Published online Feb 19, 2022. doi: 10.5498/wjp.v12.i2.236
Peer-review started: February 26, 2021
First decision: July 15, 2021
Revised: July 29, 2021
Accepted: November 25, 2021
Article in press: December 25, 2021
Published online: February 19, 2022
Processing time: 355 Days and 20.6 Hours
Drug-induced stuttering (DIS) is a type of neurogenic stuttering (NS). Although DIS has not been reported as frequently as other cases of NS in the literature, it is not a negligible adverse drug reaction (ADR) which can significantly affect the quality of life if not treated. This literature review aims to evaluate the epidemiological and clinical characteristics of DIS and suggests some pathophysiological mechanisms for this ADR. Relevant English-language reports in Google Scholar, PubMed, Web of Science, and Scopus were identified and assessed without time restriction. Finally, a total of 62 reports were included. Twenty-seven drugs caused 86 episodes of stuttering in 82 cases. The most episodes of DIS were related to antipsychotic drugs (57%), mostly including clozapine, followed by central nervous system agents (11.6%) and anticonvulsant drugs (9.3%). The majority of the cases were male and between the ages of 31 and 40 years. Repetitions were the most frequent core manifestations of DIS. In 55.8% of the episodes of DIS, the offending drug was withdrawn to manage stuttering, which resulted in significant improvement or complete relief of stuttering in all cases. Based on the suggested pathophysiological mechanisms for developmental stuttering and neurotransmitters dysfunctions involved in speech dysfluency, it seems that the abnormalities of several neurotransmitters, especially dopamine and glutamate, in different circuits and areas of the brain, including cortico-basal ganglia-thalamocortical loop and white matter fiber tracts, may be engaged in the pathogenesis of DIS.
Core Tip: Stuttering has two main types, developmental and acquired stuttering. Acquired stuttering is a manifestation of psychogenic or neurogenic disorders. Neurogenic stuttering is caused by brain injury, stroke, drugs, etc. Because most drugs inducing stuttering are used in the management of psychiatric and/or neurologic disorders, clinicians may merely attribute a new-onset stuttering to the worsening of the underlying disorder and neglect drugs as the causes of stuttering. Therefore, in this review, reports of drug-induced stuttering (DIS) are collected to provide information about epidemiological and clinical characteristics of DIS. Moreover, some pathophysiological changes are proposed as the underlying mechanisms of DIS.
- Citation: Nikvarz N, Sabouri S. Drug-induced stuttering: A comprehensive literature review. World J Psychiatry 2022; 12(2): 236-263
- URL: https://www.wjgnet.com/2220-3206/full/v12/i2/236.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i2.236
Speech production is a complex process involving various areas of the brain. Stuttering is a fluency disorder classified as developmental or acquired stuttering. Developmental stuttering, which is mentioned as childhood-onset fluency disorder in the diagnostic and statistical manual of mental disorders, fifth edition[1], often manifests between the ages of 2 and 6 years and spontaneously remits in most cases[2,3]. Acquired stuttering which has a secondary cause can occur in both children and adults. There are two types of acquired stuttering, psychogenic and neurogenic stuttering (NS). NS is caused by the traumatic brain injury, stroke, neurodegenerative disorders like Parkinson’s disease (PD) and multiple sclerosis, seizure disorders, drugs, etc.[4]. In contrast to the other cases of NS, in which injuries to the brain areas involved in the speech production result in neuroanatomical and neurochemical abnormalities leading to stuttering, in the cases of drug-induced stuttering (DIS), short intervals between the initiation of culprit drug and the occurrence of stuttering and also between the dose reduction or discontinuation of the drug and the relief of stuttering suggest that DIS may be caused merely by neurochemical changes in the brain[5].
Although DIS has not been reported as frequently as other cases of NS in the literature, it is not a rare and negligible adverse drug reaction as Trenque et al[6] have reported that 724 individual case safety reports (ICSRs) containing the lowest level terms “stuttering” or “stutter” have been registered in Vigibase, the world health organization international pharmacovigilance database, up to May 31, 2020. The aim of this review is to describe the reported cases of DIS, including their demographic characteristics, medical history, predominant manifestations of stuttering, and the interventions done to manage stuttering and propose some probable pathophysiological mechanisms of this type of NS.
The electronic databases Google Scholar, PubMed, Web of Science, and Scopus were searched by two reviewers without time limitation to find the relevant data. The keywords “stutter”, “stuttering”, “speech dysfluency”, “drug-induced stuttering”, “medication-induced stuttering”, “psychotropics” “antipsychotics”, “antiepileptics”, “antiseizure drug”, “anticonvulsants”, “antidepressants”, “clozapine”, and “mood stabilizers” were used as search terms. The references of published articles were also examined to find any additional relevant reports. Case reports and case series were included. The reports whose full texts were not available and those being written in non-English language were excluded. According to the above-mentioned inclusion and exclusion criteria, 63 articles reporting DIS in 82 cases were considered in this review.
Twenty-seven drugs caused 86 episodes of stuttering in 82 cases. In four cases, two drugs caused stuttering[8-11]. Of 86 episodes of DIS, 49 (57%) were caused by antipsychotic drugs, followed by 10 (11.6%) by central nervous system (CNS) agents and 8 (9.3%) by anticonvulsant drugs (Table 1). As mentioned above, Trenque et al[6] have done a disproportionality analysis using reports registered in Vigibase to estimate the association between exposure to a drug and occurrence of stuttering. Of 22632669 reports registered in this database, 724 ICSRs contained the lowest level term “stuttering” or “stutter”. Consistent with our findings, the most reports of stuttering were related to clozapine (n = 40), pregabalin (n = 33) methylphenidate (n = 27), adalimumab (n = 26), and olanzapine (n = 25)[6]. The results of the disproportionality analysis done by Trenque et al[6] showed that the following drugs had the highest reported odds ratio: Methylphenidate (19.57; 95%CI: 13.3-28.7); topiramate (12.48; 95%CI: 7.1-22.1); olanzapine (11.98; 95%CI: 8-17.9); golimumab (10.25; 95%CI: 5.5-19.1); clozapine (8.44; 95%CI: 6.1-11.6); and pregabalin (8.36; 95%CI: 5.9-11.9).
| Therapeutic category | Number of episodes of stuttering, n = 86 (%) |
| Antipsychotics | |
| Clozapine | 30 (34.9) |
| Olanzapine | 8 (9.3) |
| Risperidone | 4 (4.6) |
| Aripiprazole | 3 (3.5) |
| Trifluoperazine | 1 (1.2) |
| Chlorpromazine | 1 (1.2) |
| Fluphenazine | 1 (1.2) |
| Levomepromazine | 1 (1.2) |
| Anticonvulsants | |
| Phenytoin | 2 (2.3) |
| Divalproex | 2 (2.3) |
| Pregabalin | 2 (2.3) |
| Gabapentin | 1 (1.2) |
| Lamotrigine | 1 (1.2) |
| Central nervous system agents | |
| Methylphenidate | 3 (3.5) |
| Memantine | 2 (2.3) |
| Levodopa | 4 (4.6) |
| Dextroamphetamine and amphetamine salts (Adderall®) | 1 (1.2) |
| Atomoxetine | 1 (1.2) |
| Pemoline | 1 (1.2) |
| Antidepressants | |
| Sertraline | 3 (3.5) |
| Bupropion | 3 (3.5) |
| Desipramine | 1 (1.2) |
| Bipolar agents | |
| Lithium | 3 (3.5) |
| Respiratory tract agents | |
| Theophylline | 4 (4.6) |
| Anxiolytics | |
| Alprazolam | 1 (1.2) |
| Antineoplastics | |
| Methotrexate | 1 (1.2) |
| Pyrethrin | 1 (1.2) |
| Total | 86 (100) |
Twenty-eight (34.6%) cases were female. Most patients were in the age range of 31 to 40 years. Fifteen cases were less than 12 years old. Gender and age of a patient were not reported in one case report[12] (Table 2).
The primary or core behaviors of stuttering include sound, syllables, and monosyllabic whole-word repetitions, sound prolongations, and speech blocks[4]. The core behaviors were described only in 40 cases. Repetitions (n = 42) were the most frequent primary behavior, followed by blockages (n = 21) and sound prolongations (n = 16). Twenty-one cases had more than one type of primary behavior (Table 3).
| Offending drug (dosage) | Patients’ gender/age (yr) | Main indication of drug administration | Concomitant medications (dosage) | Onset/aggravation of stuttering | Primary behaviors | Concomitant symptoms | Management, response | Recurrence of stuttering after medication resumption | Concomitant disorders | Ref. |
| Adderall XR® (20 mg/d) | Male/10 | ADHA | No other drugs | Within two weeks after the initiation of Adderall XR® | Single word and syllable repetitions and audible/silent sound prolongations | Increased tic behaviors, increased levels of social anxiety and communication related frustration | DC of Adderall XR® and start of atomoxetine (10 mg/d), significant reduction of stuttering | NR | Developmental stuttering, Tourette Syndrome, allergies, chronic ear infections, frequent phonic and motor tics | Donaher et al[82] |
| Alprazolam (1 mg) | Female/22 | Anxiety and depression | No other drugs | Shortly after increasing the dose | Not restricted to initial syllables, occurred on small grammatical words and substantive words, persisted during singing, not associated with secondary symptomatology such as facial grimacing or fist clenching | A right carotid bruit and a grade II/IV systolic murmur without a click, Minimal late systolic mitral valve prolapse and mild stenosis of both internal carotid arteries | DC of alprazolam, complete relief after two days | Within one hour after a single morning dose of 0.5 mg alprazolam, stuttering started, then 10 to 12 h later it was stopped. Stuttering did not happen with placebo | No history of speech dysfluency | Elliott et al[83] |
| Aripiprazole (2 mg/d) | Male/8 | ADHD combined-type | Atomoxetine (25 mg/d) | After 10 d of starting aripiprazole | NR | NR | DC of Aripiprazole, complete relief | NR | Developmental stuttering | Ünay et al[84] |
| Aripiprazole (10 mg/d) | Male/11 | Mild intellectual disability | No other drugs | 4 wk after increasing the dose to 10 mg/d | NR | Addition of clonazepam 0.75 mg/d, no improvement. Reduction of aripiprazole dose to 5 mg, complete relief over 10 d | NR | Increasing the dose to 10 mg resulted in re-emergence of stuttering which responded to DC of aripirazole | No history of speech dysfluency | Naguy et al[85] |
| Atomoxetine (started at 25 mg/d and gradually increased to 40 mg/d) | Male/14 | ADHD | No other drugs | Three weeks after the initiation of atomoxetine | NR | NR | Dose reduction to 25 mg/d, no improvement. DC of atomoxetine and initiation of methylphenidate, complete relief of atomoxetine-induced stuttering and considerable reduction of developmental stuttering | NR | Developmental stuttering since the age of 7 yr. ADHD predominantly inattentive | Cicek et al[86] |
| Bupropion (SR) 150 mg BID | Female/59 | Major depressive disorder | No other drugs | Four days after starting the drug | Sound prolongations, silent blocking, word production with excess physical tension, and monosyllabic whole-word repetitions. The stuttering was anxiogenic and restricted to initial syllables | Slight finger dysdiadochokinesia | DC of bupropion, complete relief of stuttering after 2 d | NR | No history of speech dysfluency | Fetterolf et al[78] |
| Bupropion SR (300 mg/d) | Male/38 | Major depressive disorder | No other drugs | Two days after increasing the dosage from 150 to 300 mg/d | Involuntary silent pauses or blocks, repetitions, prolongations of sounds, syllables, and words, affected rhythm of speech | NR | DC of bupropion, complete relief of stuttering | Re-administration of bupropion 150 mg after 1 wk caused stammering, and the drug was stopped immediately | A history of occasional smoking, no history of speech dysfluency | Bhatia et al[79] |
| Bupropion XL (300 mg/d) | Male/53 | Depression | No other drugs | After increasing the dosage of Bupropion | Difficulty starting words and repetition of syllables | NR | Administration of 5 mg oral haloperidol, stuttering was improved after 3 h and completely relieved after 7 h | Medication was continued | No history of speech dysfluency | McAllister et al[80] |
| Clozapine (up to 400 mg/d) | Female/32 | Paranoid schizophrenia | No other drugs | 4 wk after the initiation of clozapine | NR | Pharyngeal dystonia and buccolingual and facial dyskinesia associated with laryngeal dystonia | DC of clozapine, complete relief after 5 d | Clozapine was reintroduced at 100 mg/d. All symptoms reoccurred and relieved by clozapine cessation | History of neuroleptic-induced parkinsonism but not concomitant with dysarthria, no history of speech dysfluency | Thomas et al[63] |
| Clozapine (was initiated at 400 mg/d and gradually increased to 900 mg/d) | Female/28 | schizoaffective disorder | No other drugs | Shortly after the initiation of clozapine at 400 mg/d and not relieved during the gradual increase in the dosage to 900 mg/d | NR | NR | Dosage reduction to ≤ 700 mg/d, complete relief | The dose was not increased again | No history of speech dysfluency | Ebeling et al[87] |
| Clozapine (450-750 mg/d) | Female/49 | Psychosis | No other drugs | Stuttering was initiated when the clozapine dosage was increased to 700 mg/d | NR | Generalized seizure followed by myoclonic jerks of her arms at the clozapine dosage of 750 mg/d | The addition of phenytoin and then sodium valproate and the reduction of clozapine dosage to 600 mg/d, complete relief | Clozapine was continued at 600 mg/d in addition to sodium valproate 900 mg/d with no recurrence of stuttering | History of neuroleptic- induced acute dystonia, no history of speech dysfluency | Supprian et al[59] |
| Clozapine (300 mg/d) | Male/28 | Paranoid schizophrenia | No other drugs | Stuttering was initiated when the dosage of clozapine was increased from 150 mg to 300 mg/d and worsened with further increases in the clozapine dosage | NR | Generalized tonic colonic seizure at 425 mg/d along with the increased severity of stuttering | The reduction in the dosage of clozapine to 200 mg/d and addition of sodium valproate, significant improvement | The clozapine dosage was increased to 300 mg/d, but stuttering was not reoccurred albeit in the presence of sodium valproate 800 mg/d | No history of speech dysfluency | Duggal et al[64] |
| Clozapine (300 mg/d) | Male/57 | Schizoaffective disorder | Lithium (900 mg/d), sodium valproate (600 mg/d) | Four days after the initiation of clozapine | NR | NR | Dose reduction and DC of clozapine, complete relief after 7 d | NR | History of alcohol dependency, diabetes mellitus, no history of speech dysfluency | Bar et al[15] |
| Clozapine (up to 500 mg/d) | Not mentioned | Schizophrenia | No other drugs | A few days after the initiation of clozapine at 300 mg/d | NR | Myoclonic jerks at night and facial tics | Addition of sodium valproate, significant improvement, reducing the dosage of clozapine from 500 to 300 mg/d, complete relief | Clozapine was not discontinued | No history of speech dysfluency | Begum et al[11] |
| Clozapine (700 mg/d) | Female/33 | Schizophrenia | No other drugs | After reaching the daily dose to 700 mg (interval was not reported) | NR | Facial tics, seizure (seizure was initiated after the occurrence of stuttering) | Reduction in the dosage of clozapine to 600 mg/d, remarkable improvement, addition of sodium valproate to control seizure, no effect on stuttering | Clozapine was not discontinued | No history of speech dysfluency | Hallahan et al[58] |
| Clozapine (300 mg/d) | Female/34 | Schizophrenia | No other drugs | 2 wk after the initiation of clozapine | NR | Orofacial dyskinesia | Clozapine dosage reduction to 50 mg/d, complete relief | Clozapine was not discontinued | No history of speech dysfluency | Hallahan et al[58] |
| Clozapine (50-125 mg) | Male/62 | Delusional disorder | No other drugs | NR | Unsustained phonation, hesitation, irregular articulatory break down, sound repetition (not related to any specific sound, occurred at irregular word positions) | Orofacial dyskinesia, laryngeal and pharyngeal tardive dystonia, harsh and strangulated voice | Addition of tetrabenazine, patient could not tolerate the clozapine dosages more than 100 mg/d, DC of clozapine, complete relief | Clozapine was not restarted | No history of speech dysfluency | Lyall et al[9] |
| Risperidone and then clozapine (450 mg/d and 75 mg/d) | Male/55 | Schizophrenia | No other drugs | NR | Occasional blocking, prolongation on word-initial sounds and repetitions of speech elements including one-syllable words at the beginning of his speech utterances | Stammering and unusual limb and trunk movements related to risperidone, belching, persistent hiccupping, worsening of the facial tic, and the orofacial dyskinesia involving the lips and tongue related to clozapine | Risperidone-induced stuttering: NR, the first episode of clozapine-induced stuttering, dose reduction to 125 mg/d and cessation of clozapine; significant improvement and complete relief of stuttering; the second time of clozapine-induced stuttering: addition of sodium valproate, considerable improvement | Clozapine was restarted at 75 mg/d, recurrence of stuttering, the addition of sodium valproate, 600 mg/d, significant improvement in the stuttering | History of head injury resulting in problems with executive functioning and a significant discrepancy, between the patient’s verbal and performance IQ, making various clicking noises and blowing sounds when speaking before the initiation of antipsychotic drugs | Lyall et al[9] |
| Clozapine (up to 600 mg/d) | Male/35 | Schizotypal personality disorder | No other drugs | At clozapine dosage of 250 mg/d and progressive worsening with dose escalation | NR | NR | Reducing the dosage of clozapine to 200 mg/d, complete relief | Clozapine was continued at 200 mg/d without causing stuttering | History of trifluoperazine-induced truncal dystonia, no history of speech dysfluency | Krishnakanth et al[88] |
| Clozapine (200 mg/d) | Male/24 | Paranoid schizophrenia | No other drugs | After increase in the dosage of clozapine to 200 mg/d | NR | NR | DC of clozapine, complete relief | Clozapine was not restarted, amisulpiride was started and did not cause stuttering | No history of speech dysfluency | Krishnakanth et al[88] |
| Clozapine (250 mg/d) | Male/23 | Paranoid schizophrenia | No other drugs | At clozapine dosage of 250 mg/d (interval was not reported) | NR | NR | Clozapine dosage reduction to 150 mg/d, complete relief | Clozapine was not discontinued | History of neuroleptic-induced tardive dyskinesia, no history of speech dysfluency | Krishnakanth et al[88] |
| Clozapine (350 mg/d) | Male/15 | Undifferentiated schizophrenia | Clomipramine (225 mg/d) | Three years after the initiation of clozapine and clomipramine | Repetitions of syllables and transient accelerations of speech rate | Involuntary paroxysmal perioral movements, facial tic-like movements, myoclonic jerks of the upper limbs, GTC seizure | Addition of valproic acid at 500 mg/d, complete relief of stuttering within days | Clozapine was continued with valproic acid without reoccurrence of seizure and speech dysfluency during 2 yrs of follow-up | Symptoms of obsessive-compulsive disorder, no history of epilepsy or speech dysfluency | Horga et al[66] |
| Clozapine (up to 250 mg/d) | Male/29 | Undifferentiated schizophrenia | No other drugs | After the clozapine dosage titration from 137.5 mg/d to 150 mg/d | Frequent repetition and prolongation of syllables or words with frequent hesitations, blocking and pauses | No focal dystonia or any evidence of seizure-like activity | Reducing and splitting the dose of clozapine to 50 mg in morning and 75 mg at night, improvement of stuttering | Reoccurrence of stuttering at clozapine dosage of 250 mg/d, improvement of stuttering after dose reduction to 225 mg/d, a later increase in the dosage to 300 mg/d did not cause recurrence of stuttering | History of antipsychotic-induced extrapyramidal symptoms, no history of speech dysfluency | Grover et al[61] |
| Clozapine (400 mg/d) | Female/33 | Severe MDD with psychotic features | No other drugs | Stuttering was started after increasing the dosage of clozapine to 400 mg/d and worsened when the dosage was increase to 450 mg/d | Excessive prolongation of syllables or words | Sialorrhea | Addition of benztropine, no improvement. Reduction of the dosage of clozapine to 350 mg/d, complete relief | Stuttering recurred 16 d after increasing the clozapine dosage to 400 mg/d, but completely relieved after dosage reduction to 300 mg/d | None | Kumar et al[89] |
| Clozapine (up to 650 mg/d) | Male/32 | Paranoid schizophrenia | Sertraline (300 mg/d), lamotrigine (500 mg/d), haloperidol (4 mg/d), clonazepam (1 mg/d) | Noticeable stuttering at clozapine dosages of ≥ 600 mg/d | Expressive speech dysfluency with hesitancy and frequent pauses | Involuntary twitching of muscles of jaw | Clozapine dose reduction by 25 mg, improvement of stuttering | Clozapine was not discontinued | No history of speech dysfluency | Murphy et al[20] |
| Clozapine (400 mg/d) | Male/43 | Schizoaffective disorder | Paroxetine (20 mg/d) | Stuttering became noticeable when the clozapine daily dose was increased to more than 350 mg | Expressive speech dysfluency | NR | Clozapine dose reduction by 50 mg, improvement of stuttering | Clozapine was not discontinued | No history of speech dysfluency | Murphy et al[20] |
| Clozapine (450 mg/d) | Male/33 | Paranoid schizophrenia | No other drugs | Stuttering was developed during the initiation and dose titration of clozapine | Intermittent stuttering of speech | NR | Reducing the rate of dose titration, improvement of stuttering | Clozapine was not discontinued | No history of speech dysfluency | Murphy et al[20] |
| Clozapine (up to 300 mg/d) | Female/46 | Delusional disorder | No other drugs | Stuttering was developed during the initiation and dose titration of clozapine | Hesitancy with specific syllables | Orofacial dyskinesia | Clozapine dose reduction to 50 mg, improvement of stuttering | Clozapine was not discontinued | No history of speech dysfluency | Murphy et al[20] |
| Clozapine (325 mg/d) | Male/67 | Schizoaffective disorder | Duloxetine (60 mg/d), hyoscine (30 mg/d), aripiprazole (10 mg/d) | Stuttering was developed during the initiation and dose titration of clozapine | Expressive speech dysfluency | Orofacial twitching, upper limb jerking, hypersalivation | Reducing the rate of clozapine dose titration, improvement of stuttering | The clozapine dose was increased again to control psychotic symptoms, but nothing about the recurrence of stuttering was reported | Hearing impairment, hypertension | Murphy et al[20] |
| Clozapine (650 mg) | Female/63 | Paranoid schizophrenia | Amisulpride 200 mg/d, amitriptyline 25 mg/d, paroxetine 20 mg/d, zopiclone 3.75 mg/d | Stuttering was developed on a stable dose of clozapine | Expressive speech dysfluency with hesitancy | NR | Reducing the dose of clozapine by 50 mg, no improvement | Clozapine at 650 mg/d was recommenced, but authors did not report its effects on the recurrence of stuttering | No history of speech dysfluency | Murphy et al[20] |
| Clozapine (100 mg), aripiprazole (7.5 mg/d) | Female/21 | Schizophrenia | No other drugs | At clozapine dosage of 100 mg/d and aripiprazole dosage of 7.5 mg/d | NR | NR | Reduction of the dose of clozapine and addition of aripiprazole (5 mg/d), complete relief. Reduction of the dose of aripiprazole from 5 to 7.5 mg/d, complete relief | The drugs were not discontinued | Turner syndrome, no history of speech dysfluency | Ertekin et al[8] |
| Clozapine (gradually increased to 450 mg/d) | Male/16 | Schizoaffective disorder | Citalopram (NR), clonazepam (NR), atenolol (NR), lithium (NR) | Approximately 22 d after increasing the clozapine dosage to 400 mg/d | Persistent stuttering (difficulties with the pronunciation of letters “I,” “D,” and “T”) | Orofacial dyskinesia with perioral twitching (started at clozapine dosage of 350 mg/d), microseizure according to EEG (at clozapine dosage of 400 mg/d) | Substituting lithium with divalproex sodium, improvement in stuttering 4 wk after receiving divalproex sodium at 500 mg BID | Clozapine was not discontinued because of its considerable therapeutic effects | History of type 1 DM, DKA with episodic hallucinations, GERD, cerebral contusion, occasional cocaine use, anxiety-induced intermittent stuttering, family history of stuttering | Rachamallu et al[62] |
| Clozapine (up to 600 mg/d) | Female/22 | Schizophrenia | Fluoxetine (60 mg/d) | Stuttering was developed after the clozapine dose escalation to 300 mg/d | NR | NR | Reduction in the clozapine dose and initiation of ECT, minimal improvement | Clozapine was not discontinued | NR | Das et al[19] |
| Clozapine (450 mg/d) | Man/in early 40s | NR | No other drugs | After increasing the clozapine daily dose from 400 mg to 450 mg | NR | Marked increase in seizure activity | DC of clozapine, nothing was clearly reported by the authors | NR | NR | Kranidiotis et al[24] |
| Clozapine (200 mg/d) | Male/38 | Schizophrenia | No other drugs | Stuttering was evident at 200 mg/d and became so disabling at 350 mg/d | NR | NR | Dose reduction of clozapine and addition of amisulpiride and BDZ, reduction of stuttering, DC of clozapine, complete relief | Clozapine was not restarted | NR | Kranidiotis et al[24] |
| Clozapine (300 mg BID) | Male/57 | Paranoid schizophrenia | Risperidone, IM injection (37.5 mg every 2 wk), Risperidone, oral (1.5 mg/d which increased to 2 mg BID on admission) | Two days after admission (the dosage of clozapine, 300 mg BID, was not changed on admission) | NR | Orofacial and extremities myoclonic jerks, drop attacks | Clozapine dosage reduction to 100 mg BID, resolution of stuttering within two days | The patient was discharged on clozapine 150 mg BID, but author reported nothing about stuttering at follow-up | History of COPD, hypertension, DM, and chronic back pain, cigarette smoking | Chochol et al[60] |
| Clozapine (125 mg/d) | Male/29 | Schizophrenia | No other drugs | A few days after titrating the clozapine dosage to 125 mg/d | Frequent repetitions of words that included broken words | NR | Reducing the clozapine dosage to 100 mg/d, significant improvement | Clozapine dosage was not re-escalated | No history of speech dysfluency | Nagendrappa et al[90] |
| Clozapine (up to 200 mg/d) | female/25 | Schizophrenia | No other drugs | At clozapine dosage dose of 150 mg/d (interval was not mentioned) | NR | Tonic-clonic epileptic seizure | DC of clozapine and start of amisulpiride and biperiden, complete relief of stuttering and seizure | Clozapine was not rechallenged | No history of speech dysfluency | Gica et al[65] |
| Divalproex sodium (600 mg/d) | Male/45 | Affective instability and irritability | Citalopram (30 mg/d), promazine (100 mg/d) | Four days after initiation of divalproex sodium | Sound repetitions and prolongations (not restricted to the initial syllable and caused pronounced difficulty in starting and completing his sentences) | NR | DC of divalproex, complete relief after 3 d | Divalproex sodium was not restarted | A 10-yr history of post-traumatic stress disorder and alcoholism, no history of speech dysfluency | Aukst-Margetić et al[91] |
| Divalproex sodium (1500 mg/d in divided dose) | Male/56 | Bipolar affective disorder | Olanzapine (10 mg/d), lorazepam (4 mg/d, gradually stopped along with increase in the dose of divalproex) | Two weeks after increasing the dosage of divalproex from 1000 to 1500 mg/d | A moderately pressured speech, articulation of speech, alterations in intensity and timings of utterance segments, Involuntary repetitions and prolongations of sounds, syllables, words or phrases, involuntary silent pauses or blocks | NR | DC of divalproex, instant amelioration of the stuttering | Re-initiation of the drug after one week caused resurgence of symptoms, so the drug was stopped | No history of speech dysfluency | Mukherj et al[92] |
| Desipramin (300 mg/d) | Male/28 | Dystimia, primary type, major depression | Doxepin (50 mg at bed time) | Two months after increasing the dosage of desipramine | Stuttering with difficulty in articulation | Minimal dryness of mouth before stuttering, myoclonic jerking (twitching movements around his jaw) concomitant with stuttering | DC of both drugs, complete relief after 48 h | Twenty-four hours after restarting both drugs stuttering happened again, the desipramin dosage was decreased to 250 mg/d, but stuttering was persisted occasionally, on 4 different occasions, desipramin was discontinued and stuttering was solved within 24-48 h; an increase in the doxepin dosage to 200 mg at bed time had not resulted in stuttering | Opiate and alcohol dependence in remission, retinal detachment and ruptured disc and chronic back pain in the past, no history of speech dysfluency | Masand et al[93] |
| Fluphenazine (up to 50 mg/d) | Male/35 | Schizophrenia | Benztropine mesylate (4 mg/d) | 12 d after increasing fluphenazine dosage to 50 mg/d | NR | EPS | Dosage reduction to 30 mg/d, complete relief | Increasing the dosage of fluphenazine to 40 mg/d caused stuttering recurrence | No history of speech dysfluency | Nurnberg et al[10] |
| Gabapentin (NR) | Female/58 | Intractable seizure | Phenytoin (NR) | NR | NR | NR | DC of gabapentin, relief after 4 d | NR | No history of speech dysfluency | Nissani et al[94] |
| Lamotrigine (up to 5 mg/kg/d) | Female/5 | BECTS | Valproic acid (30 mg/kg/d) | Stuttering was initiated after increasing the dosage of lamotrigine to 5 mg/kg/d | NR | Frequent diurnal absence seizures, poor concentration and forgetfulness, clumsiness and poor coordination, emotional lability, dysarthria, and slurred speech | DC of lamotrigine, speech improvement in a couple of days | Lamotrigine was not rechallenged | NR | Catania et al[95] |
| Levodopa/carbidopa (100/25 mg TID) | Male/44 | PD | NR | Patient had a history of PDS, and stuttering was exacerbated during on periods, 1 h after levodopa/carbidopa intake | NR | Dyskinesia during drug-on phases and akinesia, bradykinesia, resting tremors, and rigidity in drug-off phases | The severity of stuttering return to baseline during levodopa-off periods | Levodopa was not discontinued | PDS | Anderson et al[25] |
| Levodopa (200 mg/d) | Male/72 | PD | None | Nearly one month after increasing the dosage to 200 mg/d | NR | Palilalia, speech freezing | DC of levodopa and initiation of pramipexole, return to the baseline level of dysfluency | Reinitiating levodopa caused stuttering | Speech dysfluency due to PD | Louis et al[22] |
| Levodopa (up to 1000 mg/d) | Male/42 | PD | Pergolide (1.5 mg/d), quetiapine (50 mg at bed time) | After increasing the levodopa dosage to 300 mg/d | Pressured speech and sound repetition | Palilalia, speech freezing | NR | NR | None | Louis et al[22] |
| Levodopa 600 mg/d | Male/57 | PD | Cabergoline (4 mg/d), selegeline (10 mg/d), amantadine (300 mg/d) | Patient had a history of PDS, and stuttering was exacerbated during on phases after levodopa consumption | Speech repetitions and speech blocks | Speech problems associated with PD including hypokinetic dysarthria and hypophonia occurred during levodopa-off phases | Severity of stuttering return to baseline during levodopa-off periods | Levodopa was not discontinued | PDS | Burghause et al[26] |
| Levomepromazine (50 mg at bed time) | Male/65 | Bipolar disorder | Quetiapine (NR), valproate semisodium (NR), zolpidem, moxonidin (NR), propafenone (NR), insulin (NR) | Five days after the initiation of levomepromazin | NR | NR | DC of Levomepromazin, complete relief three days later | Levomepromazin was not recommenced | History of drug induced EPS, supraventricular tachycardia, type 2 DM, HTN, and mild cognitive impairment | Margeticet al[96,97] |
| Lithium (1200 mg at bed time) | Male/48 | Bipolar affective disorder | Fluoxetine (20 mg/d) | One month after the initiation of lithium | Worsening his developmental stuttering, a repetitive word stutter that severely limited his verbal communication ability | Lightheadedness, hand tremor | Tapering off lithium, stuttering returned to baseline within a few weeks | Valproic acid (2750 mg/d) was started instead of lithium | PDS, depression | Netski et al[98] |
| Lithium (900 mg twice daily) | Male/10 | Bipolar disorder | Risperidone (4 mg bed time), clonidine (0.1 mg 3 times daily), melatonin (3 mg at bed time), famotidine (20 mg BID) | Two days after increasing the dose of lithium, stuttering was worsened | Syllable repetitions, occurred only at the beginning of sentences | NR | Dose adjustment of lithium to 600 mg in the morning and 900 mg at night, stuttering returned to baseline after 2 d | Lithium was not discontinued | History of developmental stuttering, bipolar disorder not otherwise specified, ADHD, and conduct disorder | Gulack et al[99] |
| Lithium (the dose was not mentioned, but lithium was used for a long time) | Female/86 | Bipolar disorder | Donepezil (NR), primidone (NR), risperidone (NR) | After a chronic use of lithium, stuttering was started and stayed for 3 more mo. The lithium level was elevated (2.0 mmol/L) | Starting a few words fluently, then repeating syllables and words and terminating the sentence abruptly | NR | DC of lithium, complete relief of stuttering after two weeks | Lithium was not restarted | Past medical history of dementia and epilepsy, no history of speech dysfluency | Sabillo et al[100] |
| Memantine (10 mg/d) | Male/9 | Autistic disorder | No other drugs | After increasing the dose | Deterioration of primary behaviors of developmental stuttering includingsound repetition, and sound prolongation on first and middle vowels, and difficulty for starting to speak. His parents explained that the child could only start to speak after a deep and audible breath | NR | Reduction of memantine dosage to 7.5 mg/d, improvement of acquired stuttering after several days. DC of memantine, stuttering was reduced to baseline after 3 wk | Risperidone was used instead | Developmental stuttering | Alaghband-Rad et al[17] |
| Memantine (5 mg/d) | Male/4 | Autism | No other drugs | After increasing the dose | The difficulty was with the start of the speech and the child could only start to speak after a deep and audible breath | NR | The drug was continued at the same dose as the difficulty was tolerable, and gradually was increased to 7.5 mg/d, relief of speech difficulty | Medication was continued, and its dose was gradually increased | No history of speech dysfluency | Alaghband-Rad et al[17] |
| Methotrexate (cumulative dose of 62.5 mg, IT) | Female/22 | Pre-B acute lymphoblastic leukemia | NR | After achieving cumulative dose of 62.5 mg (26 d after initiating IT MTX) | NR | Dysphasia progressed to aphasia, mild headache, low-grade fever, behavioral problems | Three months after initiation of symptoms (no intervention was described) | NR | No history of speech dysfluency | Shuster et al[21] |
| Methylphenidate (10 mg/d) | Male/7 | ADHD | No other drugs | 10 d after the initiation of the drug | Sound prolongations, silent blocking, word production with excess physical tension, monosyllabic whole-word repetitions | NR | DC of methylphenidate, speech returned to normal after 1 wk | Atomoxetine was used instead | No history of speech dysfluency | Alpaslan et al[101] |
| Methylphenidate (5 mg in the morning and 5 mgat noon) | Male/7 | ADHD | No other drugs | One day after drug initiation | Troubles during the pronouncing the first syllables and repetitions of some syllables | NR | DC of Methylphenidate, improvement after 10 d | Methylphenidate was restarted at 10 mg in the morning and5 mg at noon. After 10 d, stuttering was returned | NR | Copur et al[102] |
| Methylphenidate (2.5 mg BID) and pemoline (9.375 mg/d) after DC of methylphenidate | Girl/3 | Pervasive hyperactivity | None | Three days after starting methylphenidate, four days after starting pemoline | Repetition of the first syllable of word which gradually worsened | NR | DC of methylphenidate, relief of stuttering, DC of pemoline, relief of stuttering | Methylphenidate and pemoline were not restarted | NR | Burd et al[7] |
| Olanzapine (15 mg/d) | Male/56 | Depression | Intrathecal morphine (7.5 mg/d), clomipramine (225 mg/d) | Four days after the initiation of clozapine | Constant word repetition (acquired) | NR | DC of olanzapine, complete relief after two days | NR | Chronic pain syndrome, no history of speech dysfluency | Bar et al[15] |
| Olanzapine (7.5-10 mg/d) | Male/72 | Psychotic depression | Clomipramine (50-150 mg/d) | 3 wk after the initiation of olanzapine | Repetition and retention of first syllables and prolongation of phonemes | NR | DC of olanzapine, complete relief after 5 d | NR | Brain cortical atrophy, no history of speech dysfluency | Bar et al[15] |
| Olanzapine (5 mg/d) | Female/36 | Manic episode | Sodium valproate (300 mg/d), prednisolone (75 mg/d) | 7 d after the initiation of olanzapine | Repetition of syllables and words | NR | DC of olanzapine, complete relief after 4 d | NR | Ulcerative colitis and celiac disease, no history of speech dysfluency | Bar et al[15] |
| Olanzapine (10 mg/d) | Female/43 | Schizophrenia | No other drugs | Approximately 21 d after the initiation of olanzapine | Repetition of first syllables and word prolongation | NR | DC of olanzapine, complete relief after 3-5 d | NR | Mild cluttering at the age of 19 | Bar et al[15] |
| Olanzapine (2.5 mg/d) | Female/51 | Depression | Sertraline (100 mg/d), promethazine (50 mg at night); both was started 14 wk before initiation of olanzapine | 14 d after the initiation of olanzapine | Blocking of speech and prolongation of phonemes | NR | Increase in olanzapine dose to 5 mg/d, relief of stuttering during the next weeks | Olanzapine was not discontinued | Symmetrical cerebellar hypoplasia and generalized cortical atrophy, no history of speech dysfluency | Bar et al[15] |
| Olanzapine (10 mg/d) | Male/42 | Schizophrenia | Zopiclone (7.5 mg/d) | Two days after the initiation of olanzapine | Difficulty in articulating words properly | NR | DC of olanzapine, complete relief after two days | NR (patient was not followed-up) | A fall without loss of consciousness 2 d before initiation of stuttering, no history of speech dysfluency | Bar et al[15] |
| Olanzapine (10 mg/d) | Male/42 | Paranoid ideation | Venlafaxine (150 mg/d), promazine (200 mg/d) | Four days after the initiation of olanzapine | Repetition and retention of first syllables and prolongation of phonemes | NR | DC of olanzapine, complete relief after two days | PTSD, adjustment disorders, no history of speech dysfluency | Lasic et al[103] | |
| Olanzapine (10 mg/d) | Male/21 | Psychotic disorder | No other drugs | Three days after the initiation of olanzapine | disturbance in the fluency and time patterning of speech, repetition of sounds and syllables, blocking between words | NR | DC of olanzapine and start of quetiapine, complete relief after three days | Olanzapine was not restarted | No history of speech dysfluency | Asan et al[104] |
| Phenytoin (200 mg/d) | Male/42 | Seizure due to head injury | No other drugs | Shortly after initiation of phenytoin | Predominantly part-word repetitions and prolongation | Abnormality of speech muscle fine motor control | Addition of CBZ and gradual DC of phenytoin, sustain decrease in the frequency of dysfluencies and improved motor performance | Phenytoin was not restarted | No history of speech dysfluency | Mcclean et al[105] |
| Phenytoin (20 mg/kg LD and 5 mg/kg/d MD) | Male/3 | GTC seizure due to head trauma | No other drugs | 10 d after the initiation of phenytoin | NR | NR | DC of phenytoin and initiation of sodium valproate, complete relief 10 d after DC of phenytoin | Phenytoin was not rechallenged | No history of speech dysfluency | Ekici et al[106] |
| Pregabalin (75 mg twice daily) | Female/31 | Complex regional pain syndrome | No other drugs | After taking the second dose of pregabalin on the first day | A slurred speech | NR | DC of pregabalin, complete relief after one week | Pregabalin was not restarted | No history of speech dysfluency | Giray et al[107] |
| Pregabalin (75 mg twice daily) | Female/68 | Herpes zoster | Acyclovir (800 mg five times daily) | Three days after the initiation of pregabalin | NR | Frequent blepharospasm | DC of pregabalin;alleviated of symptoms after four days and complete relief after one week | A 75 mg pregabalin capsule consumption after 4 wk resulted in stuttering and frequent blepharospasm | No history of speech dysfluency | Ge et al[108] |
| Pyrethrin product containing 0.33% pyrethrum extract and 4% piperonyl butoxide (3 times overa period of 12 d left on the scalp for 10 min) | Female/2 (the child’s mother, who was breastfeeding her atleast one time per day, were receiving this topical product) | Repeated episodes of head lice | No other drugs | Two days after the last period of mother’s treatment | An acute onset of stuttering especially at the initiation of the speech | An increase in clumsiness, slight erythematous rash ofapproximately 3 cm × 2 cm on the occiput of the scalp | Six weeks postexposure | Pyrethrin was not repeated | No history of speech dysfluency | Hammond et al[81] |
| Risperidone (4 mg/d, then 8 mg/d) | Male/32 | Aggravated psychotic disorder | Lorazepam (1 mg/d) | Stuttering was initiated after the dose increase to 4 mg/d, and worsened 16 d after the dose increase to 8 mg/d | Severe sound repetitions and interjections in a way that it was difficult to understand his words | Slight akathisia-like symptoms such as anxiety and restlessness (not prominent) | No action, stuttering diminished 23 d later | He continued taking risperidone at 8 mg/d with only a slight stuttering | A 10-yr history of Schizophrenia. His friend during junior high school was a stutter, and the patient used to mimic his stuttering. He began stuttering at that time for 1 yr | Lee et al[16] |
| Risperidone (4 mg) | Female/48 | Psychosis | Lorazepam (1 mg PRN), procyclidine (5 mg BID for treatment of EPS) | 11 d after taking risperidone | Repetitions in the speech, pausing within a word and her speech, an excess of physical tension in the speech | NR | A little decrease in risperidone dose, a bit reduction in stuttering | Risperidone was not discontinued | No history of speech dysfluency | Yadav et al[18] |
| Risperidone (at a dose of 1 mg/d for 2 yr) | Male/21 | Behavioral disorder | No other drugs | After chronic treatment with low-dose of risperidone | Prolongation of sounds, hearable blocks, repetitions of single-syllable words | NR | No action, stuttering was decreased to a minimal level after 17 d | Risperidone was not discontinued | Moderate mental retardation because of perinatal asphyxia, no history of speech dysfluency | İnci et al[23] |
| Sertraline (100 mg daily) | Male/36 | Major depression | Alprazolam (0.25 mg 3 TID) | Two weeks after increasing the dosage from 50 to 100 mg/d | Normal vocabulary, decreased rate of speech, normal tone, interrupted words | NR | DC of serteraline, speech problem resolved after one day | Medication was not restarted. Later, administering phenelzine, imipramine, and fluoxetine caused milder speech hesitancy | No history of speech dysfluency | Makela et al[109] |
| Sertraline (50 mg daily) | Female/32 | Recurrent depression | No other drugs | During the third week of starting the drug¸ stuttering occurred and worsened over a 3-d period | Difficulty in starting and completing the sentences | Feeling nervous, increased restlessness, and insomnia two days before the onset of stuttering | DC of sertraline, complete relief of stuttering after 3 d | Previously, patient has received sertraline and experienced stuttering, so discontinued the medication. Medication was not restarted. Desipramine was started and did not cause stuttering | No history of speech dysfluency | Christensen et al[76] |
| Sertraline (150 mg daily) | Female/22 | Bulimia nervosa, anorexia nervosa, posttraumatic stress disorder, recurrent depression, panic disorder | Clonazepam (0.5 mg QID), trimethoprim-sulfamethoxazole (BID) | One week after increasing the dosage of sertraline | NR | Hyperreflexia and mild tremulousness, generalized muscle twitching (myoclonus), restlessness, and mild confusion | DC of sertraline and Antibiotic, gradual normalization of speech over two to three days | Seven days after restarting sertraline at 50 mg/d, stuttering and other symptoms returned, then the drug was discontinued | No history of speech dysfluency | Brewerton et al[77] |
| Theophylline (200 mg BID to 100 mg QID) | Male/the age of the onset of theophylline-induced stuttering was not reported, but it surely occurred when he was between 1.5 and 4 yr old | Asthma | Nothing was clearly mentioned | The patient only experienced stuttering during the autumn when he was receiving theophylline for the management of asthma attacks | Repeating whole words, six or seven times usually at the beginnings of the sentences, no dysfluency while singing | Being tense, havinginsomnia, and be frustrated by his speech problem | DC of theophylline at the end of autumn before age 4 yr, complete relief of stuttering. Changing the dosage from 100 mg QID to 200 mg BID at age 4 yr, complete relief after 7 d with no recurrence of stuttering | The patients had stuttered each time that he was on Theophyllineregimen 200 mg BID | No history of speech dysfluency | Rosenfield et al[110] |
| Theophylline (130 mg TID and sometimes QID) | Female/6.5 | Asthma | Metaproterenol sulphate (PRN) | Within a few days after increasing the theophylline dosage to 130 mg TID | Multiple repetitions of the word "I", especially at the beginning of sentences, she could speak better when speak more slowly. Stuttering was worse when she was excited | NR | DC of theophylline, complete relief within two days | Resumption of theophylline resulted in the recurrence of stuttering which responded to drug withdrawal. Several months after the discontinuation of theophylline, the drug was resumed without causing any dysfluency | No history of speech dysfluency | Rosenfield et al[110] |
| Theophylline 200 mg BID to 200 mg TID | Male/4 yr and 3 mo | Asthma | Beclomethasone dipropionate and Theo-Dur sprinkle (200 mg BID) (at age 4 yr and 4 mo). Addition of metaproterenol sulphate, isoetharine HCL andatropine (at age 4 yr and 10 mo, DC of all drugs and initiation of cromolyn capsules (20 mg TID) (at age 5 yr) | Nine months after the initiation of theophylline | Repeating "ah, ah, ah" in the middle of sentences, stuttering was worse when he was excited | Anxiety, sleep problems | Withdrawal of theophylline at age 5 yr, complete relief within two weeks | After complete relief of stuttering, the patient only received theophylline during asthma attacks and experienced no stuttering | No history of speech dysfluency | Rosenfield et al[110] |
| Theophylline (400 mg BID) | Male/73 | A long-standing chronic obstructive lung disease secondary to pneumoconiosis | Steroids and ranitidine as well as being on oxygen | One month after the introduction of theophylline | An intense tonic-clonic stuttering without any extrapyramidal components | NR | DC of theophylline, stuttering was diminished within 48 h | Theophylline was readministered 2 wk later at the same dosage, and the same speech disorder recurred within a few daysand persisted until treatment was stopped | No history of speech dysfluency | Gerard et al[111] |
| 1. Trifluoperazine (30 mg/d) | Male/40 | Schizophrenia | Trihexyphenidyl (5 mg/d) | 1. Four days after increasing the trifluoperazine dosage to 30 mg/d | NR | NR | 1. Increasing dosage of trihexyphenidyl, no improvement. DC of trifluoperazine, complete relief | Increasing the dosage of chlorpromazine to 700 mg/d caused the return of stuttering; reducing the dosage of chlorpromazine to 400 mg/d caused cessation of stuttering | No history of speech dysfluency | Nurnberg et al[10] |
Individuals with developmental stuttering and persistent developmental stuttering (PDS) have anxiety related to stuttering[4]. However, patients with acquired stuttering may be annoyed by stuttering but do not experience anxiety. None of the reports included in this review described the psychological presentations of patients when producing a dysfluent speech.
Individuals with developmental stuttering[4,13] as well as patients with NS[13-15] often develop secondary behaviors including eye blinking, facial grimacing, interjections, word or phrase substitution, etc. The secondary behaviors are acts that are learnt in long term to cope with stuttering[13]. In the cases included in the present review, the secondary behaviors related to the stuttering were not reported. It may not be unusual because in comparison to the developmental stuttering, secondary behaviors are less prominent in the acquired stuttering[13]. Furthermore, we believe that since the development of the secondary behaviors requires a sufficient period, it is unlikely that patients with DIS have enough time to present these behaviors because interventions such as dose reduction or discontinuation of the culprit drug are carried out as soon as possible, which result in the complete relief or significant improvement of stuttering.
Therapeutic measures that resulted in significant improvement or complete relief of stuttering were drug withdrawal in 48 (55.8%) episodes, dose reduction in 18 (21%), addition of a new drug in three (3.6%), addition of a new drug besides dose reduction of the culprit drug in three (3.5%), and slow dose titration in two (2.3%). Moreover, in three (3.5%) episodes, the stuttering spontaneously remitted despite continuation of the offending drug with no dose reduction[16-18]. However, three (3.5%) episodes did not respond to the dose reduction[19-21]. For four (4.6%) episodes, the authors did not describe the actions taken to manage stuttering[22-25]. In two (2.3%) cases with PDS and PD, stuttering was exacerbated during levodopa-on periods, and levodopa was not discontinued[26,27] (Table 3).
In this section, the abnormalities suggested in the pathogenesis of developmental stuttering and PDS are described. Then, we propose some mechanisms for DIS based on the underlying impairments involved in the pathogenesis of PDS and developmental stuttering.
For producing a fluent speech, orofacial as well as respiratory muscles must work properly. Different areas of the brain including several parts of the cerebral cortex and subcortical structures such as the basal ganglia (BG) and cerebellum are involved in speech production. Functional abnormalities of each part of these areas may cause speech dysfluency. It has been suggested that dysfunction in the different parts and networks of cerebral cortex engaged in speech production[28], the impairments in the neural connections between the cerebral cortex, the BG, and the thalamus, which are called cortico-BG-thalamocortical circuit (CBTC)[28,29], and/or the dysfunction of the BG are involved in the pathogenesis of developmental stuttering[28,29].
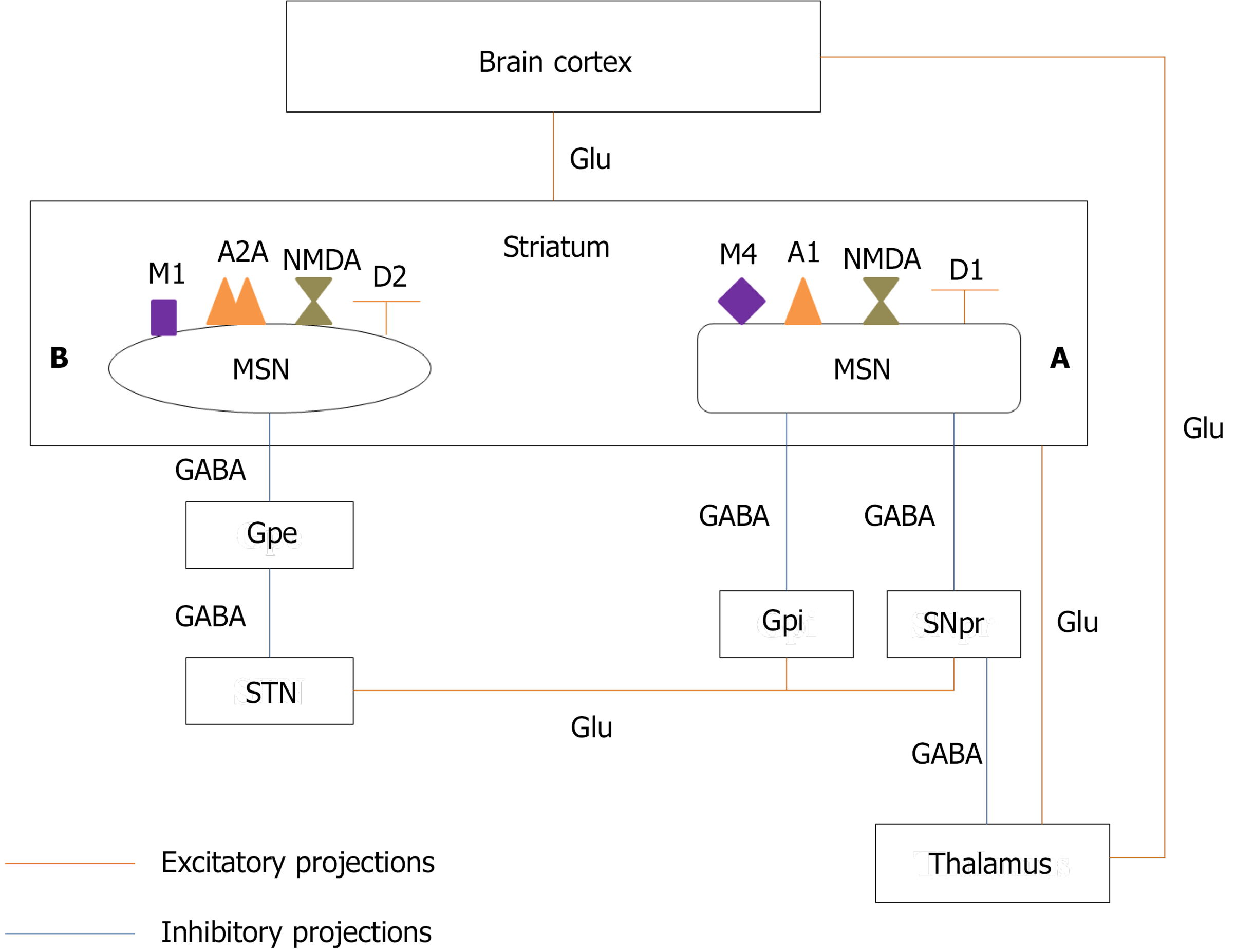
Several neural circuits are engaged in the process of speech production. One of these circuits that has received much attention in the pathogenesis of stuttering is CBTC[28,29]. The BG consist of input, intrinsic, and output nuclei. The input nuclei receive information from different parts of the brain, especially the cerebral cortex, the thalamus, and the substantia nigra, and send signals to the intrinsic nuclei for further processing and then to the output nuclei. The output nuclei relay signals to the thalamus which sends signals back to the part of the cerebral cortex from which the primary signal was originated. The input nuclei consist of the putamen and the caudate nucleus, collectively named the striatum. The intrinsic nuclei consist of the external segment of the globus pallidus (GPe), the subthalamic nucleus (STN), and the substantia nigra pars compacta (SNpc). The internal segment of the globus pallidus (GPi) and the substantia nigra pars reticulata (SNpr) make the output nuclei[30].
The striatum has two types of neurons including gamma-aminobutyric acid-ergic (GABAergic) medium-sized spiny neurons (MSNs) representing 90%-95% of the striatal neurons and GABA
The signals that are received by the input nuclei of BG are transmitted via two pathways: Direct and indirect. The activation of the direct pathway stimulates the cerebral cortex and therefore activates the right motor program while the activation of the indirect pathway inhibits the cerebral cortex and therefore, all other competing motor programs[2,28].
In the direct pathway, MSNs, which have N-methyl-D-aspartate (NMDA) glutamate receptors, D1 dopamine receptors, A1 adenosine receptors, and M4 muscarinic receptors[34] and release gamma-aminobutyric acid (GABA), substance P, and dynorphin[35], project to the SNpr and GPi, the output nucleus of BG. MSNs are stimulated by glutamatergic projections from the cerebral cortex. The activated MSNs release GABA, which inhibits the output nucleus. GABAergic projections from the output nucleus inhibit the glutamatergic neurons of the thalamus. Therefore, the inhibition of the output nucleus by MSNs disinhibits thalamic neurons which ultimately activate the cerebral cortex and increase locomotor activity[35] (Figure 1).
MSNs in the indirect pathway, which have NMDA glutamate receptors, D2 dopamine receptors, A2A adenosine receptors, and M1 muscarinic receptors[34] and release GABA and enkephalin, project to the output nucleus through the GPe and STN. The GPe has GABAergic neurons that inhibit STN neurons which are glutamatergic cells. The release of glutamate because of the activation of the cerebral cortex stimulates MSNs in the indirect pathway to release GABA. GABA inhibits the GPe and therefore disinhibits the STN. Release of glutamate from the STN stimulates the inhibitory GABAergic neurons of the output nucleus, which inhibits thalamic neurons and therefore cerebral cortex, resulting in decreased locomotor activity (Figure 1). It has been proposed that the direct and indirect pathways have interactions with each other, and their integration and balanced activation during movement selection is required for the proper execution of motor programs[36,37].
As mentioned above, D1, A1, and M4 receptors are colocalized on MSNs in the direct pathway[34]. The stimulation of D1 receptors activates MSNs in the direct pathway and stimulates this pathway[28]. Blocking A1 and M4 receptors facilitates the dopamine neurotransmission. Therefore, A1 and M4 antagonists also stimulate the direct pathway[34]. Neurons of the indirect pathway have D2, A2A, and M1 receptors[34]. The activation of D2 receptors inhibits the indirect pathway[28]. A2A and M1 receptors antagonists also increase the dopamine signaling and therefore suppress the indirect pathway. Since these two pathways have a cross talk and coordinated activity, disturbing their coordination can cause movement disorders such as dystonia, dyskinesia, and stuttering. Therefore, both increased and decreased dopamine neurotransmission in the striatum may cause stuttering by impairing the balance between the direct and indirect pathways.
Other changes that can affect connectivity between different areas of the brain involved in the speech motor control are the white matter abnormalities. The white matter tracts, bundles of myelinated axons, relay signals between different areas of the brain and therefore coordinate their communication and function[38]. Several in vitro and animal studies and a small number of human studies have found multiple neurotransmitters, including glutamate, adenosine, GABA, glycine, dopamine, serotonin, acetyl choline, histamine, norepinephrine, and substance P, and their receptors in the white matter. It has been proposed that glutamate and purine signaling have the most prominent effects on the white matter functioning; however, it seems that the white matter requires a coordinated action of all of these neurotransmitters for conduction of action potentials and maintaining signal integrity through very long signal transmission axonal pathways[38]. Different studies in adults[33,39] and adolescents and young people[40] with developmental stuttering demonstrated the reduced integrity of the white matter fiber tracts. It may be proposed that an agent that impairs the normal activity of one or some of the neurotransmitters in the white matter may impair signal transmission between different areas of the brain engaged in speech motor control and result in stuttering. Moreover, in some psychiatric disorders such as schizophrenia and bipolar disorder, one of the pathological findings in the CNS is myelin loss or disruption[41]. Of cases included in the present review, 25, 5, and 6 had schizophrenia, schizoaffective disorder, and bipolar disorder, respectively. As a result, it can be suggested that drugs disrupting normal neurotransmitter balance in the white matter, which already has an underlying impairment in these patients, may exacerbate white matter dysfunction.
Evidence that supports the role of the dopamine excess in the pathophysiology of stuttering includes the reduction of stuttering by antipsychotic drugs, which are dopamine blockers, such as haloperidol, risperidone, olanzapine, aripiprazole, and asenapine[42], the finding of Wu et al[43] that showed the excessive striatal dopamine activity and increased uptake of fluoro-l-3, 4-dihydroxy-phenylalanine, a precursor of dopamine, in several parts of the brain in persons who stutter in comparison to healthy controls, and computational modeling of stuttering by Civier et al[44]. Furthermore, it has been shown that children aged 2.5-3 years, the age of onset of developmental stuttering in most children, have more density of D2 than D1 receptors and therefore low D1/D2 density in the striatum in comparison to older children. Therefore, drugs like haloperidol, which is a highly selective D2 antagonist, decrease this D1/D2 imbalance and the severity of stuttering. Besides the above-mentioned studies conducted in persons with developmental stuttering, cases of exacerbation of stuttering by levodopa in patients with PD[23,26,27] also propose a role for dopamine excess in the pathogenesis of NS. Chang et al[28] have suggested that the inhibition of the indirect pathway in the states of dopamine excess decreases the suppressing effect of this pathway on the competing motor programs. Therefore, choosing correct motor program over incorrect ones becomes difficult, which could ultimately delay the initiation of the right motor program. This delay may cause speech blockage or sound prolongation. Furthermore, in this situation, the proper signal that originated from the direct pathway and stimulates the right motor program may be initiated but suffers premature termination which may lead to the repetitions[28].
In conclusion, drugs such as levodopa, a precursor of dopamine, methylphenidate that increases the extracellular level of dopamine in the striatum[45], amphetamines that increase the release of catecholamines mainly dopamine and nerve-end particles (NEP) from presynaptic nerves and inhibit the reuptake of dopamine and NEP into presynaptic neurons[46], and phenytoin that has been proposed as a dopamine enhancer in the BG pathways may cause stuttering by increasing the dopamine neurotransmission in BG[47,48].
Reduced dopamine neurotransmission in the striatum also can cause stuttering. For example, some patients with PD, the disorder that is mainly characterized by the dopamine depletion from BG, experience new-onset NS[49], exacerbation of PDS[26,27], or re-emergence of developmental stuttering[50]. Chang et al[28] proposed that in this state, the decreased excitation of the direct pathway results in reduced stimulation of the correct motor program and its ability to compete other motor programs. This also may result in unstable or delayed production of signals initiating the right speech motor program.
Although some studies have shown the relative efficacy of antipsychotic drugs in the treatment of stuttering[42], there are case reports of antipsychotics-induced stuttering. All studies that reported efficacy of antipsychotic drugs in reducing stuttering were conducted in patients with PDS. However, all cases of antipsychotics-induced stuttering had a psychotic disorder, which was schizophrenia in the majority of them. Elevated dopamine levels and excessive dopamine activity in the striatum are present in both developmental stuttering[42,43] and schizophrenia. Therefore, the opposite effects of dopamine blockers in these disorders, improving stuttering in some cases of developmental stuttering but causing stuttering in some patients with schizophrenia, indicate that effects of these drugs on the dopamine activity in other parts of the brain and on other neurotransmitters may be responsible for their different effects on speech motor control. Furthermore, abnormalities in the brain of patients with psychotic disorders are extensive and are not comparable to persons with developmental stuttering. These differences also may justify why a dopamine blocker can be a therapeutic option in PDS but a causative agent of stuttering in the psychotic disorders.
However, it should be noted that studies by Fish et al[51] and Langova et al[52] demonstrated that not all persons with developmental stuttering respond to the dopamine blockers. In the study conducted by Fish et al[51], 14 out of 28 persons who stuttered Prader-Willi syndrome (PWS) and received amphetamine experienced improvement in stuttering while two got worse. Of 12 PWS who did not improve by amphetamine, eight got better on trifluoperazine, a D2 blocker. Four participants did not show any improvement neither by each medication nor by their combination. Similarly, in the study conducted by Langova et al[52], 88% of PWS got better on phenmetrazine, a stimulant, while 67% deteriorated using chlorpromazine. These findings have led to the suggestion of the hypothesis that persons with developmental stuttering may be classified as the dopamine blocker-responsive or stimulant-responsive[29].
In the present review and study conducted by Trenque et al[6], the majority of cases of DIS were caused by clozapine. Regarding the effects of antipsychotics on dopamine receptors, all antipsychotics except than clozapine, olanzapine, ziprasidone, and asenapine have a higher affinity for D2 receptors than D1 receptors. Clozapine, olanzapine, ziprasidone, and asenapine equally block both D1 and D2 receptors. Furthermore, clozapine and asenapine have a lower affinity for dopamine receptors in comparison to olanzapine and ziprasidone[53]. Clozapine is one of the most effective antipsychotic drugs[54]; however, because of its serious side effects such as agranulocytosis, seizure, and cardiovascular adverse effects[55], clozapine is considered as one of the last options in the treatment of schizophrenia and other psychotic disorders. Therefore, many patients had received several antipsychotics prior to the initiation of clozapine. Long-term blockage of D2 receptors while sparing D1 receptors caused by other antipsychotics results in the supersensitivity of D2 receptors[56]. The affinity of clozapine for blocking D2 receptors is lower than that of many other antipsychotics[53]. Therefore, after the initiation of clozapine, decreased D2 blocking combined with the supersensitivity of D2 receptors creates a state of increased D2 stimulation which finally inhibits the indirect pathway. On the other hand, antagonizing the D1 receptors inhibits the direct pathway. The inhibition of the indirect pathway impairs the suppression of the competing motor programs. Besides, the inhibition of the direct pathway decreases cerebral cortex stimulation and locomotor activity, which causes difficulties in initiating next segment in a movement sequence like speaking. Clozapine also is a 5HT2a and 5HT2c receptor blocker[55]. Reducing the serotonin neurotransmission increases the dopamine transmission in the prefrontal cortex, which may also cause stuttering. Although clozapine is a potent M1 antagonist[55], the effect that facilitates the dopamine neurotransmission in the direct pathway, the final result of antagonizing all above-mentioned receptors is the inhibition of both direct and indirect pathways and therefore impaired speech motor control. Moreover, changing the normal function of dopamine, serotonin, acetylcholine, and norepinephrine, by blocking α1 receptors, and histamine, by blocking H1 receptors[55], can disturb normal functions of the white matter and cause stuttering.
Other antipsychotics that are included in the present review are olanzapine, risperidone, aripiprazole, chlorpromazine, fluphenazine, and trifluoperazine. Olanzapine is very similar to clozapine regarding the affinity for different receptors including D1, D2, 5HT2A, 5HT2C, M1, α1, and H1 receptors[57]. Other antipsychotics such as risperidone, chlorpromazine, and fluphenazine are potent inhibitors of D2 receptors without any considerable effect on D1 receptors. Chlorpromazine also is a potent M1 antagonist while risperidone and fluphenazine have no considerable effect on M1 receptor. The efficacy of risperidone in antagonizing 5HT receptors is comparable to that of olanzapine and more than the efficacy of chlorpromazine, fluphenazine, and trifluoperazine. Aripiprazole is a partial agonist of D2 and 5HT1A receptors and antagonist of 5HT2A receptor[57]. We suggest that these antipsychotics can cause extrapyramidal side effects (EPS) which may manifest as stuttering as well as other movement disorders by impairing the balanced and coordinated activity of the direct and indirect pathways. Furthermore, increasing the dopamine neurotransmission in the prefrontal cortex by blocking the serotonin effects as well as disturbing neurotransmitters’ functioning in the white matter can be the other underlying mechanisms of the antipsychotics-induced stuttering.
The following section is focused on clozapine as the most prevalent cause of DIS.
Clozapine is the drug with most reports of inducing a new episode of stuttering or worsening pre-existing stuttering both in the present review and analysis carried out by Trenque et al[6]. In the cases included in this review, clozapine induced stuttering in a wide variety of dosages ranging from 50 mg/d[10] to 700 mg/d[58,59]. However, in most cases, clozapine caused stuttering at the daily doses of 250 mg to 450 mg. It seems that stuttering is a dose-dependent adverse effect of clozapine as in 13 (43.3%) cases stuttering was significantly improved or completely vanished following dose reduction (Table 3).
We have suggested likely mechanisms of clozapine-induced stuttering in the previous section. Furthermore, based on the concomitant signs and symptoms that patients experienced with stuttering, it has been proposed that clozapine-induced stuttering may be a manifestation of the movement disorders such as focal segmental dystonia in orofacial muscles, akathisia, or dyskinesia[10,21,58,60-63] or a seizure disorder.
Although clozapine is an antipsychotic with a low potential for causing EPS[55], of 30 cases, seven experienced a type of movement disorder concomitant with stuttering[10,21,58]. Grover et al[61] reported a case who experienced clozapine-induced stuttering and had a history of EPS associated with other antipsychotics. Although clozapine did not cause other manifestations of EPS, they proposed that stuttering might be a symptom of movement disorders induced by clozapine. Concerning the management of clozapine-induced stuttering the dose reduction or withdrawal of clozapine resulted in significant improvement or complete relief of both stuttering and the movement disorders in two cases[58]. In contrast, in one case reported by Lyall et al[10], substitution of clozapine with zuclopenthixol decanoate relieved stuttering but not dyskinetic movements, and restarting clozapine resulted in reoccurrence of stuttering which responded to sodium valproate despite no electroencephalogram (EEG) abnormality. For four cases, the authors did not report whether their intervention improved the movement disorders in addition to stuttering or not[10,21].
Regarding the other likely mechanisms of clozapine-induced stuttering, it has been suggested that stuttering may be a manifestation of seizure. Clozapine-induced stuttering was associated with seizure or EEG abnormalities without typical symptoms of seizure in nine out of 30 case reports[12,25,59,60,62-66], and stuttering was significantly improved or completely relieved by addition of sodium valproate and the dose reduction of clozapine in five cases[12,59,62,64,66]. In the other four cases[25,60,63,65], anticonvulsant drugs were not tried, but discontinuation or dose reduction of clozapine resulted in complete relief of stuttering in three cases[60,63,65]. In contrast, one of the cases reported by Hallahan et al[58] experienced seizure after the development of stuttering. After the addition of sodium valproate, the patient had no seizure, but stuttering did not improve. It is worth mentioning that three cases had stuttering with both movement disorders and EEG abnormalities[60,62,63], which may demonstrate that some movement disorders induced by clozapine may be due to the epileptiform activity in the brain rather than EPS.
Collectively, the mechanism of clozapine-induced stuttering is multifactorial. Any of the following impairments or a combination of them can be a cause of clozapine-induced stuttering: Imbalance between direct and indirect pathways in the BG which may cause just stuttering or other movement disorders in addition to stuttering, abnormalities in the white matter neurotransmitters, and seizure or EEG abnormalities.
Finally, we suggest that in the cases of clozapine-induced stuttering at first, clinicians must do the electroencephalography to rule out any abnormal electrical activity of the brain which may progress to convulsion. If the patient has seizure or any abnormalities in EEG, the addition of sodium valproate is recommended. Even Varma et al[67] have recommended that one of the indications of initiating sodium valproate in patients receiving clozapine is stuttering with or without any types of seizure or EEG abnormalities. After ruling out the abnormal electrical brain activities, considering stuttering as an EPS and typical management of these side effects or dose reduction or discontinuation of clozapine may be considered if stuttering is annoying the patient.
As described above, as the input nucleus of the BG, the striatum receives glutamatergic projection from different parts of the cerebral cortex and thalamus. Furthermore, the STN stimulates inhibitory GABAergic neurons of the output nuclei of the BG by releasing glutamate. All ionotropic NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, and kainite, and metabotropic glutamate receptors, mGlu1-8, are expressed in the BG, and glutamate is one of the important neurotransmitters that mediate signal transmission in the BG motor circuit. In addition, the computational modeling of stuttering[44] and the data of diffusion tensor imaging obtained from children who stutter[44] indicate that there is an impaired connectivity between different parts of the cerebral cortex and striatum in developmental stuttering. This impaired connection causes that BG cannot optimally detect cognitive and sensory motor context to terminate the previous phoneme and initiate the next phoneme[29] in a timely manner to produce a fluent speech. Therefore, drugs like memantine by inhibiting NMDA receptors[68] and pregabalin, gabapentin, and lamotrigine by inhibiting the release of glutamate[69,70] change the normal function of glutamate in corticostriatal pathway or in the different parts of the BG motor circuit can impair balance between the direct and indirect pathways and cause motor abnormalities like stuttering. Moreover, glutamate is the main neurotransmitter in the white matter and antagonizing its effects also disturbs signal transmission through white matter fiber tracts.
Theophylline increases dopamine release and transmission by inhibiting GABA receptors on the SNpc and adenosine receptors on MSNs of both direct and indirect pathways. Theophylline also increases glutamate release. Therefore, it can disturb the normal balance between the mentioned neurotransmitters in the BG and cause stuttering. Another mechanism may be impairing the normal function of neurotransmitters in the white matter by increasing glutamate, the main neurotransmitter of the white matter[5].
Although the main mechanism of action of valproate is the blockage of voltage-dependent sodium channels[71], it has several other mechanisms of action that justify its broad anticonvulsant activity, effects in the prophylaxis of migraine headache, and mood stabilizing properties. Animal studies in rats have demonstrated that valproate increases GABA concentration in both the striatum and substantia nigra, but its effect is more pronounced in the substantia nigra[72]. Valproate also increases the firing pattern and frequency of neurons of the SNpr[73]. We propose that an increased level of GABA in the substantia nigra increases its inhibitory effects on the thalamus through the direct pathway, and therefore, reduces the brain cortex stimulation to execute the desired movement which may cause stuttering.
Atomoxetine is a selective NEP reuptake inhibitor. It increases the extracellular concentrations of NEP and dopamine in the prefrontal cortex[45]. However, studies that examined the effects of atomoxetine on the concentration of dopamine in the striatum obtained opposite results[45,74]. Because of uncertainty about the effect of atomoxetine on the dopamine levels in the striatum, we do not focus on dopamine as a mediator of likely effects of atomoxetine on speech motor control and stuttering. A study measured the blood oxygenation level dependent response using pharmacological magnetic resonance imaging in different regions of the rat brain following acute administration of atomoxetine. That study showed that atomoxetine increased SNpr and STN activity in the BG[75]. These increased activities decrease the stimulatory activity of the thalamocortical pathway. Therefore, we suggest that because of the decreased stimulation of the cerebral cortex, the favorable motor program is not executed, which can result in the inappropriate activation of the orofacial muscles and cause stuttering.
It is proposed that sertraline-induced stuttering may be related to the serotonergic inhibition of the dopaminergic neurons. The cell bodies of these neurons are located in the ventral tegmental area. Therefore, inhibition of the dopamine pathways in the nigrostriatum can be considered as a mechanism of promoting stuttering by sertraline or selective serotonin reuptake inhibitors drugs in general[76,77].
Bupropion is able to increase dopamine levels in the prefrontal cortex, which may cause stuttering[78-80].
It seems that the stuttering induced by a topical pyrethrin product in a child is related to its neurotoxicity since the metabolism of pyrethrin in children is slow. The product also had contained piperonyl butoxide, which can inhibit the hepatic metabolism of the compound and potentiate the toxicity[81].
In this review, 82 cases of DIS were collected. Most cases were related to antipsychotic drugs. Similar to the developmental stuttering, the majority of persons who experienced an episode of DIS were male. The repetitions followed by speech blocks were the most frequent core manifestations of stuttering. In 55.8% of cases, drug withdrawal was the therapeutic measure that was used to manage the stuttering.
Although we tried to provide a complete feature of the epidemiological and clinical characteristics of DIS, much information such as the core behaviors of stuttering, the interval between the initiation or increase in the dose of offending medications and the occurrence of stuttering and between the drug withdrawal or dose reduction and the improvement of stuttering, and concurrent psychological symptoms with stuttering was not reported in several cases. As a result, future cases of DIS must be reported with more detailed information since these data give others a comprehensive feature of this type of the NS.
By focusing on the cortico-BG-thalamocortical loop and the white matter fiber tracts and their neurotransmitters such as dopamine and glutamate, we suggest some likely mechanisms for DIS. However, dysfunctions in other areas of the brain like the cerebral cortex and cerebellum and other neurotransmitters are not addressed in this review. In addition, we consider stuttering as a speech motor disorder, but cognitive and sensory disorders may also play roles in the pathogenesis of DIS. Therefore, it is suggested that these subjects should be considered in the future papers discussing the underlying mechanisms of DIS. In spite of many hypotheses that can be proposed for the pathogenesis of DIS, experimental studies will provide the most robust evidence in this field. Since advanced brain imaging facilities may not be available in every setting where clinicians encounter a case of DIS to find the areas of the brain that act abnormally, animal studies evaluating the changes in the functions of the brain and different neurotransmitters are required to shed a light on the underlying mechanisms of DIS.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmad DS, Tran MT S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Wang JL
| 1. | Black DW, Grant JE. DSM-5® guidebook: the essential companion to the diagnostic and statistical manual of mental disorders: American Psychiatric Pub, 2014. |
| 2. | Craig-McQuaide A, Akram H, Zrinzo L, Tripoliti E. A review of brain circuitries involved in stuttering. Front Hum Neurosci. 2014;8:884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 3. | Neef NE, Anwander A, Friederici AD. The Neurobiological Grounding of Persistent Stuttering: from Structure to Function. Curr Neurol Neurosci Rep. 2015;15:63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Ashurst JV, Wasson MN. Developmental and persistent developmental stuttering: an overview for primary care physicians. J Am Osteopath Assoc. 2011;111:576-580. [PubMed] |
| 5. | Movsessian P. Neuropharmacology of theophylline induced stuttering: the role of dopamine, adenosine and GABA. Med Hypotheses. 2005;64:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Trenque T, Morel A, Trenque A, Azzouz B. Drug induced stuttering: pharmacovigilance data. Expert Opin Drug Saf. 2021;20:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 7. | Burd L, Kerbeshian J. Stuttering and stimulants. J Clin Psychopharmacol. 1991;11:72-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Ertekin H, Ertekin YH, Sahin B, Yayla S, Turkyilmaz E, Kara M. Clozapine and Aripiprazole-Induced Stuttering: A Case Report of Turner Syndrome with Schizophrenia. Klinik Psikofarmakoloji Bülteni-Bulletin Clin Psychopharmacol. 2016;26:422-425. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Lyall M, Pryor A, Murray K. Clozapine and speech dysfluency: two case reports. Psychiatr Bull. 2007;31:16-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Nurnberg HG, Greenwald B. Stuttering: an unusual side effect of phenothiazines. Am J Psychiatry. 1981;138:386-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Begum M. Clozapine-induced stuttering, facial tics and myoclonic seizures: a case report. Aust N Z J Psychiatry. 2005;39:202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Prasse JE, Kikano GE. Stuttering: an overview. Am Fam Physician. 2008;77:1271-1276. [PubMed] |
| 13. | Tani T, Sakai Y. Stuttering after right cerebellar infarction: a case study. J Fluency Disord. 2010;35:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Theys C, Van Wieringen A, Tuyls L, De Nil L. Acquired stuttering in a 16-year-old boy. J Neurolinguist. 2009;22:427-435. [DOI] [Full Text] |
| 15. | Bär KJ, Häger F, Sauer H. Olanzapine- and clozapine-induced stuttering. A case series. Pharmacopsychiatry. 2004;37:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Lee HJ, Lee HS, Kim L, Lee MS, Suh KY, Kwak DI. A case of risperidone-induced stuttering. J Clin Psychopharmacol. 2001;21:115-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Alaghband-Rad J, Nikvarz N, Tehrani-Doost M, Ghaeli P. Memantine-induced speech problems in two patients with autistic disorder. Daru. 2013;21:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Yadav DS. Risperidone induced stuttering. Gen Hosp Psychiatry. 2010;32:559.e9-559.10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Das S, Manjunatha N, Thirthali J. Clozapine-induced Weight Loss and Stuttering in a Patient with Schizophrenia. Indian J Psychol Med. 2018;40:385-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Murphy R, Gallagher A, Sharma K, Ali T, Lewis E, Murray I, Hallahan B. Clozapine-induced stuttering: an estimate of prevalence in the west of Ireland. Ther Adv Psychopharmacol. 2015;5:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Shuster J. Methotrexate Neurotoxicity Causes Speech Problem; Severe, Irreversible Sensory Neuropathy Due to Long-Term Use of Linezolid; Hypomania with Topiramate; Bortezomib-Induced Hepatitis; Steroid Dementia–An Overlooked Diagnosis? Hosp Pharm. 2005;40:383-386. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Louis ED, Winfield L, Fahn S, Ford B. Speech dysfluency exacerbated by levodopa in Parkinson's disease. Mov Disord. 2001;16:562-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Atay İM, Tanritanir B, Akpinar A, Demirdaş A. A Case of Risperidone Induced Stuttering as a Paradox. Noro Psikiyatr Ars. 2014;51:403-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Kranidiotis L, Thomas S. Clozapine-induced speech dysfluency: further cases. Psychiatr Bull. 2007;31:191-191. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Anderson JM, Hughes JD, Rothi LJ, Crucian GP, Heilman KM. Developmental stuttering and Parkinson's disease: the effects of levodopa treatment. J Neurol Neurosurg Psychiatry. 1999;66:776-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Burghaus L, Hilker R, Thiel A, Galldiks N, Lehnhardt FG, Zaro-Weber O, Sturm V, Heiss WD. Deep brain stimulation of the subthalamic nucleus reversibly deteriorates stuttering in advanced Parkinson's disease. J Neural Transm (Vienna). 2006;113:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Chang SE, Guenther FH. Involvement of the Cortico-Basal Ganglia-Thalamocortical Loop in Developmental Stuttering. Front Psychol. 2019;10:3088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 28. | Alm PA. Stuttering and the basal ganglia circuits: a critical review of possible relations. J Commun Disord. 2004;37:325-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 347] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 29. | Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med. 2012;2:a009621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 490] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 30. | Leisman G, Braun-Benjamin O, Melillo R. Cognitive-motor interactions of the basal ganglia in development. Front Syst Neurosci. 2014;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 31. | Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 351] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 32. | Connally EL, Ward D, Howell P, Watkins KE. Disrupted white matter in language and motor tracts in developmental stuttering. Brain Lang. 2014;131:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Silkis I. The cortico-basal ganglia-thalamocortical circuit with synaptic plasticity. II. Mechanism of synergistic modulation of thalamic activity via the direct and indirect pathways through the basal ganglia. Biosystems. 2001;59:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Galvan A, Kuwajima M, Smith Y. Glutamate and GABA receptors and transporters in the basal ganglia: what does their subsynaptic localization reveal about their function? Neuroscience. 2006;143:351-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci. 2014;17:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 551] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 36. | Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci. 2005;6:787-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 37. | Butt AM, Fern RF, Matute C. Neurotransmitter signaling in white matter. Glia. 2014;62:1762-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 38. | Kronfeld-Duenias V, Amir O, Ezrati-Vinacour R, Civier O, Ben-Shachar M. The frontal aslant tract underlies speech fluency in persistent developmental stuttering. Brain Struct Funct. 2016;221:365-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 39. | Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2008;131:50-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 263] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 40. | Haroutunian V, Katsel P, Roussos P, Davis KL, Altshuler LL, Bartzokis G. Myelination, oligodendrocytes, and serious mental illness. Glia. 2014;62:1856-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 41. | Maguire GA, Yeh CY, Ito BS. Overview of the diagnosis and treatment of stuttering. J Exp Clin Med. 2012;4:92-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Wu JC, Maguire G, Riley G, Lee A, Keator D, Tang C, Fallon J, Najafi A. Increased dopamine activity associated with stuttering. Neuroreport. 1997;8:767-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | Civier O, Bullock D, Max L, Guenther FH. Computational modeling of stuttering caused by impairments in a basal ganglia thalamo-cortical circuit involved in syllable selection and initiation. Brain Lang. 2013;126:263-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 44. | Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 826] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 45. | Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 501] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 46. | García-Ramos R, Moreno Ramos T, Villarejo Galende A, Porta Etessam J. Phenytoin-induced acute orofacial dyskinesia. Neurologia. 2013;28:193-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Zaatreh M, Tennison M, D'Cruz O, Beach RL. Anticonvulsants-induced chorea: a role for pharmacodynamic drug interaction? Seizure. 2001;10:596-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Toft M, Dietrichs E. Aggravated stuttering following subthalamic deep brain stimulation in Parkinson's disease-two cases. BMC Neurol. 2011;11:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Shahed J, Jankovic J. Re-emergence of childhood stuttering in Parkinson's disease: a hypothesis. Mov Disord. 2001;16:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Fish CH, Bowling E. Stuttering. The effect of treatment with D-amphetamine and a tranquilizing agent, trifluoperazine. A preliminary report on an uncontrolled study. Calif Med. 1965;103:337-339. [PubMed] |
| 51. | Langova J, Moravek M. Some Results of Experimental Examinations among Stutterers and Clutterers. Folia Phoniatr (Basel). 1964;16:290-296. [PubMed] |
| 52. | Perez-Costas E, Melendez-Ferro M, Roberts RC. Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. J Neurochem. 2010;113:287-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 53. | Rampino A, Marakhovskaia A, Soares-Silva T, Torretta S, Veneziani F, Beaulieu JM. Antipsychotic Drug Responsiveness and Dopamine Receptor Signaling; Old Players and New Prospects. Front Psychiatry. 2018;9:702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Essali A, Al-Haj Haasan N, Li C, Rathbone J. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev. 2009;2009:CD000059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 55. | Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. CMAJ. 2005;172:1703-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 56. | Yin J, Barr AM, Ramos-Miguel A, Procyshyn RM. Antipsychotic Induced Dopamine Supersensitivity Psychosis: A Comprehensive Review. Curr Neuropharmacol. 2017;15:174-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 57. | Alldredge BK, Corelli RL, Ernst ME, Guglielmo BJ, Jacobson PA, Kradjan WA, Williams BR. Koda-kimble and Young's applied therapeutics: the clinical use of drugs. 10th ed. Philadelphia, USA: Wolters Kluwer Health Adis (ESP), 2013: 1931-1932. |
| 58. | Hallahan B, Murray I, Doyle PG. Clozapine induced stuttering. Ir J Psychol Med. 2007;24:121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Supprian T, Retz W, Deckert J. Clozapine-induced stuttering: epileptic brain activity? Am J Psychiatry. 1999;156:1663-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Chochol MD, Kataria L, OʼRourke MC, Lamotte G. Clozapine-Associated Myoclonus and Stuttering Secondary to Smoking Cessation and Drug Interaction: A Case Report. J Clin Psychopharmacol. 2019;39:275-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Grover S, Verma AK, Nebhinani N. Clozapine-induced stuttering: a case report and analysis of similar case reports in the literature. Gen Hosp Psychiatry. 2012;34:703.e1-703.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Rachamallu V, Haq A, Song MM, Aligeti M. Clozapine-Induced Microseizures, Orofacial Dyskinesia, and Speech Dysfluency in an Adolescent with Treatment Resistant Early Onset Schizophrenia on Concurrent Lithium Therapy. Case Rep Psychiatry. 2017;2017:7359095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 63. | Thomas P, Lalaux N, Vaiva G, Goudemand M. Dose-dependent stuttering and dystonia in a patient taking clozapine. Am J Psychiatry. 1994;151:1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Duggal HS, Jagadheesan K, Nizamie SH. Clozapine-induced stuttering and seizures. Am J Psychiatry. 2002;159:315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Gica S, Kiliç C, Karamustafalioglu N. Clozapine-Associated Stuttering: A Case Report. Am J Ther. 2020;27:e624-e627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Horga G, Horga A, Baeza I, Castro-Fornieles J, Lázaro L, Pons A. Drug-induced speech dysfluency and myoclonus preceding generalized tonic-clonic seizures in an adolescent male with schizophrenia. J Child Adolesc Psychopharmacol. 2010;20:233-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Varma S, Bishara D, Besag FM, Taylor D. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1:47-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 68. | Johnson JW, Kotermanski SE. Mechanism of action of memantine. Curr Opin Pharmacol. 2006;6:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 340] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 69. | Lapidus KA, Soleimani L, Murrough JW. Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatr Dis Treat. 2013;9:1101-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 413] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 71. | Johannessen CU. Mechanisms of action of valproate: a commentatory. Neurochem Int. 2000;37:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 296] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 72. | Löscher W. Valproate enhances GABA turnover in the substantia nigra. Brain Res. 1989;501:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Töllner K, Wolf S, Löscher W, Gernert M. The anticonvulsant response to valproate in kindled rats is correlated with its effect on neuronal firing in the substantia nigra pars reticulata: a new mechanism of pharmacoresistance. J Neurosci. 2011;31:16423-16434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Easton N, Shah YB, Marshall FH, Fone KC, Marsden CA. Guanfacine produces differential effects in frontal cortex compared with striatum: assessed by phMRI BOLD contrast. Psychopharmacology (Berl). 2006;189:369-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Easton N, Marshall F, Fone K, Marsden C. Atomoxetine produces changes in cortico-basal thalamic loop circuits: assessed by phMRI BOLD contrast. Neuropharmacology. 2007;52:812-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Christensen RC, Byerly MJ, McElroy RA. A case of sertraline-induced stuttering. J Clin Psychopharmacol. 1996;16:92-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 77. | Brewerton TD, Markowitz JS, Keller SG, Cochrane CE. Stuttering with sertraline. J Clin Psychiatry. 1996;57:90-91. [PubMed] |
| 78. | Fetterolf F, Marceau M. A case of bupropion-induced stuttering. Gen Hosp Psychiatry. 2013;35:574.e7-574.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Bhatia MS. Bupropion-Induced Stuttering. Prim Care Companion CNS Disord. 2015;17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 80. | McAllister MW, Woodhall DM. Bupropion-induced stuttering treated with haloperidol. Clin Toxicol (Phila). 2016;54:603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 81. | Hammond K, Leikin JB. Topical pyrethrin toxicity leading to acute-onset stuttering in a toddler. Am J Ther. 2008;15:323-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Donaher J, Healey EC, Zobell A. The effects of ADHD medication changes on a child who stutters. Perspect Fluency Fluency Disord. 2009;19:95-98. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Elliott RL, Thomas BJ. A case report of alprazolam-induced stuttering. J Clin Psychopharmacol. 1985;5:159-160. [PubMed] |
| 84. | Ünay M, Adanır AS, Özatalay E. Aripiprazole-induced stuttering in an 8 year-old boy with ADHD. Klin Psikofarmakol Bul. 2018;28:230-231. |
| 85. | Naguy A, Moodliar S, Elsori DH, Alamiri B. Dose-Dependent Aripiprazole-Induced Stuttering in a Child With Mild Intellectual Disability. Am J Ther. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 86. | Cicek AU. Aggravating influence of atomoxetine on the severity of stuttering and its successful treatment with methylphenidate: a case report. Dusunen Adam. 2020;33:210-213. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Ebeling TA, Compton AD, Albright DW. Clozapine-induced stuttering. Am J Psychiatry. 1997;154:1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 88. | Krishnakanth M, Haridas Phutane V, Muralidharan K. Clozapine-induced stuttering: a case series. Prim Care Companion J Clin Psychiatry. 2008;10:333-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 89. | Kumar T, Kathpal A, Longshore CT. Dose dependent stuttering with clozapine: a case report. Asian J Psychiatr. 2013;6:178-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Nagendrappa S, Sreeraj VS, Venkatasubramanian G. "I Stopped Hearing Voices, Started to Stutter" - A Case of Clozapine-Induced Stuttering. Indian J Psychol Med. 2019;41:97-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 91. | Aukst-Margetić B, Margetić B. Stuttering as a side-effect of divalproex sodium. Psychiatry Clin Neurosci. 2008;62:748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 92. | Mukherjee S, Sen S, Chatterjee SS, Biswas A, Tripathi SK. Divalproex-induced stuttering: A rare case report. Eur J Psychol Educat Studies. 2015;2:25. [DOI] [Full Text] |
| 93. | Masand P. Desipramine-induced oral-pharyngeal disturbances: stuttering and jaw myoclonus. J Clin Psychopharmacol. 1992;12:444-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 94. | Nissani M, Sanchez EA. Stuttering caused by gabapentin. Ann Intern Med. 1997;126:410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 95. | Catania S, Cross H, de Sousa C, Boyd S. Paradoxic reaction to lamotrigine in a child with benign focal epilepsy of childhood with centrotemporal spikes. Epilepsia. 1999;40:1657-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Margetic B. Stuttering (first report) in an elderly patient: case report. Reactions. 2009;1257:27-28. |
| 97. | Margetic B, Aukst-Margetic B, Krajinovic B. A case of stuttering during treatment with levomepromazine. Psychopharmacol Bull. 2009;42:8-10. [PubMed] |
| 98. | Netski AL, Piasecki M. Lithium-induced exacerbation of stutter. Ann Pharmacother. 2001;35:961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Gulack BC, Puri NV, Kim WJ. Stutter exacerbated by lithium in a pediatric patient with bipolar disorder. Ann Pharmacother. 2011;45:e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 100. | Sabillo S, Samala RV, Ciocon JO. A stuttering discovery of lithium toxicity. J Am Med Dir Assoc. 2012;13:660-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 101. | Alpaslan AH, Coşkun KŞ, Kocak U, Gorücü Y. Stuttering Associated With the Use of Short-Acting Oral Methylphenidate. J Clin Psychopharmacol. 2015;35:739-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 102. | Copur M, Copur S. Emergence of stuttering in an attention deficit hyperactivity disorder patient treated with methylphenidate. Dusunen Adam. 2018;31:222-224. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 103. | Lasić D, Cvitanović MŽ, Krnić S, Uglešić B. Olanzapine induced stuttering: a case report. Psychiatr Danub. 2016;28:299-300. [PubMed] |
| 104. | Asan O, Yaylaci ET, Okay IT, Goka E. A case of stuttering due to olanzapine treatment. Dusunen Adam J Psychiatry Neurolog Sci. 2018;31:405. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 105. | McClean MD, McLean Jr A. Case report of stuttering acquired in association with phenytoin use for post-head-injury seizures. J Fluency Disord. 1985;10:241-255. [DOI] [Full Text] |
| 106. | Ekici MA, Ekici A, Ozdemir O. Phenytoin-induced stuttering: an extremely rare association. Pediatr Neurol. 2013;49:e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 107. | Giray E, Şanal Toprak C, Saçaklidir R, Gündüz OH. Pregabalin-Associated Stuttering in a Patient With Complex Regional Pain Syndrome: A Case Report. J Clin Psychopharmacol. 2016;36:740-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | Ge L, Li A, Wang N, Li P, Xin H, Li W. Pregabalin-associated stuttering and frequent blepharospasm: case report and review. Daru. 2020;28:815-818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 109. | Makela EH, Sullivan P, Taylor M. Sertraline and speech blockage. J Clin Psychopharmacol. 1994;14:432-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 110. | Rosenfield DB, McCarthy M, McKinney K, Viswanath NS, Nudelman HB. Stuttering induced by theophylline. Ear Nose Throat J. 1994;73:914, 918-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 111. | Gérard JM, Delecluse F, Robience Y. Theophylline-induced stuttering. Mov Disord. 1998;13:847-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |