INTRODUCTION
Traumatic brain injury (TBI) has been consistently connected to a broad spectrum of pathologies including post-injury cognitive and sensory deficits[1]. According to the Centers for Disease Control and Prevention’s (CDC) most recent statistics, TBI is responsible for approximately 2.9 million hospitalizations, emergency department (ED) visits, and deaths in the United States alone[2,3]. Within those 2.9 million cases, nearly 1 million of them were in children[2]. TBI related ED visits rose 53% from early 2000’s to mid-2010’s, and most signs point to these numbers continuing their annual rise in the future with only minor fluctuations[2,3]. The age group with the highest rates for TBI are ages 75 and older[2]. Additionally, the leading cause of TBI related deaths change for each age bracket. For ages 0 to 4, the leading cause for TBI related death was homicide. For ages 15-24, 25-34, and greater than 75 years, the leading cause was motor vehicle accidents. The leading cause for 45-64 years old is intentional self-harm. Finally, falls accounted for the highest rate of TBI related death for age 65-74[2].
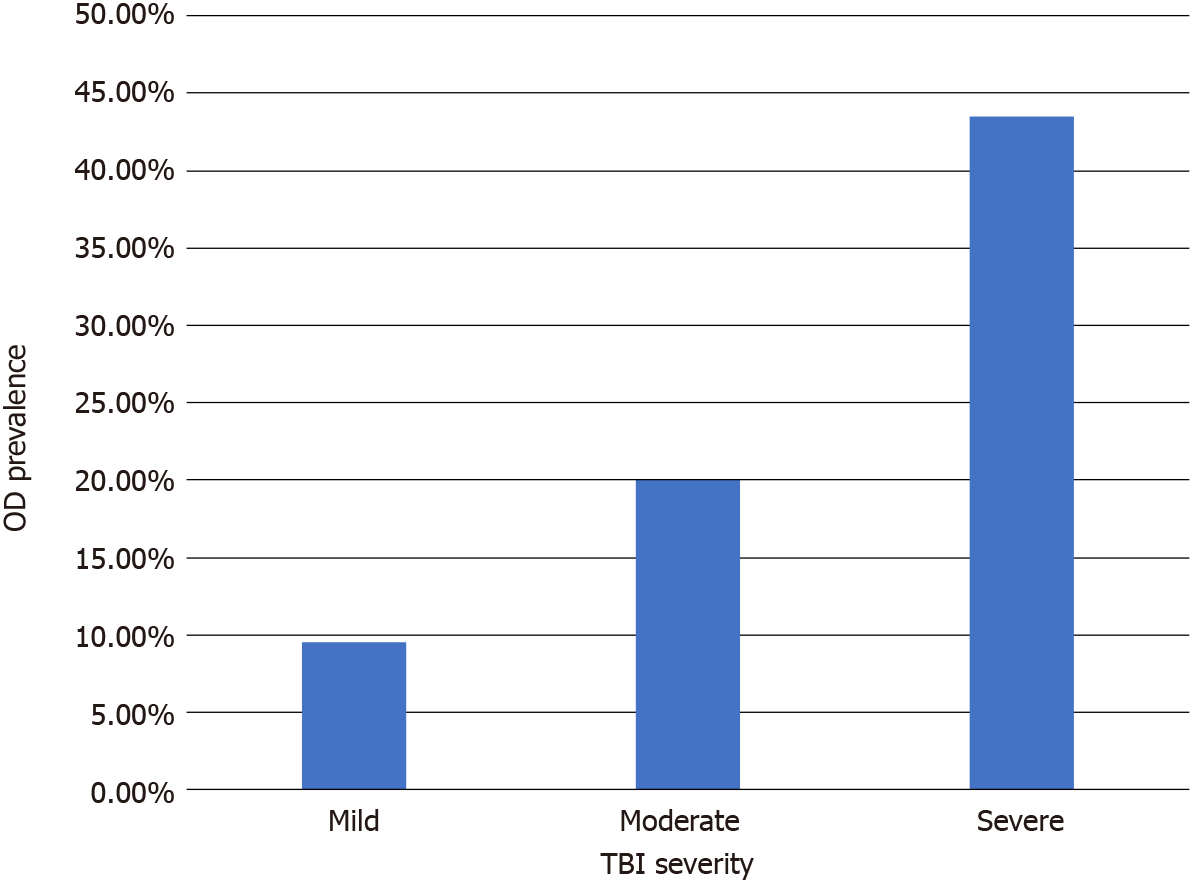
TBI can range from mild effects, such as transient mental status changes, to lasting deficits in motor skills, sensory perception, and cognitive ability[1,2,4]. Because of the wide range in severity of TBI symptoms, there is an extensive amount of literature and research within the scientific community into the etiology, pathophysiology, symptoms, and treatment options for TBI. However, an often-overlooked side effect which can be attributed to TBI is olfactory disturbance (OD). Due to the mechanism and the biophysics of acutely rapid acceleration immediately followed by deceleration associated with TBI (coup-contrecoup injury), the olfactory bulbs and the olfactory epithelium are at high risk for damage via exogenous trauma induced shearing[5,6]; as the severity of the TBI increases, so does the risk of OD. Recent literature shows OD incidence rates (specifically anosmia) of 9.5% for mild TBI, 20% for moderate TBI, and 43.5% for severe TBI[5].
When attempting to understand the cause-and-effect relationship of TBI’s direct association with OD, it is important to discern the role of head trauma as one of the main origins[7,8]. As noted in the extent literature, there are varying degrees of dysfunction encompassed by the TBI diagnosis, many of which depend on the localization of injury[9]. This has been found to be the case with OD as well, given that not all parts of the brain known to play a role in the process of olfaction are isolated to one specific area[8,10]. In instances of cerebral neurotrauma, there is a greater chance of damage to the olfactory bulb and other anterior structures, such as the olfactory cortex (OFC)[11,12]. If the trauma is more severe and diffuse in nature, there is a greater chance of disruption to sensory integration pathways, located in more posterior sections of the brain[13]. A common result of damage to the olfactory integration chain often manifests as parosmia, a dysfunction in the specificity of smell detection[14]. The replacement of normal, appetizing food aromas with repugnant odors, more associated with malodorous substances[14], is an applicable example. There is no one exclusive type of impairment or location of damage across all TBI patients[15]. Given the strong association of OD with TBI, we can conclude that multiple variants of OD can exist, particularly when considering the effects of coup-contrecoup injury[7,11,14,16].
The types of dysfunction classified as OD can range from a simple difficulty in recognizing aromas, to further disruptive forms of parosmia, and even complete anosmia or absence of olfactory perception[7,14,16]. There are multiple mechanisms for post-traumatic OD. The most common etiology, however, is related to the “anterior-posterior movement” of the brain in relation to the interior of the skull itself, from prior mentioned coup-contrecoup injury[7,11].
OD’s severity can vary greatly, typically categorized as either total loss of smell (anosmia), decreased smell sensitivity (hyposmia), or altered sense of smell (dysosmia/parosmia). OD is a prevalent condition in the United States, with an estimated rate of 10%-15% in adults over the age of 40 having disrupted smell in any given year[5,17]. When there is a sensory disturbance as prevalent as OD, we must also assess other potential subsequent problems that can come secondary to OD. A significant association to OD is depression and related disorders. Both depression and OD have neuroanatomical structures that contain common layers[18]. However, the similarities between these anatomical substrates lead to more complications in clinical differentials. Not only can depression be secondary to OD, but the inverse can also be true. In addition to developing depression post-OD, olfactory functioning can also be negatively impacted by depressive states[18], rendering this “chicken or the egg” phenomenon a challenge in accurate clinical differentials.
TBI is also associated with an increased risk of depressive symptoms. Depression has been a well-known clinical sequelae of TBI and the literature on this topic has been established for decades[19]. Additional literature also has been illustrating how clinicians can improve their diagnostic clarity for major depressive disorder (MDD), as well as how much loss of consciousness (LOC) can play a role in the severity of MDD symptoms and overall cognitive functioning[20,21]. While the estimated rate of depression in TBI patients does vary greatly (anywhere from 6%-76%), the literature has established that the incidence of MDD within the TBI population is significantly higher than the general population[21-23]. Nonetheless, given the wide range of current prevalence estimates, continued pursuit of controlled studies to address said variance is needed.
TBI AND SENSORY LOSS
As supported by the literature, the volume, or amount of gray matter and associated neurological connections within the olfactory bulb, has been associated with varying levels of functionality[9]. In patients with a decreased olfactory bulb volume, there is a strong correlation with decreased olfactory function, as evidenced by poor performance on various multi-modal examinations[14,24]. This trend of decreased olfactory capacity is augmented by the presence of TBI, or more specifically, its location and severity[14,15]. As noted previously, in patients with more diffuse neurocognitive injury, there is an increased chance of greater complexity in OD, relative to frontal lobe damage and resulting anosmia[14].
Incidence rates for complete anosmia after TBI range from 9.5%-43.5% and are highly correlated with the severity of the TBI[5]. This is compared to the approximately 20 million adults over age forty in the United States who suffer from OD[25].
In considering TBI as a primary source of OD[7,8], it is estimated that an approximate one-year recovery period exists, where the patient has the potential to gradually regain any function that was otherwise lost or impaired by their TBI. This is however, also often followed by a plateau of functional re-acquisition[15]. Similar to the global phenomenon witnessed in TBI patients, individuals with OD, specifically, may gradually regain their olfactory functionality[26,27]. This has been hypothesized to be related to the synaptogenesis of nerve cells connected to the areas damaged by the TBI, such as the olfactory bulb or OFC[28].
The olfactory system is a complex network of systems that make up one of the oldest sensory modalities of mammals[29]. The olfactory epithelium is found on the superior medial vertical lamellae of the superior turbinates, and is made up of three different types of cells: Basal, supporting, and olfactory receptor[29,30]. The olfactory system experiences consistent turnover and regeneration of its adult neurons[29]. A key part of the olfactory mechanism is the olfactory bulb, which receives the signals from the olfactory epithelial cells and continues the communication process for olfaction to be executed[30,31]. As mentioned prior, when the nerve fibers of the olfactory receptor cells are sheared or stretched from the coup-contrecoup injury, they are no longer connected to the olfactory bulb[30,31]. This leads to the disruption of sensory signals within the olfactory pathway, resulting in anosmia[5,25,30].
The epithelial cells present within the olfactory system are the only known neuron group that possess the ability to regenerate when damaged, contingent to the integrity of the olfactory bulbs[25,29]. Costanzo[31] states that “the olfactory epithelium retains its capacity to undergo neurogenesis long after development”, and that the degree of neuronal recovery varies with the severity of the injury. There are multiple barriers to effective regeneration, including spatial challenges from disruption of axon sheath alignments, fibrosis over the olfactory bulb, and/or broken synaptic sites[31]. With multiple challenges, it becomes exponentially complex for the neurons to reconnect to the olfactory bulbs. This likely explains a correlation between the severity of the TBI and the difficulty of functional recovery of olfactory senses (Figure 1).
Figure 1 Olfactory disturbance prevalence by traumatic brain injury severity.
OD: Olfactory disturbance; TBI: Traumatic brain injury.
Currently, many modalities and tools exist to identify the various forms of neurological disruption. Psychophysical and electrophysiological assessments, in addition to neuroimaging techniques, are among the tools used to diagnose OD[32-35]. In recent years, however, a more advanced form of imaging has garnered interest for its ability to scan restricted areas of the brain with more precision[32]. Known as diffusion tensor imaging (DTI), has already demonstrated itself to be a useful tool, capable of providing enhanced insight into the etiopathogenesis of the neurological disruption associated with OD[13].
DTI employs fractional anisotropy, the indices of the diffusion of water molecules across white matter tracts in the brain[13,32], in comparison to more established, de facto neuroimaging. This technique provides a more detailed, in vivo quantification of nerve fila and white matter microstructure, down to the individual nerve fibers[36,37]. In terms of its relevance to OD, medical professionals now possess the ability to illustrate the severance of nerve fibers from the olfactory bulb or other essential areas that play a role in the sensory integration of olfaction[12,13,32]. This enables the visualization of minute or gradual regrowth that is often missed by other forms of neuroimaging[13].
A 2017 study by Bonanno et al[13] focused on the role that DTI may play in the treatment of TBI and related OD; more specifically, the discrepancy between MRI and DTI were highlighted[13]. Both of the imaging techniques were performed within the same time period, first at baseline shortly after the TBI and then again, at 1-year status/post[13]. MRI demonstrated the presence of encephalomalacia, while the DTI scan was remarkably able to reveal significant axonal regrowth in both the left and right hemispheres[13,38]. This was demonstrated by as much as a 142% increase in average length of fiber tracts within the right hemisphere alone, relative to a baseline scan performed shortly after the TBI[13]. A 112% increase in the overall number of fiber tracts was also observed[13]. While DTI may be an effective tool in tracking white matter changes in the brain, additional studies utilizing the imaging technique for the examination of OD, in particular, are needed[13].
SENSORY LOSS AND DEPRESSION
Depression is common in the population, with estimates that 8%-12% of individuals in the United States will be affected by depression at least once in their lifetime[39]. The 2017 study published by the National Survey on Drug Use and Health revealed that the prevalence of a major depressive episode in adult females (8.7%) was significantly higher than adult males (5.3%) when accounting for sex, and that the 18-25 age bracket is home to the highest prevalence rate (13.1%) when accounting for age. In addition, individuals who reported two or more races had the highest rates (11.3%) when accounting for race[40]. In 2017 alone, 4.5% of the United States adult population experienced at least one major depressive episode with severe impairment, which also made up approximately 64% of major depressive episodes[40]. These statistical trends continue when looking at adolescents, but all of the prevalence rates are increased[40]. This shows that depression is still a prevalent issue in society and most people who are experiencing depressive episodes have severe impairment.
The literature also illustrate links between sensory loss, specifically olfactory functioning, and various mental health conditions including schizophrenia[17,39]. Deems et al[41] studied the relationship between depression scores and OD patients revealed that sensory dysfunction influences quality of life[41]. Subsequent studies have been conducted to explore the relationship between depression and OD. However, most of these studies have shown mixed results, which is likely related to inconsistency within the study populations[17,39,42]. While some studies focused on people reporting primary olfactory loss, others included patients with primary depression.
Despite the need for ongoing research to clarify the exact relationship between OD and depression, we can still deduce many things from this relationship. We know that when the olfactory bulb suffers from reduced input, there is a clear negative relationship with neurotransmitter concentration, leading to the potential disturbance of emotional functioning[18]. Additionally, olfactory functions are highly involved in emotion and memory due to signal exchanges from the olfactory bulb to the hippocampus and amygdala[39]. There is also a correlation between OD and depression symptoms that is mediated by the severity of the loss of smell[17]. Based on longitudinal studies, there is a predictive relationship between one month follow up parosmia scores and six month follow up depression and anxiety scores[42]. It has also been demonstrated that treatment for OD can lead to a decrease in depression scores, even if more replication is needed to increase confidence in that claim[39]. There is also evidence for clinicians to include both affective and olfactory assessments in TBI cases, as it can aid in the prediction of affective outcomes[42]. In summary, there is a bidirectional relationship between sensory loss and depression.
In addition, the recent coronavirus disease 2019 (COVID-19) pandemic has led to some further development in research, especially in the context of sensory loss. It is well known to most that sensory loss, primarily smell and taste, is among the more prevalent symptoms of COVID-19[43]. Other neurological injuries can also occur following COVID-19 infection, including strokes, that can impair affective and sensory functioning[44]. While researches on reinfection rates, mortality predictors, and electrocardiogram readings are still being investigated, there are affective concerns that should continue to be addressed[45-47]. Within the context of affective distress with known COVID-19 related symptoms including anosmia, self-care remains critical prior to regain of function back to baseline[48]. It is worth investigating whether this COVID-19 related sensory loss will hold the same link between olfactory sensory loss and long-term affective distress as discussed above.
TBI AND DEPRESSION
TBI accounted for nearly 3 million ED visits in 2014, which was up from 1.2 million in 2006, and has been experiencing steady increases in its annual death rate and hospitalization rates[1,9]. Recent estimates show that the annual financial cost of TBI associated problems is more than 56 billion dollars[22]. In addition to this great financial cost, there is also a high risk of developing symptoms of depression, impaired life satisfaction, and various chronic disabilities[2,19-23,49]. Once the age bracket where TBI is most common (age 75+) is compared to the prevalence rates of depression (ages 18-25), it is easy to see these risky age groups tend not to overlap[2,23,40]. Despite this, we are seeing an increase in depressive rates within TBI patients which tend to be significantly different than the general population rates. Depression also negatively impacts proper recovery trajectory after a TBI[19].
Within TBI patients, some experience a LOC and others do not lose consciousness, with the odds of experiencing a TBI with LOC increasing based on several factors, including TBI severity and various biological and social factors[20,22,50]. One study that analyzed the 2014 data from the Ohio Behavioral Risk Factor Surveillance System showed that 21% of adults reported to have at least one TBI with LOC in their lifetime[50]. That same study showed that various factors associated with an increased reporting of TBI with LOC included lower income, being male, age, and unemployment status[50]. Additional studies have shown that TBI (both with and without LOC) contributes to increased depressive symptoms, lower cognitive functioning, and risk for lifelong neuropsychiatric concerns[20].
It is critical for clinicians to better understand the relationship between TBI and MDD in order to better serve their patients and assist the patients on their road to recovery. Even though many physicians are aware of the link between these two diagnoses, the diagnosis of MDD when a patient is experiencing disturbed mood after a TBI remains a more complex concern. Due to the additional diagnostic criteria of MDD and the differential diagnosis between MDD and the other depressive disorders, many physicians can easily misdiagnose a TBI patient’s affective concerns[21]. More research is needed in this area to show how to qualitatively and quantitatively improve the accuracy of mood disturbance diagnoses within TBI patient population.
CONCLUSION
As one of the world’s leading causes of death and disability, TBI is a highly complex clinical phenomenon. With the bidirectionality of sensory loss and depression, as they are among some of the more common clinical sequelae of TBI, these clinical presentations themselves further complicate posttraumatic prognosis and related treatment planning. In milder injuries, said symptoms can be assessed to assist in differentials, prognostic implications, and guidance for acute therapeutic regimen. In more moderate and severe injuries with permanent injury sequelae, said symptom assessments can also assist with long term care planning, along with guided therapeutics for quality of life improvement. As the literature continues its pursuit in the study of TBI mechanisms, it remains imperative that direct and peripheral symptoms like olfaction and related gustation, affective distress, and their bidirectional implications are continuously investigated, beyond the traditional pathophysiological mechanisms. As this study was formatted as a focused topic narrative review to investigate TBI related sequelae, the study is limited in the quantity of systematically reviewing the TBI variables highlighted in this study. Recommendations for future studies include systematic analyses and review of the literature, meta-analyses, and controlled studies to further assess the bidirectionality of sensory loss and affective distress in TBI.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mehraeen E S-Editor: Fan JR L-Editor: A P-Editor: Fan JR