Published online Oct 22, 2018. doi: 10.5497/wjp.v7.i1.1
Peer-review started: July 29, 2018
First decision: September 3, 2018
Revised: September 5, 2018
Accepted: October 12, 2018
Article in press: October 13, 2018
Published online: October 22, 2018
Processing time: 86 Days and 23.4 Hours
Fibromyalgia is characterized by the primary symptoms of persistent diffuse pain, fatigue, sleep disturbance and cognitive dysfunction. Persistent pain conditions, such as fibromyalgia, are often refractory to current available therapies. An involvement of K+ channels in the pathophysiology of fibromyalgia is emerging and supported by drug treatments for this condition exhibiting action at these molecular processes. K+ channels constitute potential novel target candidates for pain therapy offering peripheral and/or central actions. The Kv7 channel activators, flupirtine and retigabine, have exhibited pharmacological profiles compatible to the requirements needed for use as a therapeutic approach to fibromyalgia. Clinical trials to address the multidimensional challenges of fibromyalgia with flupirtine and retigabine will provide important insight to the role of K+ channels in this condition.
Core tip: Fibromyalgia a multifaceted disorder remains a major unmet condition with current therapies providing limited control. An involvement of potassium channels in the pathophysiology of fibromyalgia and the related symptoms is emerging, and Kv7 channel activators, such as flupirtine and retigabine, exhibit pharmacological profiles compatible with the requirements of therapeutic approaches to fibromyalgia. Focused clinical trials with flupirtine and retigabine will provide important insight to the role of potassium channels and the utility of Kv7 channel activators in fibromyalgia.
- Citation: Lawson K. Kv7 channels a potential therapeutic target in fibromyalgia: A hypothesis. World J Pharmacology 2018; 7(1): 1-9
- URL: https://www.wjgnet.com/2220-3192/full/v7/i1/1.htm
- DOI: https://dx.doi.org/10.5497/wjp.v7.i1.1
Adequate relief with current pharmacological approaches for persistent pain is often unmet, with up to two-thirds of patients remaining refractory to treatment and reporting dissatisfaction with current therapies[1]. Further, the drug treatments are often liable to various central side effects because therapeutic targets are often within the central nervous system (CNS)[2]. Persistent pain remains a significant unmet medical need as a consequence of the understanding of the underlying mechanisms involved in the pathophysiology being limited with the complex interplay among those mechanisms having not been resolved. Preclinical research investigations of several potential targets for drug discovery involving mechanisms associated with the development and maintenance of persistent pain are offering urgently needed novel approaches for analgesic drug design[2].
Potassium (K+) channels, a diverse and widely distributed family of ion channels, play a crucial role in the cellular physiology of neuronal activity throughout the nervous system, and have a prominent involvement in nociceptive processing, particularly in regulating peripheral hyperexcitability[3]. As a consequence of differential channel subtype expression and function in the nociceptive pathways, K+ channels are important regulators of a number of physiological processes, such as membrane potential, peripheral nerve terminal action potential initiation, firing adaption in excitable tissues, axonal conduction and neurotransmitter release. Activation of K+ channels in the peripheral and CNS has been associated with a range of anti-nociceptive drugs[4]. The anti-nociception induced by agonists of G-protein-coupled receptors such as α2-adrenoceptors, opioid, GABAB, muscarinic M2, adenosine, serotonin and cannabinoid receptors has been associated with the opening of particular K+ channel subtypes[4,5]. Mutations in K+ channel genes leading to complete or partial loss of function through dominant-negative suppression or subtle functional disturbances have been suggested to contribute to altered physiological processing in nociceptive pathways or inherited pain syndromes[6,7]. Ionic mechanisms underlying persistent pain are often mediated by the upregulation or enhancement of depolarizing ion channels[8]. Consequently depolarizing ion channels have often been the focus of research for novel analgesics, with studies into the role of K+ channels as therapeutic targets in pain being less abundant. K+ channel activators constitute interesting candidates as novel analgesics which depending on the channel subtype targeted can demonstrate peripheral and/or central actions[9-11].
Voltage-gated K+ channels on the basis of homology and ability to assemble into hetero-multimeric channels have been divided into several Kv subfamilies (Kv1 to Kv12)[12]. The Kv7 subfamily encompasses five members termed Kv7.1 to Kv7.5, of which Kv7.2-Kv7.5 channels are expressed and distributed throughout peripheral nerves and the CNS, and have been investigated as novel drug targets for the treatment of neuronal hyper-excitability disorders[13-15]. Kv7.1 is expressed in cardiac muscle. Nociceptive pathways contain Kv7 channel subunits with expression in both peripheral and central systems, such as central terminals of primary afferents, dorsal horn neurons, and motor neurons within the spinal cord[15]. A decrease in Kv7 channel expression has been reported to contribute to an increased excitability of sensory neurones in the peripheral nervous system, such as Adelta and C fibres, following injury[16,17]. This change in Kv7 channels which is linked to a down regulation of the relevant genes is a feature of remodelling of the injured nerves and neighbouring uninjured fibres leading to neuropathic pain. Further, in models of nerve injury associated with peripheral neuromas an excitability compensating increase in Kv7.2 channels has been observed[18]. Kv7 channels are also inhibited by the inflammatory mediator bradykinin, and stimulation of protease-activated receptor 2 and Mas-related G-protein coupled receptor member D contributing to neuronal excitability and inflammatory pain[19]. Importantly the channel expression and function in these pathologies are reduced, but not abolished. Thus, Kv7 channels play a fundamental role in the regulation of neuronal excitability, as would be observed in persistent pain conditions and recovering or enhancing the activation of these channels would lead to suppression of aberrant neuronal activity.
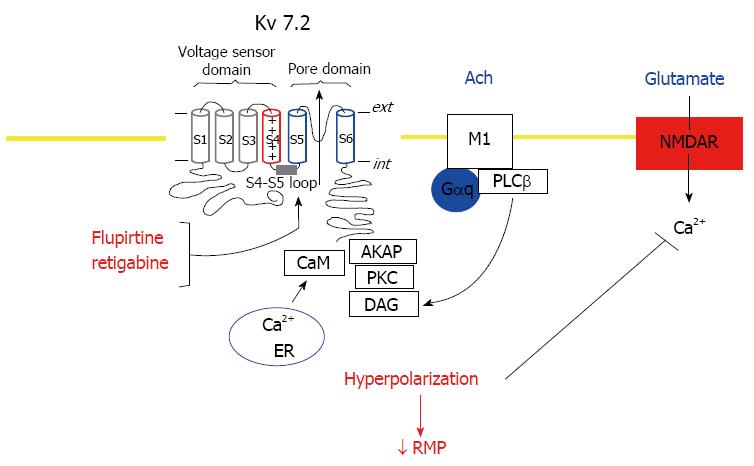
A complex formed of Kv7.2 and Kv7.3 subunits is a molecular correlate of the M-current, a voltage-sensitive K+ current which is inhibited by stimulation of muscarinic acetylcholine receptors and controls neuronal excitability[20] (Figure 1). The subthreshold membrane potential is stabilized towards the potassium equilibrium potential following activation of the Kv7.2/Kv7.3 channel complex resulting in reduced neuronal firing. Expression of Kv7.2 and Kv7.3 channels has been reported in key locations in the pain pathway, including the thalamus, cerebral cortex and the nociceptive dorsal root ganglia neurons in the spinal cord[21]. Thalamic Kv7.2 and Kv7.3 channel activation increases the occurrence of burst firing of thalamocortical neurons which may interfere with tonic action potential generation which is fundamental for relaying sensory stimuli to the cortex[22]. Thus, activation of thalamic Kv channels leads to delays in behavioural and electrophysiological correlates of pain responses[22]. Kv7.3 subunits can also co-assemble with Kv7.4 or Kv7.5 subunits to produce K+ currents with properties similar to those of the M-currents[23,24]. Further, Kv7 channel activity associated with suppression of hyperactivity of the amygdala and the dorsal raphe nucleus is linked to an anxiolytic effect[25,26]. Dysfunction of Kv7 channels within the hippocampal function is linked to memory deficit and reduction of channels has been reported to mediate age-dependent memory decline[27].
Thus, utility of activators of Kv7 channels as a treatment approach in pain conditions, which are often refractory to current available therapies, is consistent with the expression and function of Kv7 channel subunits in peripheral and central nociceptive pathways such as the central terminals of primary afferents, dorsal horn neurons, and motor neurons within the spinal cord[10,15,21].
The triaminopyridines, flupirtine and retigabine, activate all Kv subunits, except Kv7.1, by stabilizing the channel in the open confirmation with a resulting negative shift in the membrane potential[28-31] (Figure 1). Although flupirtine and retigabine express limited selectivity between the Kv7 subunits, Kv7.3 appears to be more sensitive to their actions. The binding site of retigabine is situated within the cytosolic region of the S5 transmembrane domain of Kv7 channels which involves a critical tryptophan residue (W236 in Kv7.2)[29,30] (Figure 1). Structure-activity relationship studies have produced compounds with greater potency and subunit selectivity than retigabine. PF-05020182 and NS15370 are 10-30 fold more potent than retigabine at Kv7.2-Kv7.5 channels, whilst RL648_81 is 15 times more potent than retigabine at Kv7.2/Kv7.3 channels but does not affect Kv7.4 and Kv7.5 and ICA-069673 displays selectivity for Kv7.2 over Kv7.3[32-35]. The benzanilide ICA-27243 demonstrates selectivity for Kv7.2/Kv7.3 channels over Kv7.4 or Kv7.3/Kv7.5 heteromultimers and has a binding site within the Kv7 channels distinct from that of retigabine[36]. Identification that Kv7 channels are activated by flupirtine, retigabine and related analogues has also led to the investigation of a range of new chemical scaffolds exhibiting activation properties. Acrylamides such as (S)-1 and (S)-2 appear to share the same site of action and thereby the same specificity profile as retigabine[37]. A QO series which activate all Kv7 channels except Kv7.3 and a benzimidazoles series which are selective for Kv7.2 over Kv7.3, Kv7.4 and Kv7.5 do not require the residue W236 to exhibit activation properties[38,39].
The availability of compounds offering different selectivity profiles will be valuable in the understanding of the importance of the Kv7 channel subtypes as targets for the management of pain conditions. In addition, the lack of discrimination between Kv7 subunits may be responsible for “on-target” side effects.
Persistent diffuse pain, fatigue, sleep disturbance and cognitive dysfunction are the primary characteristic symptoms of fibromyalgia[40]. The American College of Rheumatology (ACR) 1990 criteria of widespread pain (for at least 3 mo) in all 4 quadrants of the body and pain in 11 of 18 tender point sites have been used to classify fibromyalgia[41]. Revisions of the criteria were introduced in 2010 to include the assessment of somatic symptom (sleep disturbance, cognitive disturbance and fatigue) severity and widespread pain, thereby reflecting the range of symptoms and avoid reliance on tender points[42]. Further revision in 2016, comparing the 2010 criteria with the ACR 1990 classification, limits potential misclassification and introduced the use of a fibromyalgia symptom scale[43]. A worldwide prevalence of fibromyalgia has been reported to be 0.4%-8% of the population based on the application of the ACR 1990 criteria with the condition being 7 times more common in females than males[44,45]. Recognition of fibromyalgia is often complicated by the occurrence of co-morbidities exhibiting similar symptoms[40,46,47].
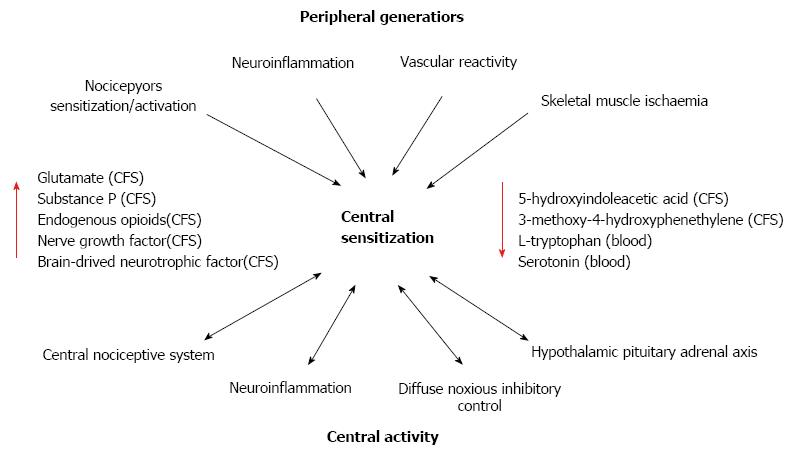
Alteration of sensory processing in the brain, disturbances in neurotransmitters such as glutamate, substance P, dopamine and serotonin, reduced reactivity of the hypothalamus-pituitary-adrenal axis to stress, increased pro-inflammatory and reduced anti-inflammatory cytokine profiles, and small fiber pathology have been associated with the pathophysiology of fibromyalgia[40,46,47]. Central sensitization (CS) is believed to underlie the neuronal excitability associated with the amplified responses of the CNS to peripheral input observed in patients with fibromyalgia[40,46,47] (Figure 1). The peripheral sensory generators reported to play a role in the heightened activity of the CNS leading to the range of symptoms include nerve pathologies, neuro-inflammation, skeletal muscle abnormalities and ischaemia[48,49]. The CS in patients with fibromyalgia reflects an altered neurotransmitter (glutamine, serotonin, substance P, dopamine) functioning and possible neuroplasticity leading to augmented sensory processing consistent with an enhanced excitation and reduced inhibition within the CNS[48]. Systemic stress-related effects have also been proposed to enhance or underlie the symptoms of fibromyalgia due to alterations in the hypothalamic pituitary adrenal axis (HPA), and autonomic and cardiovascular systems[40,47,48].
Pharmacological and non-pharmacological therapeutic approaches, as with many persistent pain conditions, are often required as treatments of the challenges associated with fibromyalgia[40]. Drug therapies, however, often involve an empiric approach resulting in a focus towards individual symptoms, in particular pain[40,48]. The lowering of levels of pronociceptive excitatory neurotransmission or/and increasing antinociceptive neurotransmission in the CNS is a primary aim of many of the pharmacological treatments, consequently the management of symptoms other than pain is often limited. The current options as therapeutic approaches of fibromyalgia include drugs, usually given as oral therapies, that target serotonin and noradrenaline levels, e.g., tricyclic antidepressants, serotonin and noradrenaline reuptake inhibitors, or voltage-gated calcium channel α2delta subunit ligands, e.g., gabapentin and pregabalin[40,48] (Table 1). The role of peripheral sensory generators within the pathophysiology of fibromyalgia would support the use of topical medicines as a treatment approach. Topical treatments would limit the occurrence of adverse effects or toxicity that may be related to oral therapy of drugs such as retigabine and flupirtine.
| Drug class | Drug |
| Tricyclic antidepressants | Amitriptyline |
| Serotonin-noradrenaline re-uptake inhibitors | Duloxetine |
| Milnacipran | |
| Selective serotonin re-uptake inhibitor | Fluoxetine |
| Citalopram | |
| Dopamine receptor agonist | Pramipexole |
| α2delta | Gabapentin |
| Pregabalin | |
| Analgesics | Dihydrocodeine |
| Morphine | |
| Tramadol | |
| Paracetamol |
Characteristic symptoms of fibromyalgia demonstrate commonality with certain clinical features consistent with altered functioning of K+ channels that present in channelopathies (i.e., conditions as a consequence of channel mutations)[50]. For example, persistent and neuropathic pain are associated with acquired and inherited channelopathies involving altered expression and/or activity of voltage-gated K+ channels (Kv)[21,51]. Kv channels have been proposed to underlie the pathogenesis of neuromyotonia, where the clinical features of fatigue, insomnia and skeletal muscle hyperactivity can be observed[52]. Further, several of the commonly used treatments of fibromyalgia have been reported to alter K+ channel activity.
The persistent pain attributed to fibromyalgia has been proposed to possibly belong to a spectrum of hyperexcitability disorders caused by autoantibodies targeting voltage-gated potassium channel (VGKC) complexes and the implicated autoimmunity[53-55]. A positive VGKC-complex immunoglobulin G status, and specifically Contactin-associated protein 2 (Caspr 2)-IgG sero-positivity, correlated significantly with pain prevalence in a range of persistent pain conditions which included fibromyalgia. In the VGKC-complex seropositive patients, immune modulation therapy was reported to evoke an improvement in pain[53-55]. Consequently, a diagnostic method and therapeutic approach for fibromyalgia related to an anti-VGKC complex antibody has been proposed[56].
The sleep disturbance in patients with fibromyalgia is characterised by a high incidence of alpha-delta sleep resulting from the intrusion of alpha activity into the delta activity that occurs during slow-wave sleep[57]. Alpha-delta sleep has been suggested to arise following alterations in conductance of K+ currents leading to selective depolarization of thalamocortical cells or of the entire somato-sensory thalamus[58]. Delta sleep can be restored from alpha-delta sleep by simultaneously increasing K+ currents and GABAB currents. The alpha-delta sleep may exacerbate and/or be the source of pain in patients with fibromyalgia[57]. An abnormal thalamic activity and a lower stimulus threshold for the activation of the pain pathway have been associated with fibromyalgia[58]. The incidence of alpha-delta sleep in fibromyalgia has been shown to be reduced by sodium oxybate which acts on K+ channels, GABAB currents, and a non-specific ionic current[59,60]. The restoration of delta sleep by sodium oxybate is associated with modulation of molecular targets in the thalamocortical cells[58].
Thus, drugs with actions on K+ currents within the peripheral nervous system and the CNS, could be effective treatments of the multifaceted disorder fibromyalgia that involves peripheral sensory generators and multiple brain circuits.
The Kv7 channel activators flupirtine and retigabine exhibit pharmacological profiles that are consistent with the management of symptoms of fibromyalgia. Flupirtine and retigabine exert analgesic properties by activation of Kv7 channels leading to hyperpolarization of neuronal membranes, indirectly reducing N-methyl-D-aspartate (NMDA) receptor activity[13,61,62]. Affinity of flupirtine for NMDA receptors has not been demonstrated, however the drug by Kv7 channel activation suppressed glutamate-induced rise in cortical neuron Ca2+ levels consistent with indirect NMDA receptor antagonism[63-65]. In addition to restoration of normal sensitivity of over-excitable nociceptive pathways, flupirtine and retigabine have been shown to inhibit the stimulation of nociceptive neurons by inflammatory mediators such as bradykinin[19,66,67]. Consequently effective analgesia of persistent pain by flupirtine has been demonstrated in conditions such as musculoskeletal pain, postoperative pain, migraine and neuralgia[61,62,68]. Retigabine has been shown to evoke analgesic efficacy in preclinical pain models of temporomandibular joint pain, visceral pain, bradykinin-induced hind-paw pain and carrageenan-induced hyperalgesia systemic[13,66,69,70]. Further, retigabine selectivity reduces the activity of axotomized Adelta/C fibres, but not uninjured axons and human C-fibre axons, and suppresses responses to dorsal root stimulation[71-73]. The analgesic and anti-allodynic effects exhibited by retigabine in neuropathic pain models are comparable to those of the treatments of fibromyalgia, tramadol and gabapentin[74,75].
Flupirtine also evokes a reduction in skeletal muscle rigidity and akinesia by the suppression of spinal mono- and polysynaptic reflexes mediated by NMDA receptors[76,77]. The muscle relaxant and analgesic properties of flupirtine are demonstrated in the same dose range, and thus would be applicable treatment of the pain and muscle stiffness observed with fibromyalgia[40,47,48]. NMDA receptors, particularly those within the dorsal horn of the spinal cord, are fundamental in nociceptive transmission and synaptic plasticity, and may play a role in CS[78]. In patients with fibromyalgia glutamate levels are elevated in key pain-processing areas of the brain, which change in response to treatment that reduce pain[79,80]. The heightened activity of glutamatergic transmission may also be responsible for raised cerebrospinal levels of nerve growth factor and brain-derived neurotrophic factor reported in FM patients[81,82]. Mechanisms that regulate NMDA receptor activity, such as Kv channels, phosphorylation sites, and interacting kinases (e.g., casein kinase 2, Src-NADH dehydrogenase) offer an alternative therapeutic target for the management of glutamatergic processes that may play a role in the pathophysiology of fibromyalgia[78].
Flupirtine has also been shown to prevent acute stress-induced impairment of spatial memory retrieval and hippocampal long-term potentiation[83]. Activation of Kv7 channels by flupirtine reduced stress-induced activation of glycogen synthase kinase-3β which appears to be responsible for impaired memory formation[83]. Thus, flupirtine could target the symptoms of fibromyalgia that are associated with stress-related effects, such as cognitive dysfunction.
Preliminary evidence from an open-label study supports the use of flupirtine as a treatment approach in patients with fibromyalgia where a reduction of pain, sleep disturbance, fatigue and depressive symptoms was observed[84]. Retigabine has been proposed as a treatment of neuropathic pain and fibromyalgia and in a phase IIa clinical trial for the treatment of post-herpetic neuralgia improvements in pain scores and Patient Global Impression of Change scores were observed but not statistically analysed[85]. During the post-herpetic neuralgia trial, but not in epilepsy trials, of retigabine proteinuria was unexpectedly reported which may have influenced continuation of the study[86]. Controlled clinical studies in fibromyalgia however are required for the confirmation of the utility of flupirtine and retigabine.
The expression of Kv7 channel subunits throughout the CNS and their involvement in various central processes however raises the potential of adverse effects and limitations to activators of this molecular target. Development of Kv channel activators exhibiting subunit selectivity (e.g., Kv7.2/7.3) could avoid possible centrally or peripherally generated unwanted effects. For example, benzimidazole derivatives have been synthesized that lack activity at Kv7.4, the main Kv7 channel expressed in vascular smooth muscle[87]. The unwanted effects related to central actions of Kv7 activators could also be contained by the availability of openers that do not cross the blood-brain barrier and target peripherally located Kv7 channels[3] (Figure 2).
Fibromyalgia is a multifaceted disorder that remains a major unmet medical need with current therapies being limited in the control of the condition. The physiological changes responsible for the diverse symptoms characteristic of fibromyalgia support the need to target multiple events to evoke effective therapeutic control. Consequently, a standard approach for the treatment of fibromyalgia is combination therapy involving drugs and non-pharmacological therapies that act through diverse mechanisms. An involvement of K+ channels in the pathophysiology of fibromyalgia and the related symptoms is emerging and supported by drug treatments for this condition exhibiting action at these molecular targets. The important role of Kv7 channels as regulators of many physiological processes has generated interest in these molecule targets for the development of drugs that would be relevant to the treatment of fibromyalgia. The distribution of Kv7 subunits, in both the CNS and PNS, is consistent with the physiological components implicated in the pathophysiology of fibromyalgia and the Kv7 channel activators, flupirtine and retigabine, have exhibited pharmacological profiles in preclinical and clinical studies compatible to the requirements needed for use as a therapeutic approach. The complexity of the pathophysiology of fibromyalgia however involves several components consequently the contribution of K channels and activators of these targets may be limited. Thus, Kv7 activators, such as flupirtine and retigabine or related drugs, may not provide complete resolution of the symptoms of fibromyalgia, but may offer an additional treatment approach to those currently available. Outcomes from preclinical studies and clinical trials in other pain states, however, may not be reliable predictors for efficacy in the multidimensional challenges of fibromyalgia where specific investigations of K channel drugs are required and focused clinical trials are awaited.
Manuscript source: Invited Manuscript
Specialty type: Pharmacology and Pharmacy
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Adams JD, Yanev SG S- Editor: Cui LJ L- Editor: A E- Editor: Wu YXJ
| 1. | van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. Br J Anaesth. 2013;111:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 403] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 2. | Gilron I, Dickenson AH. Emerging drugs for neuropathic pain. Expert Opin Emerg Drugs. 2014;19:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Tsantoulas C, McMahon SB. Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends Neurosci. 2014;37:146-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 4. | Ocaña M, Cendán CM, Cobos EJ, Entrena JM, Baeyens JM. Potassium channels and pain: present realities and future opportunities. Eur J Pharmacol. 2004;500:203-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | North RA. Twelfth Gaddum memorial lecture. Drug receptors and the inhibition of nerve cells. Br J Pharmacol. 1989;98:13-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 262] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Raouf R, Quick K, Wood JN. Pain as a channelopathy. J Clin Invest. 2010;120:3745-3752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Mathie A. Ion channels as novel therapeutic targets in the treatment of pain. J Pharm Pharmacol. 2010;62:1089-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2363] [Cited by in RCA: 2798] [Article Influence: 174.9] [Reference Citation Analysis (0)] |
| 9. | Lawson K. Potassium Channels as Targets for the Management of Pain. CNS Agents Med Chem. 2006;6:119-128. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Du X, Gamper N. Potassium channels in peripheral pain pathways: expression, function and therapeutic potential. Curr Neuropharmacol. 2013;11:621-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Busserolles J, Tsantoulas C, Eschalier A, López García JA. Potassium channels in neuropathic pain: advances, challenges, and emerging ideas. Pain. 2016;157 Suppl 1:S7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, Covarriubias M, Desir GV, Furuichi K, Ganetzky B. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev. 2003;55:583-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 224] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Passmore GM, Selyanko AA, Mistry M, Al-Qatari M, Marsh SJ, Matthews EA, Dickenson AH, Brown TA, Burbidge SA, Main M. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci. 2003;23:7227-7236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 265] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Gribkoff VK. The therapeutic potential of neuronal K V 7 (KCNQ) channel modulators: an update. Expert Opin Ther Targets. 2008;12:565-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Rivera-Arconada I, Roza C, Lopez-Garcia JA. Enhancing m currents: a way out for neuropathic pain? Front Mol Neurosci. 2009;2:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Linley JE, Rose K, Patil M, Robertson B, Akopian AN, Gamper N. Inhibition of M current in sensory neurons by exogenous proteases: a signaling pathway mediating inflammatory nociception. J Neurosci. 2008;28:11240-11249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Zheng Q, Fang D, Liu M, Cai J, Wan Y, Han JS, Xing GG. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain. 2013;154:434-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Roza C, Castillejo S, Lopez-García JA. Accumulation of Kv7.2 channels in putative ectopic transduction zones of mice nerve-end neuromas. Mol Pain. 2011;7:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Du X, Gao H, Jaffe D, Zhang H, Gamper N. M-type K+ channels in peripheral nociceptive pathways. Br J Pharmacol. 2018;175:2158-2172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 1000] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 21. | Wang JJ, Li Y. KCNQ potassium channels in sensory system and neural circuits. Acta Pharmacol Sin. 2016;37:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Cerina M, Szkudlarek HJ, Coulon P, Meuth P, Kanyshkova T, Nguyen XV, Göbel K, Seidenbecher T, Meuth SG, Pape HC. Thalamic Kv 7 channels: pharmacological properties and activity control during noxious signal processing. Br J Pharmacol. 2015;172:3126-3140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Lerche C, Scherer CR, Seebohm G, Derst C, Wei AD, Busch AE, Steinmeyer K. Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J Biol Chem. 2000;275:22395-22400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 214] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem. 2000;275:24089-24095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 318] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 25. | Korsgaard MP, Hartz BP, Brown WD, Ahring PK, Strøbaek D, Mirza NR. Anxiolytic effects of Maxipost (BMS-204352) and retigabine via activation of neuronal Kv7 channels. J Pharmacol Exp Ther. 2005;314:282-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Hansen HH, Waroux O, Seutin V, Jentsch TJ, Aznar S, Mikkelsen JD. Kv7 channels: interaction with dopaminergic and serotonergic neurotransmission in the CNS. J Physiol. 2008;586:1823-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Cavaliere S, Malik BR, Hodge JJ. KCNQ channels regulate age-related memory impairment. PLoS One. 2013;8:e62445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci. 2001;21:5535-5545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 316] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 29. | Wuttke TV, Seebohm G, Bail S, Maljevic S, Lerche H. The new anticonvulsant retigabine favors voltage-dependent opening of the Kv7.2 (KCNQ2) channel by binding to its activation gate. Mol Pharmacol. 2005;67:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 200] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Schenzer A, Friedrich T, Pusch M, Saftig P, Jentsch TJ, Grötzinger J, Schwake M. Molecular determinants of KCNQ (Kv7) K+ channel sensitivity to the anticonvulsant retigabine. J Neurosci. 2005;25:5051-5060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Tatulian L, Brown DA. Effect of the KCNQ potassium channel opener retigabine on single KCNQ2/3 channels expressed in CHO cells. J Physiol. 2003;549:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Davoren JE, Claffey MM, Snow SL, Reese MR, Arora G, Butler CR, Boscoe BP, Chenard L, DeNinno SL, Drozda SE. Discovery of a novel Kv7 channel opener as a treatment for epilepsy. Bioorg Med Chem Lett. 2015;25:4941-4944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Dalby-Brown W, Jessen C, Hougaard C, Jensen ML, Jacobsen TA, Nielsen KS, Erichsen HK, Grunnet M, Ahring PK, Christophersen P. Characterization of a novel high-potency positive modulator of K(v)7 channels. Eur J Pharmacol. 2013;709:52-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Kumar M, Reed N, Liu R, Aizenman E, Wipf P, Tzounopoulos T. Synthesis and Evaluation of Potent KCNQ2/3-Specific Channel Activators. Mol Pharmacol. 2016;89:667-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Wang AW, Yang R, Kurata HT. Sequence determinants of subtype-specific actions of KCNQ channel openers. J Physiol. 2017;595:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Wickenden AD, Krajewski JL, London B, Wagoner PK, Wilson WA, Clark S, Roeloffs R, McNaughton-Smith G, Rigdon GC. N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide (ICA-27243): a novel, selective KCNQ2/Q3 potassium channel activator. Mol Pharmacol. 2008;73:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Bentzen BH, Schmitt N, Calloe K, Dalby Brown W, Grunnet M, Olesen SP. The acrylamide (S)-1 differentially affects Kv7 (KCNQ) potassium channels. Neuropharmacology. 2006;51:1068-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Zhang F, Mi Y, Qi JL, Li JW, Si M, Guan BC, Du XN, An HL, Zhang HL. Modulation of K(v)7 potassium channels by a novel opener pyrazolo[1,5-a]pyrimidin-7(4H)-one compound QO-58. Br J Pharmacol. 2013;168:1030-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Zhang D, Thimmapaya R, Zhang XF, Anderson DJ, Baranowski JL, Scanio M, Perez-Medrano A, Peddi S, Wang Z, Patel JR. KCNQ2/3 openers show differential selectivity and site of action across multiple KCNQ channels. J Neurosci Methods. 2011;200:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Lawson K. Emerging pharmacological strategies for the treatment of fibromyalgia. World J Pharmacol. 2017;6:1-10. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 41. | Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5950] [Cited by in RCA: 5740] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 42. | Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 2565] [Article Influence: 171.0] [Reference Citation Analysis (0)] |
| 43. | Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Häuser W, Fluß E, Choy E, Kosek E, Amris K, Branco J. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76:318-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 827] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 44. | Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17:356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 509] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 45. | Jones GT, Atzeni F, Beasley M, Flüß E, Sarzi-Puttini P, Macfarlane GJ. The prevalence of fibromyalgia in the general population: a comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol. 2015;67:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 46. | Häuser W, Ablin J, Fitzcharles MA, Littlejohn G, Luciano JV, Usui C, Walitt B. Fibromyalgia. Nat Rev Dis Primers. 2015;1:15022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 298] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 47. | Borchers AT, Gershwin ME. Fibromyalgia: A Critical and Comprehensive Review. Clin Rev Allergy Immunol. 2015;49:100-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 48. | Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 1062] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 49. | Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, Casanova-Molla J, Reiners K, Sommer C. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 340] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 50. | Lawson K. Are the symptoms of Fibromyalgia Syndrome related to abnormal potassium channel function? Myalgies Intl. 2002;2:25-30. |
| 51. | Cregg R, Momin A, Rugiero F, Wood JN, Zhao J. Pain channelopathies. J Physiol. 2010;588:1897-1904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Maddison P. Neuromyotonia. Clin Neurophysiol. 2006;117:2118-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Klein CJ, Lennon VA, Aston PA, McKeon A, Pittock SJ. Chronic pain as a manifestation of potassium channel-complex autoimmunity. Neurology. 2012;79:1136-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 54. | Tamburin S, Borg K, Caro XJ, Jann S, Clark AJ, Magrinelli F, Sobue G, Werhagen L, Zanette G, Koike H. Immunoglobulin g for the treatment of chronic pain: report of an expert workshop. Pain Med. 2014;15:1072-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Goebel A, Netal S, Schedel R, Sprotte G. Human pooled immunoglobulin in the treatment of chronic pain syndromes. Pain Med. 2002;3:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Yamano Y, Nishioka K, inventor;Axis , Inc . Inc., assignee. Diagnostic agent, diagnostic method and therapeutic agent for fibromyalgia. 2013-08-01, United States patent US20130196980. . |
| 57. | Roizenblatt S, Moldofsky H, Benedito-Silva AA, Tufik S. Alpha sleep characteristics in fibromyalgia. Arthritis Rheum. 2001;44:222-230. [PubMed] [DOI] [Full Text] |
| 58. | Vijayan S, Klerman EB, Adler GK, Kopell NJ. Thalamic mechanisms underlying alpha-delta sleep with implications for fibromyalgia. J Neurophysiol. 2015;114:1923-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Madden TE, Johnson SW. Gamma-hydroxybutyrate is a GABAB receptor agonist that increases a potassium conductance in rat ventral tegmental dopamine neurons. J Pharmacol Exp Ther. 1998;287:261-265. [PubMed] |
| 60. | Schweitzer P, Roberto M, Madamba SG, Siggins GR. gamma-hydroxybutyrate increases a potassium current and decreases the H-current in hippocampal neurons via GABAB receptors. J Pharmacol Exp Ther. 2004;311:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Devulder J. Flupirtine in pain management: pharmacological properties and clinical use. CNS Drugs. 2010;24:867-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Szelenyi I. Flupirtine, a re-discovered drug, revisited. Inflamm Res. 2013;62:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 63. | Osborne NN, Cazevieille C, Wood JP, Nash MS, Pergande G, Block F, Kosinski C, Schwarz M. Flupirtine, a nonopioid centrally acting analgesic, acts as an NMDA antagonist. Gen Pharmacol. 1998;30:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Block F, Pergande G, Schwarz M. Flupirtine reduces functional deficits and neuronal damage after global ischemia in rats. Brain Res. 1997;754:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Rupalla K, Cao W, Krieglstein J. Flupirtine protects neurons against excitotoxic or ischemic damage and inhibits the increase in cytosolic Ca2+ concentration. Eur J Pharmacol. 1995;294:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, Gamper N. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl- channels. J Clin Invest. 2010;120:1240-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 67. | Linley JE, Pettinger L, Huang D, Gamper N. M channel enhancers and physiological M channel block. J Physiol. 2012;590:793-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Mastronardi P, D’Onofrio M, Scanni E, Pinto M, Frontespezi S, Ceccarelli MG, Bianchi F, Mazzarella B. Analgesic activity of flupirtine maleate: a controlled double-blind study with diclofenac sodium in orthopaedics. J Int Med Res. 1988;16:338-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Hirano K, Kuratani K, Fujiyoshi M, Tashiro N, Hayashi E, Kinoshita M. Kv7.2-7.5 voltage-gated potassium channel (KCNQ2-5) opener, retigabine, reduces capsaicin-induced visceral pain in mice. Neurosci Lett. 2007;413:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Xu W, Wu Y, Bi Y, Tan L, Gan Y, Wang K. Activation of voltage-gated KCNQ/Kv7 channels by anticonvulsant retigabine attenuates mechanical allodynia of inflammatory temporomandibular joint in rats. Mol Pain. 2010;6:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 71. | Rivera-Arconada I, Martinez-Gomez J, Lopez-Garcia JA. M-current modulators alter rat spinal nociceptive transmission: an electrophysiological study in vitro. Neuropharmacology. 2004;46:598-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Roza C, Lopez-Garcia JA. Retigabine, the specific KCNQ channel opener, blocks ectopic discharges in axotomized sensory fibres. Pain. 2008;138:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 73. | Lang PM, Fleckenstein J, Passmore GM, Brown DA, Grafe P. Retigabine reduces the excitability of unmyelinated peripheral human axons. Neuropharmacology. 2008;54:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Blackburn-Munro G, Jensen BS. The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. Eur J Pharmacol. 2003;460:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Dost R, Rostock A, Rundfeldt C. The anti-hyperalgesic activity of retigabine is mediated by KCNQ potassium channel activation. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Schmidt WJ, Schuster G, Wacker E, Pergande G. Antiparkinsonian and other motor effects of flupirtine alone and in combination with dopaminergic drugs. Eur J Pharmacol. 1997;327:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | Schwarz M, Nolden-Koch M, Purr J, Pergande G, Block F. Antiparkinsonian effect of flupirtine in monoamine-depleted rats. J Neural Transm (Vienna). 1996;103:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Zhou HY, Chen SR, Pan HL. Targeting N-methyl-D-aspartate receptors for treatment of neuropathic pain. Expert Rev Clin Pharmacol. 2011;4:379-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 79. | Foerster BR, Nascimento TD, DeBoer M, Bender MA, Rice IC, Truong DQ, Bikson M, Clauw DJ, Zubieta JK, Harris RE. Excitatory and inhibitory brain metabolites as targets of motor cortex transcranial direct current stimulation therapy and predictors of its efficacy in fibromyalgia. Arthritis Rheumatol. 2015;67:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 80. | Harte SE, Clauw DJ, Napadow V, Harris RE. Pressure Pain Sensitivity and Insular Combined Glutamate and Glutamine (Glx) Are Associated with Subsequent Clinical Response to Sham But Not Traditional Acupuncture in Patients Who Have Chronic Pain. Med Acupunct. 2013;25:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Laske C, Stransky E, Eschweiler GW, Klein R, Wittorf A, Leyhe T, Richartz E, Köhler N, Bartels M, Buchkremer G. Increased BDNF serum concentration in fibromyalgia with or without depression or antidepressants. J Psychiatr Res. 2007;41:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146-3152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 83. | Li C, Huang P, Lu Q, Zhou M, Guo L, Xu X. KCNQ/Kv7 channel activator flupirtine protects against acute stress-induced impairments of spatial memory retrieval and hippocampal LTP in rats. Neuroscience. 2014;280:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Stoll AL. Fibromyalgia symptoms relieved by flupirtine: an open-label case series. Psychosomatics. 2000;41:371-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 85. | Lineberry D. Safety/Efficacy Study of Retigabine vs Placebo in Post-Herpetic Neuralgia (PHN). [accessed 2018 July 24] In: ClinicalTrials.gov [Internet]. Bethesda (MD): US National Library of Medicine.. Available from: https://clinicaltrials.gov/ct2/show/NCT00612105 ClinicalTrials.gov Identifier: NCT00612105. |
| 86. | Brickel N, Derossett S, Buraglio M, Evans C, Jones S. Investigation of the impact of urine handling procedures on interpretation of urinalysis findings and product safety in subjects treated with ezogabine. Ther Clin Risk Manag. 2013;9:207-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Ng FL, Davis AJ, Jepps TA, Harhun MI, Yeung SY, Wan A, Reddy M, Melville D, Nardi A, Khong TK. Expression and function of the K+ channel KCNQ genes in human arteries. Br J Pharmacol. 2011;162:42-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |