Published online Feb 19, 2021. doi: 10.5498/wjp.v11.i2.35
Peer-review started: October 13, 2020
First decision: December 4, 2020
Revised: December 9, 2020
Accepted: December 24, 2020
Article in press: December 24, 2020
Published online: February 19, 2021
Processing time: 104 Days and 22.9 Hours
Major depressive disorder (MDD) is a highly disabling psychiatric syndrome associated with deficits of specific subpopulations of cortical GABAergic interneurons; however, the underlying molecular mechanism remains unknown. Type 3 adenylyl cyclase (ADCY3, AC3), which is important for neuronal excitability, has been implicated in MDD in a genome-wide association study in humans. Moreover, a study reported that ablation of AC3 in mice caused similar symptoms as MDD patients.
To determine if disruption of the AC3 gene in different subtypes of GABAergic interneurons of mice causes depression-like behaviors.
Using immunohistochemistry, we investigated the expression of AC3 in two major subtypes GABAergic interneurons: Somatostatin-positive (SST+) and parvalbumin-positive (PV+) neurons. Genetic manipulations were used to selectively disrupt AC3 expression in SST+ or PV+ interneurons. A series of behavior tests including rotarod test, open field test (OFT), elevated plus maze test (EPM), forced swimming test (FST), and tail suspension test (TST) were used to evaluate the motor ability, anxiety- and depression- like behaviors, respectively.
Our results indicate that approximately 90.41% of SST+ and 91.22% of PV+ interneurons express AC3. After ablation of AC3 in SST+ interneurons, the mice spent comparable time in the center area in OFT, but significantly less time in the open arms and low frequency of entries to the open arms in EPM. Furthermore, these mice showed prolonged immobility in FST and more freezing in TST. However, there were no significant changes in these behaviors after specific disruption of AC3 in PV+ interneurons.
This study indicates that ablation of AC3 in SST+ interneurons of mice increases anxiety- and depression-like behaviors in mice, supporting the general hypothesis that decreased AC3 activity may play a role in human depression.
Core Tip: Dysfunction of cortical GABAergic interneurons are thought to contribute to the pathophysiology of stress-related psychiatric disorders. Little is known about the regulation of GABAergic interneurons in depression. Type 3 adenylyl cyclase (AC3) is important for the neuronal excitability and was reported as a top-ranked gene in major depressive disorder (MDD). Here, we found that majority of somatostatin-positive (SST+) and parvalbumin-positive (PV+) GABAergic interneurons express AC3. Selective disruption of AC3 in SST+ but not PV+ interneurons caused anxiety- and depression-like behaviors. Our data suggest that AC3 in specific subtype interneurons played a key role in the etiology of depression, providing new insights for therapeutic interventions for the treatment of MDD.
- Citation: Yang XY, Ma ZL, Storm DR, Cao H, Zhang YQ. Selective ablation of type 3 adenylyl cyclase in somatostatin-positive interneurons produces anxiety- and depression-like behaviors in mice. World J Psychiatr 2021; 11(2): 35-49
- URL: https://www.wjgnet.com/2220-3206/full/v11/i2/35.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i2.35
Major depressive disorder (MDD) is a severe psychiatric disorder that affects up to one in six individuals worldwide[1]. Despite long-lasting efforts to explore the etiology and pathophysiology of depression, the underlying neurobiological mechanisms are not well defined. Current antidepressant pharmacological treatments are mainly focused on monoaminergic neurotransmission, which is based on the traditional catecholamine and serotonin hypotheses of mental disorders[2,3]. However, only approximately 33% of patients experience remission after pharmacological treatments, and up to one-third of these patients have a poor response to regular antidepressant treatments[4,5]. Therefore, it is important to identify new drug target sites for treatment of depression.
Recent evidence suggests that the functional interaction between the glutamatergic excitatory and GABAergic inhibitory connectivity is altered during depression and chronic stress exposure[6-8] suggesting that impairment of γ-aminobutyric acid (GABA) neurotransmission may contribute to the neurobiology of MDD[9,10]. The cortical GABAergic interneurons are divided into different subtypes according to their morphology, electrophysiological properties, and expression of calcium-binding proteins and neuropeptides[11]. Most research concerning GABAergic interneurons in depression and anxiety has focused on two major subtypes expressing somatostatin (SST) or parvalbumin (PV), which make up approximately 70% of GABAergic inhibitory neurons[12,13]. Studies focusing on SST in MDD suggest a reduction of SST in several corticolimbic brain regions in postmortem MDD patients, including the dorsolateral prefrontal cortex, subgeual anterior cingulate cortex (sgACC), and amygdala[14-16]. Mice lacking SST (SST global knockout mice) display similar behavioral and molecular features as human depression. This includes increased circulating corticosterone, elevated anxiety/depressive-like behaviors, and reduced gene expression of brain-derived neurotrophic factor (BDNF), cortistatin, and glutamate decarboxylase 67 (GAD67)[17]. However, there is less evidence for consistent alterations of PV+ interneurons in MDD. Most studies have reported that there are no significant changes in PV expression in either MDD subjects[18-21] or rodent models[21-24]. Several studies have indicated decreased PV expression in tissues of post-mortem MDD patients[21] or in rodent models[20,25]. By contrast, increased PV expression has been reported when female mice are exposed to unpredictable chronic stress for 2 wk[26]. Since SST+ and PV+ interneurons differentially target specific cellular domains (distal dendritic vs perisomatic regions) of pyramidal neurons and other interneurons, it is reasonable to believe that they might play different roles in the pathology of MDD.
Type 3 adenylyl cyclase (AC3, ADCY3), is widely expressed in primary cilia in neurons of the central nervous system[27]. AC3 is stimulated by Gs-coupled receptors and inhibited by Gi-coupled receptors and Ca2+/calmodulin-dependent protein kinase II[28]. In 2012, a genome-wide association study in humans implicated the ADCY3 gene in MDD[29]. Moreover, a study published by Chen et al[30] showed that AC3-/- mice have similar symptoms as MDD patients. This includes increased rapid eye movement sleep time, anosphrasia, and anxiety and -depression-like behaviors.
However, there is little information on how decreases in AC3 activity contribute to the pathophysiological process of MDD. In this study, we took advantage of several transgenic mice lines. We bred AC3flox/flox mice with subtype-specific GABAergic interneuron Cre lines in which the Cre recombinase (Cre) is expressed under the control of SST or PV promoters to explore the roles of AC3 in SST+ and PV+ interneurons in the development of anxiety and depression mood disorders.
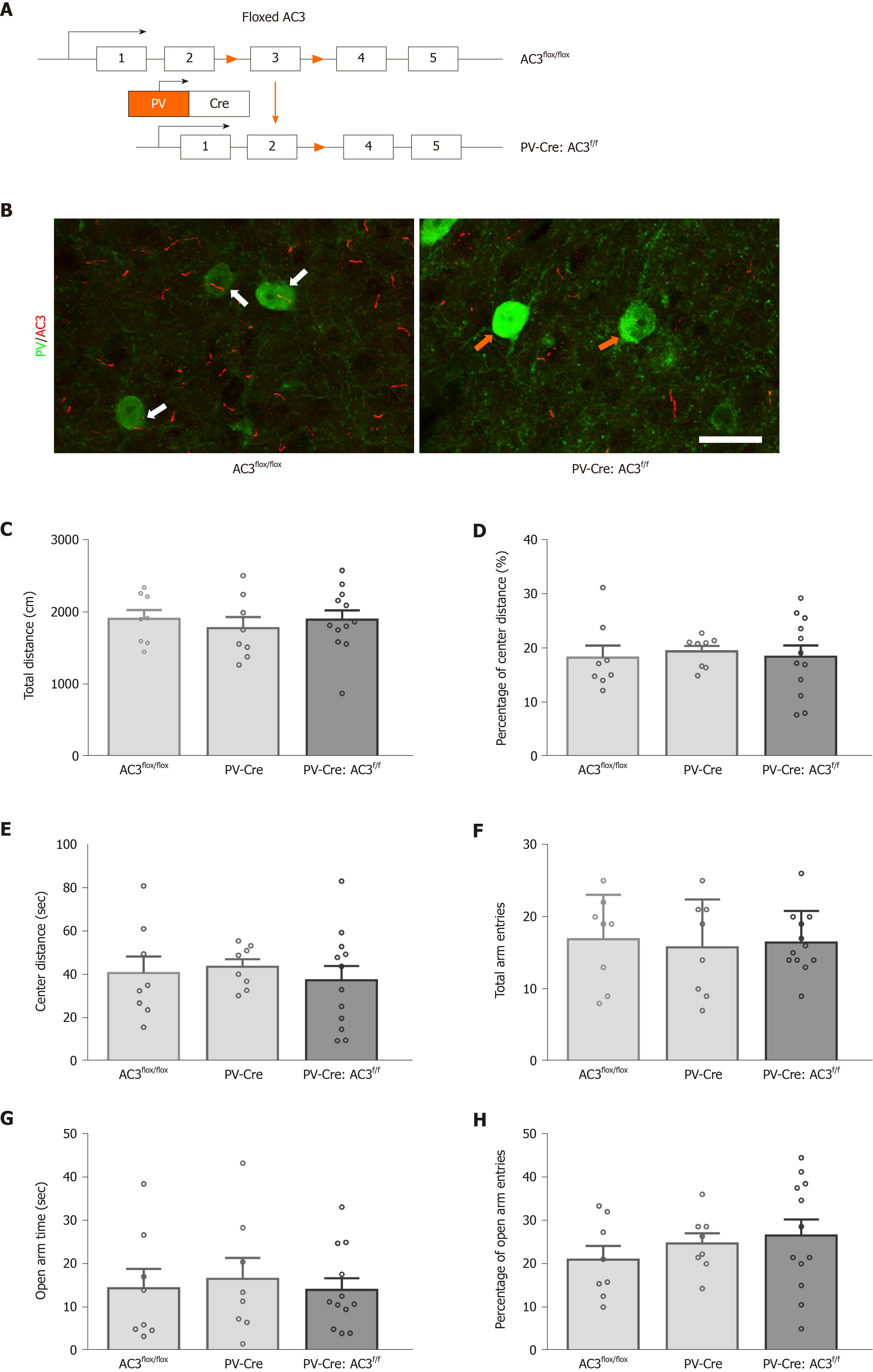
Male C57BL/6J mice were obtained from the Shanghai Experimental Animal Center of the Chinese Academy of sciences. AC3flox/flox mice were generated as previously reported[30]. SST-Cre mice (JAX013044), PV-Cre mice (JAX008069), and Ai14 mice (JAX007914) were purchased from Jackson Laboratory (Bar Harbor, ME, United States). All mice were backcrossed into the C57BL/6J genetic background for at least six generations. Mice were genotyped using their tail tissue after cracking by lysis reagent (Direct PCR Lysis Reagent [mouse tail], QIAGEN, Hilden, Germany) and proteinase K (Proteinase K, QIAGEN). Seventy-eight male mice used in behavioral analysis were age 8-12 wk old with comparable body weight. Eleven male adult mice were used for immunohistochemistry. All mice were group-housed (3-6 per cage) and maintained on the a 12/12 h light/dark cycle, 50% humidity, at 23.1 °C with access to food and water ad libitum. All experimental procedures were designed to minimize pain or discomfort to the animals, approved by the Experimental Animal Ethics Committee of Shanghai Medical College and the Institutional Animal Care & Use Committee (IACUC) of Fudan University (Permit No. SYXK2009-0082; Shanghai, China) and followed the ethical guidelines of the International Association for the Study of Pain regarding the use of laboratory animals.
Mice were handled at least 3 d by the experimenters before the behavioral tests, and habituated to the test room for 30 min before behavioral test. All behavioral tests were performed by investigators blind to genotypes.
The rotarod test was performed as previously described[31]. Briefly, the mice were placed on an accelerating rotarod (IITC Life Science, Woodland Hills, CA, United States) (4-40 rpm acceleration in 5 min) for 4 consecutive days. Training consisted of 4 trials per day at 5 min intervals. The trial ended when the mouse fell off the roller. We calculated the average latency to fall from the last two trials performed on the last day.
The apparatus consisted of a square box (40 cm × 40 cm × 30 cm, length × width × height) with blue floor and without a celling. Both the box and floor were made of Perspex plates. During testing, the floor was separated into 16 small squares of equal area. The four squares in the center were designated the central area, and the others are called the limbic area. The light level was maintained at approximately 25 lux in the open field area. The test began when the mice were put in the center of the square. After free movement for 5 min, the mice were returned to their home cages. Video tracking software (Ethovision XT v11.5; Noldus Information Technology Inc., Leesburg, VA, United States) was used to record the distance traveled and time spent in each zone.
The elevated plus maze consisted of a central platform (6 cm, 6 cm), two open arms (30 cm, 6 cm) and two closed arms (30 cm, 6 cm) with protective walls 15 cm high. The apparatus was elevated 50 cm above the floor and was placed in a room with an illumination of 30 lux. Mice were put on the central platform facing an open arm, and allowed to freely explore the maze for 5 min. The traces were recorded by the video tracking system (Ethovision XT v11.5; Noldus BV). The time spent exploring time in the open arm and the frequency of entries to the open arm were calculated by Video Tracking software (Ethovision XT v11.5; Noldus BV).
Mice swam individually in a cylindrical glass container (diameter: 18 cm, height: 50 cm, depth: 12 cm) filled with water (24.1 °C). The test comprised two sessions: A training session (10 min) on the first day and a test session (5 min) on the next day, which were recorded by a video camera. Immobility in mice was defined as no active movements except for single limb paddling to keep the head and nose above the water.
The apparatus for the tail suspension test (TST) was a white plexi-glass box with a hook on the ceiling of the box. The mice were suspended 15 cm above the floor on the hook with a tape at 2 cm proximal to the tail tip. The test session lasted 6 min and was recorded with a video camera. The freezing time was scored for the last 4 min of the session.
Mice were deeply anesthetized with urethane (1.5 g/kg) and perfused transcardially with normal saline followed by 40 g/L paraformaldehyde in phosphate-buffered saline (PBS). The brains were collected, post-fixed in the same fixative for additional 4 h at 4 °C, and dehydrated in graded sucrose solutions (10 g/L, 20 g/L, 30 g/L). Continuous coronal sections of brains (35 μm) were prepared on a cryostat (Leica, Germany) and stored at -20 °C. Sections were washed with 0.01 mol/L PBS and blocked in 10% donkey serum in 0.01 mol/L PBS, pH 7.4, with 0.3% Triton X-100 for 2 h at room temperature (RT). For triple immunostaining of SST/AC3/NeuN, SST-Cre:Aif/f reporter mice were used. Sections were incubated with a mixture of rabbit anti-AC3 (1:1000, SC-588; Santa Cruz, Dallas, TX, United States) and mouse anti-NeuN (1:1000, MAB377; Millipore, Burlington, MA, United States), followed by the corresponding secondary antibodies (1:200, Alexa Fluor 647 donkey anti-rabbit, 711-605-152, Jackson Laboratory; 1:200, Alexa Fluor 488 donkey anti-mouse, A-21202, Invitrogen, Carlsbad, CA, United States) for 2 h at 4 °C. For triple immunostaining of PV/AC3/DAPI, the sections were incubated with a mixture of rabbit anti-AC3 and mouse anti-PV (1:500, P3088; Sigma, St. Louis, MO, United States) overnight at 4 °C, followed by their corresponding secondary antibodies (1:200, Alexa Fluor 546 donkey anti-rabbit, A-10040, Invitrogen; 1:200, Alexa Fluor 488 donkey anti-mouse, A-21202, Invitrogen) for 2 h at 4 °C. The nuclei were counterstained with DAPI (1:20000, 62248; Thermo Fisher Scientific, Waltham, MA, United States) for 3 min at RT. All sections were coverslipped with a mixture of 50% glycerin in PBS. Images were captured with a confocal laser-scanning microscope (FV1000; Olympus, Tokyo, Japan). The interval between two adjacent focal planes was 2 μm (z-stack). Image analysis was performed by Photoshop or FV10-ASW 1.7 Viewer software (Olympus).
Brain sections (14 μm) were cut using a cryostat and mounted onto Colorfrost Plus slides (Thermo Fisher Scientific). In situ hybridization (ISH) was performed using the RNAscope system (Advanced Cell Diagnostics, Newark, CA, United States) according to the manufacturer’s protocol. Sections were pretreated with hydrogen peroxide for 10 min at RT, followed by target-retrieval solution and Protease III using RNAscope 2.5 Universal Pretreatment Reagents (#322381; Advanced Cell Diagnostics). Then the commercial probe for AC3 (#478071-C1) was used. After hybridization, the RNAscope Multiplex Fluorescent Detection Kit v2 (#323110) was used to amplify signal. After ISH, sections were labeled with anti-SST antibody (goat, 1:500, sc-7819; Santa Cruz), followed by its corresponding secondary antibody (Alexa Fluor 488 donkey anti-goat, 1:200, A-11055; Invitrogen). Images were captured with a confocal laser-scanning microscope (Model FV1000; Olympus).
All data are presented as the mean ± standard error of the mean and were analyzed using Prism 7.0 (GraphPad Software, San Diego, CA, United States). Comparisons between two groups were performed using the two-sided Student’s t-test. One way analysis of variance (ANOVA) followed by post hoc Tukey’s multiple comparisons test was used for data comparison between three groups. P < 0.05 was considered statistically significant.
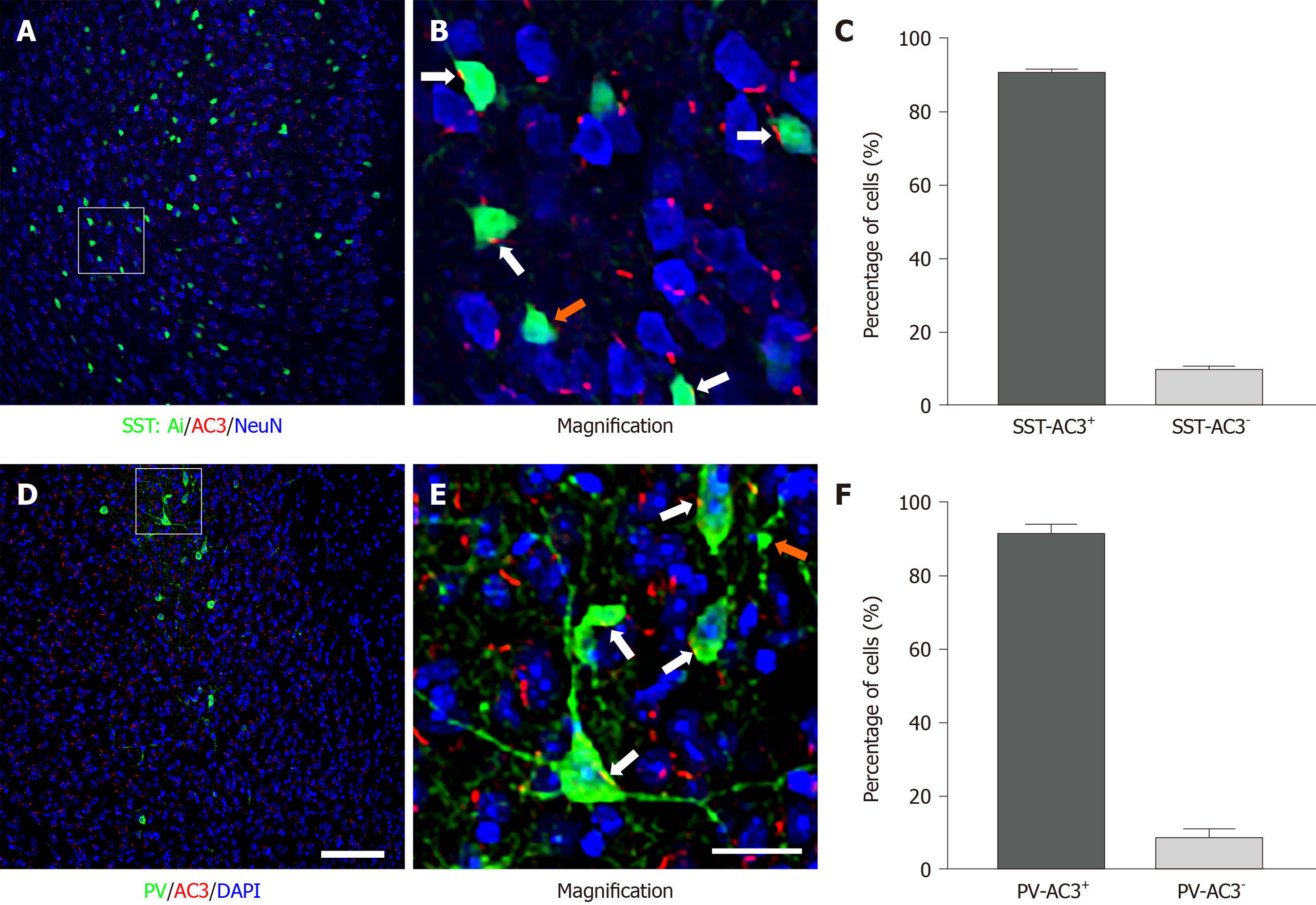
As a starting point, we examined the expression of AC3 in two major subtypes of cortical GABAergic interneurons. As shown in Figure 1, AC3 immunostaining was extensively distributed in the brain area examined (Figure 1A and D). Consistent with previous reports[27], the high magnification of imaging showed that AC3 was exclusively expressed in primary cilia with a rod-like shape (Figure 1B and E). To observe the expression of AC3 in SST+ interneurons, we bred SST-Ai14f/f transgenic mice in which SST+ interneurons were labeled with the tdTomato fluorescent protein carried by the Ai14 gene. Statistical analysis showed that approximately 90.41% of SST+ interneurons expressed AC3 (Figure 1C). We also examined the expression of AC3 in PV+ interneurons using double immunostaining. Similar to SST+, about 91.22% of PV+ interneurons expressed AC3 (Figure 1F). Our results indicated that the majority of SST+ and PV+ GABAergic interneurons express AC3.
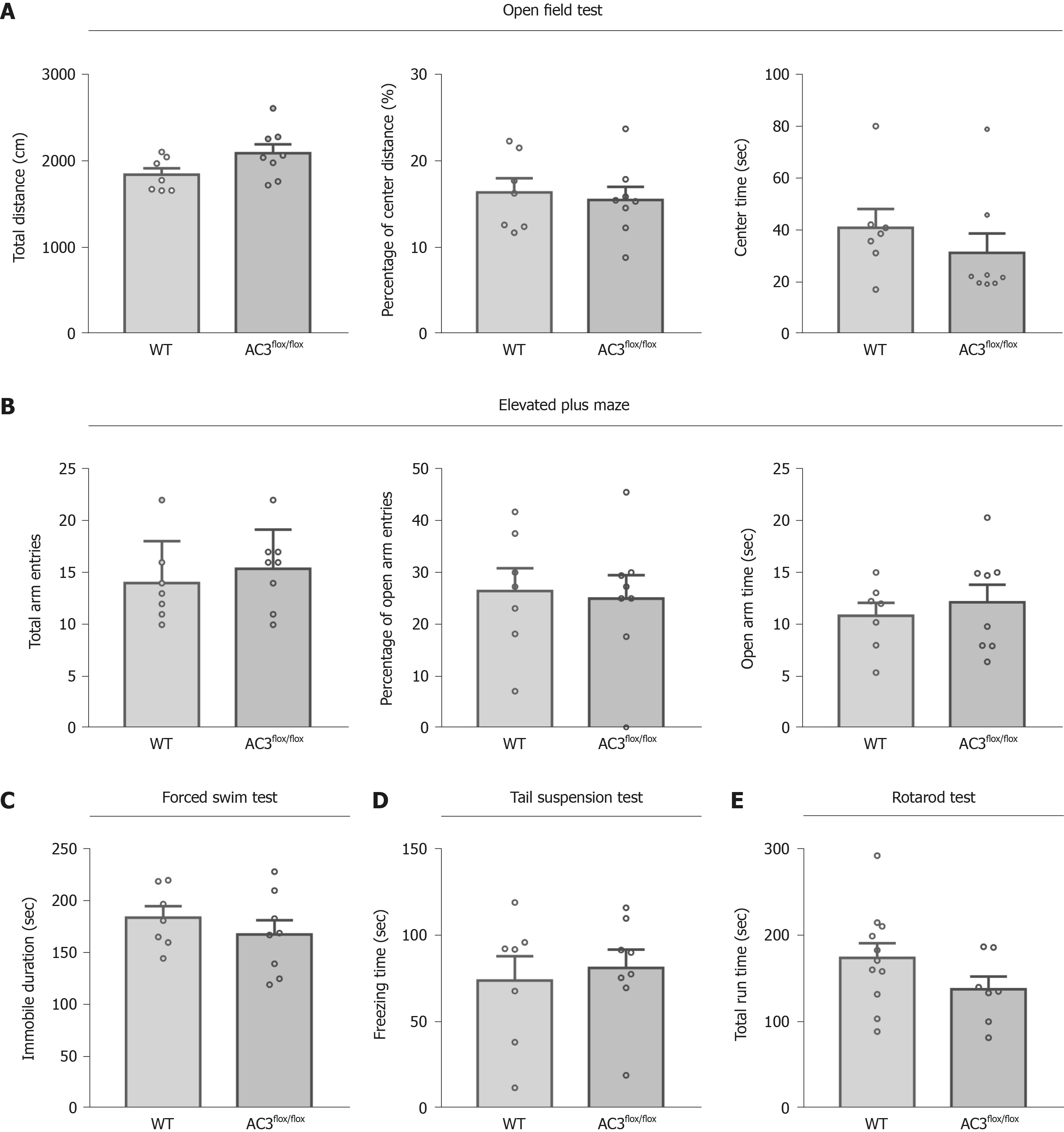
To explore the functional role of AC3 in specific cell type interneurons, SST-Cre mice, PV-Cre mice, and AC3flox/flox mice in which exon 3 of the ADCY3 gene was flanked by two loxP sites were used. We assessed a series of general behaviors of AC3flox/flox mice that served as controls. As shown in Figure 2, the behaviors of AC3flox/flox mice analyzed in the open field test (OFT) (Figure 2A), elevated plus maze (Figure 2B), forced swimming test (FST) (Figure 2C), TST (Figure 2D) and rotarod test (Figure 2E) were largely indistinguishable from wild-type mice, indicating that the floxed AC3 mice showed normal emotion-related behaviors and motor ability.
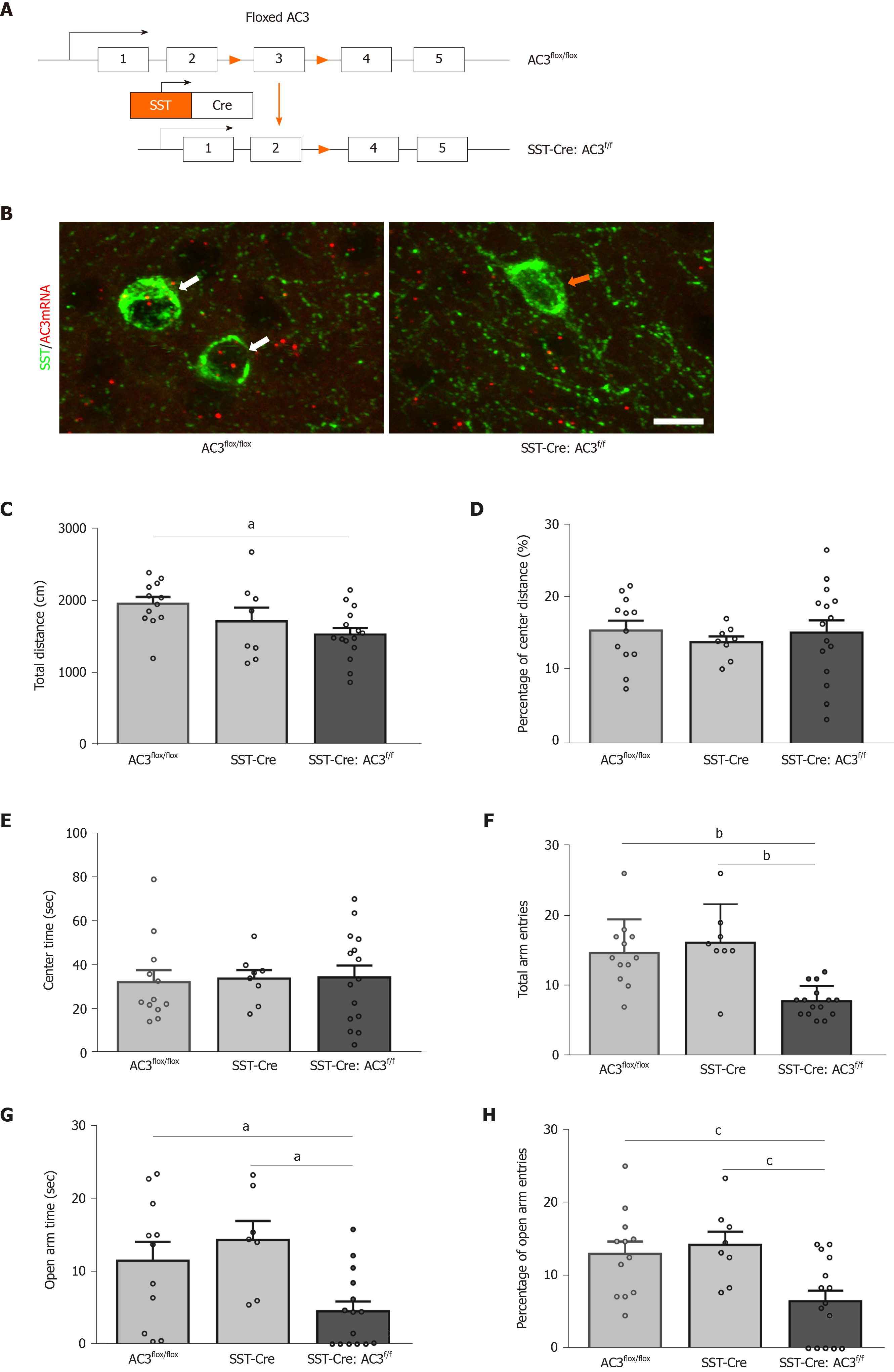
To characterize the functional role of AC3 in SST+ neurons, we generated SST-Cre:AC3f/f mice by breeding SST-Cre mice and AC3flox/flox mice to selective delete AC3 from SST+ interneurons (Figure 3A). As expected, the immunoreactivity of ACDY3 transcript examined by RNAscope IST disappeared in SST+ interneurons in SST-Cre:AC3f/f mice but not in AC3flox/flox mice (Figure 3B). Then we subjected SST-Cre:AC3f/f mice to test for anxiety- and depression-like behaviors. In the OFT, although the SST-Cre:AC3f/f mice moved less distance in the apparatus compared with the other two groups (one-way ANOVA, F(2, 32) = 3.871, P = 0.0312; Figure 3C), the SST-Cre:AC3f/f mice did not show an obvious difference in the ratio of moving distance in central area and the center area duration (one-way ANOVA, F(2, 32) = 0.2423, P = 0.7863, Figure 3D; one-way ANOVA, F(2, 32) = 0.0471, P = 0.9541, Figure 3E). In the elevated plus maze test, the total entry frequency of both arms in SST-Cre:AC3f/f mice was significantly lower than that in the AC3flox/flox mice and SST-Cre mice (one-way ANOVA, F(2, 32) = 14.62, P < 0.0001; Figure 3F). Further analysis revealed that SST-Cre:AC3f/f mice spent less time in the open arms (one-way ANOVA, F(2, 32) = 6.052, P = 0.0062; Figure 3G) and a decreased percentage of open arms entries (one-way ANOVA, F(2, 32) = 6.768, P = 0.0035; Figure 3H) relative to AC3flox/flox mice and SST-Cre mice.
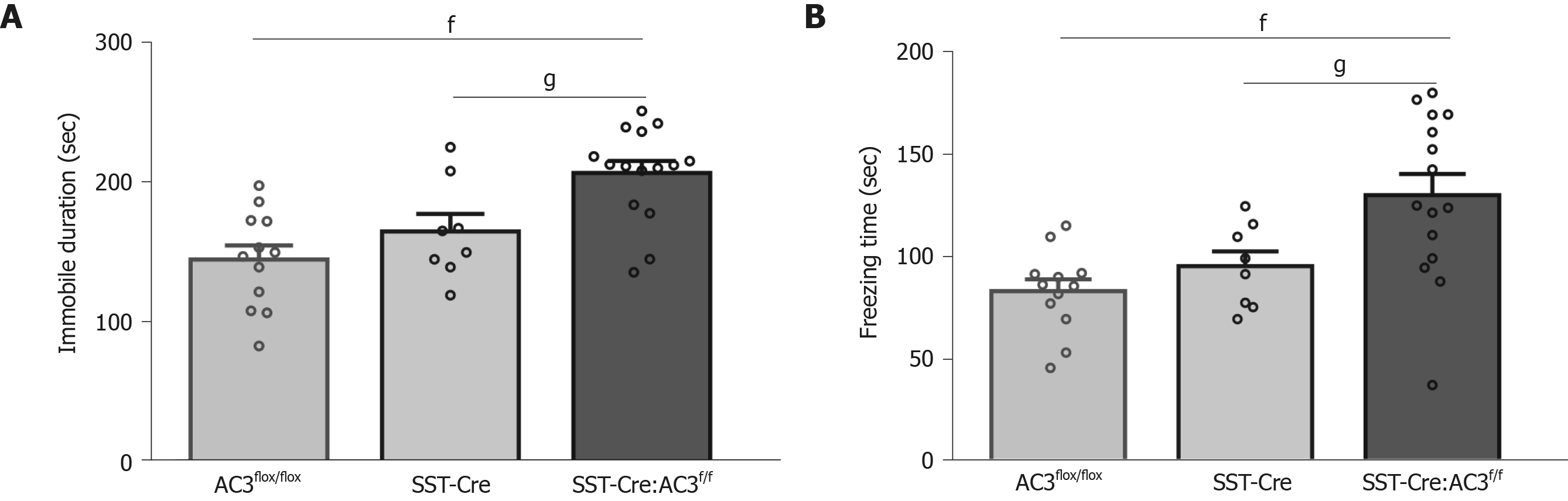
We also used the FST and TST to evaluate the depression level of the mice, since anxiety and depression are common comorbid psychiatric disorders among patients. SST-Cre:AC3f/f mice showed increased immobility time in the FST (one-way ANOVA, F(2, 32) = 11.32, P = 0.0002; Figure 4A), and prolonged freezing time in TST (one-way ANOVA, F(2, 32) = 8.509, P = 0.0011; Figure 4B) compared with AC3flox/flox mice and SST-Cre mice. These data suggest that the SST-Cre:AC3f/f mice exhibit depression-like behaviors in two different test paradigms. There was no significant difference between the AC3flox/flox group and SST-Cre mice in these behavioral tests.
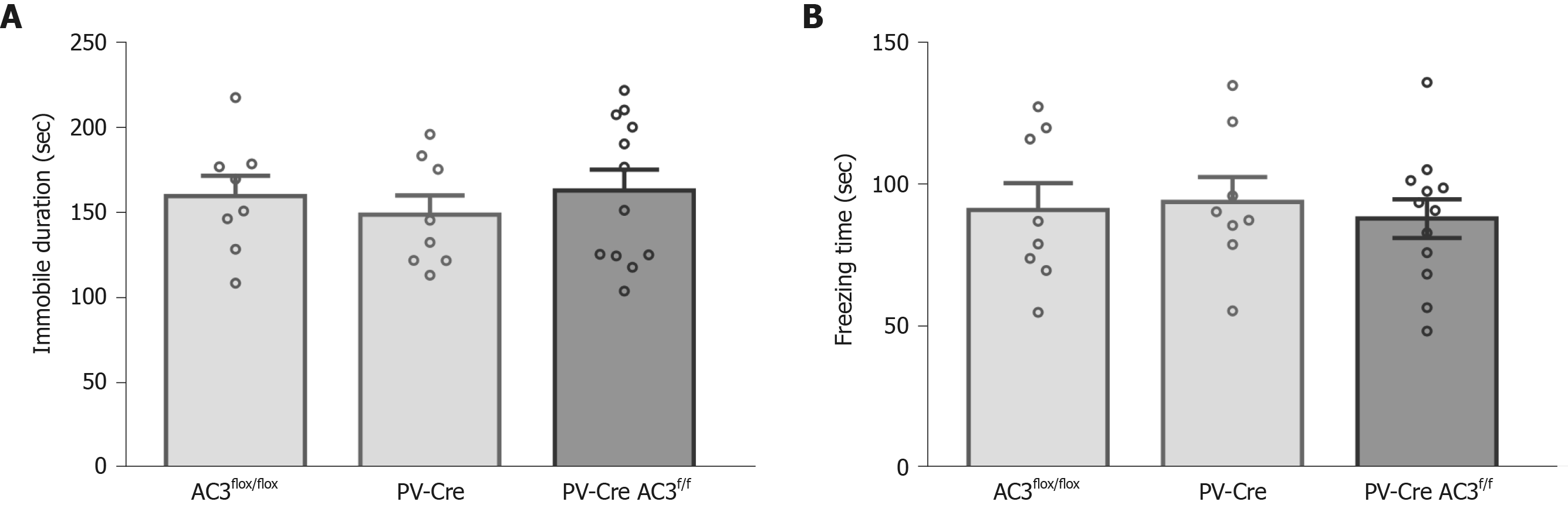
PV+ interneurons are another major group of GABAergic interneurons which are thought to play a vital role in mood disorders. Whether ablation of AC3 in PV+ interneurons influences anxiety- or depression-like behaviors was unknown. Here, AC3flox/flox mice were crossed with the PV-Cre mice for specific disruption of AC3 expression in the PV+ interneurons (Figure 5A). Ablation of AC3 expression in PV+ GABAergic interneurons in PV-Cre:AC3f/f mice, but not in control AC3flox/flox mice, was confirmed by double immunostaining (Figure 5B). Then we examined PV-Cre:AC3f/f mice for anxiety- and depression-like behaviors. The PV-Cre:AC3f/f mice moved a similar total distance and percentage of central distance (one-way ANOVA, F(2, 25) = 0.2446, P = 0.7849, Figure 5C; one-way ANOVA, F(2, 25) = 0.08463, P = 0.9191, Figure 5D) and spent a similar period of time in the center arena of the open field (one-way ANOVA, F(2, 25) = 0.246, P = 0.7838; Figure 5E) either to control AC3flox/flox or PV-Cre mice. These mice also spent comparable time in the open arms (one-way ANOVA, F(2, 25) = 0.1231, P = 0.8847; Figure 5G) and the entries to both arms and the frequency of entries to the open arms is similar compared with AC3flox/flox mice and PV-Cre mice (one-way ANOVA, F(2, 25) = 0.082, P = 0.9219, Figure 5F; one-way ANOVA, F(2, 25) = 0.7036, P = 0.5044, Figure 5H). Also, there was no significant differences in the immobility in the FST (one-way ANOVA, F(2, 25) = 0.3572, P = 0.7032, Figure 6A) or the freezing time in the tail-suspension test when selective deletion of AC3 in PV+ interneurons (one-way ANOVA, F(2, 25) = 0.136, P = 0.8735, Figure 6B). Taken together, PV-Cre:AC3f/f mice did not exhibit a pro-anxiety or pro-depression phenotype.
In this study, we determined if deletion of AC3 expression in two major GABAergic interneurons increases anxiety- and depression-related behaviors in mice. Our results indicated that the majority of SST+ and PV+ interneurons express AC3. Selective ablation of AC3 in SST+ but not PV+ interneurons induced anxiety- and depression-like behaviors, suggesting that loss of AC3 in the SST+ interneurons may play an important role in the development of anxiety and depression in humans.
The balance between excitation and inhibition of neuronal circuit in the brain is interrupted in several neuropsychiatric disorders including MDD[7]. Recent evidence has shown that a deficit of inhibitory synaptic transmission contributes to the pathophysiology and development of MDD. Two major GABAergic interneurons, SST+ and PV+ cells, are subjects of interest in MDD. SST+ interneuron synapses on the dendritic tufts of the nearby pyramidal neurons and is critical for gating the pattern of functional connectivity inputs[32]. In contrast to SST+ interneurons, PV+ GABAergic interneurons mainly target the soma of pyramidal neurons, thereby regulating action potential firing and promoting synchronization of electrical activity[33]. So, it is perhaps not so surprising that these two types of GABAergic interneurons play specific roles in different kinds of psychiatry disorders owing to their distinct electrical, neurochemical and structural properties. Our study indicates that the mice with selective ablation of AC3 from SST+ but not PV+ interneurons showed an anxiety- and depression-like behaviors, supporting the notion that specific subpopulation of GABAergic interneurons are functionally different. Our data are also consistent with several previous findings. For example, studies of brain samples from MDD corpses found that SST neurons were significantly attenuated and that expression of SST mRNA was reduced in multiple brain areas[14,34]. However, less obvious changes in PV+ interneurons in MDD patients were reported[18-20] although a reduced SgACC PV expression has been reported[21]. Moreover, contributions of SST+ but not PV+ interneurons to the pathophysiology of MDD has been directly supported through genetic and pharmacological manipulations in rodents. SST global knockout mice showed increased anxiety- and depression-like behaviors, elevated corticosterone level and decreased expression of BDNF, GAD67 and cortistatin genes expression[17]. However, selective activation of PV+ interneurons in the dentate gyrus of the hippocampus did not affect depression-like behavior in the TST[35]. Furthermore, mice with reduced BDNF expression (heterozygous deletion of BDNF gene [BDNF+/-] or targeted disruption of exon IV [BDNFKIV]) exhibited depressive-like behaviors and a robust and significant decrease of SST but not changes in PV expression[21]. Taken collectively, these previous studies and ours strongly indicated that central SST+ interneurons, as one type of susceptible GABAergic interneurons during MDD, participated in the pathophysiology of anxiety- and depression-related disorders.
Previous studies from mice and humans have suggested a role for deficiencies in adenylyl cyclase and cAMP in depression and antidepressant responses[36,37]. However, the relationship with adenylyl cyclase and depression is more complex because of the diversity of adenylyl cyclase isoforms. A notable finding of present study is that SST-Cre:AC3f/f mice produced anxiety- and depression-like behaviors after selective disruption of AC3 in SST+ interneurons, suggesting that reduction of AC3 expressed in SST+ interneurons may contribute to MDD. The data in this study is also confirmed with our previous studies showing that disruption of AC3 gene globally, conditionally, or targeted to forebrain caused depression-like behaviors in transgenic mice[30]. We do not know why loss of AC3, which is expressed in the primary cilia of CNS neurons, caused depression in mice. However, ablation of type III adenylyl cyclase in mice causes reduced neuronal activity and altered sleep patterns[30]. This suggests the interesting possibility that the depression-like behavior seen with AC3-/- mice might be caused by a chronically decreased SST+ neuronal excitability, subsequently impairment of GABAergic inhibitory synaptic input from SST+ interneurons to pyramid cells, which is consistent with the GABAergic deficit hypothesis of MDD.
In summary, primary cilia in SST+ interneurons may sense extracellular signals through non-synaptic transmission and transduce it into the neurons. Then the cellular signals may regulate by AC3 in primary cilia and effect on the activity of SST+ interneurons. The changes in SST+ interneurons may further affect the downstream neurons and modulate anxiety- and depression-like behaviors in mice.
Major depressive disorder (MDD) is a highly disabling and phenotypically heterogeneous psychiatric syndrome that is among the leading contributors to social and economic burden.
Studies from patients and animal models suggest a key role for functional imbalances between the excitatory and inhibitory neurotransmitters in the central nervous system. Somatostatin-positive (SST+) and parvalbumin-positive (PV+) neurons are two major GABAergic interneurons and play roles in excitation/inhibition balance. Type 3 adenylyl cyclase (AC3) has been reported as a top-ranked gene in MDD and our previous study indicated that AC3 globally knockout mice showed depression-like behaviors. We hope to know whether AC3 in these two subtypes of interneurons contributes to the pathophysiological process of depression.
To determine whether ablation of AC3 gene in different subtypes of GABAergic interneurons of mice produces depression-like behaviors.
Immunohistochemistry, genetic manipulations, and a series of behavior tests were used in this study.
We found that selective disruption of AC3 in SST+ but not PV+ interneurons caused anxiety- and depression-like behaviors.
AC3 in SST+ interneurons play a key role in the etiology of depression.
The AC3-dependent molecular mechanisms in SST+ interneuron underlying MDD will be further explored.
We thank Dr. Qin GY for the biostatistics check.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Chinese Association for Physiological Sciences, No. M063100060M; Chinese Society for Neuroscience, No. S4209100204M.
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Contreras CM S-Editor: Huang P L-Editor: Filipodia P-Editor: Li JH
| 1. | Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095-3105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5389] [Cited by in RCA: 5372] [Article Influence: 244.2] [Reference Citation Analysis (0)] |
| 2. | Bauer M, Bschor T, Pfennig A, Whybrow PC, Angst J, Versiani M, Möller HJ; WFSBP Task Force on Unipolar Depressive Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Unipolar Depressive Disorders in Primary Care. World J Biol Psychiatry. 2007;8:67-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 3. | Nutt DJ. The neuropharmacology of serotonin and noradrenaline in depression. Int Clin Psychopharmacol. 2002;17 Suppl 1:S1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Aguglia E, Biggio G, Signorelli MS, Mencacci C; Steering Committee on behalf of the STIMA-D Investigators. Italian Study on Depressive Disorders (STudio Italiano MAlattia Depressiva, or STIMA-D): a nationwide snapshot of the status of treatment for major depression. Pharmacopsychiatry. 2014;47:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 2691] [Article Influence: 141.6] [Reference Citation Analysis (0)] |
| 6. | Duman RS, Sanacora G, Krystal JH. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron. 2019;102:75-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 651] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 7. | Fogaça MV, Duman RS. Cortical GABAergic Dysfunction in Stress and Depression: New Insights for Therapeutic Interventions. Front Cell Neurosci. 2019;13:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 240] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 8. | Ghosal S, Hare B, Duman RS. Prefrontal Cortex GABAergic Deficits and Circuit Dysfunction in the Pathophysiology and Treatment of Chronic Stress and Depression. Curr Opin Behav Sci. 2017;14:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 9. | Fee C, Banasr M, Sibille E. Somatostatin-Positive Gamma-Aminobutyric Acid Interneuron Deficits in Depression: Cortical Microcircuit and Therapeutic Perspectives. Biol Psychiatry. 2017;82:549-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 10. | Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 645] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 11. | Oliva AA Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354-3368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 335] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | DeFelipe J, López-Cruz PL, Benavides-Piccione R, Bielza C, Larrañaga P, Anderson S, Burkhalter A, Cauli B, Fairén A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, González-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvárday Z, Kubota Y, Lewis DA, Marín O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JL, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamás G, Thomson A, Wang Y, Yuste R, Ascoli GA. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013;14:202-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 602] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 13. | Yavorska I, Wehr M. Somatostatin-Expressing Inhibitory Interneurons in Cortical Circuits. Front Neural Circuits. 2016;10:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, Tseng GC, Lewis DA, Sibille E. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17:1130-1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 15. | Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Tripp A, Kota RS, Lewis DA, Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2011;42:116-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Lin LC, Sibille E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry. 2015;20:377-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 18. | Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 298] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Cotter D, Landau S, Beasley C, Stevenson R, Chana G, MacMillan L, Everall I. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry. 2002;51:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Rajkowska G, O'Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 321] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 21. | Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 208] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Banasr M, Lepack A, Fee C, Duric V, Maldonado-Aviles J, DiLeone R, Sibille E, Duman RS, Sanacora G. Characterization of GABAergic marker expression in the chronic unpredictable stress model of depression. Chronic Stress (Thousand Oaks). 2017;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Nowak B, Zadrożna M, Ossowska G, Sowa-Kućma M, Gruca P, Papp M, Dybała M, Pilc A, Nowak G. Alterations in hippocampal calcium-binding neurons induced by stress models of depression: a preliminary assessment. Pharmacol Rep. 2010;62:1204-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Zadrożna M, Nowak B, Łasoń-Tyburkiewicz M, Wolak M, Sowa-Kućma M, Papp M, Ossowska G, Pilc A, Nowak G. Different pattern of changes in calcium binding proteins immunoreactivity in the medial prefrontal cortex of rats exposed to stress models of depression. Pharmacol Rep. 2011;63:1539-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Czéh B, Vardya I, Varga Z, Febbraro F, Csabai D, Martis LS, Højgaard K, Henningsen K, Bouzinova EV, Miseta A, Jensen K, Wiborg O. Long-Term Stress Disrupts the Structural and Functional Integrity of GABAergic Neuronal Networks in the Medial Prefrontal Cortex of Rats. Front Cell Neurosci. 2018;12:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 26. | Shepard R, Coutellier L. Changes in the Prefrontal Glutamatergic and Parvalbumin Systems of Mice Exposed to Unpredictable Chronic Stress. Mol Neurobiol. 2018;55:2591-2602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 27. | Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505:562-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 255] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 28. | Nicol X, Gaspar P. Routes to cAMP: shaping neuronal connectivity with distinct adenylate cyclases. Eur J Neurosci. 2014;39:1742-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Wray NR, Pergadia ML, Blackwood DH, Penninx BW, Gordon SD, Nyholt DR, Ripke S, MacIntyre DJ, McGhee KA, Maclean AW, Smit JH, Hottenga JJ, Willemsen G, Middeldorp CM, de Geus EJ, Lewis CM, McGuffin P, Hickie IB, van den Oord EJ, Liu JZ, Macgregor S, McEvoy BP, Byrne EM, Medland SE, Statham DJ, Henders AK, Heath AC, Montgomery GW, Martin NG, Boomsma DI, Madden PA, Sullivan PF. Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol Psychiatry. 2012;17:36-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 336] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 30. | Chen X, Luo J, Leng Y, Yang Y, Zweifel LS, Palmiter RD, Storm DR. Ablation of Type III Adenylyl Cyclase in Mice Causes Reduced Neuronal Activity, Altered Sleep Pattern, and Depression-like Phenotypes. Biol Psychiatry. 2016;80:836-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Cao VY, Ye Y, Mastwal S, Ren M, Coon M, Liu Q, Costa RM, Wang KH. Motor Learning Consolidates Arc-Expressing Neuronal Ensembles in Secondary Motor Cortex. Neuron. 2015;86:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 32. | Chiu CQ, Martenson JS, Yamazaki M, Natsume R, Sakimura K, Tomita S, Tavalin SJ, Higley MJ. Input-Specific NMDAR-Dependent Potentiation of Dendritic GABAergic Inhibition. Neuron 2018; 97: 368-377. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 33. | Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1916] [Cited by in RCA: 2154] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 34. | Douillard-Guilloux G, Lewis D, Seney ML, Sibille E. Decrease in somatostatin-positive cell density in the amygdala of females with major depression. Depress Anxiety. 2017;34:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Zou D, Chen L, Deng D, Jiang D, Dong F, McSweeney C, Zhou Y, Liu L, Chen G, Wu Y, Mao Y. DREADD in parvalbumin interneurons of the dentate gyrus modulates anxiety, social interaction and memory extinction. Curr Mol Med. 2016;16:91-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Hines LM, Tabakoff B; WHO/ISBRA Study on State and Trait Markers of Alcohol Use and Dependence Investigators. Platelet adenylyl cyclase activity: a biological marker for major depression and recent drug use. Biol Psychiatry. 2005;58:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Rasenick MM. Depression and Adenylyl Cyclase: Sorting Out the Signals. Biol Psychiatry. 2016;80:812-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |