Published online Sep 9, 2016. doi: 10.5497/wjp.v5.i3.59
Peer-review started: April 11, 2016
First decision: July 21, 2016
Revised: July 26, 2016
Accepted: August 30, 2016
Article in press: August 31, 2016
Published online: September 9, 2016
Processing time: 47 Days and 20.7 Hours
To investigate physicochemical stability of sevoflurane in dimethyl sulfoxide using gas chromatography with a flame ionization detector and nuclear magnetic resonance (NMR).
Undiluted sevoflurane, plus dilutions 1:2, 1:5, 1:10, 1:25, and 1:50 in dimethyl sulfoxide were prepared in a vertical laminar flow cabinet class II type B and stored at different temperatures (23 °C, 6 °C, and -10 °C) for 45 d. Sterile 1 mL polypropylene amber syringes to minimize light degradation, caps and needles were used. The presence of sevoflurane and its degradation products in the samples was determined by gas chromatography with flame ionization detector (260 °C, 40 min), and by 1H, 19F, and proton-decoupled 19F nuclear magnetic resonance.
The gas chromatography analysis showed sevoflurane and dimethyl sulfoxide (DMSO) retention times were 2.7 and 13.0 min, respectively. Pure DMSO injection into the column resulted in two additional peaks at 2.1 and 2.8 min. The same sevoflurane peak at 2.7 min was observed in all the dilutions at -10 °C, 4 °C and 25 °C. The NMR spectra showed signals consistent with the sevoflurane structure in all the dilutions at -10 °C, 4 °C and 25 °C. In the 1H spectrum, two signals corresponding to the sevoflurane molecule were observed at 5.12 and 4.16 parts per million (ppm). In the 19F-NMR spectrum, two signals were observed at chemical shift -76.77 ppm and chemical shift -157.13 ppm. In the 19F{1H}-NMR CPD, two signals were observed at chemical shift -76.77 ppm and chemical shift -157.13 ppm. The first one showed a doublet (JF-F = 3.1 Hz) which integrated by six fluorine nuclei from the hexafluoro-isopropyl group. The second signal was integrated by a fluorine atom and showed a septuplet (JF-F = 3.1 Hz).
This study shows that different concentrations of sevoflurane in dimethyl sulfoxide retain their chemical composition after exposure to different temperatures for a period of 45 d.
Core tip: Direct topical application of anesthetic sevoflurane has recently shown beneficial properties in the management of chronic vascular ulcers. A more convenient formulation could be obtained using solutions of sevoflurane in a miscible solvent such as dimethyl sulfoxide. However, no study has yet assessed the physicochemical and pharmaceutical stability of these formulations. Different concentrations of sevoflurane in dimethyl sulfoxide were stored over 45 d at -10 °C, 4 °C and 25 °C and assayed by gas chromatography with a flame ionization detector and nuclear magnetic resonance, showing that molecular structures remained unaltered after exposure to a range of temperatures.
- Citation: Fernández-Ginés FD, García-Muñoz S, Mateo-Carrasco H, Rincón-Cervera M&, Cortiñas-Sáenz M, Morales-Molina JA, Fernández-Sánchez C, Expósito-López JM, Rodríguez-García I. Innovate combination of sevoflurane dilution in dimethyl sulfoxide: A stability study by gas chromatography and nuclear magnetic resonance. World J Pharmacology 2016; 5(3): 59-67
- URL: https://www.wjgnet.com/2220-3192/full/v5/i3/59.htm
- DOI: https://dx.doi.org/10.5497/wjp.v5.i3.59
Sevoflurane [1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy) propane] is a highly-fluorinated methyl-isopropyl ether-derived molecule. Chemically, it is a sweet-smelling, non-flammable, colorless substance used as an inhaled anesthetic in the induction and maintenance of general anesthesia in adult and pediatric patients[1]. It has been documented that sevoflurane instillation into skin ulcers has a rapid, intense, and durable anesthetic effect. Despite the existence of some favorable safety data, uncertainty exists on the direct effects of topical undiluted sevoflurane due to its high concentration[2-4].
Dimethyl sulfoxide (DMSO, C2H6OS) is a polar aprotic solvent able to solubilize sevoflurane. Chemically, it is a low-volatile, transparent, colorless, hygroscopic substance with wide applications as topical pharmaceutical vehicle because of its ability to penetrate biological membranes. It has shown analgesic, healing, oxygen free-radical scavenger, and antimicrobial properties after topical application[5-11].
As both sevoflurane and DMSO have shown usefulness and safety in preliminary studies in topical administration for the treatment of ulcers, we designed a study aimed at assessing the physicochemical stability of several sevoflurane concentrations in DMSO at different temperatures over 45 d. Stability was assessed using gas chromatography with a flame ionization detector (GC-FID) and nuclear magnetic resonance (NMR). To our knowledge, no similar study on the stability of sevoflurane in DMSO has been reported in the literature.
Sevoflurane’s stability was defined as the maintenance of its physicochemical, microbiological, and biopharmaceutical properties within the specified range during its lifespan, under the influence of several ambient factors such as temperature, humidity, and light exposure[12].
Sevorane® 100% v/v (AbbVie®, Campoverde di Aprilia, Italy) was used in the preparation of dilutions using DMSO 99% (Fagron Ibérica, Terrassa, Spain) as a solvent. Six different sevoflurane-in-DMSO dilutions were used: Undiluted sevoflurane, and dilutions 1:2, 1:5, 1:10, 1:25, and 1:50. One milliliter aliquots were prepared as follows: 0.02 mL of sevoflurane and 0.98 mL of DMSO (1:50), 0.04 mL of sevoflurane and 0.96 mL of DMSO (1:25), 0.9 mL of sevoflurane and 0.1 mL of DMSO (1:10), 0.2 mL of sevoflurane and 0.8 mL of DMSO (1:5), and 0.5 mL of sevoflurane and 0.5 mL of DMSO. Dilutions were prepared in a vertical laminar flow cabinet class II type B (Telstar® AH-100, Terrassa, Spain), using sterile 1 mL polypropylene amber syringes to minimize light degradation, caps and needles. Fifteen syringes divided into three sets were prepared, each set consisting of five syringes with different sevoflurane concentrations. The required volume of sevoflurane was drawn up into the syringe and the volume was made up to 1 mL with DMSO. Syringes were shaken gently to allow homogenization of the dilution, secured with caps to prevent product volatilization and sealed in opaque and isotherm polystyrene boxes with freeze blocks inside.
The first set of syringes was stored in a dry sealed box stored in a refrigerator at 4 °C ± 2 °C. The second set of syringes was stored in a locked cupboard at room temperature 23 °C ± 2 °C. Finally the third set was stored in a freezer at -10 °C ± 2 °C. Temperature was monitored with digital minimum/maximum thermometers.
All dilutions were examined for changes in color (against white and black backgrounds), viscosity, and formation of precipitates at the time of preparation and at weekly interval.
Fifty microgram aliquots from each solution were plated on Müeller-Hinton agar enriched with 5% sheep blood (Remel®, Lenexa, Kansas, United States), using a spiral plating device (Microbiology International®, Rockville, Maryland, United States). Plates were incubated at 37 °C, and the number of colonies growing on the plate was counted after 24 h.
Samples were subjected to treatment with anhydrous sodium sulfate to remove any traces of humidity. One gram of sodium sulfate was added to each sample, vortex-mixed for 1 min and then filtered through 45 μm nylon microfilters. One microliter of each sample was then injected into a gas chromatographer model Focus GC® with a capillary column Omegawax® 250 (30 m, 0.25 mm inner diameter, 0.15 µm film thickness) (Supelco®, Bellefonte, Pasadena, United States) coupled with a flame ionization detector (FID) (Thermo Electron®, Cambridge, United Kingdom). The temperature program was set as follows: 1 min at 40 °C, heating at 10 °C/min up to 260 °C, and maintenance at 260 °C for 40 min. The injector temperature was 250 °C and the detector temperature was 270 °C. Nitrogen was used as carrier gas at a rate of 1 mL/min and with a split flow of 20:1. Sevorane® and pure DMSO were analyzed separately following the same procedure in order to obtain a reference for the retention time of sevoflurane and DMSO, as well as to detect the presence of any manufacturing-derived impurity prior to the mixing and storage of the dilutions. The method was validated and applied in accordance to the European Pharmacopoeia.
NMR spectra were acquired using a Bruker Avance DRX 300 MHz® spectrometer equipped with a 5 mm single-axis z-gradient quattro nucleus probe (Bruker Biospin GmbH, Rheinstetten, Germany). A capillary filled with hexadeuterated DMSO (DMSO-d6) was inserted into each NMR tube to obtain a lock signal. All NMR experiments were performed at room temperature. 1H-NMR, coupled 19F-NMR, and proton-decoupled 19F{1H}-NMR using WALTZ-16 composite pulse decoupling sequence (19F-CPD) spectra were obtained. CPD allows the saturation of the proton channel frequency whilst acquiring the fluorine spectra. This halts the heteronuclear 19F-proton coupling and allows 19F-19F homonuclear coupling only, which is used to confirm an observed 1H-19F coupling. Spectral widths were 3000 and 3700 Hz, respectively. Eight scans were accumulated for 1H and thirty-two for 19F, with an acquisition time of 2.73 and 2.00 s, respectively.
No significant physical or microbiological changes were observed at the storage temperatures after 45 d. Neither color or viscosity changes, nor formation of precipitates were observed at any temperature. At 4 °C, dilutions 1:10, 1:25, and 1:50 preserved their liquid, colorless state, whereas at -10 °C only the undiluted sevoflurane and the 1:2 dilution were liquid. No microbial growth was observed after 24 or 48 h cultures in any sample.
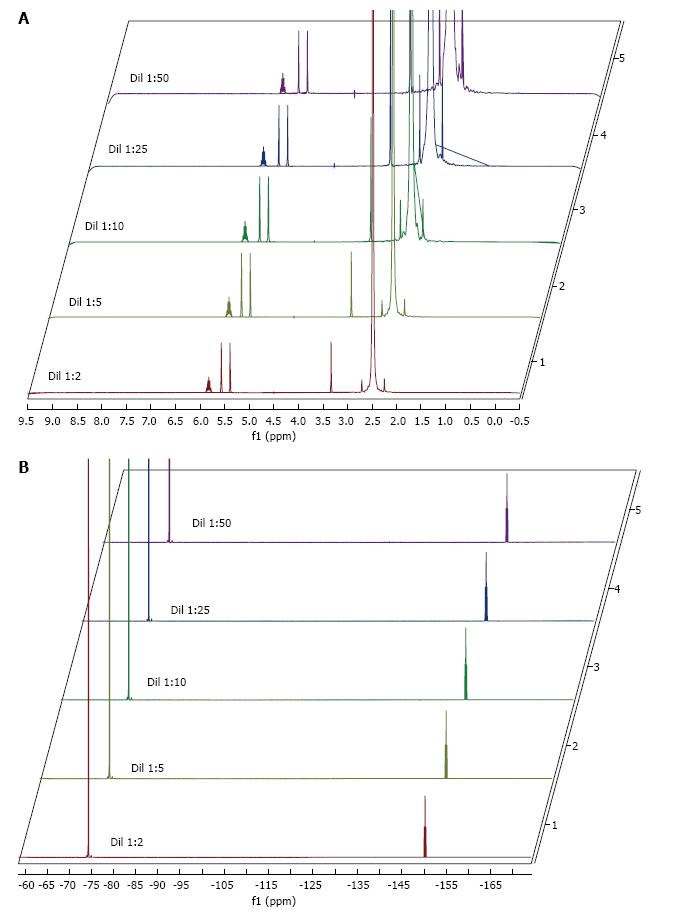
Sevoflurane and DMSO retention times were 2.7 and 13.0 min, respectively. Pure DMSO injection into the column resulted in two additional peaks at 2.1 and 2.8 min. These were probably due to manufacturing-derived volatile impurities, most likely dimethyl sulphide (starting material) and dimethyl sulphone (by-product), the levels of both impurities were 0.08%. The same peaks were found across all the dilutions of sevoflurane kept at different temperatures (Figure 1). The temperature was maintained at 260 °C up to 40 min and the chromatogram baseline was amplified in order to search for possible degradation by-products.
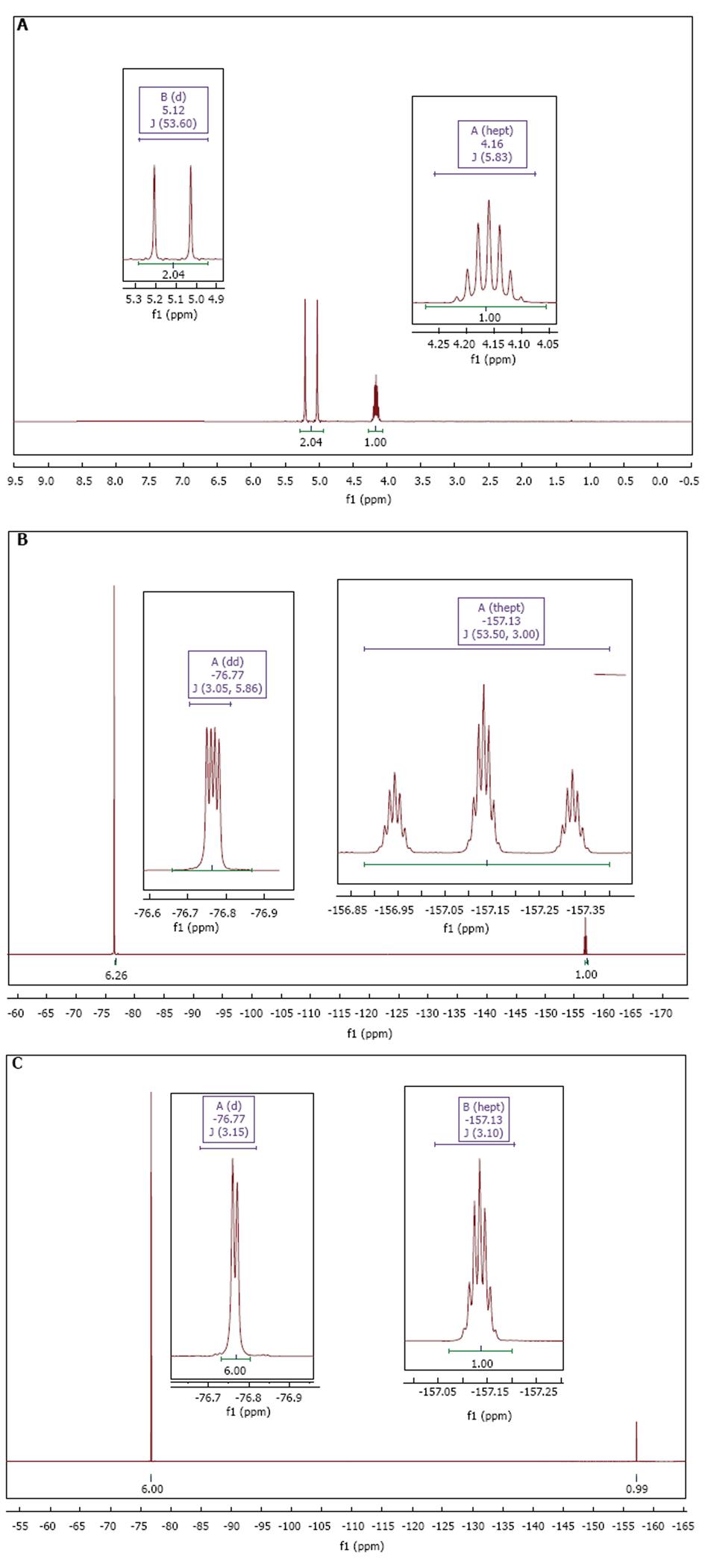
The 1H, 19F and 19F{1H}-NMR reference spectra acquired from undiluted sevoflurane are shown in Figure 2. The 1H and 19F-NMR spectra of all the dilutions kept at different temperatures are represented in Figure 3.
In the 1H spectrum, two signals corresponding to the sevoflurane molecule were observed at 5.12 and 4.16 parts per million (ppm5). The signal at 5.12 ppm was integrated by two protons with a doublet multiplicity (coupling constant JH-F = 53.6 Hz), which was assigned to the protons of the fluoromethoxy group (-OCH2F). The signal at 4.16 ppm was a septet that integrated one proton (coupling constant JH-F = 5.8 Hz), which was assigned to the proton from the hexafluoro-isopropyl group (CF3)2CH-.
In the 19F-NMR spectrum, two signals were observed at -76.77 ppm and -157.13 ppm. The signal at -76.77 ppm was integrated by six fluorine atoms, consisting of a double-doublet with coupling constants J1 = 5.9 Hz and J2= 3.1 Hz, corresponding to the hexafluoro-isopropyl group (CF3)2CH-. The multiplicity in this case was due to the coupling of six fluorine atoms with the only proton of the group (J1F-H = 5.9 Hz); there was a long range coupling with the fluorine atom from the fluoromethoxy group (J2F-F = 3.1 Hz). The signal at -157.13 ppm integrated for one fluorine nucleus and was a triple-heptuplet with coupling constants J1 = 53.5 Hz and J2 = 3 Hz, corresponding to the fluorine atom from the fluoromethoxy group (-OCH2F). The multiplicity could be explained due to the coupling between the fluorine atom and two adjacent protons (J1F-H = 53.5Hz), and a long-range five-bond coupling between the fluorine nucleus in the fluoromethoxy group and the (CF3)2CH- fluorine nuclei (J5F-F = 3.0 Hz).
In the 19F NMR CPD, two signals were observed at -76.77 ppm and -157.13 ppm. The first one showed a doublet (JF-F = 3.1 Hz) which integrated by six fluorine nuclei from the hexafluoroisopropyl group. The second signal was integrated by a fluorine atom and showed a septuplet (JF-F = 3.1 Hz).
This study shows that different concentrations of sevoflurane in DMSO (ranging from the undiluted product to a 1:50 dilution) preserve their chemical structure after exposure to a range of temperatures (-10 °C, 4 °C, and 25 °C) over 45 d. No sevoflurane by-products were detected in any of the samples. To our knowledge, the stability of sevoflurane solutions in DMSO has never been reported in the literature.
Sevoflurane is a poly-fluorinated methyl-isopropyl ether-derivative commonly used in the induction and maintenance of general anesthesia in both adult and pediatric patients[1]. Recent evidence suggests it might aid healing and mitigate pain associated with vascular ulcers[2-4]. The selection of DMSO as a vehicle for sevoflurane responds to both pharmaceutical and pharmacological needs: It is a polar solvent chemically compatible with sevoflurane over a wide range of concentrations. Additionally, some studies suggest it might possess some analgesic, hydroxyl free-radical scavenger, healing, and antimicrobial properties after topical application[7-11].
Amber polypropylene syringes and caps were used for the preparation and conservation of the aliquots. This responds to the known reactivity between sevoflurane and glass surfaces, which leads to the production of hydrofluoric acid (HF). According to this, an increase in HF concentration would be directly related to the degree of degradation of sevoflurane[13,14].
In this study, two main analytical techniques were used to assess the integrity and physicochemical stability of sevoflurane dilutions: GC-FID and NMR. GC-FID constitutes the gold-standard for the analysis of volatile substances such as sevoflurane[15,16]. This technique combines a high analytical sensibility in the range of parts per billion with a universal response to any compound suffering ionization in the hydrogen flame and with its quantitative capabilities, having a long linear range and low signal-to-noise ratio. NMR is based on the measure of the resonance frequency of active nuclei with spin different from zero (such as 1H and 19F) of a molecule in the presence of a high magnetic field. Given that sevoflurane contains seven fluorine atoms in its structure, combined NMR measuring of 19F and 1H nuclei is an ideal option for the characterization of both sevoflurane and any eventual by-product[17-21]. In the case of finding such breakdown compounds, it would be feasible to quantify the amount of degradation by any of the two techniques, preferably by GC. In addition, GC and NMR are analytical techniques recommended by the European and the American Pharmacopoeias, as well as by the International Conference on Harmonization guidelines (ICH) to study the chemical stability of drugs[22-24]. In the GC analysis, the chromatogram of each sample was compared with those of the reference commercial sevoflurane and the DMSO used. The levels of both impurities observed, dimethyl sulphide (starting material) and dimethyl sulphone (by-product), were 0.08%, DMSO impurities are controlled in the PhE at 0.1%[25]. No additional peaks other than those previously present were detected even after a chromatographic run of 40 min raising the oven temperature to 260 °C. The chromatogram baseline was amplified and searched for such impurities and the results were consistent in all the samples with the absence of degradation.
In the NMR study, sevoflurane signals were observed in both 1H and 19F spectra, regardless of the temperature conditions. This confirms the structural integrity of the sevoflurane molecule at a range of temperatures and concentrations, including the undiluted product, as well as the absence of other sevoflurane breakdown products.
Caveats associated with these findings rely on the need for longer-term stability assays in order to evaluate the effects of temperature on sevoflurane at different times. Additionally, the effects of light exposure were not assessed.
In conclusion, this study confirms that the chemical structure of sevoflurane structure remains stable both diluted in DMSO at different concentrations and in pure state when subjected to a range of temperatures longer than a month in polypropylene syringes. These findings warrant further investigation, particularly in light of its potential applications in the management of pain and healing associated to skin ulcers.
Thanks to Torrecárdenas Hospital and University of Almería. The results presented in this study are part of the doctoral thesis of F. Dámaso Fernández Ginés, which is currently being carried out at the University of Granada, within the Doctoral Program in Pharmacy.
Direct topical application of anesthetic sevoflurane has recently shown beneficial properties in the management of chronic vascular ulcers. A more convenient formulation could be obtained using solutions of sevoflurane in a miscible solvent such as dimethyl sulfoxide. However, no study has yet assessed the physicochemical and pharmaceutical stability of these formulations.
The treatment of skin ulcer and structural drug analysis by nuclear magnetic resonance have progressed immensely in recent years. Assessing the physicochemical stability of sevoflurane in dimethyl sulfoxide (DMSO) solutions becomes essential in view of the potential therapeutic applications of such solutions in the management of vascular ulcers.
The present study shows that different sevoflurane in DMSO solutions are stable from a physicochemical and pharmaceutical point of view at a range of temperatures for up to 45 d. This has important implications in the formulation of new pharmaceutical forms aimed at improving the management of vascular ulcers.
The data in this study suggested that both drugs can be preloaded in polypropylene syringes and stored at different temperatures for the topical management of chronic vascular ulcers.
Vascular ulcers are a type of skin ulcer characterized by a painful sore (usually of the lower leg), accompanied by disintegration of tissue which mostly affects the epidermis, often portions of the dermis, or even subcutaneous fat. Its management requires the generalized use of topical and systemic analgesia. However, these agents often cause severe systemic adverse effects. Some recent evidence suggests that topical instillation of sevoflurane on the vascular ulcer has a rapid, intense, and durable anesthetic and healing effect.
The study is detailed and well-made and the paper accurately written. The results reported in this study have been obtained by gas chromatography and NMR experiments, clearly and precisely described. This paper is worthy of publication.
Manuscript source: Invited manuscript
Specialty type: Pharmacology and pharmacy
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Guaragna A, Yanev S S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Technical data sheet. AbbVie, FT-61451. 2007. [accessed Apr 23]. Sevorane® 2015. Available from: https://www.medicines.org.uk/emc/medicine/49. |
| 2. | Gerónimo-Pardo M, Martínez-Monsalve A, Martínez-Serrano M. Analgesic effect of topical sevoflurane on venous leg ulcer with intractable pain. Schattauer. 2011;2:95-97 Available from: http://phlebo.schattauer.de/de/inhalt/archiv/issue/1396/manuscript/16058/download.html. |
| 3. | Martínez A, Gerónimo M. Sevoflurano como anestésico local en herida isquémica de paciente cardiópata con insuficiencia respiratoria secundaria a morfina. Heridas y Cicatrización. 2011;6:46-49. |
| 4. | Gerónimo M, Martínez M, Martínez A, Rueda JL. Usos alternativos del sevoflurano. Efecto analgésico tópico. Rev Electron Anestesia R. 2012;4:181. |
| 5. | Capriotti K, Capriotti JA. Dimethyl sulfoxide: history, chemistry, and clinical utility in dermatology. J Clin Aesthet Dermatol. 2012;5:24-26. [PubMed] |
| 6. | Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D. Systematic reviews of wound care management: (2). Dressings and topical agents used in the healing of chronic wounds. Health Technol Assess. 1999;3:1-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Miranda-Tirado R. Dimethyl sulfoxide therapy in chronic skin ulcers. Ann N Y Acad Sci. 1975;243:408-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 8. | Lishner M, Lang R, Kedar I, Ravid M. Treatment of diabetic perforating ulcers (mal perforant) with local dimethylsulfoxide. J Am Geriatr Soc. 1985;33:41-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Duimel-Peeters IGP, Houwing RH, Teunissen CP, Berger MPF, Snoeckx V, Halfens RJG. A systematic review of the efficacy of topical skin application of dimethyl sulfoxide on wound healing and as an anti-inflammatory drug. Wounds UK. 2003;15:361-370 Available from: https://www.researchgate.net/publication/258120940_A_Systematic_Review_of_the_Efficacy_of_Topical_Skin_Application_of_Dimethyl_Sulfoxide_on_Wound_Healing_and_as_an_Anti-Inflammatory_Drug. |
| 10. | Celen O, Yildirim E, Berberoğlu U. Prevention of wound edge necrosis by local application of dimethylsulfoxide. Acta Chir Belg. 2005;105:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Gaspar M, Bovaira M, Carrera-Hueso FJ, Querol M, Jiménez A, Moreno L. [Efficacy of a topical treatment protocol with dimethyl sulfoxide 50% in type 1 complex regional pain syndrome]. Farm Hosp. 2012;36:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Note for Guidance on Declaration of Storage Conditions for Medicinal Products in the Product Particulars (Annex to Note for Guidance on Stability Testing of New Active Substances and Medicinal Products) and Active Substances (Annex to Note for Guidance on Stability of Existing Active Substances and Related Finished Products). CPMP/QWP/609/96 Rev 1 (Adoption by CPMP April 2003). London: EMEA 2003; Available from: http://www.rsihata.com/updateguidance/emea/old/060996en.pdf. |
| 13. | Callan CM. Maker follows up on sevoflurane problem. J Clin Monit. 1997;13:335. [PubMed] |
| 14. | Kharasch ED, Subbarao GN, Cromack KR, Stephens DA, Saltarelli MD. Sevoflurane formulation water content influences degradation by Lewis acids in vaporizers. Anesth Analg. 2009;108:1796-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Bakshi M, Singh S. Development of validated stability-indicating assay methods--critical review. J Pharm Biomed Anal. 2002;28:1011-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 382] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 16. | Yamakage M, Hirata N, Saijo H, Satoh JI, Namiki A. Analysis of the composition of ‘original’ and generic sevoflurane in routine use. Br J Anaesth. 2007;99:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Holzgrabe U, Diehl BW, Wawer I. NMR spectroscopy in pharmacy. J Pharm Biomed Anal. 1998;17:557-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Evers AS, Dubois BW. 19F-nuclear magnetic resonance spectroscopy. Its use in defining molecular sites of anesthetic action. Ann N Y Acad Sci. 1991;625:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Orhan H, Commandeur JN, Sahin G, Aypar U, Sahin A, Vermeulen NP. Use of 19F-nuclear magnetic resonance and gas chromatography-electron capture detection in the quantitative analysis of fluorine-containing metabolites in urine of sevoflurane-anaesthetized patients. Xenobiotica. 2004;34:301-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Xu Y, Tang P, Zhang W, Firestone L, Winter PM. Fluorine-19 nuclear magnetic resonance imaging and spectroscopy of sevoflurane uptake, distribution, and elimination in rat brain. Anesthesiology. 1995;83:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Cardona D, Castaño MED, Saldarriaga CN, Quiñones W, Torres RLF, Echeverri F. Application of nuclear magnetic resonance (NMR) in drugs analysis. Vitae. 2003;10:80-88 Available from: https://www.researchgate.net/publication/237025733_APLICACION_DE_LA_RESONANCIA_MAGNETICA_NUCLEAR_RMN_EN_EL_ANALISIS_DE_MEDICAMENTOS. |
| 22. | Council of Europe (COE). European Pharmacopoeia 2007. Strasbourg: Council of Europe 2007; . |
| 23. | The United States Pharmacopoeia. 31st ed. Rockville, MD: United States Pharmacopoeial Convention (USP) 2007; . |
| 24. | Singh J. International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. J Pharmacol Pharmacother. 2015;6:185-187. [PubMed] |
| 25. | Council of Europe (COE). European Pharmacopoeia 2007. 6th ed. Method 2.4.24, Identification and control of residual solvents. Strasbourg: Council of Europe 2007; . |