Published online Jan 19, 2022. doi: 10.5498/wjp.v12.i1.151
Peer-review started: March 30, 2021
First decision: July 15, 2021
Revised: July 30, 2021
Accepted: November 25, 2021
Article in press: November 25, 2021
Published online: January 19, 2022
Processing time: 293 Days and 11.9 Hours
The capacity of posttraumatic stress disorder (PTSD) to occur with delayed onset has been documented in several systematic reviews and meta-analyses. Neurobiological models of PTSD may provide insight into the mechanisms underlying the progressive increase in PTSD symptoms over time as well as into occasional occurrences of long-delayed PTSD with few prodromal symptoms.
To obtain an overview of key concepts explaining and types of evidence supporting neurobiological underpinnings of delayed PTSD.
A scoping review of studies reporting neurobiological findings relevant to delayed PTSD was performed, which included 38 studies in the qualitative synthesis.
Neurobiological mechanisms underlying PTSD symptoms, onset, and course involve several interconnected systems. Neural mechanisms involve the neurocircuitry of fear, comprising several structures, such as the hippocampus, amygdala, and prefrontal cortex, that are amenable to time-dependent increases in activity through sensitization and kindling. Neural network models explain generalization of the fear response. Neuroendocrine mechanisms consist of autonomic nervous system and hypothalamic-pituitary-adrenocortical axis responses, both of which may be involved in sensitization to stress. Neuroinflammatory mechanisms are characterized by immune activation, which is sometimes due to the effects of traumatic brain injury. Finally, neurobehavioral/contextual mechanisms involve the effects of intervening stressors and mental and physical disorder comorbidi
Thus, delayed PTSD may result from multiple underlying neurobiological mechanisms that may influence the likelihood of developing prodromal symptoms preceding the onset of full-blown PTSD.
Core Tip: Multiple neurobiological mechanisms underlying delayed expression of posttraumatic stress disorder contribute to sensitization, kindling, and generalization leading to increasing symptoms, through epigenetic, neuroinflammatory, neuroendocrine, and neural interactions.
- Citation: Smid GE, Lind J, Bonde JP. Neurobiological mechanisms underlying delayed expression of posttraumatic stress disorder: A scoping review. World J Psychiatry 2022; 12(1): 151-168
- URL: https://www.wjgnet.com/2220-3206/full/v12/i1/151.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i1.151
Posttraumatic stress disorder (PTSD) with delayed expression (also known as delayed PTSD or delayed-onset PTSD) is a diagnostic category that applies to people who first meet the criteria for a PTSD diagnosis at least 6 mo following exposure to a traumatic event[1]. While the majority of people who develop PTSD do so within the first wk or mo following the traumatic encounter, a significant minority of people with PTSD present delayed expression of the disorder[2-4]. Since the inclusion of the PTSD diagnosis in the Diagnostic and Statistical Manual of Mental Disorders (DSM) in 1980, a delayed category has been discerned[5]. Subsequently, the capacity of PTSD to occur with delayed expression has been documented in several systematic reviews and meta-analyses[2-4]. A proper understanding of the neurobiological basis for delayed expression of PTSD is clinically useful since it has implications for diagnostic assessment in both treatment and forensic settings and in the context of litigation. Specifically, neurobiological models of PTSD may explain variability in the progressive increase in PTSD symptoms over time following exposure to trauma that characterizes PTSD with delayed expression. Neurobiological mechanisms and systems are likely to play a central role in determining the duration of the prodromal phase, the presence of prodromal symptoms and mental and physical disorder comorbidities.
Because delayed expression is the exception rather than the rule, neurobiological mechanisms underlying delayed PTSD have received limited research attention. We therefore conducted a scoping review to obtain an overview of key concepts explaining and types of evidence supporting neurobiological underpinnings of delayed PTSD. The research questions were to determine what role neurobiological mechanisms have in the delayed expression of PTSD, and how neurobiological mechanisms contribute to explaining the occurrence of delayed PTSD following a long asymptomatic interval.
Since our aim was to provide an overview of key concepts and types of evidence, we performed a scoping review[6]. We searched for publications examining the role of neurobiological mechanisms in developing delayed PTSD. The PRISMA scoping reviews checklist[7] was used to ensure correct reporting. We did not register the review protocol. The search was performed in early December 2020 in the following databases (all in the Ovid platform): PsycINFO; Ovid Medline ALL, Ovid Evidence Based Medicine Reviews (EBM Reviews - Cochrane Database of Systematic Reviews and EBM Reviews - Database of Abstracts of Reviews of Effects) and Embase. We based the search strategy on the two research questions and several lead articles (which we presented to Ovid Citation Analyzer to harvest search terms). We built a search strategy in PsycINFO (Ovid), which we then adapted to the other databases. The full search strategy for PsycINFO can be found in Supplementary Table 1. The search terms were grouped into clusters. For Question 1 (the role of neurobiological mechanisms in developing delayed PTSD), we had these clusters: etiology and neurobiological factors (sets 1 through 5), late-onset PTSD (set 6), and study types (sets 8 through 11). For Question 2 (delayed PTSD with a long-term asymptomatic interval), we used the following clusters: late-onset PTSD (set 6), remission and asymptomatic periods (set 7) and study types (sets 8 through 11). These clusters were combined using Boolean operators, and the combined clusters for question 1 (set 12) and question 2 (set 13) were combined. The search results were imported into Endnote and deduplicated using the method outlined elsewhere[8]. Supplementary Table 2 shows the number of items retrieved in each search system, the number of duplicates and thus the new articles collected. These articles were then screened for inclusion in this review.
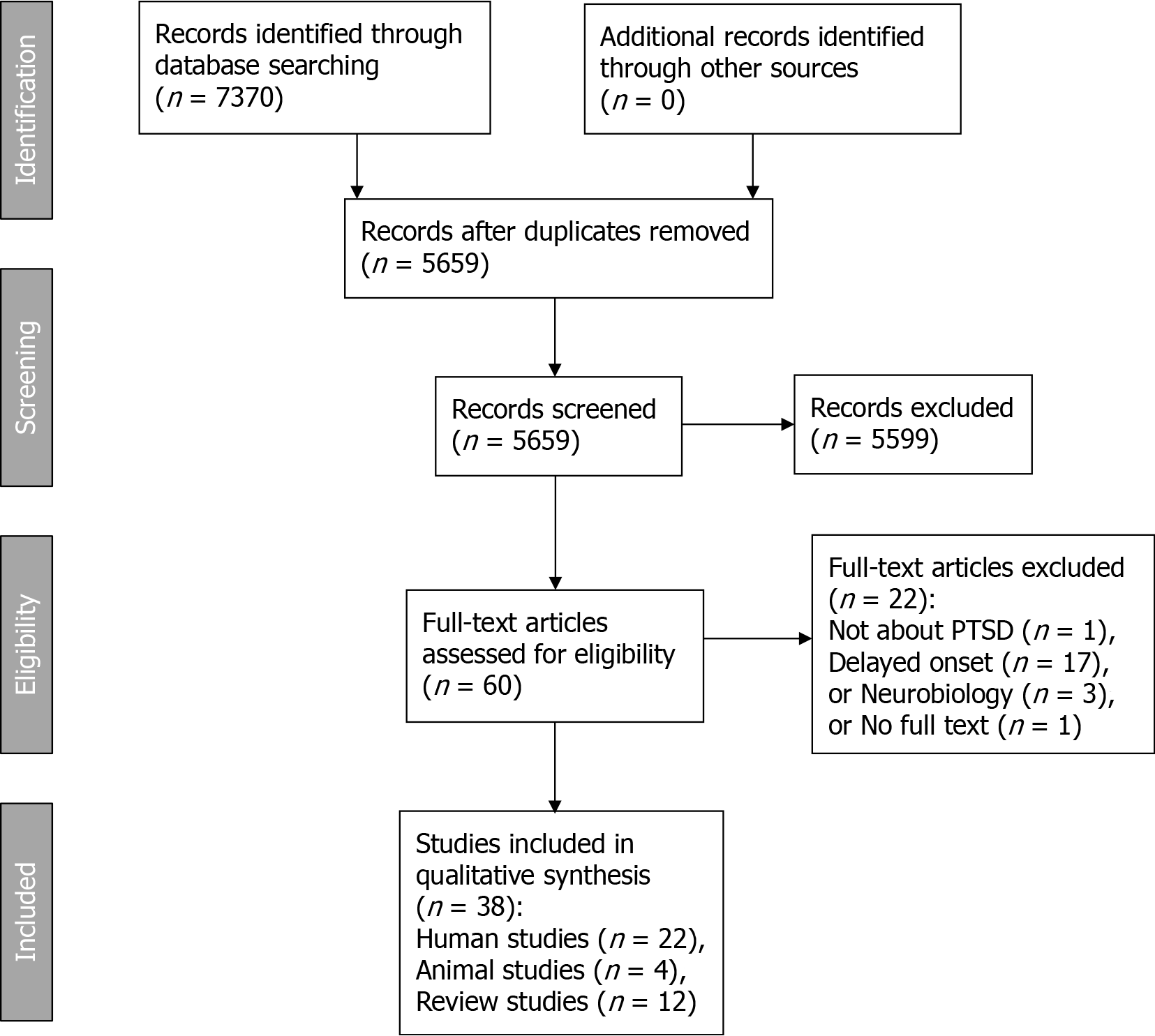
To screen and select articles, we used Rayyan, a web-based program for systematic reviews[9]. The screening process consisted of three phases: (1) Stepwise inclusion of records based on titles and abstracts with the aid of automatic Rayyan keywords for inclusion; (2) Manual inclusion and exclusion of records based on the inclusion and exclusion criteria; and (3) Manual exclusion of articles based on the full text. Records were eligible if they were: (1) About trauma and PTSD; (2) About delayed onset; (3) About neurobiology; and (4) About causal mechanisms or risk factors. Therefore, records were excluded if they reported studies that were: (1) Not about trauma and PTSD; (2) Not about delayed onset; (3) Not about neurobiology; or (4) Not about causal mechanisms or risk factors. Additionally, duplicate items were excluded. During the first phase, the references including the keywords in Table 1 were retained. In the second phase, two researchers (GS and JL) independently reviewed the titles and abstracts of the remaining 438 items, which had been preselected based on the keywords. In this phase, we used the same criteria as earlier, as outlined in Table 2. After this phase, 60 articles were screened based on the full text by the first author. Twenty-two articles were excluded, thus arriving at a final selection of 38 articles. Figure 1 shows a PRISMA diagram for an overview of the process.
| Criterion | Input for this criterion | Automatic keyword screening (Rayyan): Used keywords for include | Included | Thus excluded |
| 1 | 5659 | Trauma; traumas; traumatic; traumatized; traumatised; posttraumatic; PTSD | 5194 | 465 |
| 2 | 5194 | Asymptomatic; bridging; delayed; dormant; emerge; emerges; emerging; increase; increases; increasing; interval; late; latency; latent; onset; progression; progressive; symptom-free | 2287 | 2907 |
| 3 | 2287 | Adrenal; adrenalin; allostatic; ANS; autonomous; biochemical; biological; biology; biomarker; biomarkers; brain; cell; ceruleus; chemokine; coeruleus; cortex; cortisol; corticosteroids; corticosteroid; CT; cytokine; cytokines; DNA; epicortisol; epigenetic; epigenomic; epinephrine; frontal; genetic; hippocampus; hippocampal; HPA; hydrocortisone; hypothalamic; hypothalamus; imaging; immune; immunological; inflammation; LC; marker; markers; MRI; NE; nervous; neurobiological; neurobiology; neuroimaging; noradrenalin; norepinephrine; parasympathetic; PET; phenotype; phenotypical; pituitary; PNS; prefrontal; psychobiological; psychobiology; SNS; SPECT; stem; sympathetic | 716 | 1571 |
| 4 | 716 | Amnesia; amnesic; amnestic; cause; causal; dissociation; dissociative; factor; mechanism; mechanisms; predictor; protective; risk; sensitisation; sensitised; sensitization; sensitized; stage; staging; susceptibility; trigger; vulnerability | 455 | 261 |
| 455 | Deduplication | 438 | 17 |
| Criterion | Input for this criterion | Manual title and abstract screening: Record refers to | Inclusion | Exclusion |
| 1 | 438 | Trauma and PTSD | 308 | 130 |
| 2 | 308 | Delayed onset | 73 | 235 |
| 3 | 73 | Neurobiology | 62 | 11 |
| 4 | 62 | Causal mechanisms or risk factors | 60 | 2 |
| 60 | Full-text articles assessed for eligibility | 60 |
The selected articles were divided into human studies (n = 22), animal studies (n = 4), and review studies (n = 12). From human studies, we abstracted the following data: population (N), the type of trauma or stressor, assessment times, type of PTSD assessment, prevalence of PTSD and delayed PTSD, and neurobiological observation methods. From animal studies, we abstracted the following data: types of animals (N), type of trauma or stressor, assessment times, observed anxiety and delayed effects, and neurobiological observation methods. From review studies, we abstracted the included study types (human and/or animal) and summarized the review focus. An overview of the included human, animal, and review studies is presented in Tables 3, 4 and 5, respectively. A detailed overview of human studies is provided in Supplementary Table 3.
| Ref. | Population (n) | Trauma/stressor | Assessment times |
| Admon et al[14], 2013 | Soldiers (33) | Treating a fellow soldier with severe combat injury | Pre-deployment and 18 mo later |
| Alway et al[30],2016 | TBI patients (85) | Motor vehicle accidents (76.5%), other accidents, assaults | 6 mo, 1-, 2-, 3-, and 4-yr post-injury |
| Bryant et al[29], 2009 | Traumatic injury patients with no (708) or mild TBI (459) | Transport accident, assault, fall, work injury, other injury | During hospital admission and at 3 mo post-injury |
| Bryant et al[28], 2013 | Road traffic accident survivors admitted to trauma hospital (1084) | Transport accident, assault, fall, work injury, other injury | During hospital admission and at 3-, 12-, and 24 mos post-injury |
| Busso et al[21], 2014 | Adolescents exposed to bombing (78) | Terrorist attack at the 2013 Boston marathon | 1 year prior to trauma (n = 44), 4-6 wk posttrauma (n = 78) |
| Cacciaglia et al[13], 2017 | Healthy rescue ambulance workers (18), non-exposed matched controls (18) | Exposed group: vehicle accident (41%), traumatic loss of a loved one, domestic violence, childhood abuse | Cross-sectional; trauma occurred a mean of 7.41 yr ago |
| Chase et al[39], 2015 | Help-seeking veterans (16) and family members (10) | Exposure to blast during employment to combat-intense settings | Cross-sectional; > 7 yr after exposure |
| Do Prado et al[31], 2017 | Adolescents with childhood trauma (30), controls without history of early life stress (27) | Sexual abuse, physical abuse, emotional abuse, physical neglect, emotional neglect | Cross-sectional; maltreatment ended > 12 mo ago |
| Gandubert et al[19], 2016 | Emergency room patients (123) | Physical assault, sexual assault, serious accident, other | During the first week and at 1-, 4-, and 12 mos post-trauma |
| Gil et al[35], 2005 | Traumatic brain injury patients (120) | Traffic accident | < 1 week, 3 mo, and 6 mo later |
| Glenn et al[27], 2017 | Soldiers deployed to Afghanistan (852) | Combat experience, difficult living and working environment | 4 wk before and 22 wk after deployment |
| Jung et al[47], 2019 | Community-dwelling women (nurses) (50020) | Various self-reported on Brief Trauma Questionnaire | Biennial from enrollment |
| Monfort and Trehel[44], 2017 | 93-year-old veteran (1) | WW II combat experiences | 65 years later |
| Roy et al[36], 2015 | Combat veterans without PTSD, depression, or post-concussive syndrome < 2 mo after return (81) | Deployment to Iraq or Afghanistan > 3 mo | < 2 mo after return, 3, 6, and 12 mo |
| Smid et al[26], 2015 | Deployed soldiers (693) | 4 mo deployment to Afghanistan | 2 mo prior to deployment and 1-, 6-, 12-, and 24 mo following deployment |
| Solomon and Mikulincer[42], 2006 | Combat veterans with combat stress reaction (CSR) (131) or without (83) | 1982 Lebanon War | 1, 2, 3, and 20 yr after the war |
| Solomon et al[41], 2017 | Ex-prisoners of war (101), combat controls (15) | 1973 Yom Kippur War | 18, 30, 35, 42 yr after the war |
| Stein et al[43], 2013 | Community-dwelling (25,018) | Lifetime exposure to 27 traumatic events | Cross-sectional |
| Uddin et al[32], 2010 | PTSD-affected (23) and -unaffected individuals (77) from large sample | Lifetime exposure to 19 traumatic events | Cross-sectional |
| Vaiva et al[20], 2005 | Hospitalized traumatology patients (78) | Road traffic accident | 1 and 6 wk, 12 mo |
| Wang et al[33], 2015 | Blunt chest trauma patients (57) | Motor vehicle accidents (61.4%), falls, other accidents | 1, 3, 6 mo |
| Waszczuk et al[46], 2020 | First responders (1490) | Working at the World Trade Center site, New York following the 9/11, 2001 terrorist attacks | Mean = 7.75 monitoring visits per 1.49 yr, PTSD diagnosis at 12 yr |
| Ref. | Animals (n) | Trauma/stressor | Assessment times | Anxiety and delayed effects | Neurobiological observation methods |
| Ardi et al[15], 2014 | Rats: naïve (12), swim (12), swim + reminder (R) (12), UWT (12), UWT + R (12) | Rats were given daily 1-minute swim trials for 5 days. On day 6, ‘swim’ rats had an additional swim trial, and ‘UWT’ rats were swimming and then held underwater for 30 s using a net. On day 7, rats from the ‘reminder’ groups were exposed to 30 s of swimming | Following the ‘reminder’, rats were tested after 30 min.; ‘swim’ and ‘UWT’ rats were tested on day 7 | Undergoing UWT results in reduced exploration in the open field even 24 h after the trauma compared to ‘swim’ and ‘naïve’ groups. Exposure to the reminder resulted in significantly enhanced anxiety behavior | electrophysiological recordings of hippocampal dentate gyrus GABA-ergic local circuit activity: paired-pulse inhibition (reflecting feedback inhibition), frequency-dependent inhibition (reflecting feed-forward inhibition), long-term potentiation; biochemical analysis: amygdala extracellular-signal-regulated kinase activity |
| Justice et al[45], 2015 | Mice: wild type controls (43) and PTSD-group (65), Alzheimer’s Disease model controls (76) and PTSD-group (145) | Mice in the PTSD group were immobilized for 2h on boards with tape in a brightly lit area. For the reminder, the procedure was repeated during 15 min. | 2–3 mo and 6–12 mo | Animals displayed elevated anxiety and slightly elevated startle amplitudes | resting and peak plasma corticosteroid levels, cerebrospinal fluid beta-amyloid levels |
| Serova et al[25], 2019 | Rats: 1 wk following stress (57), 2 wk following stress (42), controls (56) | Rats were immobilized for 2 h on a board by taping the limbs and restricting motion of the head, then subjected to forced swim for 20 min. | 1 or 2 wk following stress | At 1 week, 17.5%, and at 2 wk, 57.1% of animals displayed severe anxiety | Gene expression in the mediobasal hypothalamus and locus coeruleus (LC), immunohistochemistry |
| Wilson et al[34], 2013 | Rats: PTSD-group (10), controls (10) | PTSD group rats were secured in plexiglas cylinders and placed in a cage with a cat for one hour on days 1 and 11 of a 31-day stress regimen, and their cage cohort was changed daily | day 0, day 12, day 31 | The PTSD group displayed significantly higher anxiety than the control group, and significantly diminished growthrate over the 31-day stress period | Growth, plasma (corticosterone), adrenal glands (weight, oxidative stress), and hippocampus, amygdala, and pre-frontal cortex (oxidative stress and inflammatory markers: interleukin-1β, NALP3-inflammosome, glyceraldehyde 3-phosphate dehydrogenase) |
| Ref. | Study types | Review focus |
| Admon et al[11], 2013 | Human | Reviews predisposing and acquired neural abnormalities that can be discerned based on PTSD neuroimaging studies that include genetic, environmental, twin, and prospective data |
| Belda et al[22], 2015 | Animal | Reviews sensitization: A phenomenon whereby exposure to a particular stimulus triggers a state of hyperresponsiveness |
| Kim et al[18], 2019 | Human, animal | Reviews influences of chronic exposure to stress on the immune system, resulting in increased proinflammatory cytokine levels. Focuses on changes in the amygdala, hippocampus, PFC, and insula, that are particularly influenced by excess cytokines |
| McFarlane[17], 2000 | Human | Focuses on people who develop PTSD de novo, i.e., without preexisting disorder at the time of the traumatic event that may have acted as a risk factor to the onset of PTSD |
| McFarlane[23], 2010 | Human | Examines the issue of the timing of the onset of PTSD following exposure to traumatic events |
| McFarlane et al[16], 2002 | Human | Reviews the knowledge from neural networks to model a framework for exploring the relationship between neurobiology, cognition, and behavior in PTSD |
| McFarlane et al[40], 2017 | Human | Argues that major advances in the biological treatments of PTSD depend on a more sophisticated classification of PTSD that acknowledges the heterogeneity of this condition |
| Michopoulos et al[24], 2015 | Human | Reviews putative PTSD biomarkers with specific emphasis on the interaction between neurobiological influences on disease risk and symptom progression |
| Smid et al[38], 2003 | Human | Reviews risk factors for delayed PTSD, including combat trauma, stressful events after the trauma and previous emotional problems |
| Soreq[37], 2010 | Human, animal | Reviews effects that are often reported yr after prophylactic treatment with cholinesterase inhibitors for protection under threat of chemical warfare, e.g., during the Gulf War, and their similarity to symptoms of PTSD |
| Wilker and Kolassa[10], 2013 | Human, animal | Reviews genetic risk factors in PTSD etiology from the perspective of a psychobiological model, which proposes that intrusive memories, the core PTSD symptom, result from the formation of an associative neural fear network, which stores sensory-perceptual representations of traumatic memories |
| Zovkic et al[12], 2013 | Human, animal | Discusses epigenetic regulation of PTSD in human studies and in animal models and ways in which these models can be expanded. Reviews the literature that directly addresses the involvement of epigenetics in PTSD and puts it into the broader context of epigenetics in stress and fear learning |
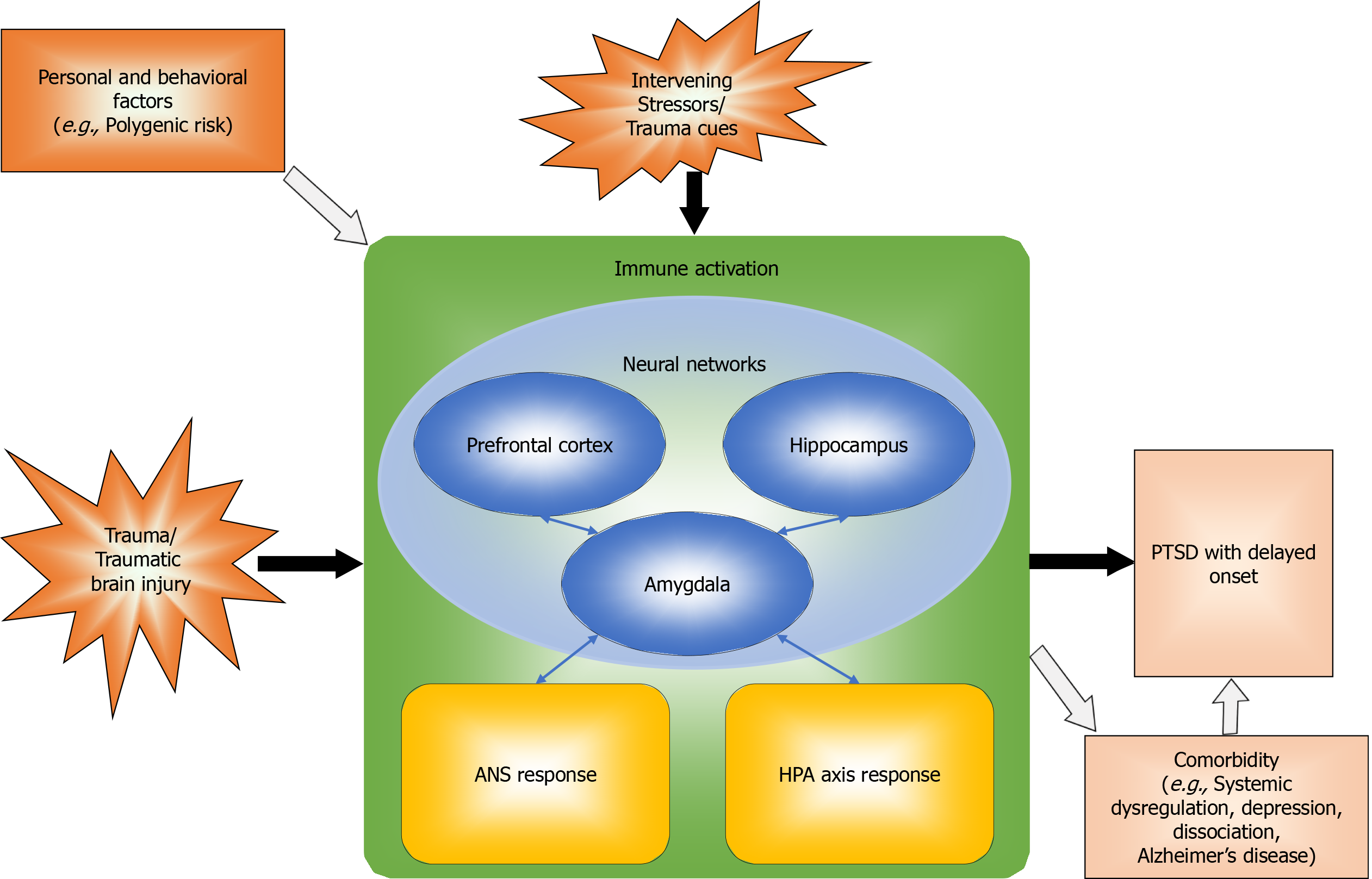
The included studies reported three types of neurobiological mechanisms, specifically neural, neuroendocrine and neuroinflammatory mechanisms. In addition, included studies reported neurobehavioral/contextual pathways. The following sections will summarize the findings for each of these types of mechanisms and pathways. An overview of the interconnected neural, neuroendocrine, neuroinflammatory, and neurobehavioral/contextual systems is presented in Figure 2.
Studies of neural mechanisms of PTSD have focused on identifying brain structures involved in fear learning and modeling structural and functional characteristics underlying PTSD symptoms, onset, and course.
Animal research has identified the amygdala, medial prefrontal cortex (PFC), and hippocampus (the so-called limbofrontal neurocircuitry of fear) as the key regions involved in the acquisition, regulation, and extinction of conditioned fear[10].
Amygdala: The most consistent functional abnormality in human PTSD studies is increased amygdalar responsiveness to emotional stimuli, which may or may not be trauma specific. A hyperactive amygdala has been associated with the heightened fear and hyperarousal of patients with PTSD[11]. Consolidation of cued and contextual fear conditioning has been found to be mediated by epigenetic modifications in the amygdala[12].
In a study of healthy trauma-exposed rescue ambulance workers, an increased volume in the left amygdala was found. Left amygdalar volumes positively correlated with suppressed morning salivary cortisol concentrations[13], suggesting amygdalar involvement in sensitized neuroendocrine responses (see below).
Hippocampus: Guided by evidence from animal studies demonstrating how stress can have a destructive effect on the hippocampus, a brain structure critical for learning and memory, studies in humans have frequently reported reduced hippocampal volume in patients with PTSD[11]. An abnormal hippocampus has been suggested to mediate PTSD-related deficits in the appreciation of safe contexts and contextual memory[11]. Indeed, epigenetic modifications in the hippocampus have been shown to be specifically involved in mediating contextual fear learning[12]. In deployed soldiers, reductions in hippocampal volume and connectivity with the ventromedial PFC from pre- to postdeployment were found to be related to concurrent increases in PTSD symptoms, whereas predeployment low hippocampal volume was not associated with outcomes in terms of PTSD symptoms[14]. However, this latter finding should not be regarded as contradicting prior evidence from homozygotic twins suggesting that smaller hippocampal volume is a predisposing risk factor for PTSD[11,14]. Probably, the effects of predisposing and acquired neural abnormalities are interrelated, such that reduced hippocampal volume may predispose to PTSD, yet at the same time development of PTSD can cause a secondary loss of hippocampal volume over time[11].
Animal research on hippocampal involvement in fear learning revealed time frames related to hippocampal fear memory processing. Consolidation of hippocampus-dependent fear memory can be evaluated 24 h after training, whereas consolidation of remote memory becomes evident at least 7 days after training, when memories from the hippocampus have been integrated for maintenance in the cortex[12]. In animal models of PTSD based on fear conditioning following shock exposure, exposure to a novel, neutral tone provides an index of fear sensitization[12]. Importantly, the expression of sensitized fear to a novel auditory cue has been found to increase with time after shock exposure[12].
A study in rats following underwater trauma exposure and subsequent exposure to a trauma reminder one week later[15] demonstrated the impact of trauma reminders on both the hippocampus and amygdala. While exposure to underwater trauma resulted in increased local circuit inhibitory feedback activity in the hippocampal dentate gyrus, exposure to the trauma reminder resulted in an additional increase in local circuit inhibitory feed-forward activity. Reminder exposure also resulted in impaired hippocampal dentate gyrus long-term potentiation and amygdalar extracellular-signal-regulated-kinase-2 (ERK2) activation, supporting the notion that under emotional conditions, the amygdala modulates stress-induced alterations in the dentate gyrus through the modulation of hippocampal ERK signaling[15].
Prefrontal cortex: The PFC has been found to display abnormal function and structure in PTSD patients. Specifically, aspects of the medial sections of the medial PFC and anterior cingulate cortex (ACC) have been associated with patients’ deficits in emotional regulation[11]. Connectivity studies, either functional or structural, have shown deficient connectivity between the amygdala and/or the hippocampus to the frontal lobe, which could contribute to difficulties that patients with PTSD have in integrating cognitive control over the emotional neural system[11]. Abnormal structure of the amygdala and dorsal ACC and their heightened responsivity to emotionally negative stimuli may represent predisposing neural abnormalities that increase the likelihood of developing PTSD following exposure to trauma[11]. Reduced volumes in medial PFC structures (specifically, the rostral ACC, ventromedial PFC, and orbitofrontal cortex), as well as reduced ventromedial PFC connectivity with the hippocampus, if acquired following exposure to trauma, may lead to PTSD susceptibility[11].
According to neural network models of PTSD, the structure of the neural networks involved in the processing of traumatic memories becomes progressively modified by the repeated replay of these memories through iterative learning, top-down activation and pruning[16]. Noradrenergic neurons play a central role in coordinating the interaction of multiple cortical regions, and functional alterations in the noradrenergic system leading to dysfunctional modulation of working memory in PTSD contribute to intrusive traumatic recollection[16]. Modifications of neural networks have a secondary effect of kindling in the hippocampus that further moderates the individual's sensitivity to a range of stressors[16]. The fear network model assumes that every new trauma activates the same memory structure, given that different traumatic experiences share important elements[10]. With each new traumatic event, sensory-perceptional elements are added, and the interconnections of the emerging fear network strengthen. With multiple traumatic events, the network will contain conflicting contextual information from different events. Hence, the memory for context weakens with increasing traumatic load, followed by the emergence of intrusive symptoms[10]. An individual exposed to sequential trauma who has developed PTSD after a particular traumatic event may develop intrusive and distressing memories of previously experienced traumatic events that had not previously led to symptoms. Due to the pruning of dendrites, inappropriate fusion of memory networks occurs, which explains how a particular traumatic event can serve as an activator of previous traumatic memories[16]. Furthermore, the triggers for intrusive traumatic memories become increasingly more subtle and generalized[16].
Neuroendocrine mechanisms involve both neural and endocrine (hormonal) components. Two major neuroendocrine systems have been implicated in the stress response, one involving the autonomic nervous system and the other involving the hypothalamic-pituitary-adrenocortical (HPA) axis. Below, findings relevant to delayed PTSD regarding both neuroendocrine systems are summarized.
The autonomic nervous system responses to stressor exposure involves activation of neurons in the locus coeruleus (LC), the major noradrenergic (norepinephrinergic) nucleus of the brain involved in the regulation of arousal and autonomic activity, leading to adrenalin (epinephrine) release via the sympatho-adrenal medullary pathway. Autonomic hyperarousal is a risk factor predicting the course of PTSD[17]. Decreased activity of the parasympathetic nervous system, along with increased activity of the sympathetic nervous system, have been observed in PTSD[18]. In emergency room patients, the waist-to-hip ratio and systolic blood pressure were associated with PTSD 4 and 12 mo later; both measures are biomarkers of autonomic nervous system responses. A higher level of overnight urinary norepinephrine predicted PTSD at 4 mo, and body mass index at baseline was associated with a 12 mo PTSD diagnosis[19]. Gamma amino-butyric acid (GABA) is inversely associated with the intensity and duration of the central hyperadrenergic response in times of high stress. In hospitalized traumatology patients, lower GABA levels one week after hospitalization predicted PTSD 12 mo later. Among victims without PTSD at 6 wk, 80% of subjects with GABA levels below 0.20 nmol/mL developed delayed-onset PTSD[20]. Adolescents who were exposed to a terrorist bombing attack and who had had high levels of sympathetic reactivity during a Trier Social Stress Test (specifically, shorter preejection period on impedance cardiography) preceding the attack exhibited an elevated risk for PTSD symptoms compared to adolescents with low sympathetic reactivity in a context of low levels of exposure to media coverage of the attacks. In the context of high levels of media exposure, youth with high and low sympathetic reactivity exhibited equally high levels of PTSD symptoms. Thus, adolescents with low sympathetic reactivity developed PTSD symptoms only following high exposure to media coverage of the attack[21]. These results suggest that in the absence of pre-exposure vulnerability for PTSD, additional superimposed stress may be needed to trigger PTSD symptom onset.
The HPA axis response to stressor exposure in humans involves activation of neurons in the hypothalamus that secrete releasing hormones, such as corticotrophin releasing hormone (CRH), that act on the pituitary to promote the secretion of adrenocorticotropic hormone (ACTH), which in turn acts on the adrenal cortex to initiate the synthesis and release of glucocorticoid hormones, specifically cortisol. Exposure to systemic or high-intensity emotional stressors is followed by HPA axis sensitization. In some studies, with acute immune stressors, HPA sensitization appears to develop over time (incubation), but most studies find a strong initial sensitization that progressively declines over the days[22]. HPA axis cross-sensitization to heterotypic stressors is best observed with short duration (5-15 min) novel challenging stressors[22]. In addition to HPA axis (cross-) sensitization, behavioral sensitization, reflected in different types of animal tests related to fear conditioning and anxiety-like behavior, can be observed, especially following the imposition of a new, brief stressor. Behavioral sensitization appears to persist longer than that of the HPA axis, suggesting long-term latent effects of the initial exposure[22].
In the domain of the HPA axis, the process of sensitization explains why individuals with PTSD become unusually reactive to stress, which is manifested as exaggerated behavioral and biological responses to environmental challenge[23]. A study of healthy trauma-exposed rescue ambulance workers reported hyposuppression of salivary cortisol in a dexamethasone challenge test. HPA axis sensitization appeared to be associated with amygdalar activity. Specifically, left amygdalar enlargement correlated with suppressed morning salivary cortisol[13]. These findings suggest that asymptomatic, trauma-exposed individuals develop neurobiological features similar to those in patients with PTSD. However, it is unclear whether and how prior trauma exposure affects the time of onset of PTSD symptoms following exposure to subsequent traumatic events.
Emerging genetic and epigenetic findings related to PTSD risk vs resilience have focused on modulators of HPA axis function prior to and following trauma[24]. In a study of gene expression in HPA axis-related brain regions in rats exposed to a single prolonged stressor, the percentage of animals displaying severe anxiety increased strongly from 17.5% at one week to 57.1% two wk after stress. This single prolonged stressor elicited time-dependent changes in gene expression for CRH and neuropeptide Y (NPY) systems in the locus coeruleus and hypothalamus. The locus coeruleus displayed prolonged activation, with enhanced gene expression for CRH receptor 1 and reduced gene expression for NPY and Y2 receptors. In the mediobasal hypothalamus, sustained increased CRH gene expression was found, but there was a flip in alterations of gene expression for glucocorticoid receptor (GR), FK506 binding protein 5 (FKBP5) and NPY receptor at two wk compared to one week. Although gene expression for GR and FKBP5 was increased over levels in unstressed rats at 1 wk, it was downregulated by 2 wk. Similarly, robust increases in Y2 receptor and Y5 receptor gene expression were observed at 1 wk, but after 2 wk, only the Y5 receptor differed from the unstressed levels and was downregulated to half the levels that were observed in unstressed rats[25]. These findings illustrate the cascade of neurobiological alterations underlying progressive symptom development following trauma.
An accumulating body of evidence suggests that cytokines play a role in processes such as fear learning and memory that are involved in the pathogenesis of PTSD[18,26,27]. High levels of proinflammatory cytokines associated with injury, inflammation, and severe psychological stress have been shown to exert direct detrimental effects on memory functioning and neural plasticity[18,26]. Studies of traumatic brain injury (TBI) survivors have yielded additional insights into PTSD progression over time[28-30].
Different mechanisms have been suggested to underlie immune activation following exposure to psychological trauma. In adolescents exposed to childhood maltreatment[31], evidence of immune activation and proinflammatory profiles was found, as well as more circulating lymphocyte subsets associated with cell activation and signs of early immunological aging. Underlying mechanisms included enhanced activation of both mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NFκB) signaling pathways and partial resistance to glucocorticoids, specifically, decreased lymphocyte sensitivity to dexamethasone[31]. In a cross-sectional study of PTSD-affected and PTSD-unaffected individuals, peripheral epigenomic and cytomegalovirus immune response profiles associated with PTSD were consistent with traumatic events inducing downstream alterations in immune function by reducing methylation levels of immune-related genes[32]. In hospitalized blunt chest trauma patients, transfusion, injury severity, and high-mobility group box 1 Levels, a key late mediator of systemic inflammation after one week, were predictive of PTSD at 6 mo, including delayed PTSD[33].
In soldiers reporting high combat stress exposure, both high mitogen-stimulated T-cell cytokine production and high innate cytokine production were associated with increases in PTSD symptoms in response to postdeployment stressful life events. In soldiers exposed to low combat stress and those with low cytokine production, postdeployment stressful life events were not associated with increases in PTSD symptoms. The effects of postdeployment stressful life events on the course of PTSD symptoms after a return from deployment largely depended on combat stress exposure as well as immune reactivity following return from deployment. High combat exposure does not by itself lead to increased sensitivity to postdeployment stressful life events but only in the presence of immune activation, as evidenced by high T-cell and innate cytokine production. Additionally, immune activation by itself is not sufficient to lead to increased reactivity to stressful life events, but only following high combat stress exposure. These findings suggest both beneficial and detrimental effects of high cytokine production, depending on the subsequent occurrence of stressful life events[26].
Heightened activity of the immune system may cause alterations in the structure and function of brain regions such as the amygdala, hippocampus, and PFC through changes in the levels of serotonin and kynurenine pathway metabolites and direct neurotoxic effects of cytokines[18]. Neurotoxic cytokine signaling may occur in conjunction with the production of reactive oxygen species (ROS). A study using a predator exposure/psychosocial stress animal model of PTSD found that proinflammatory cytokines and ROS were elevated in the amygdala, hippocampus, and PFC of the rat brain, indicating increased oxidative stress and inflammation. In addition, oxidative stress and inflammation were elevated systemically, as evidenced by increased ROS and proinflammatory cytokines in the adrenal glands and circulating blood. Importantly, within-group comparisons in the PTSD group of rats demons
Studies of TBI survivors contradict the idea that TBI and PTSD are nonoverlapping conditions, as the loss of consciousness and amnesia associated with TBI have been shown not to protect against PTSD. In a study of hospitalized trauma survivors with mild TBI, of the 55 participants with memory of the traumatic event, 13 (23%) developed PTSD, and of the 65 participants without memory of the traumatic event, there were still 4 (6%) who developed PTSD[35]. In another study of TBI patients, the duration of posttraumatic amnesia (PTA) did not significantly differ between participants with acute and delayed-onset PTSD[30]. In a prospective cohort study of traumatic injury patients, mild TBI patients were even more likely to develop PTSD at 3 mo following injury than non-TBI patients. No associations were found between the duration of PTA and PTSD symptoms, but longer PTA was associated with less severe intrusive memories at baseline[29]. The absence of early intrusive symptoms may be associated with an increased likelihood of delayed PTSD. Indeed, in long-term prospective follow-up assessments in the same cohort, in the group with no PTSD at 3 mo, PTSD severity at 24 mo was predicted by PTSD severity during hospitalization, the presence of mild TBI, and the number of days spent in the hospital[28].
In soldiers deployed to Afghanistan, TBI was found to be associated with alterations in fear learning and extinction[27]. Experiencing multiple TBIs within a 2- to 3-year time frame exacerbated conditioned fear, and elevated learned fear contributed to the risk for PTSD after TBI[27]. In combat veterans who had been deployed to Iraq or Afghanistan for over 3 mo and who had no PTSD, depression, or postconcussive syndrome within 2 mo after return, independent predictors of PTSD after one year were single nucleotide polymorphisms in the genes coding for two proteins related to neuronal recovery: myelin basic protein and brain-derived neurotrophic factor; MBP and BDNF may work in concert to protect against or enhance recovery from brain injury, thereby mediating the risk of long-term mechanical and psychological injury[36]. Additional predictive factors were elevated resting state connectivity on functional MRI between the right amygdala and left superior temporal gyrus and reduced volume on MRI of the right superior longitudinal fasciculus tract, connecting the frontal lobe with the parieto-temporal brain regions[36]. Elevated resting activity in the auditory cortex, which is part of the superior temporal gyrus, may prime the brain for enhanced vulnerability to sensory impressions.
In addition to traumatic brain injury, exposure to noxious agents may accompany psychological trauma, particularly in combat-exposed soldiers. An example was the prophylactic treatment with cholinesterase inhibitors, e.g., as prescribed during the Gulf War for protection of soldiers under threat of chemical warfare[37]. Robust elevations in acetylcholine levels occur in both PTSD and during treatment with cholinesterase inhibitors. Acetylcholine interactions with receptors induce, through a calcium-dependent mechanism, the early immediate transcription factor c-Fos. This parallels the immediate stress response, since the expression of c-Fos is drastically elevated within minutes under stress[37]. Long-term, persistent brain changes occur at a very slow pace, sometimes over years. The initial phase of the feedback response probably leads to delayed cascades of the transcription of relevant genes[37].
In a review of studies of delayed PTSD[38], several prospective studies provided evidence for stressful life events in the period following exposure to trauma to increase the risk of delayed PTSD. In a prospective cohort study of traumatic injury patients, in participants who had no PTSD at 3 mo, PTSD severity at 24 mo was predicted by the number of adverse life events after the 3 mo assessment, accounting for as much as 9% of the variance[28]. In deployed soldiers, the effects of postdeployment stressful life events on the course of PTSD symptoms after return from deployment have been shown to depend on combat stress exposure as well as immune reactivity following return from deployment[26]. Postdeployment stressors occur in a context of readjustment to civilian life that may be complicated by a gradual unfolding of symptoms. In a study of veterans and their family members following deployment-relayed traumatic brain injury[39], participants reported that veterans "downplayed" their injuries and later "detached" themselves from friends, family, and communities and "denied" or were "oblivious" to their circumstances until a "wake-up call" pushed them to "get help." Most veterans said that they simply did not see that anything was wrong, while others usually attributed their issues, at least initially, to aging, stress, being tired or overworked or as a part of readjusting to civilian life[39].
Mental and physical disorder comorbidities may need to be considered as potentially impacting the onset and course of PTSD.
Systemic dysregulation: Based on prospective studies providing substantial evidence about biological abnormalities that precede the full-blown disorder, PTSD has been conceptualized as a systemic disorder characterized by metabolic and immune dysregulations that are reflected in increased rates of cardiovascular and autoimmune disease[40]. A staging model of PTSD has been proposed[40] with the initial stages being characterized by: (1) Asymptomatic downregulation of glucocorticoid receptor sensitivity and increased amygdalar reactivity; (2) Undifferentiated symptoms of mild anxiety and distress, inflammatory cytokine activation, and decreased response inhibition in the frontal cognitive systems; and (3) Subsyndromal distress with some behavioral and functional decline, increased physiological reactivity to trauma-related stimuli and startle response, and prolonged autonomic arousal on provocation[40]. A staging model implies that although an array of putative biomarkers associated with PTSD risk and symptom progression have been identified across distinct biological domains, specific biomarkers might be relevant at one time point, e.g., heart rate immediately following trauma exposure, and not at another[24]. The severity of exposure to traumatic stress may be crucial in determining the risk of systemic comorbidity. In a long-term prospective study of combat soldiers and prisoners of war (POWs)[41], ex-POWs were almost 3 times more likely to develop metabolic syndrome than combat-exposed controls; blood levels of CRP were abnormally high in a large percentage of ex-POWs and were related to the level of physical and psychological stressors experienced during captivity. Chronic and delayed PTSD trajectories were associated with elevated CRP levels and metabolic syndrome[41].
Combat stress reaction: In a long-term prospective study of combat veterans with or without a combat stress reaction (CSR) diagnosis following participation in frontline battles with no indication of serious physical injury and other psychiatric disorders, CSR increased the risk of chronic but not delayed PTSD. Delayed PTSD, defined as onset at 2, 3, and/or 20 years after nonendorsement at year 1, was endorsed by 23.8% (n = 20) of the no-CSR group and 16.1% (n = 21) of the CSR group[42].
Dissociation: Peritraumatic dissociation, i.e., experiences of depersonalization or derealization during exposure to a traumatic event, has been suggested to predict the course of PTSD[17]. Dissociative amnesia, i.e., awareness of ‘time loss’, may occur following exposure to trauma and may exist in delayed PTSD prior to the onset of symptoms[38]. In a large cross-sectional survey of dissociative symptoms in people with PTSD[43], depersonalization and derealization were associated with high incidence of re-experiencing symptoms. Dissociation among people with PTSD has been associated with childhood onset, exposure to a high number of prior traumatic events, and childhood adversities and is not related to trauma type[43].
Depression: Depressive disorders represent stress-responsive syndromes that often cooccur with PTSD, and PTSD and depressive disorders share several overlapping symptoms. A sensitization process to depressive states has been described, which predicts that with recurrent episodes of depression, there will be a progressive diminution of the role of environmental stressors[23]. The concepts of sensitization and kindling have been extensively studied in PTSD and a range of other psychiatric disorders and highlight the commonality of etiological mechanisms, particularly with depressive disorders[23].
Alzheimer’s disease: A case report[44] described a WW2 veteran who, following several asymptomatic decades of successful adaptation to traumatic memories, developed Alzheimer’s disease and the associated cognitive autonomy loss, which subsequently led to the emergence of late-onset posttraumatic stress disorder. Animal research has provided evidence that stress biology interacts with biological mechanisms underlying neurodegenerative disease to produce comorbidities such as late-life PTSD. In a mouse model of Alzheimer’s disease[45], exposure to PTSD-like inducing trauma elevated cerebrospinal fluid beta-amyloid levels in both the short (1–2 mo) and long term (6–12 mo), and Alzheimer’s disease model mice displayed a stronger PTSD-like phenotype after trauma exposure than wild-type mice. An increase in beta-amyloid production was shown to directly activate corticotropin-releasing factor neurons to exacerbate HPA axis responses. Increased beta-amyloid levels might not only accelerate AD pathogenesis, leading to exacerbated amyloid plaque deposition, but also exacerbate chronic changes in behavior and corticosteroid regulation, resulting in a higher incidence of PTSD[45].
Other behavioral and polygenic risk factors: Polygenic risk scores based on multiple genetic variants known to contribute to psychopathology were calculated for individuals with European ancestry in a large, long-term prospective follow-up study of first responders working at the World Trade Center site (New York) following the 9/11/2001 terrorist attacks[46]. Re-experiencing, generalized anxiety, and schizophrenia polygenic risk scores were predictive of a severe PTSD symptom trajectory characterized by increasing incidence of chronic symptoms over the course of 17 years, and a depression polygenic risk score predicted a diagnosis of PTSD[46]. In a very large sample of community-dwelling women participating in the Nurses’ Health Study[47], time spent viewing TV was analyzed in relation to the onset of PTSD symptoms following exposure to trauma. Among women who developed PTSD during follow-up, a significantly steeper increase in time spent viewing TV occurred prior to the onset of PTSD symptoms compared to women who did not go on to develop PTSD symptoms following trauma exposure. Women with high PTSD symptoms reported more TV viewing than trauma-unexposed women. TV viewing following trauma exposure may therefore be a marker of vulnerability for developing PTSD and a consequence of having PTSD[47].
The neurobiological mechanisms underlying PTSD symptoms, onset, and course are heterogeneous, as they involve several interconnected systems. Studies of each of these underlying systems support their involvement in delayed reactions and/or the capacity for time-dependent increases in system reactivity. Neural mechanisms involve the neurocircuitry subserving fear conditioning, including but not limited to the hippocampus, amygdala, and prefrontal cortex. Studies in both humans and animal models consistently show time-dependent increases in activity within the neurocircuitry of fear. Neural network models emphasize the effects of iterative learning, pruning, and top-down coordination on generalization of the fear response and progressive symptom development in PTSD. Neuroendocrine mechanisms consist of autonomic nervous system responses and HPA axis responses, both of which contribute to hyperresponsiveness and sensitization to stress. Neuroinflammatory mechanisms involve immune activation due to massive psychological stress and/or the effects of traumatic brain injury, with crosstalk between the immune and endocrine systems and neurotoxic effects of excess immune system activity contributing to long-standing and delayed neuroinflammatory reactions. Finally, neurobehavioral/ contextual mechanisms involve the effects of intervening stressors, multiple traumatic exposures, and mental and physical disorder comorbidities on delayed manifestations of remote traumatic exposure.
Crucial concepts emerging from the study of neurobiological mechanisms of delayed PTSD include sensitization, kindling, and generalization. Exposure to traumatic stressors may increase an individual’s reactivity to subsequent stressors, a process that has been termed stress sensitization. The progressive development of symptoms of PTSD after exposure to traumatic events may be based on either neural, neuroendocrine, or neuroinflammatory sensitization to stress or combinations of these mechanisms. Heterogeneity in sensitization mechanisms may underlie differences with regard to the duration of the prodromal phase and/or the presence of prodromal symptoms.
Sensitization in conjunction with kindling may be linked to PTSD. Sensitization refers to externally induced reactions, e.g., flashbacks of traumatic events induced by subsequent exposure to a similar stressor, whereas kindling refers to spontaneous activity occurring in the absence of an apparent cue. Kindling may follow sensitization, when reactions are triggered by progressively less severe stressors over time and eventually occur spontaneously. Finally, generalization that may result from the pruning of dendrites within neural fear networks leads to increased responsiveness to increasingly less specific contextual cues and cross sensitization to heterotypic stressors.
Long delayed effects of traumatic exposures are likely to also involve neurobehavioral and contextual mechanisms. Exposure to specific stressful life events resembling remote traumatic events may trigger specific memories that initiate a cascade of neurobiological dysregulation characteristic of PTSD. Indeed, the impact of repeated stressor exposures and contextual reminders has been demonstrated across several human and animal studies. The effects of stressors or trauma reminders may operate in concert with comorbid mental or physical disease and the associated sense of vulnerability that could increase the salience of the triggering event(s).
These findings have implications for diagnostic assessment in both treatment and forensic settings. Delayed expression of trauma- and stressor-related disorders requires careful individual assessment of the trauma history, intervening stressors, and development of symptoms of mental and physical disorders. In addition to PTSD, other specific trauma- and stressor-related disorders and mental and physical disorder comorbidities need to be evaluated with regard to the potential causal link between traumatic exposure and delayed symptoms, while taking into account the frequently substantial etiological overlap.
Subthreshold PTSD symptoms may indicate clinically significant distress and functional impairment. Findings from a Korean cross-sectional study among 45,698 active firefighters indicated that the presence of subthreshold PTSD symptoms was associated with suicidal behavior, depression, alcohol use problems, and functional impairment[48]. Assessment of a history of TBI is mandatory in help-seeking, trauma-exposed individuals, specifically in soldiers and veterans, who are at increased risk of PTSD with delayed expression[2-4]. Foreseeable stressors and resource losses, including unemployment and physical impairments, may be an effective target for secondary prevention of psychological distress. Pharmacological prevention of PTSD following exposure to potentially traumatic events is not generally recommended, and there is insufficient evidence to recommend selective, indicated pharmacological prevention[49], with the possible exception of hydrocortisone[50], a corticosteroid drug with immunosuppressive effects. Our data provide support for exploring the preventive potential of normalizing immune reactivity by pharmacological means.
Limitations of the current review need to be considered. These include the methodological limitations of some of the included studies, such as small sample sizes that may prevent studies from obtaining sufficient statistical power to detect associations relevant to delayed PTSD, the use of self-report measures possibly leading to response biases, memory bias in studies that rely on retrospective reporting, and limited durations of follow-up that prevented the detection of long-delayed cases. Indeed, there is a paucity of long-term prospective follow-up studies investigating the impact of intervening stressors on delayed PTSD onset. Limitations of the current review include the selection of studies addressing delayed expression of PTSD and neuro
In conclusion, the capacity of PTSD to occur with delayed onset may result from the interaction of an array of underlying neurobiological mechanisms that may influence the likelihood of manifesting prodromal symptoms preceding the onset of full-blown PTSD. Highly specific contextual reminders, stressful life events or vulnerability associated with comorbid physical or mental disease may trigger the exacerbation of previously contained distress associated with traumatic memories.
Posttraumatic stress disorder (PTSD) with delayed expression occurs in people who develop PTSD at least six mo following exposure to a potentially traumatic event. During the prodromal phase or delay interval between the traumatic event and the onset of the disorder, subthreshold symptoms are often present, although long delay intervals without prodromal symptoms have rarely been reported. This study reviews neurobiological mechanisms underpinning the occurrence of a prodromal phase with or without prodromal symptoms.
Delayed expression of PTSD may present diagnostic challenges in clinical settings as well as in litigation contexts. Insight in neurobiological mechanisms is crucial to optimize diagnostic assessment and management.
To identify and characterize neurobiological mechanisms and pathways underlying delayed expression of PTSD and to obtain an overview of types of supporting evidence.
We performed a scoping review of neurobiological studies in humans and animals and reviews of such studies. Records were eligible if they reported about studies on trauma and PTSD, delayed onset, neurobiology, and causal mechanisms or risk factors.
Following the search and selection, 38 studies were included in the review. Neural, neuroendocrine, and neuroinflammatory mechanisms have been implicated in progressive PTSD symptom expression over time. Neurobehavioral and contextual pathways complement these mechanisms.
A variety of interconnected systems underlies the heterogeneity in PTSD symptom expression over time, contributing to sensitization, kindling, and generalization.
Delayed expression of trauma- and stressor-related disorders requires careful individual assessment of the trauma history, intervening stressors, and development of symptoms. Assessment of a history of TBI is mandatory in help-seeking, trauma-exposed individuals, specifically in soldiers and veterans, as this may be associated with symptom progression over time. Efforts to avert foreseeable stressors and resource losses may contribute to secondary prevention of psychological distress. Future research should explore the preventive potential of normalizing immune reactivity by pharmacological means.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Michaels TI S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC, American Psychiatric Association, 2013. [DOI] [Full Text] |
| 2. | Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164:1319-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 230] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 3. | Smid GE, Mooren TT, van der Mast RC, Gersons BP, Kleber RJ. Delayed posttraumatic stress disorder: systematic review, meta-analysis, and meta-regression analysis of prospective studies. J Clin Psychiatry. 2009;70:1572-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Utzon-Frank N, Breinegaard N, Bertelsen M, Borritz M, Eller NH, Nordentoft M, Olesen K, Rod NH, Rugulies R, Bonde JP. Occurrence of delayed-onset post-traumatic stress disorder: a systematic review and meta-analysis of prospective studies. Scand J Work Environ Health. 2014;40:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Third Edition. Washington, DC, American Psychiatric Association, 1980. |
| 6. | Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? BMC Med Res Methodol. 2018;18:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5738] [Cited by in RCA: 4607] [Article Influence: 658.1] [Reference Citation Analysis (0)] |
| 7. | Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, wk L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22118] [Cited by in RCA: 18175] [Article Influence: 2596.4] [Reference Citation Analysis (1)] |
| 8. | Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 1168] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 9. | Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 11787] [Article Influence: 1309.7] [Reference Citation Analysis (1)] |
| 10. | Wilker S, Kolassa IT. The formation of a neural fear network in posttraumatic stress disorder: Insights from molecular genetics. Clinical Psychological Science. 2013;1:452-469. [DOI] [Full Text] |
| 11. | Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 12. | Zovkic IB, Meadows JP, Kaas GA, Sweatt JD. Interindividual Variability in Stress Susceptibility: A Role for Epigenetic Mechanisms in PTSD. Front Psychiatry. 2013;4:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Cacciaglia R, Nees F, Grimm O, Ridder S, Pohlack ST, Diener SJ, Liebscher C, Flor H. Trauma exposure relates to heightened stress, altered amygdala morphology and deficient extinction learning: Implications for psychopathology. Psychoneuroendocrinology. 2017;76:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Admon R, Leykin D, Lubin G, Engert V, Andrews J, Pruessner J, Hendler T. Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Hum Brain Mapp. 2013;34:2808-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Ardi Z, Ritov G, Lucas M, Richter-Levin G. The effects of a reminder of underwater trauma on behaviour and memory-related mechanisms in the rat dentate gyrus. Int J Neuropsychopharmacol. 2014;17:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | McFarlane AC, Yehuda R, Clark CR. Biologic models of traumatic memories and post-traumatic stress disorder. The role of neural networks. Psychiatr Clin North Am. 2002;25:253-270, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | McFarlane AC. Posttraumatic stress disorder: a model of the longitudinal course and the role of risk factors. J Clin Psychiatry. 2000;61 Suppl 5:15-20; discussion 21. [PubMed] |
| 18. | Kim YK, Amidfar M, Won E. A review on inflammatory cytokine-induced alterations of the brain as potential neural biomarkers in post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Gandubert C, Scali J, Ancelin ML, Carrière I, Dupuy AM, Bagnolini G, Ritchie K, Sebanne M, Martrille L, Baccino E, Hermès A, Attal J, Chaudieu I. Biological and psychological predictors of posttraumatic stress disorder onset and chronicity. A one-year prospective study. Neurobiol Stress. 2016;3:61-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Vaiva G, Boss V, Molenda S, Rosenstrauch C, Ducrocq F, Fontaine M. Taux plasmatiques de GABA: au decours d'un psychotrauma et survenue de troubles psychotraumatiques. Revue Francophone du Stress et du Trauma;. 5:131-139. |
| 21. | Busso DS, McLaughlin KA, Sheridan MA. Media exposure and sympathetic nervous system reactivity predict PTSD symptoms after the Boston marathon bombings. Depress Anxiety. 2014;31:551-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Belda X, Fuentes S, Daviu N, Nadal R, Armario A. Stress-induced sensitization: the hypothalamic-pituitary-adrenal axis and beyond. Stress. 2015;18:269-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | McFarlane A. The delayed and cumulative consequences of traumatic stress: Challenges and issues in compensation settings. Psychological Injury and Law. 2010;3:100-110. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Michopoulos V, Norrholm SD, Jovanovic T. Diagnostic Biomarkers for Posttraumatic Stress Disorder: Promising Horizons from Translational Neuroscience Research. Biol Psychiatry. 2015;78:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Serova LI, Nwokafor C, Van Bockstaele EJ, Reyes BAS, Lin X, Sabban EL. Single prolonged stress PTSD model triggers progressive severity of anxiety, altered gene expression in locus coeruleus and hypothalamus and effected sensitivity to NPY. Eur Neuropsychopharmacol. 2019;29:482-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Smid GE, van Zuiden M, Geuze E, Kavelaars A, Heijnen CJ, Vermetten E. Cytokine production as a putative biological mechanism underlying stress sensitization in high combat exposed soldiers. Psychoneuroendocrinology. 2015;51:534-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Glenn DE, Acheson DT, Geyer MA, Nievergelt CM, Baker DG, Risbrough VB; MRS-II Team. Fear learning alterations after traumatic brain injury and their role in development of posttraumatic stress symptoms. Depress Anxiety. 2017;34:723-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Bryant RA, O'Donnell ML, Creamer M, McFarlane AC, Silove D. A multisite analysis of the fluctuating course of posttraumatic stress disorder. JAMA Psychiatry. 2013;70:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Bryant RA, Creamer M, O'Donnell M, Silove D, Clark CR, McFarlane AC. Post-traumatic amnesia and the nature of post-traumatic stress disorder after mild traumatic brain injury. J Int Neuropsychol Soc. 2009;15:862-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Alway Y, Gould KR, McKay A, Johnston L, Ponsford J. The Evolution of Post-Traumatic Stress Disorder following Moderate-to-Severe Traumatic Brain Injury. J Neurotrauma. 2016;33:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | do Prado CH, Grassi-Oliveira R, Daruy-Filho L, Wieck A, Bauer ME. Evidence for Immune Activation and Resistance to Glucocorticoids Following Childhood Maltreatment in Adolescents Without Psychopathology. Neuropsychopharmacology. 2017;42:2272-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, Goldmann E, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2010;107:9470-9475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 366] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 33. | Wang XW, Karki A, Du DY, Zhao XJ, Xiang XY, Lu ZQ. Plasma levels of high mobility group box 1 increase in patients with posttraumatic stress disorder after severe blunt chest trauma: a prospective cohort study. J Surg Res. 2015;193:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS One. 2013;8:e76146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Gil S, Caspi Y, Ben-Ari IZ, Koren D, Klein E. Does memory of a traumatic event increase the risk for posttraumatic stress disorder in patients with traumatic brain injury? Am J Psychiatry. 2005;162:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Roy MJ, Costanzo M, Gill J, Leaman S, Law W, Ndiongue R, Taylor P, Kim HS, Bieler GS, Garge N, Rapp PE, Keyser D, Nathan D, Xydakis M, Pham D, Wassermann E. Predictors of Neurocognitive Syndromes in Combat Veterans. Cureus. 2015;7:e293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Soreq H. Gulf War syndrome, psychological and chemical stressors. In: G Fink, editor. Encyclopedia of Stress (Second Edition). New York: Academic Press; 2007. [DOI] [Full Text] |
| 38. | Smid GE, Van Der Mast RC, Gersons BPR. De uitgestelde posttraumatische stressstoornis. Tijdschrift voor Psychiatrie. 2003;45:265-276. |
| 39. | Chase RP, McMahon SA, Winch PJ. Injury careers after blast exposure among combat veterans deployed to Iraq or Afghanistan. Soc Sci Med. 2015;147:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | McFarlane AC, Lawrence-Wood E, Van Hooff M, Malhi GS, Yehuda R. The Need to Take a Staging Approach to the Biological Mechanisms of PTSD and its Treatment. Curr Psychiatry Rep. 2017;19:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Solomon Z, Levin Y, Assayag EB, Furman O, Shenhar-Tsarfaty S, Berliner S, Ohry A. The Implication of Combat Stress and PTSD Trajectories in Metabolic Syndrome and Elevated C-Reactive Protein Levels: A Longitudinal Study. J Clin Psychiatry. 2017;78:e1180-e1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Solomon Z, Mikulincer M. Trajectories of PTSD: a 20-year longitudinal study. Am J Psychiatry. 2006;163:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Stein DJ, Koenen KC, Friedman MJ, Hill E, McLaughlin KA, Petukhova M, Ruscio AM, Shahly V, Spiegel D, Borges G, Bunting B, Caldas-de-Almeida JM, de Girolamo G, Demyttenaere K, Florescu S, Haro JM, Karam EG, Kovess-Masfety V, Lee S, Matschinger H, Mladenova M, Posada-Villa J, Tachimori H, Viana MC, Kessler RC. Dissociation in posttraumatic stress disorder: evidence from the world mental health surveys. Biol Psychiatry. 2013;73:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 44. | Monfort E, Trehel G. État de stress post-traumatique consécutif à une maladie d'Alzheimer: émergence d'une pathologie sous-jacente dans le très grand âge [Post-traumatic stress disorder secondary to Alzheimer's disease: Emergence of an underlying pathology in the oldest old]. Annales Medico-Psychologiques. 2017;175:776-780. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Justice NJ, Huang L, Tian JB, Cole A, Pruski M, Hunt AJ Jr, Flores R, Zhu MX, Arenkiel BR, Zheng H. Posttraumatic stress disorder-like induction elevates β-amyloid levels, which directly activates corticotropin-releasing factor neurons to exacerbate stress responses. J Neurosci. 2015;35:2612-2623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 46. | Waszczuk MA, Docherty AR, Shabalin AA, Miao J, Yang X, Kuan PF, Bromet E, Kotov R, Luft BJ. Polygenic prediction of PTSD trajectories in 9/11 responders. Psychol Med. 2020;1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Jung SJ, Winning A, Roberts AL, Nishimi K, Chen Q, Gilsanz P, Sumner JA, Fernandez CA, Rimm EB, Kubzansky LD, Koenen KC. Posttraumatic stress disorder symptoms and television viewing patterns in the Nurses' Health Study II: A longitudinal analysis. PLoS One. 2019;14:e0213441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Kim JI, Oh S, Park H, Min B, Kim JH. The prevalence and clinical impairment of subthreshold PTSD using DSM-5 criteria in a national sample of Korean firefighters. Depress Anxiety. 2020;37:375-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Department of Veterans Affairs/Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder. Washington, DC, Department of Veterans Affairs / Department of Defense, 2017. [cited 20 February 2021]. Available from: https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf. |
| 50. | International Society for Traumatic Stress Studies. ISTSS Posttraumatic Stress Disorder Prevention and Treatment Guidelines: Methodology and Recommendations. Chicago, Il, ISTSS, 2018. [cited 20 February 2021]. Available from: https://istss.org/getattachment/Treating-Trauma/New-ISTSS-Prevention-and-Treatment-Guidelines/ISTSS_PreventionTreatmentGuidelines_FNL-March-19-2019.pdf.aspx. |