Published online Feb 19, 2022. doi: 10.5498/wjp.v12.i2.298
Peer-review started: June 28, 2021
First decision: September 5, 2021
Revised: September 24, 2021
Accepted: January 17, 2022
Article in press: January 17, 2022
Published online: February 19, 2022
Processing time: 233 Days and 19.8 Hours
Antipsychotic medications such as risperidone, olanzapine and aripiprazole are used to treat psychological and behavioural symptoms among dementia patients. Current evidence indicate prescription rates for antipsychotics vary and wider consensus to evaluate clinical epidemiological outcomes is limited.
To investigate the potential impact of atypical antipsychotics on the mortality of patients with dementia.
A retrospective clinical cohort study was developed to review United Kingdom Clinical Record Interactive Search system based data between January 1, 2013 to December 31, 2017. A descriptive statistical method was used to analyse the data. Mini Mental State Examination (MMSE) scores were used to assess the severity and stage of disease progression. A cox proportional hazards model was developed to evaluate the relationship between survival following diagnosis and other variables.
A total of 1692 patients were identified using natural language processing of which, 587 were prescribed olanzapine, quetiapine or risperidone (common group) whilst 893 (control group) were not prescribed any antipsychotics. Patients prescribed olanzapine showed an increased risk of death [hazard ratio (HR) = 1.32; 95% confidence interval (CI): 1.08-1.60; P < 0.01], as did those with risperidone (HR = 1.35; 95%CI: 1.18-1.54; P < 0.001). Patients prescribed quetiapine showed no significant association (HR = 1.09; 95%CI: 0.90-1.34; P = 0.38). Factors associated with a lower risk of death were: High MMSE score at diagnosis (HR = 0.72; 95%CI: 0.62-0.83; P < 0.001), identifying as female (HR = 0.73; 95%CI: 0.64-0.82; P < 0.001), and being of a White-British ethnic group (HR = 0.82; 95%CI: 0.72-0.94; P < 0.01).
A significant mortality risk was identified among those prescribed olanzapine and risperidone which contradicts previous findings although the study designs used were different. Comprehensive research should be conducted to better assess clinical epidemiological outcomes associated with diagnosis and therapies to improve clinical management of these patients.
Core Tip: Antipsychotic medication is widely prescribed to patients with dementia displaying neuropsychiatric symptoms. Treatment with olanzapine and risperidone was associated with an increased mortality risk. In comparison, quetiapine showed a relatively lower, non-significant association with the mortality risk in those with dementia. Clinicians need to be aware of the potential heterogeneous relationship between dementias, antipsychotic medication, and mortality when creating a psychopharmacological treatment plan for their patients.
- Citation: Phiri P, Engelthaler T, Carr H, Delanerolle G, Holmes C, Rathod S. Associated mortality risk of atypical antipsychotic medication in individuals with dementia. World J Psychiatry 2022; 12(2): 298-307
- URL: https://www.wjgnet.com/2220-3206/full/v12/i2/298.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i2.298
Antipsychotic prescribing in older adults must be made with caution as there are age related changes in pharmacokinetics and pharmacodynamics that can result in an increased sensitivity to drugs and their side effects. This is evident in both typical and atypical antipsychotic prescribing within this population. Thus, Landi et al[1] demonstrated a 47% elevation of falls in elderly adults being prescribed typical antipsychotics, whilst atypical antipsychotic drugs have been associated with higher hospitalisation rates with acute kidney injury[2] and an increased 90 d risk of non-vertebral osteoporotic fracture, hip fracture and various other fractures and falls[3]. Similarly, the concerns around the safety and effectiveness of aripiprazole, olanzapine, quetiapine and risperidone (four specific antipsychotics) have been also raised for older patients in a clinical trial setting[4].
Antipsychotics are also used to treat anxiety, agitation and psychotic experiences presenting in dementia, the majority of whom are elderly. Patients with dementia are considered particularly vulnerable to the effects of antipsychotics[5]. In particular, those using antipsychotics extensively would have an increased susceptibility to venous thrombolytic episodes, hip fractures and strokes[6].
Due to the perceived risk of mortality and of side-effects, typical antipsychotics have begun to be replaced by atypicals in the last decade[5]. However, whilst there appears strong evidence for an associated risk of adverse events of both typical and atypical antipsychotic medication in the elderly, and in those with dementia, the evidence around increased mortality is less clear. Some of the literature suggests that there is an increased morality risk in dementia patients from both typical and atypical antipsychotics accounting for an additional 1800 deaths per year[5]. However, others have argued that typical antipsychotics have a greater mortality risk than atypicals for those individuals with dementia[7] yet a meta-analysis of all cause dementia, indicated that there was a small increased risk of death from atypical antipsychotics compared to those on a placebo[8]. Furthermore, a retrospective study on a cohort of vascular dementia patients have found that there was no significant increases of mortality risk with those exposed to atypical antipsychotics to those with no exposure[9].
The present study investigates the risk of antipsychotics on mortality in all forms of dementia including vascular dementia. We hope this will help inform clinical practice and contribute to the development of training packages on prescribing antipsychotics in dementia.
A retrospective clinical cohort study was designed to review data gathered over a 5-year period (January 1, 2013 to December 31, 2017) in a National Health Service (NHS) setting. The aim of the study was to investigate the potential impact of atypical antipsychotics on the mortality of patients with dementia. Health Research Authority (HRA) provided guidance to the Akrivia Health and all data controllers that neither ethics nor HRA approval (legal & governance) is required for the establishment of the Clinical Record Interactive Search (CRIS) system or using de-identified data (from the system) for research purposes in March 2020. Local approvals were obtained from the Southern Health NHS Foundation Trust (SHFT) patient-led oversight committee.
The CRIS platform was used to identify suitable participants for this study as per the inclusion/exclusion criteria. Patient records in the SHFT database were filtered to only include those: Older than 30 years at the beginning of the study period (January 1, 2013); having a first diagnosis of either Alzheimer’s disease (G30), vascular dementia (F01), frontotemporal dementia (G31.0), unspecified dementia (F03) or dementia in other diseases (F02); have been assigned this first diagnosis between January 1, 2013 and December 31, 2016; and to never had a diagnosis of either schizophrenia (F20), schizoaffective disorder (F25) or bipolar disorder (F31). A total of 1770 patients were deemed eligible for this study.
Akrivia Health provides the CRIS system to analyse de-identified data from the Southern Health NHS Foundation Trust Electronic Health Records (EHR). There are currently 14 NHS Mental Health Foundation Trusts in the United Kingdom using CRIS with 3.2 million anonymised patients’ record. Each site has its own CRIS access port that ingests data from their own EHRs that is managed within a robust governance model in the form of an independent oversight committee. The SHFT CRIS system includes records of the Trust’s patients except those that have opted out from having their de-identified records used for research and evaluation purposes that could improve clinical benefit. The accessible data include notes that are written by clinicians as a report on a patient’s progress, including comments on medication. The CRIS platform extracts the free text (progress notes) in a de-identified format to enable researchers with appropriate approvals to conduct research. Given the scale of the cohort, it was not feasible to compile a medication history manually. Natural language processing (NLP) was employed to identify medications within the patient’s notes using the Med-7 algorithm[10]. This data was used to refine the cohort into three groups; medication group prescribed olanzapine, quetiapine or risperidone, comparison group (not prescribed any antipsychotic), and exclusion group (prescribed an antipsychotic other than olanzapine, quetiapine or risperidone). Additional variables were obtained from CRIS, including: Mortality status, date of death, age at diagnosis, gender, and ethnicity.
The CRIS database supports the Med-7 NLP algorithm[10]. The algorithm indicates phrases with medications. Both the de-identified patient electronic healthcare records and the Med-7 medication outputs were searched using Structured Query Language and the relevant data tables were then exported into Python 3.8[11]. Python was then used to carry out all the analyses and generate the figures, using the following packages: Pandas[12], Numpy[13], Lifelines[14] and Matplotlib[15].
To assess the relationship between survival since diagnosis and the other variables, a cox proportional hazard (CPH) model was built. This model used the ‘death flag’ as an event of interest, ‘survival since diagnosis’ as the duration, ‘age at diagnosis’ as a continuous covariate, and one-hot encoded covariates of ‘gender’, ‘ethnicity’ and ‘MMSE Score’.
CPH models assume the time-independence of the proportional hazards, consequently assuming the hazard ratios (HR) are constant with time. In our case, a violation of this assumption would mean the HR are dependent on the time since diagnosis. For example, a specific medication could be associated with a temporary survival risk but be relatively safe in the long-term (or vice versa). Rulli et al[16] provide a detailed explanation of this issue. Particular care should be taken when comparing the results of multiple studies (i.e., including our study in an aggregate), as the time dependence of results may vary across datasets.
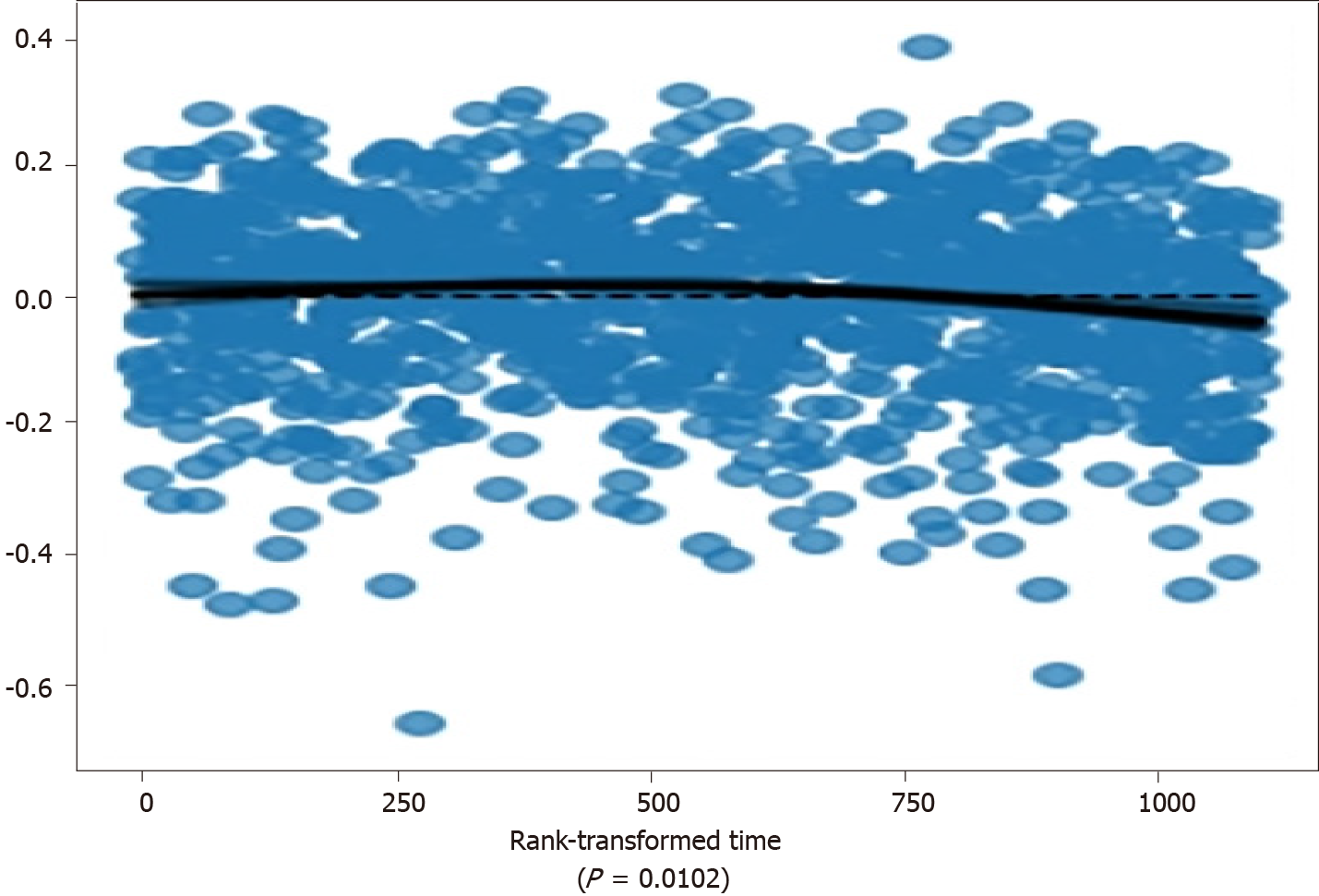
The time-independence assumption of the proportional hazards was tested using scaled Schoenfeld residuals and a rank transformation of time[17-18]. The statistical significance of the deviation from time-independence was calculated using an approximation developed by Davidson-Pilon[14] with a P-value threshold of 0.01. All variables were above the threshold, with ‘age at diagnosis’ having a P-value of 0.0102 (see Figure 1). To address the possible violation, we model the CPH as a stratified model, using ‘age at diagnosis’ as a stratifying variable, separating patients into 5-year strata intervals.
The NLP algorithm identified a total of 1692 patients with at least one medication entry. Of these, 587 patients were prescribed either olanzapine, quetiapine or risperidone (medication group), 893 were not prescribed any antipsychotic medication (comparison group) and 290 were prescribed an antipsychotic other than olanzapine, quetiapine and risperidone (exclusion group). Olanzapine was prescribed to 155 patients, quetiapine to 144 and risperidone to 450 patients. There were 153 patients who were prescribed at least two of the three antipsychotics over the study period. The demographic profiles and the MMSE scores for the study groups are shown in Table 1.
| Demographic | Category | Total | Medication group | Comparison group |
| Number of patients | 1480 | 587 | 893 | |
| Age (mean ± SD) | 82.6 ± 8.1 | 81.7 ± 8.4 | 83.3 ± 7.9 | |
| Gender | Male | 714 (48.2%) | 294 (50.1%) | 420 (47.0%) |
| Female | 766 (51.8%) | 293 (49.9%) | 473 (53.0%) | |
| Other/NA | 0 | 0 | 0 | |
| Ethnicity | White-British | 1033 (69.8%) | 451 (76.8%) | 582 (65.2%) |
| White-Irish | 5 (< 1%) | 4 (< 1%) | 1 (< 1%) | |
| White-Any other | 17 (1.1%) | 6 (1.0%) | 11 (1.2%) | |
| Mixed-White and Asian | 1 (< 1%) | 1 (< 1%) | 0 (< 1%) | |
| Asian-Indian | 6 (< 1%) | 2 (< 1%) | 4 (< 1%) | |
| Asian-Bangladeshi | 1 (< 1%) | 0 (< 1%) | 1 (< 1%) | |
| Asian-Any other | 10 (< 1%) | 5 (< 1%) | 5 (< 1%) | |
| Black-Caribbean | 2 (< 1%) | 1 (< 1%) | 1 (< 1%) | |
| Black-African | 2 (< 1%) | 1 (< 1%) | 1 (< 1%) | |
| Any other ethnic group | 2 (< 1%) | 2 (< 1%) | 0 (< 1%) | |
| Not stated/NA | 401 (27.1%) | 114 (19.4%) | 287 (32.1%) | |
| MMSE | Number of patients with MMSE | 608 (41.1%) | 226 (38.5%) | 382 (42.8%) |
| Number of patients without MMSE | 876 (58.9%) | 361 (61.5%) | 511 (57.2%) | |
| MMSE score | 20-30 | 369 (60.7%) | 101 (44.7%) | 268 (70.2%) |
| 10-19 | 199 (23.7%) | 98 (43.4%) | 101 (26.4%) | |
| < 10 | 40 (6.56%) | 27 (11.9%) | 13 (3.4%) |
MMSE scores from the time of first diagnosis were also obtained using NLP (regular expression search). Patient records were followed for up to 5 years after the first diagnosis, retrieving the date of death if present.
1097 (74%) patients had a recorded death within 5 years of their first diagnosis (i.e., patients with a ‘death flag’). For these patients, the ‘survival since diagnosis’ was calculated, representing the duration in months between the first diagnosis and the date of death. The mean survival since diagnosis was 26.7 mo (SD = 19.9).
The stratified CPH model had a concordance of 0.60, with six of the included covariates showing a significant HR. The variable-level results are listed in Table 2. Specifically, patients prescribed olanzapine showed an increased risk of death within the study period [HR = 1.32; 95% confidence interval (CI): 1.08-1.60; P < 0.01]. Those prescribed risperidone showed a similar increased risk of death (HR = 1.35; 95%CI: 1.18-1.54; P < 0.001). Quetiapine showed no significant association with an increased risk of death (HR = 1.09; 95%CI: 0.90-1.34; P = 0.38).
| Covariate | Total | Alive | Dead | Hazard ratio | P value |
| Medications | |||||
| Olanzapine | 155 | 33 (21.3%) | 122 (78.7%) | 1.32a (1.08-1.60) | < 0.01 |
| Quetiapine | 144 | 29 (20.1%) | 115 (79.9%) | 1.09 (0.90-1.34) | 0.38 |
| Risperidone | 450 | 82 (18.2%) | 368 (81.8%) | 1.35b (1.18-1.54) | < 0.001 |
| Gender | |||||
| Male | 714 | 165 (23.1%) | 549 (76.9%) | Baseline | |
| Female | 766 | 218 (28.5%) | 548 (71.5%) | 0.73b (0.64-0.82) | < 0.001 |
| Ethnicity | |||||
| White-British | 1033 | 275 (26.6%) | 758 (73.4%) | 0.82a (0.72-0.94) | < 0.01 |
| White-Irish | 5 | 2 (40.0%) | 3 (60.0%) | 0.51 (0.16-1.62) | 0.26 |
| White-Any other | 17 | 6 (35.3%) | 11 (64.7%) | 0.62 (0.34-1.13) | 0.12 |
| Mixed-White and Asian | - | - | - | - | - |
| Asian-Indian | 6 | 1 (16.7%) | 5 (83.3%) | 1.49 (0.61-3.63) | 0.38 |
| Asian-Bangladeshi | - | - | - | - | - |
| Asian-Any other | 10 | 6 (60.0%) | 4 (40.0%) | 0.17a (0.05-0.53) | < 0.01 |
| Black-Caribbean | - | - | - | - | - |
| Black-African | - | - | - | - | - |
| Any other ethnic group | - | - | - | - | - |
| MMSE score | |||||
| 20-30 | 369 | 123 (33.3%) | 246 (66.7%) | 0.72b (0.62-0.83) | < 0.001 |
| 10-19 | 199 | 45 (22.6%) | 154 (77.4%) | 0.87 (0.73-1.04) | 0.12 |
| < 10 | 40 | 11 (27.5%) | 29 (72.5%) | 0.81 (0.56-1.19) | 0.28 |
Patients with a high MMSE score (20-30) at diagnosis showed a lower risk of death (HR = 0.72; 95%CI: 0.62-0.83; P < 0.001). Interestingly, the MMSE Score HR always trend in a negative direction, suggesting that patients with any mention of an MMSE score in their clinical notes, regardless of its value, have a decreased risk of death. To better understand this effect, a follow-up CPH model was built, with ‘MMSE Missing’ as a covariate instead of the ‘MMSE Score’ groups. In this model, patients who do not have any mention of an MMSE score in their clinical notes (n = 872) show a significantly higher risk of death (HR = 1.30; 95%CI: 1.14-1.47; P < 0.001).
Those identifying as female (n = 766) had a significantly lower HR (HR = 0.73; 95%CI: 0.64-0.82; P < 0.001) than those identifying as male (n = 714). Patients of the White-British ethnicity showed a significantly lower risk of death (HR = 0.82; 95%CI: 0.72-0.94; P < 0.01), suggesting better outcomes for patients in this group.
The results show a significantly higher mortality risk for those prescribed olanzapine and risperidone. This supports previous findings of Gerhard et al[19], who showed that quetiapine had a lower mortality risk than risperidone, while olanzapine had a similar mortality rate to risperidone within the elderly population. Gerhard et al[19] argued that their findings could be due to less variance in dosing of quetiapine. In addition, higher doses of both olanzapine and risperidone were thought to have been linked to a higher risk of mortality.
Aside from dosing, the differences in mortality rate could be due to the risk of cerebrovascular events. Risperidone and olanzapine have been associated with greater risks of cerebrovascular events[20-24]. The mechanism by which risperidone and olanzapine may increase the risk of cerebrovascular adverse events could be related to high levels of prolactin. Olanzapine and risperidone have been associated with high levels of prolactin[25-26]. High levels of prolactin have been associated with cerebrovascular events[27]. Furthermore, hyperprolactinaemia has been reported to frequently complicate antipsychotic treatment[28].
It is worth noting that risperidone has not been reported to cause anticholinergic side effects in the elderly unlike other atypicals[29]. Within this population, anti-psychotics are used to treat agitation and psychotic phenomenon often presented in dementia. Olanzapine and risperidone as atypical antipsychotics are commonly prescribed due to their favourable side-effect and safer metabolic profiles[5,30] age related changes in pharmacokinetics and pharmacodynamics can lead to increased sensitivity to drugs and their side effects[31] consequently impacting on mortality rates.
Polypharmacy is another facet observed within this population of patients that could attribute to the findings of our study. A recent scoping review on the sex and gender differences in polypharmacy in this population could support this theory[32] notably for women with dementia, in comparison to men[32]. Similarly, dementia is implicated in the increased risk of polypharmacy within the elderly population with rates varying from over 65 years taking from 6 medications to more than 10 medications in those older than 85 years across the world.
This study results contradict the previous findings of Sultana et al[9], who found no increase in risk hazard across olanzapine, quetiapine and risperidone, there are several differences in our study design that may account for the differing outcomes.
The cohort in the present study covers five different International Classification of Diseases diagnosis sub-groups (G30, F01, G31.0, F03, F02), rather than vascular dementia (F01) exclusively. As such, the present results are representative of the shared patterns observed across differing dementias. Patients with Alzheimer’s disease (G30) are known to show an increased mortality risk associated with long-term antipsychotic use[33]. This is a plausible finding observed across the dementia diagnoses, in particular among vascular dementia patients. A direct comparison of the individual dementia diagnosis sub-groups could assist establishing the homogeneity/heterogeneity of the mortality risk effect in future studies.
The geographical differences between the Southampton and South London population also play a vital role in our findings, given the variations in ethnicities and races. The non-medication results are comparable across both studies with women demonstrating a lower risk in comparison to men. In addition, the Caucasian group demonstrated a relatively lower risk compared to most other groups. Consistent with other studies[34], patients with high MMSE scores were also associated with lower risk of mortality. This may either mean the MMSE test is not used in patients with advanced dementias, or that there are systematic patterns due to missing data issues within electronic healthcare records in primary and secondary care organizations. These possible theories could be substantiated with prospective research studies.
A study design using de-identified EHR has implicit strengths and limitations. The study provides a direct look into patient-level effects without influencing the clinical trajectory of the participants. Similarly, this design enables the analysis of the whole patient population in the NHS Trust (except for those opting out of NHS research) which would be prohibitively time consuming using traditional patient recruitment methods. The use of NLP allows for the estimation of prescribed medication despite the fact that this information is rarely recorded in a structured format.
The strengths of using CRIS is that key features of the older adult population could be reviewed for this disease. Validating an original dataset by way of a secondary independent analysis is valuable to further future research within this area. On the other hand, it is important to appreciate that any retrospective EHR study is descriptive in nature. It is representative only of the cohort at hand, and any attempts at generalization should be accompanied by a robust theoretical underpinning of the observed effects. These are out of the scope of the current study, whereby the presented results aim to stimulate areas of further research, not inform clinical practice.
However, to strengthen the outcomes of this study, it was not feasible to develop an aggregated dataset which would have benefited the outcomes of this study. It is therefore recognized; future research should consider expanding the data collection during patient visits to better understand key clinical features and standardized scores in relation to the disease. A key data limitation is the under-representation of certain ethnic groups. Specifically, the ‘White-British’ group accounts for 95.7% of the patients who have an ethnicity on record. This makes it impossible to accurately estimate any ethnicity-related effects of the model, especially in the ethnicity groups that only include 1-5 patients. Gianfrancesco et al[35] provide a useful discussion on the potential bias associated with underrepresentation in EHRs. To investigate these effects, studies may benefit from specifically approaching the under-represented groups in order to generate more balanced cohorts.
A further limitation is that the method used within our paper is used in limited research papers due to differences with data gathering time points which impact the patients at risks at differing time points. Parmar et al[36] demonstrated similar methods could be used to estimate censored data along with a number of events at specific intervals although, the limitation with this is further assumptions would be made to generate estimates. The correlation tests based on Schoenfeld residuals is a positive step to assess the proportionality of hazards in standard cox models. Pseudo-likelihood was used to define Schoenfeld residuals at event times. Additionally, Kaplan-Meier estimates could have been completed if the event times and a ranking system was available at the point at which the dataset was furthered to assess the performance in a better way. Similarly, it would be beneficial to conduct simulation studies to address this issue although, this is a step to be completed as part of future research.
The study showed an increased mortality risk associated with olanzapine and risperidone whilst quetiapine showed a relatively statistically insignificant association. This study reports a heterogeneous relationship between dementias, antipsychotic medication, and mortality, with some medication classes being more problematic than others. Antipsychotic use especially in the elderly population with dementia should only be prescribed when absolutely necessary given that such medication related adverse effects remain a significant source of mental and physical distress. Evidentiary argument implicates long-term antipsychotic use to progressive reduction in brain volume. As such, regulatory warnings from the Food and Drug Administration and the European Medicines Agency on antipsychotics in population seem to be ineffective as usage has increased. Future comprehensive investigation is imperative, especially in understanding how the sub-diagnoses of dementias differ in their medication interactions and the effect of biological differences in sex and ethnicity that many intervene and further elucidate our findings. Further investigation to better assess clinical epidemiological outcomes associated with diagnosis and non-pharmacological therapies to improve clinical management of these patients is warranted.
Antipsychotic medication is widely prescribed to patients with dementia displaying neuropsychiatric symptoms. The present study investigated the risk of antipsychotics on mortality in all forms of dementia including vascular dementia. It is anticipated the findings will help inform clinical practice and contribute to the development of training packages on prescribing antipsychotics in dementia.
Antipsychotic prescribing in older adults must be made with caution as there are age related changes in pharmacokinetics and pharmacodynamics that can result in an increased sensitivity to drugs and their side effects. Similarly, the concerns around the safety and effectiveness of aripiprazole, olanzapine, quetiapine and risperidone (four specific antipsychotics) have been also raised for older patients in a clinical trial setting. Usage of antipsychotics in this population has increased despite regulatory warnings from the Food and Drug Administration and the European Medicines Agency.
This study was developed with a primary objective to evaluate the impact of atypical antipsychotics associated with mortality in a dementia cohort.
A retrospective clinical cohort study was designed to review data from electrical health records (RIO system) gathered over a 5-year period (January 1, 2013 to December 31, 2017) in a National Health Service setting.
Treatment with olanzapine and risperidone was associated with an increased mortality risk. In comparison, olanzapine showed a relatively lower non-significant association with the mortality risk in those with dementia.
Clinicians within primary and secondary care need to be aware of the potential heterogeneous relationship between dementia, antipsychotic medication and mortality when creating a psychopharmacological treatment plan for their patients.
Future comprehensive investigation is imperative, especially in understanding how the sub-diagnoses of dementias differ in their medication interactions and the effect of biological differences in sex and ethnicity that many intervene and further elucidate our findings.
This study was sponsored by Southern Health NHS Foundation Trust. The team would like to thank Matthew Broadbent and Megan Pritchard from the South London and Maudsley Biomedical Research Centre for giving us permission to replicate the original study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: NMC, 98I1393E; BABCP, 060632; Royal College of Nursing, 1495194.
Specialty type: Psychiatry
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pavón L, Wang MZ S-Editor: Wang JJ L-Editor: A P-Editor: WangJJ
| 1. | Landi F, Onder G, Cesari M, Barillaro C, Russo A, Bernabei R; Silver Network Home Care Study Group. Psychotropic medications and risk for falls among community-dwelling frail older people: an observational study. J Gerontol A Biol Sci Med Sci. 2005;60:622-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Hwang YJ, Dixon SN, Reiss JP, Wald R, Parikh CR, Gandhi S, Shariff SZ, Pannu N, Nash DM, Rehman F, Garg AX. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population-based cohort study. Ann Intern Med. 2014;161:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | Fraser LA, Liu K, Naylor KL, Hwang YJ, Dixon SN, Shariff SZ, Garg AX. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med. 2015;175:450-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Jeste DV, Maglione JE. Atypical antipsychotics for older adults: are they safe and effective as we once thought? J Comp Eff Res. 2013;2:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Banerjee S. The use of antipsychotic medication for people with dementia: time for action. A report for the Minister of State for Care Services. [cited 27 May 2021]. Available from: http://psychrights.org/Research/Digest/NLPs/BanerjeeReportOnGeriatricNeurolepticUse.pdf. |
| 6. | Dennis M, Shine L, John A, Marchant A, McGregor J, Lyons RA, Brophy S. Risk of Adverse Outcomes for Older People with Dementia Prescribed Antipsychotic Medication: A Population Based e-Cohort Study. Neurol Ther. 2017;6:57-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 7. | Gill SS, Bronskill SE, Normand SL, Anderson GM, Sykora K, Lam K, Bell CM, Lee PE, Fischer HD, Herrmann N, Gurwitz JH, Rochon PA. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 420] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 8. | Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1111] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 9. | Sultana J, Chang CK, Hayes RD, Broadbent M, Stewart R, Corbett A, Ballard C. Associations between risk of mortality and atypical antipsychotic use in vascular dementia: a clinical cohort study. Int J Geriatr Psychiatry. 2014;29:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Kormilitzin A, Vaci N, Liu Q, Nevado-Holgado A. Med7: A transferable clinical natural language processing model for electronic health records. Artif Intell Med. 2021;118:102086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 11. | Python. Python 3.8.3. [cited 26 May 2021]. Available from: https://www.python.org/downloads/release/python-383/. |
| 12. | McKinney W & PyData Development Team. Pandas: powerful Python data analysis toolkit. [cited 27 May 2021]. Available from: https://pandas.pydata.org/pandas-docs/version/0.25.3/pandas.pdf. |
| 13. | Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, Wieser E, Taylor J, Berg S, Smith NJ, Kern R, Picus M, Hoyer S, van Kerkwijk MH, Brett M, Haldane A, Del Río JF, Wiebe M, Peterson P, Gérard-Marchant P, Sheppard K, Reddy T, Weckesser W, Abbasi H, Gohlke C, Oliphant TE. Array programming with NumPy. Nature. 2020;585:357-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4365] [Cited by in RCA: 4738] [Article Influence: 947.6] [Reference Citation Analysis (0)] |
| 14. | Testing the proportional hazard assumptions. [cited 27 May 2021]. Available from: https://lifelines.readthedocs.io/en/latest/jupyter_notebooks/Proportional%20hazard%20assumption.html. |
| 15. | Hunter JD. Matplotlib: A 2D graphics environment. Cum Sci Eng. 2017;9:90-95. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14295] [Cited by in RCA: 14678] [Article Influence: 815.4] [Reference Citation Analysis (0)] |
| 16. | Rulli E, Ghilotti F, Biagioli E, Porcu L, Marabese M, D'Incalci M, Bellocco R, Torri V. Assessment of proportional hazard assumption in aggregate data: a systematic review on statistical methodology in clinical trials using time-to-event endpoint. Br J Cancer. 2018;119:1456-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239-241. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1700] [Cited by in RCA: 1728] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 18. | Park S, Hendry DJ. Reassessing Schoenfeld residual tests of proportional hazards in political science event history analyses. Am J Poli Sci. 2015;59:1072-1087. [DOI] [Full Text] |
| 19. | Gerhard T, Huybrechts K, Olfson M, Schneeweiss S, Bobo WV, Doraiswamy PM, Devanand DP, Lucas JA, Huang C, Malka ES, Levin R, Crystal S. Comparative mortality risks of antipsychotic medications in community-dwelling older adults. Br J Psychiatry. 2014;205:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Chatterjee S, Chen H, Johnson ML, Aparasu RR. Comparative risk of cerebrovascular adverse events in community-dwelling older adults using risperidone, olanzapine and quetiapine: a multiple propensity score-adjusted retrospective cohort study. Drugs Aging. 2012;29:807-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ. 2002;167:1269-1270. [PubMed] |
| 22. | Wooltorton E. Olanzapine (Zyprexa): increased incidence of cerebrovascular events in dementia trials. CMAJ. 2004;170:1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. A controlled, open-label study. J Neurol. 2005;252:1186-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Layton D, Harris S, Wilton LV, Shakir SA. Comparison of incidence rates of cerebrovascular accidents and transient ischaemic attacks in observational cohort studies of patients prescribed risperidone, quetiapine or olanzapine in general practice in England including patients with dementia. J Psychopharmacol. 2005;19:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Yang F, Chen L, Fang X, Zheng K, Zhu C, Xu C, Zhang C, Tang W. Influence of olanzapine on serum prolactin levels and BMI in female patients with schizophrenia. Neuropsychiatr Dis Treat. 2018;14:3373-3379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Aboraya A, Fullen JE, Ponieman BL, Makela EH, Latocha M. Hyperprolactinemia associated with risperidone: a case report and review of literature. Psychiatry (Edgmont). 2004;1:29-31. [PubMed] |
| 27. | Tripathi SK, Kamble P, Muddeshwar MG. Serum prolactin level in patients of ischemic stroke. Int J Contemp Med Res. 2016;3:3459-3460. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Bushe C, Shaw M. Prevalence of hyperprolactinaemia in a naturalistic cohort of schizophrenia and bipolar outpatients during treatment with typical and atypical antipsychotics. J Psychopharmacol. 2007;21:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Mintzer J, Burns A. Anticholinergic side-effects of drugs in elderly people. J R Soc Med. 2000;93:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 223] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Solmi M, Murru A, Pacchiarotti I, Undurraga J, Veronese N, Fornaro M, Stubbs B, Monaco F, Vieta E, Seeman MV, Correll CU, Carvalho AF. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 287] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 31. | Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1106] [Cited by in RCA: 1139] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 32. | Trenaman SC, Rideout M, Andrew MK. Sex and gender differences in polypharmacy in persons with dementia: A scoping review. SAGE Open Med. 2019;7:2050312119845715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Ballard C, Hanney ML, Theodoulou M, Douglas S, McShane R, Kossakowski K, Gill R, Juszczak E, Yu LM, Jacoby R; DART-AD investigators. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol. 2009;8:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 358] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 34. | Schultz-Larsen K, Rahmanfard N, Kreiner S, Avlund K, Holst C. Cognitive impairment as assessed by a short form of MMSE was predictive of mortality. J Clin Epidemiol. 2008;61:1227-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Gianfrancesco MA, Tamang S, Yazdany J, Schmajuk G. Potential Biases in Machine Learning Algorithms Using Electronic Health Record Data. JAMA Intern Med. 2018;178:1544-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 657] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 36. | Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815-2834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |