Published online Jan 30, 2019. doi: 10.5497/wjp.v8.i2.14
Peer-review started: November 14, 2018
First decision: December 18, 2018
Revised: January 20, 2019
Accepted: January 28, 2019
Article in press: January 28, 2019
Published online: January 30, 2019
Processing time: 78 Days and 21.3 Hours
Tobacco smoking is a global public health threat causing several illnesses including cardiovascular disease (Myocardial infarction), cerebrovascular disease (Stroke), peripheral vascular disease (Claudication), chronic obstructive pulmonary disease, asthma, reduced female infertility, sexual dysfunction in men, different types of cancer and many other diseases. It has been estimated in 2015 that approximately 1.3 billion people smoke, around the globe. Use of medications among smokers is more common, nowadays. This review is aimed to identify the medications affected by smoking, involving Cytochrome P450 (CYP) and uridine diphosphate-glucuronosyltransferases (UGTs) enzymes and Nicotine. Polycyclic aromatic hydrocarbons (PAHs) of tobacco smoke have been associated with the induction of CYP enzymes such as CYP1A1, CYP1A2 and possibly CYP2E1 and UGT enzymes. The drugs metabolized by CYP1A1, CYP1A2, CYP2E1 and UGT enzymes might be affected by tobacco smoking and the smokers taking medications metabolized by those enzymes, may need higher doses due to decreased plasma concentrations through enhanced induction by PAHs of tobacco smoke. The prescribers and the pharmacists are required to be aware of medications affected by tobacco smoking to prevent the toxicity-associated complications during smoking cessation.
Core tip: Use of medications among smokers is more common, nowadays. This review is aimed to identify the medications affected by smoking, involving cytochrome P450 (CYP) and uridine diphosphate-glucuronosyltransferases (UGTs) enzymes and Nicotine. The drugs metabolized by CYP1A1, CYP1A2, CYP2E1 and UGT enzymes might be affected by tobacco smoking and the smokers taking medications metabolized by those enzymes, may need higher doses due to decreased plasma concentrations through accelerated metabolism by Polycyclic aromatic hydrocarbons of tobacco smoke. The prescribers and the pharmacists are required to be aware of medications affected by tobacco smoking to prevent the toxicity-associated complications during smoking cessation.
- Citation: Maideen NMP. Tobacco smoking and its drug interactions with comedications involving CYP and UGT enzymes and nicotine. World J Pharmacol 2019; 8(2): 14-25
- URL: https://www.wjgnet.com/2220-3192/full/v8/i2/14.htm
- DOI: https://dx.doi.org/10.5497/wjp.v8.i2.14
Tobacco use is a global public health threat causing several illnesses including coronary heart disease, cancers of organs such as lungs, mouth, throat, esophagus, pancreas, etc., chronic obstructive pulmonary disease, asthma and premature deaths. The World Health Organization (WHO) estimated that over 7 million people die of tobacco related diseases, annually and it has been projected to kill 10 million users of tobacco a year, by 2030[1]. Tobacco products are categorized majorly as smokeless tobacco and smoked tobacco. Various forms of smokeless tobacco include chewing tobacco, snus, moist snuff, dry snuff, gutka, loose leaf, twist and plug[2] while the smoked tobacco products include cigarettes, cigars, bidis, kreteks, pipe tobaccos and water pipe tobaccos[3].
In 2015, it has been estimated that approximately 1.3 billion people smoke, around the globe, and almost 80 percent of smokers live in Low or middle income countries (LMICs) such as Bangladesh, India, Indonesia, etc[4]. In addition, over 8 million smokers are expected to be killed due to tobacco smoking every year globally, by the year 2030. Generally, tobacco smokers are expected to die 10 years earlier than non-smokers do.
Tobacco smoking is associated with various conditions such as cardiovascular disease [Myocardial infarction (MI)], cerebrovascular disease (Stroke), peripheral vascular disease (Claudication), chronic obstructive pulmonary disease, asthma, reduced female infertility, sexual dysfunction in men, different types of cancer and many other diseases[5]. Maternal smoking during pregnancy affect the offspring in many ways including low birth weight, premature birth, still birth, fetal death, infant death, congenital heart defects, CNS effects, and respiratory complications[6] while smoking related negative outcomes in mothers include placental abruption, placenta previa, premature rupture of membranes and ectopic pregnancy[7].
Smoking can affect non-smokers through second hand smoke (SHS) and third hand smoke which can induce many health complications as the voluntary smoking does. SHS is the mixture of sidestream smoke (SSS) (-85%) and exhaled mainstream smoke (MSS) (-15%). SHS is also known as passive smoking, involuntary smoking or environmental tobacco smoke and SHS is reported to be associated with negative health effects such as increased incidence of cancers, asthma, respiratory infections, reduced lung growth in children, and many others[8]. Thirdhand smoke (THS) is the residue of chemicals emitted from SHS, adhered to indoor surfaces like walls, furniture, carpet, blankets, and toys, and reemitted into the air[9].
It has been estimated that tobacco smoke may contain 7357 chemical constituents[10] including hazardous chemicals like polycyclic aromatic hydrocarbons (PAHs), ammonia, aromatic amines, phenols, carbonyls, hydrocyanic acid, and N-nitrosamines[11] as a complex mixture of gases and particulate matter. The gaseous part of smoke contains the constituents such as Carbon monoxide, Hydrogen cyanide, and Aldehydes while the particulate matter containing Nicotine, PAHs, tars, pigments, trace elements, nitrosamines and insecticides[12].
Interference of effects of one drug by the comedications or tobacco smoke is termed “Drug interaction”. Increased incidence of adverse effects or decreased therapeutic efficacy of a drug resulting from a drug interaction is called “Adverse drug interaction”[13,14]. As the number of comedications increases, the rate of drug interactions also increases[15].
The cytochrome P450 (CYP) enzymes are hemoproteins, which are responsible for the metabolism of drugs and detoxification of xenobiotics. These enzymes are involved in the metabolism of drugs occurring through phase I (Oxidation) reactions. The drugs or other substances (herbs, nutients, supplements or tobacco smoke) inhibiting or inducing CYP enzymes, determine drug interactions[16]. PAHs of tobacco smoke have been associated with the induction of CYP enzymes such as CYP1A1, CYP1A2 and possibly CYP2E1[17].
Uridine 5’-diphospho-glucuronosyltransferases [Uridine diphosphate (UDP)-glucuronosyltransferases, UGTs] are the family of enzymes catalyzing glucuronidation (Phase II (conjugative) reactions[18]. PAHs of tobacco smoke have also been associated with the possible induction of UDP glucuronyltransferase (UGT) enzyme[19].
The databases such as Medline/PMC/PubMed, Google Scholar, Science Direct, Cochrane Library, Directory of open access journals (DOAJ) and reference lists were searched to identify related articles using the keywords such as Drug interactions, Tobacco Smoke, CYP enzymes, UGT enzymes and Nicotine.
Pharmacokinetic drug interactions associated with tobacco smoking are mediated through the stimulation of CYP and UGT enzymes while the pharmacodynamic interactions are mediated by Nicotine.
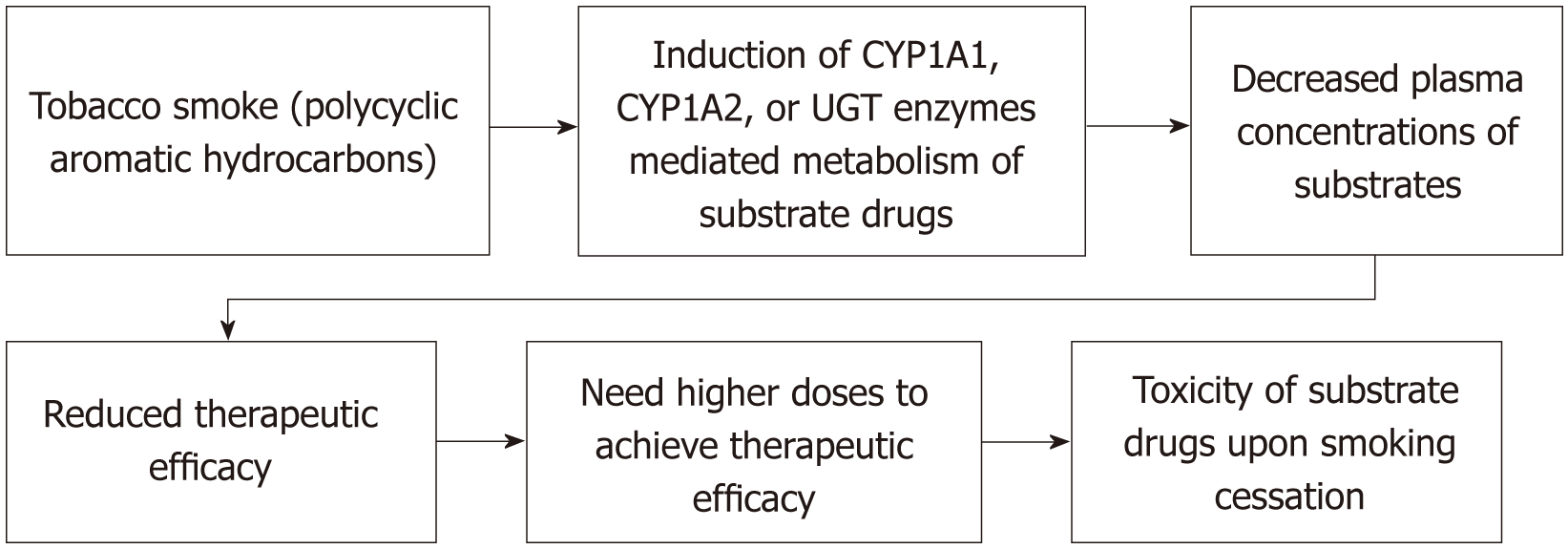
The drugs metabolized by CYP1A1, CYP1A2, CYP2E1 and UGT enzymes might be affected by tobacco smoking and the smokers taking medications metabolized by those enzymes, may need higher doses due to decreased plasma concentrations through enhanced induction by PAHs of tobacco smoke (Figure 1).
The second-generation antipsychotics or atypical antipsychotics such as clozapine and olanzapine are very much useful in the treatment of schizophrenia compared to typical antipsychotics[20]. Clozapine[21] and olanzapine[22] are primarily metabolized by CYP1A2 enzyme. It has also been reported that clozapine is metabolized by UDP‐glucuronosyltransferase 1A1 (UGT1A1) and UGT1A4[23] while olanzapine is metabolized by UGT1A4[24].
Approximately 70%-80% of patients with schizophrenia smoke[25] and the plasma concentrations of clozapine[26] and olanzapine[27] could be lowered in smokers as the PAHs of tobacco smoke enhancing their metabolism mediated by CYP1A2 and UGT enzymes. Smoking cessation may elevate the risk of toxicity in patients taking clozapine[28,29] and olanzapine[30]. The habitual smokers taking clozapine or olanzapine, should be monitored after smoking cessation for symptoms related to their toxicity. The symptoms of clozapine toxicity[31] include confusion, tachycardia, miosis, hyperthermia and leukocytosis and olanzapine toxicity[28] include extrapyramidal symptoms. The patients stopped smoking may need dosage reduction of clozapine and olanzapine[32].
The plasma concentrations of haloperidol found decreased in smokers and it is recommended to monitor the patients taking haloperidol while starting or stopping smoking[33].
The clearance of Chlorpromazine has been increased by Cigarette smoking[34] and the abrupt cessation of smoking resulted in worsening of adverse effects of chlorpromazine[35].
Tobacco smoking affects the bioavailability of antidepressants metabolized by CYP1A2 enzyme including Fluvoxamine, Duloxetine, Mirtazapine and Imipramine.
Fluvoxamine is an antidepressant, which belongs to Selective Serotonin Reuptake Inhibitor (SSRI) category. It has been reported to be metabolized by CYP enzymes like CYP1A2 and CYP2D6[36]. Tobacco smoking may decrease the serum concentrations of fluvoxamine through the induction of CYP1A2-mediated metabolism[37].
Duloxetine is categorized as a selective serotonin and norepinephrine reuptake inhibitor (SNRI) antidepressant. The CYP enzymes such as CYP1A2 and CYP2D6 are involved in the metabolism of duloxetine[38]. PAHs of tobacco smoke decreases bioavailability of duloxetine by increasing the expression of CYP1A2 and metabolism of duloxetine[39].
Mirtazapine is an atypical antidepressant and it is categorized as Tetracyclic antidepressant. The metabolism of mirtazapine is known to be mediated by CYP enzymes like CYP1A2, CYP2D6, and CYP3A4[40]. In addition, UGT enzymes also found to be involved in the metabolism of mirtazapine to some extent[41]. The serum levels of mirtazapine could be reduced in smokers due to the induction of metabolism mediated by CYP1A2 and UGT enzymes.
Imipramine is a tricyclic antidepressant and it is known to be metabolized primarily by CYP2C19 enzyme and by CYP1A2 enzyme to a smaller extent[42]. Required doses of Imipramine might be increased in Smokers due to the induction CYP1A2-mediated metabolism[43].
Theophylline is used to treat patients with airway diseases such as asthma and chronic obstructive pulmonary disease (COPD) that are poorly controlled by bronchodilators, as an add-on therapy[44]. Theophylline is effective as oral therapy and Aminophylline (Ethylenediamine salt of theophylline) is suitable for intravenous route.
CYP1A2 is known to be the major enzyme involved in the metabolism of theophylline and aminophylline[45]. The plasma levels of theophylline was lesser in smokers[46] as the PAHs of tobacco smoke accelerate the CYP1A2-mediated metabolism of theophylline. It has been reported that the plasma concentrations of theophylline was also decreased by secondhand smoke in adults[47] and in children[48] as the PAHs of sidestream smoke may induce the CYP1A2-mediated metabolism of theophylline.
The smokers may need higher doses of theophylline to compensate higher rate of clearance and smoking cessation in patients taking theophylline may result in theophylline toxicity as it has a narrow therapeutic index. The patients should be advised to seek medical attention if they develop the symptoms of theophylline toxicity such as seizures, hypotension, palpitations, nausea, vomiting, diarrhea, and others[49].
Caffeine is metabolized predominantly by CYP1A2 enzyme[50]. The CYP1A2-mediated metabolism of caffeine is enhanced in smokers[51] and the regular smokers may consume more coffee or other caffeinated drinks due to increased clearance of caffeine[52].
Riociguat is the first-in-class soluble guanylate cyclase (sGC) stimulator and it is useful to treat the patients with pulmonary hypertension[53]. Riociguat is metabolized mainly by CYP enzymes including CYP1A1, CYP3A4, CYP3A5, CYP2C8, and CYP2J2[54].
The plasma concentrations of riociguat was reduced in smokers[55,56], as the PAHs content of tobacco smoke can induce CYP1A1-mediated metabolism of riociguat. The smokers may need higher doses of riociguat and it is recommended to do dosage adjustment of riociguat in patients stopped smoking.
Erlotinib is an inhibitor of tyrosine kinase activity of Epidermal Growth Factor (EGF) receptor and it is approved to treat non-small-cell lung cancer[57]. The metabolism of Erlotinib is mediated by CYP3A4 and CYP1A2 enzymes[58].
The plasma concentrations of Erlotinib have been decreased significantly in smokers which might have occurred due to enhanced CYP1A2-mediated metabolism of Erlotinib by tobacco smoke[59].
Tacrine is a centrally acting cholinesterase inhibitor and it is approved for the treatment of Alzheimer’s disease[60,61]. Tacrine is known to be metabolized by CYP1A2 enzyme[62]. The serum conentrations of Tacrine might be decreased in smokers due to CYP1A2-mediated metabolism[63].
Warfarin is an anticoagulant drug used widely to prevent thromboembolic events. It is a racemic mixture of two enantiomers including R-Warfarin and S-Warfarin. The R-warfarin is metabolized primarily by CYP1A2[64].
Tobacco smoke may induce CYP1A2-mediated metabolism of R-Warfarin and decrease its efficacy. Smoking cessation may require close monitoring of International Normalised Ratio (INR) of patients taking warfarin[65].
Propranolol is an antagonist of adrenergic beta receptors and it may be useful to treat various conditions including hypertension, angina pectoris, migraine, essential tremor, and many others. Propranolol has been identified as a substrate of CYP1A2 and CYP2D6 enzymes[66].
The plasma concentrations of propranolol was diminished by smoking[67] and it was noted higher in patients stopped smoking[68].
Ropinirole is a dopamine agonist and it is approved for the treatment of Parkinson's disease and restless legs syndrome[69]. Ropinirole is metabolised principally by CYP1A2 enzyme[70] and the plasma concentrations of Ropinirole may be decreased in smokers due to enhanced CYP1A2-mediated metabolism.
Smoking cessation in a patient taking Ropinirole resulted in increased rate of adverse effects such as excessive sweating at night, disturbed sleep with increased awakenings for several nights in a row[71]. The dosage adjustments of Ropinirole may be required during smoking cessation.
Mexiletine is a class 1B antiarrhythmic drug and it is a substrate of CYP1A2 enzyme[72]. PAHs of tobacco smoke may induce the CYP1A2-mediated metabolism of mexiletine and it has been reported that the oral clearance of mexiletine was enhanced by tobacco smoking[73].
Frovatriptan is an agonist of 5-hydroxytryptamine (5-HT) receptors and it is effectively used in the acute management of migraine and the prevention of menstrual migraines[74]. Frovatriptan is principally metabolized by the CYP1A2 isoenzyme[75]. The plasma concentrations of Frovatriptan was slightly decreased in tobacco smokers[76].
Zolmitriptan helps the patients with migraine by exhibiting agonistic activity on 5-HT receptors[77]. It is primarily metabolised by CYP1A2 enzyme[78]. PAHs of tobacco smoke may decrease plasma concentrations of Zolmitriptan by inducing the CYP1A2-mediated metabolism.
Alosetron is a potent and selective blocker of 5-HT3 receptors and it is approved to treat the women with irritable bowel syndrome (IBS) having diarrhea as predominant bowel symptom[79]. Alosetron is metabolised by various CYP enzymes including CYP1A2[80]. Tobacco smoke may induce the CYP1A2-mediated metabolism of alosetron.
Flutamide is a nonsteroidal antiandrogen drug and it is used widely to treat carcinoma of prostate[81]. CYP1A2 enzyme is involved principally in the metabolism of flutamide[82]. CYP1A2-mediated metabolism of flutamide might be induced by PAHs of tobacco smoke.
Melatonin is a hormone, which regulates sleep-wake cycle and it is produced by the pineal gland. Exogenous melatonin is used as a dietary supplement to manage sleep disorders[83]. The CYP enzymes including CYP1A2 and CYP1A1 are involved in the metabolism of exogenous melatonin[84]. The plasma concentrations of exogenous melatonin was decreased in smokers[85].
Ramelteon is an agonist of melatonin receptors and it is approved to treat insomnia[86]. Ramelteon is metabolised primarily by CYP1A2 enzyme[87]. It has been postulated that smoking may decrease the levels of ramelteon by inducing CYP1A2-mediated metabolism.
Tasimelteon is an agonist of melatonin receptors (MT1 and MT2) and it is approved to treat non-24-hour sleep-wake disorder (N24HSWD). It is metabolised extensively by CYP1A2 and CYP3A4 enzymes[88]. The exposure of tasimelteon was 40% decreased in cigarette smokers[89].
Rasagiline is a potent monoamine oxidase-B (MAO-B) inhibitor and it is indicated in the treatment of Parkinson’s disease. It is metabolised mainly by CYP1A2 enzyme[90]. The plasma concentrations of Rasagiline could be decreased in heavy smokers due to PAHs of tobacco smoke induced CYP1A2-mediated metabolism[91]. heavy smokers, there is also a risk that rasagiline, AUC could be decreased due to induction of CYP1A2.
Tizanidine is a centrally acting α2 adrenergic receptor agonist and it is widely used as a skeletal muscle relaxant, to treat painful muscle spasms and spasticity[92]. Tizanidine is substantially metabolised by CYP1A2 enzyme[93,94]. The plasma concentrations and effects of Tizanidine could be decreased by smoking and the male smokers may require higher than average doses[95].
Triamterene is a potassium-sparing diuretic and it is approved to treat hypertension and edema. Triamterene is exclusively metabolised by CYP1A2 enzyme[96]. Cigarette smoking may induce the CYP1A2-mediated metabolism of triamterene and decrease its plasma concentrations.
Ropivacaine is an effective local anaesthetic which can be administered through epidural, intrathecal and other routes[97]. Ropivacaine is metabolised extensively by CYP1A2 enzyme[98] and it was noted that tobacco smoking increased the CYP1A2-mediated metabolism of ropivacaine[99].
Methadone is a synthetic opioid drug used to treat chronic pain. It is metabolized mainly by CYP3A4 and also by CYP1A2 and CYP2D6 enzymes in lesser extent[100]. PAHs of tobacco can induce the CYP1A2-mediated metabolism of methadone and decrease its plasma concentrations.
Methadone toxicity (Decreased respirations and altered mental status) has been reported in a patient stopped smoking while being on methadone maintenance dose to treat chronic back pain[101]. The prevalence of smoking is higher (85%-98%) in patients taking methadone as a maintenance therapy[102]. Monitor the symptoms of methadone toxicity when the patients stopped smoking and the dose of methadone may need to be adjusted.
Tobacco smoke can increase the hepatic clearance of orally administered estrogens and reduce the therapeutic efficacy of hormonal replacement therapy (HRT) such as reduction of hot flashes, osteoporosis, urogenital symptoms and cholesterol. The smoking women should be recommended to use transdermal HRT that bypasses hepatic metabolism[103].
The pharmacodynamic drug interactions of cigarette smoking are mediated mainly by nicotine, which is a major active constituent of tobacco smoke. Nicotine comprises approximately 1.5% of tobacco weight in each cigarette[104].
Combined hormonal contraceptives (CHCs) contain both an estrogen and a progestin. CHCs are available as oral pills, injectables, patches and vaginal rings. The use of CHCs is associated with elevated risk of venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE), and arterial diseases including MI and stroke[105]. The women using CHCs containing levonorgestrel or norgestimate are at lowered risk of VTE compared to their peers using CHCs containing desogestrel, cyproterone, gestodene or drospirenone[106]. The hypertensive women using CHCs may be at heightened risk of arterial diseases[107].
Cigarette smoking is also associated with increased risk of arterial diseases and VTE[108]. Smoking may cause VTE through nicotine-induced generation of platelet-dependent thrombin[109] and smoking-related cardiovascular diseases[110]. Tobacco smoking can induce the atherosclerotic changes and hypercoagulability resulting in adverse cardiovascular events[111].
The risk of VTE[112] and ischemic stroke and MI[113] is elevated in women smokers using CHCs. Administration of CHCs in women older than 35 years and smoking more than 15 cigarettes a day, is contraindicated due to heightened risk of arterial adverse events[114].
It has been estimated that approximately 27% of smoking women of reproductive age in the United States, use oral contraceptives concurrently[115]. The women smokers using CHCs should be advised to quit smoking or to use progestin-only pills or other contraceptive methods.
Nicotine content of cigarette smoke can induce small airway inflammation[116] and decrease the sensitivity of inhaled corticosteroids in asthmatic patients[117,118]. The patients with chronic asthma and with the habit of regular smoking should be advised to quit smoking or be recommended to use antiasthmatic drugs other than inhaled corticosteroids[119].
Benzodiazepines (BZDs) are effective sedative, hypnotic and anxiolytic drugs and they include alprazolam, chlordiazepoxide, diazepam, lorazepam, temazepam, triazolam, and oxazepam[120]. Nicotine induces the release of various neurotransmitters including acetylcholine, dopamine, serotonin, glutamate, and others through the binding to presynaptic nicotinic acetylcholine receptors (nAChRs) in the brain[121].
Attenuated sedation has been observed in patients taking BZDs and smoking concurrently[122]. Excessive central nervous system depression may occur when the patients stop smoking while taking BZDs.
Opioids are the drugs used to treat moderate to severe pain and they act on opioid receptors. Smoking is associated with hyperalgesia due to desensitization of nAChR[123] and it was noticed that the pain relief provided by opioid analgesics was less in smokers[124].
The smokers needed to be administered with higher doses of morphine, pethidine (meperidine) and propoxyphene[125] and the smokers requiring opioid analgesics should be advised to quit smoking[126].
Nicotine content of cigarette smoke increases the sympathetic activity and rises the blood pressure acutely[127] and it was reported that the blood pressure elevated persistently due to heavy smoking[128]. Cigarette smoking is associated with higher arterial stiffness leading to cardiovascular diseases[129,130]. Pulse wave velocity (PWV) helps to measure the arterial stiffness and the stiffer arteries have higher values of PWV[131]. The beneficial effects of amlodipine on PWV was delayed by long-term cigarette smoking[132].
Smoking increases the risk of vascular diseases through various mechanisms including elevated levels of oxidized low-density lipoprotein, triglycerides (TG), packed cell volume (PCV) and fibrinogen, reduced high-density lipoprotein (HDL) levels, increased carotid artery intima-media thickness (IMT), enhanced arterial stiffness, increased insulin resistance and intensified endothelial damage. It has been reported that smoking decreased the beneficial effects of statins on the reduction of morbidity and mortality associated with ischemic heart disease[133].
Tobacco smoking is very common around the globe and most of the smokers are living in LMICs. Tobacco smoking including SHS and third hand smoke is associated with various health hazards. Tobacco smoke contains many chemicals including PAHs which involves in majority of pharmacokinetic interactions of smoking. PAHs of tobacco smoke have been associated with the induction of CYP enzymes such as CYP1A1, CYP1A2 and possibly CYP2E1.
The smokers may need higher doses of drugs such as clozapine, olanzapine, haloperidol, chlorpromazine, fluvoxamine, duloxetine, mirtazapine, imipramine, theophylline, aminophylline, caffeine, riociguat, erlotinib, tacrine, warfarin, propranolol, Ropinirole, mexiletine, Frovatriptan, zolmitriptan, alosetron, flutamide, melatonin, Ramelteon, Tasimelteon, Rasagiline, Tizanidine, triamterene, ropivacaine, methadone and oral estrogens (Hormonal replacent therapy) due to enhanced CYP1A-mediated metabolism and upon smoking cessation they need to be monitored for toxicity of drugs and the dosage adjustments to be done if needed.
Smoking can also interact pharmacodynamically with the drugs including CHCs, inhaled corticosteroids, BZDs, opioids, antihypetensives, antihyperlipidemics and alcohol. The clinicians should be aware of the drugs affected by smoking, to prevent adverse effects especially at the time of smoking cessation.
Manuscript source: Unsolicited manuscript
Specialty type: Pharmacology and pharmacy
Country of origin: United Arab Emirates
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fernandez-Perez L, Sabatier J S- Editor: Cui LJ L- Editor: A E- Editor: Bian YN
| 1. | Jha P, Chaloupka FJ, Moore J, Gajalakshmi V, Gupta PC, Peck R, Asma S, Zatonski W, Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P. Tobacco Addiction 2006. . [PubMed] [DOI] [Full Text] |
| 2. | Ammann JR, Lovejoy KS, Walters MJ, Holman MR. A Survey of N'-Nitrosonornicotine (NNN) and Total Water Content in Select Smokeless Tobacco Products Purchased in the United States in 2015. J Agric Food Chem. 2016;64:4400-4406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Kasza KA, Bansal-Travers M, O'Connor RJ, Compton WM, Kettermann A, Borek N, Fong GT, Cummings KM, Hyland AJ. Cigarette smokers' use of unconventional tobacco products and associations with quitting activity: findings from the ITC-4 U.S. cohort. Nicotine Tob Res. 2014;16:672-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Jha P, MacLennan M, Chaloupka FJ, Yurekli A, Ramasundarahettige C, Palipudi K, Zatońksi W, Asma S, Gupta PC. Global Hazards of Tobacco and the Benefits of Smoking Cessation and Tobacco Taxes. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. Source Cancer: Disease Control Priorities, Third Edition (Volume 3). Washington (DC): The International Bank for Reconstruction and Development/The World Bank; 2015. . [PubMed] [DOI] [Full Text] |
| 5. | Hossain S, Hossain S, Ahmed F, Islam R, Sikder T, Rahman A. Prevalence of Tobacco Smoking and Factors Associated with the Initiation of Smoking among University Students in Dhaka, Bangladesh. Cent Asian J Glob Health. 2017;6:244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 6. | Berlin I, Oncken C. Maternal Smoking During Pregnancy and Negative Health Outcomes in the Offspring. Nicotine Tob Res. 2018;20:663-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Bauld L, Oncken C. Smoking in Pregnancy: An Ongoing Challenge. Nicotine Tob Res. 2017;19:495-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Hikita N, Haruna M, Matsuzaki M, Sasagawa E, Murata M, Oidovsuren O, Yura A. Prevalence and risk factors of secondhand smoke (SHS) exposure among pregnant women in Mongolia. Sci Rep. 2017;7:16426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Northrup TF, Jacob P, Benowitz NL, Hoh E, Quintana PJ, Hovell MF, Matt GE, Stotts AL. Thirdhand Smoke: State of the Science and a Call for Policy Expansion. Public Health Rep. 2016;131:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Borgerding M, Klus H. Analysis of complex mixtures--cigarette smoke. Exp Toxicol Pathol. 2005;57 Suppl 1:43-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Hoffmann D, Hoffmann I. The changing cigarette, 1950-1995. J Toxicol Environ Health. 1997;50:307-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 533] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 12. | Dawson GW, Vestal RE. Smoking and drug metabolism. Pharmacol Ther. 1981;15:207-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Pakkir Maideen NM, Manavalan G, Balasubramanian K. Drug interactions of meglitinide antidiabetics involving CYP enzymes and OATP1B1 transporter. Ther Adv Endocrinol Metab. 2018;9:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Maideen NM, Balasubramaniam R. Pharmacologically relevant drug interactions of sulfonylurea antidiabetics with common herbs. J Herbmed Pharmacol. 2018;7:200-210. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Pakkir Maideen NM, Jumale A, Balasubramaniam R. Drug Interactions of Metformin Involving Drug Transporter Proteins. Adv Pharm Bull. 2017;7:501-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | McDonnell AM, Dang CH. Basic review of the cytochrome p450 system. J Adv Pract Oncol. 2013;4:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | O'Malley M, King AN, Conte M, Ellingrod VL, Ramnath N. Effects of cigarette smoking on metabolism and effectiveness of systemic therapy for lung cancer. J Thorac Oncol. 2014;9:917-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 18. | Meech R, Mackenzie PI. Structure and function of uridine diphosphate glucuronosyltransferases. Clin Exp Pharmacol Physiol. 1997;24:907-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 166] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Villard PH, Herber R, Sérée EM, Attolini L, Magdalou J, Lacarelle B. Effect of cigarette smoke on UDP-glucuronosyltransferase activity and cytochrome P450 content in liver, lung and kidney microsomes in mice. Pharmacol Toxicol. 1998;82:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 20. | Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1686] [Cited by in RCA: 1762] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 21. | Gee S, Dixon T, Docherty M, Shergill SS. Optimising plasma levels of clozapine during metabolic interactions: a review and case report with adjunct rifampicin treatment. BMC Psychiatry. 2015;15:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM. Olanzapine. Pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet. 1999;37:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 380] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 23. | Erickson-Ridout KK, Sun D, Lazarus P. Glucuronidation of the second-generation antipsychotic clozapine and its active metabolite N-desmethylclozapine. Potential importance of the UGT1A1 A(TA)₇TAA and UGT1A4 L48V polymorphisms. Pharmacogenet Genomics. 2012;22:561-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Linnet K. Glucuronidation of olanzapine by cDNA-expressed human UDP-glucuronosyltransferases and human liver microsomes. Hum Psychopharmacol. 2002;17:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Winterer G. Why do patients with schizophrenia smoke? Curr Opin Psychiatry. 2010;23:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | van der Weide J, Steijns LS, van Weelden MJ. The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics. 2003;13:169-172. [PubMed] |
| 27. | Bigos KL, Pollock BG, Coley KC, Miller DD, Marder SR, Aravagiri M, Kirshner MA, Schneider LS, Bies RR. Sex, race, and smoking impact olanzapine exposure. J Clin Pharmacol. 2008;48:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Bondolfi G, Morel F, Crettol S, Rachid F, Baumann P, Eap CB. Increased clozapine plasma concentrations and side effects induced by smoking cessation in 2 CYP1A2 genotyped patients. Ther Drug Monit. 2005;27:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Lowe EJ, Ackman ML. Impact of tobacco smoking cessation on stable clozapine or olanzapine treatment. Ann Pharmacother. 2010;44:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Kamış GZ, Ayhan Y, Basar K, Özer S, Anıl Yağcıoğlu AE. A case of clozapine intoxication presenting with atypical NMS symptoms. Int J Neuropsychopharmacol. 2014;17:819-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Zullino DF, Delessert D, Eap CB, Preisig M, Baumann P. Tobacco and cannabis smoking cessation can lead to intoxication with clozapine or olanzapine. Int Clin Psychopharmacol. 2002;17:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Haslemo T, Eikeseth PH, Tanum L, Molden E, Refsum H. The effect of variable cigarette consumption on the interaction with clozapine and olanzapine. Eur J Clin Pharmacol. 2006;62:1049-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Jann MW, Saklad SR, Ereshefsky L, Richards AL, Harrington CA, Davis CM. Effects of smoking on haloperidol and reduced haloperidol plasma concentrations and haloperidol clearance. Psychopharmacology (Berl). 1986;90:468-470. [PubMed] |
| 34. | Chetty M, Miller R, Moodley SV. Smoking and body weight influence the clearance of chlorpromazine. Eur J Clin Pharmacol. 1994;46:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Stimmel GL, Falloon IR. Chlorpromazine plasma levels, adverse effects, and tobacco smoking: case report. J Clin Psychiatry. 1983;44:420-422. [PubMed] |
| 36. | Oliveira P, Ribeiro J, Donato H, Madeira N. Smoking and antidepressants pharmacokinetics: a systematic review. Ann Gen Psychiatry. 2017;16:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 37. | Spigset O, Carleborg L, Hedenmalm K, Dahlqvist R. Effect of cigarette smoking on fluvoxamine pharmacokinetics in humans. Clin Pharmacol Ther. 1995;58:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 38. | Fink E. Health behavior from the gender perspective--The concept of "doing genders" and the perspective of intersectionality as an explanation. Gesundheitswesen. 2015;77:880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Fric M, Pfuhlmann B, Laux G, Riederer P, Distler G, Artmann S, Wohlschläger M, Liebmann M, Deckert J. The influence of smoking on the serum level of duloxetine. Pharmacopsychiatry. 2008;41:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 372] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 41. | Lind AB, Reis M, Bengtsson F, Jonzier-Perey M, Powell Golay K, Ahlner J, Baumann P, Dahl ML. Steady-state concentrations of mirtazapine, N-desmethylmirtazapine, 8-hydroxymirtazapine and their enantiomers in relation to cytochrome P450 2D6 genotype, age and smoking behaviour. Clin Pharmacokinet. 2009;48:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Ramey K, Ma JD, Best BM, Atayee RS, Morello CM. Variability in metabolism of imipramine and desipramine using urinary excretion data. J Anal Toxicol. 2014;38:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 43. | Desai HD, Seabolt J, Jann MW. Smoking in patients receiving psychotropic medications: a pharmacokinetic perspective. CNS Drugs. 2001;15:469-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 139] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Barnes PJ. Theophylline. Am J Respir Crit Care Med. 2013;188:901-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 45. | Ha HR, Chen J, Freiburghaus AU, Follath F. Metabolism of theophylline by cDNA-expressed human cytochromes P-450. Br J Clin Pharmacol. 1995;39:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 118] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Crowley JJ, Cusack BJ, Jue SG, Koup JR, Park BK, Vestal RE. Aging and drug interactions. II. Effect of phenytoin and smoking on the oxidation of theophylline and cortisol in healthy men. J Pharmacol Exp Ther. 1988;245:513-523. [PubMed] |
| 47. | Matsunga SK, Plezia PM, Karol MD, Katz MD, Camilli AE, Benowitz NL. Effects of passive smoking on theophylline clearance. Clin Pharmacol Ther. 1989;46:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 48. | Mayo PR. Effect of passive smoking on theophylline clearance in children. Ther Drug Monit. 2001;23:503-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Greene SC, Halmer T, Carey JM, Rissmiller BJ, Musick MA. Theophylline toxicity: An old poisoning for a new generation of physicians. Turk J Emerg Med. 2018;18:37-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Nehlig A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol Rev. 2018;70:384-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 331] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 51. | Murphy TL, McIvor C, Yap A, Cooksley WG, Halliday JW, Powell LW. The effect of smoking on caffeine elimination: implications for its use as a semiquantitative test of liver function. Clin Exp Pharmacol Physiol. 1988;15:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Parsons WD, Neims AH. Effect of smoking on caffeine clearance. Clin Pharmacol Ther. 1978;24:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 205] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Lian TY, Jiang X, Jing ZC. Riociguat: a soluble guanylate cyclase stimulator for the treatment of pulmonary hypertension. Drug Des Devel Ther. 2017;11:1195-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Frey R, Becker C, Saleh S, Unger S, van der Mey D, Mück W. Clinical Pharmacokinetic and Pharmacodynamic Profile of Riociguat. Clin Pharmacokinet. 2018;57:647-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 55. | Frey R, Becker C, Unger S, Schmidt A, Wensing G, Mück W. Assessment of the effects of hepatic impairment and smoking on the pharmacokinetics of a single oral dose of the soluble guanylate cyclase stimulator riociguat (BAY 63-2521). Pulm Circ. 2016;6:S5-S14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 56. | Saleh S, Becker C, Frey R, Mück W. Population pharmacokinetics and the pharmacokinetic/pharmacodynamic relationship of riociguat in patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension. Pulm Circ. 2016;6:S86-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Schettino C, Bareschino MA, Ricci V, Ciardiello F. Erlotinib: an EGF receptor tyrosine kinase inhibitor in non-small-cell lung cancer treatment. Expert Rev Respir Med. 2008;2:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Hamilton M, Wolf JL, Zborowski D, Lu J, Lum BL, Ding K, Clark GM, Rakhit A, Seymour L, Ptaszynski AM, Rusk J. Tarceva™(erlotinib) exposure/effects (EE) analysis from a phase III study in advanced NSCLC: effect of smoking on the PK of erlotinib. Cancer Research. 2005;65. |
| 59. | Hamilton M, Wolf JL, Rusk J, Beard SE, Clark GM, Witt K, Cagnoni PJ. Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res. 2006;12:2166-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 60. | Eagger SA, Harvey RJ. Tacrine and other anticholinesterase drugs in dementia. Current Opinion in Psychiatry. 1995;8:264-267. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 61. | Qizilbash N, Birks J, López-Arrieta J, Lewington S, Szeto S. Tacrine for Alzheimer's disease. The Cochrane Library. 2000;2:CD000202. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Larsen JT, Hansen LL, Spigset O, Brøsen K. Fluvoxamine is a potent inhibitor of tacrine metabolism in vivo. Eur J Clin Pharmacol. 1999;55:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Defilippi JL, Crismon ML. Drug interactions with cholinesterase inhibitors. Drugs Aging. 2003;20:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol Ther. 1997;73:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 579] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 65. | Nathisuwan S, Dilokthornsakul P, Chaiyakunapruk N, Morarai T, Yodting T, Piriyachananusorn N. Assessing evidence of interaction between smoking and warfarin: a systematic review and meta-analysis. Chest. 2011;139:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (2)] |
| 66. | Masubuchi Y, Hosokawa S, Horie T, Suzuki T, Ohmori S, Kitada M, Narimatsu S. Cytochrome P450 isozymes involved in propranolol metabolism in human liver microsomes. The role of CYP2D6 as ring-hydroxylase and CYP1A2 as N-desisopropylase. Drug Metab Dispos. 1994;22:909-915. [PubMed] |
| 67. | Deanfield J, Wright C, Krikler S, Ribeiro P, Fox K. Cigarette smoking and the treatment of angina with propranolol, atenolol, and nifedipine. N Engl J Med. 1984;310:951-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 48] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Fox K, Deanfield J, Krikler S, Ribeiro P, Wright C. The interaction of cigarette smoking and beta-adrenoceptor blockade. Br J Clin Pharmacol. 1984;17 Suppl 1:92S-93S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 69. | Kushida CA. Ropinirole for the treatment of restless legs syndrome. Neuropsychiatr Dis Treat. 2006;2:407-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Kaye CM, Nicholls B. Clinical pharmacokinetics of ropinirole. Clin Pharmacokinet. 2000;39:243-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 71. | Juergens TM. Adverse effects of ropinirole-treated restless leg syndrome (RLS) during smoking cessation. J Clin Sleep Med. 2008;4:371-372. [PubMed] |
| 72. | Nakajima M, Kobayashi K, Shimada N, Tokudome S, Yamamoto T, Kuroiwa Y. Involvement of CYP1A2 in mexiletine metabolism. Br J Clin Pharmacol. 1998;46:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Grech-Bélanger O, Gilbert M, Turgeon J, LeBlanc PP. Effect of cigarette smoking on mexiletine kinetics. Clin Pharmacol Ther. 1985;37:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Allais G, Benedetto C. A review of the use of frovatriptan in the treatment of menstrually related migraine. Ther Adv Neurol Disord. 2013;6:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Buchan P, Keywood C, Wade A, Ward C. Clinical pharmacokinetics of frovatriptan. Headache. J Head and Face Pain. 2002;42:54-62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Buchan P, Wade A, Ward C, Oliver SD, Stewart AJ, Freestone S. Frovatriptan: a review of drug-drug interactions. Headache. 2002;42 Suppl 2:S63-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Rolan P. Potential drug interactions with the novel antimigraine compound zolmitriptan (Zomig™, 311C90). Cephalalgia. 1997;17:21-27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Wild MJ, McKillop D, Butters CJ. Determination of the human cytochrome P450 isoforms involved in the metabolism of zolmitriptan. Xenobiotica. 1999;29:847-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Ismail IM, Andrew PD, Cholerton J, Roberts AD, Dear GJ, Taylor S, Koch KM, Saynor DA. Characterization of the metabolites of alosetron in experimental animals and human. Xenobiotica. 2005;35:131-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 80. | Koch KM, Ricci BM, Hedayetullah NS, Jewell D, Kersey KE. Effect of alosetron on theophylline pharmacokinetics. Br J Clin Pharmacol. 2001;52:596-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 81. | Matsuzaki Y, Nagai D, Ichimura E, Goda R, Tomura A, Doi M, Nishikawa K. Metabolism and hepatic toxicity of flutamide in cytochrome P450 1A2 knockout SV129 mice. J Gastroenterol. 2006;41:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 82. | Shet MS, McPhaul M, Fisher CW, Stallings NR, Estabrook RW. Metabolism of the antiandrogenic drug (Flutamide) by human CYP1A2. Drug Metab Dispos. 1997;25:1298-1303. [PubMed] |
| 83. | Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, Baker G, Klassen TP, Vohra S. The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis. J Gen Intern Med. 2005;20:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 234] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 84. | Ma X, Idle JR, Krausz KW, Gonzalez FJ. Metabolism of melatonin by human cytochromes p450. Drug Metab Dispos. 2005;33:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 85. | Ursing C, von Bahr C, Brismar K, Röjdmark S. Influence of cigarette smoking on melatonin levels in man. Eur J Clin Pharmacol. 2005;61:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Spadoni G, Bedini A, Lucarini S, Mor M, Rivara S. Pharmacokinetic and pharmacodynamic evaluation of ramelteon : an insomnia therapy. Expert Opin Drug Metab Toxicol. 2015;11:1145-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Obach RS, Ryder TF. Metabolism of ramelteon in human liver microsomes and correlation with the effect of fluvoxamine on ramelteon pharmacokinetics. Drug Metab Dispos. 2010;38:1381-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 88. | Bonacci JM, Venci JV, Gandhi MA. Tasimelteon (Hetlioz™): A New Melatonin Receptor Agonist for the Treatment of Non-24-Hour Sleep-Wake Disorder. J Pharm Pract. 2015;28:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 89. | Adams KS, Breden Crouse EL. Melatonin agonists in the management of sleep disorders: A focus on ramelteon and tasimelteon. Mental Health Clinician. 2014;4:59-64. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 90. | Nayak L, Henchcliffe C. Rasagiline in treatment of Parkinson's disease. Neuropsychiatr Dis Treat. 2008;4:23-32. [PubMed] |
| 91. | Chen JJ, Swope DM. Clinical pharmacology of rasagiline: a novel, second-generation propargylamine for the treatment of Parkinson disease. J Clin Pharmacol. 2005;45:878-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 92. | Granfors MT, Backman JT, Laitila J, Neuvonen PJ. Tizanidine is mainly metabolized by cytochrome p450 1A2 in vitro. Br J Clin Pharmacol. 2004;57:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Granfors MT, Backman JT, Neuvonen M, Ahonen J, Neuvonen PJ. Fluvoxamine drastically increases concentrations and effects of tizanidine: a potentially hazardous interaction. Clin Pharmacol Ther. 2004;75:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 94. | Granfors MT, Backman JT, Neuvonen M, Neuvonen PJ. Ciprofloxacin greatly increases concentrations and hypotensive effect of tizanidine by inhibiting its cytochrome P450 1A2-mediated presystemic metabolism. Clin Pharmacol Ther. 2004;76:598-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 95. | Backman JT, Schröder MT, Neuvonen PJ. Effects of gender and moderate smoking on the pharmacokinetics and effects of the CYP1A2 substrate tizanidine. Eur J Clin Pharmacol. 2008;64:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Fuhr U, Kober S, Zaigler M, Mutschler E, Spahn-Langguth H. Rate-limiting biotransformation of triamterene is mediated by CYP1A2. Int J Clin Pharmacol Ther. 2005;43:327-334. [PubMed] |
| 97. | Kuthiala G, Chaudhary G. Ropivacaine: A review of its pharmacology and clinical use. Indian J Anaesth. 2011;55:104-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 98. | Arlander E, Ekström G, Alm C, Carrillo JA, Bielenstein M, Böttiger Y, Bertilsson L, Gustafsson LL. Metabolism of ropivacaine in humans is mediated by CYP1A2 and to a minor extent by CYP3A4: an interaction study with fluvoxamine and ketoconazole as in vivo inhibitors. Clin Pharmacol Ther. 1998;64:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 99. | Jokinen MJ, Olkkola KT, Ahonen J, Neuvonen PJ. Effect of rifampin and tobacco smoking on the pharmacokinetics of ropivacaine. Clin Pharmacol Ther. 2001;70:344-350. [PubMed] |
| 100. | Weschules DJ, Bain KT, Richeimer S. Actual and potential drug interactions associated with methadone. Pain Med. 2008;9:315-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 101. | Wahawisan J, Kolluru S, Nguyen T, Molina C, Speake J. Methadone toxicity due to smoking cessation--a case report on the drug-drug interaction involving cytochrome P450 isoenzyme 1A2. Ann Pharmacother. 2011;45:e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Elkader AK, Brands B, Selby P, Sproule BA. Methadone-nicotine interactions in methadone maintenance treatment patients. J Clin Psychopharmacol. 2009;29:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 103. | Mueck AO, Seeger H. Smoking, estradiol metabolism and hormone replacement therapy. Arzneimittelforschung. 2003;53:1-11. [PubMed] |
| 104. | Taghavi S, Khashyarmanesh Z, Moalemzadeh-Haghighi H, Nassirli H, Eshraghi P, Jalali N, Hassanzadeh-Khayyat M. Nicotine content of domestic cigarettes, imported cigarettes and pipe tobacco in iran. Addict Health. 2012;4:28-35. [PubMed] |
| 105. | Peragallo Urrutia R, Coeytaux RR, McBroom AJ, Gierisch JM, Havrilesky LJ, Moorman PG, Lowery WJ, Dinan M, Hasselblad V, Sanders GD, Myers ER. Risk of acute thromboembolic events with oral contraceptive use: a systematic review and meta-analysis. Obstet Gynecol. 2013;122:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 106. | Vinogradova Y, Coupland C, Hippisley-Cox J. Use of combined oral contraceptives and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2015;350:h2135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 107. | Brynhildsen J. Combined hormonal contraceptives: prescribing patterns, compliance, and benefits versus risks. Ther Adv Drug Saf. 2014;5:201-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 108. | Enga KF, Braekkan SK, Hansen-Krone IJ, le Cessie S, Rosendaal FR, Hansen JB. Cigarette smoking and the risk of venous thromboembolism: the Tromsø Study. J Thromb Haemost. 2012;10:2068-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 109. | Tapson VF. The role of smoking in coagulation and thromboembolism in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 110. | Blondon M, Wiggins KL, McKnight B, Psaty BM, Rice KM, Heckbert SR, Smith NL. The association of smoking with venous thrombosis in women. A population-based, case-control study. Thromb Haemost. 2013;109:891-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Salahuddin S, Prabhakaran D, Roy A. Pathophysiological Mechanisms of Tobacco-Related CVD. Glob Heart. 2012;7:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 112. | Pomp ER, Rosendaal FR, Doggen CJ. Smoking increases the risk of venous thrombosis and acts synergistically with oral contraceptive use. Am J Hematol. 2008;83:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 113. | Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190:S5-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 114. | Schiff I, Bell WR, Davis V, Kessler CM, Meyers C, Nakajima S, Sexton BJ. Oral contraceptives and smoking, current considerations: recommendations of a consensus panel. Am J Obstet Gynecol. 1999;180:S383-S384. [PubMed] |
| 115. | McClave AK, Hogue CJ, Brunner Huber LR, Ehrlich AC. Cigarette smoking women of reproductive age who use oral contraceptives: results from the 2002 and 2004 behavioral risk factor surveillance systems. Womens Health Issues. 2010;20:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 116. | Hosseinzadeh A, Thompson PR, Segal BH, Urban CF. Nicotine induces neutrophil extracellular traps. J Leukoc Biol. 2016;100:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 117. | Invernizzi G, Ruprecht A, De Marco C, Mazza R, Nicolini G, Boffi R. Inhaled steroid/tobacco smoke particle interactions: a new light on steroid resistance. Respir Res. 2009;10:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 118. | Polosa R, Thomson NC. Smoking and asthma: dangerous liaisons. Eur Respir J. 2013;41:716-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 259] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 119. | Thomson NC, Spears M. The influence of smoking on the treatment response in patients with asthma. Curr Opin Allergy Clin Immunol. 2005;5:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 120. | Guina J, Merrill B. Benzodiazepines I: Upping the Care on Downers: The Evidence of Risks, Benefits and Alternatives. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 121. | Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl). 2010;210:453-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 485] [Cited by in RCA: 453] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 122. | Pomerleau OF. Nicotine and the central nervous system: biobehavioral effects of cigarette smoking. Am J Med. 1992;93:2S-7S. [PubMed] |
| 123. | Qiu YM, Liu YT, Li ST. Tramadol requirements may need to be increased for the perioperative management of pain in smokers. Med Hypotheses. 2011;77:1071-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 124. | Ackerman WE. The effect of cigarette smoking on hydrocodone efficacy in chronic pain patients. J Ark Med Soc. 2012;109:90-93. [PubMed] |
| 125. | Miller LG. Recent developments in the study of the effects of cigarette smoking on clinical pharmacokinetics and clinical pharmacodynamics. Clin Pharmacokinet. 1989;17:90-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 126. | Yoon JH, Lane SD, Weaver MF. Opioid Analgesics and Nicotine: More Than Blowing Smoke. J Pain Palliat Care Pharmacother. 2015;29:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 127. | Virdis A, Giannarelli C, Neves MF, Taddei S, Ghiadoni L. Cigarette smoking and hypertension. Curr Pharm Des. 2010;16:2518-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 281] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 128. | Groppelli A, Giorgi DM, Omboni S, Parati G, Mancia G. Persistent blood pressure increase induced by heavy smoking. J Hypertens. 1992;10:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 234] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 129. | Doonan RJ, Hausvater A, Scallan C, Mikhailidis DP, Pilote L, Daskalopoulou SS. The effect of smoking on arterial stiffness. Hypertens Res. 2010;33:398-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 130. | Tomiyama H, Hashimoto H, Tanaka H, Matsumoto C, Odaira M, Yamada J, Yoshida M, Shiina K, Nagata M, Yamashina A. Continuous smoking and progression of arterial stiffening: a prospective study. J Am Coll Cardiol. 2010;55:1979-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 131. | Townsend RR. Arterial Stiffness: Recommendations and Standardization. Pulse (Basel). 2017;4:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 132. | Matsui Y, Kario K, Ishikawa J, Hoshide S, Eguchi K, Shimada K. Smoking and antihypertensive medication: interaction between blood pressure reduction and arterial stiffness. Hypertens Res. 2005;28:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 133. | Milionis HJ, Rizos E, Mikhailidis DP. Smoking diminishes the beneficial effect of statins: observations from the landmark trials. Angiology. 2001;52:575-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 134. | Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacological interactions between nicotine and ethanol. Nicotine Tob Res. 2004;6:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 135. | Mello NK, Mendelson JH, Palmieri SL. Cigarette smoking by women: interactions with alcohol use. Psychopharmacology (Berl). 1987;93:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 136. | Zacny JP. Behavioral aspects of alcohol-tobacco interactions. Recent Dev Alcohol. 1990;8:205-219. [PubMed] |