Published online Dec 19, 2022. doi: 10.5498/wjp.v12.i12.1356
Peer-review started: August 23, 2022
First decision: October 21, 2022
Revised: November 2, 2022
Accepted: November 21, 2022
Article in press: November 21, 2022
Published online: December 19, 2022
Processing time: 115 Days and 7.6 Hours
Parkinson's disease (PD) is a neurodegenerative disorder caused by the loss of dopaminergic neurons in the substantia nigra, resulting in clinical symptoms, including bradykinesia, resting tremor, rigidity, and postural instability. The pathophysiological changes in PD are inextricably linked to the subcortical structures. Shape analysis is a method for quantifying the volume or surface morphology of structures using magnetic resonance imaging. In this review, we discuss the recent advances in morphological analysis techniques for studying the subcortical structures in PD in vivo. This approach includes available pipelines for volume and shape analysis, focusing on the morphological features of volume and surface area.
Core Tip: Parkinson's disease (PD) is a neurodegenerative disorder caused by the loss of dopaminergic neurons in the substantia nigra, resulting in clinical symptoms, including bradykinesia, resting tremor, rigidity, and postural instability. The pathophysiological changes in PD are inextricably linked to the subcortical structures. Shape analysis is a method for quantifying the volume or surface morphology of structures using magnetic resonance imaging. In this review, we discuss the recent advances in morphological analysis techniques for studying the subcortical structures in PD in vivo. This approach includes available pipelines for volume and shape analysis, focusing on the morphological features of volume and surface area.
- Citation: Deng JH, Zhang HW, Liu XL, Deng HZ, Lin F. Morphological changes in Parkinson's disease based on magnetic resonance imaging: A mini-review of subcortical structures segmentation and shape analysis. World J Psychiatry 2022; 12(12): 1356-1366
- URL: https://www.wjgnet.com/2220-3206/full/v12/i12/1356.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i12.1356
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer's disease. It is primarily caused by the loss of dopaminergic neurons in the substantia nigra. The classical clinical symptoms of PD include movement symptoms such as bradykinesia, resting tremor, rigidity, and postural instability. Recent studies have shown that symptoms of PD extend beyond motricity and include cognitive and neuropsychiatric symptoms. Non-motor symptoms can be identified at all stages, even before the appearance of motor symptoms[1]. In addition to clinical markers, PD biomarkers include neuroimaging, genetic, and biochemical markers[2]. This review focuses primarily on the use of neuroimaging in PD.
The main pathological features of PD are the degeneration of dopaminergic neurons in the substantia nigra and deposition of Lewy bodies, leading to pathophysiological changes in the downstream basal ganglia circuits. The basal ganglia system includes the striatum, globus pallidus, and structures with functional connections to the striatum, including the subthalamic nucleus, substantia nigra, and red nucleus.
Magnetic resonance imaging (MRI) is one of the most useful noninvasive techniques for examining intracranial structures, showing macroscopic alterations of the subcortical structures, and can visualize their volume and surface morphology. Therefore, MRI-based morphological analysis of the subcortical structures has the potential to be a prominent diagnostic neuroimaging marker for PD. This review focuses on the shape analysis of the striatum, thalamus, and hippocampus, which has been mostly discussed in previous studies.
A literature search was conducted for relevant studies using four databases: PubMed, Web of Science, Google Scholar, and Scopus. The key search terms in the different combinations were “Parkinson’s disease, shape analysis, subcortical structures, striatum, thalamus, and hippocampus.” The final search was conducted on October 25, 2022.
The inclusion criteria were the studies that included: (1) A background or introduction on PD; (2) the clinical criteria of PD; (3) an introduction to methods of the subcortical structure segmentation; (4) shape analysis of the subcortical or cortical structures; and (5) data utilization of structural MRI sequences.
We excluded studies based on the following exclusion criteria: (1) Articles published in languages other than English; (2) animal model or theoretical articles; (3) studies with a sample size of < 10 patients; (4) studies whose methodology did not involve volumetric or shape analysis; and (5) review or meta-analysis articles of shape analysis.
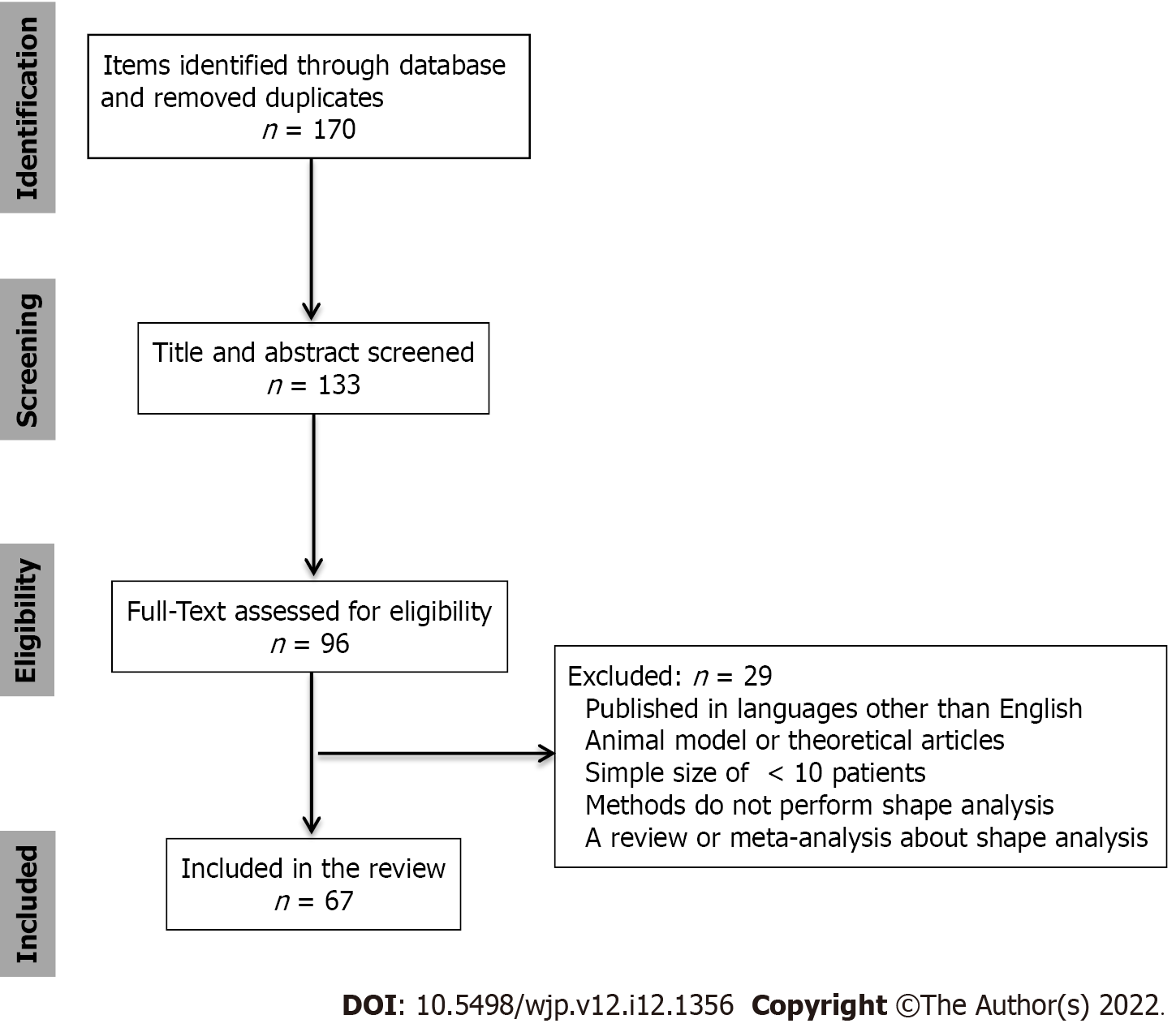
Figure 1 shows a flowchart of the study selection. This review included 69 references, of which 2 provided a background/introduction on PD, 5 referred to the segmentation methods, and 62 to the morphology of the subcortical or cortical structures in PD. Subcortical structures mainly included the striatum, thalamus, and hippocampus. Further information on the structures and morphological changes is provided in Table 1.
| Subcortical structures | Ref. | Segmentation methods | Analysis type | Results |
| Striatum | ||||
| Geng et al[12], 2006; Pitcher et al[10], 2012; Owens-Walton et al[11], 2018 | Manual | Volume | Reduced volume of bilateral caudate and putamen nuclei | |
| Sterling et al[13], 2013 | Semi-automatic | Volume | Reduced volume of bilateral caudate and putamen nuclei | |
| Geevarghese et al[15], 2015; Vasconcellos et al[17], 2018; Tanner et al[16], 2017; Melzer et al[30], 2012 | Automatic | Volume | Reduced volume of bilateral caudate nuclei | |
| Oltra et al[35], 2022 | Automatic | Volume | Reduced volume of bilateral caudate nuclei (with RBD) | |
| Lee et al[14], 2014; Garg et al[20], 2015 | Automatic | Volume | Reduced volume of bilateral putamen nuclei | |
| Garg et al[20], 2015 | Automatic | Volume | Reduced volume of right putamen nuclei | |
| Kamps et al[33], 2019 | Automatic | Volume | Reduced volume of right putamen nuclei (with RBD severity) | |
| Kluger et al[34], 2019 | Automatic | Volume | Reduced volume of dorsal striatum (with fatigue) | |
| Messina et al[18], 2011; Menke et al[19], 2014; Nemmi et al[21], 2015; Khan et al[22], 2019; Gong et al[32], 2019 | Automatic | Volume | No significant difference in bilateral striatum | |
| Chung et al[31], 2017 | Automatic | Volume | Locally reduction of right caudate nuclei | |
| Devignes et al[28], 2021 | Automatic | Shape | Locally reduction of left caudate nuclei (with cognition) | |
| Garg et al[20], 2015 | Automatic | Shape | Locally reduction of right putamen nuclei | |
| Gong et al[32], 2019 | Automatic | Shape | Locally reduction of bilateral caudate and right putamen nuclei (with RBD) | |
| Tanner et al[16], 2017 | Automatic | Shape | Locally reduction of the lateral and medial caudate nuclei | |
| Sterling et al[13], 2013 | Semi-Automatic | Shape | Locally reduction of the head and dorsal body of caudate nuclei | |
| Nemmi et al[21], 2015 | Automatic | Shape | Locally reduction of the medial surface of left caudate nuclei (with the right UPDRS) | |
| Tanner et al[16], 2017 | Automatic | Shape | Locally reduction of the medial surface of putamen nuclei | |
| Sterling et al[13], 2013 | Semi- Automatic | Shape | Locally reduction of the caudal and ventrolateral putamen nuclei | |
| Sigirli et al[23], 2021 | Automatic | Shape | Locally reduction of the middle-posterior of right putamen nuclei | |
| Lee et al[14], 2014 | Automatic | Shape | Locally reduction of the posterolateral and ventromedial putamen nuclei | |
| Nemmi et al[21], 2015 | Automatic | Shape | Locally reduction of the lateral and medial posterior putamen nuclei (with UPDRS) | |
| Khan et al[22], 2019 | Automatic | Shape | Locally reduction of the caudal-motor and rostral-motor sub-regions | |
| Thalamus | ||||
| McKeown et al[43], 2008 | Manual | Volume | No significant difference | |
| Garg et al[20], 2015 | Automatic | Volume | Significant difference | |
| Vasconcellos et al[17], 2018; Mak et al[26], 2014; Sivaranjini et al[27], 2021; Foo et al[45], 2017 | Automatic | Volume | Reduced volume of bilateral thalamus | |
| Niccolini et al[46], 2019 | Automatic | Volume | Reduced volume of bilateral thalamus (with non-motor symptom) | |
| Kamps et al[33], 2019 | Automatic | Volume | Reduced volume of left thalamus (with RBD) | |
| Chen et al[44], 2020 | Automatic | Volume | Increased volume (20) of right subnuclei | |
| Chen et al[44], 2020 | Automatic | Volume | Increased volume (21), reduced volume (2) of left subnuclei | |
| Kaya et al[40], 2019 | Manual | Shape | Locally reduction of the dorsolateral of bilateral STN | |
| Devignes et al[28], 2021 | Automatic | Shape | Locally reduction of right thalamus (with cognition) | |
| Chung et al[31], 2017 | Automatic | Shape | Locally reduction of bilateral thalamus (with cognition) | |
| McKeown et al[43], 2008 | Automatic | Shape | Locally reduction of the dorsal surface of bilateral thalamus | |
| Garg et al[20], 2015 | Automatic | Shape | Net-inward and outward deformation of left thalamus | |
| Hippocampus | ||||
| Wang et al[55] , 2018 | Automatic | Volume | Reduced volume of right hippocampus | |
| Chen et al[56], 2016 | Automatic | Density | Reduced density of left hippocampus | |
| Geevarghese et al[15], 2015 | Automatic | Volume | Reduced volume of left hippocampus (with cognition) | |
| Lee et al[14], 2014; Tanner et al[16], 2017; Radziunas et al[53], 2018; Melzer et al[30], 2012 | Automatic | Volume | Reduced volume of bilateral hippocampus | |
| Vasconcellos et al[17], 2018 | Automatic | Volume | Reduced volume of bilateral hippocampus (with disease duration) | |
| Camlidag et al[68], 2014; Xu et al[59], 2020 | Automatic | Volume | Reduced volume of bilateral hippocampus (with cognition) | |
| van Mierlo et al[64], 2015 | Automatic | Volume | Reduced volume of bilateral hippocampus (with depression) | |
| Rahayel[63], 2019 | Automatic | Volume | Reduced volume of bilateral hippocampus (with REM-RBD) | |
| Wilson et al[54], 2019 | Automatic | Volume | Reduced volume of bilateral hippocampus (with cognition, motor and disease duration) | |
| Luo et al[60], 2021 | Automatic | Volume | Reduced volume of subfields (with cognition) | |
| Uribe et al[61], 2018 | Automatic | Volume | Reduced volume of subfields, especially CA1 | |
| Becker et al[62], 2021 | Automatic | Volume | Reduced volume of CA1 (with cognition) | |
| Xu et al[59], 2020 | Automatic | Volume | Reduced volume of subiculum, CA2/3, CA4, ML and right GC-DG | |
| Park et al[57], 2019 | Automatic | Volume | Volume asymmetry, especially in CA4-DG and CA2-3 | |
| Tanner et al[16], 2017 | Automatic | Shape | Locally reduction in the head and CA1 bilaterally | |
| Devignes et al[28], 2021 | Automatic | Shape | Locally reduction of right hippocampus (with cognition) |
The Movement Disorders Society (MDS) has proposed the main diagnostic criteria for PD in clinical settings[3]. The recent version of the MDS diagnostic criteria considers three stages in the progression of PD: Preclinical, prodromal, and clinical. Clinical PD can be diagnosed when typical motor symptoms occur. Neurodegeneration may occur in patients with PD before they reach the clinical stage[3]. Previous studies have been mostly conducted based on clinical diagnosis; therefore, this review focuses on PD in the clinical stage. The striatum is one of the most affected structures in the nigrostriatal pathway because of the degeneration of dopaminergic neurons. In addition to the striatum, neurons in the substantia nigra project to other basal nuclei, such as the pallidum, substantia nigra, and thalamic nucleus basalis. A decrease in dopamine levels may cause the structural and morphological changes observed in PD.
MRI allows noninvasive observation of morphological changes in the subcortical structures in patients with PD to find changes in neuroimaging characteristics. Hence, it may help in clinical intervention, especially in the preclinical or prodromal stages of the disease. However, the naked eye cannot identify subtle changes in structures; hence, quantitative analysis using a computer may help determine the presence or absence of morphological changes in these structures. Segmentation of subcortical structures based on the images is the prerequisite to performing an accurate analysis. The following sections describe the common segmentation methods and the results of morphological analyses of the subcortical structures obtained from previous studies.
Both manual and automatic segmentation have been used in recent studies. Manual segmentation, usually the gold-standard approach for automatic segmentation, is a tedious and time-consuming task that depends on the subjectivity of the physician. Therefore, many investigators have used publicly available automated segmentation software for efficiency and objectivity. Automatic segmentation methods include voxel-based morphometry (VBM) and surface-based morphometry (SBM). The tools used for segmentation in most studies include FSL and FreeSurfer, among others. The FIRST software, distributed with the FSL package, is a tool that employs manually labeled image data to offer anatomical training information for 15 different subcortical regions using 336 manually labeled T1-weighted MRI images[4]. FreeSurfer is a suite of tools for extensive automated analysis of key features in the human brain that can be used in most MRI sequences and provides an accurate geometric surface model[5]. By minimizing the difference between the original image and the converted target image, large deformation diffeomorphic metric mapping (LDDMM) creates a differential homogenous transformation that has its own inherent smoothness and simulated displacement size. It is often applied in the object-matching segment of medical imaging data processing[6]. This review focuses on the morphological analysis of subcortical structures in PD using the techniques mentioned above in recent years.
Several scholars have compared the effects of manual and automatic segmentation. For the hippocampus and amygdala, segmentation using VBM and FreeSurfer is performed at a level comparable to manual segmentation[7]. In another study, automated segmentation revealed different degrees of variability in the subcortical structures compared to manual segmentation, with particularly pronounced differences found in the FreeSurfer and FSL pipelines for the pallidum and thalamus[8]. From these studies, it can be seen that the efficiency of automatic segmentation is comparable to that of manual segmentation. Automatic methods save more time and display better segmentation results, which could be used in the shape analysis of the subcortical structures in patients with PD.
The striatum is a critical component of the brain that controls the motor, reward, and executive functions, and dopamine serves as an important mediator[9]. Decreased dopamine levels have the greatest impact on striatal structures in patients with PD. Several studies have segmented the striatum by manual segmentation of T1-weighted MRI images for its morphology, showing that the volume of the caudate nucleus or putamen was smaller in patients than in normal controls[10,11]. In addition, studies using automatic segmentation showed the same results as those using manual segmentation of the volume of the caudate nucleus and putamen[12-17]. However, some studies have found no significant difference in striatum volume between patients with PD and normal controls[18-21]. Studies that performed further surface morphometric analyses under automated shape analyses showed: (1) A regional contraction of the posterolateral and ventromedial putamen bilaterally in patients with PD[14]; (2) areas of local atrophy in the lateral and medial posterior parts of the bilateral putamen; (3) atrophy locally on the medial surface of the left caudate nucleus[21]; and (4) a reduction in the volume and an inward displacement of the surface of the caudal motor striatum[22]. Studies using other machine learning methods have also found local atrophy in the caudate and putamen nuclei, including the caudal portion of the putamen or the middle-posterior putamen and the head of the caudate[13,23]. A study attempted to distinguish different stages of PD based solely on the shape analysis of the bilateral caudate nucleus and putamen through an automated process, with balanced accuracies in the range of 59%-85%[24].
Dysfunction of the basal ganglia plays a key role in developing motor and non-motor symptoms in PD[25]. When exploring the relationship between volume and symptoms, several studies have shown that greater atrophy of the caudate and putamen in PD is usually associated with more severe motor symptoms and cognitive impairment[11,17,26-28]. Additionally, some correlation analyses did not find a significant correlation between striatal volume and cognitive or motor symptoms[10].
Local morphological analyses provided more details; local atrophy in the left putamen and thalamus correlated with the right Unified Parkinson Disease Rating Scale (UPDRS) motor scale score, which is the most widely used scale for the clinical studies of PD[21,29]. A previous study identified PD with mild cognitive impairment (PD-MCI) with limited atrophy of the right putamen[30]. When PD-MCI converted to dementia, smaller local shape volumes were found in the right caudate nucleus of the patients compared to that of patients with PD-MCI who did not convert[31]. In addition, logistic regression analysis indicated that the local shape volumes in the right caudate nucleus were significant independent predictors of conversion to dementia in patients with PD-MCI. Distinct structural changes in the caudate and/or putamen are associated with performance in the attention or working memory domain, fatigue, the severity of rapid eye movement (REM) sleep behavior disorder (RBD), and excessive daytime sleepiness[26,32-35].
Specifically, volume atrophy of the left caudate nucleus or right putamen was found to be more pronounced in the patient cohort[11,23], which may be due to disease lateralization. Previous studies have shown that the decrease in dopamine capacity in the striatum is more pronounced in the contralateral hemisphere on the side with more severe clinical symptoms of PD[36]. It has been suggested that the onset of motor symptoms may always occur in one limb, and morphological analysis has revealed a greater degree of striatal atrophy on the contralateral side of the limb where motor symptoms occur[16]. Local deformation of the posterior side of the putamen has been reported in several articles. According to the literature, the posterior putamen is directly related to the sensorimotor cortex and is preferentially affected; dopamine depletion is mainly located in this region of the basal ganglia[10,23,37,38]. Therefore, we can also infer that the morphological changes in PD can be detected using MRI. Furthermore, we may be able to assess the severity of some symptoms, such as cognitive function in patients with PD, and provide timely interventions for clinical treatment.
The thalamus is composed of several nuclei that regulate various motor and sensory functions and is usually divided into seven nuclei: The anterior, lateral, ventral, intralaminar, medial, and posterior nuclear groups and the reticular nucleus. Among the nuclei of the thalamus, the ventral thalamus, also known as the subthalamic nucleus (STN), plays an important role in extrinsic inputs reaching the basal ganglia circuitry[39]. A study calculated the morphological changes in the STN and found statistically significant differences in the shape of bilateral STN between the PD and control groups, with the largest deformation site located in the dorsolateral parts of bilateral STNs[40]. Patriat et al[41] showed that the volume of STN was smaller in PD patients compared to healthy controls, which was further validated in the field of 7T MRI. Although thalamic degeneration may represent a site of dopaminergic degeneration in PD, the thalamus is also influenced by hyperactivity in glutamatergic signaling, which may be caused by the loss of dopaminergic neurons in the substantia nigra and striatum[42]. Thus, various morphological changes occur in the thalamus of patients with PD. Furthermore, several studies on structural and functional imaging have identified morphological or functional changes in the thalamus in patients with PD. Using manual segmentation, scholars found no significant difference in the thalamus volume between patients with PD and healthy controls[43]. They used spherical harmonic-based representation methods and detected significant differences in shape[43]. A previous study subdivided the left and right thalamus into 25 subnuclei using automatic methods. It was detected that 21 of the left and 20 of the right thalamic subnuclei had increased volume, accompanied by atrophy in two left subnuclei[44].
More studies have been conducted to correlate thalamic shape changes with clinical symptoms. Nemmi et al[21] found a significant correlation between local atrophy of the right thalamus and the UPDRS using FSL scripts. However, one study found that surface morphological changes in the thalamus were not associated with disease severity in UPDRS using FreeSurfer segmentation with LDDMM alignment[20]. This may be due to differences in segmentation methods and cohort sizes, and the influence of glutamatergic neurons on thalamic morphology requires further investigation.
Moreover, most studies have concluded that altered thalamic morphology is associated with non-motor symptoms. Several studies have found a relationship between reduced thalamic volume and poor cognitive function in patients with PD[17,26-28,45]. A more detailed correlation analysis showed that the local shape volume of the bilateral thalamus was a significant independent predictor of the conversion of MCI to dementia. However, the local shape volume of the thalamus was associated with semantic fluency and attentional composite scores[31]. In addition, some scholars have found that the severity of other non-motor symptoms in patients with PD is associated with more pronounced thalamic atrophy. Furthermore, they found that such non-motor symptoms include sleep, fatigue, gastrointestinal dysfunction, and REM-RBD[32,46].
The thalamus, one of the output nuclei of the basal ganglia, is markedly affected by dopaminergic and glutamatergic neuronal degeneration. For living subjects, imaging is potentially one of the most practical tools to detect changes in the thalamus. Precise shape analysis shows that the thalamus in PD undergoes major or minor changes. Compared to manual measurements, accurate automated measurements reflect more pronounced variation and more detailed results. Because of the varying progression of neuronal degeneration, thalamus shape analysis in patients with PD presents differently. Hence, future studies using the same methods and similar cohort sizes may show better consistency. Moreover, several studies have demonstrated the relevance of shape alterations and symptoms, especially non-motor symptoms, probably because the thalamic subnuclei play an important role in the transmission of dopaminergic neuronal pathways. However, the sequence in which the onset of symptoms and the changes within the thalamus occur is still unclear. In addition, abnormal STN activity may be associated with motor dysfunction in PD; however, further studies are needed to confirm the relationship between STN shape changes and motor symptoms.
As a subcortical structure, the hippocampus is an important brain region that carries the body's cognitive functions and is closely related to learning ability, memory, and emotion regulation. Cognitive impairment is frequently seen in PD; thus, the hippocampus may be an imaging marker of cognitive impairment[47]. Scholars have found a reduction in hippocampal gray matter density or thickness through automatic methods in the elderly or patients with cognitive impairment, especially in the CA1, which is one of the four hippocampal subfields called the cornu ammonis[48-52]. Several studies on hippocampal morphology have been conducted in patients with PD and normal controls. Using automatic shape analysis, some studies have shown smaller hippocampal volumes in patients with PD than in controls[16,17,30,53-55]. There were also reduced local volumes of the hippocampus in patients with cognitive impairment compared with those without cognitive impairment, including the subfields CA1-4[28,30,31,54-62]. Studies have shown that the development of REM-RBD and depression may be associated with a smaller hippocampal volume[33,63,64]. This suggests a close relationship between hippocampal atrophy and cognitive function, in which the CA1 may be one of the most notable subfields.
The hippocampus is the main source of cholinergic input to the cerebral cortex, and most studies have shown that the hippocampal volume shrinks in patients with PD. Hippocampal shape analysis has focused on non-motor symptoms in PD, primarily the cognitive function, which matches the function of the hippocampus. The relationship between hippocampal atrophy and cognitive decline has been confirmed in patients with PD in the majority of studies. However, recent studies mostly showed volume results; thus, the surface morphological analysis may be able to link hippocampal subregions to specific symptoms of cognitive impairment further. The relationship between morphological changes and other symptoms, such as REM-RBD and depression, warrants further investigation.
Furthermore, a large number of studies are also using these automated pipelines to analyze cortical structures in PD. Cerebral cortices are key to human activity and may be altered as a result of unusual activity in PD, such as thinning. Most studies have found atrophy in various parts of the cortex in patients with cognitive impairment. In a longitudinal study, Garcia-Diaz et al[65] confirmed the thinning of cortical thickness in PD patients with cognitive impairment vs those without. Among some symptoms related to the cerebral cortex, Vignando et al[66] reported a general reduction in occipital, parietal, temporal, frontal, and limbic cortical thickness in patients experiencing hallucinations. Changes in visuospatial and visual supraperceptual impairment also correlated with cortical thinning in occipital, parietal, and temporal regions in the study by Garcia-Diaz et al[65]. As for motor symptoms, through the calculation of surface area in a study of PD gait disorders, Wei et al[67] found that the larger the surface areas of the left lateral temporal cortex and right inferior parietal cortex, the worse the gait performance.
This review focuses on the results of patients on 3T instruments, and participants were scanned using a 1.5T MRI instrument and used manual planar measurements, revealing that the normalized STN and red nuclei volumes were larger in patients with PD than in controls[68]. Similarly, 7TMRI imaging revealed atrophy of the overall prefrontal cortex and hippocampus, as well as a reduction in STN volume, for patients with PD[41,69]. Although current studies on 7TMRI have focused only on volumetric rather than morphological changes, higher resolution instruments can help us to detect finer structural changes and conduct more structural studies.
Methods for the shape analysis of subcortical structures based on MRI data are becoming increasingly diverse and refined, allowing even minor changes to be detected. This study has reviewed previous research on the application of these techniques in PD. In contrast to manual measurements, most studies employ computational methods to maintain objectivity. Volume atrophy can be found in most structures, including the subcortical and cortical areas. Surface-based morphometry detects structural changes that can be associated with clinical symptoms. We found that pathophysiological changes in PD are closely associated with changes in the subcortical structures and that different sub-structural alterations are consistent with specific clinical phenotypes. Therefore, the shape analysis of the subcortical structures can be used as an imaging biological indicator of PD, helping to explain associated clinical symptoms.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Gokce E, Turkey S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Pfeiffer RF. Non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord. 2016;22 Suppl 1:S119-S122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 366] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 2. | Delenclos M, Jones DR, McLean PJ, Uitti RJ. Biomarkers in Parkinson's disease: Advances and strategies. Parkinsonism Relat Disord. 2016;22 Suppl 1:S106-S110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, Gasser T, Goetz CG, Halliday G, Joseph L, Lang AE, Liepelt-Scarfone I, Litvan I, Marek K, Obeso J, Oertel W, Olanow CW, Poewe W, Stern M, Deuschl G. MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2015;30:1600-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 911] [Article Influence: 101.2] [Reference Citation Analysis (0)] |
| 4. | Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1995] [Cited by in RCA: 1761] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 5. | Fischl B. FreeSurfer. Neuroimage. 2012;62:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4452] [Cited by in RCA: 6013] [Article Influence: 462.5] [Reference Citation Analysis (0)] |
| 6. | Beg MF, Miller MI, Trouvé A, Younes L. Computing large deformation metric mappings via geodesic flows of diffeomorphisms. Int J Comput Vis. 2005;61:139-57. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1031] [Cited by in RCA: 702] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 7. | Grimm O, Pohlack S, Cacciaglia R, Winkelmann T, Plichta MM, Demirakca T, Flor H. Amygdalar and hippocampal volume: A comparison between manual segmentation, Freesurfer and VBM. J Neurosci Methods. 2015;253:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Makowski C, Béland S, Kostopoulos P, Bhagwat N, Devenyi GA, Malla AK, Joober R, Lepage M, Chakravarty MM. Evaluating accuracy of striatal, pallidal, and thalamic segmentation methods: Comparing automated approaches to manual delineation. Neuroimage. 2018;170:182-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Grillner S, Robertson B, Stephenson-Jones M. The evolutionary origin of the vertebrate basal ganglia and its role in action selection. J Physiol. 2013;591:5425-5431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Pitcher TL, Melzer TR, Macaskill MR, Graham CF, Livingston L, Keenan RJ, Watts R, Dalrymple-Alford JC, Anderson TJ. Reduced striatal volumes in Parkinson's disease: a magnetic resonance imaging study. Transl Neurodegener. 2012;1:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Owens-Walton C, Jakabek D, Li X, Wilkes FA, Walterfang M, Velakoulis D, van Westen D, Looi JCL, Hansson O. Striatal changes in Parkinson disease: An investigation of morphology, functional connectivity and their relationship to clinical symptoms. Psychiatry Res Neuroimaging. 2018;275:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Geng DY, Li YX, Zee CS. Magnetic resonance imaging-based volumetric analysis of basal ganglia nuclei and substantia nigra in patients with Parkinson's disease. Neurosurgery. 2006;58:256-62; discussion 256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Sterling NW, Du G, Lewis MM, Dimaio C, Kong L, Eslinger PJ, Styner M, Huang X. Striatal shape in Parkinson's disease. Neurobiol Aging. 2013;34:2510-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Lee HM, Kwon KY, Kim MJ, Jang JW, Suh SI, Koh SB, Kim JH. Subcortical grey matter changes in untreated, early stage Parkinson's disease without dementia. Parkinsonism Relat Disord. 2014;20:622-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Geevarghese R, Lumsden DE, Hulse N, Samuel M, Ashkan K. Subcortical structure volumes and correlation to clinical variables in Parkinson's disease. J Neuroimaging. 2015;25:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Tanner JJ, McFarland NR, Price CC. Striatal and Hippocampal Atrophy in Idiopathic Parkinson's Disease Patients without Dementia: A Morphometric Analysis. Front Neurol. 2017;8:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Vasconcellos LF, Pereira JS, Adachi M, Greca D, Cruz M, Malak AL, Charchat-Fichman H. Volumetric brain analysis as a predictor of a worse cognitive outcome in Parkinson's disease. J Psychiatr Res. 2018;102:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Messina D, Cerasa A, Condino F, Arabia G, Novellino F, Nicoletti G, Salsone M, Morelli M, Lanza PL, Quattrone A. Patterns of brain atrophy in Parkinson's disease, progressive supranuclear palsy and multiple system atrophy. Parkinsonism Relat Disord. 2011;17:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Menke RA, Szewczyk-Krolikowski K, Jbabdi S, Jenkinson M, Talbot K, Mackay CE, Hu M. Comprehensive morphometry of subcortical grey matter structures in early-stage Parkinson's disease. Hum Brain Mapp. 2014;35:1681-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Garg A, Appel-Cresswell S, Popuri K, McKeown MJ, Beg MF. Morphological alterations in the caudate, putamen, pallidum, and thalamus in Parkinson's disease. Front Neurosci. 2015;9:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Nemmi F, Sabatini U, Rascol O, Péran P. Parkinson's disease and local atrophy in subcortical nuclei: insight from shape analysis. Neurobiol Aging. 2015;36:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Khan AR, Hiebert NM, Vo A, Wang BT, Owen AM, Seergobin KN, MacDonald PA. Biomarkers of Parkinson's disease: Striatal sub-regional structural morphometry and diffusion MRI. Neuroimage Clin. 2019;21:101597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Sigirli D, Ozdemir ST, Erer S, Sahin I, Ercan I, Ozpar R, Orun MO, Hakyemez B. Statistical shape analysis of putamen in early-onset Parkinson's disease. Clin Neurol Neurosurg. 2021;209:106936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 24. | Peralta M, Baxter JSH, Khan AR, Haegelen C, Jannin P. Striatal shape alteration as a staging biomarker for Parkinson's Disease. Neuroimage Clin. 2020;27:102272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Wu T, Wang J, Wang C, Hallett M, Zang Y, Wu X, Chan P. Basal ganglia circuits changes in Parkinson's disease patients. Neurosci Lett. 2012;524:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Mak E, Bergsland N, Dwyer MG, Zivadinov R, Kandiah N. Subcortical atrophy is associated with cognitive impairment in mild Parkinson disease: a combined investigation of volumetric changes, cortical thickness, and vertex-based shape analysis. AJNR Am J Neuroradiol. 2014;35:2257-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Sivaranjini S, Sujatha CM. Morphological analysis of subcortical structures for assessment of cognitive dysfunction in Parkinson's disease using multi-atlas based segmentation. Cogn Neurodyn. 2021;15:835-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Devignes Q, Viard R, Betrouni N, Carey G, Kuchcinski G, Defebvre L, Leentjens AFG, Lopes R, Dujardin K. Posterior Cortical Cognitive Deficits Are Associated With Structural Brain Alterations in Mild Cognitive Impairment in Parkinson's Disease. Front Aging Neurosci. 2021;13:668559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2015;30:1591-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2724] [Cited by in RCA: 4707] [Article Influence: 523.0] [Reference Citation Analysis (0)] |
| 30. | Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, Dalrymple-Alford JC, Anderson TJ. Grey matter atrophy in cognitively impaired Parkinson's disease. J Neurol Neurosurg Psychiatry. 2012;83:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 31. | Chung SJ, Shin JH, Cho KH, Lee Y, Sohn YH, Seong JK, Lee PH. Subcortical shape analysis of progressive mild cognitive impairment in Parkinson's disease. Mov Disord. 2017;32:1447-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Gong L, Li H, Yang D, Peng Y, Liu D, Zhong M, Zhang B, Xu R, Kang J. Striatum Shape Hypertrophy in Early Stage Parkinson's Disease With Excessive Daytime Sleepiness. Front Neurosci. 2019;13:1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Kamps S, van den Heuvel OA, van der Werf YD, Berendse HW, Weintraub D, Vriend C. Smaller subcortical volume in Parkinson patients with rapid eye movement sleep behavior disorder. Brain Imaging Behav. 2019;13:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Kluger BM, Zhao Q, Tanner JJ, Schwab NA, Levy SA, Burke SE, Huang H, Ding M, Price C. Structural brain correlates of fatigue in older adults with and without Parkinson's disease. Neuroimage Clin. 2019;22:101730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Oltra J, Segura B, Uribe C, Monté-Rubio GC, Campabadal A, Inguanzo A, Pardo J, Marti MJ, Compta Y, Valldeoriola F, Iranzo A, Junque C. Sex differences in brain atrophy and cognitive impairment in Parkinson's disease patients with and without probable rapid eye movement sleep behavior disorder. J Neurol. 2022;269:1591-1599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Haaxma CA, Helmich RC, Borm GF, Kappelle AC, Horstink MW, Bloem BR. Side of symptom onset affects motor dysfunction in Parkinson's disease. Neuroscience. 2010;170:1282-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1421] [Cited by in RCA: 1440] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 38. | O'Neill J, Schuff N, Marks WJ Jr, Feiwell R, Aminoff MJ, Weiner MW. Quantitative 1H magnetic resonance spectroscopy and MRI of Parkinson's disease. Mov Disord. 2002;17:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Jahanshahi M, Obeso I, Baunez C, Alegre M, Krack P. Parkinson's disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30:128-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 40. | Kaya MO, Ozturk S, Ercan I, Gonen M, Serhat Erol F, Kocabicak E. Statistical Shape Analysis of Subthalamic Nucleus in Patients with Parkinson Disease. World Neurosurg. 2019;126:e835-e841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Patriat R, Niederer J, Kaplan J, Amundsen Huffmaster S, Petrucci M, Eberly L, Harel N, MacKinnon C. Morphological changes in the subthalamic nucleus of people with mild-to-moderate Parkinson's disease: a 7T MRI study. Sci Rep. 2020;10:8785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Calon F, Rajput AH, Hornykiewicz O, Bédard PJ, Di Paolo T. Levodopa-induced motor complications are associated with alterations of glutamate receptors in Parkinson's disease. Neurobiol Dis. 2003;14:404-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | McKeown MJ, Uthama A, Abugharbieh R, Palmer S, Lewis M, Huang X. Shape (but not volume) changes in the thalami in Parkinson disease. BMC Neurol. 2008;8:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Chen Y, Zhu G, Liu D, Liu Y, Yuan T, Zhang X, Jiang Y, Du T, Zhang J. The morphology of thalamic subnuclei in Parkinson's disease and the effects of machine learning on disease diagnosis and clinical evaluation. J Neurol Sci. 2020;411:116721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Foo H, Mak E, Yong TT, Wen MC, Chander RJ, Au WL, Sitoh YY, Tan LC, Kandiah N. Progression of subcortical atrophy in mild Parkinson's disease and its impact on cognition. Eur J Neurol. 2017;24:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 46. | Niccolini F, Wilson H, Giordano B, Diamantopoulos K, Pagano G, Chaudhuri KR, Politis M. Sleep disturbances and gastrointestinal dysfunction are associated with thalamic atrophy in Parkinson's disease. BMC Neurosci. 2019;20:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Li H, Jia X, Qi Z, Fan X, Ma T, Pang R, Ni H, Li CR, Lu J, Li K. Disrupted Functional Connectivity of Cornu Ammonis Subregions in Amnestic Mild Cognitive Impairment: A Longitudinal Resting-State fMRI Study. Front Hum Neurosci. 2018;12:413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Schmidt-Wilcke T, Poljansky S, Hierlmeier S, Hausner J, Ibach B. Memory performance correlates with gray matter density in the ento-/perirhinal cortex and posterior hippocampus in patients with mild cognitive impairment and healthy controls--a voxel based morphometry study. Neuroimage. 2009;47:1914-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Lee P, Ryoo H, Park J, Jeong Y; Alzheimer's Disease Neuroimaging Initiative. Morphological and Microstructural Changes of the Hippocampus in Early MCI: A Study Utilizing the Alzheimer's Disease Neuroimaging Initiative Database. J Clin Neurol. 2017;13:144-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | McIntosh EC, Jacobson A, Kemmotsu N, Pongpipat E, Green E, Haase L, Murphy C. Does medial temporal lobe thickness mediate the association between risk factor burden and memory performance in middle-aged or older adults with metabolic syndrome? Neurosci Lett. 2017;636:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Shim G, Choi KY, Kim D, Suh SI, Lee S, Jeong HG, Jeong B. Predicting neurocognitive function with hippocampal volumes and DTI metrics in patients with Alzheimer's dementia and mild cognitive impairment. Brain Behav. 2017;7:e00766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Fogwe LA, Reddy V, Mesfin FB. Neuroanatomy, hippocampus. StatPearls Treasure Island (FL): StatPearls Publishing, 2022. Available from: http://www.ncbi.nlm.nih.gov/books/NBK482171/. |
| 53. | Radziunas A, Deltuva VP, Tamasauskas A, Gleizniene R, Pranckeviciene A, Petrikonis K, Bunevicius A. Brain MRI morphometric analysis in Parkinson's disease patients with sleep disturbances. BMC Neurol. 2018;18:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Wilson H, Niccolini F, Pellicano C, Politis M. Cortical thinning across Parkinson's disease stages and clinical correlates. J Neurol Sci. 2019;398:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 55. | Wang L, Nie K, Zhao X, Feng S, Xie S, He X, Ma G, Wang L, Huang Z, Huang B, Zhang Y. Characteristics of gray matter morphological change in Parkinson’s disease patients with semantic abstract reasoning deficits. Neurosci Lett. 2018;673:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Chen FX, Kang DZ, Chen FY, Liu Y, Wu G, Li X, Yu LH, Lin YX, Lin ZY. Gray matter atrophy associated with mild cognitive impairment in Parkinson's disease. Neurosci Lett. 2016;617:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Park JW, Lee CN, Sim Y, Ham HK, Tae WS, Kim BJ. Automated Subfield Volumetric Analysis of Hippocampus in Patients with Drug-Naïve Nondementia Parkinson's Disease. Parkinsons Dis. 2019;2019:8254263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 58. | Filippi M, Canu E, Donzuso G, Stojkovic T, Basaia S, Stankovic I, Tomic A, Markovic V, Petrovic I, Stefanova E, Kostic VS, Agosta F. Tracking Cortical Changes Throughout Cognitive Decline in Parkinson's Disease. Mov Disord. 2020;35:1987-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 59. | Xu R, Hu X, Jiang X, Zhang Y, Wang J, Zeng X. Longitudinal volume changes of hippocampal subfields and cognitive decline in Parkinson's disease. Quant Imaging Med Surg. 2020;10:220-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 60. | Luo C, Gao Y, Hu N, Wei X, Xiao Y, Wang W, Lui S, Gong Q. Distinct hippocampal subfield atrophy in Parkinson’s disease regarding motor subtypes. Parkinsonism Relat Disord. 2021;93:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Uribe C, Segura B, Baggio HC, Campabadal A, Abos A, Compta Y, Marti MJ, Valldeoriola F, Bargallo N, Junque C. Differential Progression of Regional Hippocampal Atrophy in Aging and Parkinson’s Disease. Front Aging Neurosci. 2018;10:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Becker S, Granert O, Timmers M, Pilotto A, Van Nueten L, Roeben B, Salvadore G, Galpern WR, Streffer J, Scheffler K, Maetzler W, Berg D, Liepelt-Scarfone I. Association of Hippocampal Subfields, CSF Biomarkers, and Cognition in Patients With Parkinson Disease Without Dementia. Neurology. 2021;96:e904-e915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Rahayel S, Gaubert M, Postuma RB, Montplaisir J, Carrier J, Monchi O, Rémillard-Pelchat D, Bourgouin PA, Panisset M, Chouinard S, Joubert S, Gagnon JF. Brain atrophy in Parkinson's disease with polysomnography-confirmed REM sleep behavior disorder. Sleep. 2019;42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 64. | van Mierlo TJ, Chung C, Foncke EM, Berendse HW, van den Heuvel OA. Depressive symptoms in Parkinson's disease are related to decreased hippocampus and amygdala volume. Mov Disord. 2015;30:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 65. | Garcia-Diaz AI, Segura B, Baggio HC, Uribe C, Campabadal A, Abos A, Marti MJ, Valldeoriola F, Compta Y, Bargallo N, Junque C. Cortical thinning correlates of changes in visuospatial and visuoperceptual performance in Parkinson's disease: A 4-year follow-up. Parkinsonism Relat Disord. 2018;46:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 66. | Vignando M, Ffytche D, Lewis SJG, Lee PH, Chung SJ, Weil RS, Hu MT, Mackay CE, Griffanti L, Pins D, Dujardin K, Jardri R, Taylor JP, Firbank M, McAlonan G, Mak HKF, Ho SL, Mehta MA. Mapping brain structural differences and neuroreceptor correlates in Parkinson's disease visual hallucinations. Nat Commun. 2022;13:519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Wei X, Wang Z, Zhang M, Li M, Chen YC, Lv H, Tuo H, Yang Z, Ba F. Brain Surface Area Alterations Correlate With Gait Impairments in Parkinson's Disease. Front Aging Neurosci. 2022;14:806026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Camlidag I, Kocabicak E, Sahin B, Jahanshahi A, Incesu L, Aygun D, Yildiz O, Temel Y, Belet U. Volumetric analysis of the subthalamic and red nuclei based on magnetic resonance imaging in patients with Parkinson's disease. Int J Neurosci. 2014;124:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Oh BH, Moon HC, Kim A, Kim HJ, Cheong CJ, Park YS. Prefrontal and hippocampal atrophy using 7-tesla magnetic resonance imaging in patients with Parkinson's disease. Acta Radiol Open. 2021;10:2058460120988097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |