Published online Jul 19, 2021. doi: 10.5498/wjp.v11.i7.347
Peer-review started: February 11, 2021
First decision: March 16, 2021
Revised: March 18, 2021
Accepted: May 24, 2021
Article in press: May 24, 2021
Published online: July 19, 2021
Processing time: 153 Days and 22.8 Hours
Evolving data show a variable expression of clinical neurological manifestations in patients suffering with coronavirus disease 2019 (COVID-19) from early disease onset. The most frequent symptoms and signs are fatigue, dizziness, impaired consciousness, ageusia, anosmia, radicular pain, and headache, as well as others. Based on the high number of series of cases reported, there is evidence for the implication of the immune system in the pathological mechanism of COVID-19. Although the exact role of the immunological mechanism is not elucidated, two main mechanisms are suggested which implicate the direct effect of severe acute respiratory syndrome coronavirus 2 infection in the central nervous system and neuroinflammation. In the context of neurological manifestations associated with COVID-19, neuropsychiatric disorders show an exacerbation and are described by symptoms and signs such as depression, anxiety, mood alterations, psychosis, post-traumatic stress disorder, delirium, and cognitive impairment, which appear to be common in COVID-19 survivors. A worsened score on psychopathological measures is seen in those with a history of psychiatric comorbidities. We review the neuropsychiatric manifestations associated with COVID-19 and some critical aspects of the innate and adaptive immune system involved in mental health disorders occurring in COVID-19.
Core Tip: Severe acute respiratory syndrome coronavirus 2 infects the central nervous system and drives neuroinflammation. In coronavirus disease 2019 (COVID-19) patients, neuropsychiatric disorders are showing an exacerbation and are described by symptoms and signs such as depression, anxiety, mood alterations, psychosis, post-traumatic stress disorder, delirium, and cognitive impairments. Some critical aspects of the innate and adaptive immune system are also involved in mental health disorders occurring in COVID-19.
- Citation: Robinson-Agramonte MA, Gonçalves CA, Noris-García E, Préndes Rivero N, Brigida AL, Schultz S, Siniscalco D, García García RJ. Impact of SARS-CoV-2 on neuropsychiatric disorders. World J Psychiatr 2021; 11(7): 347-354
- URL: https://www.wjgnet.com/2220-3206/full/v11/i7/347.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i7.347
The Coronavirus Disease 2019 (COVID-19) pandemic, at the time of this publication, has shown a tendency to a reduction in contagiousness globally; however, the fire has not gone out. Reports of the World Health Organization and Johns Hopkins University confirm as of March 18, 2021 that there have been 121 214 686 cases diagnosed around the world and 2 680 740 deaths as consequence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is accepted that this infection affects the nervous system in various ways as the virus is deeply neurotropic and neuroinvasive[1]. It will be necessary to understand the post-infectious manifestations of COVID-19 to guide long-term management of neurodevelopmental and neuropsychiatric diseases.
Data derived from published clinical papers and case reports indicate that the main clinical manifestations of COVID-19 include anosmia, ageusia, central respiratory failure, stroke, acute inflammatory demyelinating polyneuropathy, toxic metabolic encephalopathy, headache, myalgia, myelitis, ataxia, and others[2,3]. As suggested earlier, in the acute phase, COVID-19 is a potential causal factor of neuropsychiatric manifestations, such as encephalopathy, psychosis, insomnia, and mood changes[4].
The neuropsychiatric manifestations of both viral infection per se and secondary to the host neuroinflammatory reaction are attributed to: (1) Microglial activation[5,6]; (2) An imbalance of central neurotransmitters, such as noradrenaline, epinephrine, and serotonin (with potential implication in neuropsychiatric disorders); and (3) A disruption of the blood–brain barrier (BBB) leading to peripheral immune cell transmigration into the central nervous system (CNS)[7]. This manuscript reviews core critical aspects of neuropsychiatric disorders and COVID-19 while focusing on the immunopathology and related clinical symptoms.
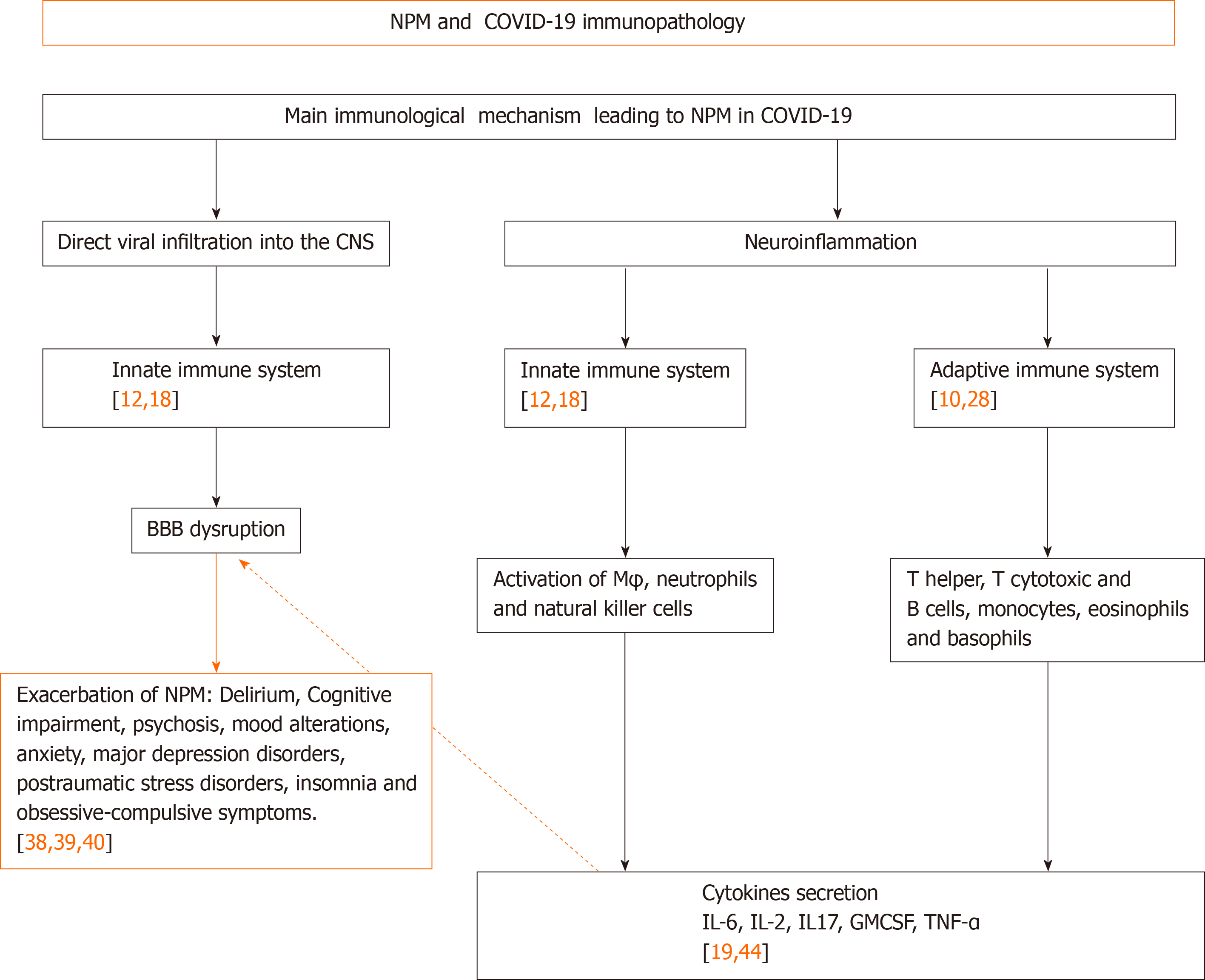
The neuroinvasive potential of coronavirus has been reported in SARS-CoV-1 patients and experimental animals[6], and it was also speculated to include SARS-CoV-2 in relation to the routes and mechanisms of CoV neurotropism[8,9]. Potential mechanisms of neuropsychiatric manifestations in COVID-19 are discussed from different viewpoints[10-13]; however, we will focus this short review on two main core mechanisms in support of CNS involvement in mental health associated with COVID-19: Direct viral infiltration into the CNS and neuroinflammation with related neuropsychiatric manifestations (Figure 1).
It is well-known that viral infection takes place by recognition and binding of SARS-CoV-2 virus-host receptor, specifically angiotensin-converting enzyme 2 (ACE-2), which is expressed in multiple tissues within the human body, including the CNS[1]. In the CNS, infection has been identified in glial cells and endothelial cells of blood vessels in the brain. In brain blood vessels, it has been demonstrated that it can induce disruption of the BBB and increase permeability[14-16].
One pathway for CoV invading the CNS is through synaptic routes of nerve cells, which seems to infect CNS retrograde via peripheral sensory nerves[17]. On the other hand, the olfactory nerve cells seem to be a feasible route for direct CNS infection, facilitated by the ACE-2 receptor expressed in olfactory epithelial cells[1]. Although this mechanism does not have a full consensus from various research groups, several pieces of evidence confirm that after cell infection by CoVs, death can be caused by autophagy, apoptosis, pyroptosis, or by elimination via innate immune cells[17,18] (Figure 1).
It has been found that following SARS-CoV-2 infection, activation of both the innate and adaptive arms of the immune system are induced toward an uncontrolled systemic response with the intervention of a non-specific immune mechanism involving activated macrophages, neutrophils, and natural killer cells, as well as an adaptive immune mechanism with a relevant effector function mediated by dendritic cells and lymphocytes. The adaptive mechanism includes T helper cells (CD4), T cytotoxic cells (CD8) and B cells, which is followed by exaggerated pro-inflammatory cytokine release from these effector cells, such as interleukin (IL)-1b, IL-6, IL-10, IL-12, interferons (IFN)-alpha, IFN-gamma, tumor necrosis factor (TNF)-alpha, transforming growth factor-beta, and chemokines (CCL2, CCL3, CCL5, CXCL8, y/o CXCL10). This begins the so-called "cytokine storm", which is critical for the multi-organ failure leading to high lethality observed in affected patients[12,19-22].
The neuroinflammation caused by SARS-CoV-2, secondary to the cytokine storm and immune cells reactivation, also becomes an additional effect of CoV infection[21]. In this context, several pathways have been discussed regarding the strong immune response in humans secondary to SARS-CoV-2 infection[22]. A marked reduction in absolute count of T cells, monocytes, eosinophils, and basophils has been found during this infection, with a main impact on the absolute decrease of T cells, memory T helper and regulatory cells[23,24].
Besides the explosive cytokine release syndrome and the macrophage activation syndrome co-existing in SARS-CoV-2 infection and affecting the CNS, there is also a strong increase in pro-inflammatory cytokines, such as IL-6, IL-2, IL-17, granulocyte-colony stimulating factor and TNF[24]. Inflammatory conditions can also induce the increase of IL-1, IL-6 and TNF soluble mediators that might facilitate major BBB permeability[25], as they have been responsible for neurological manifestations such as encephalitis, CNS demyelination and neuropsychiatric disorders[26].
Even in the absence of SARS-CoV-2 CNS infiltration, a transmigration of peripheral cytokines derived from the systemic host antiviral response may also take place to induce neuropsychiatric symptoms by the neuro-inflammatory response and BBB disruption. This may be caused by the peripheral effector immune cells migrating into the CNS, as well as local impairment of the neurotransmission system[27,28]. High levels of neurotransmitters, mainly noradrenaline, epinephrine, and serotonin, have been associated with psychological manifestations, such as depression, anxiety, and post-traumatic stress disorder[20].
It seems possible that the hypothesis of cytokine storm being associated with psychiatric manifestation induction is an indirect consequence of the hyperinflammation. Previous studies regarding pandemics caused by respiratory viruses suggest that diverse occurrence of neuropsychiatric symptoms can take place during or after the acute infection, which may be underlined by the presence of persistent cognitive deficits.
In the COVID-19 acute phase, besides the psychosocial stressor factors, it has been argued that the infection underlying the pathophysiology of neuropsychiatric manifestations, such as encephalopathy, psychosis, insomnia, and mood changes, post-traumatic stress disorder, panic attacks, and anxiety, mostly seen in health care workers and survivors of SARS-CoV infection, have been mainly attributed to viral infection per se and secondary to the host immune response. In this way, direct viral infiltration of the CNS can trigger an inflammatory reaction at the brain level leading to local microglial activation, which in turn induces demyelinating processes that are one of the primary causes of encephalopathy. In the absence of direct viral infiltration, a peripheral cytokine storm causing an imbalance of neurotransmitters within the CNS has been implicated in neuropsychiatric manifestations. This cytokine storm induces a neuroinflammatory response causing disruption of the BBB, leading to peripheral immune cell transmigration into the brain and, in turn, causes imbalances in neurotransmission.
It continues to be a challenge for researchers to elucidate the real core mechanisms of COVID-19 associated neuropsychiatric complications due to general findings caused by SARS-CoV-2 infection. This may be difficult to distinguish from the encephalopathy arising from systemic infection without affecting brain tissue[29]. Evidence such as the increase in IL-6 has been linked to high mortality in patients with COVID-19[19], similar to the increase in this cytokine observed in other neuropsychiatric disorders such as schizophrenia and depression[19].
Other arguments underlining the immunological mechanism identify that peripheral myeloid cells are also infected by CoV[5], which can be recruited to transmigrate to the CNS under an increased BBB permeability. Virus-infected monocytes in the CNS can promote microglial activation as well as induce neuropsychiatric symptoms[5,27,28]. The role of microglial activation in schizophrenia and autism is well known[30,31,32]. Other mechanisms implicating mental disease in COVID-19 are the close inter-relation between the systemic compartment and the brain[7] (Figure 1).
Since the initial phase of the COVID-19 pandemic, a large number of studies have shown the clinical symptoms and signs of the infection. These principally include fever, cough, sore throat, dyspnea, nausea, diarrhea, and fatigue[27,33]. However, numerous reports also reveal an accumulation of frequent neurological symptoms in COVID-19 positive patients, such as dizziness, ageusia, fatigue, headache, impaired consciousness, and anosmia[34-36].
Parallel to the neurological events, evidence is growing for neuropsychiatric disorders that are also reported as secondary complications of SARS-CoV-2 infection[37], which will be made clear in the few years. In this context, an exacerbation of mental health disorders has been described in COVID-19 that include delirium, cognitive impairment, mood alterations, and psychosis[38-40]. Delirium occurs in 90% of COVID-19 cases, while cognitive disorder is also considered a direct consequence of CNS infection by SARS-CoV-2[39]. Anxiety, depression, post-traumatic stress disorder, insomnia, and obsessive-compulsive symptomatology, mainly in females, appear to be quite common in COVID-19 survivors and coworkers with worsened scores on psychopathological measures in those with a history of psychiatric comorbidities. In addition, hypoxemia, a frequent clinical finding in COVID-19, can also produce mental health impairment[38]. Thus, a consequence of acute respiratory syndrome and associated relative hypoxia also shows worsening of attention, executive function, and verbal memory[41].
An exaggerated immune response, under a dysregulated cytokine network, occurring during the COVID-19 pandemic would also drive symptoms of visceral stress with an impact on mental health[38,42]. In this line of thinking, authors have defined a relationship between COVID-19 disease severity, somatic and psychiatric symptoms, and cytokine levels in patients positive for SARS-CoV-2 infection. There is also some evidence that patients with comorbidities and immunosuppression are more susceptible to developing psychiatric disorders, such as cognitive impairment, anxiety, and depression[43,44].
Considering the trajectory of the occurrence of COVID-19 around the world, from its beginnings in China in December 2019, it is clear that not only patients but also their family and the normal population are victims of the psychosocial impacts derived from this pandemic[42,45]. From this viewpoint, it is clearly necessary to investigate the behavioral aspects of this disease, either in the short- or long-term, to identify a more valuable strategy to control the transmission by carriers and assess the impact of COVID-19, not only in affected patients, but also in the general population. We are proposing a diagram regarding the impact of the immunological mechanism involved in SARS-CoV-2 infection by the occurrence of neuropsychiatric manifestation as a tool of acknowledgment of the way the disease progresses which could allow better management of the disease and prevention of the consequences in the short- or long-term.
The long-term neuropsychiatric consequences of SARS-CoV-2 infection have been demonstrated in a fraction of cases; however, they appear to be significant for the future due to the global burden of COVID-19. In this context, to understand the evolution and specificity of neuropsychiatric outcomes stemming from SARS-CoV-2 infection and to elucidate the pathogenic mechanisms involved in these events should be useful in targeting critical interventions for the prevention of mental disorders derived from COVID-19. Nevertheless, the work of psychologists and psychiatrists concentrated on handling the psyche at clinics and paraclinics looking for more effective evidence of recovery from the potential neurological consequences will not be enough. It will be necessary to develop programs and strategies to achieve a more humanist medical approach to promote the resilience of the individuals affected as well as their parents to avoid the long-term occurrence of mental disorders due to the lost connection between the body and the soul. This is the neurobiological substrate in the development of neuropsychiatric disorders as a consequence of COVID-19. Finally, an earlier understanding of the characteristics of neuropsychiatric outcomes stemming from SARS-CoV-2 infection and their pathogenic mechanisms will be necessary as an intervention target to avoid the sub-acute or chronic neuropsychiatric consequences of SARS-CoV-2 infection.
According to the numerous articles published since the beginning of the COVID-19 pandemic, it could be surmised that the accumulated knowledge would be enough to understand many of the events occurring in this illness. However, there is not enough data to understand the long-term consequences of this disease. The neuropsychiatric dysfunctions as part of the group of disorders unknown in the evolutionary context of COVID-19 could also be the result of an exaggerated host response against SARS-CoV-2 infection. The impact on the permeability of the BBB which facilitates the migration of immune cells to the CNS and their deleterious effect on neural function, followed by deregulation of the cytokine network that affects mental health, are manifested more frequently by anxiety, cognitive impairment and major depressive disorder. More studies will continue to be necessary to address this topic.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: Cuba
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morenikeji OB S-Editor: Liu M L-Editor: Webster JR P-Editor: Li JH
| 1. | Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74:8913-8921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 380] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 2. | Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2289] [Cited by in RCA: 2550] [Article Influence: 510.0] [Reference Citation Analysis (2)] |
| 3. | Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1284] [Cited by in RCA: 1522] [Article Influence: 304.4] [Reference Citation Analysis (0)] |
| 4. | Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Brain Behav Immun. 2020;87:34-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 665] [Cited by in RCA: 620] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 5. | Wohleb ES, Delpech JC. Dynamic cross-talk between microglia and peripheral monocytes underlies stress-induced neuroinflammation and behavioral consequences. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, Talbot PJ. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses. 2019;12:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 590] [Cited by in RCA: 707] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 7. | Dantzer R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol Rev. 2018;98:477-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 623] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 8. | Vavougios GD. Host proteases as determinants of coronaviral neurotropism and virulence. Brain Behav Immun. 2020;87:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1219] [Cited by in RCA: 1255] [Article Influence: 251.0] [Reference Citation Analysis (0)] |
| 10. | Gómez-Rial J, Currás-Tuala MJ, Rivero-Calle I, Gómez-Carballa A, Cebey-López M, Rodríguez-Tenreiro C, Dacosta-Urbieta A, Rivero-Velasco C, Rodríguez-Núñez N, Trastoy-Pena R, Rodríguez-García J, Salas A, Martinón-Torres F. Increased Serum Levels of sCD14 and sCD163 Indicate a Preponderant Role for Monocytes in COVID-19 Immunopathology. Front Immunol. 2020;11:560381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 11. | Xiang YT, Zhao YJ, Liu ZH, Li XH, Zhao N, Cheung T, Ng CH. The COVID-19 outbreak and psychiatric hospitals in China: managing challenges through mental health service reform. Int J Biol Sci. 2020;16:1741-1744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 12. | Chen Y, Wang J, Liu C, Su L, Zhang D, Fan J, Yang Y, Xiao M, Xie J, Xu Y, Li Y, Zhang S. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol Med. 2020;26:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 13. | Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1415] [Cited by in RCA: 1444] [Article Influence: 288.8] [Reference Citation Analysis (0)] |
| 14. | Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem Neurosci. 2020;11:995-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1435] [Cited by in RCA: 1413] [Article Influence: 282.6] [Reference Citation Analysis (0)] |
| 15. | Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1709] [Cited by in RCA: 1732] [Article Influence: 346.4] [Reference Citation Analysis (0)] |
| 16. | Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1522] [Cited by in RCA: 1712] [Article Influence: 342.4] [Reference Citation Analysis (0)] |
| 17. | Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of A case. Acta Neurol Taiwan. 2006;15:26-28. [PubMed] |
| 18. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4584] [Article Influence: 916.8] [Reference Citation Analysis (0)] |
| 19. | Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1228] [Cited by in RCA: 1219] [Article Influence: 243.8] [Reference Citation Analysis (0)] |
| 20. | Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020;11:1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1044] [Cited by in RCA: 1097] [Article Influence: 219.4] [Reference Citation Analysis (0)] |
| 21. | Accinelli RA, Zhang Xu CM, Ju Wang JD, Yachachin-Chávez JM, Cáceres-Pizarro JA, Tafur-Bances KB, Flores-Tejada RG, Paiva-Andrade ADC. COVID-19: la pandemia por el nuevo virus SARS-CoV-2. Rev Peru Med Exp Salud Publica. 2020;37:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Bae YS, Shin EC, Bae YS, Van Eden W. Editorial: Stress and Immunity. Front Immunol. 2019;10:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J Med Virol. 2020;92:1733-1734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 378] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 24. | Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 3341] [Article Influence: 668.2] [Reference Citation Analysis (0)] |
| 25. | Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264-7275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 963] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 26. | Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, Muñoz-Ruiz M, McKenzie DR, Hayday TS, Francos-Quijorna I, Kamdar S, Joseph M, Davies D, Davis R, Jennings A, Zlatareva I, Vantourout P, Wu Y, Sofra V, Cano F, Greco M, Theodoridis E, Freedman JD, Gee S, Chan JNE, Ryan S, Bugallo-Blanco E, Peterson P, Kisand K, Haljasmägi L, Chadli L, Moingeon P, Martinez L, Merrick B, Bisnauthsing K, Brooks K, Ibrahim MAA, Mason J, Lopez Gomez F, Babalola K, Abdul-Jawad S, Cason J, Mant C, Seow J, Graham C, Doores KJ, Di Rosa F, Edgeworth J, Shankar-Hari M, Hayday AC. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020;26:1623-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 682] [Article Influence: 136.4] [Reference Citation Analysis (0)] |
| 27. | Pereira A. Long-Term Neurological Threats of COVID-19: A Call to Update the Thinking About the Outcomes of the Coronavirus Pandemic. Front Neurol. 2020;11:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci. 2014;8:447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 29. | Inchausti F, MacBeth A, Hasson-Ohayon I, Dimaggio G. Psychological Intervention and COVID-19: What We Know So Far and What We Can Do. J Contemp Psychother. 2020;1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 30. | Robinson-Agramonte MA, González Fraguela ME, Bergado Rosado ME. Autism, Developmentand Neural Plasticity. Robinson Agramonte MA, editor. Translational Approaches to Autism Spectrum Disorder. Heidelberg: Springer, 2015: 119-135. [DOI] [Full Text] |
| 31. | Kazlauskas N, Robinson Agramonte MA, Mara Depino A. Neuroinflammation in Animal Models of Autism. Robinson Agramonte MA, editor. Translational Approaches to Autism Spectrum Disorder. Heidelberg: Springer, 2015: 137-153. [DOI] [Full Text] |
| 32. | Amaicha Mara Depino A, Robinson Agramonte MA. Understanding on Neuroimmunology in Autism Spectrum Disorder. Robinson Agramonte MA, editor. Translational Approaches toAutism Spectrum Disorder. Heidelberg: Springer, 2015: 155-180. [DOI] [Full Text] |
| 33. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18878] [Article Influence: 3775.6] [Reference Citation Analysis (7)] |
| 34. | Lee SM, Kang WS, Cho AR, Kim T, Park JK. Psychological impact of the 2015 MERS outbreak on hospital workers and quarantined hemodialysis patients. Compr Psychiatry. 2018;87:123-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 646] [Cited by in RCA: 638] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 35. | Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Xiao W, Wang YN, Zhong MH, Li CH, Li GC, Liu HG. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 856] [Article Influence: 171.2] [Reference Citation Analysis (0)] |
| 36. | Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and Ageusia: Common Findings in COVID-19 Patients. Laryngoscope. 2020;130:1787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 467] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 37. | Bao Y, Sun Y, Meng S, Shi J, Lu L. 2019-nCoV epidemic: address mental health care to empower society. Lancet. 2020;395:e37-e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1076] [Cited by in RCA: 1009] [Article Influence: 201.8] [Reference Citation Analysis (0)] |
| 38. | Carvalho PMM, Moreira MM, de Oliveira MNA, Landim JMM, Neto MLR. The psychiatric impact of the novel coronavirus outbreak. Psychiatry Res. 2020;286:112902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 39. | Goularte JF, Serafim SD, Colombo R, Hogg B, Caldieraro MA, Rosa AR. COVID-19 and mental health in Brazil: Psychiatric symptoms in the general population. J Psychiatr Res. 2021;132:32-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 40. | Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F, Rovere-Querini P; COVID-19 BioB Outpatient Clinic Study group; Benedetti F. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 967] [Cited by in RCA: 1016] [Article Influence: 203.2] [Reference Citation Analysis (0)] |
| 41. | Ardila A, Lahiri D. Executive dysfunction in COVID-19 patients. Diabetes Metab Syndr. 2020;14:1377-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Noris-García E, Robinson-Agramonte MA. Psycho-neuro-immuno-endocrinology and COVID-19. Revista Electrónica Dr. Zoilo E. Marinello Vidaurreta. 2021;46. |
| 43. | Klok FA, Boon GJAM, Barco S, Endres M, Geelhoed JJM, Knauss S, Rezek SA, Spruit MA, Vehreschild J, Siegerink B. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56:2001494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 377] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 44. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration; UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6752] [Article Influence: 1350.4] [Reference Citation Analysis (0)] |
| 45. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17648] [Article Influence: 3529.6] [Reference Citation Analysis (0)] |