Published online Jul 19, 2021. doi: 10.5498/wjp.v11.i7.355
Peer-review started: February 25, 2021
First decision: April 20, 2021
Revised: April 22, 2021
Accepted: June 22, 2021
Article in press: June 22, 2021
Published online: July 19, 2021
Processing time: 139 Days and 19.1 Hours
The dopamine hypothesis of how antipsychotic drugs exert their beneficial effect in psychotic illness has an interesting history that dates back to 1950. This hypothesis is not to be confused with the dopamine hypothesis of schizophrenia; the aim of the latter is to explain the etiology of schizophrenia. The present review does not deal with schizophrenia but, rather, with the historical development of our current understanding of the dopamine-associated actions of the drugs that reduce the symptoms of psychosis. This historical review begins with the serendipitous discovery of chlorpromazine, a drug synthesized around a chemical core that initially served to produce man-made dyes. This molecular core subsequently contributed to the chemistry of antihistamines. It was with the aim of producing a superior antihistamine that chlorpromazine was synthesized; instead, it revolutionized the treatment of psychosis. The first hypothesis of how this drug worked was that it induced hypothermia, a cooling of the body that led to a tranquilization of the mind. The new, at the time, discoveries of the presence of chemical transmitters in the brain soon steered investigations away from a temperature-related hypothesis toward questioning how this drug, and other drugs with similar properties and effects, modulated endogenous neurotransmission. As a result, over the years, researchers from around the world have begun to progressively learn what antipsychotic drugs do in the brain.
Core Tip: This history starts with the synthesis of chlorpromazine in 1950 and traces the steps taken to discover how this drug, and related drugs, work to reduce, sometimes to reverse, the delusions and hallucinations associated with psychosis. The task to understand how these drugs work in the brain continues, as many unknowns remain.
- Citation: Seeman MV. History of the dopamine hypothesis of antipsychotic action. World J Psychiatr 2021; 11(7): 355-364
- URL: https://www.wjgnet.com/2220-3206/full/v11/i7/355.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i7.355
The synthesis of chlorpromazine in 1950 marks the beginning of modern psychopharmacology. While the clinical usefulness of this drug was almost immediately recognized, it took another 20 years to begin to uncover its mode of action. This review covers the history of these years and the steps that were taken to arrive at the dopamine hypothesis of antipsychotic drug action. It more briefly also outlines how this hypothesis has fared over the ensuing years (Table 1).
| Year | Major Advances |
| 1950 | Synthesis of chlorpromazine[3] |
| 1952 | Preliminary evidence of antipsychotic effect of chlorpromazine[6,7,11] |
| 1958 | Synthesis of haloperidol[16] |
| 1960 | Parkinson basal ganglia are deficient in dopamine[19] |
| 1963 | Neuroleptics raise level of monoamine metabolites[18] |
| 1966 | Neuroleptics may antagonize dopamine receptors[26] |
| 1971 | 2 nmol haloperidol in plasma effective in psychosis[34] |
| 1974 | Synthesis of (+-) butaclamol[36] |
| 1975 | Tritiated haloperidol binds DA receptors[38] |
| 1975 | Effective neuroleptic dose correlates with D2 block[39] |
| 1979 | Multiple dopamine receptors[54] |
| 1984 | Bimodal D2 distribution in schizophrenia[45] |
| 1984 | High and low affinity states for D2[58] |
| 1988 | Cloning of the D2 receptor[63] |
| 1988 | In vivo imaging of D2 occupancy[47] |
| 1990 | Cloning of D3[67] |
| 1999 | Fast-off theory[71] |
| 2000 | Multiple genetic variants of D2 receptor[73-75] |
| 2000 | Impact of the D3 receptor[81] |
| 2005 | Impact of other neurotransmitter receptors[83] |
| 2010 | Impact of receptor heterodimers[85] |
| 2017 | Impact of D2 high affinity state[77] |
| 2021 | Structure and specificities of D1, D2 signaling complexes[79] |
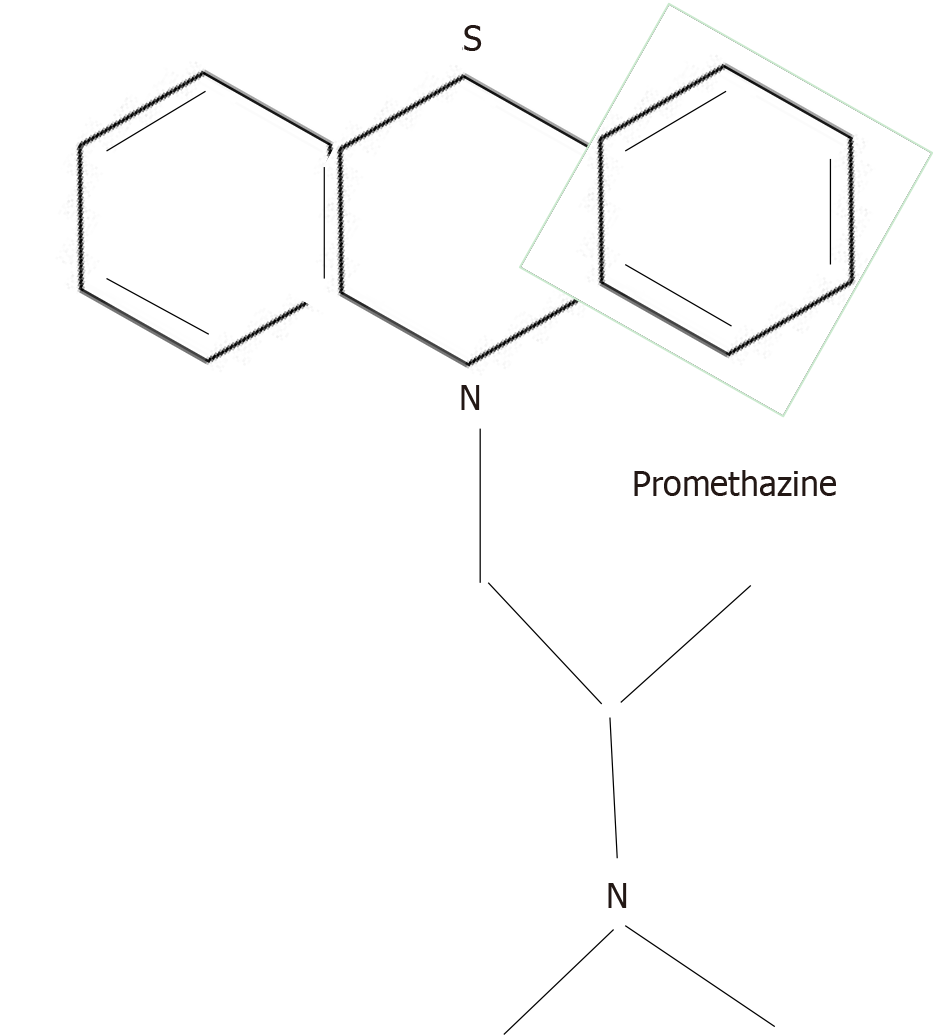
Prior to the availability of chlorpromazine, many drugs had been used in psychiatry to tranquilize agitated patients, but this was the first psychoactive agent to not only calm patients, but also to decrease the intensity of their psychotic symptoms. Synthesized by Paul Charpentier of the French pharmaceutical company Rhône-Poulenc, chlorpromazine was the product of a long process that began in the mid-1800s, starting out as a search for a method to synthesize dyes. This search led, in 1883, to the identification by August Bernthsen of a molecular structure, which he called a phenothiazine nucleus, around which later generations of chemists began to make antihistamine drugs for the treatment of allergies. In 1947, the pharmaceutical company, Rhône-Poulenc, produced promethazine, a first generation antihistamine[1] (Figure 1).
Promethazine induced hypothermia in laboratory animals so, in 1949, French military surgeon, Laborit[2] tried giving it to soldiers in order to lower their body temperature and prevent shock before, during, and after surgical operations. He noted that promethazine induced a “euphoric quietude” in his soldiers[2].
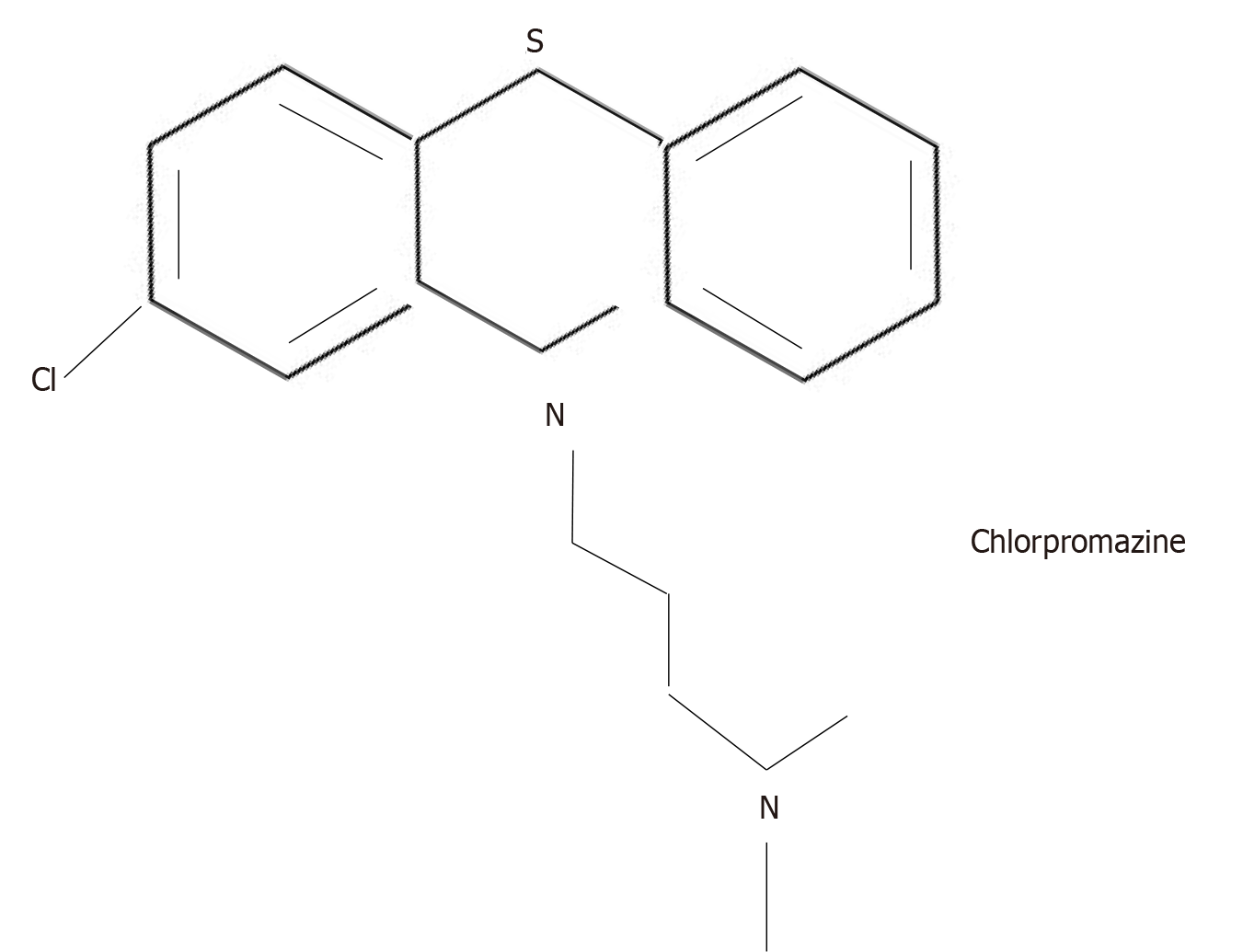
Hoping to increase the potency of promethazine, on December 11, 1950, Charpentier et al[3] introduced a chlorine atom into one of the rings of promethazine (Figure 2).
The new drug was called RP (for Rhône-Poulenc) 4560. It was tested on rats and produced “detachment, slow reaction to stimuli, and a decrease in initiative.” The compound was again sent for human trials to Laborit, now at the Val de-Grâce Hospital in Paris, and, because of its psychological effects in animals, to a variety of French psychiatrists as well.
The first published report of its effect in humans was by Laborit and his team in February 1952. They reported that the drug calmed anxious patients without producing oversedation[4]. Three psychiatrists reported on the effect of the drug one month later[5]. This team concluded that 50-100 mg of RP4560, diluted in a glucose solution and given intravenously to patients with mania, kept them calm for 3 to 18 h, as long as an analgesic or a barbiturate was administered concurrently.
In May 1952, psychiatrists Delay and Deniker[6] published their observation on the soothing effect of the drug in patients with psychosis[6]. In June, this team once again reported positive results[7]. Going on the theory that the drug worked by cooling the body, a therapeutic intervention commonly used by psychiatrists at the time[8-10], in July, Delay et al[11] published more detailed results of 8 cases treated with RP4560[11]. They diagnosed six of these patients as suffering from acute mania, one was said to show “excited delirium.” One patient showed “recurrent excitement” and was described as having “trouble thinking” and using “over rationalization.” In today’s classification systems, this patient might be diagnosed with schizophrenia. In this clinical group’s hands, RP4560 was administered without adjuncts, by injection, with oral tablets substituted for injections usually by the 10th treatment day. The results were remarkable–calm was induced in all patients, with minimum sedation. This created such a sensation in psychiatric circles that, by November 1952, the drug had become available by prescription in France[12].
The pharmaceutical firm Smith Klein and French bought the American rights to the drug and, in 1954, received Food and Drug Administration approval to market it in the United States under the name, Thorazine. The advertisement in the May 1954 issue of the American Journal of Psychiatry read: “Thorazine is useful in controlling anxiety, tension, agitation, confusion, delirium, or hostility, whether occurring in schizophrenic, manic-depressive, toxic, or functional states.” [1]. In France and also in Canada[13-15], the drug was called Largactil. Since it was mainly used in hospitalized patients who, for the most part, suffered from psychotic disorders, it swiftly gained a worldwide reputation for being able to reverse the symptoms of psychosis.
The phenothiazine core molecule was malleable and comparably easy to copy so that compounds with very similar efficacy were readily produced. By 1964, a variety of pharmaceutical companies had synthesized and marketed their own phenothiazines: promazine, triflupromazine, methoxypromazine, trifluoperazine, fluphenazine, thioridazine, and prochlorperazine. In 1958, haloperidol, a non-phenothiazine drug [a butyrophenone synthesized on the base of an opioid analgesic, meperidine (Demerol)] was created by pharmaceutical genius, Paul Janssen. This compound proved to be more potent against delusions and hallucinations than the phenothiazines[16].
Both phenothiazines and butyrophenones were initially called ‘major tranquilizers’ to distinguish them from the ‘minor tranquilizer,’ meprobamate[17], which was being widely marketed at the time for anxiety.
Searching for the mode of action of major tranquilizers, in 1963, Carlsson and Londqvist[18] reported that this category of drugs increased the level of metabolites of catecholamines. They suggested ‘‘. . . that chlorpromazine and haloperidol block monoaminergic receptors in brain . . . .” It was not possible at the time to selectively distinguish among alpha-adrenoceptors, beta-adrenoceptors, and dopamine receptors.
There were, nevertheless, several reasons to believe that it was the dopamine pathway that was involved. Firstly, the clinical side effects of chlorpromazine and haloperidol were tremor, rigidity, and akinesia -e.g. Parkinsonian signs and, by then, Parkinson’s disease had been linked to a deficiency of dopamine[19]. Secondly, it had already been suggested that dopamine-mimetic drugs such as amphetamine acted via dopamine receptors[20] and that amphetamines could induce schizophrenia-like psychotic symptoms in patients[21,22]. Disulfiram (Antabuse), in clinical use to prevent alcohol addiction, was known to inhibits dopamine beta-hydroxylase, the enzyme that converts dopamine to noradrenaline, and this drug, too, was capable of inducing psychosis[23].
There were also reports of chlorpromazine accelerating the turnover of dopamine[24]. Van Rossum[25] had noticed in 1965 that major tranquilizers (or neuroleptics as they were called by this time), though not, as was first thought, particularly antiadrenergic, were, instead, potent amphetamine antagonists[25].
The significance of dopamine for the action of antipsychotic drugs, a breakthrough often attributed to Arvid Carlsson (who did not seriously consider dopamine in the context of the action of these drugs until much later) was first formulated by Jacques van Rossum. In 1966, van Rossum[26] hypothesized that dopamine receptor blockade was a likely explanation for the mechanism of action of this group of drugs. He referred to neuroleptics as the first available dopamine antagonists. In 1967, van Rossum[27] wrote: “When the hypothesis of dopamine blockade by neuroleptic agents can be further substantiated, it may have far going consequences for the pathophysiology of schizophrenia. Overstimulation of dopamine receptors could then be part of the aetiology. Obviously such an overstimulation might be caused by overproduction of dopamine, production of substances with dopamine actions (methoxy derivatives), abnormal susceptibility of the receptors, etc.”[27].
Van Rossum, thus, formulated two dopamine hypotheses (1) The hypothesis that dopamine receptor blockade was responsible for the antipsychotic effects of drugs like chlorpromazine, haloperidol, and similar drugs; and (2) The hypothesis that an excess of dopamine might be part of the etiology of schizophrenia. These two distinct hypotheses are often conflated[28].
They are conflated because, in the 1970s, it was hoped that the discovery of how neuroleptics work could lead to understanding the nature of schizophrenia itself. Based on the fact that amphetamine releases dopamine and amphetamine-induced psychosis is clinically very similar to an acute episode of schizophrenia[29], van Rossum[27] thought that schizophrenia might be due to an overproduction of dopamine[27].
From today’s standpoint, overactivity of dopamine, while possibly explaining the hallucinations and delusions of schizophrenia, does not shed light on the more fundamental negative and cognitive symptoms of schizophrenia. Over the years, many attempts have been made to elaborate and expand on van Rossum’s dopamine hypothesis of schizophrenia to account for symptoms other than delusions and hallucinations[30,31]. It remains the case, however, that schizophrenia is too multifaceted and heterogeneous a disorder to be fully explained by dopamine overactivity alone. That being said, it is still possible that the secretion and transmission of dopamine serves as a final common pathway to the expression of specific schizophrenia symptoms[32].
Van Rossum’s first hypothesis-that neuroleptics (today referred to as antipsychotics) exert their effect through dopamine receptors–has enjoyed a longer life than his second. In 2020, Kaar et al[33], in their review of mechanisms underlying clinical response to antipsychotics, conclude that “all currently licensed antipsychotic drugs show appreciable binding to dopamine D2 receptors at therapeutic doses, and this action is core to their therapeutic action.”
Van Rossum[25-27] had pointed out that overstimulation by dopamine could result from a number of potential causes, from overproduction by the secreting cell to oversensitivity of receptors on the post-synaptic cell. By extension, antipsychotic drugs could theoretically act by blocking dopamine synthesis or secretion or by interfering with its transport across the synapse or by blocking membrane receptors.
In 1971, Zingales[34] reported that the concentration of haloperidol in the plasma of treated patients was approximately 3 nanograms per millilitre of plasma (3 nmol)[34]. Because over 90% of haloperidol in plasma is bound to plasma proteins, the actual free concentration that enters the brain would then have to be approximately 1 nmol. This was a problem for the radioactive tagging needed in the search for specific targets of haloperidol action. The classical way to find a drug target was to tag the drug with a radioactive marker. In this case, however, because the drug needed to be diluted down to 1 nmol and still have enough radioactivity left for the experiment to succeed, the radioactive label had to be extra powerful. No such label existed at the time.
In November 1971, Philip Seeman, a Toronto physician/pharmacologist, asked Paul Janssen to persuade the company, I.R.E. Belgique, to prepare radioactive haloperidol at a high specificity of 10.5 Curies per millimole, which the company succeeded in doing in 1974.
True drug receptor targets have to take up the radioactively labelled ligand; a second important criterion in identifying a specific site of action is stereoselectivity–the configuration of the relevant molecule must fit the configuration of the target[35]. Seeman obtained mirror image antipsychotic molecules (+butaclamol and -butaclamol), the first one active, the second inactive[36,37]. A specific antipsychotic target was confirmed when the site was blocked by +butaclamol to a significantly greater degree than it was by -butaclamol. This more or less settled the identity of the antipsychotic receptor. A further step was to see which of the endogenous neurotransmitters had the most affinity for this location. When tested against noradrenaline, acetylcholine, serotonin, and dopamine, dopamine proved to be the most potent. This meant that the antipsychotic receptor was a dopamine receptor[38].
Soon after discovering the receptor, Seeman et al[39,40] showed that the published clinical doses of all antipsychotic drugs available at the time, regardless of their molecular structure, directly correlated with their ability to displace radioactive haloperidol[39,40]. This graph has recently been called “the most famous graph in schizophrenia therapeutics.”[41]. The findings from the Seeman laboratory were soon confirmed by binding studies from other labs[42-44]. A further confirmatory finding was that treatment with antipsychotic drugs increased the density of dopamine receptors in post mortem brain tissue of individuals with schizophrenia[45].
In vivo molecular imaging studies were not initially available but, when they were, they were eventually able to confirm striatal dopamine D2 receptor blockade at clinically effective doses of all antipsychotic drugs, including first and second-generation agents and dopamine partial agonists[46-52]. The initial molecular imaging studies in patients with treated schizophrenia suggested a therapeutic window (relatively good response without unacceptable extrapyramidal adverse effects) of between 60% and 80% D2 receptor occupancy. The definition of ‘response,’ of course, varies and non-response did not necessarily correlate with low occupancy rates[53].
Since then, the field of dopamine receptors has considerably expanded[54]. The receptor labeled by [3H] haloperidol was called D2[55,56], because, by the mid 1980s, five dopamine receptors, all belonging to a G-protein coupled set of receptors, had been isolated. Today, D1 and D5 are known to stimulate the cyclic adenosine monophosphate signaling pathway through G_s G-proteins, whereas D2R, D3, and D4 inhibit this signal via G_i/o G-proteins[57]. Moreover, each of these receptors can exist, as can all G-protein linked receptors, in a state of high or low affinity for their ligand[58,59]. Of the 5 known dopamine receptors, D1, D4, and D5 were cloned in the Seeman laboratory[60-62].
The dopamine D2 receptor was cloned in 1988 in the Civelli lab[63,64]. In 1989, Grandy et al[65] used in situ hybridization to map the gene to the 11q22–q23 junction.
Because of the excellent correlation between the affinity for striatal D2 receptors and the average clinical dose of antipsychotic drugs given to patients with schizophrenia, there was at first general agreement that all effective antipsychotic drugs must act by not only blocking dopamine D2 receptors in the striatum, but also blocking them in the mesolimbic system, where symptoms of psychosis are thought to originate[66].
In 1990, the D3 receptor (closely related to D2) was cloned in the laboratory of Jean-Claude Schwartz[67].
Soon after, the 2nd generation antipsychotic drugs were brought to market, and they appeared to have much lower affinity for the D2/D3 receptors (they induced far fewer extrapyramidal symptoms) but to be just as potent against psychotic symptoms (delusions and hallucinations) as the older drugs. Clozapine, in particular, the best antipsychotic in that patients resistant to all other drugs often respond when prescribed clozapine, attached to many neurotransmitter receptors besides D2/D3[68]. Many of the new drugs[69], including clozapine[70] had affinity for serotonin 2AR, which was thought explain their much lower relative rate of extrapyramidal effects.
Another explanation was that the antipsychotics drugs that do not elicit extrapyramidal symptoms, such as clozapine and quetiapine, bind to the D2 receptor more loosely than dopamine itself so that endogenous dopamine displaces them very quickly from the target receptor. Drugs that bind most tightly to the D2 receptor (chlorpromazine, trifluoperazine, fluphenazine, haloperidol, risperidone) stay on the receptor for 20-30 min and it is this long continuous occupation that may be responsible for parkinsonism[71,72]. This explanation suggests that, though a certain threshold percentage of D2 receptors still need to be bound in order to obtain an antipsychotic effect, the binding need not be of long duration. ‘Hit and run’ or ‘fast-off’ binding is able to prevent some of the adverse effects while still maintaining efficacy against psychosis.
An unresolved continuing problem with respect to antipsychotic drug action is that at least one third of patients with schizophrenia do not respond to drugs that block D2, whether transiently or for long periods, whether with or without serotonin 2A binding. One possible explanation is that individuals inherit different genetic variants of the D2 receptor[73-76], and that these variants determine response. Since the functional state of the dopamine receptor in the anterior pituitary[58], and perhaps everywhere in the brain[77], is its high affinity form, it is perhaps the relative duration of time that these receptors spend in their various affinity states that determines the extent of clinical response. It has been hypothesized that an interaction between D1 and D2 receptors influences the time spent in the high affinity functional state[78]. Every year, more knowledge accumulates about the signaling complexes of D1 and D2 receptors[79] and new radioactive ligands are available that bind specifically to high affinity sites[80].
Although binding to the D2 receptor continues to be considered as the cornerstone of antipsychotic action, the original hypothesis has undergone several refinements, such as the acknowledgement that other dopamine receptors as well as other neurotransmitter receptors play a part[81-84]. There now exist effective antipsychotic drugs that defy the earlier established D2 receptor occupancy threshold, which makes it difficult to attribute antipsychotic effect to any single neurotransmitter receptor[85-87].
Dopamine D2 receptor blockade remains necessary in order to obtain antipsychotic response in most patients. Individuals differ, however, and it remains possible, even probable, that specific subgroups of patients showing psychotic symptoms may respond most robustly to pharmaceutical agents that mainly affect brain chemical transmitters other than dopamine. Pimavanserin, for instance, a serotonin 2A receptor antagonist, has had some success in treating the psychosis associated with Parkinson’s disease, a condition of dopamine deficiency[88], but the Food and Drug Administration in the United States has recently found it insufficiently effective for the psychosis associated with Alzheimer’s dementia. Differently caused psychoses may respond to differently configured drugs. Looking for the mechanism of action of drugs for psychosis continues, and, as new mechanisms are found, the secrets of the multiple causes of psychotic disorders may be decoded.
Thank you to Dr. Gary Remington for suggestions and editing.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soriano-Ursúa MA S-Editor: Fan JR L-Editor: A P-Editor: Wang LYT
| 1. | Braslow JT, Marder SR. History of psychopharmacology. Annu Rev Clin Psychol. 2019;15:25-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Laborit H. [Neuro-vegetative therapy for shock and post-traumatic disease]. Presse Med. 1949;58:138-140. [PubMed] |
| 3. | Charpentier P, Gailliot P, Jacob R, Gaudechon J, Buisson P. [Research on substituted dimethylaminopropyl-N phenothiazines]. C R Acad Sci III (Paris). 1952;235:59-60. |
| 4. | Laborit H, Huguenard P, Alluaume R. [A new vegetative stabilizer; RP4560]. Presse Med. 1952;60:206-208. [PubMed] |
| 5. | Hamon, Paraire, Velluz. [Effect of RP4560 on maniacal agitation]. Ann Med Psychol (Paris). 1952;110:331-335. [PubMed] |
| 6. | Delay J, Deniker P. [Prolonged and continuous treatment of psychosis by a neuroleptic method solely derived from hibernotherapy]. CR Congr Méd Alién Neurol. 1952;50:497-502. |
| 7. | Delay J, Deniker P, Harl JM. [Therapeutic use in psychiatry of phenothiazine of central elective action (RP4560 )]. Ann Med Psychol (Paris). 1952;110:112-117. [PubMed] |
| 8. | Broggi E. [Results of the national convention held at Vercelli on September 24-25 on artificial hibernation, prolonged sleep and tranquilizing agents in neuropsychiatry]. Encephale. 1956;45:1035-1041. [PubMed] |
| 9. | Navarrane P, Picard P. [Some reflections on the applications of artificial hibernation in neuro-psychiatry]. Encephale. 1956;45:456-457. [PubMed] |
| 10. | Neveu P. [Hibernation, emergency treatment of syndromes of decompensated shock in psychiatry]. Encephale. 1956;45:377-381. [PubMed] |
| 11. | Delay J, Deniker P, Harl JM. [Therapeutic method derived from hibernotherapy in excitation and agitation states]. Ann Med Psychol (Paris). 1952;110:267-273. [PubMed] |
| 12. | Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3:495-500. [PubMed] |
| 13. | Koeppe R. Largactil—some preliminary clinical observations. Meeting of the Ontario Neuropsychiatric Association at Ontario Hospital; 27 November 1953; Whitby (ON), 1953. |
| 14. | Lehmann HE, Hanrahan GE. Chlorpromazine; new inhibiting agent for psychomotor excitement and manic states. AMA Arch Neurol Psychiatry. 1954;71:227-237. [PubMed] |
| 15. | Saucier R, Fischmeister LV. L’emploi du Largactil en psychiatrie. [The use of Largactil in psychiatry]. Saguenay Médical. 1954;3:226-231. |
| 16. | Janssen PA, Van de Westeringh C, Jageneau AH, Demoen PJ, Hermans BK, Van Daele GH, Schellekens KH, Van Der Eycken CA. Chemistry and pharmacology of CNS depressants related to 4-(4-hydroxy-phenylpiperidino)butyrophenone. I. Synthesis and screening data in mice. J Med Pharm Chem. 1959;1:281-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Berger FM. The pharmacological properties of 2-methyl-2-n-propyl-1,3-propanediol dicarbamate (Miltown), a new interneuronal blocking agent. J Pharmacol Exp Ther. 1954;112:413-423. [PubMed] |
| 18. | Carlsson A, Londqvist M. Effect of chlorpromazine or haloperidol on formation of 3methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol (Copenh). 1963;20:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1590] [Cited by in RCA: 1330] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 19. | Ehringer H, Hornykiewicz O. [Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system]. Klin Wochenschr. 1960;38:1236-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1470] [Cited by in RCA: 1262] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 20. | Van Rossum JM, Hurkmans AT. Mechanism of action of psychomotor stimulant drugs. significance of dopamine in locomotor stimulant action. Int J Neuropharmacol. 1964;3:227-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 21. | Beamish P, Kilioh LG. Psychoses due to amphetamine consumption. J Ment Sci. 1960;106:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Bennett AE, Mckeever LG, Turk RE. Psychotic reactions during tetraethylthiuramdisulfide (antabuse) therapy. J Am Med Assoc. 1951;145:483-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Da Prada M, Pletscher A. Acceleration of the cerebral dopamine turnover by chlorpromazine. Experientia. 1966;22:465-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 69] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | van Rossum JM. Different types of sympathomimetic alpha-receptors. J Pharm Pharmacol. 1965;17:202-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 91] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | van Rossum JM. The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch Int Pharmacodyn Ther. 1966;160:492-494. [PubMed] |
| 27. | van Rossum JM. In: Proceedings of the fifth international congress of the Collegium Internationale neuro-psycho-pharmacologicum, Washington, DC. March 1966 (Brill H. ed.), Excerpta Medica Foundation, Amsterdam, 1967: 321-329.. |
| 28. | Kendler KS, Schaffner KF. The dopamine hypothesis of schizophrenia: An historical and philosophical analysis. Philosophy, psychiatry, psychology. Johns Hopkins University Press. 2011;18:41-63. [DOI] [Full Text] |
| 29. | Snyder SH. Amphetamine psychosis: a "model" schizophrenia mediated by catecholamines. Am J Psychiatry. 1973;130:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 383] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1223] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 31. | Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2033] [Cited by in RCA: 1825] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 32. | Seeman P. All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS Neurosci Ther. 2011;17:118-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 33. | Kaar SJ, Natesan S, McCutcheon R, Howes OD. Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. 2020;172:107704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 224] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 34. | Zingales IA. A gas chromatographic method for the determination of haloperidol in human plasma. J Chromatogr. 1971;54:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | McConathy J, Owens MJ. Stereochemistry in Drug Action. Prim Care Companion J Clin Psychiatry. 2003;5:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Humber LG, Bruderlein F. Butaclamol hydrochloride, a novel neuroleptic agent. Part I Synthesis and stereochemistry. Proceedings of the 167th American Chemical Society Meeting for Medicinal Chemistry, abstract #5, American Chemical Society, Washington. DC, 1974.. |
| 37. | Voith K. Butaclamol hydrochloride, a novel neuroleptic agent. Part II Pharmacology. Proceedings of the 167th American Chemical Society Meeting for Medicinal Chemistry, abstract #6, American Chemical Society, Washington, DC, 1974.. |
| 38. | Seeman P, Chau-Wong M, Tedesco J, Wong K. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Natl Acad Sci USA. 1975;72:4376-4380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 382] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 39. | Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 569] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 40. | Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1198] [Cited by in RCA: 1035] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 41. | Tricklebank MD, Tamminga C, Grottick A, Llorca PM, Gatti McArthur S, Martel JC. Editorial: Dopaminergic Alterations in Schizophrenia. Front Neurosci. 2021;15:663245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Burt DR, Creese I, Snyder SH. Properties of [3H]haloperidol and [3H]dopamine binding associated with dopamine receptors in calf brain membranes. Mol Pharmacol. 1976;12:800-812. [PubMed] |
| 43. | Creese I, Burt DR, Snyder SH. Dopamine receptor binding: differentiation of agonist and antagonist states with 3H-dopamine and 3H-haloperidol. Life Sci. 1975;17:933-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 275] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 1424] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 45. | Seeman P, Ulpian C, Bergeron C, Riederer P, Jellinger K, Gabriel E, Reynolds GP, Tourtellotte WW. Bimodal distribution of dopamine receptor densities in brains of schizophrenics. Science. 1984;225:728-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 226] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Farde L, Nordström AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 844] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 47. | Farde L, Wiesel FA, Halldin C, Sedvall G. Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry. 1988;45:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 404] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 48. | Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 146] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Kapur S, Zipursky R, Roy P, Jones C, Remington G, Reed K, Houle S. The relationship between D2 receptor occupancy and plasma levels on low dose oral haloperidol: a PET study. Psychopharmacology (Berl). 1997;131:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Nordström AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C, Uppfeldt G. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry. 1993;33:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 317] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 51. | Pilowsky LS, Costa DC, Ell PJ, Murray RM, Verhoeff NP, Kerwin RW. Antipsychotic medication, D2 dopamine receptor blockade and clinical response: a 123I IBZM SPET (single photon emission tomography) study. Psychol Med. 1993;23:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Stone JM, Davis JM, Leucht S, Pilowsky LS. Cortical dopamine D2/D3 receptors are a common site of action for antipsychotic drugs--an original patient data meta-analysis of the SPECT and PET in vivo receptor imaging literature. Schizophr Bull. 2009;35:789-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Wolkin A, Barouche F, Wolf AP, Rotrosen J, Fowler JS, Shiue CY, Cooper TB, Brodie JD. Dopamine blockade and clinical response: evidence for two biological subgroups of schizophrenia. Am J Psychiatry. 1989;146:905-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 145] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | Seeman P. Nomenclature of central and peripheral dopaminergic sites and receptors. Biochem Pharmacol. 1982;31:2563-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3032] [Cited by in RCA: 2793] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 56. | Spano PF, Govoni S, Trabucchi M. Studies on the pharmacological properties of dopamine receptors in various areas of the central nervous system. Adv Biochem Psychopharmacol. 1978;19:155-165. [PubMed] |
| 57. | Martel JC, Gatti McArthur S. Dopamine receptor subtypes, Physiology and pharmacology: New ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 58. | George SR, Watanabe M, Di Paolo T, Falardeau P, Labrie F, Seeman P. The functional state of the dopamine receptor in the anterior pituitary is in the high affinity form. Endocrinology. 1985;117:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Wreggett KA, Seeman P. Agonist high - and low-affinity states of the D2-dopamine receptor in calf brain. Partial conversion by guanine nucleotide. Mol Pharmacol. 1984;25:10-17. [PubMed] |
| 60. | Sunahara RK, Niznik HB, Weiner DM, Stormann TM, Brann MR, Kennedy JL, Gelernter JE, Rozmahel R, Yang YL, Israel Y. Human dopamine D1 receptor encoded by an intronless gene on chromosome 5. Nature. 1990;347:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 330] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 61. | Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 1390] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 62. | Sunahara RK, Guan HC, O'Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HH, Niznik HB. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350:614-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 780] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 63. | Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988;336:783-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 834] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 64. | Grandy DK, Marchionni MA, Makam H, Stofko RE, Alfano M, Frothingham L, Fischer JB, Burke-Howie KJ, Bunzow JR, Server AC. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc Natl Acad Sci U S A. 1989;86:9762-9766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 314] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Grandy DK, Litt M, Allen L, Bunzow JR, Marchionni M, Makam H, Reed L, Magenis RE, Civelli O. The human dopamine D2 receptor gene is located on chromosome 11 at q22-q23 and identifies a TaqI RFLP. Am J Hum Genet. 1989;45:778-785. [PubMed] |
| 66. | Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophr Bull. 1976;2:19-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 570] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 67. | Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2036] [Cited by in RCA: 1964] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 68. | Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17:263-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 256] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 69. | Meltzer HY, Massey BW. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr Opin Pharmacol. 2011;11:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 70. | Steward LJ, Kennedy MD, Morris BJ, Pratt JA. The atypical antipsychotic drug clozapine enhances chronic PCP-induced regulation of prefrontal cortex 5-HT2A receptors. Neuropharmacology. 2004;47:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 71. | Seeman P, Tallerico T. Rapid release of antipsychotic drugs from dopamine D2 receptors: an explanation for low receptor occupancy and early clinical relapse upon withdrawal of clozapine or quetiapine. Am J Psychiatry. 1999;156:876-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 150] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 72. | Vauquelin G, Bostoen S, Vanderheyden P, Seeman P. Clozapine, atypical antipsychotics, and the benefits of fast-off D2 dopamine receptor antagonism. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:337-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Hearn MG, Ren Y, McBride EW, Reveillaud I, Beinborn M, Kopin AS. A Drosophila dopamine 2-like receptor: Molecular characterization and identification of multiple alternatively spliced variants. Proc Natl Acad Sci U S A. 2002;99:14554-14559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 74. | Mi H, Thomas PD, Ring HZ, Jiang R, Sangkuhl K, Klein TE, Altman RB. PharmGKB summary: dopamine receptor D2. Pharmacogenet Genomics. 2011;21:350-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 75. | Seeman P, Nam D, Ulpian C, Liu IS, Tallerico T. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Thompson MD, Siminovitch KA, Cole DEC. G Protein-coupled receptor pharmacogenetics. In: Yan Q. (ed) Pharmacogenomics in drug discovery and development. Methods in molecular biology. Humana Press. 2008;448:139-185. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Kubota M, Nagashima T, Takano H, Kodaka F, Fujiwara H, Takahata K, Moriguchi S, Kimura Y, Higuchi M, Okubo Y, Takahashi H, Ito H, Suhara T. Affinity States of Striatal Dopamine D2 Receptors in Antipsychotic-Free Patients with Schizophrenia. Int J Neuropsychopharmacol. 2017;20:928-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Seeman P, Sunahara RK, Niznik HB. Receptor-receptor link in membranes revealed by ligand competition: example for dopamine D1 and D2 receptors. Synapse. 1994;17:62-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Zhuang Y, Xu P, Mao C, Wang L, Krumm B, Zhou XE, Huang S, Liu H, Cheng X, Huang XP, Shen DD, Xu T, Liu YF, Wang Y, Guo J, Jiang Y, Jiang H, Melcher K, Roth BL, Zhang Y, Zhang C, Xu HE. Structural insights into the human D1 and D2 dopamine receptor signaling complexes. Cell 2021; 184: 931-942. e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 80. | Subburaju S, Sromek AW, Seeman P, Neumeyer JL. The high affinity dopamine D2 receptor agonist MCL-536: A new tool for studying dopaminergic contribution to neurological disorders. ACS Chem Neurosci. 2021;12:1428-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Schwartz JC, Diaz J, Pilon C, Sokoloff P. Possible implications of the dopamine D(3) receptor in schizophrenia and in antipsychotic drug actions. Brain Res Brain Res Rev. 2000;31:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 82. | Girgis RR, Slifstein M, D'Souza D, Lee Y, Periclou A, Ghahramani P, Laszlovszky I, Durgam S, Adham N, Nabulsi N, Huang Y, Carson RE, Kiss B, Kapás M, Abi-Dargham A, Rakhit A. Preferential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [(11)C]-(+)-PHNO. Psychopharmacology (Berl). 2016;233:3503-3512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 83. | Laruelle M, Frankle WG, Narendran R, Kegeles LS, Abi-Dargham A. Mechanism of action of antipsychotic drugs: from dopamine D(2) receptor antagonism to glutamate NMDA facilitation. Clin Ther. 2005;27 Suppl A:S16-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 84. | Maksymetz J, Moran SP, Conn PJ. Targeting metabotropic glutamate receptors for novel treatments of schizophrenia. Mol Brain. 2017;10:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 85. | Borroto-Escuela DO, Romero-Fernandez W, Tarakanov AO, Marcellino D, Ciruela F, Agnati LF, Fuxe K. Dopamine D2 and 5-hydroxytryptamine 5-HT(₂A) receptors assemble into functionally interacting heteromers. Biochem Biophys Res Commun. 2010;401:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 86. | Borroto-Escuela DO, Romero-Fernandez W, Narvaez M, Oflijan J, Agnati LF, Fuxe K. Hallucinogenic 5-HT2AR agonists LSD and DOI enhance dopamine D2R protomer recognition and signaling of D2-5-HT2A heteroreceptor complexes. Biochem Biophys Res Commun. 2014;443:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | Lukasiewicz S, Polit A, Kędracka-Krok S, Wędzony K, Maćkowiak M, Dziedzicka-Wasylewska M. Hetero-dimerization of serotonin 5-HT(2A) and dopamine D(2) receptors. Biochim Biophys Acta. 2010;1803:1347-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 88. | Baltzersen OB, Meltzer HY, Frokjaer VG, Raghava JM, Baandrup L, Fagerlund B, Larsson HBW, Fibiger HC, Glenthøj BY, Knudsen GM, Ebdrup BH. Identification of a serotonin 2A receptor subtype of schizophrenia spectrum disorders with pimavanserin: The Sub-Sero Proof-of-Concept Trial Protocol. Front Pharmacol. 2020;11:591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |