with ARFID do not present with the concerns about weight and body shape typically associated with AN[8], they are susceptible to the same medical complications[4,9].
Approximately 92% of individuals affected by AN are female[10], but all genders, sexual orientations and ethnicities are affected[11]. The most common age of onset is 15-25 years[12]. The age of onset appears to be decreasing[6,13,14]. The incidence is low in children aged 4-11 years, but it increases significantly with age above 11 years[6]. The restricting subtype of AN is associated with earlier onset and greater likelihood of crossover to the binge-eating/purging subtype[15]. Onset after the age of 30 years is rare[13,16].
The estimated prevalence of AN among young females is 0.3%[13,17], and it affects up to 4% of females and 0.2% of males during their lifetime[17,18].
Time-trend data suggest that the incidence of AN in Europe increased from the 1930s to the 1970s. This might have been due to improvements in the detection rate of persons with AN, but it might also reflect a true increase. In the 1960s another beauty ideal became more widely adopted, as represented by very thin models such as the supermodel Twiggy. It appears that the incidence of AN in Europe was stable from 1970 into the 21st century[19], but the global incidence of AN appears to be increasing, particularly in Asia and the Middle East[20,21]. However, the incidence of AN remains low in Africa and among African American females in the USA, in Latin America, and among Hispanics/Latinos in the USA[19]. These observations may reflect both genetic and cultural etiological factors. A large-scale national health survey in South Africa revealed that despite a high mean BMI of 29.0 kg/m2, more black African females were happy with their current weight and fewer attempted to lose weight, compared with females of other ethnicities[19,22]. A study involving the Caribbean Island of Curacao found no cases of AN among the mainly black population, while the incidence in the white population was similar to that in the United States and the Netherlands. That study was performed when the cultural influence of North America and Europe was increasing with the development of an affluent minority and relatively poor majority[23]. Studies involving Hispanics/Latinos in the United States found that they had fewer concerns about weight gain than did their non-Hispanic white peers, leading to fewer cases of AN[24]. This seems to be related to a body ideal of a “curvier” shape and higher body weight compared with the ideals in Western countries[25,26]. Case series of males with AN in Western societies indicate that they display many of the same characteristics and clinical course as females with AN[27]. AN in males has been reported in non-Western societies, but few cross-cultural data are available on the incidence and prevalence of AN among males[28,29].
The etiology of AN is complex and involves genetic and neurobiological factors[30], and a range of psychological risk factors has been identified, such as childhood anxiety disorders, trauma (e.g., sexual assault, physical abuse, neglect), early feeding problems, temperamental traits such as inhibition, perfectionism and harm avoidance[31]. In addition, living in a society in which a high value is placed on thinness, including occupations that require a lean physique and perfectionism (e.g., sports and modelling), seems to be associated with an increased risk of AN[1,32-34]. Genetic studies indicate that genes coding for metabolic factors seem to play an important role in the development and maintenance of AN. The aspects of AN as a metabo-psychiatric disorder are further discussed in the section below on emerging therapies and outstanding research issues.
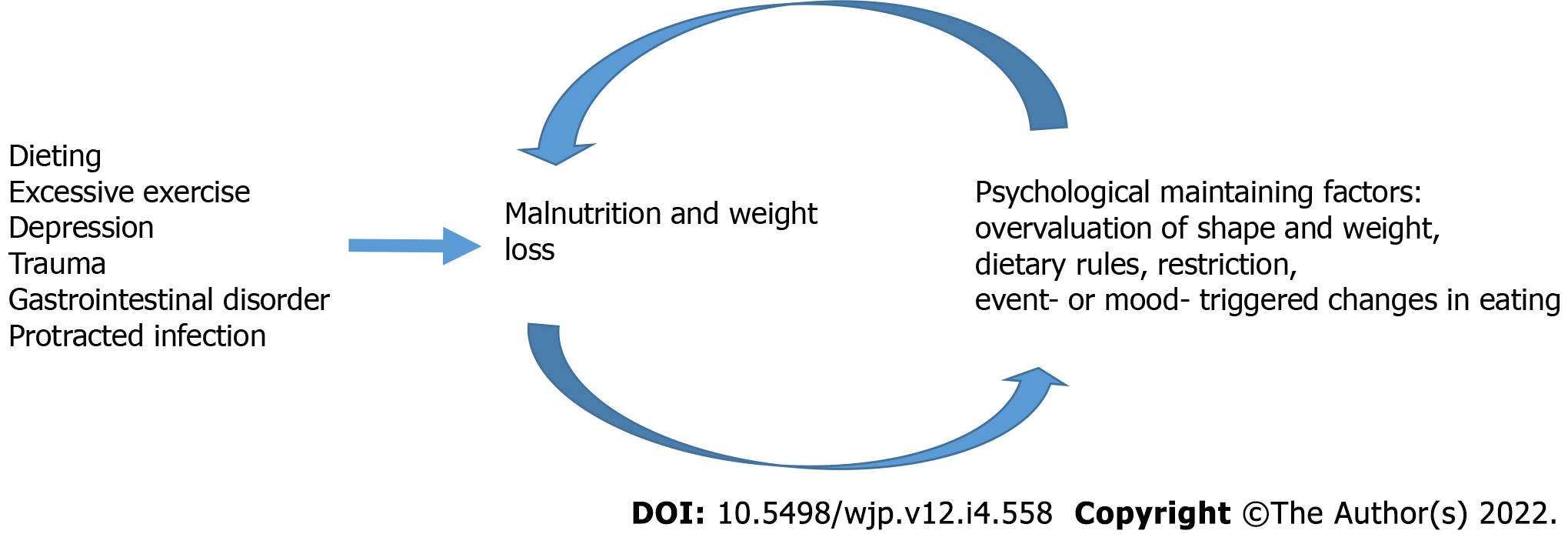
Most AN patients report that they started losing weight by voluntary dieting, but in some patients the weight loss is caused by depression, trauma, excessive exercise, a gastrointestinal disorder or protracted infection. If the initial weight loss is voluntary, the patient usually has a positive experience during the first mo. However, over time they will find it increasingly difficult to eat normally, and a normal meal can induce discomfort, anxiety or even panic reactions. The patient gradually becomes preoccupied with body weight and body shape. Negative experiences from trying to eat normally lead patients to eating too little. Food intake is further decreased in times of stress, and even everyday stressful experiences may induce a further reduction in food intake. Thus, patients enter a vicious cycle of reduced food intake with increasing overvaluation of shape and weight, and further reduction of food intake. Treatment becomes more difficult when these psychological maintaining mechanisms are established. The vicious cycle appears to include both psychological and somatic factors that are closely related to nutritional status (see Figure 1).
Research into the primary prevention of eating disorders (EDs) is in its early stages. A meta-analysis[35] concluded that there are promising strategies for universal prevention (targeting whole populations) as well as the selective prevention of EDs (targeting individuals showing specified risk factors), but not for indicated prevention (early detection and intervention for individuals with symptoms below the diagnostic level of EDs). However, most studies were found to have a high risk of bias, which demonstrates the methodological challenges of this research. The picture is even more complicated when looking specifically at AN. Stice et al[36] reviewed known risk factors to target in preventive interventions. They highlighted the challenge that risk factors predicting other EDs (body dissatisfaction, negative affect, thin-ideal internalization, perceived pressure for thinness, dieting and family support deficits) do not consistently predict AN. Existing prevention programs tend to target these risk factors, and therefore they might be less effective in preventing AN. The only risk factor that spans the full spectrum of EDs, including AN, is impaired psychosocial functioning. An additional risk factor for AN is low BMI. This situation prompted Stice et al[36] to argue that AN-specific preventive strategies should target psychosocial functioning and healthy weight gain. However, previous universal prevention programs have demonstrated little or no effect on the prevalence of AN. A long-term effectiveness study of a school-based primary prevention program for AN in Germany found an effect on body self-esteem but not on disordered eating. However, that study exhibited implementation difficulties, including problems with maintaining the sample size[37]. Primary prevention in smaller populations with a high risk of an ED seems to exert some effects. An intervention designed to prevent the onset of EDs among adolescent athletes through a 1-year intervention program prevented the onset of EDs and reduced symptoms associated with EDs relative to assessment-only control athletes. However, the small number of ED patients in that study prevented a subgroup analysis of the effect on the prevalence of AN[36,38].
A recent study reviewed the evidence for the early detection of individuals fulfilling diagnostic criteria, which is an aspect of secondary prevention[39]. Those authors found evidence that educational interventions targeting professionals (e.g., from medical, educational or sports environments) are somewhat effective. The case for early detection is very strong, since many studies have demonstrated that the risk of severe and enduring anorexia nervosa (SE-AN) increases with the duration of untreated illness[40]. Until more evidence on primary prevention of AN is available, it seems prudent to focus efforts and healthcare costs on early detection and ease of access to treatment.
Rates of recovery from AN at 1- to 2-year follow-ups with the best available treatments lie in the range of 13-50% across age groups[41,42]. Among the patients who complete psychotherapy the relapse rates are ranging from 9% to 52%, with most studies finding rates higher than 25%[43]. The average duration of illness with AN is about 6 years[12]. The long-term course is heterogeneous, with 20-year longitudinal studies finding that 30%-60% of patients will experience full remission, while 20% will have enduring illness and the remainder will have residual symptoms[43,44].
In a meta-analysis of 36 studies published between 1966 and 2010, the standardized mortality ratio for patients with AN (the percentage of observed deaths among patients with AN divided by the percentage of expected deaths in the population of origin) was 5.9[7]. One in five individuals with AN who died had committed suicide[7]. However, these data were often derived from patients admitted to hospitals, and early intervention and active engagement might have reduced the prevalence, need for hospitalization and mortality[4,14]. No mortality was observed in a cohort of 51 patients with AN recruited from all individuals born in 1985 in Gothenburg, Sweden who were followed for 30 years[45]. Although these observations were made in a small cohort of young patients, they might indicate that early intervention and structured follow-up are associated with low mortality.
Both psychiatric and somatic comorbidities are common in AN. The most common psychiatric comorbidities are mood and anxiety disorders, obsessive-compulsive disorders, personality disorders, substance-use disorders and neurodevelopmental disorders such as autism spectrum disorder or attention-deficit hyperactivity disorder[4]. Comorbid disorders tend to worsen the prognosis of AN because they interfere with treatment response[46-49]. Suicidal behaviors and ideation are markedly increased in patients with ED[50,51]. Type 1 diabetes is sometimes a challenging comorbidity, since the omission of insulin in order to lose weight can induce severe complications, including recurring ketoacidosis and rapid development of neurological, retinal and renal complications, and is associated with a significantly increased mortality rate compared with AN without type 1 diabetes[52].
Since most patients with AN should be treated as outpatients, the assessment should determine whether outpatient treatment is safe. A clinical interview is essential for risk assessment. Ascertaining the duration and severity of the patient’s ED may help to identify likely complications. Assessment of nutrition should include information about the intake of bread and similar thiamine-containing nutrients, the intake of meat and fish and other zinc-containing nutrients, and whether the patient has a varied or monotonous diet with the associated risk of multiple deficiencies[53,54]. Information about physical capacity compared with friends or relatives of the same age should be obtained. The clinical interview should also assess whether the patient has excessive exercise, vomiting and use of laxatives or other medications, including those that aim to increase metabolism (e.g., thyroxine), or herbs or other substances that may have metabolic or diuretic effects[55].
The presence of purging behaviors is sometimes difficult to assess, and corroborative sources of data should be obtained whenever possible. Information about past eating disorder treatment including previously diagnosed complications is also valuable[50]. Anamnestic information regarding attacks of dizziness, syncope, or near-syncope warrants the acquisition of more detailed anamnestic information about possible arrythmia and other causes of the attacks such as hypoglycemia or hypotension. Information regarding exercise (especially excessive exercise), vomiting or other purging activity, pulse rate during or before the attack, and data on altered medication can shed light on possible underlying mechanisms. In particular, recent onset of symptoms suggestive of cardiac arrythmia is important because refeeding might alter the electrolyte balance and further worsen unstable arrythmia. Chest pain and attacks of dyspnea could be related to pneumothorax or cardiovascular disease. The malnutrition in AN is associated with pulmonary changes that may predispose to spontaneous pneumothorax[56]. Patients with severe or extreme AN may have several potentially lethal complications, and these patients should be assessed by a physician with experience in extreme AN before outpatient treatment is commenced.
A physical examination should be performed in a sufficiently warm room and undressing should be performed gradually and respectfully to allow examination of the chest with auscultation of lungs and heart, including searching for systolic murmur in the left axilla that indicates mitral-valve insufficiency. Gentle palpation of the abdomen can give valuable information on the location of any abdominal pain, and whether the pain is referred. Palpation and percussion can reveal distension of the GI tract and other parts of abdomen including tendency to gastric retention. Arms and legs should be examined to assess the peripheral circulation, dehydration, peripheral oedema and pitting. Balance can be assessed by asking the patient to stand on one foot, and simple tests of coordination can reveal the risks of falling and fractures. Problems with coordination and balance can be related to malnutrition-induced cerebellar dysfunction or proximal myopathy, which is a common problem in severe malnutrition. This myopathy can be significantly increased during refeeding and can be associated with increased risk of falling[34].
It might be difficult to measure the blood pressure properly due to thin arms, but in patients with symptoms of orthostatic hypotension, valuable information can be revealed by using a blood-pressure cuff for children. Palpation of the pulse can give information about the heart rate, pulse pressure and dehydration, and the tendency for severe hypotension, which causes a very thin pulse wave with relative tachycardia[57]. Resting tachycardia is unusual and may be indicative of a superimposed infection or other complication[58]. The usual signs of infection (fever and elevated white blood cell count) may not be present in AN. A lower threshold to evaluate for an infection should be followed[57]. Examination of the teeth can reveal erosions, indicating possible vomiting with risk of electrolyte disturbances[59].
Blood tests in AN (especially restricting AN) can be normal or close to normal even when the patient is at risk of lethal arrythmia or other severe complications of treatment. Therefore, using blood tests alone without comparing with the clinical picture is not adequate for risk assessment. Assessment of patients with extreme AN (BMI < 15 kg/m2) should be performed in collaboration with a physician with experience in extreme AN as this group of patients may have a large number of complications[57,60].
Outpatient psychotherapy is the mainstay of treatment for AN, as it is less costly and disruptive than other, more intensive levels of care[61,62]. A proportion of patients will need inpatient psychotherapy or supportive care. Research data to guide choices among types of psychotherapy for outpatient and inpatient treatment are limited and disputed[1]. AN remains difficult to manage since patients are often challenging to engage, and outcomes are often poor, even in those who agree to commence treatment[61]. However, over the past 20 years there has been empiric support for the efficacy of several treatments, mainly in the outpatient setting, and thanks to an improved understanding of the psychological mechanisms that maintain them, manualized treatments for children, adolescents and adults with AN have been developed.
The decision regarding whether to proceed with outpatient treatment or to transfer a patient for inpatient therapy may be difficult, especially in non-specialist or general psychiatry settings. The feasibility of outpatient psychotherapy requires containment of concerns regarding the short- and long-term somatic consequences of malnutrition and monitoring of medical safety needs. The patient’s medical and psychiatric stability, AN severity, age and duration of illness must be considered during treatment decision-making[34]. Psychopharmacological medications are generally ineffective for promoting weight gain, reducing AN-related depressive symptoms or preventing relapse in AN[1]. However, there is some preliminary evidence for the use of atypical antipsychotics for adolescents to support the acute phase of renourishment[63]. Overall, medication plays a very limited role in the treatment of AN[1,64-66].
Early intervention improves outcomes, and so the rapid commencement of specialized treatment for EDs is essential[4,67,68]. The duration of untreated AN before treatment initiation varies, but multiple studies have found that adults and adolescents had AN for a mean of 30 mo before treatment was initiated[4]. The First Episode Rapid Early Intervention for Eating Disorders (FREED) study found that the duration of untreated AN in patients aged 16-25 years could be significantly reduced by implementing an early intervention service model and care pathway for young adults with EDs[69]. In addition, the proportion of patients taking up treatment was significantly higher among FREED patients than among those who received treatment as usual (TAU)[69].
Family-based treatment (FBT) is the most empirically supported intervention for children and adolescents with AN[4,29,70,71]. In general, FBT does not align with a particular therapeutic approach, but instead integrates techniques from a variety of schools of psychotherapy, including systemic, strategic, narrative and structural types of family therapy[72]. The overall philosophy of FBT is to empower parents to help their child to overcome a disease that is beyond his/her own control. The family is viewed as a resource and the child or adolescent with AN is seen as embedded in the family and temporarily regressed, and parental involvement in therapy plays a pivotal role in treatment success[72].
Six randomized, controlled trials (RCTs) have assessed the efficacy of manualized FBTs for AN in adolescents. Manualized FBTs have been compared with a single individual therapy (i.e. adolescent-focused therapy) in only one RCT. In that trial, FBT was not significantly more efficacious than was adolescent-focused therapy at the end of treatment, but it was more effective for facilitating full remission at follow-up[61,73]. The findings of a Cochrane review suggest that the evidence favoring family-based interventions over standard treatment or other psychological approaches is not robust[74]. This opinion has been criticized partly because studies of questionable validity were not excluded from the Cochrane review and because the study had inadequate statistical power[75]. FBT is recommended in several clinical guidelines[29,62,76] on the basis of evidence of remission rate, faster weight gain and less reliance on the young patient’s own motivation and ability to change their symptomatic behaviors.
Several modifications of standard FBT have been tested. One of these is Parent-Focused Family Therapy, a type of FBT in which most sessions only involve the parents. According to one high-quality RCT, this modality is as effective as traditional FBT, where the family is seen together[29,77]. Another modality of family therapy that has been adapted to treat AN is multifamily therapy, which draws on the conceptual principles of FBT, applying them to groups of typically five to seven families in extended whole-day sessions, initially over four consecutive days. The presence of other families and the intensity of the contact creates a powerful treatment context wherein families learn from each other, share their experiences and gain multiple perspectives on the problems they face. The group context also helps to reduce the sense of isolation and stigmatization that is often experienced by families living with an ED[78]. Another high-quality RCT compared systemic family therapy with FBT and found no significant differences in remission rates; however, the rate of weight gain was greater and the need for hospitalization was significantly lower in the FBT group[79].
While parental involvement is effective and often necessary to bring about changes in children and adolescents with AN, it does come at a considerable cost for the families. Parents may find the task of renourishment in the face of strong emotional reactions from their child daunting[80,81], and the intensive care required may be an economic burden for parents since it may require them to take time off from work. Regardless of which mode of FBT is chosen, monitoring of the patient’s somatic condition is necessary in order for parents to know that their child is safe while they struggle to learn how to manage, for example meal support, and for the family therapist to support the agency of the parents. Furthermore, although parents receiving FBT are supported to rely on their own experience when making feeding choices, they may need dietary advice on how to increase the energy density of the meals they provide to affected children. Consequently, easy access to multidisciplinary support helps parents and therapists to provide effective outpatient psychotherapy. It is important that all team members, e.g., the physician in charge of somatic assessment, understand the principles of parent empowerment, as described by Katzman et al[82].
Data to guide choices among types of psychotherapy for adults remain inadequate and disputed[1]. The guideline from the National Institute for Health and Care Excellence (NICE) on the recognition and treatment of EDs recommends that the first-line treatments for adults consist of structured individual therapies that focus on EDs, including individual cognitive behavior therapy (CBT) with an eating disorder focus (CBT-ED), Maudsley Model of Anorexia Nervosa Treatment for Adults or Specialist Supportive Clinical Management (SSCM) (36). These therapies have been evaluated in large-scale trials, which have revealed little or no difference in efficacy between them[4,83-86]. All of these therapies lead to considerable improvements in body weight and reductions in AN symptoms[4]. CBT-ED is the most widely used manualized individual psychotherapy for adult patients, with enhanced CBT for EDs (CBT-E) as described by Fairburn probably being the most widely disseminated CBT-ED for AN[87].
The most common age of onset of EDs is in adolescence and young adulthood, but the clinical services for adolescents and adults are separate in some countries[4]. This means that patients and their families are often obliged to change treatments when the adolescent patient is transferred to adult services[61]. The choice of treatment should be related to the needs of the patients and their families. For adolescents, FBT is the current leading empirically supported intervention for AN[4,62]. NICE has recently recommended the use of CBT-ED in children and young people when family therapy is unacceptable, contraindicated or ineffective[62]. This recommendation was supported by promising results demonstrated by the application of CBT-E adapted for adolescents with EDs[72]. A recent systematic review found that outpatient CBT-E was well accepted by adolescent patients with AN; it was completed by about two-thirds of participants and produced improvements in eating-disorder psychopathology and general psychopathology, and remission from AN was achieved by about 50% of patients at the 12-mo follow-up[88].
Some of the differences and similarities of FBT vs individual psychotherapy were discussed in a recent conceptual comparison of FBT and CBT-E[72]. Briefly, parental involvement in FBT is vitally important for the ultimate success of the treatment. In CBT-E, parental involvement is useful but not essential[72], with their role being simply to support the implementation of the one-to-one treatment. Both types of treatment address adolescent development, but in FBT the adolescent is not viewed as being in control of his/her behavior (i.e. the ED is considered to be controlling the adolescent). This is corrected in the first phase of the treatment by improving the parental control over eating[72]. In CBT-E, the adolescent is helped to learn how to control his/her behavior, and parents may help and support the adolescent in taking control[72]. In FBT, the adolescent is initially not actively involved and plays a more passive role, although their role becomes more active in the second and third phases of the treatment, while in CBT-E the adolescent is encouraged from the beginning to become actively involved in the treatment[72]. CBT-E for adolescents does not use directives or coercive procedures. The patients are never asked to work on issues that they do not consider to be a problem, as that would tend to increase their resistance to change. The key strategy of CBT-E is to create a formulation of the main mechanisms maintaining their individual eating problems, and actively involve the patient in the decision to address them, including their low weight. If they do not reach the conclusion that they have a problem to address, the treatment cannot begin or must be suspended.
A lack of insight and motivation for change in the young person is one of the main reasons why FBT is often preferred by both healthcare services and parents. However, most adolescents are able to reach the conclusion that they have a problem to address if they are introduced to CBT-E by a trained psychotherapist[61,72,89]. Once the patient is engaged in the process of change, their personal eating-disorder psychopathology (outlined in the formulation) is addressed via a flexible series of sequential cognitive behavioral procedures and strategies, integrated with progressive patient education[61]. Despite several differences, the general strategy that is common to FBT and CBT-E is to address the maintaining mechanism of the eating-disorder psychopathology, especially undereating, as opposed to exploring any potential causes of the eating-disorder psychopathology. Both treatments take an agnostic view of the cause of the illness; that is, no assumptions are made about the potential origins of EDs[72].
Some clinical services still do not provide patients with evidence-based psychological treatments, or else they rely on therapists who deviate from the established protocols. The dissemination of FBT, CBT-E and other evidence-based treatments needs to be promoted. Web-centered training programs designed to enable simultaneous training of large numbers of therapists in different countries is a potential solution[61].
In DSM-5, severe AN is defined as AN with a BMI of 15.00-15.99 kg/m2, while extreme AN is defined as a BMI < 15.00 kg/m2 (see Table 1)[2]. Most of the studies on outpatient treatment of AN have included patients with mild or moderate AN. However, some studies have shown that outpatient treatment can be a valid alternative to inpatient treatment in cases of severe or extreme AN if the patient is medically stable[83,90,91]. Outpatient treatment must be safe, otherwise concerns regarding the medical risks will become the focus, rendering psychotherapy difficult or impossible to perform. When a patient is medically stable there is no significant risk of dangerous complications during therapy. These issues are discussed in more detail below in the section on medical management and in the treatment section.
In a case series of 30 patients aged ≥ 17 years with a mean BMI of 15.1 kg/m2 (range 12.82-15.99) at baseline, 66% completed outpatient CBT-E and demonstrated both considerable weight gain and reduced psychopathology at the end of treatment[90]. Among the 20 patients who completed the treatment, 11 (55%) were classified as having a “full response”, corresponding to BMI ≥ 18.5 kg/m2 combined with a global score on the Eating Disorder Examination Questionnaire (EDE-Q) of less than 1 SD above the community mean[87]. Moreover, among the 9 patients with BMI < 18.5 kg/m2, 7 (35%) had a BMI that was classified as being of mild severity (≥ 17.0 kg/m2 according to the DSM-5) and 2 (10%) had a BMI of moderate severity (16.00-16.99 kg/m2), while no patient was classified as having severe or extreme AN. Changes remained stable at the 1-year follow-up, and no severe complications were observed in the study[90]. These findings indicate that outpatient CBT-E is a valid alternative to inpatient treatment for severe and extreme AN when the patient is medically stable.
A substantial subgroup of patients with AN develop SE-AN[92]. This is currently a rather ill-defined patient population. SE-AN is characterized by: (1) A persistent state of dietary restriction, underweight and overvaluation of weight/shape with functional impairment; (2) Duration longer than 3 years; and (3) Exposure to at least two appropriately delivered evidence-based treatments[41,93]. It is difficult to define what an appropriate treatment is, and a duration of longer than 3 years is very common among patients with AN. In addition, the criteria for recovery from AN remain unclear, so this population is potentially very large. In a 22-year follow-up study of 246 patients with AN and bulimia nervosa, the patients were assessed at 9 years and at 22-25 years after inclusion. Approximately half of those with AN who had not recovered by 9 years progressed to recovery by the 22-year follow-up[94]. These findings argue against the implementation of palliative care for individuals with SE-AN. At present only one formal RCT has been published on the treatment of patients with SE-AN. In that study, 63 patients with an AN duration of at least 7 years were randomized to receive either CBT or SSCM, both adapted for the treatment of SE-AN and both in an outpatient setting. A very low attrition rate was observed, and small effects on the BMI and quality of life were detected in both treatment groups[95]. Raykos et al[96] compared illness severity and duration with outcome among 134 patients with SE-AN who received CBT-E in an outpatient setting and found that the illness severity and duration had no effect on outcome. In an inpatient study by Calugi and colleagues, 66 adult patients were divided into groups according to their illness duration: ≤ 7 or > 7 years. All patients received inpatient intensive CBT-E as described by Dalle Grave[97], and the two groups showed similar improvements in BMI and eating-disorder symptoms at the end of treatment and at the 12-mo follow-up[98]. Thus, there appears to be either a weak or no association between AN duration and the effect of treatment among patients with SE-AN[99]. Although the findings of several studies indicate that these patients can benefit from psychotherapy, many with SE-AN are not provided with a treatment program when they seek care[41]. Their presence in an eating-disorder unit can exert complicated effects on the milieu, with a significant proportion of SE-AN patients reporting having experienced coercive efforts to increase their body weight[41]. The poor understanding and paucity of treatments for SE-AN has been described as a crisis in the field of EDs[41].
During the first weeks of refeeding the patient’s blood levels of phosphate may drop significantly with an increased risk of cardiac arrhythmia. If not treated promptly in a patient with severe or extreme AN, critical hypophosphatemia may quickly ensue, which can lead to cardiac arrythmia, refeeding syndrome with heart failure, respiratory failure and central nervous system symptoms[50,100]. The clinical interview should include efforts to detect the risk of a sudden intake of large amounts of food, as part of binge-eating/purging AN. The BMI at the start of treatment and the food intake during the preceding 10 d indicate risk of significant hypophosphatemia during the first weeks of refeeding[101,102]. Gradual increase of food intake during the first weeks of treatment significantly decreases the risk of significant hypophosphatemia. The AN-related inhibition of food intake will usually cause slow increase in the food intake in outpatient treatment. Mild hypophosphatemia can be treated with oral supplements, while significant hypophosphatemia is treated with intravenous phosphate. Patients with AN and a significant risk of severe hypophosphatemia should be treated as inpatient during the first weeks of refeeding.
Loss of potassium is usually caused by vomiting or other purging, but significant hypokalemia may also be seen as part of a refeeding reaction caused by increased insulin release induced by food intake. The insulin induces an intracellular flux of potassium with concomitant hypokalemia. Hypokalemia is associated with risk of cardiac arrythmia. Potassium intake the last hours before blood sampling may result in falsely increased potassium readings. Moderate hypokalemia is treated with oral supplements, while severe hypokalemia is treated with intravenous infusion. Patients with recurrent hypokalemia would usually not be regarded as sufficiently stable for outpatient therapy, partly because refeeding can exacerbate the condition[103].
In purging, gastric acids are lost and alkalosis may be the consequence. Venous base excess can provide valuable information about alkalosis due to purging. As acid-base disturbances tend to develop before hypokalemia, venous base measurements may be more sensitive to purging than are blood levels of potassium. Alkalosis usually resolves rapidly when purging has stopped and the patient is rehydrated[103].
Significant hypoglycemia is a common problem in AN, both for the restricting and binge-eating/purging subtypes. The main symptoms are dizziness or feeling of weakness, sometimes related to physical activity or after consuming sugar-containing nutritious drinks. If sugar is absorbed quickly it may induce hyperglycemia with concomitant insulin release and hypoglycemia[104]. Severe hypoglycemia is associated with an increased risk of arrythmia. Hypoglycemia usually resolves with refeeding and is not usually a problem when normal weight is re-established. Symptomatic hypoglycemia in patients with severe or extreme AN is usually not compatible with safe outpatient psychotherapy[34,105].
Isolated moderate hyponatremia is usually of little or no clinical importance, but severe hyponatremia warrants more detailed assessment and careful inpatient treatment[106].
The glomerular filtration rate may decline over time[1,107]. Impaired renal function is frequently overlooked by physicians. Clinicians should consider collecting 24-h urine and calculate creatinine clearance to correctly assess renal function in patients with SE-AN[108].
Blood count often reveals leucopenia, granulocytopenia and mild thrombocytopenia. These low blood counts are usually moderate, have no clinical significance and typically resolve with refeeding. In rare cases extreme granulocytopenia or extreme thrombocytopenia indicate the need for inpatient care to manage risk of infection or bleeding[109,110]. Moderate anemia is common. It may be normocytic or macrocytic, even though vitamin B12 and folate may be normal[34]. Sometimes iron supplement is necessary, but the condition typically resolves with refeeding.
Several vitamin deficiencies are common in AN. Specifically, deficiencies in fat-soluble vitamins such as vitamin D are common[111]. Routine supplementation with age-appropriate oral multi-vitamin and multi-mineral supplement is recommended[62]. Usually, vitamin D and calcium would be included in these supplements.
Patients with a low intake of bread or other grain products may be at risk of developing thiamine deficiency. Severe thiamine deficiency can be fatal. Patients with suspected thiamine deficiency should be advised to take oral supplements or injections[112,113].
Magnesium deficiency in AN is usually related to reduced intake, but laxatives and diuretics may also cause magnesium deficiency[102]. Deficiency may cause fatigue, muscle cramps, mental problems, cardiac arrythmia and osteoporosis[114]. Mild or suspected deficiency is treated with oral supplements[102]. Deficiency in zinc may cause depressive symptoms, reduced height growth and lack of appetite. Patients with low intakes of fish and meat should take an oral multi-mineral supplement containing zinc[115].
Elevated aminotransferases are common in patients with AN. A mild increase in aminotransferases during the initial weeks of refeeding should not cause alarm or slow down the rate of refeeding[116]. While the liver enzyme values in AN can reach severe levels, a supervised increase in food intake and return to a healthy body weight usually rapidly leads to normalization of elevated aminotransferases caused by starvation and refeeding[116].
Sinus bradycardia, which is sometimes associated with orthostatic hypotension, is often observed in patients with severe or extreme AN[34]. An electrocardiogram (ECG) with corrected QT (QTc) interval measurement is usually performed to assess risk of arrythmia. When detected, a prolonged QTc interval is usually a consequence of QT- usage of interval-prolonging medications or electrolyte disturbances[117]. Most ECG abnormalities respond to adjustment of medication and electrolytes and most do not need further investigation[4].
Generally, if the clinical and biochemical assessment suggests a significant risk of arrythmia, inpatient assessment and medical stabilization is necessary before refeeding is commenced.
This serious complication affects up to 50% of patients with AN and can be associated with a life-long elevated fracture risk and the debility that ensues with spinal vertebral compression fractures, among other conditions[118]. Measuring bone mineral density is indicated if the patient has had AN for more than 1 year or amenorrhea for more than 9 or 12 mo[34]. Bone densitometry should be conducted every 2 years during the active phase of AN[34]. Bone mineral density is usually expressed as the T-score, which compares the measured score with that of healthy young adults.
Patients with AN and a T-score of -1.5 to -2.5 (osteopenia) should be advised to focus on weight restoration, and adequate vitamin D and calcium intake[119]. A T-score of less than -2.5 indicates that the patient has osteoporosis. In addition to weight restoration with resumption of normal menses, supplementation with calcium, vitamin D or bisphosphonates is often considered. Only a few studies support the utility of bisphosphonates in AN, but their usage seems to reduce the risk of future spine and hip fractures[119]. However, there are significant safety concerns regarding the use of bisphosphonates, including fetal malformation in pregnant females who are exposed to them[119]. Some studies support the use of transdermal estrogen therapy in patients with AN and osteoporosis. Several RCTs have demonstrated that oral contraceptives are not effective in the treatment of osteoporosis in patients with AN[119].
Patients who have an ED that cannot be managed safely in the outpatient setting or do not respond sufficiently to outpatient treatment are usually advised to enter hospital as a day patient or receive residential or inpatient care. Two main groups of patients are usually transferred to inpatient care: (1) Those who need inpatient psychotherapy in order to gain weight or to stabilize purging[97]; and (2) those who are unable to benefit from inpatient psychotherapy but are in need of supportive care, usually due to medical complications or suicide risk. Some guidelines also recommend inpatient treatment in cases with a BMI of < 15 kg/m2[1,120]. However, BMI alone may be of limited value as a criterion for inpatient care[83,90,91].
Various inpatient treatments for children and adolescents have been developed. In one study a family-based inpatient program was used to treat 57 patients during the period 2008-2014, and 37 patients consented to take part in a follow-up study[121]. The average length of hospital stay was 20.6 ± 13.6 wk. The average time between discharge and follow-up was 4.5 ± 1.8 years. A total of 65% of the participants had achieved a normal body weight (BMI ≥ 18.5 kg/m2) and were classified as “weight recovered” at follow-up. These findings indicate that adolescents who are unable to benefit sufficiently from FBT in the outpatient setting may benefit from a family-based inpatient program.
If outpatient treatment of an adolescent or an adult has revealed stable engagement in therapy, but the patient has been unable to obtain sufficient weight gain, inpatient intensive treatment should be considered. For example, a patient who starts outpatient CBT-E for AN but is unable to achieve sufficient weight gain can enter inpatient intensive CBT-E[122,123]. These programs usually last 13 wk. During inpatient treatment, CBT-E is used to help the patient to address their psychological maintaining mechanisms while normal weight is re-established. Transfer to day-patient care or directly to outpatient CBT-E enables the patient to meet everyday challenges without returning to eating-disorder behaviors[122]. According to a recent review intensive CBT-E for the inpatient treatment of adolescents with AN was particularly effective, with approximately 80% of patients achieving normal weight by the 12 mo follow-up[88]. These studies suggest that outcome could be improved if outpatient and inpatient treatments are applying similar psychotherapeutical methods.
Indications for hospitalization for supportive care are usually risk of arrythmia, profound hypotension or dehydration, severe electrolyte abnormalities or risk of suicide. In intensely ill patients who are unable to benefit from outpatient or inpatient psychotherapy a multidisciplinary treatment team could be the best alternative. Treatment is designed by the different team members in regular meetings. The influence of specialists of pediatrics, internal medicine or intensive care medicine can be adapted to the need of the patient[50].
The available findings on inpatient treatment of AN, which mainly come from observational cohort studies, indicate that in a large percentage of patients inpatient treatment is associated with weight restoration and improvements in eating-disorder psychopathology. But unfortunately many patients experience relapse after discharge[61,124]. Studies have indicated that 30%-50% of patients need to be rehospitalized in the first years following discharge[125].
Relapse prevention forms part of most psychotherapies, both inpatient and outpatient treatments. But relapse after the end of treatment remains a significant challenge. The usual strategy adopted for addressing relapse after inpatient treatment has been to provide some type of post-hospitalization treatment. Preliminary evidence, which remains to be validated, suggests that CBT is beneficial for patients with AN[61,126] as relapse prevention. One large trial tested Internet-based CBT added to TAU vs TAU alone in the post-hospitalization treatment phase in 258 females[127]. CBT completers had greater improvements in BMI compared with those who received TAU only. These findings indicate that the relapse-prevention effect of CBT can be delivered via the Internet.
The medical complications of AN affect all organs and systems, and are generally due to weight loss, malnutrition and purging behaviors[106,128].
Most patients with AN have the restricting subtype, but a significant proportion has binge-eating/purging AN with self-induced vomiting or the misuse of laxatives, diuretics or enemas (see Table 1). A study of laxative use among adolescents with AN found a prevalence of 12%[129]. Taking high doses of laxatives is associated with electrolyte disturbances, dehydration and secondary hyperaldosteronism, which can be most challenging during therapy due to the accumulation of water with significant weight gain and oedema[103]. This is usually addressed during psychotherapy[87]. In order to avoid sudden fluid retention with unacceptable weight gain, laxatives should be gradually decreased over a period of 1-2 wk. During the last week, the patient should start taking stool softeners such as lactulose to reduce the tendency for constipation[103]. If the patient is taking extreme doses of laxatives and has very high blood levels of aldosterone, inpatient treatment during the laxative discontinuation may be indicated. However, laxative discontinuation is usually achieved in the outpatient setting[87]. Vomiting is addressed as part of outpatient psychotherapy[87]. Due to loss of acid and potassium, acid-base disturbances and hypokalemia may occur. Hypokalemia is associated with significant risk of cardiac arrhythmia. Severe hypokalemia is an emergency and is treated with inpatient intravenous infusion of potassium[60].
Varying degrees of excessive exercise are common in AN. A French study[130] found that more than half (54%) of eating-disorder patients exercised for at least 6 h/wk. However, only a small minority of patients (5%) reported vigorous, compulsive exercise for at least 6 h/wk[18,130]. Excessive exercise is associated with increased risk of fractures[131]. Excessive exercise is sometimes used to compensate for specific episodes of perceived or actual overeating and can be regarded as being related to purging. However, a significant number of patients report that exercise is mainly used to regulate mood[132]. As it is a maintaining factor of the ED, excessive exercise is addressed in the psychotherapy[87].
Most of the medical complications associated with AN can be resolved to a normal status with weight restoration and nutritional rehabilitation. However, some complications can be fatal if not diagnosed and treated adequately and some other complications may persist and cause reduced quality of life (growth retardation, osteoporosis and renal insufficiency). The medical evaluation is aiming at detecting comorbidity and complications. As part of the comorbidity assessment and differential diagnostic considerations tests to detect coeliac disease (e.g., transglutaminase antibodies), thyroid disease (blood levels of thyroxin and thyroid stimulating hormone) and prolactinoma (blood prolactin) are usually performed.
Many patients with AN experience pain in different parts of the body. Pain in the back can be related to minor fractures in the spinal column, which are usually compression fractures. Fractures induced by minimal trauma can significantly impair the quality of life. Attacks of chest pain could indicate pneumothorax or rib fractures, which sometimes occur with minimal trauma.
Gastrointestinal (GI) complaints are especially frequent in AN, with more than 90% of patients reporting GI complaints including postprandial fullness, early satiety, abdominal distention, pain, nausea and obstipation[50,133-135]. These symptoms sometimes increase after food intake and may inhibit attempts to eat sufficiently. Potential reasons for these problems other than AN should be assessed before initiating therapy. If no specific gastrointestinal disorder is diagnosed the symptoms may be regarded as secondary to the ED. Management of the symptoms should be discussed with the patient as part of the psychotherapy[134,136].
There is an increased risk of sudden cardiac death related to malnutrition in AN[50]. Assessment of the risk of sudden cardiac death is essential when determining which patients should receive inpatient medical stabilization prior to commencement of outpatient treatment. This evaluation requires detailed medical, psychiatric, and nutritional assessments, a physical examination, and laboratory testing as described in the treatment section.
A comprehensive account of medical management is outside the scope of this review but is available in the Management of Really Sick Patients with Anorexia Nervosa (MARSIPAN)[60] and Junior MARSIPAN guideline[137]. The scientific background for basic risk assessment and medical management of the most important medical complications relevant for outpatient treatment are discussed in the clinical interview and the physical assessment section and in the treatment section.
A small proportion of patients do not appreciate the severity of their illness, even when they are in a life-threatening situation, which may interfere with their ability to make decisions about life-saving treatment[4]. In countries where compulsory care is possible, an important ethical dilemma may emerge: how many times should compulsory care be provided, and for how long should it be continued?
Those who have been in compulsory care several times sometimes need multidisciplinary treatment to survive their exacerbations. The costs for the patients, their families and the healthcare providers are significant, and sometimes patients or their healthcare providers want to discuss the possibility of ending multidisciplinary healthcare. This would raise several ethical questions. What is the prognosis if multidisciplinary treatment is continued and what quality of life can be expected? This ethical problem is growing with our increasing knowledge around how to assess medical risks and manage life-threatening complications. Patients with life-threatening AN can survive for several decades if they are brought to healthcare centers before they are at a terminal stage with irreversible life-threatening complications. The 22-year follow-up study of Eddy and colleagues found that a significant subpopulation of the patients who had not recovered by 9 years had recovered by 22 years[94]. There are insufficient data to enable a conclusion to be drawn on the probability of recovery after 20 years of SE-AN. In addition, patients with SE-AN can improve their quality of life by weight gain[138,139].
The stakeholders involved when a process towards ending treatment commences need to be determined. Yager[140] suggested that different stakeholders should be involved, including the patients and their families, healthcare providers (the entire treatment team as well as the institutional administrators and their boards), payers and policymakers. There is also a need to determine who is going to make a decision and at what level such decisions should be made[140,141]. Our poor understanding of SE-AN and the paucity of available treatments AN makes this ethical issue even more complicated[41].
The Psychiatric Genomic Consortium was established in 2007 to conduct meta- and mega-analyses of genome-wide genomic data related to psychiatric disorders. The Eating Disorders Workgroup has been a part of this consortium since 2013. Members of this workgroup have performed genome-wide association studies, which have revealed strong associations between AN and insulin sensitivity, low BMI-adjusted fasting serum insulin and several other metabolic markers[30]. The increase in insulin sensitivity was greater than what could be explained by low BMI. The authors of that report suggested that it is time for a reconceptualization of AN and that it should be regarded as a metabo-psychiatric disorder[30,142].
Several other studies support the reconceptualization of AN towards regarding it as a metabo-psychiatric disorder. Our brain and gut are linked through humoral and bidirectional neural connections that allow fast and complex interactions via the brain-gut axis[143]. Several studies indicate that decreased food intake may alter the normal interactions in the brain-gut axis, thereby enhancing the maintenance of AN[142]. GI hormones and gut microbiota may be essential participants in the regulation of the brain gut axis. Several GI hormones are released by food intake and may participate in the development of the maintaining mechanisms in AN[144]. Some of them have been shown to induce anxiety and panic attacks in animals and humans when administered in supraphysiological doses, and in susceptible patients[145-147]. Ghrelin is a peptide hormone synthesized in the enteroendocrine cells of the stomach. It is released by calorie restriction and stress to stimulate appetite and increase food intake. Food intake increases ghrelin levels in healthy individuals, whereas in AN patients it is followed by decreased levels of ghrelin. Clinical trials of the novel ghrelin receptor agonist RM-131 in the treatment of AN are currently being performed[148].
The gut microbiota influences the extraction of energy from food and body weight gain, as well as appetite, gut permeability, inflammation and complex psychological behaviors such as depression or anxiety, all of which may play roles in the development and maintenance of AN. Nutrition is one of the main factors that influence the gut microbiota. Starvation has a substantial impact on the gut microbiota[149], inducing cell death in several fast-growing bacteria, while allowing the proliferation of slowly growing bacteria and bacteria that are able to feed on indigestible fiber or the mucin layer along the gut wall. Thus, food restriction might exert its maintaining effects on AN by affecting the gut microbiota[150,151].
The malnutrition-related alterations in the gut content also induce several metabolic effects, partly mediated by short-chain fatty acids (SCFAs) produced by the gut bacteria[152]. The pathways of SCFA production are relatively well understood, with major products being acetate, propionate and butyrate[153]. Acetate production pathways are widely distributed among bacterial groups, whereas pathways for the production of propionate and butyrate appear more highly conserved and substrate-specific. It has been proposed that an elevated colonic production of SCFA could stimulate numerous hormonal and neural signals at different tissue sites that would cumulatively suppress the energy intake[154]. There is emerging evidence that the anorexigenic hormone peptide YY (PYY) plays a role in the pathogenesis of AN[155]. PYY is produced mainly in the colon, where SCFAs are produced at high levels through the fermentation of fiber by the gut microbiota[156]. SCFAs strongly stimulate the production of PYY in human enteroendocrine L-cells in the gut wall[152].
Microbiota-modulating strategies may be promising determinants of the healing process and the outcome of AN[149]. Nutritional interventions, including supplements that have the potential to influence the gut microbiota, are important research targets when developing future AN therapies, especially for patients who are unable to normalize their gut microbiota by a sufficient food intake during psychotherapy. Fecal microbiota transplantation (FMT) has been associated with significant improvement in diseases such as irritable bowel syndrome[157,158]. FMT involves transplanting the entire fecal microenvironment, including SCFAs and other substances with potential effects on food intake and brain-gut interactions[159]. The first case reports on FMT in patients with SE-AN found weight gain in one patient, but no effect on BMI in another[160,161]. Data from the ongoing pilot study “Fecal Microbiota Transplantation (FMT) in the treatment of SE-AN”[162] may provide valuable information on feasibility of FMT in patients with SE-AN. Future studies should clarify whether interventions aimed at establishing normal brain-gut interactions can reduce dropout and relapse rates among patients with AN. Tools to assess and describe the responses mediated through the brain-gut axis, including clinical, biochemical and radiological methods like functional magnetic resonance imaging[163], should be further developed. Such approaches would allow interactions between the brain and gut in AN and the possibility of treatment with brain-gut modulation to be assessed.
Other emerging therapies focus on direct effects on the brain in regulating food intake and the cognitive alterations related to AN. Transcranial direct-current stimulation is a method for directly modulating the excitability of cortical regions using small electrical currents. A pilot study that applied this method to the left dorsolateral prefrontal cortex in seven patients with AN found that the procedure was well tolerated, and was associated with modest short-term improvements in scores on eating scales in five of the patients[148]. Another non-invasive technique, repetitive transcranial magnetic stimulation, was investigated in ten patients with AN, which revealed that a single session of repetitive transcranial magnetic stimulation was well tolerated and improved feelings of fullness and anxiety[148].
Deep brain stimulation is another neuromodulatory technique that is currently under investigation for the treatment of AN[148]. It is a surgical procedure that involves the implantation of stimulating electrodes into key brain structures that are believed to drive the pathological activity associated with AN[148]. One study applied continuous stimulation of the subcallosal cingulate for 1 year to 16 patients with SE-AN, which increased BMI from 13.8 to 17.3 kg/m2[164]. However, these findings in studies of neuromodulatory techniques might be driven by a placebo effect as well as an increased motivation of patients to engage in TAU[4,165,166].
Learning models suggest that exposure-like therapy could be effective in AN. Exposure interventions to AN-related stimuli (e.g., food, body) have been tested in small trials of patients with AN[4,167,168]. Virtual-reality environments have also been used to manage food-related or body-related fears in small trials including patients with AN[4,169].
Current research activity in the field of EDs is inadequate given the cost of the problem, since 8- to 10-times more research funding is provided for depression and psychosis[4]. Several projects have aimed at producing research priorities for AN. The Canadian Eating Disorder Priority Partnership was established to identify and prioritize the ten top research priorities for females aged at least 15 years with AN, by incorporating equal input from those with lived experience, families, and healthcare professionals. Their conclusions were published 2020[170]. The top priorities identified were related to “treatment gaps” and the need for “more surveillance data”. Furthermore, a panel consisting of Australian members of the Australian and New Zealand Academy for Eating Disorders and the National Collaboration for Eating Disorders in Australia[171] were invited to take part in a survey on how important it was that each of 29 research areas received funding. The 291 responders were eating-disorder specialists, consumers/carers or affiliates (clinicians and researchers not specializing in EDs, along with participants from the industry). The top-three-ranked priorities for research funding were “accessible evidence-based treatments”, “origins of EDs” and “early detection and intervention”. Within these domains, the following research areas all received very high ratings: “early intervention at all critical risk periods”, “what to do when first line treatments don’t work”, “enhancing existing eating-disorder treatments”, “accessible services” and “early detection”[171].
These surveys support research on “early detection and intervention”, including those that aim to decrease the time from the onset of AN to the initiation of evidence-based treatment.
Studies on the transition to adult psychiatric services (“treatment gaps”) will be valuable, including those designed to determine how to ensure that the patient accepts transfer to another therapist, who will sometimes apply a different treatment approach. Comparisons of different models of organization including studies on the co-localization of adolescent and adult treatment services could be part of this research. Long term effects of outpatient vs inpatient therapy should be assessed. If a substantially larger proportion of the patients may be treated as outpatients, the problems related to coordinating outpatient and inpatient therapy can be reduced.
Studies on the effects of implementing treatment standards and therapist training suitable for therapists in decentralized treatment units may inform decisions around how to make services for early intervention more accessible.
National quality-assurance registries for EDs are being established in several countries[172], which can provide surveillance data and detect possible regional inequalities in healthcare. Studies on early intervention with optimally implemented, effective evidence-based methods would be of great value. How non-completion rates can be decreased is another essential research question to address (“enhancing existing eating-disorder treatments”). Establishing standards for practice and training appears to be of value for standardizing care and enhancing existing eating-disorder treatments[173].
In line with the aim of “enhancing existing ED treatments”, several adaptations to FBT have been published in order to enhance effectiveness[174]. Still, outstanding dilemmas need research attention. Especially, FBT places a heavy task on parents, and it may be impossible for some families to mobilize for a variety of reasons, e.g., other mental illnesses. Mental health services need guidance on how to support these families so the young person can stay home and yet receive the necessary day-by-day support. This is especially crucial in cases where the young person does not have the motivation and/or ability to initiate behavioral change that is needed to succeed in CBT-ED.
In light of the moderate success rates across all psychotherapeutic treatment for AN, two types of research might improve outcomes. First, information is needed on what works for whom, in order to choose more personalized modes of treatment. Second, research on how to maximize the effect of non-specific factors in therapy, e.g., therapeutic alliance, may provide avenues for better outcomes in the future. One example of a service development that aims to adapt specific aspects of treatment to individual needs is the “PEACE pathway” which targets individuals with AN and autism spectrum disorders[175].
Turning to the medical management of AN, the mechanisms underlying sudden death in these patients warrant further research, since data are lacking regarding the cardiac rhythm at the end of life. The most significant other knowledge gaps include the management of bone mineral density, and the GI problems in AN, as well as electrolyte regulation, mechanisms of kidney damage and refeeding syndrome[176].
Despite the significant progress that has been made in the understanding of the medical complications of AN, considerable work remains to be done. There are both research and treatment gaps, and bridging them will ultimately improve the medical treatment outcomes of patients suffering from AN[176].
Defining the diagnostic criteria is probably the most important first step in SE-AN research. In addition, the criteria for recovery from AN will help delineate the population and help to define the aims of healthcare for SE-AN. Progress in understanding how to manage the medical complications of this illness has provided the potential to significantly increase the quality of life and life expectancy in this patient population. Some of the emerging strategies might improve the results of psychotherapy, including treatment aiming at reducing attrition, relapse and thereby decreasing recruitment to the SE-AN population. Early intervention with evidence-based therapy including engagement of the large population of patients with SE-AN who is not seeking healthcare will perhaps be the most important prophylactic intervention to reduce the number of patients with SE-AN.
Outcome of AN is unacceptably poor. However, the last decades have brought several effective psychotherapies for children, adolescents and adults. For many reasons, outpatient treatment is preferable. Inpatient treatment is needed in cases of acute medical risk, severe suicidal risk and when weight gain is not obtained in outpatient treatment in spite of engagement in therapy. Due to progress in understanding of the medical complications of AN more patients can safely be treated as outpatients. The implementation of effective psychotherapies and safe outpatient medical management are valuable tools for improvement of healthcare in AN.
The authors thank Lein RK at Bergen University Library for performing the literature searches.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Norway
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li SY S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Mitchell JE, Peterson CB. Anorexia Nervosa. N Engl J Med. 2020;382:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 2. | American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). Washington DC: American Psychiatric Association, 2013. |
| 3. | Golden NH, Mehler PS. Atypical anorexia nervosa can be just as bad. Cleve Clin J Med. 2020;87:172-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Treasure J, Duarte TA, Schmidt U. Eating disorders. Lancet. 2020;395:899-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 512] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 5. | Treasure J, Zipfel S, Micali N, Wade T, Stice E, Claudino A, Schmidt U, Frank GK, Bulik CM, Wentz E. Anorexia nervosa. Nat Rev Dis Primers. 2015;1:15074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 6. | Steinhausen HC, Jensen CM. Time trends in lifetime incidence rates of first-time diagnosed anorexia nervosa and bulimia nervosa across 16 years in a Danish nationwide psychiatric registry study. Int J Eat Disord. 2015;48:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry. 2011;68:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1391] [Cited by in RCA: 1674] [Article Influence: 119.6] [Reference Citation Analysis (0)] |
| 8. | Sharp WG, Stubbs KH. Avoidant/restrictive food intake disorder: A diagnosis at the intersection of feeding and eating disorders necessitating subtype differentiation. Int J Eat Disord. 2019;52:398-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Aulinas A, Marengi DA, Galbiati F, Asanza E, Slattery M, Mancuso CJ, Wons O, Micali N, Bern E, Eddy KT, Thomas JJ, Misra M, Lawson EA. Medical comorbidities and endocrine dysfunction in low-weight females with avoidant/restrictive food intake disorder compared to anorexia nervosa and healthy controls. Int J Eat Disord. 2020;53:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Udo T, Grilo CM. Prevalence and Correlates of DSM-5-Defined Eating Disorders in a Nationally Representative Sample of U.S. Adults. Biol Psychiatry. 2018;84:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 510] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 11. | Nagata JM, Ganson KT, Austin SB. Emerging trends in eating disorders among sexual and gender minorities. Curr Opin Psychiatry. 2020;33:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 12. | Schmidt U, Adan R, Böhm I, Campbell IC, Dingemans A, Ehrlich S, Elzakkers I, Favaro A, Giel K, Harrison A, Himmerich H, Hoek HW, Herpertz-Dahlmann B, Kas MJ, Seitz J, Smeets P, Sternheim L, Tenconi E, van Elburg A, van Furth E, Zipfel S. Eating disorders: the big issue. Lancet Psychiatry. 2016;3:313-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 13. | Litmanen J, Fröjd S, Marttunen M, Isomaa R, Kaltiala-Heino R. Are eating disorders and their symptoms increasing in prevalence among adolescent population? Nord J Psychiatry. 2017;71:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Smink FR, van Hoeken D, Hoek HW. Epidemiology, course, and outcome of eating disorders. Curr Opin Psychiatry. 2013;26:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 303] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 15. | Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. Diagnostic crossover in anorexia nervosa and bulimia nervosa: implications for DSM-V. Am J Psychiatry. 2008;165:245-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 302] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 16. | Javaras KN, Runfola CD, Thornton LM, Agerbo E, Birgegård A, Norring C, Yao S, Råstam M, Larsson H, Lichtenstein P, Bulik CM. Sex- and age-specific incidence of healthcare-register-recorded eating disorders in the complete swedish 1979-2001 birth cohort. Int J Eat Disord. 2015;48:1070-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Galmiche M, Déchelotte P, Lambert G, Tavolacci MP. Prevalence of eating disorders over the 2000-2018 period: a systematic literature review. Am J Clin Nutr. 2019;109:1402-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 804] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 18. | Keski-Rahkonen A, Mustelin L. Epidemiology of eating disorders in Europe: prevalence, incidence, comorbidity, course, consequences, and risk factors. Curr Opin Psychiatry. 2016;29:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 480] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 19. | Hoek HW. Review of the worldwide epidemiology of eating disorders. Curr Opin Psychiatry. 2016;29:336-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 20. | Pike KM, Dunne PE. The rise of eating disorders in Asia: a review. J Eat Disord. 2015;3:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 21. | Schaumberg K, Welch E, Breithaupt L, Hübel C, Baker JH, Munn-Chernoff MA, Yilmaz Z, Ehrlich S, Mustelin L, Ghaderi A, Hardaway AJ, Bulik-Sullivan EC, Hedman AM, Jangmo A, Nilsson IAK, Wiklund C, Yao S, Seidel M, Bulik CM. The Science Behind the Academy for Eating Disorders' Nine Truths About Eating Disorders. Eur Eat Disord Rev. 2017;25:432-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 22. | van Hoeken D, Burns JK, Hoek HW. Epidemiology of eating disorders in Africa. Curr Opin Psychiatry. 2016;29:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Hoek HW, van Harten PN, Hermans KME, Katzman MA, Matroos GE, Susser ES. The incidence of anorexia nervosa on Curacao. Am J Psychiat. 2005;162:748-752. |
| 24. | Perez M, Ohrt TK, Hoek HW. Prevalence and treatment of eating disorders among Hispanics/Latino Americans in the United States. Curr Opin Psychiatry. 2016;29:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Kolar DR, Rodriguez DL, Chams MM, Hoek HW. Epidemiology of eating disorders in Latin America: a systematic review and meta-analysis. Curr Opin Psychiatry. 2016;29:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Schooler D, Daniels EA. "I am not a skinny toothpick and proud of it": Latina adolescents' ethnic identity and responses to mainstream media images. Body Image. 2014;11:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Carlat DJ, Camargo CA Jr, Herzog DB. Eating disorders in males: a report on 135 patients. Am J Psychiatry. 1997;154:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 224] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Sjostedt JP, Schumaker JF, Nathawat SS. Eating disorders among Indian and Australian university students. J Soc Psychol. 1998;138:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Couturier J, Isserlin L, Norris M, Spettigue W, Brouwers M, Kimber M, McVey G, Webb C, Findlay S, Bhatnagar N, Snelgrove N, Ritsma A, Preskow W, Miller C, Coelho J, Boachie A, Steinegger C, Loewen R, Loewen T, Waite E, Ford C, Bourret K, Gusella J, Geller J, LaFrance A, LeClerc A, Scarborough J, Grewal S, Jericho M, Dimitropoulos G, Pilon D. Canadian practice guidelines for the treatment of children and adolescents with eating disorders. J Eat Disord. 2020;8:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 30. | Watson HJ, Yilmaz Z, Thornton LM, Hübel C, Coleman JRI, Gaspar HA, Bryois J, Hinney A, Leppä VM, Mattheisen M, Medland SE, Ripke S, Yao S, Giusti-Rodríguez P; Anorexia Nervosa Genetics Initiative, Hanscombe KB, Purves KL; Eating Disorders Working Group of the Psychiatric Genomics Consortium, Adan RAH, Alfredsson L, Ando T, Andreassen OA, Baker JH, Berrettini WH, Boehm I, Boni C, Perica VB, Buehren K, Burghardt R, Cassina M, Cichon S, Clementi M, Cone RD, Courtet P, Crow S, Crowley JJ, Danner UN, Davis OSP, de Zwaan M, Dedoussis G, Degortes D, DeSocio JE, Dick DM, Dikeos D, Dina C, Dmitrzak-Weglarz M, Docampo E, Duncan LE, Egberts K, Ehrlich S, Escaramís G, Esko T, Estivill X, Farmer A, Favaro A, Fernández-Aranda F, Fichter MM, Fischer K, Föcker M, Foretova L, Forstner AJ, Forzan M, Franklin CS, Gallinger S, Giegling I, Giuranna J, Gonidakis F, Gorwood P, Mayora MG, Guillaume S, Guo Y, Hakonarson H, Hatzikotoulas K, Hauser J, Hebebrand J, Helder SG, Herms S, Herpertz-Dahlmann B, Herzog W, Huckins LM, Hudson JI, Imgart H, Inoko H, Janout V, Jiménez-Murcia S, Julià A, Kalsi G, Kaminská D, Kaprio J, Karhunen L, Karwautz A, Kas MJH, Kennedy JL, Keski-Rahkonen A, Kiezebrink K, Kim YR, Klareskog L, Klump KL, Knudsen GPS, La Via MC, Le Hellard S, Levitan RD, Li D, Lilenfeld L, Lin BD, Lissowska J, Luykx J, Magistretti PJ, Maj M, Mannik K, Marsal S, Marshall CR, Mattingsdal M, McDevitt S, McGuffin P, Metspalu A, Meulenbelt I, Micali N, Mitchell K, Monteleone AM, Monteleone P, Munn-Chernoff MA, Nacmias B, Navratilova M, Ntalla I, O'Toole JK, Ophoff RA, Padyukov L, Palotie A, Pantel J, Papezova H, Pinto D, Rabionet R, Raevuori A, Ramoz N, Reichborn-Kjennerud T, Ricca V, Ripatti S, Ritschel F, Roberts M, Rotondo A, Rujescu D, Rybakowski F, Santonastaso P, Scherag A, Scherer SW, Schmidt U, Schork NJ, Schosser A, Seitz J, Slachtova L, Slagboom PE, Slof-Op 't Landt MCT, Slopien A, Sorbi S, Świątkowska B, Szatkiewicz JP, Tachmazidou I, Tenconi E, Tortorella A, Tozzi F, Treasure J, Tsitsika A, Tyszkiewicz-Nwafor M, Tziouvas K, van Elburg AA, van Furth EF, Wagner G, Walton E, Widen E, Zeggini E, Zerwas S, Zipfel S, Bergen AW, Boden JM, Brandt H, Crawford S, Halmi KA, Horwood LJ, Johnson C, Kaplan AS, Kaye WH, Mitchell JE, Olsen CM, Pearson JF, Pedersen NL, Strober M, Werge T, Whiteman DC, Woodside DB, Stuber GD, Gordon S, Grove J, Henders AK, Juréus A, Kirk KM, Larsen JT, Parker R, Petersen L, Jordan J, Kennedy M, Montgomery GW, Wade TD, Birgegård A, Lichtenstein P, Norring C, Landén M, Martin NG, Mortensen PB, Sullivan PF, Breen G, Bulik CM. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet. 2019;51:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 669] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 31. | Herpertz-Dahlmann B, Seitz J, Konrad K. Aetiology of anorexia nervosa: from a "psychosomatic family model" to a neuropsychiatric disorder? Eur Arch Psychiatry Clin Neurosci. 2011;261 Suppl 2:S177-S181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Trottier K, MacDonald DE. Update on Psychological Trauma, Other Severe Adverse Experiences and Eating Disorders: State of the Research and Future Research Directions. Curr Psychiatry Rep. 2017;19:45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 33. | Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WS. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychol Bull. 2004;130:19-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 839] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 34. | Cost J, Krantz MJ, Mehler PS. Medical complications of anorexia nervosa. Cleve Clin J Med. 2020;87:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Le LK, Barendregt JJ, Hay P, Mihalopoulos C. Prevention of eating disorders: A systematic review and meta-analysis. Clin Psychol Rev. 2017;53:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 36. | Stice E, Johnson S, Turgon R. Eating Disorder Prevention. Psychiatr Clin North Am. 2019;42:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Adametz L, Richter F, Strauss B, Walther M, Wick K, Berger U. Long-term effectiveness of a school-based primary prevention program for anorexia nervosa: A 7-to 8-year follow-up. Eat Behav. 2017;25:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Martinsen M, Bahr R, Børresen R, Holme I, Pensgaard AM, Sundgot-Borgen J. Preventing eating disorders among young elite athletes: a randomized controlled trial. Med Sci Sports Exerc. 2014;46:435-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 39. | Kalindjian N, Hirot F, Stona AC, Huas C, Godart N. Early detection of eating disorders: a scoping review. Eat Weight Disord. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | Austin A, Flynn M, Richards K, Hodsoll J, Duarte TA, Robinson P, Kelly J, Schmidt U. Duration of untreated eating disorder and relationship to outcomes: A systematic review of the literature. Eur Eat Disord Rev. 2021;29:329-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 41. | Wonderlich SA, Bulik CM, Schmidt U, Steiger H, Hoek HW. Severe and enduring anorexia nervosa: Update and observations about the current clinical reality. Int J Eat Disord. 2020;53:1303-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 42. | Brockmeyer T, Friederich HC, Schmidt U. Advances in the treatment of anorexia nervosa: a review of established and emerging interventions. Psychol Med. 2018;48:1228-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 43. | Khalsa SS, Portnoff LC, McCurdy-McKinnon D, Feusner JD. What happens after treatment? J Eat Disord. 2017;5:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 226] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 44. | Treasure J, Stein D, Maguire S. Has the time come for a staging model to map the course of eating disorders from high risk to severe enduring illness? Early Interv Psychiatry. 2015;9:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 45. | Dobrescu SR, Dinkler L, Gillberg C, Råstam M, Wentz E. Anorexia nervosa: 30-year outcome. Br J Psychiatry. 2020;216:97-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 46. | Stewart CS, McEwen FS, Konstantellou A, Eisler I, Simic M. Impact of ASD Traits on Treatment Outcomes of Eating Disorders in Girls. Eur Eat Disord Rev. 2017;25:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Hughes EK, Goldschmidt AB, Labuschagne Z, Loeb KL, Sawyer SM, Le Grange D. Eating disorders with and without comorbid depression and anxiety: similarities and differences in a clinical sample of children and adolescents. Eur Eat Disord Rev. 2013;21:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 48. | Herpertz-Dahlmann B, Dempfle A, Egberts KM, Kappel V, Konrad K, Vloet JA, Bühren K. Outcome of childhood anorexia nervosa-The results of a five- to ten-year follow-up study. Int J Eat Disord. 2018;51:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 49. | Hjern A, Lindberg L, Lindblad F. Outcome and prognostic factors for adolescent female in-patients with anorexia nervosa: 9- to 14-year follow-up. Br J Psychiatry. 2006;189:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Cass K, McGuire C, Bjork I, Sobotka N, Walsh K, Mehler PS. Medical Complications of Anorexia Nervosa. Psychosomatics. 2020;61:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 51. | Goldstein A, Gvion Y. Socio-demographic and psychological risk factors for suicidal behavior among individuals with anorexia and bulimia nervosa: A systematic review. J Affect Disord. 2019;245:1149-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 52. | Winston AP. Eating Disorders and Diabetes. Curr Diab Rep. 2020;20:32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 53. | Marzola E, Nasser JA, Hashim SA, Shih PA, Kaye WH. Nutritional rehabilitation in anorexia nervosa: review of the literature and implications for treatment. BMC Psychiatry. 2013;13:290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 54. | Hanachi M, Dicembre M, Rives-Lange C, Ropers J, Bemer P, Zazzo JF, Poupon J, Dauvergne A, Melchior JC. Micronutrients Deficiencies in 374 Severely Malnourished Anorexia Nervosa Inpatients. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 55. | Trigazis L, Tennankore D, Vohra S, Katzman DK. The use of herbal remedies by adolescents with eating disorders. Int J Eat Disord. 2004;35:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Biffl WL, Narayanan V, Gaudiani JL, Mehler PS. The management of pneumothorax in patients with anorexia nervosa: A case report and review of the literature. Patient Saf Surg. 2010;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Mehler PS, Brown C. Anorexia nervosa - medical complications. J Eat Disord. 2015;3:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 58. | Krantz MJ, Mehler PS. Resting tachycardia, a warning sign in anorexia nervosa: case report. BMC Cardiovasc Disord. 2004;4:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Hermont AP, Oliveira PA, Martins CC, Paiva SM, Pordeus IA, Auad SM. Tooth erosion and eating disorders: a systematic review and meta-analysis. PLoS One. 2014;9:e111123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Robinson P, Rhys Jones W. MARSIPAN: management of really sick patients with anorexia nervosa. BJPsych Advances. 2018;24:20-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 61. | Dalle Grave R, Sartirana M, Sermattei S, Calugi S. Treatment of Eating Disorders in Adults Versus Adolescents: Similarities and Differences. Clin Ther. 2021;43:70-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 62. | Eating Disorders: recognition and treatment [Internet]. National Institute for Health and Care Excellence, London, 2017. [cited 18 May 2020.]. Available from: https://www.nice.org.uk/guidance/ng69. |
| 63. | Couturier J, Isserlin L, Spettigue W, Norris M. Psychotropic Medication for Children and Adolescents with Eating Disorders. Child Adolesc Psychiatr Clin N Am. 2019;28:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Crow SJ. Pharmacologic Treatment of Eating Disorders. Psychiatr Clin North Am. 2019;42:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 65. | Blanchet C, Guillaume S, Bat-Pitault F, Carles ME, Clarke J, Dodin V, Duriez P, Gerardin P, Hanachi-Guidoum M, Iceta S, Leger J, Segrestin B, Stheneur C, Godart N. Medication in AN: A Multidisciplinary Overview of Meta-Analyses and Systematic Reviews. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 66. | Alañón Pardo MDM, Ferrit Martín M, Calleja Hernández MÁ, Morillas Márquez F. Adherence of psychopharmacological prescriptions to clinical practice guidelines in patients with eating behavior disorders. Eur J Clin Pharmacol. 2017;73:1305-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | McClelland J, Hodsoll J, Brown A, Lang K, Boysen E, Flynn M, Mountford VA, Glennon D, Schmidt U. A pilot evaluation of a novel First Episode and Rapid Early Intervention service for Eating Disorders (FREED). Eur Eat Disord Rev. 2018;26:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 68. | Royal College of Psychiatrists. Position statement on early intervention for eating disorders, London, 2019. [cited 2019 Mar 15]. Available from: https://www.rcpsych.ac.uk/docs/default-source/improving-care/better-mh-policy/position-statements/ps03_19.pdf?sfvrsn=b1283556_2. |
| 69. | Flynn M, Austin A, Lang K, Allen K, Bassi R, Brady G, Brown A, Connan F, Franklin-Smith M, Glennon D, Grant N, Jones WR, Kali K, Koskina A, Mahony K, Mountford V, Nunes N, Schelhase M, Serpell L, Schmidt U. Assessing the impact of First Episode Rapid Early Intervention for Eating Disorders on duration of untreated eating disorder: A multi-centre quasi-experimental study. Eur Eat Disord Rev. 2021;29:458-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 70. | Lock J. Family therapy for eating disorders in youth: current confusions, advances, and new directions. Curr Opin Psychiatry. 2018;31:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 71. | Lock J, Le Grange D. Treatment Manual for Anorexia Nervosa. 2nd edition. New York: Guilford Press, 2015. |
| 72. | Dalle Grave R, Eckhardt S, Calugi S, Le Grange D. A conceptual comparison of family-based treatment and enhanced cognitive behavior therapy in the treatment of adolescents with eating disorders. J Eat Disord. 2019;7. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 73. | Lock J, Le Grange D, Agras WS, Moye A, Bryson SW, Jo B. Randomized clinical trial comparing family-based treatment with adolescent-focused individual therapy for adolescents with anorexia nervosa. Arch Gen Psychiatry. 2010;67:1025-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 695] [Cited by in RCA: 514] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 74. | Fisher CA, Skocic S, Rutherford KA, Hetrick SE. Family therapy approaches for anorexia nervosa. Cochrane Database Syst Rev. 2019;5:CD004780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 75. | Lock J, Kraemer HC, Jo B, Couturier J. When meta-analyses get it wrong: response to 'treatment outcomes for anorexia nervosa: a systematic review and meta-analysis of randomized controlled trials'. Psychol Med. 2019;49:697-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Hay P, Chinn D, Forbes D, Madden S, Newton R, Sugenor L, Touyz S, Ward W; Royal Australian and New Zealand College of Psychiatrists. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of eating disorders. Aust N Z J Psychiatry. 2014;48:977-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 374] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 77. | Le Grange D, Hughes EK, Court A, Yeo M, Crosby RD, Sawyer SM. Randomized Clinical Trial of Parent-Focused Treatment and Family-Based Treatment for Adolescent Anorexia Nervosa. J Am Acad Child Adolesc Psychiatry. 2016;55:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 78. | Carrot B, Duclos J, Barry C, Radon L, Maria AS, Kaganski I, Jeremic Z, Barton-Clegg V, Corcos M, Lasfar M, Gerardin P, Harf A, Moro MR, Blanchet C, Godart N. Multicenter randomized controlled trial on the comparison of multi-family therapy (MFT) and systemic single-family therapy (SFT) in young patients with anorexia nervosa: study protocol of the THERAFAMBEST study. Trials. 2019;20:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Agras WS, Lock J, Brandt H, Bryson SW, Dodge E, Halmi KA, Jo B, Johnson C, Kaye W, Wilfley D, Woodside B. Comparison of 2 family therapies for adolescent anorexia nervosa: a randomized parallel trial. JAMA Psychiatry. 2014;71:1279-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 80. | Scarborough J. Family-Based Therapy for Pediatric Anorexia Nervosa. The Family Journal. 2018;26:90-98. [DOI] [Full Text] |
| 81. | Wufong E, Rhodes P, Conti J. "We don't really know what else we can do": Parent experiences when adolescent distress persists after the Maudsley and family-based therapies for anorexia nervosa. J Eat Disord. 2019;7:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 82. | Katzman DK, Peebles R, Sawyer SM, Lock J, Le Grange D. The role of the pediatrician in family-based treatment for adolescent eating disorders: opportunities and challenges. J Adolesc Health. 2013;53:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. |
Byrne S, Wade T, Hay P, Touyz S, Fairburn CG, Treasure J, et al A randomised controlled trial of three psychological treatments for anorexia nervosa.
|
| 84. | Schmidt U, Ryan EG, Bartholdy S, Renwick B, Keyes A, O'Hara C, McClelland J, Lose A, Kenyon M, Dejong H, Broadbent H, Loomes R, Serpell L, Richards L, Johnson-Sabine E, Boughton N, Whitehead L, Bonin E, Beecham J, Landau S, Treasure J. Two-year follow-up of the MOSAIC trial: A multicenter randomized controlled trial comparing two psychological treatments in adult outpatients with broadly defined anorexia nervosa. Int J Eat Disord. 2016;49:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 85. | Schmidt U, Magill N, Renwick B, Keyes A, Kenyon M, Dejong H, Lose A, Broadbent H, Loomes R, Yasin H, Watson C, Ghelani S, Bonin EM, Serpell L, Richards L, Johnson-Sabine E, Boughton N, Whitehead L, Beecham J, Treasure J, Landau S. The Maudsley Outpatient Study of Treatments for Anorexia Nervosa and Related Conditions (MOSAIC): Comparison of the Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA) with specialist supportive clinical management (SSCM) in outpatients with broadly defined anorexia nervosa: A randomized controlled trial. J Consult Clin Psychol. 2015;83:796-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 86. |
Zipfel S, Wild B, Groß G, Friederich H-C, Teufel M, Schellberg D, et al Focal psychodynamic therapy, cognitive behaviour therapy, and optimised treatment as usual in outpatients with anorexia nervosa (ANTOP study): randomised controlled trial.
|
| 87. | Fairburn CG. Cognitive Behavior Therapy and Eating Disorders. London/New York: The Guilford Press, 2008. |
| 88. | Dalle Grave R, Calugi S, Sartirana M, Sermattei S, Conti M. Enhanced cognitive behaviour therapy for adolescents with eating disorders: A systematic review of current status and future perspectives. Ijedo. 2021;3:1-11. [DOI] [Full Text] |
| 89. | Dalle Grave R, Calugi S. Cognitive Behavior Therapy for Adolescents with Eating Disorders. New York: Guilford Press, 2020. |
| 90. | Calugi S, Sartirana M, Frostad S, Dalle Grave R. Enhanced cognitive behavior therapy for severe and extreme anorexia nervosa: An outpatient case series. J Eat Disord. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 91. | Fairburn CG, Cooper Z, Doll HA, O'Connor ME, Palmer RL, Dalle Grave R. Enhanced cognitive behaviour therapy for adults with anorexia nervosa: a UK-Italy study. Behav Res Ther. 2013;51:R2-R8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 92. | Zhu J, Yang Y, Touyz S, Park R, Hay P. Psychological Treatments for People With Severe and Enduring Anorexia Nervosa: A Mini Review. Frontiers in psychiatry. 2020;11. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 93. | Hay P, Touyz S. Classification challenges in the field of eating disorders: can severe and enduring anorexia nervosa be better defined? J Eat Disord. 2018;6:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 94. | Eddy KT, Tabri N, Thomas JJ, Murray HB, Keshaviah A, Hastings E, Edkins K, Krishna M, Herzog DB, Keel PK, Franko DL. Recovery From Anorexia Nervosa and Bulimia Nervosa at 22-Year Follow-Up. J Clin Psychiatry. 2017;78:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 288] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 95. | Touyz S, Le Grange D, Lacey H, Hay P, Smith R, Maguire S, Bamford B, Pike KM, Crosby RD. Treating severe and enduring anorexia nervosa: a randomized controlled trial. Psychol Med. 2013;43:2501-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 96. | Raykos BC, Erceg-Hurn DM, McEvoy PM, Fursland A, Waller G. Severe and enduring anorexia nervosa? J Consult Clin Psychol. 2018;86:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 97. | Dalle Grave R. Intensive Cognitive Behavior Therapy for Eating Disorders. New York: Nova Science Publisher, 2012. |
| 98. | Calugi S, El Ghoch M, Dalle Grave R. Intensive enhanced cognitive behavioural therapy for severe and enduring anorexia nervosa: A longitudinal outcome study. Behav Res Ther. 2017;89:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 99. | Radunz M, Keegan E, Osenk I, Wade TD. Relationship between eating disorder duration and treatment outcome: Systematic review and meta-analysis. Int J Eat Disord. 2020;53:1761-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 100. | Sachs K, Andersen D, Sommer J, Winkelman A, Mehler PS. Avoiding medical complications during the refeeding of patients with anorexia nervosa. Eat Disord. 2015;23:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Garber AK, Sawyer SM, Golden NH, Guarda AS, Katzman DK, Kohn MR, Le Grange D, Madden S, Whitelaw M, Redgrave GW. A systematic review of approaches to refeeding in patients with anorexia nervosa. Int J Eat Disord. 2016;49:293-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 102. | Winston AP. The clinical biochemistry of anorexia nervosa. Ann Clin Biochem. 2012;49:132-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 103. | Mehler PS, Krantz MJ, Sachs KV. Treatments of medical complications of anorexia nervosa and bulimia nervosa. J Eat Disord. 2015;3:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Hart S, Abraham S, Franklin RC, Twigg SM, Russell J. Hypoglycaemia following a mixed meal in eating disorder patients. Postgrad Med J. 2011;87:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 105. | Gaudiani JL, Brinton JT, Sabel AL, Rylander M, Catanach B, Mehler PS. Medical outcomes for adults hospitalized with severe anorexia nervosa: An analysis by age group. Int J Eat Disord. 2016;49:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 106. | Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Arch Intern Med. 2005;165:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 107. | Bouquegneau A, Dubois BE, Krzesinski JM, Delanaye P. Anorexia nervosa and the kidney. Am J Kidney Dis. 2012;60:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 108. | Onfiani, Carubbi, Pellegrini. Evaluating renal function and defining protein requirements in patients affected by anorexia nervosa: a case report. Ijedo. 2020;2:43-48. [DOI] [Full Text] |
| 109. | De Filippo E, Marra M, Alfinito F, Di Guglielmo ML, Majorano P, Cerciello G, De Caprio C, Contaldo F, Pasanisi F. Hematological complications in anorexia nervosa. Eur J Clin Nutr. 2016;70:1305-1308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 110. | Brown RF, Bartrop R, Beumont P, Birmingham CL. Bacterial infections in anorexia nervosa: delayed recognition increases complications. Int J Eat Disord. 2005;37:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Veronese N, Solmi M, Rizza W, Manzato E, Sergi G, Santonastaso P, Caregaro L, Favaro A, Correll CU. Vitamin D status in anorexia nervosa: A meta-analysis. Int J Eat Disord. 2015;48:803-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 112. | Oudman E, Wijnia JW, Oey MJ, van Dam MJ, Postma A. Preventing Wernicke's encephalopathy in anorexia nervosa: A systematic review. Psychiatry Clin Neurosci. 2018;72:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 113. | Sechi G, Serra A. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. The Lancet Neurology. 2007;6:442-455. [RCA] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 742] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 114. | DiNicolantonio JJ, Liu J, O'Keefe JH. Magnesium for the prevention and treatment of cardiovascular disease. Open Heart. 2018;5:e000775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 115. | Aigner M, Treasure J, Kaye W, Kasper S; WFSBP Task Force On Eating Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of eating disorders. World J Biol Psychiatry. 2011;12:400-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 116. | Rosen E, Bakshi N, Watters A, Rosen HR, Mehler PS. Hepatic Complications of Anorexia Nervosa. Dig Dis Sci. 2017;62:2977-2981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 117. | Gibson D, Watters A, Cost J, Mascolo M, Mehler PS. Extreme anorexia nervosa: medical findings, outcomes, and inferences from a retrospective cohort. J Eat Disord. 2020;8:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 118. | Bachmann KN, Fazeli PK, Lawson EA, Russell BM, Riccio AD, Meenaghan E, Gerweck AV, Eddy K, Holmes T, Goldstein M, Weigel T, Ebrahimi S, Mickley D, Gleysteen S, Bredella MA, Klibanski A, Miller KK. Comparison of hip geometry, strength, and estimated fracture risk in women with anorexia nervosa and overweight/obese women. J Clin Endocrinol Metab. 2014;99:4664-4673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 119. | Mehler PS. Clinical guidance on osteoporosis and eating disorders: the NEDA continuing education series. Eat Disord. 2019;27:471-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Resmark G, Herpertz S, Herpertz-Dahlmann B, Zeeck A. Treatment of Anorexia Nervosa-New Evidence-Based Guidelines. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 121. | Halvorsen I, Reas DL, Nilsen JV, Rø Ø. Naturalistic Outcome of Family-Based Inpatient Treatment for Adolescents with Anorexia Nervosa. Eur Eat Disord Rev. 2018;26:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 122. | Dalle Grave R, Conti M, Calugi S. Effectiveness of intensive cognitive behavioral therapy in adolescents and adults with anorexia nervosa. J Eat Disord. 2020;1-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 123. |
Frostad S, Danielsen YS, Rekkedal GÅ, Jevne C, Dalle Grave R, Rø Ø, et al Implementation of enhanced cognitive behaviour therapy (CBT-E) for adults with anorexia nervosa in an outpatient eating-disorder unit at a public hospital.
|
| 124. | Carter JC, Blackmore E, Sutandar-Pinnock K, Woodside DB. Relapse in anorexia nervosa: a survival analysis. Psychol Med. 2004;34:671-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 125. | Herzog DB, Dorer DJ, Keel PK, Selwyn SE, Ekeblad ER, Flores AT, Greenwood DN, Burwell RA, Keller MB. Recovery and relapse in anorexia and bulimia nervosa: a 7.5-year follow-up study. J Am Acad Child Adolesc Psychiatry. 1999;38:829-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 247] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 126. | Carter JC, McFarlane TL, Bewell C, Olmsted MP, Woodside DB, Kaplan AS, Crosby RD. Maintenance treatment for anorexia nervosa: a comparison of cognitive behavior therapy and treatment as usual. Int J Eat Disord. 2009;42:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 127. | Fichter MM, Quadflieg N, Nisslmüller K, Lindner S, Osen B, Huber T, Wünsch-Leiteritz W. Does internet-based prevention reduce the risk of relapse for anorexia nervosa? Behav Res Ther. 2012;50:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 128. | Gibson D, Workman C, Mehler PS. Medical Complications of Anorexia Nervosa and Bulimia Nervosa. Psychiatr Clin North Am. 2019;42:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 129. | Turner J, Batik M, Palmer LJ, Forbes D, McDermott BM. Detection and importance of laxative use in adolescents with anorexia nervosa. J Am Acad Child Psy. 2000;39:378-385. |
| 130. | Rizk M, Lalanne C, Berthoz S, Kern L; EVHAN Group, Godart N. Problematic Exercise in Anorexia Nervosa: Testing Potential Risk Factors against Different Definitions. PLoS One. 2015;10:e0143352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 131. | Misra M, Golden NH, Katzman DK. State of the art systematic review of bone disease in anorexia nervosa. Int J Eat Disord. 2016;49:276-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 132. | Bratland-Sanda S, Martinsen EW, Rosenvinge JH, Rø O, Hoffart A, Sundgot-Borgen J. Exercise dependence score in patients with longstanding eating disorders and controls: the importance of affect regulation and physical activity intensity. Eur Eat Disord Rev. 2011;19:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 133. | Hetterich L, Mack I, Giel KE, Zipfel S, Stengel A. An update on gastrointestinal disturbances in eating disorders. Mol Cell Endocrinol. 2019;497:110318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 134. | Schalla MA, Stengel A. Gastrointestinal alterations in anorexia nervosa - A systematic review. Eur Eat Disord Rev. 2019;27:447-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 135. | Mattheus HK, Wagner C, Becker K, Bühren K, Correll CU, Egberts KM, Ehrlich S, Fleischhaker C, Föcker M, Hahn F, Hebebrand J, Herpertz-Dahlmann B, Jaite C, Jenetzky E, Kaess M, Legenbauer PhD T, Pfeiffer PhD JP, Renner Md TJ, Roessner V, Schulze U, Sinzig J, Wessing I, von Gontard A. Incontinence and constipation in adolescent patients with anorexia nervosa-Results of a multicenter study from a German web-based registry for children and adolescents with anorexia nervosa. Int J Eat Disord. 2020;53:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 136. | Kessler U, Rekkedal GÅ, Rø Ø, Berentsen B, Steinsvik EK, Lied GA, Danielsen Y. Association between gastrointestinal complaints and psychopathology in patients with anorexia nervosa. Int J Eat Disord. 2020;53:532-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 137. | Marikar D, Reynolds S, Moghraby OS. Junior MARSIPAN (Management of Really Sick Patients with Anorexia Nervosa). Arch Dis Child Educ Pract Ed. 2016;101:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 138. | Bamford B, Barras C, Sly R, Stiles-Shields C, Touyz S, Le Grange D, Hay P, Crosby R, Lacey H. Eating disorder symptoms and quality of life: where should clinicians place their focus in severe and enduring anorexia nervosa? Int J Eat Disord. 2015;48:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 139. | Carney T, Yager J, Maguire S, Touyz SW. Involuntary Treatment and Quality of Life. Psychiatr Clin North Am. 2019;42:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 140. | Yager J. Managing Patients With Severe and Enduring Anorexia Nervosa: When Is Enough, Enough? J Nerv Ment Dis. 2019;208:277-282. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 141. | Yager J. The Futility of Arguing About Medical Futility in Anorexia Nervosa: The Question Is How Would You Handle Highly Specific Circumstances? Am J Bioeth. 2015;15:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 142. | Bulik CM, Flatt R, Abbaspour A, Carroll I. Reconceptualizing anorexia nervosa. Psychiatry Clin Neurosci. 2019;73:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 143. | Khlevner J, Park Y, Margolis KG. Brain-Gut Axis: Clinical Implications. Gastroenterol Clin North Am. 2018;47:727-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 144. | Misra M, Miller KK, Tsai P, Gallagher K, Lin A, Lee N, Herzog DB, Klibanski A. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 145. | Rehfeld JF. Cholecystokinin-From Local Gut Hormone to Ubiquitous Messenger. Front Endocrinol (Lausanne). 2017;8:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 146. | Eser D, Leicht G, Lutz J, Wenninger S, Kirsch V, Schüle C, Karch S, Baghai T, Pogarell O, Born C, Rupprecht R, Mulert C. Functional neuroanatomy of CCK-4-induced panic attacks in healthy volunteers. Hum Brain Mapp. 2009;30:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 147. | Bradwejn J, Koszycki D, Meterissian G. Cholecystokinin-tetrapeptide induces panic attacks in patients with panic disorder. Can J Psychiatry. 1990;35:83-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 180] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 148. | Lutter M. Emerging Treatments in Eating Disorders. Neurotherapeutics. 2017;14:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 149. | Herpertz-Dahlmann B, Seitz J, Baines J. Food matters: how the microbiome and gut-brain interaction might impact the development and course of anorexia nervosa. Eur Child Adolesc Psychiatry. 2017;26:1031-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 150. | Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 711] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 151. | Seitz J, Dahmen B, Keller L, Herpertz-Dahlmann B. Gut Feelings: How Microbiota Might Impact the Development and Course of Anorexia Nervosa. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 152. | Larraufie P, Martin-Gallausiaux C, Lapaque N, Dore J, Gribble FM, Reimann F, Blottiere HM. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 287] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 153. | Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1566] [Cited by in RCA: 2370] [Article Influence: 263.3] [Reference Citation Analysis (0)] |
| 154. | Chambers ES, Morrison DJ, Frost G. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proc Nutr Soc. 2015;74:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 155. | Chaudhri OB, Field BC, Bloom SR. Editorial: from gut to mind--hormonal satiety signals and anorexia nervosa. J Clin Endocrinol Metab. 2006;91:797-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 156. | Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221-1227. [PubMed] |
| 157. | Goll R, Johnsen PH, Hjerde E, Diab J, Valle PC, Hilpusch F, Cavanagh JP. Effects of fecal microbiota transplantation in subjects with irritable bowel syndrome are mirrored by changes in gut microbiome. Gut Microbes. 2020;12:1794263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 158. | El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. 2020;69:859-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 159. |
Johnsen PH, Hilpusch F, Cavanagh JP, Leikanger IS, Kolstad C, Valle PC, et al Faecal microbiota transplantation vs placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial.
|
| 160. | de Clercq NC, Frissen MN, Davids M, Groen AK, Nieuwdorp M. Weight Gain after Fecal Microbiota Transplantation in a Patient with Recurrent Underweight following Clinical Recovery from Anorexia Nervosa. Psychother Psychosom. 2019;88:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 161. | Prochazkova P, Roubalova R, Dvorak J, Tlaskalova-Hogenova H, Cermakova M, Tomasova P, Sediva B, Kuzma M, Bulant J, Bilej M, Hrabak P, Meisnerova E, Lambertova A, Papezova H. Microbiota, Microbial Metabolites, and Barrier Function in A Patient with Anorexia Nervosa after Fecal Microbiota Transplantation. Microorganisms. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 162. | Kimmel M. Fecal Microbiota Transplantation (FMT) in Treatment of Severe and Enduring Anorexia Nervosa. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. [cited 15 March 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT03928808. |
| 163. | Olivo G, Gaudio S, Schiöth HB. Brain and Cognitive Development in Adolescents with Anorexia Nervosa: A Systematic Review of fMRI Studies. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 164. | Lipsman N, Lam E, Volpini M, Sutandar K, Twose R, Giacobbe P, Sodums DJ, Smith GS, Woodside DB, Lozano AM. Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1 year follow-up of an open-label trial. Lancet Psychiatry. 2017;4:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 165. | Dalton B, Bartholdy S, McClelland J, Kekic M, Rennalls SJ, Werthmann J, Carter B, O'Daly OG, Campbell IC, David AS, Glennon D, Kern N, Schmidt U. Randomised controlled feasibility trial of real vs sham repetitive transcranial magnetic stimulation treatment in adults with severe and enduring anorexia nervosa: the TIARA study. BMJ Open. 2018;8:e021531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 166. | Dalton B, Bartholdy S, Campbell IC, Schmidt U. Neurostimulation in Clinical and Sub-clinical Eating Disorders: A Systematic Update of the Literature. Curr Neuropharmacol. 2018;16:1174-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 167. | Steinglass JE, Albano AM, Simpson HB, Wang Y, Zou J, Attia E, Walsh BT. Confronting fear using exposure and response prevention for anorexia nervosa: A randomized controlled pilot study. Int J Eat Disord. 2014;47:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 168. | Levinson CA, Byrne M. The fear of food measure: a novel measure for use in exposure therapy for eating disorders. Int J Eat Disord. 2015;48:271-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 169. | Clus D, Larsen ME, Lemey C, Berrouiguet S. The Use of Virtual Reality in Patients with Eating Disorders: Systematic Review. J Med Internet Res. 2018;20:e157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 170. | Obeid N, McVey G, Seale E, Preskow W, Norris ML. Cocreating research priorities for anorexia nervosa: The Canadian Eating Disorder Priority Setting Partnership. Int J Eat Disord. 2020;53:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 171. | Hart LM, Wade T. Identifying research priorities in eating disorders: A Delphi study building consensus across clinicians, researchers, consumers, and carers in Australia. Int J Eat Disord. 2020;53:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 172. | Birgegård A, Björck C, Clinton D. Quality assurance of specialised treatment of eating disorders using large-scale Internet-based collection systems: methods, results and lessons learned from designing the Stepwise database. Eur Eat Disord Rev. 2010;18:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 173. | Hurst K, Heruc G, Thornton C, Freeman J, Fursland A, Knight R, Roberts M, Shelton B, Wallis A, Wade T. ANZAED practice and training standards for mental health professionals providing eating disorder treatment. J Eat Disord. 2020;8:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 174. | Richards IL, Subar A, Touyz S, Rhodes P. Augmentative Approaches in Family-Based Treatment for Adolescents with Restrictive Eating Disorders: A Systematic Review. Eur Eat Disord Rev. 2018;26:92-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 175. | Tchanturia K, Smith K, Glennon D, Burhouse A. Towards an Improved Understanding of the Anorexia Nervosa and Autism Spectrum Comorbidity: PEACE Pathway Implementation. Front Psychiatry. 2020;11:640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 176. | Gibson D, Drabkin A, Krantz MJ, Mascolo M, Rosen E, Sachs K, Welles C, Mehler PS. Critical gaps in the medical knowledge base of eating disorders. Eat Weight Disord. 2018;23:419-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |