Peer-review started: September 22, 2015
First decision: October 30, 2015
Revised: November 26, 2015
Accepted: December 29, 2015
Article in press: January 4, 2016
Published online: March 9, 2016
Processing time: 166 Days and 23.3 Hours
Schizophrenia is a psychiatric disorder affecting approximately 1% of the population worldwide and is characterised by the presence of positive and negative symptoms and cognitive deficits. Whilst current therapeutics ameliorate positive symptoms, they are largely ineffective in improving negative symptoms and cognitive deficits. The cholinergic neurotransmitter system heavily influences cognitive function and there is evidence that implicates disruption of the central cholinergic system in schizophrenia. Historically, targeting the cholinergic system has been impeded by poor selectivity leading to intolerable side effects warranting the need to develop more targeted therapeutic compounds. In this review we will summarise evidence supporting the roles of the cholinergic system, particularly the muscarinic M1 receptor, in the pathophysiology of schizophrenia and discuss the potential of a promising new class of candidate compounds, allosteric ligands, for addressing the difficulties involved in targeting this system. The body of evidence presented here highlights the dysfunction of the cholinergic system in schizophrenia and that targeting this system by taking advantage of allosteric ligands is having clinically meaningful effect on cognitive deficits.
Core tip: Schizophrenia is a psychiatric disorder affecting approximately 1% of the world population. Current treatments inadequately redress the cognitive impairments associated with the disease. In light of that we discuss the role of the cholinergic system, in particular the muscarinic M1 receptor, in schizophrenia and cognition and how allosteric compounds are being developed to address this undertreated aspect of the disease. We also discuss and compile mutagenesis studies of the muscarinic M1 receptor and how they relate to allosteric binding and function.
- Citation: Hopper S, Udawela M, Scarr E, Dean B. Allosteric modulation of cholinergic system: Potential approach to treating cognitive deficits of schizophrenia. World J Pharmacol 2016; 5(1): 32-43
- URL: https://www.wjgnet.com/2220-3192/full/v5/i1/32.htm
- DOI: https://dx.doi.org/10.5497/wjp.v5.i1.32
Cognitive impairment is associated with multiple disease states and usually has a big impact on quality of life. Schizophrenia is a psychiatric disorder with a predominant age of onset from late teens to early adulthood that affects approximately 1% of the world population. It is diagnosed by the presence of what are characterised as positive and negative symptoms and cognitive deficits. Unlike neurological disorders no major lesion was apparent in the central nervous system (CNS) from subjects with schizophrenia[1] and therefore many of the hypotheses as to its cause have been based on the actions of drugs that either worsen or ameliorate the symptoms of the disorder.
With the serendipitous discovery that chlorpromazine had unexpected therapeutic benefit when given to psychotic individuals there was a significant effort to develop a range of what became known as first generation neuroleptic drugs[2]. Subsequently, it was determined that the ability of these drugs to reduce the severity of positive symptoms in some people with schizophrenia was directly related to the ability of first generation neuroleptic drugs to act as antagonists at the dopamine D2 receptor[3]. Understanding this mechanism led to the dopamine hypothesis of schizophrenia which states hyperactivity of the dopamine neurotransmitter system contributes to the pathophysiology of schizophrenia (reviewed in[4]).
The dopamine hypothesis was the first attempt to encapsulate the biological mechanisms underpinning schizophrenia. This hypothesis grew from an understanding that the early antipsychotics block the dopamine D2 receptor and that drugs which increase CNS dopamine function cause or worsen psychosis. The original dopamine hypothesis proposed that widespread hyperactivity of the dopamine system within the brain was the cause of psychotic symptoms but the hypothesis was subsequently refined to propose that cortical hypo-activity in addition to subcortical hyper-activity of dopamine contributed to both the psychotic and other symptoms associated with schizophrenia (reviewed in[5]). Inconsistent reports of differences in dopamine receptor levels, metabolic enzymes[6], and imaging studies have led to questioning of the dopamine hypothesis[7,8] and investigation into other possible mechanisms of disease aetiology. However, a neuroimaging report of increased amphetamine-induced dopamine release in the striatum of subjects with schizophrenia appears to reinforce a role for dopamine in the aetiology of the disorder (reviewed in[9]).
Clozapine is the archetypal drug that led to the development of a family of drugs termed second-generation neuroleptics[10]. Whilst the second-generation drugs were developed to bind to some of the receptors targeted by clozapine it is notable that none of these drugs have achieved the extended therapeutic reach of clozapine such as improving the cognitive deficits[11]. An early second generation neuroleptic drug, risperidone, was suggested to have significant benefits compared to first generation neuroleptic drugs[12] which were presumed to be due, at least in part, to its ability to antagonise the serotonin 2A receptor[13]. The clinical benefit obtained by risperidone, added to the observation that drugs such as lysergic acid diethylamide could cause psychotic symptoms by stimulating the serotonergic system[14], became the evidence to support the serotonin hypothesis of schizophrenia.
Initial interest in the serotonergic system stems from the observation that drugs affecting serotonin receptors produce psychotomimetic effects[15] and the discovery that second generation neuroleptics bind to various serotonin receptors[16,17]. These observations have led to investigation of the serotonin system both post-mortem and in vivo; a recent meta-analysis[18] demonstrated a moderately higher level of the serotonin receptor subtype 5-HT1A and a substantially lower levels of the 5-HT2A receptor subtype in the prefrontal cortex of post-mortem subjects with schizophrenia.
The glutamate hypothesis is somewhat unique as it is founded in the observation that phencyclidine could cause a broad range of symptoms in normal individuals[19] and exacerbate psychotic symptoms in patients with schizophrenia[20] but no drug targeting the glutamatergic system has proved useful in treating the disorder[21]. Further investigation has revealed elevated glutamate activity particularly in the medial prefrontal cortex and basal ganglia of un-medicated and first episode patients, demonstrated by magnetic resonance spectroscopy studies (reviewed in[22]). Of studies in post-mortem brain tissue, the most robust finding has been widespread differences in glutamatergic pyramidal neuron morphology, particularly lower numbers[23] and lengths of dendrites[24] and lower numbers of dendritic spines[25] in deep cortical layer III of the dorsolateral prefrontal cortex. There have also been mixed reports of differences in mRNA and protein levels of receptor subunits, transporters, and synthesis enzymes (post-mortem glutamatergic differences in schizophrenia reviewed in[26]).
Preceding all of these neurotransmitter hypotheses was the cholinergic hypothesis which was founded on the observation that some subjects with schizophrenia had remission or an improvement in symptom severity after coma induced by a dose of acetylcholine (ACh)[27]. Since those early experiments components of the cholinergic system, the muscarinic receptors, have been targeted by drugs such as clozapine and olanzapine that reduce the symptoms of schizophrenia[28] and drugs such as benzatropine which were used to control the extrapyramidal side effects associated with treating with first generation antipsychotic drugs[29]. Whilst these neuropsychopharmacological findings are important to consideration of the role of the cholinergic system in schizophrenia, other studies which have advanced our understanding of the cholinergic system and how it may be affected in the disorder have been important in refining the cholinergic hypotheses of schizophrenia.
The cholinergic system consists of several distinct pathways; the most well studied being the pathway that projects from the basal forebrain to most of the CNS[30]. This pathway extensively modulates the dopamine system by affecting striatal dopamine release[31]; the glutamate system by potentiating NMDA receptors[32]; and the serotonergic system via projections to the dorsal raphe nucleus where ACh inhibits the release of serotonin[33] (cholinergic interactions in schizophrenia reviewed in[34]). Whilst the modulatory interactions of the cholinergic system with other neurotransmitter systems provide mechanisms which can implicate that system in schizophrenia there are also several lines of evidence to suggest that the cortical muscarinic M1 receptor, a component of the cholinergic system, is particularly implicated in the pathophysiology of schizophrenia.
Although there have been several reviews discussing the cholinergic system and its role in schizophrenia[35,36], there have recently been some exciting advances in developing drugs to target the cholinergic system[37-39] that make a review of the area timely. This is particularly the case because the cognitive deficits of schizophrenia are largely non-responsive to current drug treatments[40] whereas preliminary data suggest that the new drugs targeting the cholinergic system will improve cognition[41]. Cognitive deficits are a core feature of schizophrenia and are made up of deficits in the domains of speed of processing, attention/vigilance, working memory, verbal learning and memory, visual learning and memory, reasoning and problem solving, and social cognition (reviewed in[42]). The impact of these deficits in people with schizophrenia is now considered the most debilitating of all the symptom domains associated with the disorder. Therefore, this review will first summarize the cholinergic system, then briefly review evidence supporting its role, particularly the muscarinic M1 receptor, in cognition and the pathophysiology of schizophrenia and finally discuss the potential of a promising new class of candidate compounds and how they target the muscarinic M1 receptor for addressing the difficulties involved in targeting this system.
ACh is a modulatory neurotransmitter[43] in the CNS and peripheral autonomic and somatic nervous systems. It is essential to the function of all branches of the peripheral nervous system[44] and has been associated with a large number of cognitive processes in the CNS. Two classes of integral membrane receptor mediate signal transmission: The ionotropic nicotinic receptors (nAChR), and the metabotropic muscarinic receptors.
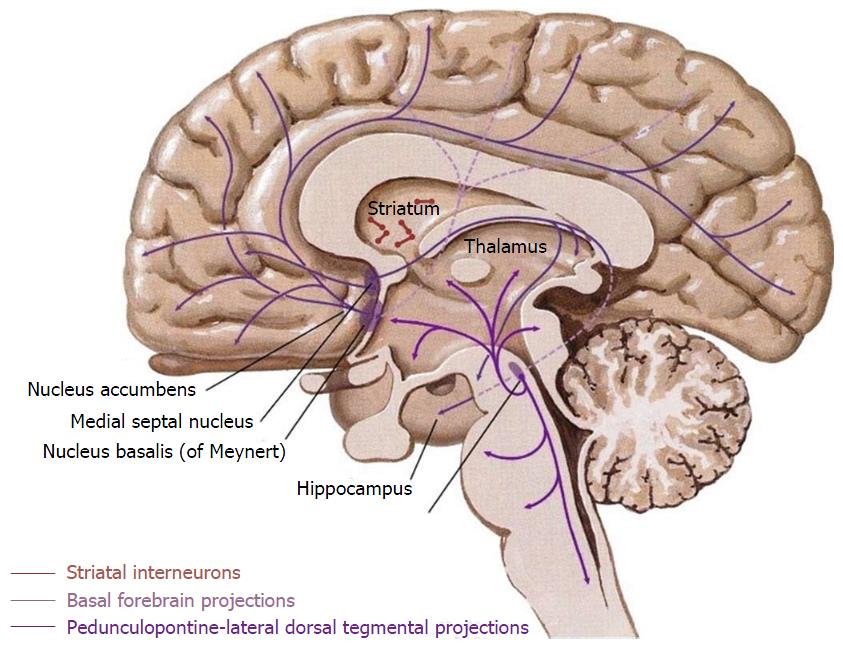
Within the CNS, cholinergic neurons are predominantly located in the basal forebrain as cortical projection neurons (reviewed in[45]) and striatal interneurons (Figure 1). The projection neurons arise from four cell groups (Ch1-4) in the basal forebrain[46] and from the pedunculopontine-lateral dorsal tegmental area in the brainstem. These cell groups are not exclusively composed of cholinergic neurons, but contain a diverse mix of other neurotransmitter systems including GABAergic, peptide transmitter, and catecholaminergic neurons. These projection neurons modulate all regions of cortex including the hippocampal formation[46]. In addition to the projection nuclei of the basal forebrain, striatal cholinergic interneurons interact heavily with the dopaminergic projections from the substantia nigra pars compacta and the ventral tegmental area (VTA)[47] (The human cholinergic brain architecture is extensively reviewed in[30]).
ACh acts in part via the family of five muscarinic (M1-5) receptors; they have a differential expression pattern throughout the human CNS[48]. Point mutation and other studies suggest that the structural position of the muscarinic receptor transmembrane domains relative to one another affect the differential binding specificity of ligands to these receptors[49,50]. Significantly, the orthosteric site on the five muscarinic receptors is highly conserved and this has presented a challenge to the development of receptor-specific drugs.
Of the five muscarinic receptors, the muscarinic M1 receptor is the predominant muscarinic receptor in all cortical areas[48] where it is located on excitatory neurons to modulate their firing, for example, by potentiating NMDA receptors[32] and at cholinergic synapses[51]. Mouse knockout and knock down studies have elucidated some of its roles in CNS function. For example, in muscarinic M1 receptor knockout mice, mitogen activated protein kinase (MAPK) signalling is impaired in cortical neuronal cultures[52] and hippocampal slices[53]. The role of MAPK in hippocampal long term potentiation[54] and neuronal plasticity[55] demonstrate a potential mechanism by which muscarinic M1 receptors modulate learning and memory. Gamma oscillations (20-80 Hz) of neuronal firing patterns are associated with memory[56], hippocampal gamma oscillations induced by muscarine, a muscarinic agonist, but not those induced by kainite, a glutamatergic kainite receptor agonist, are completely absent from muscarinic M1 receptor knockout mice[57]. Another study reported that muscarinic M1 receptor knockout mice had fewer and shorter dendrites and disrupted cortical tonotopic maps in the auditory cortex[58]. Additionally, muscarinic M1 receptor knockout mice had impaired experience dependent plasticity in the auditory cortex[59], implicating the muscarinic M1 receptor in auditory cortical organisation, sensory processing, and learning. Additionally, muscarinic M1 receptor knockout mice are deficient in working memory and memory consolidation processes where the hippocampus and cerebral cortex interact[60]. These studies together implicate muscarinic M1 receptors in various aspects of learning and memory.
In addition to their cognitive effects, muscarinic M1 receptors are implicated in the response to a number of brain-penetrant drugs in mice. Muscarinic M1 receptor knockout mice are highly resistant to seizures induced by pilocarpine, a muscarinic agonist, which does not supress the voltage dependent K+ M current in these animals. Conversely, muscarinic M2-4 receptor knockout mice display wild-type seizure response and M current suppression when administered pilocarpine[61]. As M current suppression is considered the mechanism by which pilocarpine seizures are produced[62], the muscarinic M1 receptors mediate this drug induced seizure response by modulating M currents in sympathetic neurons[61]. Muscarinic M1 receptor knockout mice displayed an elevated striatal level of extracellular dopamine and increased locomotor activity, which neuroleptic treatment attenuated[63]. In addition, the mice had an increased sensitivity to amphetamine administration, in both the locomotor response and striatal dopamine levels[63] demonstrating an interaction between the cholinergic and dopaminergic systems. This interaction is supported by the report of an increase in morphine-induced analgesia and lower rates of self-administration of morphine and cocaine in muscarinic M1 receptor knockout mice[64]. Taken together, these studies implicate the muscarinic M1 receptor in the both the response to these drugs and as a mechanism by which the cholinergic system interacts with the dopamine reward pathway.
The cholinergic system’s role in schizophrenia is supported by research involving neuropsychopharmacological agents, post-mortem brain tissue, and neuroleptic drug treatments. In addition to the these three areas of research, a landmark single photon emission computed tomography (SPECT) study showed widespread lower levels of muscarinic receptor binding in the cerebral cortex in patients with schizophrenia who were medication free compared to healthy control subjects[65].
Several neuropsychopharmacological studies provide evidence to support the role of muscarinic receptors in schizophrenia. Administering a muscarinic antagonist, scopolamine, to healthy control subjects produced deficits in spatial and working memory as well as sustained visual attention[66]. In another report this group demonstrated that scopolamine produced deficits in spatial and object working memory using the n-back paradigm[67], which is used to assess working memory function in patients with schizophrenia. Additionally, acute administration of scopolamine to healthy subjects produced deficits in maintenance of working memory[68]. Another study demonstrated higher doses of scopolamine as well as atropine and Ditran, two other muscarinic antagonists, produced hallucination, and delirium in addition to cognitive deficits in healthy control subjects[69]. Thus blocking muscarinic recptor activity mimics both positive and cognitive symptoms observed in patients with schizophrenia.
Sleep disturbances, including impaired sleep continuity, abnormal REM latency[70] and decreased slow wave sleep[71] observed in patients with schizophrenia have been associated with impaired sleep dependent memory consolidation, as well as negative symptom severity[71]. Administration of cholinergic agonists to healthy volunteers causes shortening of REM latency during sleep[72,73] and altered REM latency in a subset of patients with schizophrenia more severely than healthy control subjects[74]. These findings demonstrate that pharmacological manipulation of the cholinergic system can mimic and worsen the sleep disturbances observed in patients with schizophrenia. These studies add support to the body of evidence implicating the cholinergic system in the pathology of schizophrenia.
A role for the muscarinic M1 receptor in schizophrenia is supported by a variety of post-mortem studies investigating radioligand binding and mRNA levels in multiple brain regions (Table 1), which report a decrease in muscarinic M1/M4 receptor binding and muscarinic M1 receptor mRNA levels in multiple brain regions (reviewed in[75]). Notably, we reported a decrease in muscarinic M1[76], but not M2, M3[77] or M4[76] receptor protein in the cortex of subjects who had schizophrenia confirming that the M1 receptor is selectively decreased in people with the disorder. More recently, we[78] reported lower binding of [3H]pirenzepine ([3H]PRZ), a muscarinic M1/M4 receptor selective antagonist, in the dorsolateral prefrontal cortices from people with schizophrenia to be limited to a sub-set (approximately 25%) of subjects; these subjects had 75% lower binding to muscarinic receptors in Brodmann area (BA) 9 when compared to both healthy control subjects and other subjects with schizophrenia. These data underpin the proposal of a subgroup within schizophrenia that have a muscarinic receptor deficit schizophrenia (MRDS). This hypothesis is pertinent to the growing acceptance that schizophrenia is a syndrome of disorders that may well have differing aetiologies[79].
| Ref. | Receptor Type | Regions | Results | |
| Protein | mRNA | |||
| [114] | M1/M4 | CPu | Lower binding | |
| [115] | M2/M4 | CPu | Lower binding | |
| [116] | M1 M2 | CPu | M1 no difference; M2 below sensitivity in both SCZ and HC | |
| [117] | M1/M4 | PFC | Lower binding | |
| [76] | M1/M4 | M1 M4 | DLPFC; PCx | Lower binding and M1 mRNA levels in DLPFC; no difference in binding, lower M1 and M4 mRNA levels in PCx |
| [118] | M1 | PMC | Lower levels | |
| [119] | M1/M4 | ACC | Lower binding | |
| [120] | M2/M4 | ACC | No difference | |
| [121] | M1/M4 M2/M4 | STG | Lower M1/M4 binding non-significant; lower M2/M4 binding | |
| [77] | M2 M3 | M2 M3 | DLPFC; PCx | M2 and M3 binding and M3 mRNA no difference; M2 mRNA below sensitivity in both SCZ and HC |
| [122] | M1/M4 | M1 M4 | Hipp | Lower binding; lower M4 mRNA no difference in M1 mRNA levels |
| [123] | M1/M4 M3 | PMC | Lower M1/M4 binding; no difference in M3 binding | |
| [78] | M1 | DLPFC | Lower binding in subset of SCZ subjects | |
| [81] | M1 M3 | M1 M3 M4 | PMC | Lower M1 binding in subset of SCZ subjects; no difference in mRNA levels or M3 binding |
| [82] | M1 | DLPFC; ACC; BrA | Lower binding in subset of SCZ subjects | |
Although [3H]PRZ binds to both muscarinic M1 and M4 receptors, its selectivity for the muscarinic M1 receptor was increased under the conditions used for the study that identified MRDS[80]. Furthermore, we previously showed that muscarinic M1, but not M4, receptor mRNA and [3H]PRZ binding levels were significantly lower in BA 9 in subjects with schizophrenia[76]. These data, combined with the high abundance of muscarinic M1 receptors relative to the other muscarinic receptor types within the cerebral cortex[48], strongly implicates muscarinic M1 receptors in the pathophysiology of the MRDS group. More recent work has demonstrated lower [3H]PRZ binding in BAs 6, 10, 24, 44 and 46 in the same cohort[81,82]; these findings are consistent with the widespread loss of cortical muscarinic receptors in people with schizophrenia reported in the SPECT study[83].
Traditional antipsychotic therapies achieve their action by blockade of central dopamine D2 receptors (reviewed in[4]); unfortunately, these drugs can cause extrapyramidal side effects (EPS) such as tremors and dyskinesia similar to those seen in people with Parkinson’s disease[84]. Adjunctive anti-cholinergic agents are often used to combat EPS, due to the interaction between the dopaminergic and cholinergic systems in the basal ganglia, particularly the VTA[85]. The non-specific muscarinic antagonist procyclidine, however, exacerbated positive symptoms in patients with schizophrenia treated with the antipsychotic flupentixol[86], while the muscarinic M1 preferring antagonist biperiden worsened positive symptoms and ameliorated the negative symptoms in patients who were otherwise drug free for at least 2 wk[87]. Patients with schizophrenia who were taking benzatropine, a muscarinic antagonist, as an adjunctive therapy had impaired semantic organisation[88]. These studies show that pharmacological interventions affecting the central cholinergic tone in patients with schizophrenia has a complex effect on symptomatology, and that more specific modulation of the system may be beneficial for ameliorating the symptoms of the disorder.
A small, double-blind, placebo controlled trial of an agonist selective for the muscarinic M1 and M4 receptors, xanomeline, was conducted in a cohort of treatment resistant schizophrenia patients[39]. The xanomeline treated group showed significant improvement in both positive and negative symptoms as measured using the positive and negative syndrome scale and the brief psychiatric rating scale as well as improvements in a battery of cognitive tests, particularly in working memory and verbal and visual learning[39]. Unfortunately, peripheral adverse events were observed including vomiting, nausea and gastrointestinal distress similar to those observed in previous Alzheimer’s disease trials[89,90]; this led to the discontinuation of xanomeline. This compound highlighted two important points regarding muscarinic receptors as drug targets. Firstly, its efficacy in the schizophrenia trial[39] supports the body of evidence suggesting that muscarinic receptors, particularly muscarinic M1 and M4 receptors, are viable targets for treatment of schizophrenia. Secondly, the problem of selectivity for specific muscarinic receptors. High selectivity for individual muscarinic receptors is difficult to achieve with orthosteric ligands, due to the high homology between their orthosteric binding pockets. The side effects observed in the xanomeline trials were considered to be the result of “off target” activation of muscarinic receptors, particularly muscarinic M2 and M3 receptors[89,90]. Clearly, there is a need for ligands that are more selective, both for drug development and investigating individual receptors both in vivo and in vitro; allosteric compounds present a possible solution.
An allosteric site is a binding site on a receptor distinct from the orthosteric binding site of the endogenous ligand that can be activated by proteins or small molecules to elicit a biologic response (allosteric agonist) or modulate the response of an endogenous molecule, orthosteric agonist or orthosteric antagonist biding to the orthosteric site (allosteric modulator). Allosteric modulators increase [positive allosteric modulator (PAM)] or decrease [negative allosteric modulator (NAM)] the ability of orthosteric ligands to activate or inactivate the receptor. These terms describing allosterism are defined according to the guidelines set out by the International Union of Basic and Clinical Pharmacology[91].
In an attempt to better understand the muscarinic M1 receptor and aid drug development, site-directed mutagenesis techniques have identified amino acid residues of the muscarinic M1 receptor implicated in the binding of compounds to the orthosteric site, the allosteric site, and in the functional cooperativity between the orthosteric and allosteric sites. From these studies, the orthosteric site is considered to be a pocket deep in the transmembrane domains (TMs; reviewed in[92]). Recent X-ray crystallographic determination of the structures of the muscarinic M2[93] and M3[50] receptors has confirmed the orthosteric site is located in a pocket that forms a channel between the TMs for these receptors. The differential binding specificity of ligands to the different receptors is thought to relate to the structural position of the transmembrane domains relative to one another[49,50].
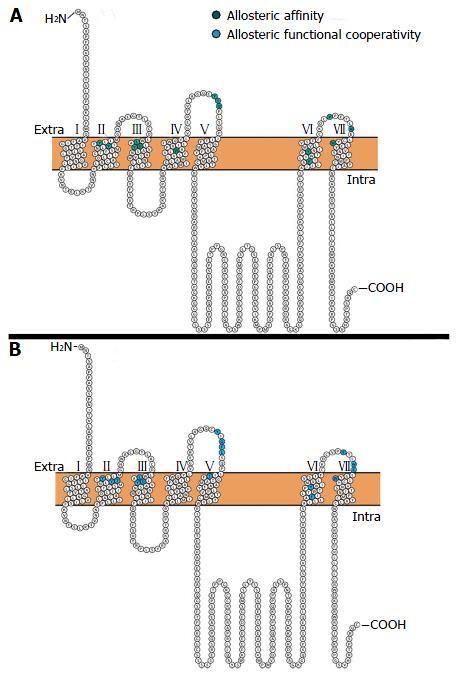
An early study characterising the allosteric site of the muscarinic M1 receptor performed alanine scanning of the extracellular loop (ECL) amino acid residues conserved across the five muscarinic receptors. The authors found that the only residue to have any appreciable effect on the binding characteristics of gallamine (NAM) was Tyr400, found in the 3rd ECL, leading them to hypothesise that the allosteric site of gallamine is close in space to the orthosteric site[94]. More recently, other residues that are implicated in binding to the allosteric site (Figure 2A) and functional cooperativity between the allosteric and orthosteric sites (Figure 2B) of the muscarinic M1 receptor were identified in the 2nd, 3rd, 4th, 6th, and 7th TMs and the 2nd ECL[95,96]. Computer modelling of the residues identified in mutagenesis studies has predicted a putative binding site which is topographically distinct from the orthosteric site, mainly composed of residues from ECL2, TMII, and TMVII[96]. Notably, these studies using 1-(4-methoxybenzyl)-4-oxo-1, 4-dihydroquinoline-3-carboxylic acid (BQCA)[96] and a structural derivative of BQCA (benzoquinazolinone 12)[95] found that mutating the Tyr400 residue completely abolished binding to the allosteric site of the muscarinic M1 receptor; in agreement with the study using gallamine. Interestingly, the residues implicated in allosteric binding to the muscarinic M1 receptor are highly conserved across all five muscarinic receptors, while residues implicated in the functional cooperativity between the orthosteric and allosteric sites are not; leading the authors to hypothesise that these residues underlie the muscarinic M1 receptor specific cooperativity of these ligands[96].
There has been considerable interest in using allosteric ligands to target GPCRs implicated in a variety of disease states (reviewed in[97,98]). Here we will discuss a number of attempts to develop compounds targeted to the allosteric site of the muscarinic M1 receptor. Early evidence for allosteric ligands came from the discovery that brucine is a weak PAM[99] and allosteric agonist of muscarinic M1 receptors[100] demonstrating that potentiation of the muscarinic M1 receptor is possible. Since this discovery, multiple allosteric agonists of muscarinic M1 receptors have been identified by high through put screening; including: AC-42[101]; 77-LH-28-1, which increased gamma oscillations, which are associated with memory[56], in rat hippocampal slices and hippocampal cell firing in vivo[102]; 1-(1’-2-methylbenzyl)-1,4’-bipiperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one (TBPB), which reversed amphetamine induced hyper-locomotion in rats[103], a model predictive of antipsychotic-like activity; LuAE51090, which improved mouse performance on the delayed alternation Y-maze[104], a model of working memory; and GSK1034702, which improved episodic memory in humans on the nicotine withdrawal model of cognitive dysfunction[38]. Additionally, [11C]GSK1034702 was clinically trialled as a positron emission tomography (PET) ligand (clinicaltrials.gov identifier: NCT00937846) to assess its blood brain barrier penetrance and distribution in vivo; unfortunately [11C]GSK1034702 was deemed unsuitable as a PET ligand for quantification of muscarinic M1 receptors in vivo[105].
Notably, Researchers at Merck Laboratories identified the first highly selective PAM of the muscarinic M1 receptor: BQCA[106]. BQCA increases ACh affinity for human cloned muscarinic M1 receptors demonstrating a selective, dose dependant potentiation of ACh’s ability to displace [3H]n-methyl scopolamine ([3H]NMS), a pan muscarinic antagonist, binding to muscarinic M1 receptors expressed in Chinese hamster ovary cells[107]. Further, BQCA has been demonstrated to potentiate muscarinic M1 receptors cloned from rhesus, dog, mouse and rat muscarinic M1 receptors as well as selectively potentiate native mouse muscarinic M1 receptors in vivo[107,108]. Recently, we demonstrated that BQCA dose dependently potentiates ACh’s ability to displace [3H]NMS in human brain homogenate and that a subset of individuals with schizophrenia with a loss of cortical muscarinic M1 receptors[78] have a decreased response to BQCA’s modulation of ACh binding[109]. Modification of this compound by chemical motif substitution[37,110] is ongoing. A selective muscarinic M1 receptor PAM based on the BQCA scaffold, 1-{[4-cyano-4-(pyridine-2-yl) piperidin-1-yl] methyl}-4-oxo-4 H-quinolizine-3-carboxylic acid (PQCA)[37], attenuated scopolamine deficits in novel object recognition, self-ordered spatial search, and the object retrieval detour tasks in rats, cynomolgus macaques, and rhesus macaques, respectively[111]. The efficacy of PQCA in these three models of cognitive function further supports the role of the muscarinic M1 receptor in cognition and demonstrates the potential of this class of compound as useful therapeutics. Additionally, Merck have taken MK-7622, a muscarinic M1 receptor PAM to phase II clinical trial (clinicaltrials.gov identifier: NCT01852110) as an adjunct therapy to an acetylcholinesterase inhibitor (donepezil, rivastigmine or galantamine) for the treatment of cognitive impairment and disease progression in Alzheimer’s disease. This highlights the promise of allosteric modulators as pro-cognitive agents.
Allosteric modulators of muscarinic M1 receptors provide a promising method for developing effective, well-tolerated therapies to redress cognitive impairment, particularly in schizophrenia and other diseases characterised by significant cognitive impairment. Historically, treating any disorder with drugs targeting muscarinic receptors has been hampered by the difficulties associated with targeting the orthosteric binding site, particularly the propensity for “off target” side effects, due to the high degree of homology between the orthosteric binding sites of the five muscarinic receptors. Fortunately, the discovery of highly selective allosteric ligands provides a potential solution to this problem and provides a unique opportunity to maintain physiological firing patterns, unattainable using orthosteric ligands. However, allosteric modulation comes with its own host of idiosyncrasies to be considered when developing ligands targeting this region of the receptors. At the level of preclinical pharmacology, allosteric compounds provide an exciting opportunity to tease out specific downstream signalling pathways, selectively targeting them to achieve highly specific fine-tuning of receptor response. Additionally, by iterative chemical substitutions of the base compound, multiple parameters can be modified to tailor compounds to specific needs, enhancing some aspects of signalling while inhibiting or not affecting others. Although these aspects of allostery provide unique opportunities, they also highlight a need for care when testing compounds and appropriate modelling paradigms, as particular effects could be overlooked or masked by classical drug screening methods. The emergence of allosteric ligands provides us with the exciting opportunity to develop well-tolerated, highly selective therapies with the ability to fine tune distinct pathways addressing subtle pathological changes in complex disease states.
P- Reviewer: Kravos M, Soriano-Ursua M, Yang JJ S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Dean B. Signal transmission, rather than reception, is the underlying neurochemical abnormality in schizophrenia. Aust N Z J Psychiatry. 2000;34:560-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Dean B, Moller HJ, Svensson TH, Geyer MA, Rujescu D, Scarr E, Millan MJ. Problems and solutions to filling the drying drug pipeline for psychiatric disorders: a report from the inaugural 2012 CINP Think Tank. Int J Neuropsychopharmacol. 2014;17:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Andén NE, Butcher SG, Corrodi H, Fuxe K, Ungerstedt U. Receptor activity and turnover of dopamine and noradrenaline after neuroleptics. Eur J Pharmacol. 1970;11:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 794] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 4. | Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 627] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 5. | Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;9:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Fan JB, Zhang CS, Gu NF, Li XW, Sun WW, Wang HY, Feng GY, St Clair D, He L. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: a large-scale association study plus meta-analysis. Biol Psychiatry. 2005;57:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Moncrieff J. A critique of the dopamine hypothesis of schizophrenia and psychosis. Harv Rev Psychiatry. 2009;17:214-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Kendler KS, Schaffner KF. The dopamine hypothesis of schizophrenia: An historical and philosophical analysis. PPP. 2011;18:41-63. [DOI] [Full Text] |
| 9. | Dean B. Neurochemistry of schizophrenia: the contribution of neuroimaging postmortem pathology and neurochemistry in schizophrenia. Curr Top Med Chem. 2012;12:2375-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Baldessarini RJ, Frankenburg FR. Clozapine. A novel antipsychotic agent. N Engl J Med. 1991;324:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 399] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 11. | Meltzer HY. Treatment-resistant schizophrenia--the role of clozapine. Curr Med Res Opin. 1997;14:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 324] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Borison RL, Pathiraja AP, Diamond BI, Meibach RC. Risperidone: clinical safety and efficacy in schizophrenia. Psychopharmacol Bull. 1992;28:213-218. [PubMed] |
| 13. | Leysen JE, Gommeren W, Eens A, de Chaffoy de Courcelles D, Stoof JC, Janssen PA. Biochemical profile of risperidone, a new antipsychotic. J Pharmacol Exp Ther. 1988;247:661-670. [PubMed] |
| 14. | Vardy MM, Kay SR. LSD psychosis or LSD-induced schizophrenia? A multimethod inquiry. Arch Gen Psychiatry. 1983;40:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Woolley DW, Shaw E. A biochemical and pharmacological suggestion about certain mental disorders. Proc Natl Acad Sci USA. 1954;40:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 306] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 16. | Leysen JE, Niemegeers CJ, Tollenaere JP, Laduron PM. Serotonergic component of neuroleptic receptors. Nature. 1978;272:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 485] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Meltzer HY, Massey BW. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr Opin Pharmacol. 2011;11:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 18. | Selvaraj S, Arnone D, Cappai A, Howes O. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci Biobehav Rev. 2014;45:233-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 19. | Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2312] [Cited by in RCA: 2384] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 20. | Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 600] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 21. | Citrome L. Unmet needs in the treatment of schizophrenia: new targets to help different symptom domains. J Clin Psychiatry. 2014;75 Suppl 1:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Poels EM, Kegeles LS, Kantrowitz JT, Javitt DC, Lieberman JA, Abi-Dargham A, Girgis RR. Glutamatergic abnormalities in schizophrenia: a review of proton MRS findings. Schizophr Res. 2014;152:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 23. | Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res. 2002;58:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1179] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 25. | Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 532] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 26. | Hu W, MacDonald ML, Elswick DE, Sweet RA. The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies. Ann N Y Acad Sci. 2015;1338:38-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 27. | Cohen LH, Thale T, Tissenbaum MJ. Acetylcholine treatment of schizophrenia. Arch Neurol Psychiatry. 1944;51:171-175. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 705] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 29. | Brunton LL, Chabner B, Knollmann BC. Goodman & gilman‘s the pharmacological basis of therapeutics. New York: McGraw-Hill 2011; 2084. |
| 30. | Mesulam MM. The cholinergic innervation of the human cerebral cortex. Prog Brain Res. 2004;145:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 31. | Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci. 2002;22:6347-6352. [PubMed] |
| 32. | Sur C, Mallorga PJ, Wittmann M, Jacobson MA, Pascarella D, Williams JB, Brandish PE, Pettibone DJ, Scolnick EM, Conn PJ. N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-D-aspartate receptor activity. Proc Natl Acad Sci USA. 2003;100:13674-13679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 236] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 33. | Koyama Y, Kayama Y. Mutual interactions among cholinergic, noradrenergic and serotonergic neurons studied by ionophoresis of these transmitters in rat brainstem nuclei. Neuroscience. 1993;55:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Scarr E, Gibbons AS, Neo J, Udawela M, Dean B. Cholinergic connectivity: it’s implications for psychiatric disorders. Front Cell Neurosci. 2013;7:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Sarter M, Lustig C, Taylor SF. Cholinergic contributions to the cognitive symptoms of schizophrenia and the viability of cholinergic treatments. Neuropharmacology. 2012;62:1544-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Sarter M, Nelson CL, Bruno JP. Cortical cholinergic transmission and cortical information processing in schizophrenia. Schizophr Bull. 2005;31:117-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Kuduk SD, Chang RK, Di Marco CN, Pitts DR, Greshock TJ, Ma L, Wittmann M, Seager MA, Koeplinger KA, Thompson CD. Discovery of a selective allosteric M1 receptor modulator with suitable development properties based on a quinolizidinone carboxylic acid scaffold. J Med Chem. 2011;54:4773-4780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Nathan PJ, Watson J, Lund J, Davies CH, Peters G, Dodds CM, Swirski B, Lawrence P, Bentley GD, O’Neill BV. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int J Neuropsychopharmacol. 2013;16:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dubé S, Mallinckrodt C, Bymaster FP, McKinzie DL, Felder CC. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165:1033-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 428] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 40. | Nielsen RE, Levander S, Kjaersdam Telléus G, Jensen SO, Østergaard Christensen T, Leucht S. Second-generation antipsychotic effect on cognition in patients with schizophrenia--a meta-analysis of randomized clinical trials. Acta Psychiatr Scand. 2015;131:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 41. | Graef S, Schönknecht P, Sabri O, Hegerl U. Cholinergic receptor subtypes and their role in cognition, emotion, and vigilance control: an overview of preclinical and clinical findings. Psychopharmacology (Berl). 2011;215:205-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 895] [Cited by in RCA: 910] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 43. | Dale H. Chemical transmission of the effects of nerve impulses. Br Med J. 1934;1:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Rang HP, Dale MM, Ritter JM, Flower RJ. Rang and dale’s pharmacology. 6th ed. Philadelphia: Churchill Livingstone Elsevier 2007; 397-399. |
| 45. | Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 497] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 46. | Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1524] [Cited by in RCA: 1524] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 47. | Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 319] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 48. | Flynn DD, Ferrari-DiLeo G, Mash DC, Levey AI. Differential regulation of molecular subtypes of muscarinic receptors in Alzheimer’s disease. J Neurochem. 1995;64:1888-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 177] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Leach K, Simms J, Sexton P, Christopoulos A. Structure-function studies of muscarinic acetylcholine receptors. In: Fryer AD, Christopoulos A and Nathanson NM Muscarinic receptors. Springer Berlin Heidelberg 2012; 29-48. |
| 50. | Kruse AC, Hu J, Pan AC, Arlow DH, Rosenbaum DM, Rosemond E, Green HF, Liu T, Chae PS, Dror RO. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature. 2012;482:552-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 621] [Cited by in RCA: 638] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 51. | Mrzljak L, Levey AI, Goldman-Rakic PS. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc Natl Acad Sci USA. 1993;90:5194-5198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 202] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 52. | Hamilton SE, Nathanson NM. The M1 receptor is required for muscarinic activation of mitogen-activated protein (MAP) kinase in murine cerebral cortical neurons. J Biol Chem. 2001;276:15850-15853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Berkeley JL, Gomeza J, Wess J, Hamilton SE, Nathanson NM, Levey AI. M1 muscarinic acetylcholine receptors activate extracellular signal-regulated kinase in CA1 pyramidal neurons in mouse hippocampal slices. Mol Cell Neurosci. 2001;18:512-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563-4572. [PubMed] |
| 55. | Kelleher RJ, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 665] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 56. | Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993;55:349-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1004] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 57. | Fisahn A, Yamada M, Duttaroy A, Gan JW, Deng CX, McBain CJ, Wess J. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron. 2002;33:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 58. | Zhang Y, Dyck RH, Hamilton SE, Nathanson NM, Yan J. Disrupted tonotopy of the auditory cortex in mice lacking M1 muscarinic acetylcholine receptor. Hear Res. 2005;201:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Zhang Y, Hamilton SE, Nathanson NM, Yan J. Decreased input-specific plasticity of the auditory cortex in mice lacking M1 muscarinic acetylcholine receptors. Cereb Cortex. 2006;16:1258-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 412] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 61. | Hamilton SE, Loose MD, Qi M, Levey AI, Hille B, McKnight GS, Idzerda RL, Nathanson NM. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc Natl Acad Sci USA. 1997;94:13311-13316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Schroeder BC, Kubisch C, Stein V, Jentsch TJ. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature. 1998;396:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 408] [Article Influence: 15.1] [Reference Citation Analysis (2)] |
| 63. | Gerber DJ, Sotnikova TD, Gainetdinov RR, Huang SY, Caron MG, Tonegawa S. Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc Natl Acad Sci USA. 2001;98:15312-15317. [PubMed] |
| 64. | Carrigan KA, Dykstra LA. Behavioral effects of morphine and cocaine in M1 muscarinic acetylcholine receptor-deficient mice. Psychopharmacology (Berl). 2007;191:985-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Raedler TJ, Knable MB, Jones DW, Urbina RA, Egan MF, Weinberger DR. Central muscarinic acetylcholine receptor availability in patients treated with clozapine. Neuropsychopharmacology. 2003;28:1531-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Ellis JR, Ellis KA, Bartholomeusz CF, Harrison BJ, Wesnes KA, Erskine FF, Vitetta L, Nathan PJ. Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol. 2006;9:175-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Green A, Ellis KA, Ellis J, Bartholomeusz CF, Ilic S, Croft RJ, Phan KL, Nathan PJ. Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacol Biochem Behav. 2005;81:575-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 68. | Mintzer MZ, Griffiths RR. Lorazepam and scopolamine: A single-dose comparison of effects on human memory and attentional processes. Exp Clin Psychopharmacol. 2003;11:56-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Ketchum JS, Sidell FR, Crowell EB, Aghajanian GK, Hayes AH. Atropine, scopolamine, and ditran: comparative pharmacology and antagonists in man. Psychopharmacologia. 1973;28:121-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 127] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Riemann D, Kammerer J, Löw H, Schmidt MH. Sleep in adolescents with primary major depression and schizophrenia: a pilot study. J Child Psychol Psychiatry. 1995;36:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Tandon R, Shipley JE, Taylor S, Greden JF, Eiser A, DeQuardo J, Goodson J. Electroencephalographic sleep abnormalities in schizophrenia. Relationship to positive/negative symptoms and prior neuroleptic treatment. Arch Gen Psychiatry. 1992;49:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 132] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Krieg JC, Berger M. REM sleep and cortisol response to the cholinergic challenge with RS 86 in normals and depressives. Acta Psychiatr Scand. 1987;76:600-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 73. | Hohagen F, Riemann D, Spiegel R, Holzhauer M, Berger M. Influence of the cholinergic agonist SDZ 210-086 on sleep in healthy subjects. Neuropsychopharmacology. 1993;9:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 74. | Riemann D, Hohagen F, Krieger S, Gann H, Müller WE, Olbrich R, Wark HJ, Bohus M, Löw H, Berger M. Cholinergic REM induction test: muscarinic supersensitivity underlies polysomnographic findings in both depression and schizophrenia. J Psychiatr Res. 1994;28:195-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2007;12:232-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 76. | Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E. Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2002;7:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 77. | Scarr E, Keriakous D, Crossland N, Dean B. No change in cortical muscarinic M2, M3 receptors or [35S]GTPgammaS binding in schizophrenia. Life Sci. 2006;78:1231-1237. [PubMed] |
| 78. | Scarr E, Cowie TF, Kanellakis S, Sundram S, Pantelis C, Dean B. Decreased cortical muscarinic receptors define a subgroup of subjects with schizophrenia. Mol Psychiatry. 2009;14:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 79. | Jablensky A. Subtyping schizophrenia: implications for genetic research. Mol Psychiatry. 2006;11:815-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 80. | Scarr E, Dean B. Muscarinic receptors: do they have a role in the pathology and treatment of schizophrenia? J Neurochem. 2008;107:1188-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Seo MS, Scarr E, Dean B. An investigation of the factors that regulate muscarinic receptor expression in schizophrenia. Schizophr Res. 2014;158:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Gibbons AS, Scarr E, Boer S, Money T, Jeon WJ, Felder C, Dean B. Widespread decreases in cortical muscarinic receptors in a subset of people with schizophrenia. Int J Neuropsychopharmacol. 2013;16:37-46. [PubMed] [DOI] [Full Text] |
| 83. | Raedler TJ, Knable MB, Jones DW, Urbina RA, Gorey JG, Lee KS, Egan MF, Coppola R, Weinberger DR. In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry. 2003;160:118-127. [PubMed] |
| 84. | Miller RJ, Hiley CR. Anti-muscarinic properties of neuroleptics and drug-induced Parkinsonism. Nature. 1974;248:596-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 273] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 85. | Gronier B, Perry KW, Rasmussen K. Activation of the mesocorticolimbic dopaminergic system by stimulation of muscarinic cholinergic receptors in the ventral tegmental area. Psychopharmacology (Berl). 2000;147:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Johnstone EC, Crow TJ, Ferrier IN, Frith CD, Owens DG, Bourne RC, Gamble SJ. Adverse effects of anticholinergic medication on positive schizophrenic symptoms. Psychol Med. 1983;13:513-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 87. | Tandon R, DeQuardo JR, Goodson J, Mann NA, Greden JF. Effect of anticholinergics on positive and negative symptoms in schizophrenia. Psychopharmacol Bull. 1992;28:297-302. [PubMed] |
| 88. | Brébion G, Bressan RA, Amador X, Malaspina D, Gorman JM. Medications and verbal memory impairment in schizophrenia: the role of anticholinergic drugs. Psychol Med. 2004;34:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Bodick NC, Offen W. S-16-4 xanomeline: The first study of the safety and efficacy of an m1 agonist in mild and moderate alzheimer’s disease. Eur Neuropsychopharm. 1995;5:205. [DOI] [Full Text] |
| 90. | Cutler NR, Sramek JJ, Seifert RD, Conrad JJ, Wardle TS, Hurley DJ, Thoren LM. A bridging study of xanomeline tartrate in alzheimers disease (AD). Biol Psychiat. 1994;35:628-628. [DOI] [Full Text] |
| 91. | Christopoulos A, Changeux JP, Catterall WA, Fabbro D, Burris TP, Cidlowski JA, Olsen RW, Peters JA, Neubig RR, Pin JP. International Union of Basic and Clinical Pharmacology. XC. multisite pharmacology: recommendations for the nomenclature of receptor allosterism and allosteric ligands. Pharmacol Rev. 2014;66:918-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 92. | Hulme EC, Lu ZL, Bee MS. Scanning mutagenesis studies of the M1 muscarinic acetylcholine receptor. Receptors Channels. 2003;9:215-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 93. | Haga K, Kruse AC, Asada H, Yurugi-Kobayashi T, Shiroishi M, Zhang C, Weis WI, Okada T, Kobilka BK, Haga T. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature. 2012;482:547-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 625] [Cited by in RCA: 634] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 94. | Matsui H, Lazareno S, Birdsall NJ. Probing of the location of the allosteric site on m1 muscarinic receptors by site-directed mutagenesis. Mol Pharmacol. 1995;47:88-98. [PubMed] |
| 95. | Abdul-Ridha A, Lane JR, Mistry SN, López L, Sexton PM, Scammells PJ, Christopoulos A, Canals M. Mechanistic insights into allosteric structure-function relationships at the M1 muscarinic acetylcholine receptor. J Biol Chem. 2014;289:33701-33711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 96. | Abdul-Ridha A, López L, Keov P, Thal DM, Mistry SN, Sexton PM, Lane JR, Canals M, Christopoulos A. Molecular determinants of allosteric modulation at the M1 muscarinic acetylcholine receptor. J Biol Chem. 2014;289:6067-6079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 97. | Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8:41-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 902] [Cited by in RCA: 866] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 98. | Dalet FG, Guadalupe TF, María Del Carmen CH, Humberto GA, Antonio SU. Insights into the structural biology of G-protein coupled receptors impacts drug design for central nervous system neurodegenerative processes. Neural Regen Res. 2013;8:2290-2302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 99. | Lazareno S, Gharagozloo P, Kuonen D, Popham A, Birdsall NJ. Subtype-selective positive cooperative interactions between brucine analogues and acetylcholine at muscarinic receptors: radioligand binding studies. Mol Pharmacol. 1998;53:573-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 83] [Reference Citation Analysis (0)] |
| 100. | Jakubík J, Bacáková L, Lisá V, el-Fakahany EE, Tucek S. Activation of muscarinic acetylcholine receptors via their allosteric binding sites. Proc Natl Acad Sci USA. 1996;93:8705-8709. [PubMed] |
| 101. | Spalding TA, Trotter C, Skjaerbaek N, Messier TL, Currier EA, Burstein ES, Li D, Hacksell U, Brann MR. Discovery of an ectopic activation site on the M(1) muscarinic receptor. Mol Pharmacol. 2002;61:1297-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 102. | Langmead CJ, Austin NE, Branch CL, Brown JT, Buchanan KA, Davies CH, Forbes IT, Fry VA, Hagan JJ, Herdon HJ. Characterization of a CNS penetrant, selective M1 muscarinic receptor agonist, 77-LH-28-1. Br J Pharmacol. 2008;154:1104-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 103. | Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, Tantawy MN, Kane AS, Bridges TM, Kennedy JP, Bradley SR. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci. 2008;28:10422-10433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 104. | Sams AG, Hentzer M, Mikkelsen GK, Larsen K, Bundgaard C, Plath N, Christoffersen CT, Bang-Andersen B. Discovery of N-{1-[3-(3-oxo-2,3-dihydrobenzo[1,4]oxazin-4-yl)propyl]piperidin-4-yl}-2-phenylacetamide (Lu AE51090): an allosteric muscarinic M1 receptor agonist with unprecedented selectivity and procognitive potential. J Med Chem. 2010;53:6386-6397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 105. | Ridler K, Cunningham V, Huiban M, Martarello L, Pampols-Maso S, Passchier J, Gunn RN, Searle G, Abi-Dargham A, Slifstein M. An evaluation of the brain distribution of [(11)C]GSK1034702, a muscarinic-1 (M 1) positive allosteric modulator in the living human brain using positron emission tomography. EJNMMI Res. 2014;4:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 106. | Marlo JE, Niswender CM, Days EL, Bridges TM, Xiang Y, Rodriguez AL, Shirey JK, Brady AE, Nalywajko T, Luo Q. Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol Pharmacol. 2009;75:577-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 107. | Ma L, Seager MA, Wittmann M, Jacobson M, Bickel D, Burno M, Jones K, Graufelds VK, Xu G, Pearson M. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci USA. 2009;106:15950-15955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 108. | Shirey JK, Brady AE, Jones PJ, Davis AA, Bridges TM, Kennedy JP, Jadhav SB, Menon UN, Xiang Z, Watson ML. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci. 2009;29:14271-14286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 109. | Dean B, Hopper S, Conn PJ, Scarr E. Changes in BQCA Allosteric Modulation of [(3)H]NMS Binding to Human Cortex within Schizophrenia and by Divalent Cations. Neuropsychopharmacology. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Kuduk SD, Chang RK, Greshock TJ, Ray WJ, Ma L, Wittmann M, Seager MA, Koeplinger KA, Thompson CD, Hartman GD. Identification of amides as carboxylic Acid surrogates for quinolizidinone-based m1 positive allosteric modulators. ACS Med Chem Lett. 2012;3:1070-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Uslaner JM, Eddins D, Puri V, Cannon CE, Sutcliffe J, Chew CS, Pearson M, Vivian JA, Chang RK, Ray WJ. The muscarinic M1 receptor positive allosteric modulator PQCA improves cognitive measures in rat, cynomolgus macaque, and rhesus macaque. Psychopharmacology (Berl). 2013;225:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 112. | Felten DL, Shetty A. Netter’s atlas of neuroscience. 2 ed. Elsevier. In: Fryer AD, Christopoulos A and Nathanson NM Muscarinic receptors. Springer Berlin Heidelberg 2009; 438. |
| 113. | Omasits U, Ahrens CH, Müller S, Wollscheid B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30:884-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 974] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 114. | Dean B, Crook JM, Opeskin K, Hill C, Keks N, Copolov DL. The density of muscarinic M1 receptors is decreased in the caudate-putamen of subjects with schizophrenia. Mol Psychiatry. 1996;1:54-58. [PubMed] |
| 115. | Crook JM, Dean B, Pavey G, Copolov D. The binding of [3H]AF-DX 384 is reduced in the caudate-putamen of subjects with schizophrenia. Life Sci. 1999;64:1761-1771. [PubMed] |
| 116. | Dean B, Crook JM, Pavey G, Opeskin K, Copolov DL. Muscarinic1 and 2 receptor mRNA in the human caudate-putamen: no change in m1 mRNA in schizophrenia. Mol Psychiatry. 2000;5:203-207. [PubMed] |
| 117. | Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Low muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: a study of Brodmann’s areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. Am J Psychiatry. 2001;158:918-925. [PubMed] |
| 118. | Mancama D, Arranz MJ, Landau S, Kerwin R. Reduced expression of the muscarinic 1 receptor cortical subtype in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2003;119B:2-6. [PubMed] |
| 119. | Zavitsanou K, Katsifis A, Mattner F, Huang XF. Investigation of m1/m4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacology. 2004;29:619-625. [PubMed] |
| 120. | Zavitsanou K, Katsifis A, Yu Y, Huang XF. M2/M4 muscarinic receptor binding in the anterior cingulate cortex in schizophrenia and mood disorders. Brain Res Bull. 2005;65:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Deng C, Huang XF. Decreased density of muscarinic receptors in the superior temporal gyrusin schizophrenia. J Neurosci Res. 2005;81:883-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Scarr E, Sundrarn S, Keriakous D, Dean B. Altered hippocampal muscarinic m4, but not m1, receptor expression from subjects with schizophrenia. Biol Psychiat. 2007;61:1161-1170. [RCA] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 123. | Dean B, Soulby A, Evin GM, Scarr E. Levels of [(3)H]pirenzepine binding in Brodmann’s area 6 from subjects with schizophrenia is not associated with changes in the transcription factor SP1 or BACE1. Schizophr Res. 2008;106:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |