Published online Dec 9, 2015. doi: 10.5497/wjp.v4.i4.265
Peer-review started: June 1, 2015
First decision: August 4, 2015
Revised: September 10, 2015
Accepted: October 16, 2015
Article in press: October 19, 2015
Published online: December 9, 2015
Processing time: 193 Days and 12 Hours
Lysophosphatidic acid (LPA) is a pleiotropic lipid mediator that promotes motility, survival, and the synthesis of chemokines/cytokines in human fibroblast-like synoviocytes (FLS) from patients with rheumatoid arthritis. LPA activates several proteins within the mitogen activated protein (MAP) kinase signaling network, including extracellular signal-regulated kinases (ERK) 1/2 and p38 MAP kinase (MAPK). Upon docking to mitogen- and stress-activated kinases (MSKs), ERK1/2 and p38 MAPK phosphorylate serine and threonine residues within its C-terminal domain and cause autophosphorylation of MSKs. Activated MSKs can then directly phosphorylate cAMP response element-binding protein (CREB) at Ser133 in FLS. Phosphorylation of CREB by MSKs is essential for the production of pro-inflammatory and anti-inflammatory cytokines. However, other downstream effectors of MSK1/2 such as nuclear factor-kappa B, histone H3, and high mobility group nucleosome binding domain 1 may also regulate gene expression in immune cells involved in disease pathogenesis. MSKs are master regulators of cell function that integrate signals induced by growth factors, pro-inflammatory cytokines, and cellular stresses, as well as those induced by LPA.
Core tip: Extracellular signal-regulated kinases 1/2 and p38 mitogen activated protein kinase cascades are activated in response to stimulation with inflammatory stimuli, including lysophosphatidic acid, and are able to activate mitogen- and stress-activated kinase (MSK) 1 and MSK2 in human synovial fibroblasts. MSKs then phosphorylate the transcription factor cAMP response element-binding protein (CREB), leading to the production of pro-inflammatory and anti-inflammatory cytokines. In addition to CREB, many other downstream effectors of MSK1/2 such as nuclear factor-kappa B, histone H3, the E3 ubiquitin ligase, Tripartite motif containing 7 and high mobility group nucleosome binding domain 1 have been reported and suggested to play important functions in immunity and disease states, including arthritis.
- Citation: Bourgoin SG, Hui W. Role of mitogen- and stress-activated kinases in inflammatory arthritis. World J Pharmacol 2015; 4(4): 265-273
- URL: https://www.wjgnet.com/2220-3192/full/v4/i4/265.htm
- DOI: https://dx.doi.org/10.5497/wjp.v4.i4.265
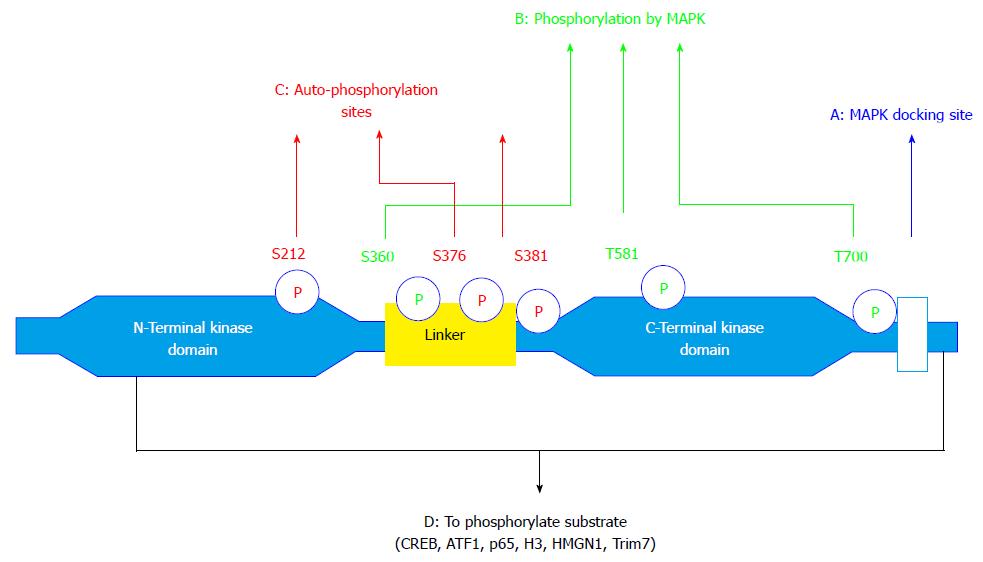
Mitogen- and stress-activated protein kinases (MSKs) were first identified as efficient cAMP response element-binding protein (CREB) kinases in 1998[1]. For the past 17 years, MSKs have been investigated thoroughly as regulators of gene expression at multiple levels[2]. The known function of MSKs is mainly phosphorylation of transcription factors, chromatin-associated proteins and ubiquitin ligase. MSKs are activated in response to mitogenic signals [e.g., serum, epidermal growth factor and fibroblast growth factor, lysophosphatidic acid (LPA)], neurotransmitters, progesterone, cellular stresses (e.g., UV-irradiation, oxidative stress, arsenite, metals and retinoic acid), and other signals from pro-inflammatory cytokines (e.g., TNF-α), as well as PAMP[3]. Through binding to G protein-coupled receptors, cytokine/chemokine and growth factor receptors, or activation of stress sensors, all these stimuli activate various mitogen activated protein kinase (MAPK) signaling pathways (p38 MAPK and ERK). Activation of ERK1/2 and p38 MAPK directly or indirectly through the phosphorylation of MSKs regulates the function of transcription factors and nuclear proteins involved in gene transcription (Figure 1). The substrates of MSKs including, CREB, ATF-1, NF-κB p65, Histone H3, and high mobility group nucleosome binding domain 1 (HMGN1), have been extensively studied and validated in cells silenced for the expression of MSK1 and/or MSK2[2]. Another protein, the E3 ubiquitin ligase named Tripartite motif containing 7 was recently reported to be a target of MSK1 using cell silencing approaches, thereby highlighting the crosstalk between different post-translational protein modifications[4]. The roles of MSKs downstream of ERK1/2 and p38 MAPK and their important functions in immunity and disease states, including arthritis, have been highlighted in the book edited by Arthur[3].
Rheumatoid arthritis (RA) is a severe, chronic and systemic inflammatory disease. Infiltration of multiple blood-derived cells (macrophages, dendritic cells, T cells, B cells, neutrophils, platelets[5]) into inflamed joints, high levels of cytokines/chemokines, production of lipid mediators and matrix metalloproteinases (MMPs) in synovial joints, synovial cell proliferation leading to synovium thickening, and pannus formation are the hallmarks of RA[6]. All these features eventually lead to cartilage dysfunction, damage to adjacent tissues, and deformation of joints with associated chronic pain. At the present time, medical therapy for RA uses conventional disease-modifying anti-rheumatic drugs such as corticosteroids and methotrexate, anti-malarials, and TNF inhibitors alone or in combination with methotrexate. New strategies targeting other cytokines, like IL-1[7,8] and IL-6[9,10], or B cell marker CD20[11] are also approved for RA treatment. Inhibitors of specific protein kinase pathways also hold potential in the treatment of chronic autoimmune diseases. More recently the JAK1/3 inhibitor tofacitinib, which suppresses inflammation driven by immune cells through inhibition of JAK/STAT, was approved by the Food and Drug Administration for the treatment of RA[12]. Although anti-cytokine therapies provide significant benefits for RA patients, there is still a substantial subset of nonresponsive patients as well as patients who cannot tolerate the current therapy[13]. Presently, researchers focus more on the cellular pathways of inflammation to search for new therapeutic targets for the treatment of autoimmune diseases such as RA[14]. MAPKs are potential targets to treat RA because of their important role in regulating cell proliferation, apoptosis, cytokine and MMP expression[15]. The functions of MSKs as important nuclear signaling kinases phosphorylated by MAPKs and regulators of inflammatory gene transcription were investigated extensively during the past decade. More extensive reviews are found elsewhere[16,17]. In this article, we will summarize current knowledge on MSK signaling in inflammatory arthritis and describe its potential roles in amplification and perpetuation of inflammation.
The kinase domains composing MSK1/2 include a C-terminal and N-terminal kinase domain, harboring several phosphorylation sites[16,17] (Figure 1). Human MSK1 can be activated by MAPK at Ser360, Thr581 and Thr700 located in the C-terminal domain[18]. Phosphorylation of the C-terminal domain induces a conformational change in MSK1, which permits autophosphorylation on Ser212, Ser376 and Ser381 by the C-terminal kinase domain and phosphorylation of MSK substrates by the N-terminal kinase domain[2,18]. Compared with ribosomal S6 kinases (RSKs), the main difference is that RSK is activated by ERK whereas MSK can be activated by both ERK and p38 MAPK through a closely related mechanism that reflects the common domain structure of MSKs and RSKs[19]. The molecular docking interaction between p38 MAPK or ERK1/2 and MSKs has been clearly highlighted previously[19,20] and will not be further discussed in this review.
The key evidence for the aberrant pathway of T cell activation in the initiation and perpetuation of RA is the association between disease pathogenesis and HLA-DRB1[21,22]. Th1 cells expressing IFNγ and TNF-α are present in RA synovial tissues[21,23]. Data from animal models of arthritis suggest that IL-17-producing CD4+ T cells (Th17 cells), also contribute to the inflammatory processes[24-26]. The p38α MAPK-MSK1/2 axis was reported to induce IL-17 synthesis by CD4+ T cells in experimental models of autoimmune diseases[27]. The absence of the msk1/2 gene resulted in the failure to produce IL-17 by murine lymphocytes isolated from lymph node and blood[27]. The potential role of MSK1/2 in the regulation of gene transcription downstream of p38α MAPK signaling in T cells is illustrated by the LAT (linker for activation of T cells) signalosome that propagates signals through branching of several signaling pathways including that of MAPK[28]. In T cells, MSK is the major kinase responsible for CREB phosphorylation in response to TCR activation, and MSK1/2 knockout mice showed reduced T cell proliferation in the presence of IL-2[29]. In this study, the authors pointed out that MSK1/2 are highly expressed in the thymus and the spleen, and that spleens from MSK1/2 knockout mice contain fewer T cells. Data for tissue-specific patterns of mRNA expression available at the Scripps Research Institute BioGPS Website and Database indicate that MSK1 (gene symbol: RPS6KA5) but not MSK2 (gene symbol: RPS6KA4) is highly expressed in CD19+ B cells, CD4+ T cells, CD8+ T cells, and CD56+ NK cells compared to other tissues and cell types. Hence, it is possible that MSK1 and MSK2 have different functions (i.e., substrate specificity) in those cells.
CD20+ B cells are enriched in the RA synovium and their functions mainly include autoantibody production, T cell interaction and cytokine production[30]. MSK1/2 deficiency has no significant effect on T cells or B cell development[29]. At present, we do not know what impact MSK1 and/or MSK2 deficiency has on T/B cell interaction, cytokine/chemokine production by B cells, and mature B cell proliferation. Mn2+ induced apoptosis of human lymphoma B cells through the activation of caspase-8[31]. This study using specific pharmacological inhibitors and dominant-negative mutants of p38α MAPK and MSK1 showed the p38α MAPK-MSK1 signaling pathway, but not Fas-associated death domain protein, drives B cell apoptosis. Nevertheless, the mechanism of how the p38α MAPK-MSK1 axis regulates B cell apoptosis is not clear given that caspase-8 does not associate with MSK1 and is not a substrate of MSK1[31]. Another study showed that TGFβ mediated apoptosis in human Burkitt lymphoma B cells through caspase-8 activation downstream of p38α MAPK, but the possible contribution of MSKs to this effect is not yet known[32].
Neutrophils constitute 90% of the cells in RA synovial fluids[33]. The crucial roles of neutrophils in inflammation, inflammatory diseases, and systemic autoimmune diseases have been thoroughly reviewed[34-38]. The main functions of neutrophils include phagocytosis, degranulation, production of antimicrobial peptides and proteins, production of reactive oxygen species, and NETosis (release of neutrophil extracellular traps)[35,39]. Khandpur et al[40] showed enhanced NETosis of circulating and synovial fluid neutrophils from RA patients, compared to those from osteoarthritis patients or healthy individuals. In neutrophils, a role for p38 MAPK has also been reported in chemotaxis[41], regulation of apoptosis[42], as well as cytokine/chemokine and MMP production[43,44]. The p38 MAPK-MSK1 axis contributes to chemokine production through CREB activation in LPS-stimulated human neutrophils[44]. In this study CREB was presumably phosphorylated by MSK1, but the data require further validation since the authors used Ro-31-8220, a non-selective inhibitor of MSK1[45] (Table 1). There is another report showing that neutrophil stimulation with sphingosine-1-phosphate (S1P) induces p38 MAPK and ERK-dependent phosphorylation of MSK1 to control the secretion of IL-8[46].
| MSK inhibitors | IC50 | Mechanism of action | Ref. |
| SB747651A | 0.5 nmol/L | Selectively targets MSK1/2 | [45,100,101] |
| Inhibits the N-terminal kinase domain of MSKs | |||
| > 300-fold selectivity over RSK1 and > 3000-fold selectivity over GSK3 | |||
| Ro-31–8220 | 8 nmol/L | Inhibitor of PKC, MSK1, RSK, S6K1, GSK3 | [85,102] |
| H89 | 120 nmol/L | Inhibitor of MSK1, S6K1 and ROCK-II, PKA | [85] |
Activation of the ERK and p38 MAPK pathways has been reported in human neutrophils stimulated with chemoattractants, pro-inflammatory cytokines, and Fcγ receptor ligands[47], and their activation is also required for the respiratory burst in TNF-α and GM-CSF primed cells[48]. As MSK1 is phosphorylated by ERK1/2 and p38 MAPK in neutrophils under certain conditions, we cannot deny a role for MSKs in the signaling pathway leading to a coordinated pattern of cytokine/chemokine gene expression induced by various stimuli.
Fibroblast-like synoviocytes (FLS) play a substantial role in many pathologic events in inflammatory arthritis. As a key component of the hyperplastic rheumatoid pannus, combined with their invasive phenotype, FLS have a major role in the initiation and perpetuation of destructive joint inflammation[49]. As passive responders, FLS in RA secrete cytokines/chemokines, lipid mediators of inflammation, a subset of extracellular matrix remodeling enzymes, and express adhesion molecules. Somatic mutations and epigenetic alterations associated with signaling anomalies may also contribute to invasive behavior, resistance to apoptosis, and production of inflammatory cytokines (reviewed in[49]). We demonstrated that Ser376 on MSK1 and possibly Ser360 on MSK2 were transiently phosphorylated in RAFLS shortly after stimulation with LPA[50] and TNF-α as well (unpublished data). A specific inhibitor of MSKs (SB747651A) or silencing of MSK1 and/or MSK2 with siRNAs significantly reduced LPA-induced chemokine secretion (IL-8 and MCP-1) and CREB phosphorylation at Ser133[50]. FLS priming with TNF-α for 8 h prior to LPA stimulation consistently increases the phosphorylation of MSK1/2 at Ser376/Ser360 (unpublished data), as well as IL-8, IL-6, and MCP-1 secretion[50,51]. These data suggest an important role for MSKs in LPA signaling which leads to inflammatory cytokine/chemokine secretion by FLS in RA. A possible explanation for transient MSK1/2 phosphorylation could be the activity of a protein phosphatase such as protein phosphatase 2Cδ, which has been reported to be phosphorylated by ERK and to associate with MSKs[52], or dephosphorylation of ERK1/2 and p38α MAPK by dual-specific phosphatase 1 (DUSP1)[53]. Further work is needed to pinpoint the phosphatases that regulate the p38α MAPK-MSK1/2 signaling axis in FLS.
To date little is known about the mechanism of how TNF-α drastically enhances the secretion of chemokines in response to bioactive lipids such as LPA and S1P. Early studies showed correlation between chemokine synthesis and increased expression of a subset of LPA and S1P receptors by cultured FLS or the lining tissue of mouse air pouch when stimulated with TNF-α[54]. Both S1P and LPA promote chemokine secretion, and p38 MAPK, ERK1/2 and Rho kinase activation in FLS[55]. Hence, increased expression of certain LPA receptors (LPA1 and LPA3) and of S1P receptors by cells exposed to an inflammatory environment may contribute, at least in part, to enhanced intracellular signals that lead to further activation of MSK and CREB.
By producing various pro-inflammatory cytokines/chemokines macrophages play a critical role in cartilage and bone destruction in inflammatory arthritis[56,57]. There is an imbalance between inflammatory and anti-inflammatory macrophages in the RA synovium[58], and more information on how macrophages contribute to RA disease activity at both the local and the systemic levels can be obtained by reference to other reviews[59-61]. LPS-mediated activation of MSK1 and MSK2 was associated with COX-2 expression and IL-1β secretion in macrophages[62]. MSK activation is not restricted to TLR4 signaling, as Pam3CSK4 (TLR1/2 agonist), lipoteichoic acid (TLR2 agonist), CpG-DNA (TLR9 agonist) and dectin-1 agonist stimulation all phosphorylated MSK1 at Thr581[63]. Inhibition of early expression of COX2 in MSK1/2 knockout macrophages was confirmed by another study, but induction of COX-2 protein and prostaglandin secretion was detected at later time points due to reduced LPS-mediated production of IL-10 and increased COX-2 mRNA stability in the absence of IL-10[64]. By regulating the CREB/ATF-1 dependent transcription of DUSP1 and of IL-10, MSK1 and MSK2 are also part of a negative feedback loop that limits TLR4-driven inflammation. Hence, the absence of this feedback loop may explain why LPS-mediated expression of TNF-α, IL-6, IL-12, and late expression of COX-2 are increased in MSK1/2 deficient macrophages[63]. In line with those studies, it was reported that stimulation of MSK1/2 knockout macrophages with zymozan particles reduced the secretion of IL-10 and increased that of IL-12[65]. So far there is no information on the role of MSK1/2 in mouse models of arthritis but it would be interesting to evaluate the impact of MSK knockdown on disease onset, severity and duration as TNF-α, IL-1β, IL-6 and chemokines are produced by various cell types including macrophages and other immune cells[66]. MSKs may limit inflammation since mice that lack MSK1 and MSK2 produce less IL-10 and IL-1 receptor antagonist, which provide crucial negative-feedback loops in response to LPS[64,67]. A study investigating genetic variations in the p38 MAPK signaling network in RA patients identified SNPs in MSK1 and MSK2 that were associated with anti-TNF treatment response[68]. Further analysis suggests that the MSK2 genetic variant has a recessive effect whereas other SNPs in proteins of the p38 MAPK signaling pathway have a dominant effect on the change in DAS28[68]. However, the impact of these SNPs on MSK protein expression and activation has not been investigated.
MSKs play a versatile role through the phosphorylation of transcription factors and nuclear proteins that up-regulate the expression of pro-inflammatory and anti-inflammatory genes including chemokines/cytokines and signaling proteins[16,17]. TNF-α, IL-6, IL-2, and IL-10 genes share in common a CRE element in the core promoter region that is required for CREB binding and gene transcription[69,70]. However, phosphorylation of CREB on Ser133 by MSKs has a greater effect on the induction of CREB-dependent immediate-early genes than that induced by protein kinase A (PKA), possibly due to differential recruitment of CREB co-activator proteins[71]. Activation of ERK1/2 also leads to histone phosphorylation and Sp1 transcription factor binding to the IL-10 promoter[72]. As validated substrates of MSKs, histone H3 and HMGN1 may also contribute to immediate-early gene expression through various mechanisms[2].
The expression of IL-8 (CXCL8) is controlled by three different mechanisms: derepression of the gene promoter; transcriptional activation of the gene by NF-κB and JUN-N-terminal protein kinase pathways; and stabilization of the mRNA by the p38 MAPK pathway[73]. In FLS from RA patients the production of IL-8 is upregulated by approximately 100-fold in response to TNF-α or IL-1β[5]. Inhibitors of p38 MAPK inhibit the functional responses to these cytokines including the production of IL-8[74]. This study suggests positive feedback loop mechanisms that lead to activation of the p38 MAPK pathway and long term IL-8 secretion, which recruit neutrophils to the inflammatory sites. We demonstrated that LPA-induced production of IL-8 is inhibited by inhibitors of p38 MAPK and MSK, as well as silencing of MSK1/2 and CREB in FLS[50,51]. The mechanism by which MSK regulates the transcription of CREB-dependent genes such as IL-8 and MCP-1 as well is not clear[71]. In RAFLS, NF-κB p65 subunit, but not C/EBP-β or AP-1, dominantly regulates IL-8 gene expression under IL-1β stimulation[75]. Whether phosphorylated CREB needs to recruit co-activation proteins (CBP/p300)[76] or synergizes with other transcription factors such as NF-κB p65 subunit[75,77], C/EBP-β[78,79], or AP-1[80-82] to regulate IL-8 expression in FLS requires further investigation.
Two MSK inhibitors have been used to study various MSK functions, including the secretion of cytokines[1,83-90]. However, these inhibitors are not selective for MSKs and inhibit many other kinases, including PKA[45]. This is a major limitation since these compounds show better selectivity for PKA which targets CREB, ATF1, RARα and nuclear factor-kappa B (NF-κB) p65 subunit shown to be phosphorylated by MSKs[2,17,91]. The selectivity of new MSK inhibitor SB-747651A was evaluated in vitro and shown to have superior selectivity for MSKs than that of H89 and Ro31-8220[45]. In this study, the authors showed that Ro31-8220 reduced LPS-induced TNF-α secretion that cannot be attributed to MSK inhibition. In contrast, inhibition of MSK in LPS-stimulated macrophages reduced IL-10 secretion and enhanced that of IL-12 as previously reported in MSK1/2 knockout cells[63,64]. In oral squamous cell carcinoma, SB-747651A was found to inhibit the phosphorylation of NF-κB p65 subunit[92]. We demonstrated that SB747651A inhibits LPA-mediated chemokine synthesis through inhibition of CREB phosphorylation[50]. SB-747651A inhibits CREB phosphorylation without affecting MSK1 phosphorylation at Thr581 (a critical site for MSK1 activation)[64]. Autophosphorylation of Ser212 and Ser376 in MSK1 is poorly affected by SB-747651 thereby suggesting that the inhibitor targets the N-terminal kinase domain of MSKs[45]. Although SB747651A showed improved selectivity, off target effects cannot be totally excluded since other kinases like RSK1, p70RSK and Rho-associated protein kinase 2 (ROCK-II) are inhibited by the compound[45].
Inhibition of p38α MAPK showed efficacy in animal models of arthritis but failed in clinical trials[93-96]. Inhibition of p38 MAPK initiates an imbalance between anti-inflammatory and pro-inflammatory processes which excludes this kinase from drug targeting in autoimmune diseases[97]. This is illustrated by the fact that MSKs, the downstream targets of p38 MAPK, differentially regulate the synthesis of two important anti-inflammatory cytokines, IL-10 and IL-12, as a negative feedback loop in inflammation[63]. From the experience of cancer therapy, monotherapy using signaling inhibitors such as MEK-ERK is not permanently effective, as cells may become resistant to the inhibitor by different mechanism[98]. As the aggressive characteristics of RA synoviocytes were viewed as reminiscent of neoplastic tissue[99], and MAPK signaling plays an important role in cell proliferation and cytokine production, it will be necessary to study well the signaling pathways downstream of MSKs to better understand their dual roles in inflammation.
Several inflammatory cytokines and lipid mediators of inflammation activate MSK1/2 signaling downstream of p38 MAPK and ERK1/2. Inhibitors of MSK1/2 have effects on different cell types involved in the pathogenesis of RA, which could provide an important advantage in treatment. Inhibitors of MSK inhibit the production of cytokines/chemokines as well as the responses induced by these pro-inflammatory mediators thereby limiting the activation and/or the recruitment of immune cells to sites of inflammation. However, as reported for other inhibitors of the MAPK pathway, targeting MSK in arthritis may have undesirable effects due to inhibition of other kinase pathways or regulation of complex positive and negative feedback loops that could induce imbalance in the production of pro-inflammatory and anti-inflammatory mediators. Screening for more selective inhibitors or developing isoform-specific inhibitors of MSK1 and MSK2 is required to establish applicability as a drug in the future. Furthermore, more research will be necessary to identify targets downstream of MSK1/2. Understanding how CREB interplays with other transcription factors such as NF-κB or other MSK-dependent pathways regulating protein stability through ubiquitinylation may enable the development of drugs that have less adverse effects for treatment of chronic inflammatory diseases.
Weili Hui is the recipient of a scholarship from the Chinese Scholarship Council. We thank Lynn Davis for her critical reading and language editing of the manuscript.
P- Reviewer: Boggaram V, Lee WH S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426-4441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 822] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 2. | Vermeulen L, Vanden Berghe W, Beck IM, De Bosscher K, Haegeman G. The versatile role of MSKs in transcriptional regulation. Trends Biochem Sci. 2009;34:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Chakraborty A, Diefenbacher ME, Mylona A, Kassel O, Behrens A. The E3 ubiquitin ligase Trim7 mediates c-Jun/AP-1 activation by Ras signalling. Nat Commun. 2015;6:6782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O’Donnell E, Farndale RW, Ware J. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 838] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 7. | Geyer M, Müller-Ladner U. Actual status of antiinterleukin-1 therapies in rheumatic diseases. Curr Opin Rheumatol. 2010;22:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Mertens M, Singh JA. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol. 2009;36:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 9. | Navarro-Millán I, Singh JA, Curtis JR. Systematic review of tocilizumab for rheumatoid arthritis: a new biologic agent targeting the interleukin-6 receptor. Clin Ther. 2012;34:788-802.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Dougados M, Kissel K, Sheeran T, Tak PP, Conaghan PG, Mola EM, Schett G, Amital H, Navarro-Sarabia F, Hou A. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis. 2013;72:43-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 11. | Jacobi AM, Dörner T. Current aspects of anti-CD20 therapy in rheumatoid arthritis. Curr Opin Pharmacol. 2010;10:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Zerbini CA, Lomonte AB. Tofacitinib for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2012;8:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Ohori M. ERK inhibitors as a potential new therapy for rheumatoid arthritis. Drug News Perspect. 2008;21:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | MacFarlane LA, Todd DJ. Kinase inhibitors: the next generation of therapies in the treatment of rheumatoid arthritis. Int J Rheum Dis. 2014;17:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Hammaker D, Sweeney S, Firestein GS. Signal transduction networks in rheumatoid arthritis. Ann Rheum Dis. 2003;62 Suppl 2:ii86-ii89. [PubMed] |
| 16. | Moens U, Kostenko S, Sveinbjørnsson B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation. Genes (Basel). 2013;4:101-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 17. | Arthur JS. MSK activation and physiological roles. Front Biosci. 2008;13:5866-5879. [PubMed] |
| 18. | McCoy CE, macdonald A, Morrice NA, Campbell DG, Deak M, Toth R, McIlrath J, Arthur JS. Identification of novel phosphorylation sites in MSK1 by precursor ion scanning MS. Biochem J. 2007;402:491-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Hauge C, Frödin M. RSK and MSK in MAP kinase signalling. J Cell Sci. 2006;119:3021-3023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1704] [Cited by in RCA: 1851] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 21. | Cope AP, Schulze-Koops H, Aringer M. The central role of T cells in rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:S4-11. [PubMed] |
| 22. | Cope AP. T cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10 Suppl 1:S1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Rönnelid J, Berg L, Rogberg S, Nilsson A, Albertsson K, Klareskog L. Production of T-cell cytokines at the single-cell level in patients with inflammatory arthritides: enhanced activity in synovial fluid compared to blood. Br J Rheumatol. 1998;37:7-14. [PubMed] |
| 24. | Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther. 2005;7:29-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 296] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 25. | Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173-6177. [PubMed] |
| 26. | Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986-5990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 390] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 27. | Commodaro AG, Bombardieri CR, Peron JP, Saito KC, Guedes PM, Hamassaki DE, Belfort RN, Rizzo LV, Belfort R, de Camargo MM. p38{alpha} MAP kinase controls IL-17 synthesis in vogt-koyanagi-harada syndrome and experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2010;51:3567-3574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded. Nat Rev Immunol. 2013;13:257-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 345] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 29. | Kaiser M, Wiggin GR, Lightfoot K, Arthur JS, Macdonald A. MSK regulate TCR-induced CREB phosphorylation but not immediate early gene transcription. Eur J Immunol. 2007;37:2583-2595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Bugatti S, Codullo V, Caporali R, Montecucco C. B cells in rheumatoid arthritis. Autoimmun Rev. 2007;6:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | El Mchichi B, Hadji A, Vazquez A, Leca G. p38 MAPK and MSK1 mediate caspase-8 activation in manganese-induced mitochondria-dependent cell death. Cell Death Differ. 2007;14:1826-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Schrantz N, Bourgeade MF, Mouhamad S, Leca G, Sharma S, Vazquez A. p38-mediated regulation of an Fas-associated death domain protein-independent pathway leading to caspase-8 activation during TGFbeta-induced apoptosis in human Burkitt lymphoma B cells BL41. Mol Biol Cell. 2001;12:3139-3151. [PubMed] |
| 33. | Weissmann G, Korchak H. Rheumatoid arthritis. The role of neutrophil activation. Inflammation. 1984;8 Suppl:S3-14. [PubMed] |
| 34. | Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford). 2010;49:1618-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 538] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 35. | Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 411] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 36. | Kaplan MJ. Role of neutrophils in systemic autoimmune diseases. Arthritis Res Ther. 2013;15:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 37. | Cascão R, Rosário HS, Souto-Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: More than simple final effectors. Autoimmun Rev. 2010;9:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 38. | Pillinger MH, Abramson SB. The neutrophil in rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21:691-714. [PubMed] |
| 39. | Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1181] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 40. | Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 975] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 41. | Kim D, Haynes CL. The role of p38 MAPK in neutrophil functions: single cell chemotaxis and surface marker expression. Analyst. 2013;138:6826-6833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Alvarado-Kristensson M, Melander F, Leandersson K, Rönnstrand L, Wernstedt C, Andersson T. p38-MAPK signals survival by phosphorylation of caspase-8 and caspase-3 in human neutrophils. J Exp Med. 2004;199:449-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 43. | Dumitru CA, Fechner MK, Hoffmann TK, Lang S, Brandau S. A novel p38-MAPK signaling axis modulates neutrophil biology in head and neck cancer. J Leukoc Biol. 2012;91:591-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Mayer TZ, Simard FA, Cloutier A, Vardhan H, Dubois CM, McDonald PP. The p38-MSK1 signaling cascade influences cytokine production through CREB and C/EBP factors in human neutrophils. J Immunol. 2013;191:4299-4307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Naqvi S, Macdonald A, McCoy CE, Darragh J, Reith AD, Arthur JS. Characterization of the cellular action of the MSK inhibitor SB-747651A. Biochem J. 2012;441:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Rahman MM, Alkhouri H, Tang F, Che W, Ge Q, Ammit AJ. Sphingosine 1-phosphate induces neutrophil chemoattractant IL-8: repression by steroids. PLoS One. 2014;9:e92466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Coxon PY, Rane MJ, Uriarte S, Powell DW, Singh S, Butt W, Chen Q, McLeish KR. MAPK-activated protein kinase-2 participates in p38 MAPK-dependent and ERK-dependent functions in human neutrophils. Cell Signal. 2003;15:993-1001. [PubMed] |
| 48. | McLeish KR, Knall C, Ward RA, Gerwins P, Coxon PY, Klein JB, Johnson GL. Activation of mitogen-activated protein kinase cascades during priming of human neutrophils by TNF-alpha and GM-CSF. J Leukoc Biol. 1998;64:537-545. [PubMed] |
| 49. | Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 702] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 50. | Zhao C, Hui W, Fernandes MJ, Poubelle PE, Bourgoin SG. Lysophosphatidic acid-induced IL-8 secretion involves MSK1 and MSK2 mediated activation of CREB1 in human fibroblast-like synoviocytes. Biochem Pharmacol. 2014;90:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Zhao C, Fernandes MJ, Prestwich GD, Turgeon M, Di Battista J, Clair T, Poubelle PE, Bourgoin SG. Regulation of lysophosphatidic acid receptor expression and function in human synoviocytes: implications for rheumatoid arthritis? Mol Pharmacol. 2008;73:587-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Doehn U, Gammeltoft S, Shen SH, Jensen CJ. p90 ribosomal S6 kinase 2 is associated with and dephosphorylated by protein phosphatase 2Cdelta. Biochem J. 2004;382:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Toh ML, Yang Y, Leech M, Santos L, Morand EF. Expression of mitogen-activated protein kinase phosphatase 1, a negative regulator of the mitogen-activated protein kinases, in rheumatoid arthritis: up-regulation by interleukin-1beta and glucocorticoids. Arthritis Rheum. 2004;50:3118-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Zhao C, Sardella A, Chun J, Poubelle PE, Fernandes MJ, Bourgoin SG. TNF-alpha promotes LPA1- and LPA3-mediated recruitment of leukocytes in vivo through CXCR2 ligand chemokines. J Lipid Res. 2011;52:1307-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Bourgoin SG, Zhao C. Autotaxin and lysophospholipids in rheumatoid arthritis. Curr Opin Investig Drugs. 2010;11:515-526. [PubMed] |
| 56. | Ma Y, Pope RM. The role of macrophages in rheumatoid arthritis. Curr Pharm Des. 2005;11:569-580. [PubMed] |
| 57. | Kinne RW, Bräuer R, Stuhlmüller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 566] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 58. | Li J, Hsu HC, Mountz JD. Managing macrophages in rheumatoid arthritis by reform or removal. Curr Rheumatol Rep. 2012;14:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 59. | Kinne RW, Stuhlmüller B, Burmester GR. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis Res Ther. 2007;9:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 60. | Torsteinsdóttir I, Arvidson NG, Hällgren R, Håkansson L. Monocyte activation in rheumatoid arthritis (RA): increased integrin, Fc gamma and complement receptor expression and the effect of glucocorticoids. Clin Exp Immunol. 1999;115:554-560. [PubMed] |
| 61. | Davignon JL, Hayder M, Baron M, Boyer JF, Constantin A, Apparailly F, Poupot R, Cantagrel A. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology (Oxford). 2013;52:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 62. | Caivano M, Cohen P. Role of mitogen-activated protein kinase cascades in mediating lipopolysaccharide-stimulated induction of cyclooxygenase-2 and IL-1 beta in RAW264 macrophages. J Immunol. 2000;164:3018-3025. [PubMed] |
| 63. | Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM, Wingate A, Monk CE, Toth R, Santos SG. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol. 2008;9:1028-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 267] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 64. | MacKenzie KF, Van Den Bosch MW, Naqvi S, Elcombe SE, McGuire VA, Reith AD, Blackshear PJ, Dean JL, Arthur JS. MSK1 and MSK2 inhibit lipopolysaccharide-induced prostaglandin production via an interleukin-10 feedback loop. Mol Cell Biol. 2013;33:1456-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Elcombe SE, Naqvi S, Van Den Bosch MW, MacKenzie KF, Cianfanelli F, Brown GD, Arthur JS. Dectin-1 regulates IL-10 production via a MSK1/2 and CREB dependent pathway and promotes the induction of regulatory macrophage markers. PLoS One. 2013;8:e60086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 66. | Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1775] [Cited by in RCA: 1805] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 67. | Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1029] [Cited by in RCA: 1363] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 68. | Coulthard LR, Taylor JC, Eyre S, Robinson JI, Wilson AG, Isaacs JD, Hyrich K, Emery P, Barton A, Barrett JH. Genetic variants within the MAP kinase signalling network and anti-TNF treatment response in rheumatoid arthritis patients. Ann Rheum Dis. 2011;70:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1869] [Cited by in RCA: 2006] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 70. | Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol. 2010;185:6413-6419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 672] [Cited by in RCA: 624] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 71. | Naqvi S, Martin KJ, Arthur JS. CREB phosphorylation at Ser133 regulates transcription via distinct mechanisms downstream of cAMP and MAPK signalling. Biochem J. 2014;458:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 72. | Zhang X, Edwards JP, Mosser DM. Dynamic and transient remodeling of the macrophage IL-10 promoter during transcription. J Immunol. 2006;177:1282-1288. [PubMed] |
| 73. | Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847-855. [PubMed] |
| 74. | Westra J, Limburg PC, de Boer P, van Rijswijk MH. Effects of RWJ 67657, a p38 mitogen activated protein kinase (MAPK) inhibitor, on the production of inflammatory mediators by rheumatoid synovial fibroblasts. Ann Rheum Dis. 2004;63:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Georganas C, Liu H, Perlman H, Hoffmann A, Thimmapaya B, Pope RM. Regulation of IL-6 and IL-8 expression in rheumatoid arthritis synovial fibroblasts: the dominant role for NF-kappa B but not C/EBP beta or c-Jun. J Immunol. 2000;165:7199-7206. [PubMed] |
| 76. | Miyazawa K, Mori A, Yamamoto K, Okudaira H. Transcriptional roles of CCAAT/enhancer binding protein-beta, nuclear factor-kappaB, and C-promoter binding factor 1 in interleukin (IL)-1beta-induced IL-6 synthesis by human rheumatoid fibroblast-like synoviocytes. J Biol Chem. 1998;273:7620-7627. [PubMed] |
| 77. | Gerritsen ME, Williams AJ, Neish AS, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA. 1997;94:2927-2932. [PubMed] |
| 78. | Gao H, Parkin S, Johnson PF, Schwartz RC. C/EBP gamma has a stimulatory role on the IL-6 and IL-8 promoters. J Biol Chem. 2002;277:38827-38837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Zwergal A, Quirling M, Saugel B, Huth KC, Sydlik C, Poli V, Neumeier D, Ziegler-Heitbrock HW, Brand K. C/EBP beta blocks p65 phosphorylation and thereby NF-kappa B-mediated transcription in TNF-tolerant cells. J Immunol. 2006;177:665-672. [PubMed] |
| 80. | Asahara H, Fujisawa K, Kobata T, Hasunuma T, Maeda T, Asanuma M, Ogawa N, Inoue H, Sumida T, Nishioka K. Direct evidence of high DNA binding activity of transcription factor AP-1 in rheumatoid arthritis synovium. Arthritis Rheum. 1997;40:912-918. [PubMed] |
| 81. | Khanjani S, Terzidou V, Johnson MR, Bennett PR. NFκB and AP-1 drive human myometrial IL8 expression. Mediators Inflamm. 2012;2012:504952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 82. | Park SH, Kim JH, Lee DH, Kang JW, Song HH, Oh SR, Yoon DY. Luteolin 8-C-β-fucopyranoside inhibits invasion and suppresses TPA-induced MMP-9 and IL-8 via ERK/AP-1 and ERK/NF-κB signaling in MCF-7 breast cancer cells. Biochimie. 2013;95:2082-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 83. | Markou T, Hadzopoulou-Cladaras M, Lazou A. Phenylephrine induces activation of CREB in adult rat cardiac myocytes through MSK1 and PKA signaling pathways. J Mol Cell Cardiol. 2004;37:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J. 2003;22:1313-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 635] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 85. | Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95-105. [PubMed] |
| 86. | Chwang WB, Arthur JS, Schumacher A, Sweatt JD. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J Neurosci. 2007;27:12732-12742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 87. | Drobic B, Pérez-Cadahía B, Yu J, Kung SK, Davie JR. Promoter chromatin remodeling of immediate-early genes is mediated through H3 phosphorylation at either serine 28 or 10 by the MSK1 multi-protein complex. Nucleic Acids Res. 2010;38:3196-3208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 88. | Beck IM, Vanden Berghe W, Vermeulen L, Bougarne N, Vander Cruyssen B, Haegeman G, De Bosscher K. Altered subcellular distribution of MSK1 induced by glucocorticoids contributes to NF-kappaB inhibition. EMBO J. 2008;27:1682-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 89. | Cho IJ, Woo NR, Shin IC, Kim SG. H89, an inhibitor of PKA and MSK, inhibits cyclic-AMP response element binding protein-mediated MAPK phosphatase-1 induction by lipopolysaccharide. Inflamm Res. 2009;58:863-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 90. | Kawaguchi M, Fujita J, Kokubu F, Huang SK, Homma T, Matsukura S, Adachi M, Hizawa N. IL-17F-induced IL-11 release in bronchial epithelial cells via MSK1-CREB pathway. Am J Physiol Lung Cell Mol Physiol. 2009;296:L804-L810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 91. | Bruck N, Vitoux D, Ferry C, Duong V, Bauer A, de Thé H, Rochette-Egly C. A coordinated phosphorylation cascade initiated by p38MAPK/MSK1 directs RARalpha to target promoters. EMBO J. 2009;28:34-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 92. | Johnson J, Shi Z, Liu Y, Stack MS. Inhibitors of NF-kappaB reverse cellular invasion and target gene upregulation in an experimental model of aggressive oral squamous cell carcinoma. Oral Oncol. 2014;50:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 93. | Revesz L, Blum E, Di Padova FE, Buhl T, Feifel R, Gram H, Hiestand P, Manning U, Rucklin G. Novel p38 inhibitors with potent oral efficacy in several models of rheumatoid arthritis. Bioorg Med Chem Lett. 2004;14:3595-3599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Genovese MC, Cohen SB, Wofsy D, Weinblatt ME, Firestein GS, Brahn E, Strand V, Baker DG, Tong SE. A 24-week, randomized, double-blind, placebo-controlled, parallel group study of the efficacy of oral SCIO-469, a p38 mitogen-activated protein kinase inhibitor, in patients with active rheumatoid arthritis. J Rheumatol. 2011;38:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 95. | Damjanov N, Kauffman RS, Spencer-Green GT. Efficacy, pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: results of two randomized, double-blind, placebo-controlled clinical studies. Arthritis Rheum. 2009;60:1232-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 96. | Kyttaris VC. Kinase inhibitors: a new class of antirheumatic drugs. Drug Des Devel Ther. 2012;6:245-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 97. | Clark AR, Dean JL, Saklatvala J. The p38 MAPK pathway mediates both antiinflammatory and proinflammatory processes: comment on the article by Damjanov and the editorial by Genovese. Arthritis Rheum. 2009;60:3513-3514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 98. | Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1220] [Cited by in RCA: 1175] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 99. | Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2468] [Cited by in RCA: 2641] [Article Influence: 120.0] [Reference Citation Analysis (0)] |
| 100. | Bamford MJ, Bailey N, Davies S, Dean DK, Francis L, Panchal TA, Parr CA, Sehmi S, Steadman JG, Takle AK. (1H-imidazo[4,5-c]pyridin-2-yl)-1,2,5-oxadiazol-3-ylamine derivatives: further optimisation as highly potent and selective MSK-1-inhibitors. Bioorg Med Chem Lett. 2005;15:3407-3411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Bamford MJ, Alberti MJ, Bailey N, Davies S, Dean DK, Gaiba A, Garland S, Harling JD, Jung DK, Panchal TA. (1H-imidazo[4,5-c]pyridin-2-yl)-1,2,5-oxadiazol-3-ylamine derivatives: a novel class of potent MSK-1-inhibitors. Bioorg Med Chem Lett. 2005;15:3402-3406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Beltman J, McCormick F, Cook SJ. The selective protein kinase C inhibitor, Ro-31-8220, inhibits mitogen-activated protein kinase phosphatase-1 (MKP-1) expression, induces c-Jun expression, and activates Jun N-terminal kinase. J Biol Chem. 1996;271:27018-27024. [PubMed] |