Published online Apr 19, 2020. doi: 10.5498/wjp.v10.i4.71
Peer-review started: December 26, 2019
First decision: February 19, 2020
Revised: March 3, 2020
Accepted: March 25, 2020
Article in press: March 25, 2020
Published online: April 19, 2020
Processing time: 112 Days and 12.6 Hours
The prevalence of post-natal depression (PND) is high in India, as it is in many other low to middle income countries. There is an urgent need to identify PND and treat the mother as early as possible. Among the many paper and pencil tests available to identify PND, the Edinburgh Postnatal Depression Scale (EPDS) is a widely used and validated measure in India. However, the summary diagnostic accuracy and clinical utility data are not available for this measure.
To establish summary data for the global diagnostic accuracy parameter as well as the clinical utility of the non-English versions of the EPDS in India.
Two researchers independently searched the PubMed, EMBASE, MEDKNOW and IndMED databases for published papers, governmental publications, conference proceedings and grey literature from 2000-2018. Seven studies that evaluated the diagnostic accuracy of EPDS in five Indian languages against DSM/ICD were included in the final analysis. Two other investigators extracted the Participants’ details, Index measures, Comparative reference measures, and Outcomes of diagnostic accuracy data, and appraised the study quality using QUADS-2. Deek’s plots were used to evaluate publication bias. We used the area under the curve of the hierarchical summary area under the receiver operating characteristic curve, with the random effect model, to summarize the global diagnostic accuracy of EPDS. Using the 2 × 2 table, we calculated positive and negative likelihood ratios. From the likelihood ratios, the Fagan’s nomogram was built for evaluating clinical utility using the Bayesian approach. We calculated the 95% confidence interval (95%CI) whenever indicated. STATA (version 15) with MIDAS and METANDI modules were used.
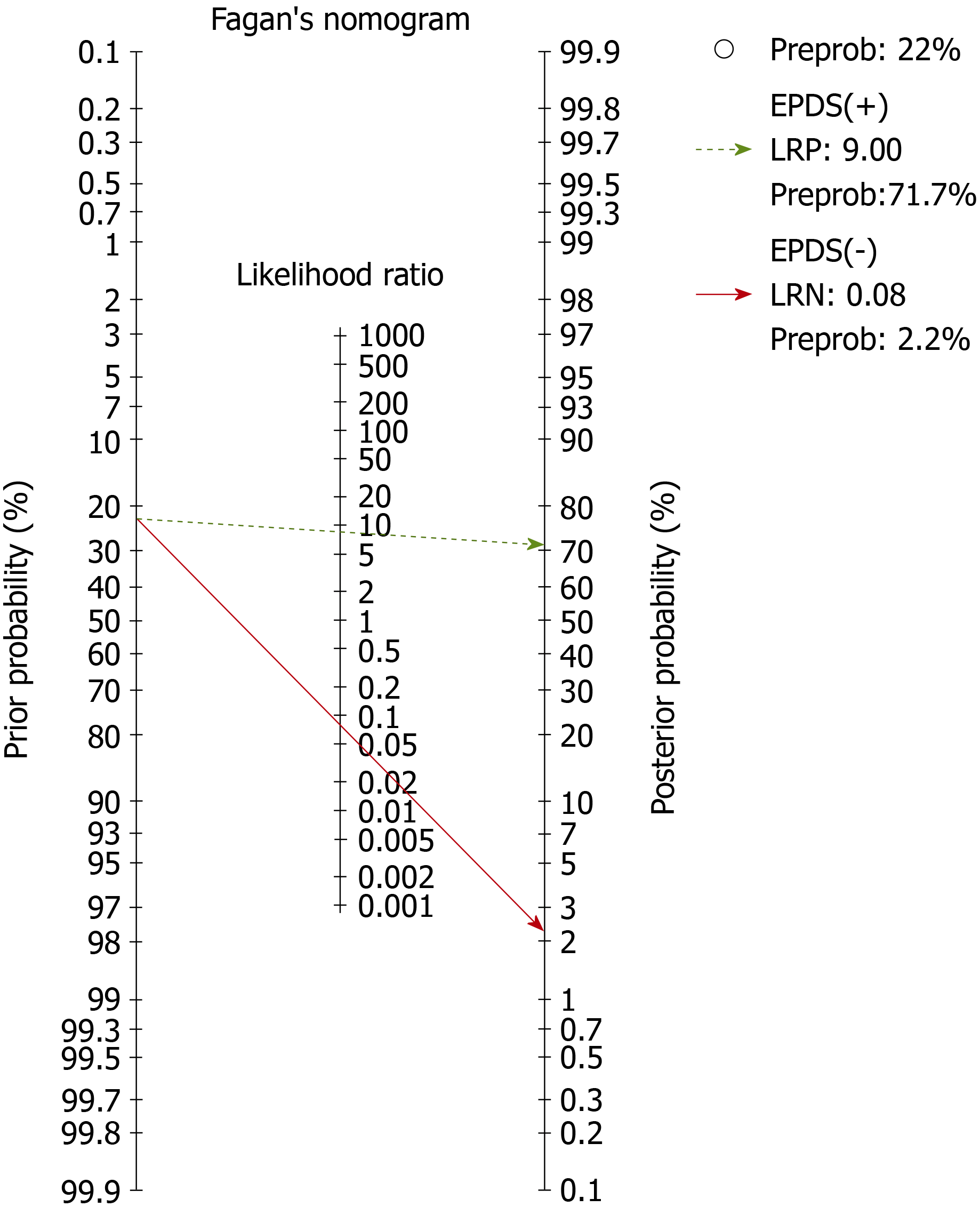
There was no publication bias. The area under the curve for EPDS was 0.97 (95%CI: 0.95-0.98). The pre-test probability for the nomogram was 22%. For a positive likelihood ratio of 9, the positive post-test probability was 72% (95%CI: 68%, 76%) and for a negative LR of 0.08, the negative post-test probability was 2% (95%CI: 1%, 3%).
In this meta-analysis, we established the summary global diagnostic parameter and clinical utility of the non-English versions of the EPDS in India. This work demonstrates that these non-English versions are accurate in their diagnosis of PND and can help clinicians in their diagnostic reasoning.
Core tip: Post-natal depression (PND) affects both the mother and baby. Currently, although one of the most common psychiatric disorders among women, early identification and treatment is underprovided in low and middle-income countries. Paper-and-pencil tests remain the primary mode of identifying PND, and the Edinburgh Postnatal Depression Scale is widely used and validated in many languages in India. This meta-analysis documents that the diagnostic parameters are good for Edinburgh Postnatal Depression Scale in India, and that its use can significantly help to scale up the services for PND.
- Citation: Russell PSS, Chikkala SM, Earnest R, Viswanathan SA, Russell S, Mammen PM. Diagnostic accuracy and clinical utility of non-English versions of Edinburgh Post-Natal Depression Scale for screening post-natal depression in India: A meta-analysis. World J Psychiatr 2020; 10(4): 71-80
- URL: https://www.wjgnet.com/2220-3206/full/v10/i4/71.htm
- DOI: https://dx.doi.org/10.5498/wjp.v10.i4.71
India is a low-middle income country with a birth rate of 20/1000 population, and the summary prevalence of Post-natal Depression (PND) was 22% in the country in 2017[1-3]. There is compelling evidence that PND is associated with morbidity and mortality in the mother-infant dyad[4,5]. Therefore, scaling-up identification and early, effective management of the identified mother-infant dyad is very much needed in India.
The Edinburgh Postnatal Depression Scale (EPDS) is an accurate screening measure[6,7] and improves the follow-up care of PND and maternal mental health[8]. In India, it is the most commonly used screening measure for PND. A recent meta-analysis demonstrates that 29 of the 38 prevalence studies on PND have used EPDS in India[3]. This measure has been translated and validated in the eight regional languages of India: Assamese, Bengali, Gujarati, Kannada, Konkani, Marathi, Punjabi and Tamil, and has been validated in both clinical and community settings in India against a variety of reference standards. The total EPDS threshold score for diagnosing PND has ranged from 6/7 to 12.5/13. Reflecting the possible effect of the varying prevalence of PND, the setting of the study, the threshold-score of EPDS, and the reference standard used or other methodological differences, the sensitivity and specificity have varied from 71%-100% and 77%-98%, respectively (further details are given in Table 1). Furthermore, good diagnostic accuracy does not always translate into good clinical utility among measures. The clinical utility of EPDS has not previously been studied in India. Therefore, because of the wide variation in diagnostic accuracy parameters, there is a need to generate summary diagnostic accuracy parameters from pooled studies for use across India, and its clinical utility needs to be demonstrated.
| PICO details | QUADAS-2 | |||||||||||||
| Risk of bias | Applicability concerns | |||||||||||||
| Ref. | Sample,n | Setting, Age, PN | EPDS language | EPDS thres-hold | Interview schedule, Reference standard | Sn | Sp | PS | IT | RS | F&T | PS | IT | RS |
| Patel et al[13], 2002 | 270 | Clinic, 18-40 yr, 6-8 wk PN | Konkani | 11/12 | CIS-R, ICD-10 | 92 | 85 | UC | L | L | UC | UC | UC | UC |
| Benjamin et al[14], 2005 | 121 | Clinic, 17-35 yr, NA-PN | Tamil | 8/9 | CIS-R, ICD-10 | 94.1 | 90.2 | UC | L | UC | UC | L | L | L |
| Werrett and Clifford[15], 2006 | 25 | Clinic, 23-40 yr; 5-8 wk, 10-14 wk PN | Punjabi | 12.5/13 | CIDI, ICD-10 | 71.4 | 93.7 | L | L | L | L | L | L | L |
| Fernandes et al[16], 2011 | 194 | Clinic, 3rd trimester | Kannada | 12/13 | MINI-Plus, DSM-IV | 100 | 84.9 | UC | L | L | UC | L | L | L |
| Desai et al[17], 2011 | 200 | Clinic, 18-35, up to 6 mo PN | Gujarati | 10.5/11 | SSI, DSM-IV | 100 | 98 | UC | L | UC | UC | L | L | L |
| Savarimuthu et al[18], 2012 | 137 | Community 21-30 yr, 4-6 wk PN | Tamil | 6/7 | CI, ICD-10 | 85.3 | 77.7 | UC | L | UC | L | L | L | L |
| Kalita et al[19], 2015 | 200 | Clinic, 18-42 yr, 6 wk PN | Assame-se | 13 | CI, ICD-10 | 88.9 | 85.3 | UC | L | UC | H | L | L | L |
| Maity et al[20], 2015 | 105 | Clinic, NA, NA | Bengali | 13 | NA | 84 | 91 | H | UC | H | H | H | H | H |
| Khapre et al[21], 2017 | 280 | Community, 25 yr median, 2 wk PN | Marathi | 12/13 | SSI, ICD-10 | 93.8 | 94.9 | UC | L | UC | UC | L | L | L |
Using this meta-analysis, we aim to fill in the lacunae in the existing literature, namely the absence of a summary global diagnostic accuracy and clinical utility parameter for use in India for non-English EPDS. Hence we: (1) Establish the summary global diagnostic accuracy of the non-English EPDS versions in India; and (2) Evaluate the clinical utility of the measure for post-natal Depression.
Two researchers (SMC and ER) independently electronically searched for relevant published studies in the PubMed, EMBASE (international database), MEDKNOW and IndMED (regional database) databases as well as hand-searched to augment the search with cross-references, published conference abstracts, Government of India publications, and grey literature from January 2000 to February 2018. We combined the search terms as follows: "diagnosis"[MeSH Terms] OR "diagnosis"[All Fields] OR "diagnostic"[All Fields]) AND accuracy[All Fields] AND ("psychiatric status rating scales"[MeSH Terms] OR ("psychiatric"[All Fields] AND "status"[All Fields] AND "rating"[All Fields] AND "scales"[All Fields]) OR "psychiatric status rating scales"[All Fields] OR ("Edinburgh"[All Fields] AND "postnatal"[All Fields] AND "depression"[All Fields] AND "scale"[All Fields]) OR "Edinburgh postnatal depression scale"[All Fields]) AND ("India"[MeSH Terms] OR "india"[All Fields].
Two other researchers (SR and SAV) extracted the required details independently, resolved any differences in extraction by consultation with another researcher (PSSR), and entered the information as electronic data. They extracted the participants, index measure, comparative reference measure and outcome of diagnostic accuracy details of each study. For a study to be included in the final analysis, it should have been conducted in India or among Indian populations, and must have compared the diagnostic accuracy of EPDS against either the Diagnostic and Statistical Manual (DSM) or International Classification of Diseases (ICD) for PND as the reference standard. Finally, each study had to report sufficient data to construct 2 × 2 tables for calculating the true positive, false positive, false negative and true negative values of EPDS against the reference standard.
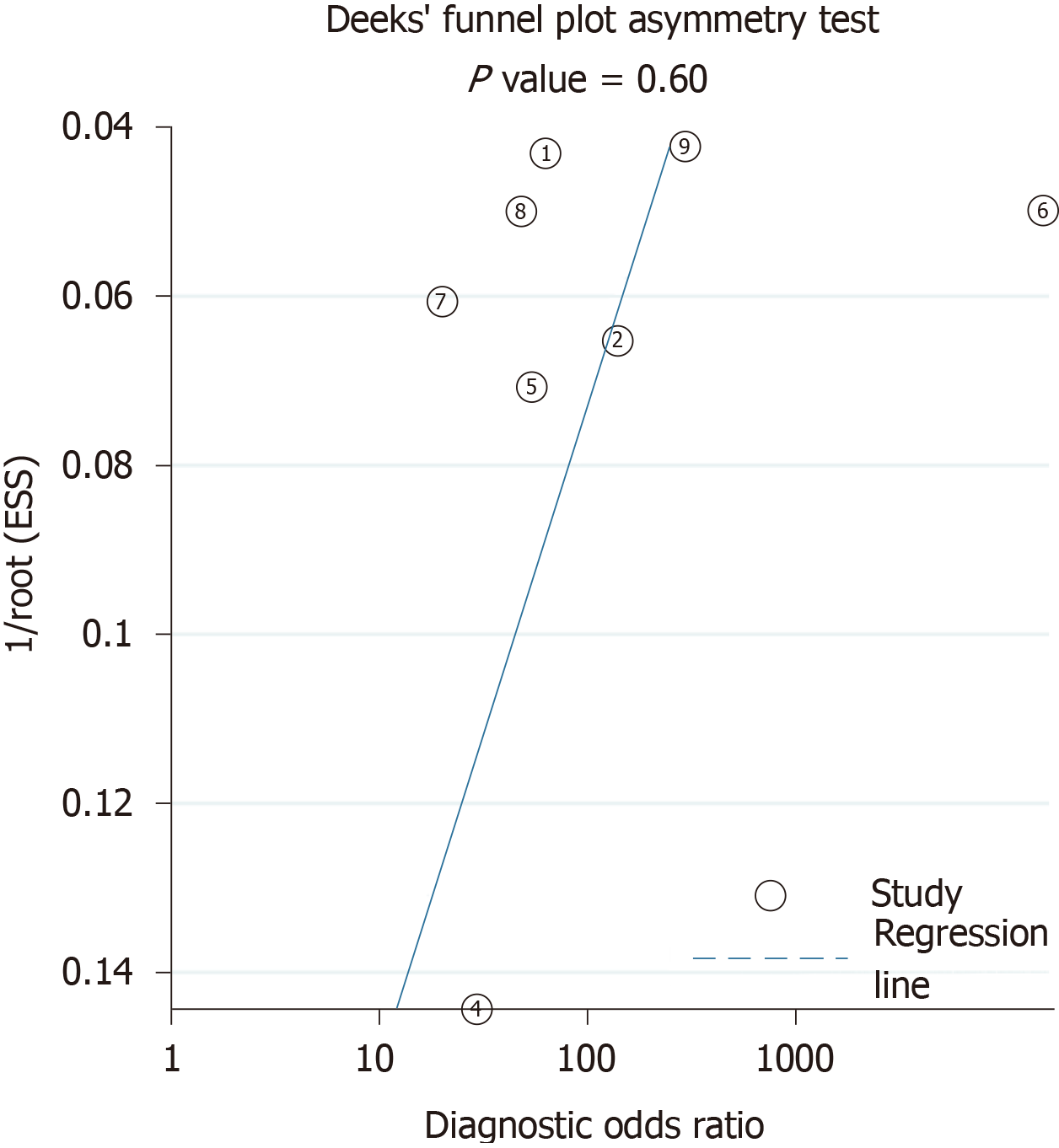
Two researchers (SAV and PMM) also appraised the quality of the studies with Quality Assessment of Diagnostic-Accuracy Studies, version 2 (QUADAS-2)[9]. We calculated the Deek’s plot for publication bias[10].
We used the Area Under the Characteristic Curve of the Hierarchical Summary Receiver Operating Curve (HSAUROC), with random effects model, to establish the global diagnostic accuracy of EPDS[11]. This was the first outcome of this meta-analysis. Using the 2 × 2 table, we calculated the positive and negative likelihood ratios (+LR and –LR, respectively). From these likelihood ratios, we evaluated the post-test probabilities of EPDS using the Fagan’s nomogram (Bayesian approach); these post-test probabilities indicating the clinical utility was the second outcome of our study[12]. We calculated the 95% confidence interval (95%CI) whenever indicated. All analyses were done at the study level and not at the participant level. The analyses were done with STATA (version 15) using the MIDAS and METANDI modules.
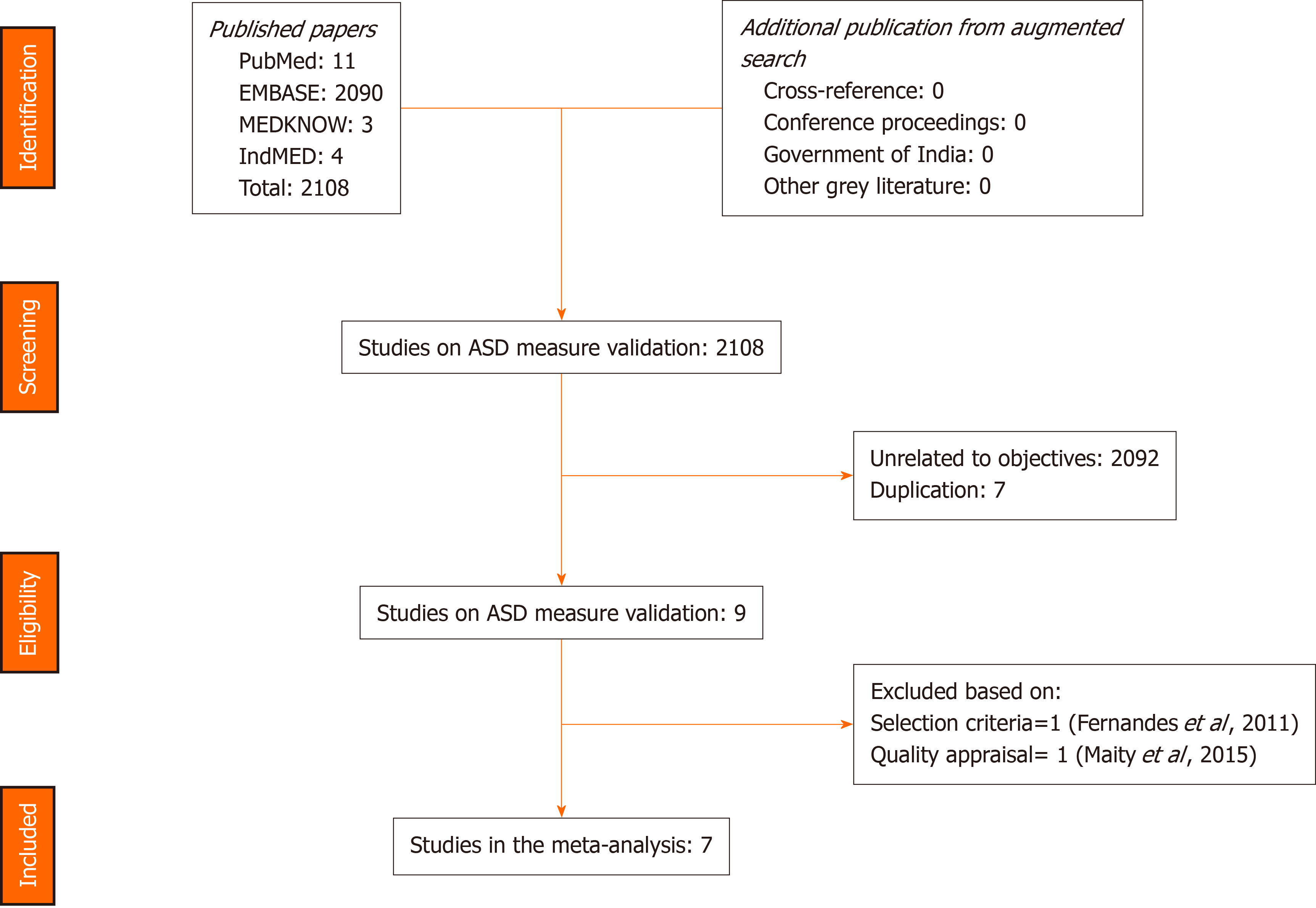
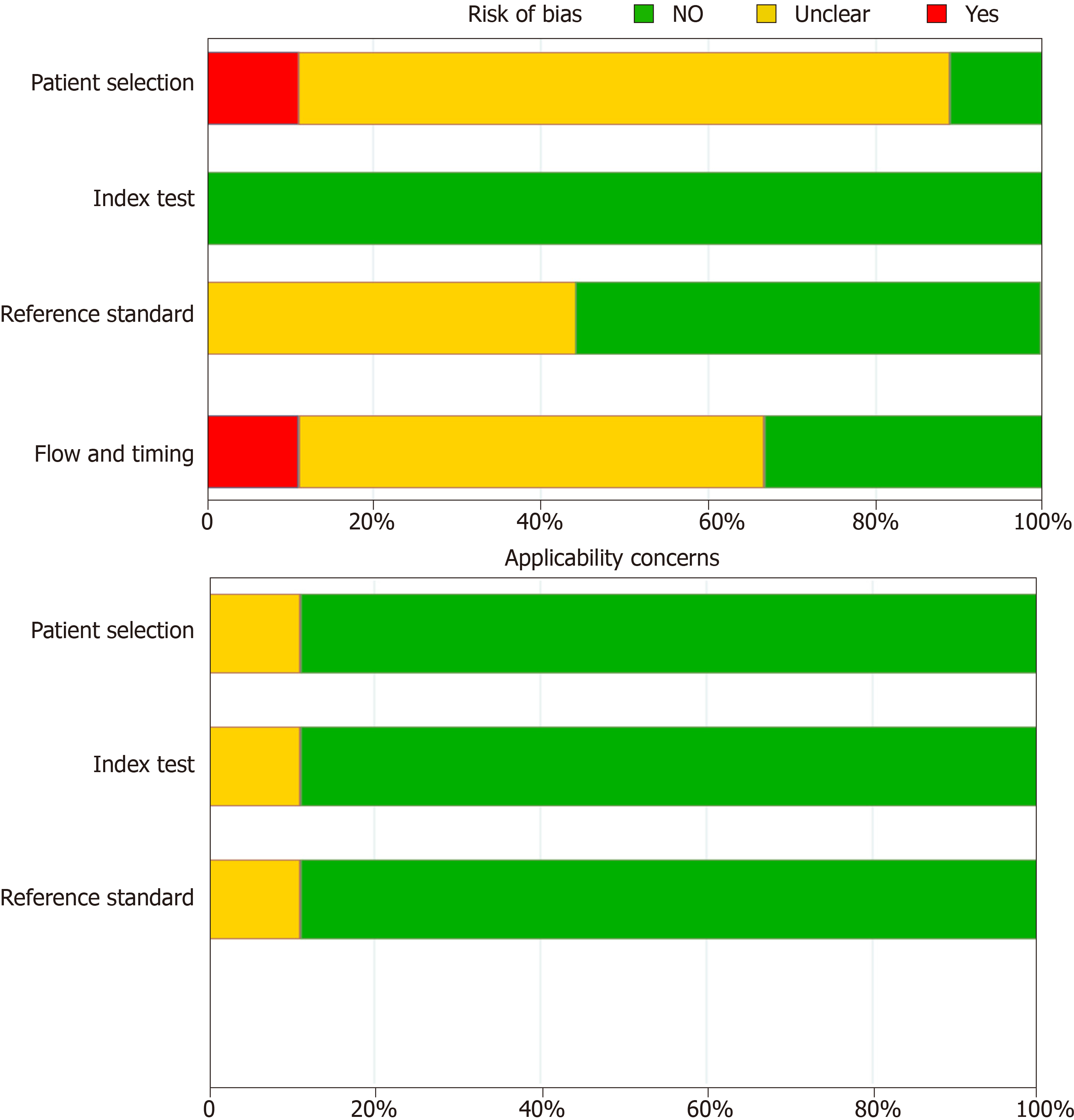
The search strategies provided 2108 titles and the diagnostic accuracy of EPDS was documented in nine studies in seven of the official languages of India[13-21]. One study in Kannada was excluded, as it included participants during their third trimester of pregnancy and not the post-natal period[16]. Another study in Bengali was excluded due to the poor quality of the study[20]. Figure 1 captures the PRISMA details, and Table 1 summarises the participants, index measure, comparative reference measure and outcome of diagnostic accuracy details and QUADAS -2 appraisal of each of the studies that were included or excluded in the final analysis (n = 1227). The QUADAS-2 appraisal demonstrated that in the risk of bias criteria, one study was rated as “at low risk of bias” across all domains. A rating of an unclear risk of bias was the most common rating across the appraisal domains. The Deek’s plot for publication bias is presented in Figure 2. In terms of applicability criteria, all seven studies were rated as applicable on all domains (Figure 3 for QUADAS-2 details). All studies had a cross-sectional design.
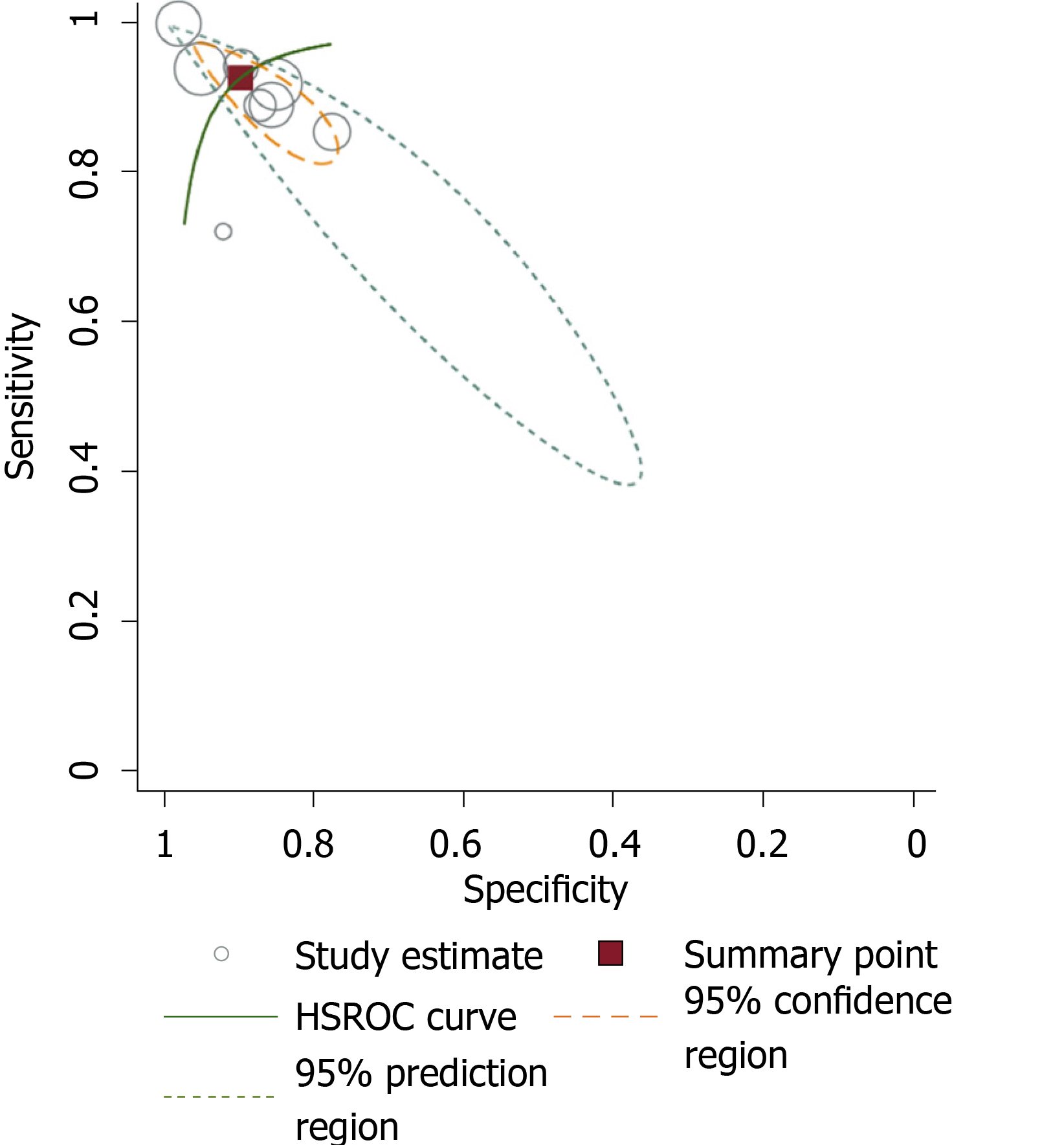
The global diagnostic accuracy of EPDS as ascertained by HSAUROC was 0.97 (95%CI: 0.95-0.98) (Figure 4). The pre-test probability for the nomogram was 22%. For a +LR of 9, the positive post-test probability was 72% (95%CI: 68%, 76%) and for a -LR of 0.08, the negative post-test probability was 2% (95%CI: 1%, 3%) (Figure 5).
Firstly, the global diagnostic accuracy of EPDS was excellent for the five different non-English versions in India. This HSAUROC value of 0.97 when converted to a more comprehensible clinical effect size of Cohen’s d or correlation coefficient r was 2.66 or 0.79, respectively[22]. This was a large effect size in the context of the diagnostic accuracy of EPDS when used as a screening measure for PND. Secondly, for the pre-test probability of 22%, the positive increment in diagnostic utility was 51% and the negative decrement was 20% for the post-test probability of EPDS. Given that the prevalence of PND in India is 22%[3], our incremental changes in post-test probability values have added considerable certainty to the diagnosis of PND when EPDS is used[12]. Thus, if a postnatal mother tests positive for EPDS, the chance she has PND increases from 22% to 72%; the clinician might therefore decide to actively engage in treatment. Conversely, if the patient tests negative, the chance of having PND decreases from 22% to 2%, and the clinician might decide not to actively treat the PND but engage instead in watchful waiting. Our finding about the diagnostic accuracy of EPDS versions is comparable with the values reviewed for the English versions in native English-speaking countries[6,7]. In comparison to some of the other selected non-English EPDS versions among African languages, the Chichewa version in Malawi, the Shona version in Zimbabwe, and the Nigerian version have relatively lower diagnostic accuracies than the summary value that is reported in this meta-analysis[23,24]. The translated version of EPDS in Afrikaans, Zulu, Tswana, Sotha, and Xhosa has demonstrated higher diagnostic accuracy for EPDS in South Africa[25]. Among European languages, the Danish version of EPDS has an Area Under the Curve of 0.96 and is comparable to our summary data[26], the Spanish version had an overall accuracy of 87.4%[27], and the French versions of EPDS has a sensitivity and specificity of 80% and 92%, and thus had lower diagnostic accuracy[28]. The other Asian language where EPDS has been validated includes Arabic[29], Chinese[30], and Japanese[31]; they have been found to have lower or similar diagnostic accuracies as in our meta-analysis.
The strengths of this study from a methodological perspective are that we followed the guidelines recommended by the Cochrane Diagnostic Test Accuracy Protocol. To present the summary of the global diagnostic accuracy of the EPDS, we used a summary line (HSROC) then summary point, as studies with various EPDS threshold values and two reference standards were analysed together. Furthermore, we anticipated the sensitivity as well as specificity of EPDS to differ widely between studies from the literature, and used the random effects model over the fixed effects model for analysis[6,7]. There was no publication or small study bias in our meta-analysis. Finally, from the policy implication standpoint, in about 69069 births expected per day in India[32], the need to identify the 22% of mothers with PND and deliver the integrated management of mother-baby dyad is a huge task. However, this can be achieved if PND is identified and EPDS is used as a valuable measure[33]. The National Mental Health Program should routinely incorporate the use of EPDS as the screening measure for PND in India through its District Mental Health approach.
In light of these findings, we conclude that the EPDS, with its many language versions and its brevity, is eminently suited for the screening of PND in India, where mental health resources are low but burden is high.
Various language versions of Edinburgh Postnatal Depression (EPDS) have been validated in India. The summary global diagnostic accuracy and clinical utility of these versions was established.
The diagnosis of postnatal depression (PND) is often missed or misdiagnosed. This affects both the mother and the baby, with significant morbidity. The widely used EPDS in India has to be proven for the early identification of PND.
The aim of this meta-analysis was to document the summary diagnostic accuracy and clinical utility of the various language versions of EPDS in India.
Seven studies were included in the analysis following the PRISMA guidelines. We used Area Under the Characteristic Curve of the Hierarchical Summary Receiver Operating Curve, with random effect model, to summarize the diagnostic accuracy of EPDS; Fagan’s nomogram was used for calculating clinical utility.
The global diagnostic accuracy of EPDS, as ascertained by Area Under the Characteristic Curve of the Hierarchical Summary Receiver Operating Curve, was 0.97 (95%CI: 0.95-0.98). For a PND prevalence of 22%, the positive post-test probability was 72% (95%CI: 68%, 76%) and the negative post-test probability was 2% (95%CI: 1%, 3%).
We established the summary global diagnostic accuracy and clinical utility of the various versions of EPDS. The EPDS is effective in the early identification of PND.
The EPDS in its various versions in India could be used for the scaling-up of PND treatment. The specific diagnostic parameters need to be further studied.
We acknowledge Professor Antonisamy B, Department of Biostatistics, Christian Medical College, Vellore for endorsing the data, analysis and conclusions of this study.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tu WJ S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Liu MY
| 1. | The World Bank Group. World Bank Open Data: Free and Open access to global development data. 2018. [cited 3 April 2018]. Available from: https://data.worldbank.org/?locations=IN-XN. html. |
| 2. | United Nations. World Population Prospects. 2017. Department of Economic and Social Affairs, Population Division. [cited 4 April 2018]. Available from: https://esa.un.org/unpd/wpp/Publications/Files/WPP2017_Wallchart.pdf. |
| 3. | Upadhyay RP, Chowdhury R, Aslyeh Salehi, Sarkar K, Singh SK, Sinha B, Pawar A, Rajalakshmi AK, Kumar A. Postpartum depression in India: a systematic review and meta-analysis. Bull World Health Organ. 2017;95:706-717C. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 4. | Chen YH, Tsai SY, Lin HC. Increased mortality risk among offspring of mothers with postnatal depression: a nationwide population-based study in Taiwan. Psychol Med. 2011;41:2287-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Parsons CE, Young KS, Rochat TJ, Kringelbach ML, Stein A. Postnatal depression and its effects on child development: a review of evidence from low- and middle-income countries. Br Med Bull. 2012;101:57-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 6. | Eberhard-Gran M, Eskild A, Tambs K, Opjordsmoen S, Samuelsen SO. Review of validation studies of the Edinburgh Postnatal Depression Scale. Acta Psychiatr Scand. 2001;104:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, Gray R. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119:350-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 716] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 8. | Leung SS, Leung C, Lam TH, Hung SF, Chan R, Yeung T, Miao M, Cheng S, Leung SH, Lau A, Lee DT. Outcome of a postnatal depression screening programme using the Edinburgh Postnatal Depression Scale: a randomized controlled trial. J Public Health (Oxf). 2011;33:292-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9589] [Article Influence: 684.9] [Reference Citation Analysis (0)] |
| 10. | Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1792] [Cited by in RCA: 2228] [Article Influence: 111.4] [Reference Citation Analysis (1)] |
| 11. | Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta-analysis of diagnostic test accuracy with few studies or sparse data. Stat Methods Med Res. 2017;26:1896-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 12. | Caraguel CG, Vanderstichel R. The two-step Fagan's nomogram: ad hoc interpretation of a diagnostic test result without calculation. Evid Based Med. 2013;18:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Patel V, Rodrigues M, DeSouza N. Gender, poverty, and postnatal depression: a study of mothers in Goa, India. Am J Psychiatry. 2002;159:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 439] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 14. | Benjamin D, Chandramohan A, Annie IK, Prasad J, Jacob KS. Validation of the Tamil version of Edinburgh post-partum depression scale. J Obstet Gynecol India. 2005;55:241-243. |
| 15. | Werrett J, Clifford C. Validation of the Punjabi version of the Edinburgh postnatal depression scale (EPDS). Int J Nurs Stud. 2006;43:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Fernandes MC, Srinivasan K, Stein AL, Menezes G, Sumithra R, Ramchandani PG. Assessing prenatal depression in the rural developing world: a comparison of two screening measures. Arch Womens Ment Health. 2011;14:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Desai N, Mehta R, Ganjiwale J. Validation of Gujarati version of Edinburg post natal depression scale among women within their first post partum year. Indian J Soc Psychiatry. 2011;27:16-23. |
| 18. | Savarimuthu RJ, Ezhilarasu P, Charles H, Balavendra A, Jacob KS. Validating the Tamil Version of the Edinburgh Postpartum Depression Scale in South Indian Setting. Indian J Cont Nsg Edn. 2012;13:27-32. |
| 19. | Kalita KN, Phookun HR, Das GC. A clinical study of postpartum depression: validation of the Edinburgh postnatal depression scale (Assamese version). Eastern J Psychiatry. 2014;11:14-18. |
| 20. | Maity C, Saha S, Sanyal D, Biswas A. The Bengali Adaptation of Edinburgh Postnatal Depression Scale. IOSR-JNHS. 2015;4:12-16. [DOI] [Full Text] |
| 21. | Khapre M, Dhande N, Mudey A. Validity and Reliability of Marathi Version of Edinburgh Postnatal Depression Scale as a Screening Tool for Post Natal Depression. Natl J Community Med. 2017;8:116-121. |
| 22. | Rice ME, Harris GT. Comparing effect sizes in follow-up studies: ROC Area, Cohen's d, and r. Law Hum Behav. 2005;29:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 664] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 23. | India Population, 2018. World Population Review. [cited 3 October 2018]. Available from: http://worldpopulationreview.com/countries/india-population/. |
| 24. | Stewart RC, Umar E, Tomenson B, Creed F. Validation of screening tools for antenatal depression in Malawi--a comparison of the Edinburgh Postnatal Depression Scale and Self Reporting Questionnaire. J Affect Disord. 2013;150:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Chibanda D, Mangezi W, Tshimanga M, Woelk G, Rusakaniko P, Stranix-Chibanda L, Midzi S, Maldonado Y, Shetty AK. Validation of the Edinburgh Postnatal Depression Scale among women in a high HIV prevalence area in urban Zimbabwe. Arch Womens Ment Health. 2010;13:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Lawrie TA, Hofmeyr GJ, de Jager M, Berk M. Validation of the Edinburgh Postnatal Depression Scale on a cohort of South African women. S Afr Med J. 1998;88:1340-1344. [PubMed] |
| 27. | Smith-Nielsen J, Matthey S, Lange T, Væver MS. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 28. | Alvarado R, Jadresic E, Guajardo V, Rojas G. First validation of a Spanish-translated version of the Edinburgh postnatal depression scale (EPDS) for use in pregnant women. A Chilean study. Arch Womens Ment Health. 2015;18:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Guedeney N, Fermanian J. Validation study of the French version of the Edinburgh Postnatal Depression Scale (EPDS): new results about use and psychometric properties. Eur Psychiatry. 1998;13:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 30. | Ghubash R, Abou-Saleh MT, Daradkeh TK. The validity of the Arabic Edinburgh Postnatal Depression Scale. Soc Psychiatry Psychiatr Epidemiol. 1997;32:474-476. [PubMed] |
| 31. | Lee DT, Yip SK, Chiu HF, Leung TY, Chan KP, Chau IO, Leung HC, Chung TK. Detecting postnatal depression in Chinese women. Validation of the Chinese version of the Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1998;172:433-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 408] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 32. | Sasaki Y, Baba T, Oyama R, Fukumoto K, Haba G, Sasaki M. Re-evaluation of the Edinburgh Postnatal Depression Scale as screening for post-partum depression in Iwate Prefecture, Japan. J Obstet Gynaecol Res. 2019;45:1876-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Stein A, Netsi E, Lawrence PJ, Granger C, Kempton C, Craske MG, Nickless A, Mollison J, Stewart DA, Rapa E, West V, Scerif G, Cooper PJ, Murray L. Mitigating the effect of persistent postnatal depression on child outcomes through an intervention to treat depression and improve parenting: a randomised controlled trial. Lancet Psychiatry. 2018;5:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |