Published online Oct 19, 2021. doi: 10.5498/wjp.v11.i10.805
Peer-review started: February 26, 2021
First decision: April 20, 2021
Revised: May 12, 2021
Accepted: August 18, 2021
Article in press: August 18, 2021
Published online: October 19, 2021
Processing time: 230 Days and 19.6 Hours
The mind is embodied; thoughts and feelings interact with states of physiological arousal and physical integrity of the body. In this context, there is mounting evidence for an association between psychiatric presentations and the expression variant connective tissue, commonly recognised as joint hypermobility. Joint hypermobility is common, frequently under-recognised, significantly impacts quality of life, and can exist in isolation or as the hallmark of hypermobility spectrum disorders (encompassing joint hypermobility syndrome and hypermobile Ehlers-Danlos syndrome). In this narrative review, we appraise the current evidence linking psychiatric disorders across the lifespan, beginning with the relatively well-established connection with anxiety, to hypermobility. We next consider emerging associations with affective illnesses, eating disorders, alongside less well researched links with personality disorders, substance misuse and psychosis. We then review related findings relevant to neurodevelopmental disorders and stress-sensitive medical conditions. With growing understanding of mind-body interactions, we discuss potential aetiopathogenetic contributions of dysautonomia, aberrant interoceptive processing, immune dysregulation and proprioceptive impairments in the context of psychosocial stressors and genetic predisposition. We examine clinical implications of these evolving findings, calling for increased awareness amongst healthcare professionals of the transdiagnostic nature of hypermobility and related disorders. A role for early screening and detection of hypermobility in those presenting with mental health and somatic symptoms is further highlighted, with a view to facilitate preventative approaches alongside longer-term holistic management strategies. Finally, suggestions are offered for directions of future scientific exploration which may be key to further delineating fundamental mind-body-brain interactions.
Core tip: The association between vulnerability to psychological or psychiatric symptoms and hypermobile joints may initially appear counterintuitive to many clinicians. However, a relationship with anxiety is consistently confirmed across multiple studies worldwide. In this narrative review, we appraise increasing evidence linking neuropsychiatric presentations to hypermobility across the lifespan, including emerging links to neurodevelopmental disorders and stress-sensitive medical conditions. We discuss pertinent mechanistic insights in the context of growing understanding of mind-body interactions. We offer direction for future research and highlight implications for clinical practice, notably roles of timely screening and detection alongside longer-term holistic management strategies.
- Citation: Sharp HEC, Critchley HD, Eccles JA. Connecting brain and body: Transdiagnostic relevance of connective tissue variants to neuropsychiatric symptom expression. World J Psychiatr 2021; 11(10): 805-820
- URL: https://www.wjgnet.com/2220-3206/full/v11/i10/805.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i10.805
There is a rapidly growing body of evidence showing a curious excess of psychiatric burden among individuals with joint hypermobility. Common constitutional variants of connective tissue often present as joint hypermobility, reflecting increased laxity of ligaments and extended movement of joints beyond typically ‘normal’ limits (otherwise described as joint hyperlaxity, being double-jointed)[1]. Internationally, joint hypermobility is found in up to 20% of the general population, and is influenced by age, sex and ethnicity; hypermobility is more frequent in children, with higher prevalence in women, and Asian and African populations[2]. Hypermobility is also highly heritable, and has been proposed to be an autosomal dominant trait with incomplete penetrance, variable expressivity and influenced by sex[3]. The epidemiological characterisation is influenced by clinical definitions and assessment tools; the 9-point Beighton score is used most frequently to assess hypermobility in both clinical and research settings, although other methods include the Hospital del Mar criteria[4], and self-administered hypermobility questionnaires.
Hypermobility is a descriptive term and might exist as an asymptomatic and isolated feature. However, neuropsychiatric symptoms are among extra-articular manifestations that frequently accompany more widespread musculoskeletal symptoms that typically co-occur with hypermobility. In these cases, hypermobility appears as one feature of a multisystemic disorder[3]. This complexity is reflected in the current debate concerning terminology and classification: A consensus group in 2017 introduced the concept of hypermobility spectrum disorders (HSD); encompassing a continuum including hypermobile Ehlers-Danlos syndrome’ (hEDS, previously EDS-HT), joint hypermobility syndrome [JHS, previously diagnosed according to the Brighton Criteria – Revised (1998)][5] and symptomatic hypermobility not fulfilling stricter criteria for other associated syndromes[6]. Diagnostic classification remains a contentious area, particularly since reliable genetic tests or physiological markers for HSD are presently lacking, so diagnosis relies solely on clinical criteria[6]. Hypermobility may also signal the presence of other heritable disorders of connective tissue including Marfan syndrome, osteogenesis imperfecta and Ehlers-Danlos syndromes which have a more clearly defined genetic basis[7]. Here, we review literature across a broad terminology referring to joint hypermobility and related syndromes.
Joint hypermobility is frequently under-recognised[3] and has a significant impact on quality of life across all age-groups[8,9]. Anxiety symptoms and disorders have a well-established link with hypermobility, yet growing evidence also now points towards associations across psychiatric diagnoses, notably with affective illnesses, increasingly with neurodevelopmental disorders and with stress-sensitive medical conditions, including fibromyalgia, myelo-encephalomyelitis/chronic fatigue syndrome (ME/CFS) and irritable bowel syndrome (IBS). Awareness of these relationships are important to enhance understanding of early risk factors to allow screening and timely intervention for vulnerable individuals. The value of such a strategy is illustrated by a recent longitudinal study, which demonstrated that hypermobile children at age 14 years were more likely to suffer from anxiety and depression by age 18 years[10]. Increasing clinicians’ awareness of the multisystemic features associated with hypermobility, especially psychiatric morbidity, will enable appropriate detection, diagnosis, and preventative intervention and can shape the implementation of effective longer-term holistic management strategies.
This paper presents a comprehensive narrative review of existent clinical knowledge and empirical evidence regarding the association of hypermobility with psychopathology. We also present a broad perspective on this expression of body-brain-mind interactions, including current mechanistic understanding, and we highlight implications for clinical practice and directions of future research.
This is a narrative review based on a comprehensive search of online databases (including MEDLINE, Embase and PsychInfo). Search terms included were ‘hypermobility’ and ‘joint laxity’ combined with each diagnostic category. This search comprised studies published from 1980 to 2021. Reference lists of identified papers were further scrutinised for additional relevant articles.
The expression of anxiety in the context of joint hypermobility was first recognised in the late 1980s. Subsequent research has repeatedly demonstrated this association, which has been the subject of several substantive reviews (for example[11]). Anxiety disorders are the most prevalent set of psychiatric illnesses[12], and hypermobility is most strongly linked to the expression of panic disorder and agoraphobia[13]. Moreover, state anxiety is a transdiagnostic symptom that is pervasive across distinct psychiatric disorders; even in non-clinical populations, hypermobility predicts elevated levels of trait and state anxiety, without necessarily reaching formal thresholds for anxiety disorder[14]. This pattern has been observed in the elderly[15] and in children, including both clinical[16,17] and non-clinical cohorts[18].
Hypermobility is becoming a recognised vulnerability factor for affective disorders, particularly depression, especially if comorbid anxiety is present[19,20]. Patients with clinically significant hypermobility (i.e., HSD/hEDS) demonstrate higher rates of depressive disorders (for example[21]), recently summarised in a meta-analysis[13] although there some inconsistencies within the literature[22]. A population-wide study, using Swedish national registries, observed a heightened risk of depression and increased rates of attempted suicide among hypermobile (EDS and HSD) individuals and their siblings[23]. Hypermobility is also associated with elevated self-report depressive symptoms in non-clinical populations[24]. There are a number of reasons why hypermobility may be linked to depression, and in many cases it is unclear whether studies have effectively controlled for anxiety, which could act as an explanatory mediator between hypermobility and depression, since these two conditions are highly comorbid. Other potential mediating factors common to both HSD/hEDS and depression include chronic pain, fatigue or impaired sleep[25].
An increased risk of bipolar affective disorder (BPAD) is also reported in individuals with ESD/HSD (relative risk, RR = 2.7)[23]. In this study, no difference in rates of schizophrenia were observed between people with and without hypermobility. Similarly, hypermobility is over-represented in patients with BPAD diagnosis attending a psychiatric outpatient setting[26]. However, further detailed studies are lacking, and the potential mediating role of other conditions, such as attention deficit hyperactivity disorder (ADHD; see below), are as yet unexplored. Thus the link between hypermobility and BPAD remains preliminary.
There are several case series and reports that describe the co-occurrence of EDS and eating disorders (reviewed in[27]). Elevated rates of hypermobility occur in both psychiatric outpatients with eating disorders[27] and in hospitalised in-patients with anorexia nervosa[28], who experienced excess gastrointestinal symptoms, orthostatic intolerance and fatigue (symptoms common to HSD/hEDS). A higher lifetime prevalence of eating disorder was also demonstrated in students with non-clinical hypermobility[29]. However, large and comprehensive epidemiological studies investigating this proposed link are lacking.
Mechanistically however, a proposed model of the relationship between eating disorders and HSD/hEDS recognises the contribution of both articular (such as temporo-mandibular disorders) and extra-articular features (including gastrointestinal problems, smell and taste abnormalities, dental problems, oral mucosal fragility) to difficult or painful eating, which reinforce dysregulated eating behaviour and associated weight loss[27,29]. Thus HSD/hEDS can plausibly contribute to, mask or even be misdiagnosed as an eating disorder.
The link between hypermobility and schizophrenia-type psychosis remains much less apparent than that seen in relation to anxiety, affective and eating disorders. One case-control study reported similar prevalence of HSD/hEDS in patients with schizophrenia and controls[30], a finding that was confirmed in a population matched cohort study[23]. Even in psychiatric outpatients, schizophrenia was reported to be negatively associated with hypermobility[26]. Nevertheless, hypermobility is implicated as a clinical marker for co-morbid anxiety in schizophrenia: Patients with comorbid hypermobility and schizophrenia express elevated rates of panic/agoraphobia disorder, exacerbating positive psychotic symptoms[31].
Only one case-control study to date has investigated personality disorder in hypermobility, revealing elevated prevalence of personality disorder in JHS (RR = 5.8), especially of the obsessive-compulsive (anankastic) subtype[21]. It was speculated that that joint instability and associated imprecision of proprioception might underlie compensatory over-controlling behaviours (in the context of anxiety), and even that unrecognised JHS can contribute to perfectionism. However, rates of hypermobility were reported elsewhere to be no different in psychiatric outpatients with or without personality disorder[26]. While a number of possible putative mechanisms might link hypermobility to personality disorder (including anxious temperament and/or neurodevelopmental conditions including as ADHD), these findings should be interpreted cautiously, as there is an obvious need for large and well powered studies.
Studies investigating addiction and substance misuse in the context of hypermobility are sparse. Early findings of elevated hypermobility scores in female chronic alcoholic patients[32] have not been replicated since. One longitudinal study revealed significantly higher prevalence of at-risk drinkers and smokers in young females with hypermobility compared to controls[33]. It is possible that alcohol and tobacco use could be overused to cope with chronic pain and anxiety (or indeed to self-medicate against ADHD symptoms, see below) and further studies should evaluate and control for these variables.
In recent years, several authors have highlighted a relationship between hypermobility and neurodevelopmental disorders, notably autism (autistic spectrum disorders, ASD), ADHD and developmental coordination disorder (DCD)[34,35]. Interest in tic disorder (Tourette syndrome) and hypermobility is also growing. Hypermobility is frequently co-morbid with neurodevelopmental disorders and may contribute to the accompanying physical behavioural and cognitive symptoms that encompass motor difficulties, sleep and feeding problems, sensory hypersensitivities, behavioural hyperactivity, inattention, dysexecutive issues, speech and language problems and social deficits. Neurodevelopmental disorders most often present in school age children, and thus may precede or overshadow recognition of comorbid HSD/hEDS[35]. Nevertheless, these two clinical entities retain their association through into adulthood[26,36]. Interestingly, however, subclinical expression of neurodevelopmental (ADHD, ASD or DCD) traits in the general population are not strongly associated with hypermobility, suggesting that the expression of this association is limited to clinical populations[37].
Autism: Evidence for an overlap between hypermobility and autism is growing. Initially described in case reports (reviewed in[38]), excess hypermobility has since been demonstrated in children with ASD (average age 4 years) in a case-control study[39], although differences in passive muscular tonicity may be a confound[40]. At a population-level, elevated rates of ASD is apparent in individuals with EDS/JHS and their siblings[23]. Both ASD and HSD/hEDS are considered heritable spectrum disorders that appear in infancy and share clinical presentations that include motor and coordination difficulties, sensory hypersensitivity/hyperalgesia, autonomic dysfunction, proprioceptive impairments and sleep disorders[34]. This has led to the suggestion that EDS/HSD might be considered as a subtype of ASD[34].
Furthermore, there has been longstanding recognition of the positive association between ASD and heritable disorders of connective tissue including Marfan’s syndrome[41] and osteogenesis imperfecta[42]. ASD is known to be associated with several genetic causes, the most common being Fragile X syndrome (caused by mutations in FMR1 gene). Up to half of patients with Fragile X syndrome are hypermobile. Soft skin, scoliosis and flat feet are common, providing further evidence for variant of collagen or related connective tissue[43]: The gene FMR1 negatively regulates protein translation, which theoretically could cause downstream effects on collagen formation[43]. More broadly, across monogenic genetic syndromes strongly associated with both ASD and hypermobility, and known genetic causes of EDS subtypes, analyses of gene interactions reveal extensive gene clustering that might represent a biological mechanisms for the observed clinical overlap[34].
ADHD: Increasing clinical awareness of the co-occurrence of ADHD and hypermobility was confirmed by two case-control studies[44,45] and a population based cohort study[23], which also demonstrated higher rates of ADHD in siblings of children with HSD/hEDS diagnosis. Co-occurrence of ADHD and HSD/hEDS is also seen in adults[36,46].
DCD: According to DSM-5[47], EDS/JHS is included both as a differential diagnosis and as a potential comorbidity of DCD (previously termed developmental dyspraxia) in acknowledgement of the functional and clinical similarities[48]. High rates of symptoms related to HSD/hEDS are found among patients with DCD diagnosis, including pain and autonomic dysfunction, and there are significant commonalities in their motor features[48].
In children with HSD/hEDS diagnoses, high rates of clumsiness, impaired coordination and dyspraxia are observed[49], and up to 55% meet DCD criteria[50,51]. Similarly, 46%-64% of children with DCD are hypermobile[52,53], the exact figure depending on appropriate use of age appropriate Beighton score cut-offs[51].
Developmental tic disorder (Tourette syndrome): Despite emergent evidence describing hypermobility in ASD, ADHD and DCD, data concerning neurodevelopmental tic disorders (such as Tourette’s syndrome) are remarkably lacking. To our knowledge, no current data have assessed this relationship in children. The first work in adults found elevated prevalence (38%) of hypermobility amongst 24 adults with Tourette syndrome[36]. Interestingly, these individuals had a high comorbidity of obsessive-compulsive disorder diagnoses (37.5%), another condition which is yet to be investigated with respect to hypermobility.
Fibromyalgia and ME/CFS: Fibromyalgia and ME/CFS are both common overlapping disorders characterised by chronic pain, fatigue, sleep disturbance, cognitive complaints, gastrointestinal disturbance and affective problems.
There is further overlap of symptomatology and clinical findings with HSD/hEDS[54]. High rates of hypermobility are present in patients with fibromyalgia both as adults[55] and children[56], and in patients with ME/CFS, again both in adults[57] and children[58]. Hypermobility is specifically linked to the expression of pain and fatigue in these patients[59]. Conversely, the majority of EDS patients suffer from marked fatigue[60]. In fact, many patients with HSD/hEDS meet Fukada criteria for diagnosis of CFS[50], and hence it is argued that an assessment for hypermobility/variant connective tissues should be an integral part of a thorough evaluation of ME/CFS[61].
IBS and functional gastrointestinal disorders: Gastrointestinal symptoms (including nausea, abdominal pain, bloating, constipation and diarrhoea) are commonly experienced by patients with HSD/hEDS diagnosis[62]. Objectively, gastrointestinal dysmotility is observed[63] and is predicted by the symptoms and signs of dysautonomia, notably the occurrence of postural tachycardia syndrome (PoTS). Interestingly, psychiatric disorders, especially mood and somatoform disorders, occur at higher rates in patients with HSD/hEDS who experience gastrointestinal dysfunction and associated abdominal pain[64].
The prevalence of hypermobility appears to be greater in patients with IBS or gastrointestinal symptoms (especially functional dyspepsia) when compared to healthy controls[65]. This represents a subgroup (even variant phenotype) of patients who experience primary gastrointestinal problems with co-existing HSD/hEDS, who also demonstrate high comorbidity with chronic pain and fibromyalgia. These patients show increased somatisation score, urinary autonomic (symptom) score and reduced pain-related quality of life[66]. High rates of hypermobility are particularly observed in patients with constipation-predominant IBS[67], consistent with perturbation of colonic sensorimotor biomechanics consequent upon variant connective tissue.
However, while in adults the association between hypermobility and functional gastrointestinal disorders appears relatively robust, in children there is increasing evidence that the prevalence of hypermobility is similar for children with IBS, functional abdominal pain or functional constipation, and for healthy controls[68-70]. Intriguingly, this suggests that, in hypermobile individuals, IBS and functional GI symptoms may develop later in life perhaps interacting with hormonal or other maturational changes that impact connective tissue compliance.
Other associated stress-sensitive medical conditions: There are reports demonstrating that HSD/hEDS is also associated with increased risk of migraine (in females)[71], and with chronic regional pain syndrome[72], chronic myofascial pelvic pain[73], and stress incontinence[74].
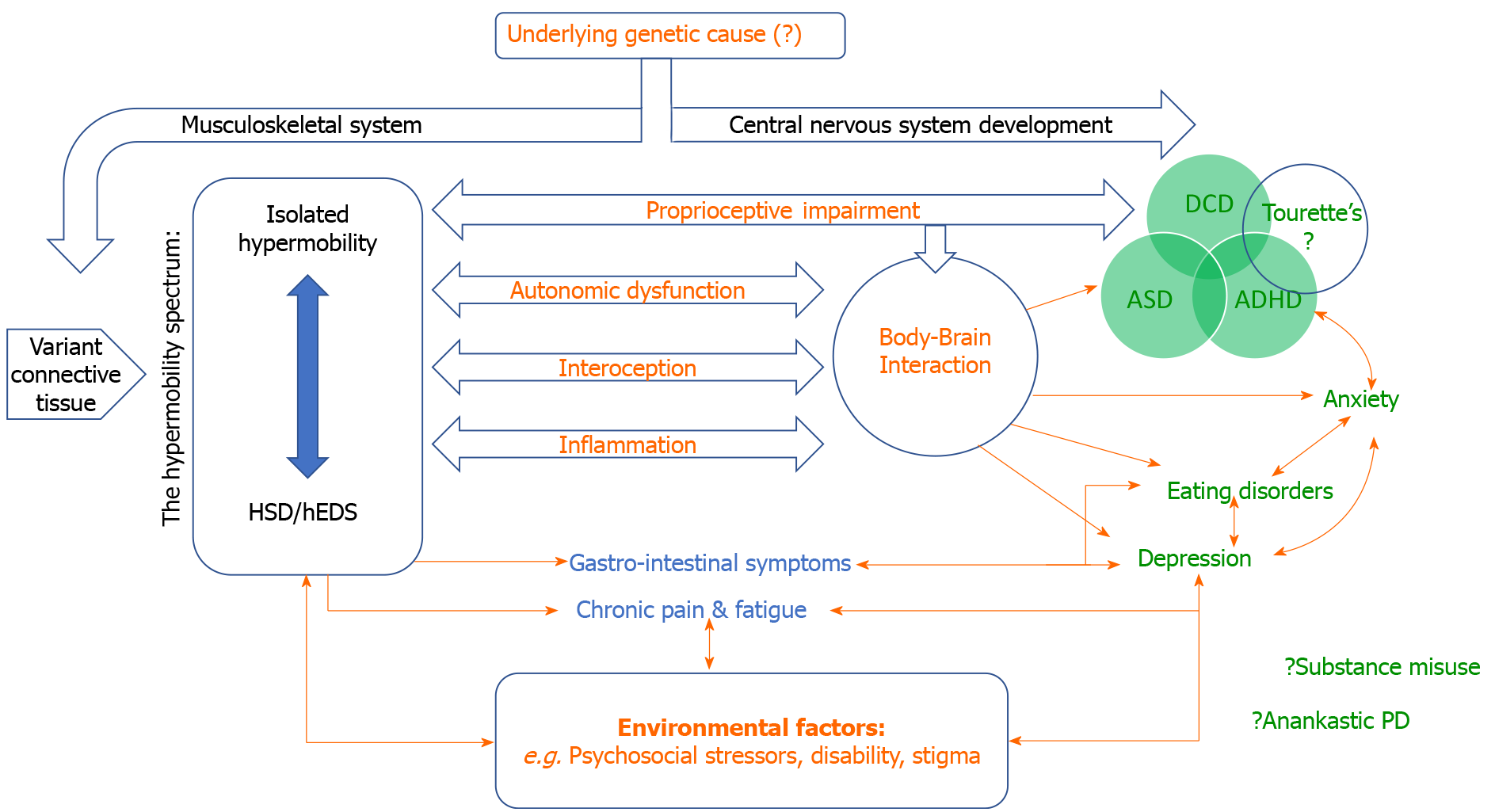
The ‘biopsychosocial approach’ in psychiatry acknowledges the contribution of interconnected and interacting influences on the development and maintenance of psychiatric symptoms and diagnoses, including biological vulnerabilities, psychological factors and dynamic social context. The associations highlighted within this review, linking psychiatric symptoms and conditions to constitutional differences in connective tissue (apparent, in part, as joint hypermobility), likely depend on multiple mechanisms, which may differ between disorders. Hypothetically, joint hypermobility may represent a non-specific signature of a general mechanism linking variant connective tissue to psychological vulnerability, manifesting in a continuum across neurodevelopmental and psychiatric disorders. Alternatively, a pre-existing pathological process (as yet undiscovered) could predispose to both psychopathology and hypermobility in parallel via independent actions on the musculoskeletal and central nervous systems (Figure 1).
Through lived experience, the presence of hypermobility from early childhood may directly underpin the development of psychiatric disorders. Excluding neurodevelopmental conditions, this makes temporal sense: The presence and consequences of hypermobility in childhood precedes the typically later emergence of psychiatric disorders in adolescence and adulthood. When symptomatic from a young age, the daily challenges of living with physical risks and difficulties associated with HDS/hEDS (including chronic pain and disability, threat of injury secondary to connective tissue fragility, restriction of social and physical activities and associated stigma) contribute to psychiatric vulnerability, avoidant behaviours and the development of anxiety and depressive symptoms[75].
Correspondingly, intense fear of pain and subsequent pain-avoidant strategies that limit movement (kinesiophobia) are common in HSD/hEDS[76]. These increase the likelihood of deconditioning and symptom progression, thereby furthering physical and psychological disability. In addition, problematic hypermobility is typically under-recognised and diagnostic delay is common[3]. Consequently, patients may experience frustration if their complaints are trivialised by healthcare professionals, adding to suffering and exclusion[75]. High anxiety and distress may drive maladaptive behaviours including tobacco/alcohol use and disordered eating[33], and the psychosocial complexity surrounding hypermobility is associated with poorer quality of life in both adults[8] and children[9]. In hEDS patients, the psychological expression of high anxiety interacts with more severe fatigue and depressive symptoms, pain catastrophising, somatosensory amplification, to predict poorer social functioning and worse general health[20].
The bidirectional interaction between pain and psychological ill-health is widely recognised; chronic pain influences psychiatric symptoms including depression and anxiety, while low mood and negative emotions increase and maintain the experience of pain[77]. In this context, psychiatric disorders in HSD/hEDS may be secondary to pain symptoms rather than a primary or parallel manifestation. Hypermobility is a risk factor for chronic widespread pain[78], and hypermobile individuals exhibit sensory hypersensitivity to nociceptive stimuli[79] and secondary central sensitisation[80]. In fact, the presence of pain, rather than the degree hypermobility, is associated with the expression of psychiatric symptoms in EDS patients[81]. Similarly, the experience of pain and gastrointestinal dysfunction, rather than connective tissue features per se, is associated with higher rates of psychiatric disorders (especially mood and somatoform conditions) in HSD/hEDS patients[64].
The relationship with pain is more nuanced; rates of psychiatric disorder are higher for HSD/hEDS than for other chronic pain conditions[13]. Thus, psychosocial theories fail to explain the full extent of all associations between hypermobility and psychopathological distress. This is particularly evident when considering neurodevelopmental disorders and in individuals with seemingly expressing sub-syndromic and isolated hypermobility. Instead, alternate theories propose that a common set of pathological mechanisms fundamentally predispose to the expression of both hypermobility and psychopathological symptoms (for example, as genetic pleiotropy).
There is interesting evidence from animals: Hypermobility is linked to exaggerated reactivity of behavioural/emotional arousal and excitability in dogs[82]. This finding suggests that the link between joint hypermobility and the affective control of bodily arousal is a universal transdiagnostic trait in mammals, influencing the expression of anxiety and behavioural responses. Moreover, such findings argue against conscious awareness and social implications of living with physical symptoms as a primary driver for the raised levels of psychopathology associated with hypermobility.
Genetic and/or early environmental influences are suggested by the increased risk of psychiatric disorders observed in the (unaffected) first-degree relatives of HSD/hEDS patients[23]. However, unlike other heritable disorders of connective tissue and EDS subtypes, genetic origins for HSD/hEDS remain poorly understood[3]. Previous identification of a genetic anomaly common to both anxiety and hypermobility, a duplication within chromosome 15, failed confirmation in subsequent studies[83]. A promising link has been noted between EDS and the gene TNXb (6p21.33), encoding the extracellular matrix glycoprotein Tenascin-XB, which is directly involved in connective tissue structure[84]. This molecule was recently identified as integral in enteric motor neurons and influencing nociceptive sensory neurons[85] and is expressed in the brain, suggesting further roles within the central nervous system. The extracellular matrix plays a critical role in both variant connective tissue and central nervous system development, and so alterations could predispose to abnormalities across central and peripheral systems[86].
HSD/hEDS frequently co-occurs with autonomic dysfunction commonly experienced as symptoms of orthostatic intolerance and, in severe cases, PoTS[87]. The diagnosis of PoTS focuses on the characteristic elevation of heart rate during postural change and accompanying orthostatic intolerance[88]. The widespread presence of variant connective tissue is implicated as one potential physiological mechanism for the overlap between HSD/hEDS and dysautonomia. Within blood vessels, more compliant connective tissue may cause abnormal vascular reactivity, notably increased venous pooling on standing, which reduces venous return. Heart rate acceleration and a secondary hyperadrenergic state would thus result[87].
There is significant phenomenological overlap between symptoms of anxiety and panic and the symptoms of autonomic disfunction in orthostatic intolerance and PoTS: Physical symptoms include palpitations, breathlessness and dizziness[88], and these themselves could trigger and amplify anxiety states or be misperceived or diagnosed as panic attacks. Enhanced bodily awareness (described below) may be both a consequence and maintaining factor for such experiences, since altered autonomic reactivity will influence emotional state and vulnerability to psychiatric disorders[89]. Autonomic dysfunction is also demonstrable in children with neurodevelopmental conditions linked to hypermobility, notably ASD[90] and ADHD[91]. Again, there is symptomatic overlap between PoTS and ASD in both affective symptoms and sensory sensitivities[34]. The causal relationship between altered autonomic function and neurodevelopmental phenotypes remains unclear but evidence for an interactive association continues to grow.
Interoception refers to the afferent signalling to brain of changes in the internal physiological state of the body, and the perception of these changes. Interoceptive signals therefore represent the sensory limb of autonomic control loops (e.g., the baroreflex), yet also reach perceptual awareness as physiological feelings including palpitations, nausea and arousal. In this way, interoceptive signals can guide motivation behaviour and are fundamental components of emotional feeling states. Central interoceptive representation and/or misinterpretation of dysregulated peripheral autonomic function may underpin the generation of a range of symptoms associated with hypermobility[92]. Dysregulated interoceptive processes are implicated in the expression of specific psychiatric symptoms, particularly anxiety[92]. Hypermobile individuals demonstrate enhanced subjective sensitivity to internal bodily sensations[93] and in more objective measures of detecting interoceptive signals. These differences are shown to mediate the relationship between state anxiety and hypermobility[94] and offer a potential treatment target for intervention.
Neuroimaging studies in hypermobile individuals reveal structural[93] and functional brain differences[94] notably within brain systems critical to emotional processing and anxiety[95], including regions such as insular cortex, that are also specifically implicated in interoceptive representation[94. However, much of this work has been conducted in sub-clinical cohorts. While findings endorse a potential autonomic/interoceptive basis to vulnerability to anxiety and other neuropsychiatric symptoms, further work is needed to map these relationships in patients with psychiatric disorders in the context of hypermobility and to dissect levels of putative interoceptive dysfunction.
Extensive bi-directional communication exists between the brain and the immune system and, increasingly, immune mechanisms are implicated in the pathogenesis of psychiatric disorders[96]. A specific association appears to exist between immune system and hypermobility: Mast cell related disorders (giving allergy-like symptoms often across multiple organ systems) are commonly reported in patients with EDS/hEDS, highlighting a deep interrelationship between connective tissue and immune function occurring across genetic, molecular and physiological levels[97]. The concurrent expression of connective tissue impairments and immune dysfunction is speculated to influence vulnerability to psychiatric disorders. This may extend to neurodevelopmental disorders[34], as recent evidence highlights immune dysregulation in ASD[98].
Abnormal proprioception is observed in both hypermobility and neurodevelopmental disorders and may account for their association[38]. Symptoms of both are present from early in childhood and appear highly heritable. Impaired proprioception particularly affects the lower limbs of children and adults with HSD/hEDS[99] and often leads to issues with coordination, balance, clumsiness and motor problems. Poor proprioception may directly account for the relationship between hypermobility and DCD[100]. Moreover, it is speculated that maintaining motor competences despite impaired proprioceptive function may overload executive functions and compete for attentional resources. This may in turn reinforce inattention in ADHD[101], and could further impact the timely acquisition of social and communication skills, thereby exacerbating ASD traits[34].
We echo other authors calling for widened awareness of the diverse manifestations of hypermobility, particularly in psychiatry[34]. A recently proposed ‘neuroconnective phenotype’ model usefully describes the symptom profiles often expressed by people with hypermobility, including behavioural, psychopathological, somatic symptoms, somatosensory symptoms and somatic illness[11]. Considering the evidence presented here, we recommend vigilance for potentially overlooked mental health symptoms within the plethora of difficulties faced by patients living with HSD/hEDS. These should be part of a holistic clinical formulation that can enable appropriate psychoeducation, referral to mental health services, prompt diagnosis and access to optimal treatments.
Alongside this, we recommend screening for hypermobility, particularly in those presenting with neurodevelopmental disorders, but also in other mental health presentations, notably of panic and anxiety, in the context of physical symptoms, since articular and extra-articular features are often present[102] (Table 1). Simple screening using a 5-point questionnaire can detect hypermobility with high sensitivity and specificity[1] (Table 2). Early assessment can prompt referral to specialist services and thus reduce delayed or misdiagnosis of HSD/hEDS. It also widens opportunities for early intervention to prevent progression of psychological as well as physical symptoms throughout the lifespan.
| Extra-articular features | Articular features | |
| In children and adolescents | Prolonged fatigue or tiring easily | Joint dislocations/subluxations (including congenital hip dislocation) |
| Poor motor coordination or ‘clumsiness’ (such as poor ball catching and poor handwriting) | Recurrent ankle sprains | |
| Chronic widespread pain or ‘growing pains’ | ||
| Delayed walking, with bottom shuffling instead of crawling | ||
| In adults | Prolonged unexplained fatigue (including ME/CFS) | Recurrent joint dislocations |
| Chronic widespread pain, particularly if unresponsive to analgesia (including fibromyalgia) | Multiple soft tissue injuries/rheumatisms | |
| Functional gastrointestinal disorders (such as IBS, functional dyspepsia, constipation) | Premature osteoarthritis | |
| Autonomic dysfunction (such as orthostatic intolerance or PoTS) | Persistent or recurrent joint pains | |
| Progressive loss of mobility secondary to pain or pain-avoidance strategies | ||
| Laxity in supporting tissues (such as hernias, varicose veins, pelvic floor dysfunction) | ||
| Soft/hyperextensible skin, unexplained striae, easy bruising |
| 1 Can you now (or could you ever) place your hands flat on the floor without bending your knees? |
| 2 Can you now (or could you ever) bend your thumb to touch your forearm? |
| 3 As a child, did you amuse your friends by contorting your body into strange shapes OR could you do the splits? |
| 4 As a child or teenager did your shoulder or kneecap dislocate on more than one occasion? |
| 5 Do you consider yourself ‘double-jointed’? |
| Answering yes to two or more questions suggests hypermobility with sensitivity 80%-85% and specificity 80%-90% |
Currently, there no specific guidelines for the psychological or pharmacological management of psychiatric conditions in patients with HSD/hEDS. Nevertheless, considering the complex difficulties faced by these patients, we recommend multidisciplinary management across mental and physical health professionals, including early involvement of physiotherapy to facilitate improvement of proprioception and bodily awareness[19].
One considerable limitation in evaluating the growing evidence base describing the association between joint hypermobility and psychiatric disorder is the variable use of assessment tools to measure hypermobility. Specific genetic tests or biomarkers do not exist. The use of assorted self-report questionnaires and distinct clinical assessment scales is liable to subjective interpretation and observer dependence, limiting reliability. Furthermore, the application of age-dependent cut-off scores remains controversial. Our review has considered articles covering the whole spectrum of hypermobility and related disorders yet highlights the need for further disentangling research.
Nevertheless, individuals with higher anxiety and more severe symptoms are more likely to seek medical attention, join patient support associations and participate in research studies, even among ‘non-clinical’ populations. Those recruited may be experiencing higher distress, and hence introduce sampling bias. Published studies may therefore overlook (and over-pathologise) the spectrum of hypermobility presentations.
There is strong evidence linking the expression of mental health disorders to hypermobility as a signature of variant connective tissue. However, future research is needed to investigate the genetic, neural and psychophysiological mechanisms for such mind-body interactions. Longitudinal studies are needed to better identify which hypermobility individuals develop psychiatric symptoms or diagnoses to delineate contributions of precipitating, perpetuating, and preventative factors and map how these progress with time. Such fresh insights offer the potential for the implementation of early preventative strategies in at-risk individuals, minimising complications in later life.
We argue that variant connective tissue contributes to the pathogenesis of mental health disorders: This likely represents an ‘endophenotype’; a measurable component along the pathway between phenotype and disease, an important emerging concept in neuropsychiatric research[103]. In this respect, hypermobility is clearly heritable, and presents with excess relative risk in relatives[23]. In addition, its role has biological plausibility, is not state dependent, and is easily testable. However, there remains much to understand, not least how hypermobility is connected with candidate genes. In this context, across psychiatric disorders the presence of hypermobility could represent a phenotypic subgroup of patients[103]. Hypermobility may also act as a clinical marker for specific target symptoms e.g., anxiety[16], as demonstrated in hypermobile patients with schizophrenia[31] or for sensory hypersensitivities, e.g., to olfactory stimuli in patients with panic disorder[104].
More research is also needed to determine if this patient subset responds differently to treatment, allowing for a ‘personalised medicine’ approach. For example, a dietary intervention (low FODMAP) for irritable bowel syndrome is reportedly more effective in hypermobile compared to non-hypermobile patients[105]. Dysfunctional coping strategies are associated with hypermobility[33], so psychological approaches addressing these could prove beneficial. Anecdotally, sensitivity to unwanted drug side-effects is high in this group. The first randomised controlled trial of a targeted non-pharmacological therapy for anxiety in the context of hypermobility is currently ongoing, comparing modified interoceptive training to standard supportive treatment in individuals with hypermobility and anxiety[106].
In conclusion, alongside the well-known link between hypermobility and anxiety, recognition of which has expanded across age groups, there is growing evidence of associations with depression, eating disorders, neurodevelopmental conditions and stress-sensitive medical conditions. There remains a paucity of evidence for links with schizophrenia and addictions. We recommend clinicians across different specialities are aware of the transdiagnostic nature of HSD/hEDS, which may present with both primary and secondary difficulties in psychological and physical domains. In particular, professionals encountering patients with mental health difficulties should consider the prospect of underlying hypermobility as a potential influence on neuropsychiatric symptom progression in this subgroup of patients.
While hypermobility and psychopathological attributes are conventionally considered distinct, their association affirms the pervasive interplay of mind, brain and body. Perceptions, emotions, cognitions and behaviours are dynamically coupled to the state of the body through both unconscious and conscious mechanisms[89]. Further investigation of the psychophysiological, cellular, molecular and genetic underpinnings of the link between hypermobility and psychiatric disorders may provide clinically relevant insights to improve recognition, identify treatment targets, and plan holistic management strategies across the lifespan that combine both psychiatric and somatic approaches.
We wish to thank Professor Rodney Grahame, Professor Christopher Mathias, Dr. Valeria Iodice, Professor Antonio Bulbena and Dr. Baeza-Velasco for their inspiration for this paper. We are also grateful to the journal’s editors and to Rosie Mulgrue for proof-reading input.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Stoyanov D S-Editor: Gong ZM L-Editor: A P-Editor: Ma YJ
| 1. | Hakim A, Grahame R. Joint hypermobility. Best Pract Res Clin Rheumatol. 2003;17:989-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Remvig L, Jensen DV, Ward RC. Epidemiology of general joint hypermobility and basis for the proposed criteria for benign joint hypermobility syndrome: review of the literature. J Rheumatol. 2007;34:804-809. [PubMed] |
| 3. | Castori M. Ehlers-danlos syndrome, hypermobility type: an underdiagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations. ISRN Dermatol. 2012;2012:751768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 4. | Bulbena A, Duró JC, Porta M, Faus S, Vallescar R, Martín-Santos R. Clinical assessment of hypermobility of joints: assembling criteria. J Rheumatol. 1992;19:115-122. [PubMed] |
| 5. | Grahame R, Bird HA, Child A. The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS). J Rheumatol. 2000;27:1777-1779. [PubMed] |
| 6. | Castori M, Tinkle B, Levy H, Grahame R, Malfait F, Hakim A. A framework for the classification of joint hypermobility and related conditions. Am J Med Genet C Semin Med Genet. 2017;175:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 354] [Article Influence: 44.3] [Reference Citation Analysis (1)] |
| 7. | Colombi M, Dordoni C, Chiarelli N, Ritelli M. Differential diagnosis and diagnostic flow chart of joint hypermobility syndrome/ehlers-danlos syndrome hypermobility type compared to other heritable connective tissue disorders. Am J Med Genet C Semin Med Genet. 2015;169C:6-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Rombaut L, Malfait F, Cools A, De Paepe A, Calders P. Musculoskeletal complaints, physical activity and health-related quality of life among patients with the Ehlers-Danlos syndrome hypermobility type. Disabil Rehabil. 2010;32:1339-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Pacey V, Tofts L, Adams RD, Munns CF, Nicholson LL. Quality of life prediction in children with joint hypermobility syndrome. J Paediatr Child Health. 2015;51:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Eccles JA, Scott HE, Davies KA, Bond R, David AS, Harrison NA, Critchley HD. Joint hypermobility and its relevance to common mental illness in adolescents: a population-based longitudinal study. medRxiv. 2020;Preprint. [DOI] [Full Text] |
| 11. | Bulbena A, Pailhez G, Bulbena-Cabré A, Mallorquí-Bagué N, Baeza-Velasco C. Joint hypermobility, anxiety and psychosomatics: two and a half decades of progress toward a new phenotype. Adv Psychosom Med. 2015;34:143-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Kessler RC, Ruscio AM, Shear K, Wittchen HU. Epidemiology of anxiety disorders. Curr Top Behav Neurosci. 2010;2:21-35. [PubMed] |
| 13. | Smith TO, Easton V, Bacon H, Jerman E, Armon K, Poland F, Macgregor AJ. The relationship between benign joint hypermobility syndrome and psychological distress: a systematic review and meta-analysis. Rheumatology (Oxford). 2014;53:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Bulbena A, Agulló A, Pailhez G, Martín-Santos R, Porta M, Guitart J, Gago J. Is joint hypermobility related to anxiety in a nonclinical population also? Psychosomatics. 2004;45:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Bulbena-Cabré A, Rojo C, Pailhez G, Buron Maso E, Martín-Lopez LM, Bulbena A. Joint hypermobility is also associated with anxiety disorders in the elderly population. Int J Geriatr Psychiatry. 2018;33:e113-e119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Bulbena-Cabre A, Duñó L, Almeda S, Batlle S, Camprodon-Rosanas E, Martín-Lopez LM, Bulbena A. Joint hypermobility is a marker for anxiety in children. Rev Psiquiatr Salud Ment (Engl Ed). 2019;12:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Javadi Parvaneh V, Modaress S, Zahed G, Rahmani K, Shiari R. Prevalence of generalized joint hypermobility in children with anxiety disorders. BMC Musculoskelet Disord. 2020;21:337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Ezpeleta L, Navarro JB, Osa N, Penelo E, Bulbena A. Joint Hypermobility Classes in 9-Year-Old Children from the General Population and Anxiety Symptoms. J Dev Behav Pediatr. 2018;39:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Bulbena A, Baeza-Velasco C, Bulbena-Cabré A, Pailhez G, Critchley H, Chopra P, Mallorquí-Bagué N, Frank C, Porges S. Psychiatric and psychological aspects in the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Baeza-Velasco C, Bourdon C, Montalescot L, de Cazotte C, Pailhez G, Bulbena A, Hamonet C. Low- and high-anxious hypermobile Ehlers-Danlos syndrome patients: comparison of psychosocial and health variables. Rheumatol Int. 2018;38:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Pasquini M, Celletti C, Berardelli I, Roselli V, Mastroeni S, Castori M, Biondi M, Camerota F. Unexpected association between joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type and obsessive-compulsive personality disorder. Rheumatol Int. 2014;34:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Gurer G, Sendur F, Gultekin BK, Ozcan ME. The anxiety between individuals with and without joint hypermobility. Europ J Psychiatry. 2010;24:205-209. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Cederlöf M, Larsson H, Lichtenstein P, Almqvist C, Serlachius E, Ludvigsson JF. Nationwide population-based cohort study of psychiatric disorders in individuals with Ehlers-Danlos syndrome or hypermobility syndrome and their siblings. BMC Psychiatry. 2016;16:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Baeza-Velasco C, Gely-Nargeot MC, Vilarrasa AB, Fenetrier C, Bravo JF. Association between psychopathological factors and joint hypermobility syndrome in a group of undergraduates from a French university. Int J Psychiatry Med. 2011;41:187-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Albayrak İ, Yilmaz H, Akkurt HE, Salli A, Karaca G. Is pain the only symptom in patients with benign joint hypermobility syndrome? Clin Rheumatol. 2015;34:1613-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Eccles JA. Hypermobility and Autonomic Hyperactivity: Relevance for the Expression of Psychiatric Symptoms. PhD Thesis, University of Brighton; 2016. Available from: https://cris.brighton.ac.uk/ws/portalfiles/portal/4755648/FINAL+ECCLES.pdf. |
| 27. | Baeza-Velasco C, Van den Bossche T, Grossin D, Hamonet C. Difficulty eating and significant weight loss in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. Eat Weight Disord. 2016;21:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Goh M, Olver J, Huang C, Millard M, O'Callaghan C. Prevalence and familial patterns of gastrointestinal symptoms, joint hypermobility and diurnal blood pressure variations in patients with anorexia nervosa. J Eat Disord. 2013;1:O45. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Baeza-Velasco C, Pailhez G, Bulbena A, Baghdadli A. Joint hypermobility and the heritable disorders of connective tissue: clinical and empirical evidence of links with psychiatry. Gen Hosp Psychiatry. 2015;37:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Pailhez G, Rodríguez A, Ariza J, Palomo AL, Bulbena A. [Somatotype and schizophrenia. A case-control study]. Actas Esp Psiquiatr. 2009;37:258-266. [PubMed] |
| 31. | Bulbena A, Sperry L, Anguiano B, Pailhez G, Gago J. Joint hypermobility in schizophrenia: a potential marker for co-morbid anxiety. Open Psychiatry J. 2007;1:31-33. [DOI] [Full Text] |
| 32. | Carlsson C, Rundgren A. Hypermobility of the joints in women alcoholics. J Stud Alcohol. 1980;41:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Baeza-Velasco C, Stoebner-Delbarre A, Cousson-Gélie F, Pailhez G, Bulbena A, Baguet F, Gély-Nargeot MC. Increased tobacco and alcohol use among women with joint hypermobility: a way to cope with anxiety? Rheumatol Int. 2015;35:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Casanova EL, Baeza-Velasco C, Buchanan CB, Casanova MF. The Relationship between Autism and Ehlers-Danlos Syndromes/Hypermobility Spectrum Disorders. J Pers Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Baeza-Velasco C, Grahame R, Bravo JF. A connective tissue disorder may underlie ESSENCE problems in childhood. Res Dev Disabil. 2017;60:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Csecs JLL, Iodice V, Rae CL, Brooke A, Simmons R, Dowell NG, Fenella Prowse, Themelis K, Critchley PD, Eccles PA. Increased rate of joint hypermobility in autism and related neurodevelopmental conditions is linked to dysautonomia and pain. medRxiv. 2020;Preprint. [DOI] [Full Text] |
| 37. | Glans M, Bejerot S, Humble MB. Generalised joint hypermobility and neurodevelopmental traits in a non-clinical adult population. BJPsych Open. 2017;3:236-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Baeza-Velasco C, Cohen D, Hamonet C, Vlamynck E, Diaz L, Cravero C, Cappe E, Guinchat V. Autism, Joint Hypermobility-Related Disorders and Pain. Front Psychiatry. 2018;9:656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Shetreat-Klein M, Shinnar S, Rapin I. Abnormalities of joint mobility and gait in children with autism spectrum disorders. Brain Dev. 2014;36:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Paquet A, Olliac B, Golse B, Vaivre-Douret L. Evaluation of neuromuscular tone phenotypes in children with autism spectrum disorder: An exploratory study. Neurophysiol Clin. 2017;47:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Blair DR, Lyttle CS, Mortensen JM, Bearden CF, Jensen AB, Khiabanian H, Melamed R, Rabadan R, Bernstam EV, Brunak S, Jensen LJ, Nicolae D, Shah NH, Grossman RL, Cox NJ, White KP, Rzhetsky A. A nondegenerate code of deleterious variants in Mendelian loci contributes to complex disease risk. Cell. 2013;155:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 42. | Balasubramanian M, Jones R, Milne E, Marshall C, Arundel P, Smith K, Bishop NJ. Autism and heritable bone fragility: A true association? Bone Rep. 2018;8:156-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Ciaccio C, Fontana L, Milani D, Tabano S, Miozzo M, Esposito S. Fragile X syndrome: a review of clinical and molecular diagnoses. Ital J Pediatr. 2017;43:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 44. | Doğan ŞK, Taner Y, Evcik D. Benign joint hypermobility syndrome in patients with attention deficit/hyperactivity disorders. Arch Rheumatol. 2011;26:187-192. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Shiari R, Saeidifard F, Zahed G. Evaluation of the prevalence of joint laxity in children with attention deficit/hyperactivity disorder. Ann Paediatr Rheumatol. 2013;7:8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Hollertz O. [Searching for a biological marker common for both ADHD and EDS]. Lakartidningen. 2012;109:41-42. [PubMed] |
| 47. | Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub; 2013. [RCA] [DOI] [Full Text] [Cited by in Crossref: 66101] [Cited by in RCA: 58173] [Article Influence: 3635.8] [Reference Citation Analysis (4)] |
| 48. | Kirby A, Davies R. Developmental Coordination Disorder and Joint Hypermobility Syndrome--overlapping disorders? Child Care Health Dev. 2007;33:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Adib N, Davies K, Grahame R, Woo P, Murray KJ. Joint hypermobility syndrome in childhood. A not so benign multisystem disorder? Rheumatology (Oxford). 2005;44:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 50. | Castori M, Dordoni C, Valiante M, Sperduti I, Ritelli M, Morlino S, Chiarelli N, Celletti C, Venturini M, Camerota F, Calzavara-Pinton P, Grammatico P, Colombi M. Nosology and inheritance pattern(s) of joint hypermobility syndrome and Ehlers-Danlos syndrome, hypermobility type: a study of intrafamilial and interfamilial variability in 23 Italian pedigrees. Am J Med Genet A. 2014;164A:3010-3020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | Piedimonte C, Penge R, Morlino S, Sperduti I, Terzani A, Giannini MT, Colombi M, Grammatico P, Cardona F, Castori M. Exploring relationships between joint hypermobility and neurodevelopment in children (4-13 years) with hereditary connective tissue disorders and developmental coordination disorder. Am J Med Genet B Neuropsychiatr Genet. 2018;177:546-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Jelsma LD, Geuze RH, Klerks MH, Niemeijer AS, Smits-Engelsman BC. The relationship between joint mobility and motor performance in children with and without the diagnosis of developmental coordination disorder. BMC Pediatr. 2013;13:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Celletti C, Mari G, Ghibellini G, Celli M, Castori M, Camerota F. Phenotypic variability in developmental coordination disorder: Clustering of generalized joint hypermobility with attention deficit/hyperactivity disorder, atypical swallowing and narrative difficulties. Am J Med Genet C Semin Med Genet. 2015;169C:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Hakim A, De Wandele I, O'Callaghan C, Pocinki A, Rowe P. Chronic fatigue in Ehlers-Danlos syndrome-Hypermobile type. Am J Med Genet C Semin Med Genet. 2017;175:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 55. | Ofluoglu D, Gunduz OH, Kul-Panza E, Guven Z. Hypermobility in women with fibromyalgia syndrome. Clin Rheumatol. 2006;25:291-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Kashikar-Zuck S, King C, Ting TV, Arnold LM. Juvenile Fibromyalgia: Different from the Adult Chronic Pain Syndrome? Curr Rheumatol Rep. 2016;18:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 57. | Bragée B, Michos A, Drum B, Fahlgren M, Szulkin R, Bertilson BC. Signs of Intracranial Hypertension, Hypermobility, and Craniocervical Obstructions in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front Neurol. 2020;11:828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 58. | Barron DF, Cohen BA, Geraghty MT, Violand R, Rowe PC. Joint hypermobility is more common in children with chronic fatigue syndrome than in healthy controls. J Pediatr. 2002;141:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Eccles JA, Thompson B, Themelis K, Amato ML, Stocks R, Pound A, Jones AM, Cipinova Z, Shah-Goodwin L, Timeyin J, Thompson CR, Batty T, Harrison NA, Critchley HD, Davies KA. Beyond bones: The relevance of variants of connective tissue (hypermobility) to fibromyalgia, ME/CFS and controversies surrounding diagnostic classification: an observational study. Clin Med (Lond). 2021;21:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 60. | Voermans NC, Knoop H, van de Kamp N, Hamel BC, Bleijenberg G, van Engelen BG. Fatigue is a frequent and clinically relevant problem in Ehlers-Danlos Syndrome. Semin Arthritis Rheum. 2010;40:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 61. | Rowe PC, Barron DF, Calkins H, Maumenee IH, Tong PY, Geraghty MT. Orthostatic intolerance and chronic fatigue syndrome associated with Ehlers-Danlos syndrome. J Pediatr. 1999;135:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 62. | Beckers AB, Keszthelyi D, Fikree A, Vork L, Masclee A, Farmer AD, Aziz Q. Gastrointestinal disorders in joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type: A review for the gastroenterologist. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 63. | Alomari M, Hitawala A, Chadalavada P, Covut F, Al Momani L, Khazaaleh S, Gosai F, Al Ashi S, Abushahin A, Schneider A. Prevalence and Predictors of Gastrointestinal Dysmotility in Patients with Hypermobile Ehlers-Danlos Syndrome: A Tertiary Care Center Experience. Cureus. 2020;12:e7881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Wasim S, Suddaby JS, Parikh M, Leylachian S, Ho B, Guerin A, So J. Pain and gastrointestinal dysfunction are significant associations with psychiatric disorders in patients with Ehlers-Danlos syndrome and hypermobility spectrum disorders: a retrospective study. Rheumatol Int. 2019;39:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Fikree A, Grahame R, Aktar R, Farmer AD, Hakim AJ, Morris JK, Knowles CH, Aziz Q. A prospective evaluation of undiagnosed joint hypermobility syndrome in patients with gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2014;12:1680-87.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 66. | Fikree A, Aktar R, Grahame R, Hakim AJ, Morris JK, Knowles CH, Aziz Q. Functional gastrointestinal disorders are associated with the joint hypermobility syndrome in secondary care: a case-control study. Neurogastroenterol Motil. 2015;27:569-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 67. | Zweig A, Schindler V, Becker AS, van Maren A, Pohl D. Higher prevalence of joint hypermobility in constipation predominant irritable bowel syndrome. Neurogastroenterol Motil. 2018;30:e13353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Shulman RJ, Self MM, Czyzewski DI, Goldberg J, Heitkemper M. The Prevalence of Hypermobility in Children with Irritable Bowel Syndrome and Functional Abdominal Pain Is Similar to that in Healthy Children. J Pediatr. 2020;222:134-140.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Saps M, Blom PJJ, Velasco-Benitez CA, Benninga MA. Functional Gastrointestinal Disorders and Joint Hypermobility: A School-based Study. J Pediatr Gastroenterol Nutr. 2018;66:387-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Khorasgani SMF, Ramezani N, Varnousfaderani NE. Joint hypermobility in children with and without functional constipation. J Res Med Sci. 2020;25:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 71. | Bendik EM, Tinkle BT, Al-shuik E, Levin L, Martin A, Thaler R, Atzinger CL, Rueger J, Martin VT. Joint hypermobility syndrome: A common clinical disorder associated with migraine in women. Cephalalgia. 2011;31:603-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Stoler JM, Oaklander AL. Patients with Ehlers Danlos syndrome and CRPS: a possible association? Pain. 2006;123:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Hastings J, Forster JE, Witzeman K. Joint Hypermobility among Female Patients Presenting with Chronic Myofascial Pelvic Pain. PM R. 2019;11:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Karan A, Isikoglu M, Aksac B, Attar E, Eskiyurt N, Yalcin O. Hypermobility syndrome in 105 women with pure urinary stress incontinence and in 105 controls. Arch Gynecol Obstet. 2004;269:89-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Baeza-Velasco C, Gély-Nargeot MC, Bulbena Vilarrasa A, Bravo JF. Joint hypermobility syndrome: problems that require psychological intervention. Rheumatol Int. 2011;31:1131-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Celletti C, Castori M, La Torre G, Camerota F. Evaluation of kinesiophobia and its correlations with pain and fatigue in joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type. Biomed Res Int. 2013;2013:580460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 77. | Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2057] [Cited by in RCA: 2371] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 78. | Tobias JH, Deere K, Palmer S, Clark EM, Clinch J. Joint hypermobility is a risk factor for musculoskeletal pain during adolescence: findings of a prospective cohort study. Arthritis Rheum. 2013;65:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 79. | Rombaut L, Scheper M, De Wandele I, De Vries J, Meeus M, Malfait F, Engelbert R, Calders P. Chronic pain in patients with the hypermobility type of Ehlers-Danlos syndrome: evidence for generalized hyperalgesia. Clin Rheumatol. 2015;34:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 80. | Di Stefano G, Celletti C, Baron R, Castori M, Di Franco M, La Cesa S, Leone C, Pepe A, Cruccu G, Truini A, Camerota F. Central sensitization as the mechanism underlying pain in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. Eur J Pain. 2016;20:1319-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Hershenfeld SA, Wasim S, McNiven V, Parikh M, Majewski P, Faghfoury H, So J. Psychiatric disorders in Ehlers-Danlos syndrome are frequent, diverse and strongly associated with pain. Rheumatol Int. 2016;36:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 82. | Bowen J, Fatjó J, Serpell JA, Bulbena-Cabré A, Leighton E, Bulbena A. First evidence for an association between joint hypermobility and excitability in a non-human species, the domestic dog. Sci Rep. 2019;9:8629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 83. | Tabiner M, Youings S, Dennis N, Baldwin D, Buis C, Mayers A, Jacobs PA, Crolla JA. Failure to find DUP25 in patients with anxiety disorders, in control individuals, or in previously reported positive control cell lines. Am J Hum Genet. 2003;72:535-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Petersen JW, Douglas JY. Tenascin-X, collagen, and Ehlers-Danlos syndrome: tenascin-X gene defects can protect against adverse cardiovascular events. Med Hypotheses. 2013;81:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Aktar R, Peiris M, Fikree A, Cibert-Goton V, Walmsley M, Tough IR, Watanabe P, Araujo EJA, Mohammed SD, Delalande JM, Bulmer DC, Scott SM, Cox HM, Voermans NC, Aziz Q, Blackshaw LA. The extracellular matrix glycoprotein tenascin-X regulates peripheral sensory and motor neurones. J Physiol. 2018;596:4237-4251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 86. | Sinibaldi L, Ursini G, Castori M. Psychopathological manifestations of joint hypermobility and joint hypermobility syndrome/ Ehlers-Danlos syndrome, hypermobility type: The link between connective tissue and psychological distress revised. Am J Med Genet C Semin Med Genet. 2015;169C:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 87. | Gazit Y, Nahir AM, Grahame R, Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med. 2003;115:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 88. | Mathias CJ, Low DA, Iodice V, Owens AP, Kirbis M, Grahame R. Postural tachycardia syndrome--current experience and concepts. Nat Rev Neurol. 2011;8:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 89. | Critchley HD, Eccles J, Garfinkel SN. Interaction between cognition, emotion, and the autonomic nervous system. Handb Clin Neurol. 2013;117:59-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 90. | Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, Porges SW. Emotion recognition in children with autism spectrum disorders: relations to eye gaze and autonomic state. J Autism Dev Disord. 2010;40:358-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 91. | Morris SSJ, Musser ED, Tenenbaum RB, Ward AR, Martinez J, Raiker JS, Coles EK, Riopelle C. Emotion Regulation via the Autonomic Nervous System in Children with Attention-Deficit/Hyperactivity Disorder (ADHD): Replication and Extension. J Abnorm Child Psychol. 2020;48:361-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 92. | Mallorquí-Bagué N, Bulbena A, Pailhez G, Garfinkel SN, Critchley HD. Mind-Body Interactions in Anxiety and Somatic Symptoms. Harv Rev Psychiatry. 2016;24:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 93. | Eccles JA, Beacher FD, Gray MA, Jones CL, Minati L, Harrison NA, Critchley HD. Brain structure and joint hypermobility: relevance to the expression of psychiatric symptoms. Br J Psychiatry. 2012;200:508-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 94. | Mallorquí-Bagué N, Garfinkel SN, Engels M, Eccles JA, Pailhez G, Bulbena A, Critchley HD. Neuroimaging and psychophysiological investigation of the link between anxiety, enhanced affective reactivity and interoception in people with joint hypermobility. Front Psychol. 2014;5:1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 95. | Mallorquí-Bagué N, Bulbena A, Roé-Vellvé N, Hoekzema E, Carmona S, Barba-Müller E, Fauquet J, Pailhez G, Vilarroya O. Emotion processing in joint hypermobility: A potential link to the neural bases of anxiety and related somatic symptoms in collagen anomalies. Eur Psychiatry. 2015;30:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Savitz J, Harrison NA. Interoception and Inflammation in Psychiatric Disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:514-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 97. | Seneviratne SL, Maitland A, Afrin L. Mast cell disorders in Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet. 2017;175:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 98. | Marchezan J, Winkler Dos Santos EGA, Deckmann I, Riesgo RDS. Immunological Dysfunction in Autism Spectrum Disorder: A Potential Target for Therapy. Neuroimmunomodulation. 2018;25:300-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 99. | Smith TO, Jerman E, Easton V, Bacon H, Armon K, Poland F, Macgregor AJ. Do people with benign joint hypermobility syndrome (BJHS) have reduced joint proprioception? Rheumatol Int. 2013;33:2709-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 100. | Ghibellini G, Brancati F, Castori M. Neurodevelopmental attributes of joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type: Update and perspectives. Am J Med Genet C Semin Med Genet. 2015;169C:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 101. | Baeza-Velasco C, Sinibaldi L, Castori M. Attention-deficit/hyperactivity disorder, joint hypermobility-related disorders and pain: expanding body-mind connections to the developmental age. Atten Defic Hyperact Disord. 2018;10:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Ross J, Grahame R. Joint hypermobility syndrome. BMJ. 2011;342:c7167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 103. | Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4048] [Cited by in RCA: 3826] [Article Influence: 173.9] [Reference Citation Analysis (0)] |
| 104. | Burón E, Bulbena A, Bulbena-Cabré A, Rosado S, Pailhez G. Both anxiety and joint laxity determine the olfactory features in panic disorder. Psychiatry Res. 2018;262:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Fragkos KC, Keetarut K, Cox A, Eady J, Emmanuel AV, Zarate-Lopez N. Joint Hypermobility Syndrome Affects Response to a Low Fermentable Oligosaccharide, Disaccharide, Monosaccharide and Polyol Diet in Irritable Bowel Syndrome Patients: A Retrospective Study. Gastroenterology Res. 2019;12:27-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Davies GD, Csecs J, Ball H, Dare J, Hosking R, Critchley HD, Grey N, Eccles JA. Altering Dynamics of Autonomic Processing Therapy (ADAPT) Trial: A Novel, Targeted Treatment for Reducing Anxiety in Joint Hypermobility. Research Square. 2021;Preprint. [DOI] [Full Text] |