Published online Dec 19, 2021. doi: 10.5498/wjp.v11.i12.1177
Peer-review started: February 4, 2021
First decision: April 21, 2021
Revised: April 30, 2021
Accepted: November 18, 2021
Article in press: November 18, 2021
Published online: December 19, 2021
Processing time: 313 Days and 23.1 Hours
Polyamines play preeminent roles in a variety of cellular functions in the central nervous system and other organs. A large body of evidence suggests that the polyamine pathway is prominently involved in the etiology and pathology of schizophrenia. Alterations in the expression and activity of polyamine metabolizing enzymes, as well as changes in the levels of the individual polyamines, their precursors and derivatives, have been measured in schizophrenia and animal models of the disease. Additionally, neuroleptic treatment has been shown to influence polyamine concentrations in brain and blood of individuals with schizophrenia. Thus, the polyamine system may appear to be a promising target for neuropharmacological treatment of schizophrenia. However, for a number of practical reasons there is currently only limited hope for a polyamine-based schizophrenia therapy.
Core Tip: This review summarizes the advancements in research on the implications of polyamines and their metabolites for schizophrenia. Evidence from clinical and experimental studies show that some members of the polyamine regulatory system are altered in schizophrenia, but no polyamine-based therapy for schizophrenia is currently available.
- Citation: Bernstein HG, Keilhoff G, Laube G, Dobrowolny H, Steiner J. Polyamines and polyamine-metabolizing enzymes in schizophrenia: Current knowledge and concepts of therapy. World J Psychiatr 2021; 11(12): 1177-1190
- URL: https://www.wjgnet.com/2220-3206/full/v11/i12/1177.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i12.1177
Schizophrenia is one of the great scourges of humanity, affecting approximately 1% of the worldwide population. It is a devastating and debilitating mental illness, which appears to result from a complex interplay between genetic and environmental risk factors. Clinically, schizophrenia may be characterized by an array of positive, negative and cognitive symptoms, which include hallucinations, delusions, disorganized speech or executive function, impaired memory and reduced cognitive abilities[1]. Unfortunately, a large percentage of patients with schizophrenia suffer from treatment-resistance. Therefore, there is an urgent need for new treatment opportunity strategies.
Among putative therapeutic targets for schizophrenia treatment are polyamines (PAs), their precursors, derivatives and conversion enzymes. Aliphatic PAs constitute a small family of polycationic molecules derived from decarboxylation of ornithine[2], which play a crucial role in the developing and mature mammalian central nervous system (CNS). The initial suggestion that these were contributing factors to schizophrenia pathology dates back to the late 1950s, when it was shown that N,N-dimethyl-p-phenylenediamine oxidation rates were increased in sera of schizophrenia patients compared to non-psychotic individuals[3,4]. Since then, numerous papers have shown that the PAs, spermine, spermidine and putrescine, as well as their metabolites, are functionally linked with schizophrenia. The goal of this article is to review the current knowledge and insights about the role of the PA system in schizophrenia. Further, we attempt to assess the suitability of PA as targets for therapeutic intervention.
Using relevant search terms we searched published literature (including doctoral theses and patents) from 1 January 1955 to 21 January 2021 in PubMed and Google Scholar. Search terms were schizophrenia in combination with one or more of the following terms: polyamines, spermine, spermidine, putrescine, agmatine, S-adenosylmethionine, acrolein, L-ornithine decarboxylase, antizyme, antizyme inhibitor, spermine oxidase, spermidine synthase, spermidine/spermine N1-acetyltransferase, polyamine oxidase, S-adenosylmethionine decarboxylase, agmatinase and agmatinase-like protein. No language restrictions were applied.
Natural PAs, spermine and spermidine, and their precursor putrescine are present at relatively high concentrations in the mammalian brain. Because of the limited transport of PAs across the blood-brain barrier, their presence in the CNS should largely result from local synthesis (described previously in[5]). Brain PA content is tightly controlled through a complex network of biosynthetic and catabolic enzymes and a recently discovered transport system. However, while there are no doubts about the brain-borne origin of the largest fraction of cerebral PA, the precise cellular locus of PA biosynthesis has been a matter of contention for several years.
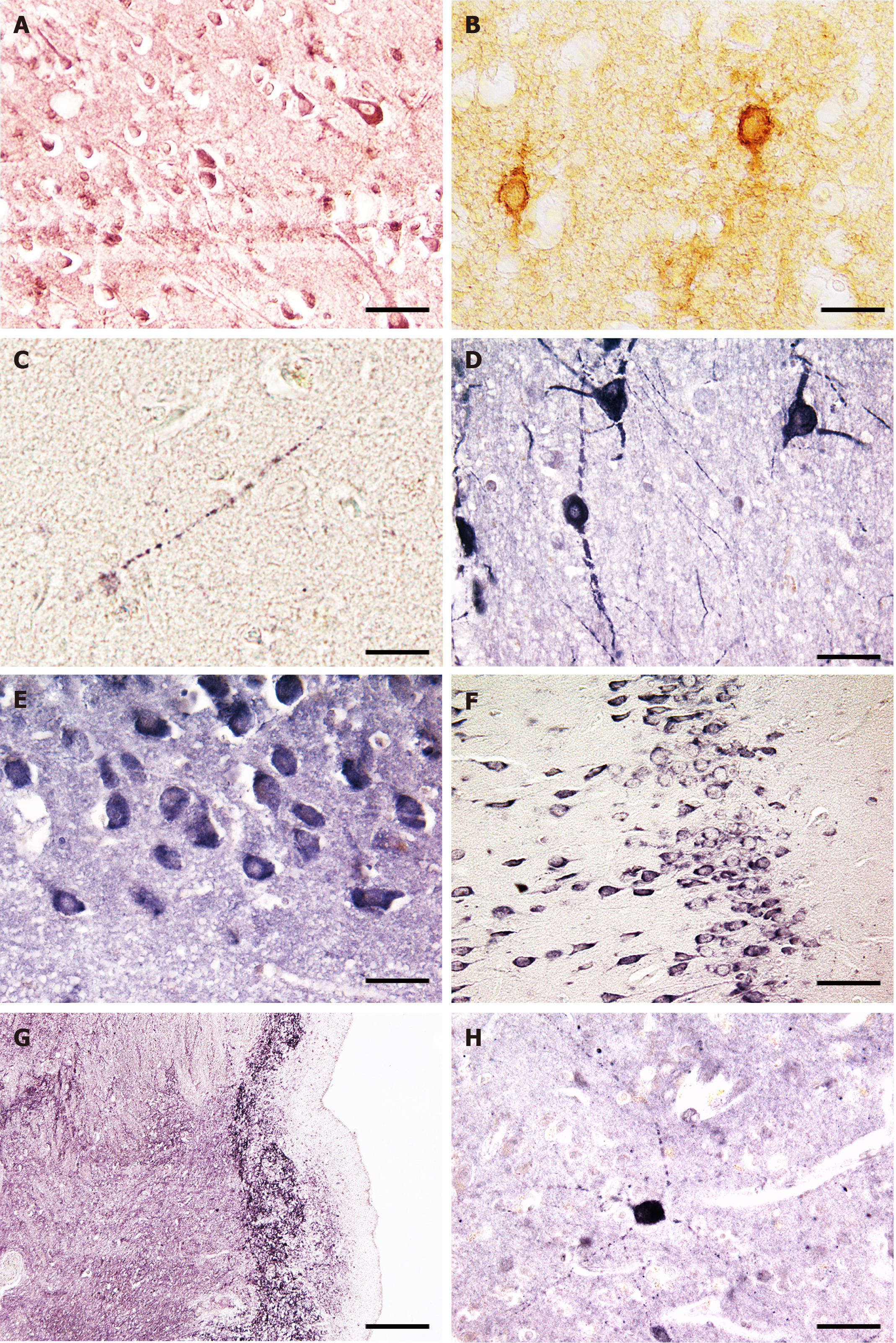
PAs are present at high concentrations both in glial cells (especially astroglia) and neurons[6-9]. Under normal conditions, neurons express L-ornithine decarboxylase (EC 4.1.1.17, ODC; Figure 1A), the rate-limiting enzyme of PA biosynthesis, which generates putrescine from ornithine[10,11]. This suggests that neurons are the primary source of newly synthesized PAs in the brain. Consequently, the observed strong astroglial immunostaining for spermidine and spermine would have to occur due to other reasons. Currently, it is certain that there is an efficient PA transport system involved in translocation of PAs from the site of synthesis in neurons to glial cells, a site of uptake, accumulation and release[9]. Indeed, various vesicular transporters for PAs have been identified[12-15], and these may function bidirectionally[9].
Interestingly, other enzymes and enzyme regulators involved in the metabolism PAs are also predominantly or exclusively located in neurons. The ODC antizyme (which binds to ODC and thereby destabilizes and inactivates the enzyme) and antizyme inhibitors 1 and 2 (AZINs, which both enhance ODC activity) have been found in neurons but not glia[16-18] (Figure 1B and C). Spermidine/spermine N1-acetyltransferase (EC 2.3.1.57, SAT1) is an enzyme responsible for PA interconversion. Its mRNA is widely distributed in neurons, with the highest concentrations found in the hippocampus and olfactory bulb[19]. The enzyme spermidine synthase (EC 2.5.1.22, which catalyzes the interconversion of S-adenosylmethionine to spermidine and 5’methylthioadenosine was localized to multiple rat brain neurons and neuropil of several brain regions [accumbens nucleus, hypothalamus, hippocampus (Figure 1E), cerebral cortex, striatum, cerebellum and others][20,21]. Spermine oxidase (EC 1.5.3.16, SMOX), which catalyzes the conversion of spermidine to spermine, is immunocytochemically detectable in many neurons of the cerebral cortex, hypothalamus, hippocampus, thalamus and cerebellum of human and rat brains (Figure 1D-F).
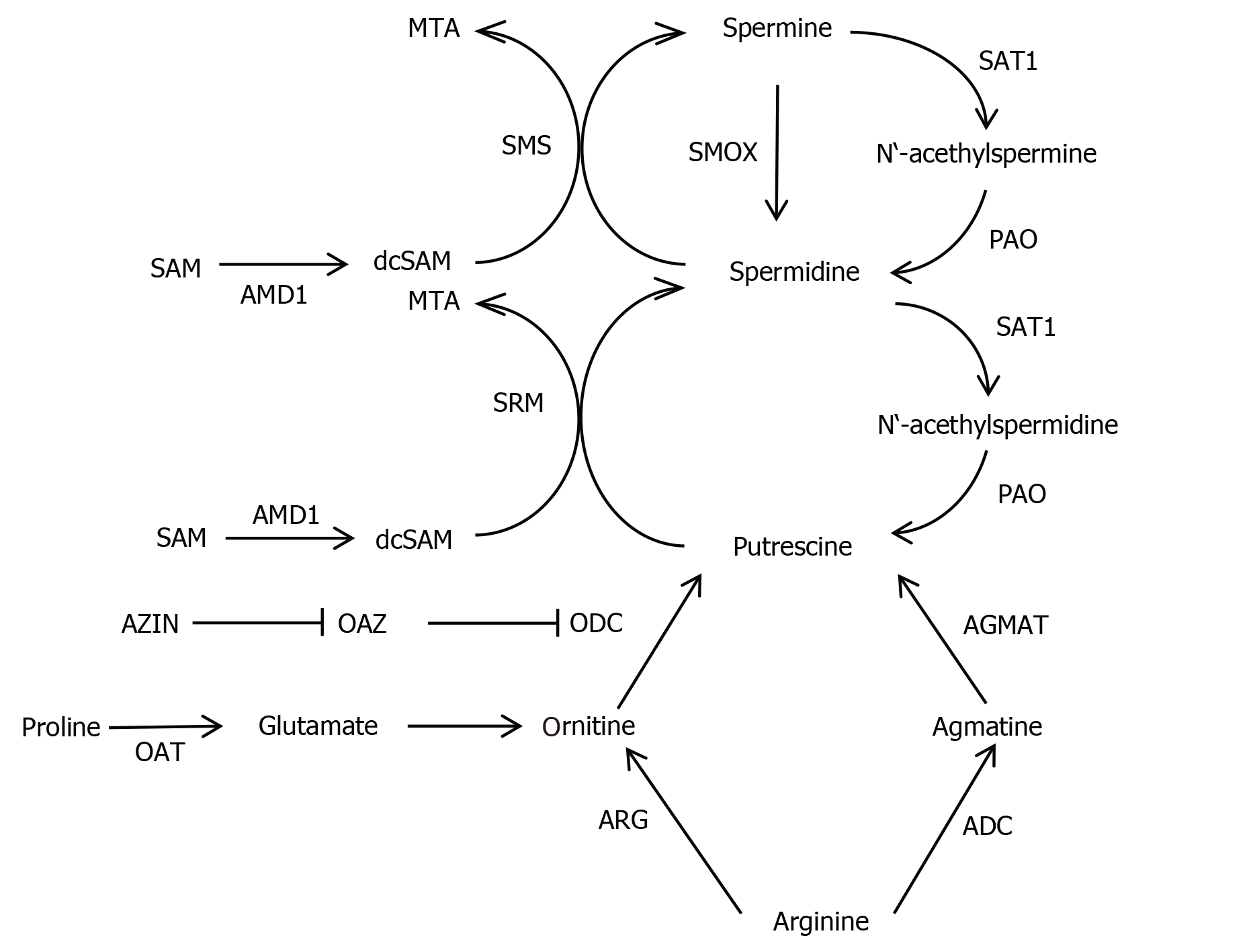
The neuromodulator agmatine, which is a precursor of putrescine, can be detected in synapses[22,23]. Agmatine is highly expressed in magnocellular hypothalamic neurons and many other nerve cell populations[23,24]. The agmatine-degrading enzyme, agmatinase (EC 3.5.3.11) was found in distinct interneurons of rat and human brain, located in cerebral cortex, hippocampus, habenula and cerebellum (Figure 1G and H)[25,26]. Interestingly, a second agmatine-metabolizing enzyme (called agmatinase-like protein) is present in rat brain neurons and astrocytes[27]. Finally, arginase (EC 3.5.3.1, ARG), which converts L-arginine into L-ornithine and urea, and arginine decarboxylase (EC 4.1.1.19, which catalyzes the conversion of L-arginine into agmatine and carbon dioxide) are widely expressed in rat brain neurons[28,29]. Thus, neurons harbor all PA synthesizing and degrading enzymes studied so far apart from the agmatinase-like protein, which is located mainly in astroglial cells[27]. However, the situation may be different in neonatal brains because recent evidence suggests that developing astroglial cells contain catalytically active ODC, synthesize PAs and release these[30]. The major reactions of the PA pathway are shown in Figure 2.
The classical PAs, spermine, spermidine and putrescine are multifunctional chemical compounds, which serve a variety of important tasks in the CNS (for overviews see[5,9,10,30-32]). PA pathways play pivotal roles in the correct development of nervous tissue. There is experimental evidence to suggest that the replication of neurons and their precursor cells are dependent on the maintenance of certain region-and time-specific PA levels[32]. Depletion of PAs through inhibition of ODC arrests brain cell maturation, disrupts neuronal migration, disturbs the outgrowth of neurites and impairs the formation of synapses[11,31,32]. In addition, PAs are prominently involved in proliferation of neonatal astrocytes[30]. In both the pre- and postnatal CNS, PAs act as intracellular growth factors. By directly binding to DNA or the rough endoplasmic reticulum, spermine and spermidine increase the rate of cell growth and control protein synthesis of brain cells[11,33].
In the mammalian CNS PAs act as important endogenous modulators of glutamate receptors and are capable of altering the functioning of N-methyl-D-aspartate (NMDA) receptors. As NMDA receptor ligands, PAs exert both activator and inhibitor effects. On the one hand, PAs can enhance NMDA receptor currents by increasing the probability of channel opening. On the other hand, spermine is able to block NMDA channels in the open state, thereby reducing or blocking NMDA receptor currents by a voltage-dependent reduction of single-channel conductance[34,35]. Another inhibitory effect of spermine is to reduce the sensitivity to glutamate (or other glutamate site agonists) at NMDA receptors composed of NMDA receptor 1/NMDA receptor 2B subunits by reducing the affinity for glutamate[34]. PAs also contribute to alterations of membrane excitability by interacting with ionotropic kainate and AMPA glutamate receptors (discussed in detail in[36]). Modulation of glutamate signaling by PA influences a variety of functional processes in the brain, ranging from regulation of neuronal and glial excitability to memory and aging[37].
Another mechanism through which PAs exert influence on membrane excitability is the blockade of outward potassium currents through, consequently so-called, inwardly-rectifying potassium channels. Inwardly-rectifying potassium channels exhibit a sharp voltage dependence and crucially contribute to maintenance of the resting membrane potential. Thus, they are involved in the regulation of bioelectrical excitation of many cell types including neurons and glial cells[9,37-40]. The basic mechanism underlying this steep voltage dependence is the channel blockade by PA and magnesium. PAs are thought to enter the inwardly-rectifying potassium channel pore via the intracellular side of the membrane and displace multiple ions during their stable binding site within the channel[40].
Lastly, PAs act as free radical scavengers and effective antioxidants. This role is unrelated to the activation of the NMDA receptor[41]. Of note, PA catabolism, which is upregulated after traumatic brain injury and other stressful situations, can be a source of toxic reactive oxygen species[41,42].
The PA agmatine, a decarboxylation product of arginine, is an endogenous ligand of imidazoline, α2-adrenergic and glutamatergic NMDA receptors[43,44]. Agmatine is a neuromodulator and neurotransmitter, which significantly contributes to the regulation of various neurotransmitters and signaling pathways (reviewed in[43-47]). Several studies have demonstrated that agmatine is involved in cognitive processes[47-50]. In addition, it performs a neuroprotective function by reducing oxidative damage, neuroinflammation and proapoptotic signaling[43,51].
In the early 1980s it was hypothesized that PA might play a central role in the etiology of schizophrenia[52]. Since then, numerous papers have appeared in support of this conjecture, while others call in question a significant role of PA in schizophrenia development and persistence.
Compared with psychically healthy individuals, tissue and body fluid concentrations of some PAs are altered in patients suffering from schizophrenia. Since single nucleotide polymorphisms in the intron region of ODC (+316 G>A) and the promoter region of the SAT1 encoding gene SAT (1415 T>C) genes are known to be associated with the expression levels of PAs[53], both gene polymorphisms might be potential genetic markers for susceptibility to schizophrenia. Only the SAT 1 gene polymorphism has been studied so far. There were no significant differences in the distribution of the genotypes of the SAT-1415 T/C single nucleotide polymorphisms between schizophrenia patients, non-psychotic psychiatric patients and healthy controls. However, a “mild association” between the C allele and psychopathology was found for the female group[54]. A translational convergent functional genomics study identified AZIN 1, the gene encoding antizyme inhibitor 1, as a candidate gene for schizophrenia (convergent functional genomics score 3.0[55]).
A comprehensive DNA microarray study revealed that the expression of AZIN 1 was reduced in almost all samples from subjects with schizophrenia. In addition, reduced cellular expression of AZIN 1 was verified by in situ hybridization of postmortem brain samples of the same schizophrenia subjects. Of note, this reduction was not a consequence of long-term neuroleptic treatment of the patients since there was only a marginal reduction of AZIN 1 expression in haloperidol treated monkeys[56]. Ornithine aminotransferase (EC 2.6.1.13) is an enzyme that has been indirectly connected with PA metabolism through catalyzing the formation of glutamate or proline from ornithine[57]. This enzyme was found to be reduced in samples from schizophrenia patients[56]. However, other studies were unable to replicate these findings. Maycox et al[58] could not identify any differentially expressed genes implicated in PA metabolism in two large schizophrenia cohorts (with more than 30000 mRNA transcripts).
Numerous communications have dealt with altered expression and/or activity of PA-metabolizing enzymes in brain tissue or blood of schizophrenia patients. Conflicting findings exist regarding polyamine oxidase(PAO). Blood plasma PAO activity was reportedly lower in acute schizophrenia patients[59,60]. This decrease in activity was unrelated to the subtype of schizophrenia (paranoid vs non-paranoid), age of onset or neuroleptic treatment[60-62]. In contrast with these findings, Dahel et al[63] and Das et al[64] found increased PAO activity in blood sera from schizophrenic patients, which was reduced by electroconvulsive therapy[63]. On the other hand, ODC activity was found to be normal in prefrontal cortex and hippocampus autopsy samples from people who suffered from schizophrenia[65], and there were no differences with regard to the number of ODC immunoreactive entorhinal cortex neurons between schizophrenia patients and controls[5]. Another study that measured SMOX activity found increased activity in sera from schizophrenia patients compared to non-psychotic controls[66], whereas AMD and SAT1 activities were unaltered in prefrontal cortex and hippocampus tissue of schizophrenia patients[65].
The density of agmatinase-containing interneurons was lower in the hippocampus of schizophrenia patients in comparison with controls[43]. Significantly increased activity of ARG (a gate keeper enzyme of PA synthesis) was observed in the cerebrospinal fluid of schizophrenia patients[67]. Lastly, increased enzyme activity and ARG II protein expression were found in postmortem brain tissue specimen in schizophrenia[68], whereas plasma ARG activity was significantly lower in schizophrenia than in controls[69].
There is evidence that gene regulation via epigenetic modifications play a major role in schizophrenia pathophysiology. However, in contrast to other mental disorders and suicide, no such modifications for PA metabolizing genes in schizophrenia have been identified[70-72].
Several reports have targeted the levels of cerebral and peripheral PA in schizophrenia patients. Elevated blood concentrations of spermine and/or spermidine have been measured in treated schizophrenia patients[73,74] and in drug-naïve cases[75], whereby long-term neuroleptic treatment was shown to reduce spermine levels[75,76]. Also, increased concentrations of spermidine and total PA were detected in fibroblasts obtained from schizophrenia patients (reviewed in[11]). So far, little information is available regarding brain PA concentrations in schizophrenia. Gilad et al[65] could not find significant alterations of PA levels in postmortem brain tissue of schizophrenia persons compared with controls. However, a more recent paper described significantly elevated levels of spermine, spermidine and putrescine in many brain regions of psychotic individuals[77].
Significantly increased agmatine concentrations were measured in blood plasma and postmortem frontal cortex tissue of individuals with first episode and chronic schizophrenia[68,78-80], and antipsychotic treatment was found to decrease blood agmatine levels[77]. In contrast to the previously mentioned work, reduced blood agmatine levels were measured in early-onset schizophrenia[81]. The amino acid L-arginine is metabolized by ARG, which is the first step in PA synthesis. Increased blood plasma arginine levels were reported in medication-naïve, first episode patients. Medication had no influence on enhanced blood concentrations in schizophrenia patients[79]. Finally, two-fold increased concentrations of S-adenosylmethionine were found in prefrontal cortex samples of schizophrenia patients compared with controls. This drastically increased brain S-adenosylmethionine content was not affected by postmortem interval or medication[82]. The main findings regarding this are summarized in Table 1.
| No significant differences in the distribution of the genotypes of the SAT-1415 T/C SNP between schizophrenia patients and healthy controls[54]. |
| CFG study identified AZIN 1 as a candidate gene for schizophrenia[55]. |
| DNA microarray and in situ hybridization studies show decreased AZIN 1 expression in schizophrenia. No influence of neuroleptic treatment[56]. |
| OAT expression is reduced in samples of schizophrenia individuals[56,57]. |
| No differently expressed genes implicated in PA metabolism in two large schizophrenia cohorts[58]. |
| PAO activity is lower in blood plasma of acute and chronic schizophrenia patients[59-62]. |
| PAO activity increased in blood sera of schizophrenic patients[64,65], reduction by electroconvulsive therapy[62]. |
| ODC activity and cellular expression is normal in brain autopsy samples from people who suffered from schizophrenia[65,66]. |
| SMOX activity is markedly higher in sera of schizophrenic patients[67]. |
| AMDI and SAT1 activities are unaltered in brain tissue of schizophrenia individuals[65]. |
| Density of AGMAT-containing interneurons is reduced in the hippocampus of schizophrenia patients[43]. |
| Increased ARG activity in the CSF of schizophrenia patients[67]. |
| Increased ARGII activity and protein expression in postmortem brain tissue in schizophrenia[68]. |
| ARG activity is lower in plasma of schizophrenia individuals[71]. |
| Elevated blood concentrations of spermine and/or spermidine in drug-naïve and treated schizophrenia patients[73-75]. |
| Long-term neuroleptic treatment reduces spermine levels[76,77]. |
| Increased concentrations of spermidine and total PA in fibroblasts from schizophrenia patients (reviewed in[11]). |
| PA levels normal in postmortem brain tissue of schizophrenia persons[65]. |
| Elevated levels of spermine, spermidine and putrescine in brains of psychotic individuals[77]. |
| Increased agmatine concentrations in blood plasma and postmortem brains of individuals with first episode and chronic schizophrenia[68,78-80]. |
| Antipsychotic treatment decreases blood agmatine levels[77]. |
| Reduced blood agmatine concentrations in early-onset schizophrenia[81]. |
| Increased concentrations of SAM in brain samples of schizophrenia patients[82]. |
Numerous studies have shown alterations of PA and PA-metabolizing enzymes in individuals who died by suicide (reviewed in[5,71,83]). However, a closer look at these studies reveals that they all provide findings from individuals who had suffered from major depression or from suicide victims with no specified psychiatric diagnosis but not from those with schizophrenia. Thus, it would be interesting to determine if schizophrenia patients who died by suicide would differ from non-suicide schizophrenia persons with regards to PA levels.
A promising approach to identify and better understand diverse schizophrenia symptoms in humans has been to investigate behavioral phenotypes in animal models of the disease[84]. Although numerous animal models of schizophrenia have been introduced so far, only a few of these have accounted for the role of PAs and their metabolites. We studied the cellular expression of ODC in rats with neonatal lesions of the ventral hippocampus and found increased immunostaining in the prefrontal, perirhinal and entorhinal cortex[85]. In addition, we found an increased density of SMOX-immunoreactive medial prefrontal neurons (unpublished findings). Increased levels of putrescine, spermidine, spermine and arginine and decreased levels of agmatine were found in the prefrontal cortex and hippocampus of male and female rat offspring after maternal immune activation[86].
Prepulse inhibition of the startle reflex response is disturbed in schizophrenia, although there are conflicting findings regarding the influence of agmatine on this effect. In one study, agmatine was reported to disrupt prepulse inhibition in rats[87], whereas another investigation found that low doses of agmatine attenuated the disruptive effects of the psychotomimetic substance phencyclidine on this response[88]. Other studies showed that agmatine depressed conditioned avoidance response and enhanced the inhibitory effect of haloperidol and olanzapine on this readout. Furthermore, agmatine attenuated apomorphine induced climbing and diminished amphetamine or ketamine induced hyperlocomotor activity[89,90]. Injection of phencyclidine, which induces psychotic symptoms in healthy individuals, was shown to also alter arginine metabolism in the rat hippocampus and prefrontal cortex[90], and withdrawal from repeated phencyclidine administration has been found to alter ARG activity as well as the concentration of arginine metabolites in rat brain[68].
Interestingly, transgenic animals, which overexpress ODC and/or SAT1, show a variety of neuroanatomical, neurochemical and behavioral peculiarities but not schizophrenia-like behavior[91]. SMOX overexpression in the neocortex in Dach-SMOX mice leads to glutamate excitotoxicity[92], which is a characteristic feature of human schizophrenia[93-95]. The main findings regarding this are summarized in Table 2.
| Neuronal expression of ODC increased in rats with neonatal lesion of the ventral hippocampus[85]. |
| Neuronal expression of SMOX increased in rats with neonatal lesion of the ventral hippocampus (unpublished). |
| Increased brain levels of putrescine, spermidine, spermine and arginine but decreased levels of agmatine were measured in rat offspring after maternal immune activation[86]. |
| Agmatine disrupts prepulse inhibition in rats[87]. |
| Agmatine attenuates the disruptive effects of phencyclidine on prepulse inhibition[88,89]. |
| Injection of phencyclidine alters arginine metabolism in rat brain[90]. |
| Withdrawal from repeated phencyclidine administration alters ARG activity and the levels of arginine metabolites in rat brain tissue[91,92]. |
| No schizophrenia-like behavior in transgenic animals that overexpress ODC and/or SAT1[91]. |
| SMOX overexpression in mice leads to glutamate excitotoxicity[91,92], a characteristic feature of “human” schizophrenia. |
PAs and their metabolites are crucial factors in a variety of functions, which are disrupted in schizophrenia. These disease-related functional impairments range from abnormal prenatal CNS development (disrupted neuronal migration and other pathological processes), impaired glutamate receptor functioning, glia pathology and immune dysregulation, to serious cognition problems and bizarre behavior. Thus, it is conceivable that altered PA supply and/or action contribute to the initiation and further progression of these impairments. More specifically, since spermine and spermidine positively influence many of these disturbed cellular mechanisms (see our considerations about PA functions), one may expect deficits in brain PA content in schizophrenia. Curiously, the opposite is the case as either increased blood and brain levels[73-75] or normal concentrations[65] of PA and ODC[81,85,87] have been reported for schizophrenia and animal disease models. Moreover, increased PA levels are not the result of neuroleptic treatment since anti-psychotics decrease PA concentrations in tissues and blood[75,76]. Thus, it seems unlikely that increased concentrations of spermine or other PAs contribute to schizophrenia pathology. In particular, there is no evidence that a PA excess is involved in impaired NMDA receptor functioning as observed in schizophrenia[34].
The situation is less evident with agmatine, which shows beneficial effects on some of the functions disrupted in schizophrenia. Increased agmatine concentrations were determined in blood plasma and post-mortem brain of individuals with first episode and chronic schizophrenia[68,76-81]. In contrast, reduced levels were measured in brain tissue of rat offspring after maternal immune activation[86]. It cannot be excluded, however, that overproduction of PA in human schizophrenia represents an attempt to compensate for certain functional losses (for example, induction and promotion of autophagy[95-97], which is abnormally reduced in schizophrenia[96]).
In addition, the possibility cannot be excluded that there may be a PA deficit in the developing CNS of future schizophrenia patients, which would contribute to disturbed prenatal brain development[11,96]. However, the latter scenario is not likely since increased concentrations of PA have been determined in rat offspring after maternal immune activation, a suitable neurodevelopmental model of the disease[86].
While the findings showing that increased PA and agmatine levels would not serve as convincing arguments in support of their involvement in schizophrenia pathology, it is possible that increased expression and enzyme activity of SMOX is a contributing factor[66]. SMOX catalyzes the oxidation of spermine to produce spermidine, hydrogen peroxide (H2O2) and 3-aminopropanal, which may spontaneously be converted to acrolein[97]. Consequently, increased oxidative stress was identified in SMOX over-expressing Dach-SMOX mice[92] which resulted in glu, tamate excitotoxicity[92,95]. Both reactive oxygen species, hydrogen peroxide and acrolein, are highly cytotoxic, as these can lead to production of massive cellular damage and pathologies[98-101]. Interestingly, in major depression and schizophrenia patients, significantly increased concentrations of protein-conjugated acrolein were measured, which could be reduced by anti-psychotic medication[101]. There is ample evidence that oxidative stress is a core feature in schizophrenia (for recent comprehensive reviews, see[101-104]). Oxidative stress is thought to be one of the mediators of progressive changes in brain structure and function in schizophrenia, which take place as schizophrenia progresses. The pathophysiological consequences include gray matter loss, myelination deficits and subsequent cognitive and functional impairment[105].
The extent that increased SMOX activity contributes to the aberrantly activated immune[104] and inflammatory[106] processes in schizophrenia remains to be established.
In schizophrenia, the brain and blood levels of PAs are normal or even increased. Hence, higher dietary intake of spermine or spermidine[43,107] cannot be a suitable approach to remove or mitigate schizophrenia symptoms. A potentially more promising approach would be to reduce SMOX expression or activity in order to diminish the generation of hydrogen peroxide and acrolein. Indeed, it has been shown recently that targeting SMOX is neuroprotective in a model of ischemic brain damage[108]. In this context, however, two questions need to be answered: (1) How to depress SMOX activity; and (2) What would be the consequences of this? In general, there are only a few approaches for a potentially successful intervention in this pathway. One of these is the application of the SMOX inhibitor N-(3-{[3-(dimethylamino)propyl] amino}propyl)-8-quinolinecarboxamide, which is under consideration for anti-cancer therapy[107]. Another one is the irreversible inhibition of SMOX by use of the PAO inhibitor MDL72527 {N1,N4-(bis(2,3-butadienyl)-1,4-butanediamine)}[108]. However, the application of both enzyme inhibitors produces a number of serious side effects. Furthermore, SMOX has an essential role in normal brain PA homeostasis (for review, see[101,107,108]), which might be disrupted by any kind of intervention. Thus, the use of these enzyme inhibitors for schizophrenia therapy does not appear to be viable. Hence, PA-based schizophrenia therapy will probably remain an unresolved issue in the foreseeable future.
The PA system plays an essential role in the brain and other organs. Over the past half century, numerous reports appeared that showed that PAs, their precursors and derivatives, as well as some PA-metabolizing enzymes, are altered in schizophrenia, thus giving rise to the possibility for new PA-based therapies. Unfortunately, there are currently no prospects for such a therapeutic intervention, given a number of currently insurmountable obstacles. Therefore, further studies are urgently needed to learn more about the relationship between the PA system and schizophrenia pathology.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo WF S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Patel KR, Cherian J, Gohil K, Atkinson D. Schizophrenia: overview and treatment options. P T. 2014;39:638-645. [PubMed] |
| 2. | Ramani D, De Bandt JP, Cynober L. Aliphatic polyamines in physiology and diseases. Clin Nutr. 2014;33:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Angel C, Leach BE, Martens S, Cohen M, Heath RG. Serum oxidation tests in schizophrenic and normal subjects; copper levels, adrenaline and N, N-dimethyl-p-phenylenediamine oxidation rates, and glutathione concentration. AMA Arch Neurol Psychiatry. 1957;78:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Aprison MH, Grosz HJ. Ascorbic acid level and lag time in oxidation of N,N dimethyl-p-phenylenediamine; correlation in sera of normal controls, psychotic patients, and animals. AMA Arch Neurol Psychiatry. 1958;79:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Fiori LM, Turecki G. Implication of the polyamine system in mental disorders. J Psychiatry Neurosci. 2008;33:102-110. [PubMed] |
| 6. | Laube G, Veh RW. Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia. 1997;19:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Fujiwara K, Bai G, Kitagawa T. Polyamine-like immunoreactivity in rat neurons. Brain Res. 1997;767:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Laube G, Bernstein HG, Wolf G, Veh RW. Differential distribution of spermidine/spermine-like immunoreactivity in neurons of the adult rat brain. J Comp Neurol. 2002;444:369-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Skatchkov SN, Antonov SM, Eaton MJ. Glia and glial polyamines. Role in brain function in health and disease. Biochem Moscow Suppl Ser A. 2016;10:73-98. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Junttila T, Hietanen-Peltola M, Rechardt L, Persson L, Hökfelt T, Pelto-Huikko M. Ornithine decarboxylase-like immunoreactivity in rat spinal motoneurons and motoric nerves. Brain Res. 1993;609:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Bernstein HG, Müller M. The cellular localization of the L-ornithine decarboxylase/polyamine system in normal and diseased central nervous systems. Prog Neurobiol. 1999;57:485-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Hiasa M, Miyaji T, Haruna Y, Takeuchi T, Harada Y, Moriyama S, Yamamoto A, Omote H, Moriyama Y. Identification of a mammalian vesicular polyamine transporter. Sci Rep. 2014;4:6836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Fredriksson R, Sreedharan S, Nordenankar K, Alsiö J, Lindberg FA, Hutchinson A, Eriksson A, Roshanbin S, Ciuculete DM, Klockars A, Todkar A, Hägglund MG, Hellsten SV, Hindlycke V, Västermark Å, Shevchenko G, Olivo G, K C, Kullander K, Moazzami A, Bergquist J, Olszewski PK, Schiöth HB. The polyamine transporter Slc18b1(VPAT) is important for both short and long time memory and for regulation of polyamine content in the brain. PLoS Genet. 2019;15:e1008455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Moriyama Y, Hatano R, Moriyama S, Uehara S. Vesicular polyamine transporter as a novel player in amine-mediated chemical transmission. Biochim Biophys Acta Biomembr. 2020;1862:183208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Sala-Rabanal M, Li DC, Dake GR, Kurata HT, Inyushin M, Skatchkov SN, Nichols CG. Polyamine transport by the polyspecific organic cation transporters OCT1, OCT2, and OCT3. Mol Pharm. 2013;10:1450-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Junttila T, Rechardt L, Hietala OA, Pelto-Huikko M. The expression of ornithine decarboxylase antizyme mRNA and protein in rat motoneurons. Neurosci Lett. 1995;197:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Greenwood MP, Greenwood M, Paton JF, Murphy D. Control of Polyamine Biosynthesis by Antizyme Inhibitor 1 Is Important for Transcriptional Regulation of Arginine Vasopressin in the Male Rat Hypothalamus. Endocrinology. 2015;156:2905-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Mäkitie LT, Kanerva K, Polvikoski T, Paetau A, Andersson LC. Brain neurons express ornithine decarboxylase-activating antizyme inhibitor 2 with accumulation in Alzheimer's disease. Brain Pathol. 2010;20:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Zoli M, Pedrazzi P, Agnati LF. Regional and cellular distribution of spermidine/spermine N1-acetyltransferase (SSAT) mRNA in the rat central nervous system. Neurosci Lett. 1996;207:13-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Krauss M, Langnaese K, Richter K, Brunk I, Wieske M, Ahnert-Hilger G, Veh RW, Laube G. Spermidine synthase is prominently expressed in the striatal patch compartment and in putative interneurones of the matrix compartment. J Neurochem. 2006;97:174-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Krauss M, Weiss T, Langnaese K, Richter K, Kowski A, Veh RW, Laube G. Cellular and subcellular rat brain spermidine synthase expression patterns suggest region-specific roles for polyamines, including cerebellar pre-synaptic function. J Neurochem. 2007;103:679-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Reis DJ, Yang XC, Milner TA. Agmatine containing axon terminals in rat hippocampus form synapses on pyramidal cells. Neurosci Lett. 1998;250:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Jing Y, Liu P, Leitch B. Region-specific changes in presynaptic agmatine and glutamate levels in the aged rat brain. Neuroscience. 2016;312:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Gorbatyuk OS, Milner TA, Wang G, Regunathan S, Reis DJ. Localization of agmatine in vasopressin and oxytocin neurons of the rat hypothalamic paraventricular and supraoptic nuclei. Exp Neurol. 2001;171:235-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Bernstein HG, Derst C, Stich C, Prüss H, Peters D, Krauss M, Bogerts B, Veh RW, Laube G. The agmatine-degrading enzyme agmatinase: a key to agmatine signaling in rat and human brain? Amino Acids. 2011;40:453-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Bernstein HG, Stich C, Jäger K, Dobrowolny H, Wick M, Steiner J, Veh R, Bogerts B, Laube G. Agmatinase, an inactivator of the putative endogenous antidepressant agmatine, is strongly upregulated in hippocampal interneurons of subjects with mood disorders. Neuropharmacology. 2012;62:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Mella C, Martínez F, de Los Angeles García M, Nualart F, Castro V, Bustos P, Carvajal N, Uribe E. Expression and localization of an agmatinase-like protein in the rat brain. Histochem Cell Biol. 2010;134:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Peters D, Berger J, Langnaese K, Derst C, Madai VI, Krauss M, Fischer KD, Veh RW, Laube G. Arginase and Arginine Decarboxylase - Where Do the Putative Gate Keepers of Polyamine Synthesis Reside in Rat Brain? PLoS One. 2013;8:e66735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Polis B, Srikanth KD, Elliott E, Gil-Henn H, Samson AO. L-Norvaline Reverses Cognitive Decline and Synaptic Loss in a Murine Model of Alzheimer's Disease. Neurotherapeutics. 2018;15:1036-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Malpica-Nieves CJ, Rivera-Aponte DE, Tejeda-Bayron FA, Mayor AM, Phanstiel O, Veh RW, Eaton MJ, Skatchkov SN. The involvement of polyamine uptake and synthesis pathways in the proliferation of neonatal astrocytes. Amino Acids. 2020;52:1169-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Li J, Doyle KM, Tatlisumak T. Polyamines in the brain: distribution, biological interactions, and their potential therapeutic role in brain ischaemia. Curr Med Chem. 2007;14:1807-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Slotkin TA, Bartolome J. Role of ornithine decarboxylase and the polyamines in nervous system development: a review. Brain Res Bull. 1986;17:307-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 193] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Giorgi PP. Polyamines and the regulation of protein synthesis in brain. Biochem J. 1972;127:6P. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Jorratt P, Hoschl C, Ovsepian SV. Endogenous antagonists of N-methyl-d-aspartate receptor in schizophrenia. Alzheimers Dement. 2021;17:888-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 634] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 36. | Bowie D. Polyamine-mediated channel block of ionotropic glutamate receptors and its regulation by auxiliary proteins. J Biol Chem. 2018;293:18789-18802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 37. | Handa AK, Fatima T, Mattoo AK. Polyamines: Bio-Molecules with Diverse Functions in Plant and Human Health and Disease. Front Chem. 2018;6:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 38. | Nichols CG, Lee SJ. Polyamines and potassium channels: A 25-year romance. J Biol Chem. 2018;293:18779-18788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | Williams K. Interactions of polyamines with ion channels. Biochem J. 1997;325 (Pt 2):289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 368] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 40. | Oliver D, Baukrowitz T, Fakler B. Polyamines as gating molecules of inward-rectifier K+ channels. Eur J Biochem. 2000;267:5824-5829. [PubMed] |
| 41. | Bellé NA, Dalmolin GD, Fonini G, Rubin MA, Rocha JB. Polyamines reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res. 2004;1008:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 202] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 42. | Pegg AE. Introduction to the Thematic Minireview Series: Sixty plus years of polyamine research. J Biol Chem. 2018;293:18681-18692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Laube G, Bernstein HG. Agmatine: multifunctional arginine metabolite and magic bullet in clinical neuroscience? Biochem J. 2017;474:2619-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Halaris A, Plietz J. Agmatine : metabolic pathway and spectrum of activity in brain. CNS Drugs. 2007;21:885-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 45. | Neis VB, Rosa PB, Olescowicz G, Rodrigues ALS. Therapeutic potential of agmatine for CNS disorders. Neurochem Int. 2017;108:318-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Uzbay T. A new target for diagnosis and treatment of CNS disorders: agmatinergic system. Curr Med Chem. 2012;19:5116-5121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Piletz JE, Aricioglu F, Cheng JT, Fairbanks CA, Gilad VH, Haenisch B, Halaris A, Hong S, Lee JE, Li J, Liu P, Molderings GJ, Rodrigues AL, Satriano J, Seong GJ, Wilcox G, Wu N, Gilad GM. Agmatine: clinical applications after 100 years in translation. Drug Discov Today. 2013;18:880-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 48. | Rushaidhi M, Jing Y, Zhang H, Liu P. Participation of hippocampal agmatine in spatial learning: an in vivo microdialysis study. Neuropharmacology. 2013;65:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Borikar SP, Dongare SI, Danao KR. Reversal of lipopolysaccharide-induced learning and memory deficits by agmatine in mice. Int J Neurosci. 2021;1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Aglawe MM, Kale MB, Rahangdale SR, Kotagale NR, Umekar MJ, Taksande BG. Agmatine improves the behavioral and cognitive impairments associated with chronic gestational ethanol exposure in rats. Brain Res Bull. 2021;167:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Kotagale NR, Taksande BG, Inamdar NN. Neuroprotective offerings by agmatine. Neurotoxicology. 2019;73:228-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 52. | Richardson-Andrews RC. A central role for the polyamines in the aetiology of schizophrenia. Med Hypotheses. 1983;11:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Coker-Gürkan A, Arisan S, Arisan ED, Unsal NP. Lack of evidence for the association of ornithine decarboxylase (+316 G>A), spermidine/spermine acetyl transferase (-1415 T>C) gene polymorphisms with calcium oxalate stone disease. Biomed Rep. 2014;2:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Bermudo-Soriano CR, Vaquero-Lorenzo C, Diaz-Hernandez M, Perez-Rodriguez MM, Fernandez-Piqueras J, Saiz-Ruiz J, Baca-Garcia E. SAT-1 -1415T/C polymorphism and susceptibility to schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:345-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Ayalew M, Le-Niculescu H, Levey DF, Jain N, Changala B, Patel SD, Winiger E, Breier A, Shekhar A, Amdur R, Koller D, Nurnberger JI, Corvin A, Geyer M, Tsuang MT, Salomon D, Schork NJ, Fanous AH, O'Donovan MC, Niculescu AB. Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Mol Psychiatry. 2012;17:887-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 315] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 56. | Middleton FA, Mirnics K, Pierri JN, Lewis DA, Levitt P. Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. J Neurosci. 2002;22:2718-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 312] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 57. | Ginguay A, Cynober L, Curis E, Nicolis I. Ornithine Aminotransferase, an Important Glutamate-Metabolizing Enzyme at the Crossroads of Multiple Metabolic Pathways. Biology (Basel). 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 58. | Maycox PR, Kelly F, Taylor A, Bates S, Reid J, Logendra R, Barnes MR, Larminie C, Jones N, Lennon M, Davies C, Hagan JJ, Scorer CA, Angelinetta C, Akbar MT, Hirsch S, Mortimer AM, Barnes TR, de Belleroche J. Analysis of gene expression in two large schizophrenia cohorts identifies multiple changes associated with nerve terminal function. Mol Psychiatry. 2009;14:1083-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 59. | Meltzer HY, Arora RC, Jackman H, Pscheidt G, Smith MD. Platelet monoamine oxidase and plasma amine oxidase in psychiatric patients. Schizophr Bull. 1980;6:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Baron M, Risch N, Levitt M, Gruen R. Genetic analysis of platelet monoamine oxidase activity in families of schizophrenic patients. J Psychiatr Res. 1985;19:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 61. | Baron M, Gruen R, Levitt M, Kane J. Neuroleptic drug effect on platelet monoamine oxidase and plasma amine oxidase in schizophrenia. Psychiatry Res. 1982;7:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Gruen R, Baron M, Levitt M, Kane J. Plasma amine oxidase and clinical features of schizophrenia. Am J Psychiatry. 1985;142:972-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 63. | Dahel KA, Al-Saffar NM, Flayeh KA. Polyamine oxidase activity in sera of depressed and schizophrenic patients after ECT treatment. Neurochem Res. 2001;26:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Das I, de Belleroche J, Essali M, Richardson-Andrews R, Hirsch S. Plasma polyamine oxidase in plasma in normal and schizophrenic subjects. Schizophr Res. 1990; 3:36 (abstr. V.D.2).. |
| 65. | Gilad GM, Gilad VH, Casanova MF, Casero RA Jr. Polyamines and their metabolizing enzymes in human frontal cortex and hippocampus: preliminary measurements in affective disorders. Biol Psychiatry. 1995;38:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Flayeh KA. Spermidine oxidase activity in serum of normal and schizophrenic subjects. Clin Chem. 1988;34:401-403. [PubMed] |
| 67. | Szilágyi AK. [Studies on arginase activity in the cerebrospinal fluid]. J Neurol Sci. 1973;18:143-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 68. | Knox LT, Jing Y, Bawazier-Edgecombe J, Collie ND, Zhang H, Liu P. Effects of withdrawal from repeated phencyclidine administration on behavioural function and brain arginine metabolism in rats. Pharmacol Biochem Behav. 2017;153:45-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Yanik M, Vural H, Kocyigit A, Tutkun H, Zoroglu SS, Herken H, Savaş HA, Köylü A, Akyol O. Is the arginine-nitric oxide pathway involved in the pathogenesis of schizophrenia? Neuropsychobiology. 2003;47:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Wockner LF, Morris CP, Noble EP, Lawford BR, Whitehall VL, Young RM, Voisey J. Brain-specific epigenetic markers of schizophrenia. Transl Psychiatry. 2015;5:e680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 71. | Gross JA, Turecki G. Suicide and the polyamine system. CNS Neurol Disord Drug Targets. 2013;12:980-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Fiori LM, Turecki G. The Role of Epigenetic Dysregulation in Suicidal Behaviors. Curr Top Behav Neurosci. 2020;46:41-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 73. | Svinarev VI. [Serum spermidine levels of schizophrenic patients]. Zh Nevropatol PsikhiatrIm S S Korsakova. 1987;87:732-734. [PubMed] |
| 74. | Das I, de Belleroche J, Adams C, Essali MA, Richardson-Andrews RC, Hirsch S. Blood polyamines in schizophrenia. Schizophr Res. 1989;2:146 (VL-2 abstract). [DOI] [Full Text] |
| 75. | Leppik L, Kriisa K, Koido K, Koch K, Kajalaid K, Haring L, Vasar E, Zilmer M. Profiling of Amino Acids and Their Derivatives Biogenic Amines Before and After Antipsychotic Treatment in First-Episode Psychosis. Front Psychiatry. 2018;9:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 76. | Das I, Ramchand CN, Gliddon A, Hirsch SR. Nitric oxide, free radicals and polyamines may have a role in the membrane pathology of schizophrenia. Neuropsychobiology. 1998;37:65-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Syatkin SP, Smirnova IP, Kuznetsova OM, Lobaeva TA, Blagonravov ML, Ryskina EA, Sokuyev RI, Shevkun NA. The levels of polyamines in autopsy materials of some structures of brain limbic system and reticular formation of patients with schizophrenia. Res J PharmaceutBiolChemSci2014; 5:1486-1490. |
| 78. | Garip B, Kayir H, Uzun O. l-Arginine metabolism before and after 10 weeks of antipsychotic treatment in first-episode psychotic patients. Schizophr Res. 2019;206:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Uzbay T, Goktalay G, Kayir H, Eker SS, Sarandol A, Oral S, Buyukuysal L, Ulusoy G, Kirli S. Increased plasma agmatine levels in patients with schizophrenia. J Psychiatr Res. 2013;47:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Liu P, Jing Y, Collie ND, Dean B, Bilkey DK, Zhang H. Altered brain arginine metabolism in schizophrenia. Transl Psychiatry. 2016;6:e871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 81. | Baytunca BM, Kalyoncu T, Özbaran B, Köse S, Öngür D, Uzbay T. Reduced blood agmatine level in early-onset schizophrenia. Schizophr Res. 2020;222:528-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 82. | Guidotti A, Ruzicka W, Grayson DR, Veldic M, Pinna G, Davis JM, Costa E. S-adenosyl methionine and DNA methyltransferase-1 mRNA overexpression in psychosis. Neuroreport. 2007;18:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 83. | Chen GG, Almeida D, Fiori L, Turecki G. Evidence of Reduced Agmatine Concentrations in the Cerebral Cortex of Suicides. Int J Neuropsychopharmacol. 2018;21:895-900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Ang MJ, Lee S, Kim JC, Kim SH, Moon C. Behavioral Tasks Evaluating Schizophrenia-like Symptoms in Animal Models: A Recent Update. Curr Neuropharmacol. 2021;19:641-664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 85. | Bernstein HG, Grecksch G, Becker A, Höllt V, Bogerts B. Cellular changes in rat brain areas associated with neonatal hippocampal damage. Neuroreport. 1999;10:2307-2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 86. | Zhang J, Jing Y, Zhang H, Bilkey DK, Liu P. Effects of maternal immune activation on brain arginine metabolism of postnatal day 2 rat offspring. Schizophr Res. 2018;192:431-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | Uzbay T, Kayir H, Goktalay G, Yildirim M. Agmatine disrupts prepulse inhibition of acoustic startle reflex in rats. J Psychopharmacol. 2010;24:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 88. | Pålsson E, Fejgin K, Wass C, Klamer D. Agmatine attenuates the disruptive effects of phencyclidine on prepulse inhibition. Eur J Pharmacol. 2008;590:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Knox LT, Jing Y, Collie ND, Zhang H, Liu P. Effects of acute phencyclidine administration on arginine metabolism in the hippocampus and prefrontal cortex in rats. Neuropharmacology. 2014;81:195-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Kotagale NR, Taksande BG, Wadhwani PJ, Palhade MW, Mendhi SM, Gawande DY, Hadole PN, Chopde CT. Psychopharmacological study of agmatine in behavioral tests of schizophrenia in rodents. Pharmacol Biochem Behav. 2012;100:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Kauppinen RA, Alhonen LI. Transgenic animals as models in the study of the neurobiological role of polyamines. Prog Neurobiol. 1995;47:545-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Pietropaoli S, Leonetti A, Cervetto C, Venturini A, Mastrantonio R, Baroli G, Persichini T, Colasanti M, Maura G, Marcoli M, Mariottini P, Cervelli M. Glutamate Excitotoxicity Linked to Spermine Oxidase Overexpression. Mol Neurobiol. 2018;55:7259-7270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Shah P, Plitman E, Iwata Y, Kim J, Nakajima S, Chan N, Brown EE, Caravaggio F, Torres E, Hahn M, Chakravarty MM, Remington G, Gerretsen P, Graff-Guerrero A. Glutamatergic neurometabolites and cortical thickness in treatment-resistant schizophrenia: Implications for glutamate-mediated excitotoxicity. J Psychiatr Res. 2020;124:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 94. | Stacchiotti A, Corsetti G. Natural Compounds and Autophagy: Allies Against Neurodegeneration. Front Cell Dev Biol. 2020;8:555409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 95. | Schneider JL, Miller AM, Woesner ME. Autophagy and Schizophrenia: A Closer Look at How Dysregulation of Neuronal Cell Homeostasis Influences the Pathogenesis of Schizophrenia. Einstein J Biol Med. 2016;31:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Volk DW, Lewis DA. Early developmental disturbances of cortical inhibitory neurons: contribution to cognitive deficits in schizophrenia. Schizophr Bull. 2014;40:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 97. | Uemura T, Takasaka T, Igarashi K, Ikegaya H. Spermine oxidase promotes bile canalicular lumen formation through acrolein production. Sci Rep. 2017;7:14841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Seiler N. Oxidation of polyamines and brain injury. Neurochem Res. 2000;25:471-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Cervelli M, Amendola R, Polticelli F, Mariottini P. Spermine oxidase: ten years after. Amino Acids. 2012;42:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 100. | Yoshida M, Kanzaki T, Mizoi M, Nakamura M, Uemura T, Mimori S, Uju Y, Sekine K, Ishii Y, Yoshimi T, Yasui R, Yasukawa A, Sato M, Okamoto S, Hisaoka T, Miura M, Kusanishi S, Murakami K, Nakano C, Mizuta Y, Mishima S, Hayakawa T, Tsukada K, Kashiwagi K, Igarashi K. Correlation between brain damage, associated biomarkers, and medication in psychiatric inpatients: A cross-sectional study. Clin Chim Acta. 2017;464:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 101. | Morris G, Walder KR, Berk M, Marx W, Walker AJ, Maes M, Puri BK. The interplay between oxidative stress and bioenergetic failure in neuropsychiatric illnesses: can we explain it and can we treat it? Mol Biol Rep. 2020;47:5587-5620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 102. | Madireddy S, Madireddy S. Regulation of Reactive Oxygen Species-Mediated Damage in the Pathogenesis of Schizophrenia. Brain Sci. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 103. | Roberts RC. Mitochondrial dysfunction in schizophrenia: With a focus on postmortem studies. Mitochondrion. 2021;56:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 104. | Magalhães PV, Dean O, Andreazza AC, Berk M, Kapczinski F. Antioxidant treatments for schizophrenia. Cochrane Database Syst Rev. 2016;2:CD008919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 105. | Fan J, Chen M, Wang X, Tian Z, Wang J, Fan D, Zeng J, Zhang K, Dai X. Targeting Smox Is Neuroprotective and Ameliorates Brain Inflammation in Cerebral Ischemia/Reperfusion Rats. Toxicol Sci. 2019;168:381-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 106. | Schwarz C, Horn N, Benson G, Wrachtrup Calzado I, Wurdack K, Pechlaner R, Grittner U, Wirth M, Flöel A. Spermidine intake is associated with cortical thickness and hippocampal volume in older adults. Neuroimage. 2020;221:117132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 107. | Sun L, Yang J, Qin Y, Wang Y, Wu H, Zhou Y, Cao C. Discovery and antitumor evaluation of novel inhibitors of spermine oxidase. J Enzyme Inhib Med Chem. 2019;34:1140-1151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 108. | Dunston TT, Khomutov MA, Gabelli SB, Stewart TM, Foley JR, Kochetkov SN, Khomutov AR, Casero RA Jr. Identification of a Novel Substrate-Derived Spermine Oxidase Inhibitor. Acta Naturae. 2020;12:140-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |