Published online Oct 19, 2021. doi: 10.5498/wjp.v11.i10.897
Peer-review started: February 27, 2021
First decision: May 5, 2021
Revised: May 18, 2021
Accepted: August 27, 2021
Article in press: August 27, 2021
Published online: October 19, 2021
Processing time: 230 Days and 7.8 Hours
Chronic insomnia affects about 6%-13% of the Canadian population. Although treatments already exist, they each have their own issues. Neurofeedback is a neuromodulation technique that specifically targets abnormal brain activity and is gaining attention as a possible insomnia treatment.
To review the latest studies pertaining to the use of neurofeedback in the treatment of insomnia.
In this non-systematic review, only experimental studies assessing the effects of neurofeedback on patients with insomnia were targeted across four bibliographic databases.
A total of 12 studies were retained. All neurofeedback studies included in this study showed a clear improvement of subjective sleep. However, data concerning objective improvement are contradictory. Most studies regarding surface and z-score neurofeedback show that neurofeedback targeting the sensorimotor rhythm in the sensorimotor cortex may help improve subjective sleep. A placebo effect seems also to be present in some studies. Several limitations were present in each study.
While studies concerning neurofeedback as a treatment for insomnia are encouraging, many methodological barriers remain to be resolved to prove its efficacy unequivocally. More studies using robust design parameters, as well as the replication of existing studies, are necessary to support neurofeedback as an effective treatment for insomnia.
Core Tip: Insomnia is a sleep disorder that is extremely prevalent in the general population. The current treatments offered tend to ignore the neurological marker of insomnia. Neurofeedback is a type of neurotherapy that is based on training one’s electrical brain activity to treat multiple ailments including insomnia. In this review, we discuss the different studies that have been published in the last few years concerning the use of neurofeedback to treat insomnia and what needs to be improved in this domain of research.
- Citation: Lambert-Beaudet F, Journault WG, Rudziavic Provençal A, Bastien CH. Neurofeedback for insomnia: Current state of research. World J Psychiatr 2021; 11(10): 897-914
- URL: https://www.wjgnet.com/2220-3206/full/v11/i10/897.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i10.897
Sleep difficulties are very common, with an estimated one-third of adults around the world being dissatisfied with their sleep[1]. Furthermore, approximately 6%-13% of the general population meets all criteria necessary for the diagnosis of insomnia disorder[1-4]. Insomnia is a sleep disorder characterized by a dissatisfaction with the quantity and/or quality of sleep[5]. This dissatisfaction must be associated with difficulty falling asleep, maintaining sleep and/or waking up early in the morning. These difficulties must be present at least 3 nights a week for the past 3 mo and must cause distress or impaired functioning[5].
Not only does insomnia causes distress, it is linked to multiple psychopathologies. In fact, there is a 40% co-occurrence between insomnia symptoms and mental illness[6]. Insomnia is also linked to multiple health problems, such respiratory and cardiac diseases[7]. Insomnia affects everyone differently, and the profile of individuals that suffer from it is very heterogeneous[8]. However, it is widely recognized that hyperarousal plays an important role in insomnia[9-12]. For example, insomnia is linked to an increased heart rate[13], increased facial muscle tension[14] and a higher rate of cortical activation[15]. According to Bonnet and Arand[16], insomnia is a hyperarousal disorder and its treatment should aim to decrease arousal.
Currently, there are two main types of treatment: Medication or cognitive behavioural therapy for insomnia (CBT-I)[17]. Medication has been shown as effective for treating insomnia, while its effects are mainly short-term[18]. Unfortunately, long-term use of medication causes undesirable side effects, such as cognitive and motor coordination problems, physical dependence and rebound insomnia[18,19].
Several studies support the effectiveness of CBT-I in reducing insomnia symptoms, such as wakefulness after sleep onset (WASO) and sleep efficiency (SE)[17,20,21]. However, there is a lack of research establishing that CBT-I leads to significant changes in sleep onset latency (SOL)[22]. It is also important to note that only 60% of individuals receiving CBT-I are considered good sleepers after treatment[23]. The literature showing that CBT-I is effective at decreasing hyperarousal is also quite scarce. In that regard, Altena et al[24] have shown a frontal hypoactivation after CBT-I, while Cervena et al[25] have shown an increase in slow waves and a slight decrease in beta activity after CBT-I.
While medication is recommended only as a short-term treatment for insomnia with non-equivocally lacking effectiveness in the long-term for treating insomnia[18], CBT-I focuses mainly on behaviors and cognition surrounding sleep[26]. Because hyperarousal has been identified as a predisposing and maintaining factor in insomnia disorder[27,28], it would be advisable to offer treatment, such as neurofeedback (NF), that aims to modulate hyperarousal and which could offer long-term benefits.
NF is a sub-category of biofeedback, a technique where one learns to modulate bodily functions, such as heart rate, through feedback[29]. To do this, biofeedback uses various stimuli corresponding to one’s physiological characteristics and presents them to the client (feedback). The client then uses this information to modify his/her physiological functioning[30]. In the case of NF, the physiological signals used and modulated are brainwaves.
NF is based on the principle of operative conditioning[31]. There are many different types of NF, but they all have in common the use of visual and/or auditory stimuli (video, music, etc.) as positive reinforcement presented when electroencephalographic (EEG) activity corresponds to the training goal. The withdrawal or modification of this stimulus is used as a negative punishment to inform the individual that his/her brain functioning is diverging from the training goal. With this basic principle, NF can both encourage and dissuade certain brainwaves[32].
The history of neurofeedback is strongly intertwined with that of sensorimotor rhythm, beginning with the studies of Sterman et al[33]. Their 1967's study, a precursor in neurofeedback research, led to the discovery of the sensorimotor rhythm (SMR), a brain state present only during periods of motor stillness. The aim of these studies was to reproduce the internal inhibition observed in Pavlov's previous studies, when presenting contradictory conditioned responses. Pavlov had observed that the modification of conditioned responses caused, in time, lethargy and even sleep in dogs. When they attempted to reproduce this effect with cats, they noticed that the suppression of a conditioned response, and thus of movement, was linked to a bursting rhythm localized at the sensorimotor cortex. This rhythm was thus named SMR (11-15 Hz)[34]. They reported that this EEG pattern strongly resembled the sleep spindles observed during sleep. In these studies, they also found that cats were able to induce this rhythm voluntarily. These cats then showed an increase in the density of sleep spindles as well as a reduction in sleep stage transitions and thus prolonged sleep[35].
Since then, research on neurofeedback as a treatment for sleep difficulties often focuses on the control of SMR. The frequencies associated with SMR vary between studies but they usually are around 12 to 15 Hz, sometimes including higher or lower frequencies. Some studies uses the inhibition of higher frequencies, such as high beta frequencies (20-35 Hz), to reduce alertness, whereas others enhance slower frequencies, from delta (less than 4 Hz) to alpha frequencies (8-12 Hz), to promote deep relaxation[36].
The various NF techniques can be divided into two broad categories: closed-loop NF and open-loop NF[37,38]. Open-loop NF aims at regulating EEG activity by using auditory and visual stimuli not representative of the EEG activity at that time. Rather, this type of NF aims at synchronizing brain activity to rhythmic external stimulus. Closed-loop NF, on the other hand, is based on real-time EEG activity to determine characteristics of the transmitted feedback (positive reinforcement or negative punishment)[39]. NF is not regulated by any governmental body. However, some organizations such as the Biofeedback Certification Institute of America and the International Society for Neurofeedback and Research offer guidelines for biofeedback training as well as certifications for clinicians. The list of NF techniques presented in the Results section is not exhaustive and refers only to those used by the studies included in this review.
This review uses a non-systematic approach to give a general idea of the literature available in the field of NF. Articles were collected on four bibliographic databases: Psycnet, PubMed, Cochrane Central Register of Controlled Trials and Web of Science. For the bibliographic searches, the term "neurofeedback" was combined with the term "insomnia" for all categories with the "AND" operator. Synonyms and Medical Subject Headings for each term were combined with the "OR" operator.
Once articles were identified, those dealing directly with the use of NF for the treatment of insomnia were extracted. Studies focusing on insomnia treatment as a comorbid disorder were included, while those papers simply mentioning sleep difficulties, without any emphasis on it, were excluded. Only scientific articles and abstracts from experimental studies were used for this study. Case studies and retrospective studies were excluded. We included all sleep-related measures and outcomes. Results pertaining to other disorders are not discussed in this review since our aim is to focus on the efficacy of NF as an insomnia treatment.
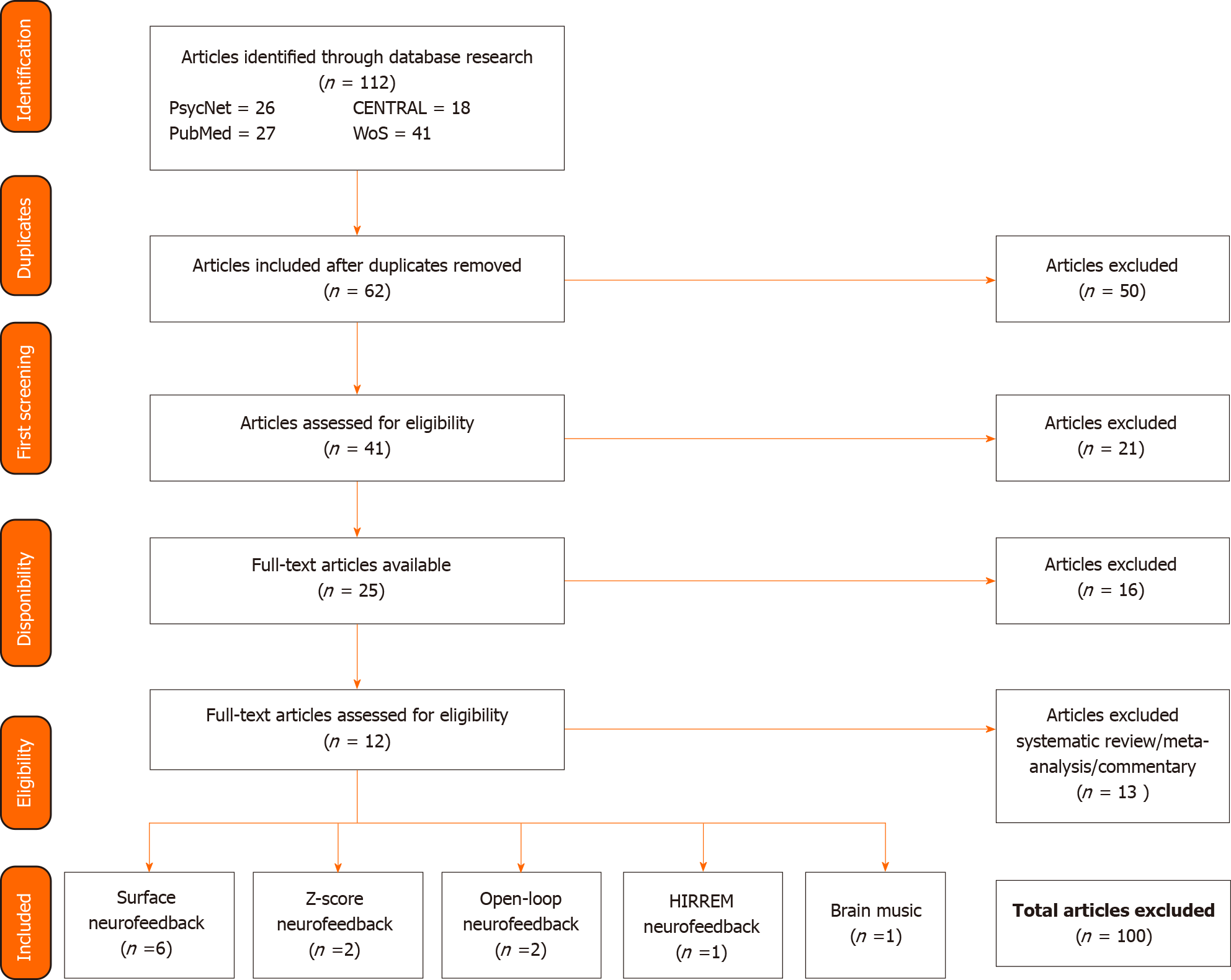
Overall, 12 articles were included in this study, including 6 on surface neurofeedback (NFS), 2 on z-score NF, 2 on open-loop NF, 1 on high-resolution, relational, resonance-based, electroencephalic mirroring (HIREEM) NF and 1 on Brain Music NF (see Figure 1 for the schematic of the bibliographical search).
NFS is a type of closed-loop NF that uses only two to four electrodes and targets a reduced number of cortical regions as well as a reduced number of brainwaves[40]. During NFS, the client will be presented with his/her own EEG activity, at a specific region, in the form of visual and/or auditory stimuli. The purpose of NFS is to increase/decrease specific frequencies at specific locations. Our review identified six studies using NF for the treatment of insomnia (see Table 1 for the study parameters and Table 2 for a summary of the results).
| Ref. | Groups | Age/sex | Electrode placement | Protocol | Sessions | Control/sham condition |
| Schabus et al[45] | 16 primary insomnia; 9 insomnia misperception; 26 sleep control; 12 NF control (criteria: PSG, PSQI, DSM-IV) | Insomnia group: 38.59 ± 11.18-year-old (range: 27- to 50-year-old) / 19 females and 6 males; Sleep control: 35.52 ± 10.63 (range: 24- to 47-year-old) / 19 females and 7 males; NF control: 26.67 ± 4.46 (range: 22- to 32-year-old) / 6 females and 6 males | C3 | Enhance: SMR (12-15 Hz) | 12 sessions NF 12 Sham (mixed); (2-4 sessions/wk); 8 5 min blocks/session | Enhance random frequencies (other than SMR rhythm) during present sessions (½ first; ½ second) |
| Schabus et al[41] | 24 primary insomnia (criteria: PSQI, SIS-D) | 34.83 ± 10.6 (range: 24- to 46-year-old) / 17 females and 7 males | C3 | Enhance: SMR (12-15 Hz) | N/A | Enhance random frequencies (other than SMR rhythm) during preset sessions |
| Cortoos et al[46] | 9 tele-NF; 8 tele-biofeedback; 12 controls | Tele-NF: 41.5-year-old / 3 females and 6 males; Tele-biofeedback: 43.8-year-old / 3 females and 5 males; Controls: 44.4-year-old/5 females and 7 males | Cz | Enhance: SMR (12-15 Hz); Inhibit: Theta (4-8 Hz) and high beta (20-30 Hz); Biofeedback: 50 Hz | 18 sessions of 1 h (2-3 sessions/wk) | Control group |
| Arns et al[49] | 51 ADHD (SMR: 27, TBR: 10, SMR + TBR: 14); 28 controls | ADHD: (range: 6- to 53-year-old) / 14 females and 37 males; Controls: (range: 21- to 64-year-old) / 15 females and 13 males | SMR: C3;Cz or C4; TBR: Fz; FCz or Cz | Enhance: SMR (12-15 Hz); TBR (20-25 Hz and 15-20 Hz) | 29-31 sessions (2-3 sessions/wk); 8 5 min blocks/session | Control group |
| Sungwon[51] | 14 participants; NF; CBT-I (criteria: ISI) | N/A | N/A | Inhibit: Beta | N/A | CBT-I |
| Shin[50] | 4 mild insomnia (Registered at a sleep clinic) | 34.8 ± 5.3-year-old/1 female and 3 males | N/A | Enhance: Theta and sigma; Inhibit: Beta | N/A | None |
| Ref. | Sleep outcomes measurements | Results |
| Schabus et al[45] | Objective: PSG; EEG; Subjective: PSQI; WHOQLA; SSS | Significant effect for NF compared to PF: (1) Higher SMR-power directly after each sessionb; and (2) Higher spindle density for the fast spindle type (C3 only)b; Significant effect for NF and PF: (1) Reduction of subjective sleep complaints after 12 sessionsb; and (2) Unspecific increase of physical quality of lifeb; Significant effect for INS compared to MP: (1) More wake timeb; and (2) Less stage 2, stage 3 and REMb; Significant effect for MP compared to INS: Higher SMR-power after each sessionb |
| Schabus et al[41] | Subjective: PSQI, Sleep Diary, WHOQLA, WMS-R, BSI | Significant effect only for NF: (1) Increase of SMR activity at C3a; (2) Decrease of the number of awakeningsa; and (3) Increase of stage 3a; Significant effect for NF and PF: (1) Reduction in subjective sleep complaintsb; and (2) Increased physical quality of lifeb |
| Cortoos et al[46] | Objective: PSG; Subjective: Sleep diary | Significant effect only for NF: (1) Decrease of SOLa and WASOa (Sleep diaries)a; and (2) Increased TSTa and SEa (Sleep diaries); Significant effect only for BF: Increase of SEa (Sleep diaries); Significant effect for NF and PF: (1) Decrease of SOLa and WASOa (PSG); and (2) Increase of REM sleep; |
| Arns et al[49] | Subjective: PSQI | Significant effect only for SMR: (1) Decrease of SOLd; (2) Decrease of SOL (40.1 min to 19.1 min)b; and (3) Correlation between the change in inattention and changes in PSQIb and SOLb; Significant effect for SMR + TBR: Decrease of SOL (25.8 min to 18.8 min)a |
| Sungwon[51] | Objective: EEG (resting state); Subjective: ISI, PSQI, DBAS | Significant effect only for NF: (1) Decrease of ISIb; (2) Changes in PSQIa; and (3) Decreased Beta power; Significant effect only for CBT-I: (1) Decrease of ISIa; (2) Changes in PSQIa; and (3) Decrease of DBASa |
| Shin[50] | Objective: EEG | Significant effect only for NF: Decreased spectral power for thetaa |
This study[41] used a counterbalance within-subject design with placebo to demonstrate whether NF can improve sleep and cognitive performance in individuals with insomnia. A single group of 24 participants with primary insomnia received 10 sessions of NF during which they had to increase the power of their SMR (12-15 Hz) at C3. They also received five placebo neurofeedback (PF) sessions where they trained the amplitude of non-SMR frequencies at random.
They found a significant increase in SMR power at C3 after NF sessions, with a non-significant increase at C4. No significant change was observed in SMR power across the PF sessions. They reported an increase in fast sleep spindles during non-rapid eye movement (referred to as NREM) but no change in slow sleep spindles. Sleep spindles are EEG waves of a frequency of 12 Hz (slow sleep spindles) to 14 Hz (fast sleep spindles) that are typical of NREM sleep, especially stage 2 sleep[42]. They are markers of the wake-sleep transition and have been theorized to play a role in learning and plasticity as well as in sleep protection by inhibiting sensory processing[43]. Slower sleep spindles have been found in sleep-maintenance insomnia and are linked to insufficient sleep pressure and thus difficulties maintaining sleep[44]. Accordingly, they observed a decrease in the number of awakenings and an increased duration of stage 3 following the NF sessions. The researchers also reported a decrease in subjective sleep complaints following NF and PF sessions, but it was not possible to determine whether this decrease was solely due to NF. On the other hand, physical quality of life improved regardless of the treatment offered, suggesting a placebo effect.
In conclusion, they found that it is possible for individuals with insomnia to increase SMR power, which allowed an objective modification of certain aspects of sleep architecture (N3, fast sleep spindles, number of awakenings). Thus, SMR NF in the sensorimotor cortex would improve sleep architecture, subjective sleep quality and physical quality of life. It is important to note that there is a strong chance that a placebo played a role in this study.
Schabus et al[45] tried to replicate their 2014 findings in a double-blind, placebo-controlled study. To do so, they used four groups: a primary insomnia group (INS: n = 16), an insomnia misperception group (MP: n = 9), a sleep control group (n = 26) and a NF control group (n = 12). The MP group was composed of individuals that met the subjective criteria for insomnia but not the objective criteria. The control groups were composed of healthy individuals. The INS and MP groups received 12 NF sessions (increase SMR) and 12 PF sessions (increase non-SMR frequencies at random) in reverse order. Polysomnographic (PSG) data were recorded before and after each condition (NF and PF) for the INS and MP group as well as an initial PSG for the sleep control group. The NF control group received only 12 NF sessions.
They found that participants had a higher SMR power after NF sessions than after PF sessions, suggesting the ability to self-regulate SMR frequencies. The MP group had higher SMR power following the NF sessions than the INS group. The NF control group also had an increase in SMR power, but that increase stabilized after one NF session. The MP group required four sessions and the INS group required six to achieve stabilization of the increase of SMR power. Contradictory to their 2014 study, no significant changes were found in sleep architecture following NF or PF sessions. The authors found that participants' subjective complaints were reduced following the NF as well as the PF sessions, regardless of group (INS/MP), with a larger decrease for participants who started with the NF sessions.
The researchers therefore suggested that INS and MP participants were able to regulate SMR frequencies following the NF sessions and that this may lead to a decrease in the subjective symptoms of insomnia. However, the subjective effects reported by this study appear quite similar to those obtained with the placebo effect and were not associated with objective changes in PSG data.
In this 2010 study, Cortoos et al[46] investigated whether NF can reduce symptoms of primary insomnia using a pretest post-test design with control group. The study consisted of 17 participants with insomnia randomly distributed in two groups for: receipt of 18 NF sessions (n = 9) or receipt of 18 biofeedback sessions (BF; n = 8). A third group of 12 good sleepers was used as a control group to compare initial PSG data. PSG data were recorded for all participants at the beginning of the study and then at the end of the NF/BF sessions for the NF and BF groups.
The NF and BF sessions were ran at home and participants were responsible for electrodes’ placement. The BF sessions consisted of relaxing the forehead muscles using information from an electromyogram. The NF group had to increase SMR (12-15 Hz) amplitude and reduce the amplitude of theta (4-8 Hz) and high beta (20-30 Hz) frequencies at Cz.
PSG data from both groups (BF; NF) showed a significant decrease in SOL and WASO. The BF group had a greater decrease in SOL (44.9%) than the NF group (39.7%), while the NF group showed a greater decrease in WASO (53.6%) than the BF group (13.2%). Both groups also had increased rapid eye movement (referred to as REM) duration. Only the NF group reported a significant increase in total sleep time (TST). The sleep diaries of the NF group also reported a significant decrease in SOL and WASO, and an increase in TST. The BF group only reported significant improvement in SE in their sleep diaries.
Cortoos et al[46] therefore concluded that NF allows for more significant sleep improvements, particularly TST, than biofeedback. Results of the BF group had a caveat since no participant reported abnormal forehead muscle tension or high somatic arousal compared to the control group, which can indicate that somatic arousal was not an issue for the participants. It is also important to note that although sleep diaries in the NF group showed an overall improvement in sleep, there was no significant change in sleep diaries completed the day after the PSG recordings compared to those completed at home. This suggests a strong influence of the environment on the results, which is in accordance with sleep studies that have shown the effects of sleep laboratories on sleep parameters[47,48].
The purpose of this study[49] was to verify the presence of sleep difficulties in individuals with attention deficit disorder (ADHD) and to test the effects of SMR and theta/beta (TBR) NF on ADHD symptoms and the associated sleep difficulties. This study used an open-label design including a control group (n = 28) and an ADHD group (n = 51). The ADHD group was divided into three groups: a group (SMR) receiving NF sessions aimed at increasing SMR (n = 27), a group (TBR) aimed at increasing beta waves outside SMR frequencies (20-25 Hz, 15-20 Hz) and inhibiting theta waves (n = 10), and a last group receiving NF sessions combining SMR and TBR treatments (n = 9). The remaining five participants of the ADHD group were used as a control group for the initial measures. On average, participants received 30 NF sessions.
SMR and TBR NF significantly decreased SOL (SMR: 40-19 min; TBR: 26-19 min). SMR NF resulted in a rapid decrease in SOL between pre-treatment and mid-treatment measurements, followed by a plateau until the end of the sessions. TBR NF showed opposite results, i.e. a non-significant decrease for the first half of treatment and then a strong significant decrease during the second half. Strangely, they found that individuals who learned to increase their SMR during SMR NF sessions reported a smaller decrease in their Pittsburgh Sleep Quality Index (PSQI) score.
In this study[50], the researcher wished to quantify the effects of NF on the brain waves of individuals suffering from insomnia. To do so, he administered a randomized series of NF (increasing theta and sigma waves and decreasing beta waves) and placebo (PF) sessions.
By comparing the NF sessions to the PF sessions, Shin found a significant decrease in theta spectral power in the NF group. Mental slowness (delta to alpha ratio) increased slightly, but not significantly, during NF sessions. This study therefore suggests that individuals experience greater fatigue during NF sessions, which could explain sleep improvements by increasing sleep pressure and thus facilitating sleep onset.
In this abstract, Sungwon[51] verified whether NF is as effective as CBT-I in treating insomnia. A total of 14 participants were randomly assigned to two groups, one receiving NF sessions and one receiving CBT-I sessions. An EEG (resting state) was recorded before and after each condition.
Participants in both groups reported significantly lower Insomnia Severity Index (ISI) and PSQI scores following treatment. In contrast, only the CBT-I group reported a significant decrease of their Dysfunctional Belief and Attitudes about Sleep scores and only the NF group showed a significant decrease in beta potency. The author therefore concluded that both treatments are effective in reducing insomnia but each in its own way. NF reduces hyperarousal, while CBT-I reduces dysfunctional thoughts about sleep.
The majority of the studies aimed at increasing SMR in the sensorimotor cortex[41,45,46,49], and the number of sessions varied between 12 to 31 sessions at a minimum of two sessions per week. A total of four studies concerning NFS used a combination of objective and subjective measures[41,45,46,50]. All studies except one used subjective measures[50]. All of the studies using subjective measures reported a subjective improvement of sleep, such as SOL[46,49], WASO[41,46], TST[46] and subjective sleep complaints[41,45]. The results of objective measures were varied, but the recurrent finding was that EEG activity was changed after NF sessions, which suggests that the participants were capable of modulating their own EEG activity[41,45,50]. These results support the idea that modulation of EEG activity is possible and that it can reduce insomnia symptoms. However, a placebo effect was noted in the Schabus[41,45]’s studies.
Z-score NF (live z-score based training, LZT) is also part of the closed-loop NF family and is based on the same principles as NFS. The main difference is that the LZT is not based on the power spectral analysis of specific brain waves but on its comparison to the population average. To do this, EEG activity is compared in real time to a normative database that accounts for age, gender and handedness to provide a score (Z-score) representing the position of the client related to the population average[40,52]. The purpose of LZT is to bring this score closer to the population average (Z = 0). This type of NF therefore aims to normalize brain activity at one or multiple cerebral regions. We included two studies in our literature review (see Tables 3 and 4).
| Ref. | Groups | Age/sex | Electrode placement | Protocol | Sessions | Sham condition |
| Hammer et al[53] | 5 SMR; 3 IND | 49.63-year-old / 5 females and 3 males | SMR: Cz and C4; IND: 4-channel (highest abnormal sites) | SMR: Increase SMR (12-15 Hz) and inhibit excessive theta (4-8 Hz) and high beta (25-30 Hz). Normalization at Cz/C4 (Amplitude, connectivity); IND: Normalization of amplitudes, coherence, asymmetry and phase lag with the highest Z-scores (> 1.96) at the 4-channels trained | Range: 9 to 15 sessions; (2 sessions/wk); 20 min/session | None |
| Perez-Elvira et al[55] | 6 participants with insomnia and/or learning disorder | Range: 16- to 30-year-old | N/A | Normalization of the highest Z-score | 20 sessions; (2 sessions/wk) | None |
| Ref. | Sleep outcomes measurements | Results |
| Hammer et al[53] | Objective: QEEG (Z-score); Subjective: PSQI, ISI | Significant effect only for SMR: Decrease z-score; Significant effect only for IND: Decreased proportion of abnormal z-scores at all 19 sitesb, especially for deltab and betab bands; Significant effect for SMR and IND: (1) Decreased score for ISIb, for PSQIb: All participants finished under the threshold for insomnia (ISI < 10; PSQI < 5, except 1 at 6); and (2) Increased TSTb; Results of the 6-9 mo follow up: 5 out of 6 participants remained below the ISI threshold for insomnia; 3 of the 5 improved during the months following the end of the treatment |
| Perez-Elvira et al[55] | Objective: QEEG; Subjective: Visual analogue scale on their symptoms | Significant effect: (1) Significant difference of 5.70% between pre- and post-QEEGs; (2) More probable to normalize then to get further away from normalizationa; and (3) Improvement of symptoms |
The purpose of this study[53] was to determine whether two z-score NF protocols, one SMR protocol and one QEEG-guided and individualized protocol, can lead to a decrease in sleep and daytime dysfunction associated with insomnia. This pilot study used a randomized, parallel-group, single-blind experimental design.
All participants received a maximum of 15 sessions. They could stop sessions early when they felt that their insomnia had been successfully treated or when they were able to achieve normalization (Z-score < 0.5) of 80% of the trained variables for 80% of the training time. The SMR group had to increase, at Cz and C4, SMR frequencies (12-15 Hz) while inhibiting theta (4-8 Hz) and high beta (25-30 Hz) waves. Participants in this group also had to ensure that amplitudes and brain connectivity at the trained regions remained closed to the database average. Participants in the individualized protocol group were given a protocol tailored to their own brain activity, based on the four brain regions with the highest level of abnormal activity (highest z-score) identified during a quantitative EEG (QEEG). QEEG is the quantification of EEG data in order to compare it to databases or to highlight specific waveform components[54]. During training, the individualized protocol group had to normalize z-scores that deviated from the average of the database of delta, theta, alpha and beta waves amplitudes present in the four regions with the highest Z-score. They also had to normalize coherence, asymmetry and lag phase.
By the end of sessions, all participants had achieved the normalization of 80% of the trained variables for 80% of the training time. On average, participants achieved normalization of 88% of the variables for 80% of the training time (range: 80%-95%). On the other hand, no participant achieved complete normalization of all trained variables. Approximately 50% of participants were able to train closer to normal in their last session, with a Z-score of ± 1.5 on all variables. All but one participant of the SMR group managed to increase their SMR Z-score at Cz and C4, but there was no overall significant increase in SMR. For participants in the individualized protocol group, the proportion of abnormal z-scores was significantly lower after treatment for all brain regions, particularly for delta and beta waves. There was no significant difference between the different regions due to the individual aspect of their training protocols.
Following training, all participants reported an ISI score below the insomnia threshold (< 10) and 7 of the 8 participants reported a PSQI score below the insomnia threshold (< 5). The eighth participant had a PSQI score of 6. Half of the participants reported a decrease in WASO. Seven of the eight participants also reported an increase in TST. Six participants completed the 6-9 mo follow-up for the ISI. Five of the six participants remained below the threshold for insomnia, with three experiencing further sleep improvements in the following months. The sixth participant reported the same score as at their initial ISI.
In conclusion, the researchers reported that the SMR protocol achieved the same results as the individualized protocol in a simpler manner. They suggested that z-score NF is effective in the treatment of insomnia and that effects may last, or even improve, in the months following the end of treatment.
Information for this study[55] is taken from a brief abstract. The aim of this study was to observe the neurometric changes generated by 20 Z-score NF sessions. To do so, authors compared the initial and final QEEGs of 6 patients from the Nepsa clinic. A significant difference in the z-scores was found between the initial and final QEEGs, i.e. Z-scores out of range of the -1 to 1 average. Participants were also more likely to decrease the Z-score (normalization) than to increase it. Authors also reported a subjective improvement in patients' symptoms, who were asked to rate them on a visual scale. No information was given on the specific symptoms improved. Researchers therefore suggested that z-score NF allows normalization of EEG activity and the alleviation of insomnia symptoms.
Only two studies on Z-score NF were included in this review. Both of them found that participants were able to normalize their EEG activity in the NF sessions by reducing the proportion of abnormal z-scores. The number of sessions varied between nine and twenty sessions, at a rate of two per week. They all found a subjective improvement in sleep following the treatment. Only one study described the subjective improvement and the authors reported an increase of TST[53]. None of the studies used a placebo group. Both studies supported the idea that normalization of EEG activity was possible through z-score NF.
High-resolution, relational, resonance-based, electroencephalic mirroring (HIRREM) NF is a closed-loop NF technique aiming at improving neurodynamic self-regulation by presenting the brain with its own oscillatory pattern through auditory stimuli[56]. To do this, EEG activity is recorded at two locations (usually contralateral) on the scalp[57]. This activity is then translated into music based on the dominant frequency of the client’s EEG activity. This creates a resonance between the music and the neural oscillations, thus encouraging self-regulation. A HIRREM session consists of several training protocols[56]. HIRREM NF thus aims at regulating the general oscillatory pattern. Only one study using this technique was used in this review (see Tables 5 and 6).
| Ref. | Groups | Age/sex | Electrode placement | Protocol | Sessions | Sham condition |
| Tegeler et al[56] | 10 HUC; 9 UC (criteria: Clinical diagnosis (clinician referral and ISI) | HUC: 41.3 ± 17.5-year-old / 8 females and 2 males; UC: 49.5 ± 8.1-year-old / 6 females and 4 males | Two-channel, changes with each protocol | Each session comprises of 4-8 protocols | 8-12 sessions of up to 90 min over 3 wk | None |
In their 2012 study, Tegeler et al[56] investigated whether HIRREM reduces insomnia symptoms. To do so, they used a randomized, unblinded, wait-list control, crossover, superiority study design. A first group (n = 10) received eight to twelve HIRREM sessions plus usual care, while a second group (n = 10) was placed on a wait-list control (usual care).
The researchers reported a significant drop of 10.3 points in ISI scores for participants following the HIRREM sessions, with 9 out of 10 participants in the HIRREM group now being just below the threshold (15 to 21) for moderate insomnia. The wait-list group also reported a significant decrease in ISI scores, but only 6 out of 10 participants scored below the threshold for moderate insomnia (< 15) after receiving HIRREM sessions. ISI scores were stable for both groups 1 mo after the last HIRREM session. Finally, they appeared to have decreased in high-frequency brainwaves power (23-36 Hz) in temporal regions (T3/T4). A power decrease in high frequency brainwaves (23-36 Hz) in temporal regions (T3/T4) was also observed for all participants. However, no information was provided as to whether this decrease was statistically significant.
Open-loop audiovisual training (AVE) is, as the name suggests, part of the open-loop NF category. It is designed to stimulate the cerebral cortex using goggles and headphones programmed to provide visual and auditory stimuli in the form of synchronous lights and auditory pulsations. Stimuli are constant, repetitive and delivered at a predetermined frequency to arouse the thalamus and the neo-cortex, where visual and auditory information will be respectively processed[38]. This stimulation is believed to synchronize neuronal activity between the thalamus and neo-cortex, which will subsequently spread to the rest of the cerebral cortex via thalamo-cortical-thalamic circuits[37,38]. A total of two articles using this technique were included in this review (see Tables 7 and 8).
| Ref. | Groups | Age/sex | Electrode placement | Protocol | Sessions | Sham condition |
| Tang et al[37] | 8 chronic insomnia (criteria: ISI) | 88 ± 8.7-year-old / 7 females and 1 male | N/A | Progressive reduction from 8 Hz to 1 Hz with flickering lights | 30 sessions of 30 min (at bedtime) over 4 wk | None |
| Tang et al[58] | 9 insomnia and chronic pain AVE; 7 insomnia and chronic pain Placebo (criteria: ISI) | AVE group: 67.2 ± 5.0-year-old / N/A; Placebo group: 69.6 ± 4.4-year-old / N/A | N/A | Progressive reduction from 10 Hz to 2 Hz with pulsing lights | 1 session | Progressive reduction from 1 to 0.2 Hz with constant dim light changing in color |
| Ref. | Sleep outcomes measurements | Results |
| Tang et al[37] | Subjective: ISI, PSQI, sleep diary | Significant effect: Decreased ISI score (15.6 ± 4.8 to 8.0 ± 6.4)b |
| Tang et al[58] | Objective: QEEG; Subjective: ISI, PSQI | Significant effect only for AVE: Increased delta frequencies’ absolute power on all 19 EEG channels; Significant difference between AVE and placebo: Delta power at Cz |
In their study, Tang et al[37] used a pretest and post-test design to test the effectiveness of a daily 30-min AVE at bedtime for 1 mo in a group of 8 elderly people with chronic insomnia. Participants were required to use an AVE device. Sound stimuli gradually decreased from 8 Hz (theta/alpha), associated with a mental state of relaxed wakefulness, to 1 Hz (delta), which is usually associated with deeper sleep.
At the end of the month, researchers observed that 63% of participants no longer met the threshold for insomnia according to scores on the ISI (ISI ≤ 7). Although there was no significant pre/post difference in the overall ISI score, authors reported a significant improvement for daytime functioning and sleep quality. Clinical parameters reported in sleep diaries (SOL, TST, WASO) were not significantly different between subjects when compared across the five measurement periods (pre-treatment and subsequent 4 wkly sessions). However, the trend between weeks suggested that AVE effects appeared right in the first week and then stabilized.
As a follow-up to their 2015 study, Tang et al[58] recruited 19 seniors to test AVE NF on EEG activity during a 30-min session. To do so, they randomly assigned participants to two groups, AVE NF (n = 9) or placebo stimulation (n = 7). The placebo stimulation session consisted of a color changing dim light provided by goggles and a monotonous tone decreasing in frequency from 1 Hz to 0.2 Hz. The AVE group received flashing light (red and green spectrum) from goggles as well as auditory pulsations gradually changing from 10 Hz (alpha) to 2 Hz (delta). A QEEG (5 min, eyes closed, resting state) was recorded before and during both AVE and placebo sessions. Results were compared to a normative database taking into account age, gender and handedness.
At initial assessment, all participants had higher absolute power for high-beta (21-34 Hz) and gamma waves (35-45 Hz) than the database. After the experimental session, participants in the AVE group had a significant increase in the absolute power of delta waves on all 19 EEG channels, while participants of the placebo group had no significant EEG power change in any band frequency. Participants of the AVE group had higher delta power at Cz after the AVE sessions than the placebo group. Specific brain regions displaying a significant increase in delta power in the AVE group were central (Cz), fronto-parietal (i.e., Fp) and occipital (i.e. O1 and O2) regions. According to the authors, this study showed that AVE NF allows for immediate changes in the EEG activity.
Both studies on AVE neurofeedback were conducted by Tang et al[58]’s research team. One study concerned the subjective improvement of sleep after 30 sessions and found that AVE neurofeedback led to a significant decrease of ISI scores. The second study concerned the objective effect of one session of AVE NF on brain activity. They found that participants presented increased delta frequencies, even when compared to a placebo group. This supports the idea that AVE NF can modulate EEG activity and improve insomnia symptoms.
Brain Music NF is a type of open-loop NF that closely resembles HIRREM. This type of NF also uses musical compositions generated from one’s brain activity. Music is created from various EEG parameters, such as frequency ratios of certain waves modifying octave selection, tempo, volume in the left or right ear, etc.[59]. Music tracks are built to correspond to two states of rest, one active and one calm, and to encourage brain functions associated with these mental states[60]. However, unlike HIRREM, the musical compositions are generated during an initial assessment, and the individual under training then listens to their own musical compositions at home, without being connected to an EEG. Thus, although the music reflects a specific brain state experienced by the individual in the past, it does not adapt to the individual's EEG fluctuations during a training session[60]. Very few papers regarding this technique were found, and only one met our inclusion criteria (see Tables 9 and 10).
| Ref. | Groups | Age/sex | Electrode placement | Protocol | Sessions | Sham condition |
| DuRousseau et al[60] | 15 OPS support; 20 first responders; 6 controls | Range 24- to 58-year-old / 13 females and 28 males | F3; F4; C3; C4 | Brain Music (1-4 Hz up to 30 Hz) | N/A | Enhance random frequencies during preset sessions |
DuRousseau et al[60] investigated whether a music-based NF treatment could improve sleep quality, mood and daytime functioning in policemen and firefighters using a pre-test and post-test design with a control group. Participants were then given a copy of their personalized composition and had to listen to it during the day for 4 wk.
The study consisted of three groups: a group of individuals working in operational support of first responders (n = 15), a group of first responders (n = 20) and a control group of first responders (n = 6). Individuals in the control group were given the composition of another participant. Results are based on a 128-item questionnaire created by the group that focuses on mood, sleep quality, insomnia level, job performance and life satisfaction.
They found an improvement in sleep quality in 90% of participants (all groups combined) but more particularly in the two experimental groups. However, since the control group also reported improvements, it is possible that a placebo effect is present or that simply listening to music before falling asleep promotes sleep. Moreover, this study is based only on a subjective measure, the 128-item questionnaire. It is therefore difficult to estimate whether changes are really due to Brain Music training and not to a placebo effect.
A majority of studies included in this review were centered on closed-loop NF, precisely NFS[41,45,46,49-51] and Z-score NF[53,55]. They mostly used a protocol aimed at increasing SMR (12-15 Hz)[41,45,46,49,53].
All 10 studies using subjective measures reported subjective improvements in sleep[37,41,45,46,49,51,53,55,56,60]. The subjective sleep characteristics that most often appeared to be improved by NF, regardless of type, are SOL[46,49], WASO[45,46] and TST[46,53]. Another subjective improvement reported by studies is subjective complaints[41,45]. It should be noted that some studies[37,51,56,60] only reported changes in sleep using general questionnaires’ scores (ISI, PSQI, etc.), without providing details on the specific sleep characteristics that had improved.
Only six out of twelve studies[41,45,46,50,55,58] used objective measures to verify sleep improvement. The objective results are very varied and do not reach a clear consensus. The PSG data were mostly used to verify sleep characteristics used in subjective measures such as TST, WASO, etc. Only a few studies used polysomnography to verify changes to sleep architecture. For example, Schabus et al[41] reported an increase in N3, whereas Cortoos et al[46] reported an increase of REM. The EEG data were mostly used to assess EEG activity right after the NF sessions. Most studies using objective measures reported a change in the brainwave-trained[41,45,50], supporting the idea that an individual is capable of regulating his/her own EEG activity. However, this change is not always sustained over time[41], which calls into question the idea of learning retention and its application in everyday life.
Results extracted from this review show that, although the use of NF seems encouraging for the treatment of insomnia, there are few studies in this field of research. Moreover, available studies often show methodological or clinical limits.
In general, NF appears to lead to improvements in sleep quality for individuals with insomnia, particularly SOL, WASO, TST, SE and subjective sleep complaints. In a few cases, improvements allowed participants to no longer meet the criteria for an insomnia diagnosis[53,56]. Studies on the objective effects of NF report conflicting results, so it is difficult to draw a clear conclusion. Still, most studies agree that objective measures of NF seem to confirm its ability to alter positively the electrical functioning of the brain during training, but it is unclear whether this effect persists in everyday life. It will be necessary for future studies to implement objective measures, such as EEG and PSG, to prove the efficacy of NF.
The most commonly used training protocol is aimed at increasing SMR oscillations in the sensorimotor cortex. The SMR is linked to a state of relaxed wakefulness as well as to NREM sleep[35,41]. The decrease in insomnia symptoms with the increase in SMR frequencies tend to support the hyperarousal model that postulates that individuals with insomnia present higher mental and physiological hyperarousal. For example, compared with good sleepers, individuals with insomnia generally present an increased power in fast EEG frequency bands during sleep[61]. By increasing SMR, NF counteracts for cortical hyperarousal associated with insomnia by attenuating the power of high-frequency EEG waves[41]. Some studies have used neuromodulation techniques, other than NF, that aim at reducing hyperarousal, such as transcranial magnetic stimulation, to treat insomnia, but the evidence remains scarce[62]. It would be interesting to verify the efficacy of NF for the treatment of other disorders that are characterized by hyperarousal such as post-traumatic stress disorder[63]. Such studies would provide additional support to the hypothesis that neuromodulation techniques, such as NF, reduce insomnia symptoms by decreasing cortical hyperarousal.
Although results of studies included in this review are encouraging for the future of NF in the treatment of insomnia, several impediments are present and obscure its effectiveness.
Lack of consensus and replication: First, there is a lack of consensus on the type of protocols to be used according to the different NF techniques. While trained brainwaves vary greatly between studies, guidelines for duration, frequency and number of sessions are necessary. For example, NF duration ranges from eight to thirty sessions at a rate of two to four sessions per week. However, some researchers suggest that up to 40 sessions of NFS and 10 sessions of LZT NF are necessary to change efficiently behavior and symptoms[64,65]. Therefore, without clear guidelines, it is challenging to replicate each study correctly. It is therefore essential for researchers to share clearly how their study was conducted and to be consistent in terms of training parameters. It is only then that researchers will be able to replicate other studies adequately.
Small sample size: NF studies generally have a small sample size. Whether this is because of the complexity of studies or the burden imposed upon participants during participation, larger sample size would be indicated to obtain reliable results that could then be generalized to the general population or the one under study. There is still undeniable advocacy towards the scientific community to run large-scale NF studies.
Insufficient placebo group: Studies with a pre-/post-test design without a placebo or even a control group are currently the standard in NF research. Studies using placebo groups often report a placebo effect that is sometimes equal to that of the one provided by NF. Reported placebo effects should be considered with caution because it is difficult to create a placebo treatment that can actually mislead participants without mistakenly producing neurophysiological neurological changes. For example, in the Schabus et al[41,45] studies, the placebo groups had to increase power in random EEG frequencies, other than those targeted by the experimental condition. Although these EEG frequencies were varied with each session to avoid long-term learning, it is difficult to argue that the placebo treatment was completely inactive. There is always the possibility of learning to modulate random EEG activity[66]. Since no alternative currently exists, the Schabus et al[41,45] studies represent the golden standard for placebo protocols. Understandably, to develop a truly inactive placebo treatment will require improvements to the ones currently used in research.
Possible bias: Sadly, only the study by Schabus et al[45] used a double-blind design. Although some studies are single-blind, it is difficult to ignore researchers’ bias of their studies’ outcomes, especially if participants are aware of the training condition. It would therefore be crucial to use the double-blind design more often.
This review also has limitations. First, we focused on studies dealing with the treatment of insomnia. Studies using the general term of ‘sleep difficulties’ were not included and it would be interesting in the future to include these studies since favorable results with the use of NF were obtained. For example, NF was found to help reduce sleep difficulties in individuals that suffered from childhood obesity[67], seizures[68], fibromyalgia[69], stroke[70], traumatic brain injury[71], post-traumatic stress[71], etc. Second, our approach was a non-systematic one. It is possible that some studies have been discarded but would have contributed to our understanding of this encouraging treatment for insomnia.
Altogether, results of research using NF as a treatment for insomnia are encouraging with respect to subjective improvements in insomnia symptoms, specifically SOL, WASO and TST. The increase in SMR frequencies coupled with a decrease of insomnia symptoms, as seen in multiple studies, seem to support the hyperarousal model of insomnia. It will be interesting to study the efficacy of NF on other disorders characterized by hyperarousal, such as post-traumatic stress disorder, as a way to confirm if this technique reduces sleep difficulties by reducing cortical hyperarousal. However, multiple improvements are necessary in NF research beforehand to create reliable studies.
According to our literature review, there is an urgent need of double-blind controlled or placebo-controlled experimental design with larger sample sizes. Once this becomes usual in NF research, the replication of studies to strengthen NF’s status as an alternative to traditional insomnia treatment will be possible.
Insomnia is one of the most common sleep disorder among adults in Canada and the treatments currently available present some limits. Neurofeedback (NF) is a technique that could offer a new way to treat insomnia through the regulation of abnormal brain activity.
In the last few years, NF has been gaining attention in the research community. However, there are only a few studies in the field of insomnia and their methods and results vary greatly. It is important to offer a consensus as to what is missing and what is successful in NF research so that future researchers can build upon what has already been done.
The goal of this review was to summarize the research that has already been done concerning the use of NF in the treatment of insomnia. This summary includes the common results and methodologies used in NF research as well as the improvements that need to be implemented.
Data from experimental studies pertaining to the use of NF as a treatment of insomnia was collected from four bibliographical database and analysed. A short summary containing the methods, the results and the conclusions for each study was provided as well as for each NF type. A general summary was presented for all the studies included in this review.
A total of 12 studies on 5 different types of NF were used in this review, including surface NF, z-score NF, open-loop NF, high-resolution relational resonance-based electroencephalic mirroring NF and Brain Music NF. All the studies reported a clear improvement of subjective sleep, but there was no consensus concerning objective sleep. Many studies suggest that training the sensorimotor rhythm in the sensorimotor cortex improves subjective sleep. However, many studies also point out a possible placebo effect. The diversity of methods used across studies hinders on the ability to replicate NF studies and create robust studies.
The findings of this study support the hyperarousal model of insomnia, by increasing sensorimotor rhythm frequencies to decrease insomnia symptoms. To verify this theory, further research should be conducted to study the efficacy of NF to treat other disorders that are characterized by hyperarousal. This review has also point out the limits that currently plague NF research such as small sample sizes, inexistent placebo group and double blind design.
This review has brought to light the need for more double-blind controlled and placebo-controlled experimental design and bigger sample size to prove the efficacy of NF as a treatment of insomnia.
The authors would like to acknowledge the contribution of Laval University for providing the resources necessary for the bibliographic searches.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Canadian Sleep Society; World Sleep Society; Sleep Research Society.
Specialty type: Psychology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vyshka G S-Editor: Ma YJ L-Editor: A P-Editor: Guo X
| 1. | Morin CM, LeBlanc M, Daley M, Gregoire JP, Mérette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 757] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 2. | Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2362] [Cited by in RCA: 2404] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 3. | Tjepkema M. Insomnia. Health Rep. 2005;17:9-25. [PubMed] |
| 4. | Morin CM, LeBlanc M, Bélanger L, Ivers H, Mérette C, Savard J. Prevalence of insomnia and its treatment in Canada. Can J Psychiatry. 2011;56:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Pub, 2013. |
| 6. | Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 858] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 7. | Bixler EO, Kales A, Soldatos CR, Kales JD, Healey S. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry. 1979;136:1257-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 445] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Morin CM, Bélanger L. Insomnie chez l’adulte. In: Les troubles du sommeil. 3rd ed. Paris: Elsevier Masson, 2019: 11–127. |
| 9. | Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53:215-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 375] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 455] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 11. | Drake CL, Pillai V, Roth T. Stress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomnia. Sleep. 2014;37:1295-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 12. | Pérusse AD, Turcotte I, St-Jean G, Ellis J, Hudon C, Bastien CH. Types of primary insomnia: is hyperarousal also present during napping? J Clin Sleep Med. 2013;9:1273-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Farina B, Dittoni S, Colicchio S, Testani E, Losurdo A, Gnoni V, Di Blasi C, Brunetti R, Contardi A, Mazza S, Della Marca G. Heart rate and heart rate variability modification in chronic insomnia patients. Behav Sleep Med. 2014;12:290-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Freedman RR, Sattler HL. Physiological and psychological factors in sleep-onset insomnia. J Abnorm Psychol. 1982;91:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 216] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia:update of the recent evidence (1998-2004). Sleep. 2006;29:1398-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 797] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 18. | National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13-15, 2005. Sleep. 2005;28:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 463] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 19. | Buscemi N, Vandermeer B, Friesen C, Bialy L, Tubman M, Ospina M, Klassen TP, Witmans M. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 288] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 20. | Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive Behavioral Therapy for Chronic Insomnia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163:191-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 643] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 21. | Geiger-Brown JM, Rogers VE, Liu W, Ludeman EM, Downton KD, Diaz-Abad M. Cognitive behavioral therapy in persons with comorbid insomnia: A meta-analysis. Sleep Med Rev. 2015;23:54-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 22. | Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2016;165:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1178] [Article Influence: 130.9] [Reference Citation Analysis (0)] |
| 23. | Harris J, Lack L, Kemp K, Wright H, Bootzin R. A randomized controlled trial of intensive sleep retraining (ISR): a brief conditioning treatment for chronic insomnia. Sleep. 2012;35:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Altena E, Van Der Werf YD, Sanz-Arigita EJ, Voorn TA, Rombouts SA, Kuijer JP, Van Someren EJ. Prefrontal hypoactivation and recovery in insomnia. Sleep. 2008;31:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Cervena K, Dauvilliers Y, Espa F, Touchon J, Matousek M, Billiard M, Besset A. Effect of cognitive behavioural therapy for insomnia on sleep architecture and sleep EEG power spectra in psychophysiological insomnia. J Sleep Res. 2004;13:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Williams J, Roth A, Vatthauer K, McCrae CS. Cognitive behavioral treatment of insomnia. Chest. 2013;143:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 474] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 28. | Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, Espie CA, Garcia-Borreguero D, Gjerstad M, Gonçalves M, Hertenstein E, Jansson-Fröjmark M, Jennum PJ, Leger D, Nissen C, Parrino L, Paunio T, Pevernagie D, Verbraecken J, Weeß HG, Wichniak A, Zavalko I, Arnardottir ES, Deleanu OC, Strazisar B, Zoetmulder M, Spiegelhalder K. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 1201] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 29. | Frank DL, Khorshid L, Kiffer JF, Moravec CS, McKee MG. Biofeedback in medicine: who, when, why and how? Ment Health Fam Med 2010; 7: 85-91. [PubMed] |
| 30. | Moss D. Biofeedback. In: Shannon S, editor. Handbook of Complementary and Alternative Therapies in Mental Health. 3rd ed. San Diego: Academic Press, 2002: 135–158. |
| 31. | Lubar J. Optimal procedures in z-score neurofeedback: Strategies for maximizing learning for surface and LORETA neurofeedback. In: Thatcher RW, Lubar J, editors. Z-score neurofeedback: Clinical Applications. Oxford: Academic Press, 2014: 41–58. |
| 32. | Thatcher RW, Lubar J. History of the scientific standards of QEEG normative database. In: Budzynski TH, Budzynski HK, Evans JR, Abarbanel A, editors. Introduction to Quantitative EEG and Neurofeedback: Advanced Theory and Applications. 2nd ed. Oxford: Academic Press, 2009: 29–63. |
| 33. | Sterman MB, Wyrwicka W. EEG correlates of sleep: evidence for separate forebrain substrates. Brain Res. 1967;6:143-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 95] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Sterman MB. Physiological origins and functional correlates of EEG rhythmic activities: implications for self-regulation. Biofeedback Self Regul. 1996;21:3-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 162] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Sterman MB, Howe RC, Macdonald LR. Facilitation of spindle-burst sleep by conditioning of electroencephalographic activity while awake. Science. 1970;167:1146-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 102] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Abhang PA, Gawali BW, Mehrotra SC. Chapter 2 - Technological Basics of EEG Recording and Operation of Apparatus. In: Abhang PA, Gawali BW, Mehrotra SC, editors. Introduction to EEG- and Speech-Based Emotion Recognition. Academic Press, 2016: 19–50. |
| 37. | Tang HY, Vitiello MV, Perlis M, Riegel B. Open-Loop Neurofeedback Audiovisual Stimulation: A Pilot Study of Its Potential for Sleep Induction in Older Adults. Appl Psychophysiol Biofeedback. 2015;40:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Collura TF, Siever D. Audio-visual entrainment in relation to mental health and EEG. In: Budzynski TH, Budzynski HK, Evans JR, Abarbanel A, editors. Introduction to quantitative EEG and neurofeedback: Advanced theory and applications. 2nd ed. Boston: Elsevier, 2008: 193–223. |
| 39. | Sitaram R, Ros T, Stoeckel L, Haller S, Scharnowski F, Lewis-Peacock J, Weiskopf N, Blefari ML, Rana M, Oblak E, Birbaumer N, Sulzer J. Closed-loop brain training: the science of neurofeedback. Nat Rev Neurosci. 2017;18:86-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 640] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 40. | Marzbani H, Marateb HR, Mansourian M. Neurofeedback: A Comprehensive Review on System Design, Methodology and Clinical Applications. Basic Clin Neurosci. 2016;7:143-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 41. | Schabus M, Heib DP, Lechinger J, Griessenberger H, Klimesch W, Pawlizki A, Kunz AB, Sterman BM, Hoedlmoser K. Enhancing sleep quality and memory in insomnia using instrumental sensorimotor rhythm conditioning. Biol Psychol. 2014;95:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 42. | Gorgoni M, D’Atri A, Scarpelli S, Ferrara M, De Gennaro L. Chapter 2 - Timing and Topography of Sleep Onset: Asynchronies and Regional Changes of Brain Activity. In: Dringenberg HC, editor. Handbook of Behavioral Neuroscience. Elsevier, 2019: 19–31. |
| 43. | Normand MP, St-Hilaire P, Bastien CH. Sleep Spindles Characteristics in Insomnia Sufferers and Their Relationship with Sleep Misperception. Neural Plast. 2016;2016:6413473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Besset A, Villemin E, Tafti M, Billiard M. Homeostatic process and sleep spindles in patients with sleep-maintenance insomnia: effect of partial (21 h) sleep deprivation. Electroencephalogr Clin Neurophysiol. 1998;107:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Schabus M, Griessenberger H, Gnjezda MT, Heib DPJ, Wislowska M, Hoedlmoser K. Better than sham? Brain. 2017;140:1041-1052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 46. | Cortoos A, De Valck E, Arns M, Breteler MH, Cluydts R. An exploratory study on the effects of tele-neurofeedback and tele-biofeedback on objective and subjective sleep in patients with primary insomnia. Appl Psychophysiol Biofeedback. 2010;35:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Pérusse AD, Koninck JD, Bastien CH. Insomnia sufferers can tolerate laboratory REM sleep dream collection and may improve their sleep perception. Int J Dream Res 2015; 8: 54–57. [DOI] [Full Text] |
| 48. | Edinger JD, Fins AI, Sullivan RJ Jr, Marsh GR, Dailey DS, Hope TV, Young M, Shaw E, Carlson D, Vasilas D. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20:1119-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 130] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Arns M, Feddema I, Kenemans JL. Differential effects of theta/beta and SMR neurofeedback in ADHD on sleep onset latency. Front Hum Neurosci. 2014;8:1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Shin H. Effects of neurofeedback on EEG and sleep measures in patients with insomnia. Sleep Med. 2017;40:e305. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 51. | Sungwon C. The comparison research of effects of neurofeedback and cognitive behavior treatment for insomnia patients. J Sleep Res. 2018;. |
| 52. | Collura TF, Guan J, Tarrant J, Bailey J, Starr F. EEG Biofeedback Case Studies Using Live Z-Score Training and a Normative Database. J Neurother. 2010;14:22-46. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Hammer BU, Colbert AP, Brown KA, Ilioi EC. Neurofeedback for insomnia: a pilot study of Z-score SMR and individualized protocols. Appl Psychophysiol Biofeedback. 2011;36:251-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Nuwer M. Assessment of digital EEG, quantitative EEG, and EEG brain mapping: report of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology. 1997;49:277-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 222] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 55. | Perez-Elvira R, Bote DJ, Guarino S, Juan M, De Leon R, Feiner T, Perez B. Neurometric Results of a Case Series Using live Z-Scores Neurofeedback. Int J Psychophysiol. 2018;131:S139-S140. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Tegeler CH, Kumar SR, Conklin D, Lee SW, Gerdes L, Turner DP, Tegeler CL, C Fidali B, Houle TT. Open label, randomized, crossover pilot trial of high-resolution, relational, resonance-based, electroencephalic mirroring to relieve insomnia. Brain Behav. 2012;2:814-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Gerdes L, Gerdes P, Lee SW, H Tegeler C. HIRREM™: a noninvasive, allostatic methodology for relaxation and auto-calibration of neural oscillations. Brain Behav. 2013;3:193-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Tang HJ, McCurry SM, Riegel B, Pike KC, Vitiello MV. Open-Loop Audiovisual Stimulation Induces Delta EEG Activity in Older Adults With Osteoarthritis Pain and Insomnia. Biol Res Nurs. 2019;21:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Levin YaI. "Brain music" in the treatment of patients with insomnia. Neurosci Behav Physiol. 1998;28:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | DuRousseau DR, Mindlin G, Insler J, Levin II. Operational Study to Evaluate Music-Based Neurotraining at Improving Sleep Quality, Mood, and Daytime Function in a First Responder Population. J Neurother. 2011;15:389-398. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Med Rev. 2001;5:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 205] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 62. | Provencher T, Charest J, Bastien C. Non-Invasive Brain Stimulation for Insomnia - A Review of Current Data and Future Implications. OBM Integr Complement Med. 2019;5:1-1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Sherin JE, Nemeroff CB. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin Neurosci. 2011;13:263-278. |
| 64. | Thatcher RW. Latest Developments in Live Z-Score Training: Symptom Check List, Phase Reset, and Loreta Z-Score Biofeedback. J Neurother. 2013;17:69-87. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Thibault RT, Lifshitz M, Raz A. Neurofeedback or neuroplacebo? Brain. 2017;140:862-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 66. | Witte M, Kober SE, Wood G. Noisy but not placebo: defining metrics for effects of neurofeedback. Brain. 2018;141:e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | Chirita-Emandi A, Puiu M. Outcomes of neurofeedback training in childhood obesity management: a pilot study. J Altern Complement Med. 2014;20:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Malkowicz D, Martinez D. Role of Quantitative Electroencephalography, Neurotherapy, and Neuroplasticity in Recovery from Neurological and Psychiatric Disorders. J Neurother. 2009;13:176-188. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 69. | Mueller HH, Donaldson CC, Nelson DV, Layman M. Treatment of fibromyalgia incorporating EEG-Driven stimulation: a clinical outcomes study. J Clin Psychol. 2001;57:933-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Cannon KB, Sherlin L, Lyle RR. Neurofeedback Efficacy in the Treatment of a 43-Year-Old Female Stroke Victim: A Case Study. J Neurother. 2010;14:107-121. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | Nelson DV, Esty ML. Neurotherapy of Traumatic Brain Injury/Post-Traumatic Stress Symptoms in Vietnam Veterans. Mil Med. 2015;180:e1111-e1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |