Published online Mar 19, 2022. doi: 10.5498/wjp.v12.i3.521
Peer-review started: September 22, 2021
First decision: November 8, 2021
Revised: December 14, 2021
Accepted: January 29, 2022
Article in press: January 29, 2022
Published online: March 19, 2022
Processing time: 177 Days and 1.6 Hours
Antipsychotic drugs remain the mainstay of schizophrenia treatment; however, their effectiveness has been questioned, and it is not possible to predict the response to a specific antipsychotic drug in an individual patient. Thus, it is important to compare the effectiveness of the various antipsychotics and search for possible response predictors.
To investigate the effectiveness of antipsychotic drugs, we examined response trajectories and predictors for belonging to different trajectory groups.
The Bergen-Stavanger-Innsbruck-Trondheim (BeSt InTro) trial compared the effectiveness of three atypical antipsychotics-amisulpride, aripiprazole, and olanzapine-in a prospective, semirandomized, rater-blind, head-to-head design. Adult participants with a schizophrenia spectrum disorder diagnosis, according to international classification of diseases, Tenth Revision (ICD-10) F20–29, were included. Participants were followed for a period of 12 mo, with assessments at baseline; after one, three and six weeks; and after three, six, nine and 12 mo. A latent class mixed model was fitted to our data. The three-trajectory model based on the Positive and Negative Syndrome Scale (PANSS) total score reduction was found to have adequate fit, and the study drugs, as well as various demographic and clinical parameters, were tested as predictors for belonging to the different trajectory groups.
Overall, 144 participants were included, and 41% completed the 12-mo study period. The largest trajectory group, consisting of 74% of participants, showed a PANSS total score reduction of 59% from baseline to 12 mo (Good response group). A trajectory group comprising 13% of participants had their PANSS total score reduced by 82.5% at 12 mo (Strong response group), while the last response trajectory group comprising 13% of the participants had a PANSS total score reduction of 13.6% (Slight response group). The largest part of the total reduction for the Good and Strong response groups occurred at six weeks of treatment, amounting to 45% and 48% reductions from baseline, respectively. The use of amisulpride predicted belonging to the Strong response group, while unemployment, depression, and negative psychotic symptoms at baseline increased the chance of belonging to the Slight response group, indicating a poor response to antipsychotic drug treatment.
Most of the participants (87%) had a good outcome after one year. Amisulpride users, more often than aripiprazole and olanzapine users, belonged to the response trajectory group with a strong response.
Core Tip: In this clinical trial of the three atypical antipsychotics amisulpride, aripiprazole, and olanzapine, we identified three trajectory groups of responses at the one-year follow-up. The majority of the study participants (87%) followed a trajectory of a good or strong response to antipsychotic drugs, while 13% showed a poor response. The use of amisulpride predicted belonging to the Strong response group. This antipsychotic should therefore be used more often in clinical practice. Unemployment, depression, and negative psychotic symptoms at baseline predicted nonresponse to antipsychotic drugs.
- Citation: Drosos P, Johnsen E, Bartz-Johannessen CA, Larsen TK, Reitan SK, Rettenbacher M, Kroken RA. Trajectories of response in schizophrenia-spectrum disorders: A one-year prospective cohort study of antipsychotic effectiveness. World J Psychiatry 2022; 12(3): 521-532
- URL: https://www.wjgnet.com/2220-3206/full/v12/i3/521.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i3.521
Antipsychotic drugs remain one of the most effective interventions for patients with schizophrenia-spectrum disorders[1], as recommended by the guidelines for schizophrenia treatment[2,3]. The choice of antipsychotic drug is based on its efficacy and side-effect profile, the patient’s personal history of previous response to antipsychotics, and the clinician’s experience with different types of antipsychotics[2,4]. Several studies exist on the efficacy of the various antipsychotic drugs in both multiple-episode and first-episode schizophrenia, including pairwise and network meta-analyses, which conclude that antipsychotic drugs are generally more efficacious than placebo[1,5,6]. However, the long-term use of antipsychotic drugs has been criticized because of the associated severe side effects, including brain structural changes[7] and metabolic abnormalities[8]. Moreover, a study showed that nonmedicated patients with schizophrenia performed better after the first three years of illness[9]. Many studies support, however, the effect of antipsychotic drugs on both symptom improvement and social function, as well as on the risk of hospitalization, mortality, and suicidality[10-12]. These results apply both to first-episode and multiple-episode treatment-resistant schizophrenia.
There are various ways to describe the effects of antipsychotic drug treatment on patients with schizophrenia. Important parameters include medication adherence and side effects, symptom improvement, and illness relapse. Clinicians and researchers in the field of schizophrenia frequently use the terms “treatment response”, “symptom remission”, and “recovery”. However, not all of these concepts have been clearly defined, and it is of high importance to agree on their definitions and rating methods to enhance the quality of clinical practice and research in schizophrenia. The first step in the progress of schizophrenia treatment is the response to antipsychotic drugs, which provides an amelioration of mostly positive psychotic symptoms and helps patients maintain stability. The second step is the remission of symptoms, where a prolonged improvement of key schizophrenia symptoms can be seen. The last and most difficult stage to achieve is recovery, where the patient enjoys functional and social autonomy, with no symptoms of schizophrenia or mild symptoms over a long period.
There remains a lack of consensus on the definition of standardized response criteria. Researchers have used different criteria based on the reduction of the Positive and Negative Syndrome Scale (PANSS)[13] total score and the Brief Psychiatric Rating Scale (BPRS)[14] score from baseline[15,16]. Various cutoffs have been used in clinical trials, from at least 20% to 30%, 40%, or 50% of the baseline score. Another issue is the clinical significance of the measured response, and researchers have proposed solving this problem by linking the PANSS and BPRS scores to Clinical Global Impression (CGI) scales[17]. They concluded that it is useful to apply both PANSS and CGI, as they measure different dimensions, and that it is possible to link PANSS scores to CGI scores. For example, the importance of a 20% reduction in PANSS score varies from the perspective of treating refractory patients vs acutely ill, nonrefractory patients[18,19]. In a study of response to antipsychotics in drug-naive patients with schizophrenia, 71% responded to second-generation antipsychotics at the one-year follow-up, with a 50% drop in baseline PANSS total score[20]. A shorter duration of untreated psychosis, compliance with medication treatment, and alcohol and other substance use were important predictors influencing response but not remission.
The course of schizophrenia is highly heterogeneous, and it has not yet been possible to predict which patient will respond adequately to which antipsychotic drug. An important aim of current research is to define predictors of medication response. A novel way to examine response is to define trajectories that describe the timeframe of symptom change. Trajectories also provide better information about the course of schizophrenia than dichotomized measures of success or failure of treatment, as the latter does not capture the complexity of treatment response.
In our study, the Bergen-Stavanger-Innsbruck-Trondheim (BeSt InTro) study, we compared the efficacy of three antipsychotic drugs-amisulpride, aripiprazole, and olanzapine-after a 12-mo follow-up[21]. The primary aim of this study was to define trajectories for the pooled 12-mo response to treatment with three different antipsychotic drugs. We then wanted to identify possible predictors for belonging to a certain response trajectory in the studied cohort.
This cohort study included participants in the BeSt InTro study, a 12-mo prospective, randomized, rater-blind, head-to-head comparison of amisulpride, aripiprazole, and olanzapine[21]. Each participant was randomized to a sequence of the examined antipsychotic drugs, for example amisulpride-olanzapine-aripiprazole or aripiprazole-amisulpride-olanzapine. The patient was offered the first drug in the randomized sequence, and this drug was the basis of the intention-to-treat (ITT) analyses. If the first drug could not be used because of previous inefficacy or tolerability issues, the patient was offered the next drug in the randomized sequence. The drug that was actually chosen was the basis of the preprotocol (PP) analyses.
Participants were followed over a period of 12 mo, and the assessment points were at baseline and then after one week, three weeks, six weeks, three months, six months, nine months, and 12 mo. The study medications were administered as oral tablets, and the dosing intervals were 50–1200 mg/d for amisulpride, 5–30 mg/d for aripiprazole, and 2.5–20 mg/d for olanzapine.
The participating study centers were in Bergen, Trondheim, and Stavanger in Norway in collaboration with the Schizophrenia Research Group in Innsbruck, Austria.
The inclusion criteria were 18 years of age or more and a diagnosis within the schizophrenia spectrum according to International Classification of Diseases, Tenth Revision (ICD-10) diagnoses F20–29. Participants should also have symptoms of ongoing psychosis as determined by a score of four or more on at least one of the following PANSS items: P1 (delusions), P3 (hallucinations), P5 (grandiosity), P6 (suspiciousness/persecution), or G9 (unusual thought content).
Exclusion criteria were the inability to understand the native language, organic psychosis due to limbic encephalitis, pregnancy or breastfeeding, hypersensitivity to the active substance or any of the excipients of the study drugs, prolactin-dependent tumors, pheochromocytoma, lactation, combination with medications that could induce torsade de pointes, and patients with known risk of narrow-angle glaucoma.
Patients’ clinical condition and capability of providing informed consent were confirmed by their attending physician or psychiatrist. All patients entering the study provided written informed consent. More information about randomization and concomitant medications can be found in the BeSt InTro primary outcome publication[21].
The primary outcome measure was the change in PANSS total score during the one-year follow-up, which corresponded to the minimum recommended time of maintenance antipsychotic drug therapy after an acute psychotic episode in patients with schizophrenia[22,23]. To compute the percentage reduction in PANSS, we subtracted 30 points, as this is the minimum score possible. To calculate response rates, we used the following formula: [(PANSS baseline-30)-(PANSS followup-30)] × 100/(PANSS baseline-30)[15].
We used the Structured Clinical Interview for the PANSS. All investigators conducting assessments were trained and calibrated by the PANSS Institute (https://panss.org/) until satisfactory interrater reliability was achieved.
Other outcome measures included the Calgary Depression Scale for Schizophrenia (CDSS), the CGI-Severity of Illness scale (CGI-S), and the Global Assessment of Functioning scale (GAF) as the average of GAF function and GAF symptom scale score[24].
A latent class mixed model (LCMM) with PANSS total score as a dependent variable, time as an independent fixed variable, and subject as a random intercept was fit to our data. The model fitting was performed in R using the LCMM package[25]. Models with a different number of latent classes and with the time variable on different functional forms were investigated. The Bayesian information criterion (BIC) and entropy were used to select the best model. Lower BIC and higher entropy values indicate a better model fit. Differences between the latent classes obtained by the LCMM model were examined. The model with three latent classes and with time represented as visit number best fit the data. We labeled the three different response groups as “Strong response group”, “Good response group” and “Slight response group”. Comparisons between response groups were performed by analyzing categorical and continuous variables with the use of chi-square tests and one-way ANOVAs in IBM SPSS Statistics (version 24). In the case of significant ANOVA tests, post hoc pairwise analyses were performed using Tukey’s test. In the antipsychotic drug use comparison among response groups, we divided the patients according to the ITT method, and post hoc pairwise analyses were conducted using Fisher’s test.
The data were also analyzed by splitting the patients into two groups: The Good and Strong response groups were merged into the “Response group”, and the Slight response group was labeled the “Nonresponse group”.
The study was approved in Norway by the Regional Committees for Medical and Health Research Ethics and the Norwegian Medicines Agency and in Austria by the Ethical Committee of the Medical University of Innsbruck and the Austrian Federal Office for Safety in Health Care (BASG).
The Department of Research and Development in Haukeland University Hospital conducted clinical monitoring according to the International Conference on Harmonisation-Good Clinical Practice (ICH-GCP) in Norway; in Austria, this was performed by the Clinical Trial Centre at the Medical University of Innsbruck.
Between October 20, 2011 and December 30, 2016, 359 participants were assessed for eligibility, and 144 were included and randomized to one of the study drugs. In total, 215 patients were excluded (107 did not meet the inclusion criteria, 82 declined to participate, and 26 for other reasons). Fifty-nine participants (41%) completed the 12-mo study period. The demographic and clinical characteristics for each response trajectory group are presented in Table 1.
| Strong response group (n = 19) | Good response group (n = 106) | Slight response group (n = 19) | Total (n = 144) | P value (3 groups) | P value (2 groups) | |
| n (%) | n (%) | n (%) | n (%) | |||
| Men | 13 (68.4) | 70 (66) | 10 (52.6) | 93 (64.6) | 0.495 | 0.242 |
| White | 14 (87.5) | 87 (87.9) | 17 (89.5) | 118 (88.1) | 0.978 | 0.837 |
| Living alone | 7 (38.9) | 48 (48) | 6 (31.6) | 61 (44.5) | 0.640 | 0.418 |
| Employed | 3 (17.6) | 32 (32) | 1 (5.3) | 36 (26.5) | 0.036 | 0.024 |
| Smokers | 13 (81.2) | 58 (61.1) | 13 (81.2) | 84 (66.1) | 0.113 | 0.172 |
| Alcohol abuse/dependence | 2 (13.3) | 8 (7.9) | 3 (15.8) | 13 (9.6) | 0.496 | 0.326 |
| Drug abuse/dependence | 4 (26.7) | 19 (18.6) | 4 (21.1) | 27 (19.9) | 0.759 | 0.888 |
| AP-naive | 5 (26.3) | 45 (42.4) | 6 (31.6) | 56 (38.9) | 0.323 | 0.483 |
| Antipsychotic drug | 0.046 | 0.023 | ||||
| Amisulpride | 9 (47.4) | 34 (32.1) | 1 (5.3) | 44 (30.6) | ||
| Aripiprazole | 4 (21.1) | 37 (34.9) | 7 (36.8) | 48 (33.3) | ||
| Olanzapine | 6 (31.6) | 35 (33) | 11 (57.9) | 52 (36.1) | ||
| Diagnosis | 0.226 | 0.428 | ||||
| Schizophrenia F20 | 15 (78.9) | 56 (52.8) | 13 (68.4) | 84 (58.3) | ||
| Schizotypal F21 | 0 (0) | 1 (0.9) | 1 (5.3) | 2 (1.4) | ||
| Delusional disorder F22 | 1 (5.3) | 18 (17) | 2 (10.5) | 21 (14.6) | ||
| Acute and transient F23 | 2 (10.5) | 16 (15.1) | 0 (0) | 21 (14.6) | ||
| Schizo-affective F25 | 1 (5.3) | 7 (6.6) | 2 (10.5) | 10 (6.9) | ||
| Other nonorganic F28 | 0 (0) | 1 (0.9) | 0 (0) | 1 (0.7) | ||
| Unspecified nonorganic F29 | 0 (0) | 7 (6.6) | 1 (5.3) | 8 (5.5) | ||
| Age | 31.7 (12.3) | 31.3 (12.7) | 33.5 (13.9) | 31.7 (12.7) | 0.798 | 0.508 |
| DUP | ||||||
| Mean weeks | 114 (207) | 101.7 (261.6) | 119 (163.1) | 105.1 (244.2) | 0.979 | 0.875 |
| Median weeks | 6 | 25 | 40 | 21 | 0.332 | 0.966 |
| Duration of AP treatment (weeks) | 21.1 (19.3) | 19.8 (20.9) | 16.2 (14.5) | 19.5 (19.9) | 0.716 | 0.436 |
| Years of education | 11.0 (1.6) | 12.6 (2.9) | 11.6 (2.3) | 12.2 (2.7) | 0.047 | 0.303 |
| CGI-S | 5.8 (0.6) | 4.8 (0.8) | 5.3 (0.7) | 5.0 (0.8) | < 0.001 | 0.061 |
| GAF | 30.6 (10.7) | 37.4 (8.7) | 32.2 (8.0) | 35.8 (9.3) | 0.002 | 0.068 |
| CDSS | 8.1 (6.4) | 6.0 (4.8) | 9.0 (4.9) | 6.7 (5.1) | 0.035 | 0.038 |
| PANSS total | 94.7 (12.2) | 72.3 (12.1) | 85.4 (15.6) | 78.4 (15.9) | < 0.001 | 0.023 |
| PANSS positive | 25.4 (5.2) | 19.8 (4) | 22.2 (4.1) | 21.2 (4.8) | < 0.001 | 0.123 |
| PANSS negative | 20.8 (6.2) | 16.2 (5.2) | 21.4 (6.1) | 17.8 (6.1) | < 0.001 | 0.006 |
| PANSS general | 48.4 (6.3) | 36.3 (6.6) | 41.8 (9) | 39.4 (8.6) | < 0.001 | 0.172 |
In the cases of missing data, the total number of patients with data available for analysis was as follows: White: 134; Living alone: 137; Employed: 136; Smokers: 127; Alcohol abuse/dependence: 135; Drug abuse/dependence: 136; DUP: 65; Years of education: 127; GAF: 143; CDSS: 135.
The number of patients with data available for analysis for DUP by group was as follows: Strong response group: 8; Good response group: 50; Slight response group: 7.
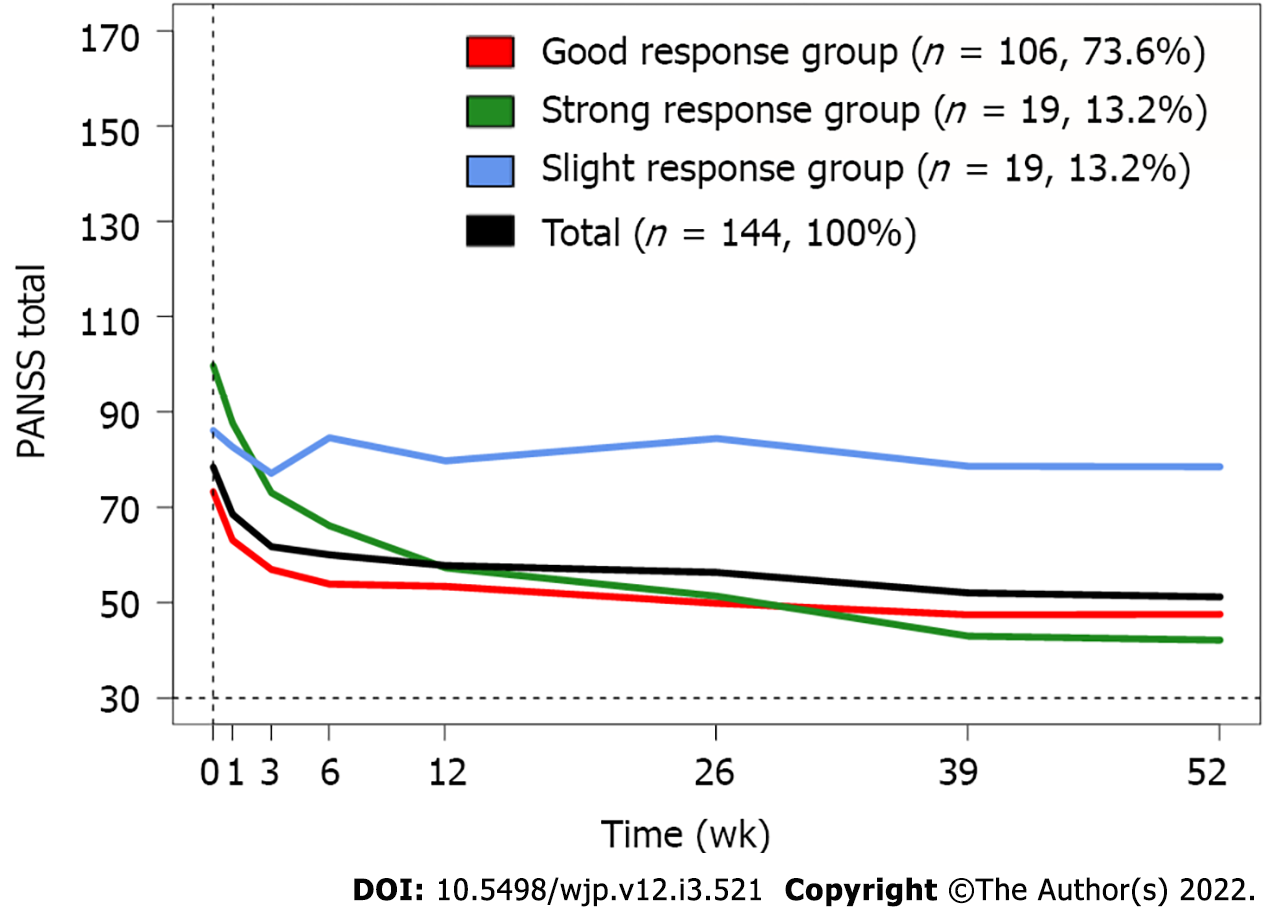
In total, participants had an average PANSS total score of 78.4 points at baseline, which was reduced by 56% after one year. In our three-trajectory model (Figure 1), a large group of patients (n = 106, 74%) (Good response group) had a 54% reduction in PANSS total score over the first 26 wk of follow-up and maintained it after one year, with a 59% total reduction (Table 2). The second group of patients (n = 19, 13%) showed the fastest response, with a 17% reduction after one week of antipsychotic treatment, and had the largest reduction in PANSS total score among the three groups, with 82.5% at one year (Strong response group). These two groups showed similar improvement of PANSS total score until the six-week follow-up (Good response group: 45% reduction, Strong response group: 48% reduction). However, after this, the Good response group had only a 15% further reduction until the one-year follow-up. In contrast, the Strong response group continued to show remarkable improvement until one year, with a 34% further reduction after the six-week follow-up. The third group of patients (n = 19, 13%) followed a trajectory of poor improvement, with a 13.6% reduction in PANSS total score over the one-year study period (Slight response group). The course of the PANSS total score in this group was quite stable throughout the entire follow-up period.
| Baseline PANSS total score | 1 wk1 | 3 wk1 | 6 wk1 | 12 wk1 | 26 wk1 | 39 wk1 | 52 wk1 | |
| Strong response group | 99.7 | 12.0 (17.2%) | 26.6 (38.2%) | 33.5 (48.1%) | 42.4 (60.8%) | 48.3 (69.3%) | 56.7 (81.3%) | 57.5 (82.5%) |
| Good response group | 73.3 | 10.2 (23.5%) | 16.3 (37.6%) | 19.3 (44.7%) | 19.8 (45.8%) | 23.4 (54%) | 25.8 (59.6%) | 25.7 (59.4%) |
| Slight response group | 86.1 | 3.5 (6.2%) | 9.0 (16%) | 1.5 (2.7%) | 6.4 (11.3%) | 1.7 (3%) | 7.5 (13.4%) | 7.6 (13.6%) |
| Total | 78.4 | 9.9 (20.5%) | 16.7 (34.5%) | 18.4 (38%) | 20.7 (42.6%) | 22.1 (45.6%) | 26.4 (54.4%) | 27.2 (56.2%) |
Patients in the three groups had different baseline average PANSS total scores. Patients in the Strong response group had the highest average PANSS total score (99.7 points), while patients in the Good response group had the lowest (73.3 points). The end point estimates, however, were quite similar, with the Strong response group ending at 42.2 points and the Good response group ending at 47.6 points. Patients in the Slight response group had an average PANSS total score of 86.1 at baseline but had a substantially higher PANSS total score than patients in both the other two groups at the six-week follow-up and until the end of the one-year follow-up (78.5 points).
In our three-trajectory model and after conducting post hoc pairwise analyses, we did not find significant differences among the trajectory groups regarding years of education or CDSS score at baseline. Having a regular job was significantly more common among patients in the Good response group than in the Slight response group after the pairwise analyses. In post hoc pairwise analyses for the GAF score at baseline, patients in the Strong response group had significantly lower GAF scores than patients in the Good response group. For the CGI-S score, patients in the Good response group had a significantly lower score at baseline than patients in both other response groups. As expected, because the grouping was based on the PANSS total score data, the PANSS total, PANSS positive, and PANSS general average scores at baseline were significantly different in all the post hoc pairwise comparisons between response groups. Patients in the Strong response group had higher PANSS total, PANSS positive and PANSS general average scores at baseline than patients in both the other response groups. For the PANSS negative score, we found significant differences between the Good response and the Strong response group and between the Good response and the Slight response group. Patients in the Slight response group had the highest PANSS negative average score at baseline, while patients in the Good response group had the lowest.
When the Strong and Slight response groups were compared in the antipsychotic drug post hoc analyses, we found significantly more patients who used amisulpride in the Strong response group (47.4% vs 5.3%). The proportion of Slight response patients in each medication group was as follows: 1/44 for amisulpride, 7/48 for aripiprazole and 11/52 for olanzapine. When these proportions were compared pairwise, we did not find a significant difference between olanzapine and aripiprazole or between amisulpride and aripiprazole. There was a statistically significant difference between olanzapine and amisulpride (i.e., a significantly higher proportion of Slight response participants in the olanzapine group than in the amisulpride group).
In the comparison between the Response and Nonresponse groups, there was a significant difference regarding employment status: More patients in the Response group had a regular job at baseline. The CDSS score at baseline was significantly higher in the Nonresponse group than in the Response group. The Nonresponse group had higher average scores in both PANSS total-86.1 vs 77.3 points-and PANSS negative-21.4 vs 17.3 points-at baseline. There was a significantly higher proportion of patients who used amisulpride in the Response group than in the Nonresponse group-43/125 compared to 1/18.
The key finding of our study is that 87% of participants followed a trajectory of good or strong response to antipsychotic drugs. This provides additional strong evidence of the efficacy of antipsychotic drugs, corresponding to current research and treatment guidelines for schizophrenia[1-3,5,6]. The largest group of patients, the Good response trajectory group, showed a 54% reduction in PANSS total score at the one-year follow-up. This percentage can be regarded as a good response to schizophrenia treatment, as a cutoff of 20%, 30%, 40%, or 50% reduction in PANSS total score has been used in clinical studies[15]. The PANSS total change of 54% corresponds to a CGI-I (improvement scale) of “much improved”[19]. The Slight response group in our study included both antipsychotic-naive patients and patients with previous exposure to antipsychotics. We know from previous research that a group of patients (approximately 13%-25%) will not respond adequately after a trial of an antipsychotic drug in their first episode of psychosis[26-28], while approximately 30% of those with chronic schizophrenia will be regarded as treatment-resistant after two failed trials with antipsychotics[29].
Another key finding of this study is the importance of response in the first six weeks of treatment, which seems to predict further response to the antipsychotic drug. Participants in the Slight response group did not show any further improvement after the first six weeks of treatment. Both the current Norwegian guidelines and the National Institute for Health and Care Excellence (NICE) guidelines suggest a trial with medication at an optimum dosage for four to six weeks[2,30]. The Maudsley guidelines propose an assessment of the adjusted dosage over two to three weeks and an antipsychotic switch if there is no effect during this period. If a partial response is detected, the clinician should continue for at least four weeks before abandoning this treatment[3]. Our data could suggest that patients without a reduction in the PANSS total score of 30% from baseline to six weeks in treatment with nonclozapine antipsychotic drugs seldom achieve sufficient response, and switching to another antipsychotic drug should be considered. On the other hand, the results from the OPTiMiSE study, a multicenter three-phase switching study in first-episode schizophrenia, concluded that switching antipsychotics did not improve clinical outcomes in patients who had not reached symptomatic remission after their first antipsychotic trial compared to continuing treatment[31]. The authors suggested an algorithm of treatment with a single antipsychotic drug for up to 10 wk, followed by the use of clozapine in patients who did not reach symptomatic remission.
There were significant differences in the distribution of the examined antipsychotic drugs among the three response groups, and our findings indicate more favorable results for amisulpride. Interestingly, amisulpride is less frequently used than aripiprazole and olanzapine. In Norway, the use of amisulpride remained stable from 2014 to 2018, and in 2018, amisulpride was used 30 times less frequently than olanzapine and 9 times less frequently than aripiprazole[32]. In the United States, amisulpride is registered for the treatment and prevention of postoperative nausea and vomiting[33] but not for schizophrenia treatment. Hence, one of the most effective drugs is not available for antipsychotic treatment. This is a strong reminder that different prescribing cultures among countries regarding the choice of drugs for schizophrenia treatment exist[4,34] and underlines the need for evidence-based clinical practice in schizophrenia treatment.
We found three variables that predicted nonresponse: Unemployment, depression, and negative psychotic symptoms. Previous studies have suggested that there may be a correlation between employment status and other types of outcomes. The causal direction, however, remains unclear[35,36]. In our study, we found that patients in the Slight response group had a significantly lower percentage of employment, both in the two-group and three-group analyses. Of the 36 participants with paid work at baseline, 35 belonged to the Response group and only one to the Nonresponse group, showing a strong predictive value of having paid work for a good symptom outcome over the 12-mo follow-up. We also found a higher level of symptoms of depression at baseline in the Nonresponse group. This corresponds with previous studies showing that depression in schizophrenia is common and associated with negative outcomes[37]. The Slight response group had the highest PANSS negative average score at baseline in both the two-group and three-group analyses. Negative psychotic symptoms are difficult to treat with the available antipsychotic drugs, which stresses the need for new therapeutic agents in schizophrenia treatment[38].
Our study (BeSt InTro) is the first head-to-head comparison of amisulpride, aripiprazole and olanzapine in a randomized, pragmatic efficacy trial. This direct comparison of these agents provides some clear advantages compared to network meta-analyses. Moreover, our study was industry-independent and rater-blind. Another strength of the study was the frequent follow-up points, particularly in the first weeks of treatment, which are quite important, as demonstrated above. Our follow-up was relatively long (12 mo), which gave an advantage compared to other response studies that examined shorter periods with antipsychotic drugs. Finally, we used well-validated instruments to describe our main parameters, such as PANSS, CGI and CDSS.
Our study has also some limitations. First, there was no placebo control; therefore, we must interpret our results with caution. Second, there was a drop-out rate of 59%, which is comparable to that found in other large randomized antipsychotic drug trials, such as the CATIE study (74% before 18 mo)[39] and the EUFEST study (41.6% before 12 mo)[40]. Furthermore, further analyses of attrition indicated that the sample after 52 wk was representative of the sample at baseline. Finally, some of our participants entered the study having tried other antipsychotic(s) previously, while the rest were antipsychotic-naive. This could have brought some bias into the interpretation of our results. Last, the vast majority of the included patients were white Europeans (88%). Our results are therefore not generalizable to all human populations.
In summary, the vast majority of our study participants had a very good outcome during the 12-mo course. The response to antipsychotic drugs after the first six weeks of treatment predicted a further course during the first year, and the use of amisulpride indicated a better response. An antipsychotic switch should be considered in patients with inadequate response (less than 30% reduction in PANSS total from baseline) after six weeks of treatment. Unemployment, depression, and negative psychotic symptoms at baseline predicted nonresponse.
It is important to compare the effectiveness of various antipsychotic agents in the treatment of schizophrenia. The Bergen-Stavanger-Innsbruck-Trondheim (BeSt InTro) study directly compared three antipsychotics (amisulpride, aripiprazole and olanzapine) in patients with schizophrenia-spectrum disorders between October 20, 2011 and December 30, 2016. The inclusion and follow-up of the patients are now completed, and the main findings have been published. In this substudy, we examined response trajectories and possible predictors for belonging to the different response groups.
Schizophrenia is a serious illness with a heterogeneous course. Pharmacological treatment with antipsychotic drugs remains the cornerstone in the treatment of schizophrenia, yet it is not possible to predict its effect on individual patients. Finding predictors of medication response can enhance the quality of schizophrenia treatment and the development of more personalized medicine.
The main objective of this substudy was to define response trajectories after a one-year follow-up for patients randomized to the three studied antipsychotics. The secondary objective was to define predictors of belonging to the different response trajectories. After realizing these objectives, we could present some suggestions for better clinical practice. We could also suggest further research on switching antipsychotics and on factors that predicted nonresponse, such as unemployment, depression, and negative psychotic symptoms.
Our study was a cohort study with data from a clinical trial of three antipsychotics in a prospective, randomized, rater-blind design. We defined response trajectories by fitting a latent class mixed model with Positive and Negative Syndrome Scale (PANSS) total as a dependent variable, time as an independent fixed variable, and subject as a random intercept to our data. We used the Bayesian information criterion and entropy to select the best model, and the model with three latent classes and with time represented as visit number best fit the data. Response trajectories provide a better picture of the course of symptoms over time and are a relatively novel way of examining response in schizophrenia.
The finding that 87% of the participants had a good or strong response to antipsychotic treatment adds to the research evidence about the general effectiveness of antipsychotic drugs. The response after the first six weeks of treatment seems to indicate further response to antipsychotics. The results indicate the need for further research on switching antipsychotics in incomplete responders to avoid delays in treatment and to enhance the quality of treatment.
Antipsychotic treatment has a good effect in a vast majority of schizophrenia-spectrum patients enrolled in a randomized drug trial. Furthermore, the six-week response seemed to predict the effects through the one-year follow-up. This can indicate an antipsychotic switch in patients without a reduction in the PANSS total score of 30% from baseline to six weeks in treatment with nonclozapine antipsychotics. Another important conclusion is the favorable results for amisulpride in comparison to aripiprazole and olanzapine, which could encourage more frequent use of this drug in schizophrenia treatment.
Future research on schizophrenia treatment should be designed to develop more personalized medicine through the identification of response predictors.
The authors wish to thank all participants for their contribution to the study, as well as the staff of the study groups in Bergen, Trondheim, Stavanger and Innsbruck for their valuable contribution to the detection, inclusion and follow-up of participants.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Norway
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ma H, Yelamanchi R S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1686] [Cited by in RCA: 1762] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 2. | NICE. Psychosis and schizophrenia in adults: Prevention and management. In: National Institute for Health and Care Excellence NICE Clinical Guideline 178. London, 2014. [cited 10 August 2021]. Available from: https://www.nice.org.uk/guidance/cg178. |
| 3. | Taylor D, Barnes T, Young A. The Μaudsley Prescribing Guidelines in Psychiatry. 13th edition. UK: Wiley Blackwell, 2018.. |
| 4. | Papageorgiou G, Cañas F, Zink M, Rossi A. Country differences in patient characteristics and treatment in schizophrenia: data from a physician-based survey in Europe. Eur Psychiatry. 2011;26:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, Arndt T, Backers L, Rothe P, Cipriani A, Davis J, Salanti G, Leucht S. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: A systematic review and network meta-analysis. Lancet 2019; 394: 939-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1211] [Cited by in RCA: 993] [Article Influence: 165.5] [Reference Citation Analysis (0)] |
| 6. | Boter H, Peuskens J, Libiger J, Fleischhacker WW, Davidson M, Galderisi S, Kahn RS; EUFEST study group. Effectiveness of antipsychotics in first-episode schizophrenia and schizophreniform disorder on response and remission: an open randomized clinical trial (EUFEST). Schizophr Res. 2009;115:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? Neurosci Biobehav Rev. 2013;37:1680-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 386] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 8. | Rotella F, Cassioli E, Calderani E, Lazzeretti L, Ragghianti B, Ricca V, Mannucci E. Long-term metabolic and cardiovascular effects of antipsychotic drugs. A meta-analysis of randomized controlled trials. Eur Neuropsychopharmacol. 2020;32:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Harrow M, Jobe TH, Faull RN, Yang J. A 20-Year multi-followup longitudinal study assessing whether antipsychotic medications contribute to work functioning in schizophrenia. Psychiatry Res. 2017;256:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Zhang C, Chen MJ, Wu GJ, Wang ZW, Rao SZ, Zhang Y, Yi ZH, Yang WM, Gao KM, Song LS. Effectiveness of Antipsychotic Drugs for 24-Month Maintenance Treatment in First-Episode Schizophrenia: Evidence From a Community-Based "Real-World" Study. J Clin Psychiatry. 2016;77:e1460-e1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Taylor M, Cavanagh J, Hodgson R, Tiihonen J. Examining the effectiveness of antipsychotic medication in first-episode psychosis. J Psychopharmacol. 2012;26:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Samara MT, Dold M, Gianatsi M, Nikolakopoulou A, Helfer B, Salanti G, Leucht S. Efficacy, Acceptability, and Tolerability of Antipsychotics in Treatment-Resistant Schizophrenia: A Network Meta-analysis. JAMA Psychiatry. 2016;73:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 13. | Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13937] [Cited by in RCA: 15647] [Article Influence: 411.8] [Reference Citation Analysis (0)] |
| 14. | Overall JE, Gorham DR. The Brief psychiatric rating scale. Psychol Rep. 1962;10:799-812. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6631] [Cited by in RCA: 6663] [Article Influence: 740.3] [Reference Citation Analysis (0)] |
| 15. | Leucht S, Davis JM, Engel RR, Kane JM, Wagenpfeil S. Defining 'response' in antipsychotic drug trials: recommendations for the use of scale-derived cutoffs. Neuropsychopharmacology. 2007;32:1903-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Leucht S. Measurements of response, remission, and recovery in schizophrenia and examples for their clinical application. J Clin Psychiatry. 2014;75 Suppl 1:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Guy W. Clinical global impressions. ECDEU assessment manual for psychopharmacology, revised (DHEW publ. No. ADM 76-338). National Institute of Mental Health, Rockville, 1976: 218-222. |
| 18. | Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res. 2005;79:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 964] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 19. | Levine SZ, Rabinowitz J, Engel R, Etschel E, Leucht S. Extrapolation between measures of symptom severity and change: an examination of the PANSS and CGI. Schizophr Res. 2008;98:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Zhang HX, Shen XL, Zhou H, Yang XM, Wang HF, Jiang KD. Predictors of response to second generation antipsychotics in drug naïve patients with schizophrenia: a 1 year follow-up study in Shanghai. Psychiatry Res. 2014;215:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Johnsen E, Kroken RA, Løberg EM, Rettenbacher M, Joa I, Larsen TK, Reitan SK, Walla B, Alisauskiene R, Anda LG, Bartz-Johannessen C, Berle JØ, Bjarke J, Fathian F, Hugdahl K, Kjelby E, Sinkeviciute I, Skrede S, Stabell L, Steen VM, Fleischhacker WW. Amisulpride, aripiprazole, and olanzapine in patients with schizophrenia-spectrum disorders (BeSt InTro): a pragmatic, rater-blind, semi-randomised trial. Lancet Psychiatry. 2020;7:945-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25:567-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 23. | Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Möller HJ; WFSBP Task force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14:2-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 285] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 24. | Karterud S, Pedersen G, Løvdahl H, Friis S. Global assessment of Functioning-Split version. Background and scoring manual. Oslo, Norway. Ullevaal University Hospital, Department of Psychiatry, 1998.. |
| 25. | Rcore Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2020.. |
| 26. | Agid O, Arenovich T, Sajeev G, Zipursky RB, Kapur S, Foussias G, Remington G. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72:1439-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 27. | Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16:505-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 28. | Robinson DG, Woerner MG, Alvir JM, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Bilder R, Goldman R, Lieberman JA. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 1999;156:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Meltzer HY. Treatment-resistant schizophrenia--the role of clozapine. Curr Med Res Opin. 1997;14:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 324] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 30. | Helsedirektoratet. Nasjonal faglig retningslinje for utredning, behandling og oppfølging av personer med psykoselidelser (National guideline for assessment, treatment and follow-up of persons with psychotic disorders). Norwegian Directorate of Health. Oslo, 2013. [cited 10 August 2021]. Available from: https://helsedirektoratet.no/retningslinjer/nasjonal-faglig-retningslinje-for-utredning-behandling-og-oppfolging-av-personer-med-psykoselidelser. |
| 31. | Kahn RS, Winter van Rossum I, Leucht S, McGuire P, Lewis SW, Leboyer M, Arango C, Dazzan P, Drake R, Heres S, Díaz-Caneja CM, Rujescu D, Weiser M, Galderisi S, Glenthøj B, Eijkemans MJC, Fleischhacker WW, Kapur S, Sommer IE; OPTiMiSE study group. Amisulpride and olanzapine followed by open-label treatment with clozapine in first-episode schizophrenia and schizophreniform disorder (OPTiMiSE): a three-phase switching study. Lancet Psychiatry. 2018;5:797-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 32. | Norwegian Institute of Public Health. Drug Consumption in Norway 2014–2018. Oslo. [cited 10 August 2021]. Available from: https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2019/Legemiddelforbruket-i-norge-2014-2018.pdf. |
| 33. | Ientile G. Amisulpride injection now available in the US for postoperative nausea and vomiting. 2020. [cited 10 August 2021]. Available from: https://www.drugtopics.com/view/amisulpride-injection-now-available-in-the-us-for-postoperative-nausea-and-vomiting. |
| 34. | Taylor DM, Werneke U. Ethnopharmacology†. Nord J Psychiatry. 2018;72:S30-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Marwaha S, Johnson S. Schizophrenia and employment - a review. Soc Psychiatry Psychiatr Epidemiol. 2004;39:337-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 554] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 36. | Bell MD, Lysaker PH, Milstein RM. Clinical benefits of paid work activity in schizophrenia. Schizophr Bull. 1996;22:51-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 75] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Kjelby E, Gjestad R, Sinkeviciute I, Kroken RA, Løberg EM, Jørgensen HA, Johnsen E. Trajectories of depressive symptoms in the acute phase of psychosis: Implications for treatment. J Psychiatr Res. 2018;103:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Möller HJ, Czobor P. Pharmacological treatment of negative symptoms in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2015;265:567-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3914] [Cited by in RCA: 3739] [Article Influence: 187.0] [Reference Citation Analysis (0)] |
| 40. | Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, Gheorghe MD, Rybakowski JK, Galderisi S, Libiger J, Hummer M, Dollfus S, López-Ibor JJ, Hranov LG, Gaebel W, Peuskens J, Lindefors N, Riecher-Rössler A, Grobbee DE; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371:1085-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 726] [Article Influence: 42.7] [Reference Citation Analysis (0)] |