Published online Jun 9, 2015. doi: 10.5497/wjp.v4.i2.168
Peer-review started: January 24, 2015
First decision: February 7, 2015
Revised: March 6, 2015
Accepted: April 1, 2015
Article in press: April 7, 2015
Published online: June 9, 2015
Processing time: 146 Days and 21.6 Hours
Tumor-targeting is becoming more and more important for cancer chemotherapy. Though many molecular-target drugs have been developed in the past two decades which shed some light on targeted tumor therapy, clinical results of those molecular-target drugs are not so encouraging especially for solid tumors, problems mostly relating to the heterogeneity and mutations of target molecules in human solid tumors. More general tumor-targeting strategy is thus anticipated. In this regard, the enhanced permeability and retention (EPR) effect which is a unique phenomenon of solid tumors based on the anatomical and pathophysiological nature of tumor blood vessels, is receiving more and more attentions. This EPR effect now served as a standard for tumor-targeted macromolecular anticancer therapy, namely nanomedicine. Many nanoplatforms have been developed as targeted drug delivery systems, including liposome, polymeric micelles, polymer conjugate, nanoparticles. Ample macromolecular drugs are now approved for clinical use or in clinical stage development, all of which by taking advantage of EPR effect, show superior in vivo pharmacokinetics and remarkable tumor selectivity, resulting in improved antitumor effects with less adverse effects. We thus believe EPR-based nanomedicine will be a solution for cancer in the future, whereas further consideration of factors involved in EPR effect and strategies to augment/improve EPR effect are warranted.
Core tip: Current cancer chemotherapy is less effective with adverse side effects, mostly due to lack of tumor-selectivity. Thus tumor-targeting is known the key for successful chemotherapy. Molecular-target therapy is such a strategy but the clinical results are disappointing probably due to the diversity of cancer-related molecules and enormous mutations. A more general tumor-targeting strategy is based on the unique physiophathological and anatomical features of solid tumors - enhanced permeability and retention (EPR) effect. Accordingly nanomedicine has been developed, with promising therapeutic potential and very less side effects. We thus believe EPR-based nanomedicine will be a solution for cancer in the future.
- Citation: Fang J. Enhanced permeability and retention effect based nanomedicine, a solution for cancer. World J Pharmacol 2015; 4(2): 168-171
- URL: https://www.wjgnet.com/2220-3192/full/v4/i2/168.htm
- DOI: https://dx.doi.org/10.5497/wjp.v4.i2.168
Cancer remains the major threat to human health in most advanced countries in the world. While surgical removal is effective to small and confined early-stage tumors, use of anticancer drugs (chemotherapy) is a less invasive option for cancer patients. Though there is more than 70-year history of chemotherapy, the clinical results of conventional chemotherapy is far from successful. The major problem is the lack of tumor selectivity of conventional anticancer drugs which are mostly small molecular drugs, namely non-selective delivery of cytotoxic drugs to normal vital organs and tissues results in less antitumor effect and severe adverse side effects. Thus, it is an urgent need to develop therapeutic strategies to selectively target tumors.
Development of molecular-target drugs is a remarkable progress in the past two decades, which usually focuses on specific genes or molecules that are highly expressed in tumors and essential for tumor growth. A successful example is imatinib, an inhibitor of the BCR/ABL oncogene product, which shows high efficacy in patients with chronic myeloid leukemia (CML) though it is not curative[1]. However, many recent clinical results using those molecular-target drugs are disappointing especially for solid tumors[2,3]. The problems probably relate to the intrinsic heterogeneity and mutations of cancer-related molecules in human solid tumors[4,5]. Namely, in most solid tumors, multiple mutated genes (10 to > 100) exist[4], different cells have distinct genetic lesions even in the same tumor[5], and the critical mutation is not always clear. Thus such a highly specific molecular approach seems to be premature or imperfect, not mentioning the toxic effects as well as enormous and inappropriate expense of these drugs.
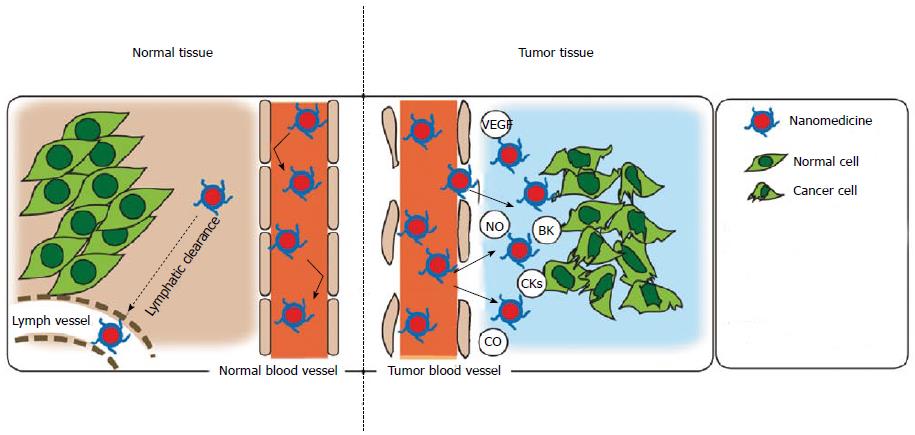
A more general tumor-targeting strategy is focusing on the unique anatomical and pathophysiological features of solid tumors leading to high vascular permeability (Table 1, Figure 1), which facilitates delivery of macromolecules (i.e., larger than 40 kDa) selectively into tumor tissues but with very less distribution in normal tissues[6]. This phenomenon is coined enhanced permeability and retention (EPR) effect that was first reported by Matsumura and Maeda in 1986[7], and is now considered a landmark principle in the development of anticancer drugs.
| Active angiogenesis and high vascular density |
| Extensive production of vascular mediators that facilitate extraravasation |
| Bradykinin |
| Nitric oxide |
| Vascular permeability factor/vascular endothelial growth factor |
| Prostaglandins |
| Collagenase (matrix metalloproteinases, or MMPs) |
| Peroxynitrite |
| Defective vascular architecture, for example, lack of smooth muscle layer cells, lack of or fewer receptors for angiotensin II, large gap in endothelial cell-cell junctions, anomalous conformation of tumor vasculature (e.g., branching or stretching) |
| Impaired lymphatic clearance of macromolecules and lipids from interstitial tissue (→ prolonged retention of these substances) |
In this concept of EPR based tumor-targeted therapy, nanotechnology is introduced in cancer chemotherapy, namely nanomedicine. Many nanoplatforms have been developed as targeted drug delivery systems, including liposome, polymeric micelles, polymer conjugate, nanoparticles. For example, Doxil, a PEGylated liposome formulation of doxorubicin, is an FDA approved drug for the treatment of Kaposi sarcoma and other cancers. Other clinically used nanomedicine includes DaunoXome (nonpegylated liposomal daunorubicin), DepoCyt (nonpegylated liposomal cytarabine), Myocet (nonpegylated liposomal doxorubicin), Oncaspar (pegylated L-asparaginase), Abraxane (albumin-based paclitaxel), and Genexol-PM (paclitaxel-containing polymeric micelles, approved in South Korea). Much more liposome, polymeric or micellar drugs are in clinical stage development[8,9]. All these macromolecular drugs, by taking advantage of EPR effect, show superior in vivo pharmacokinetics and remarkable tumor selectivity, resulting in improved antitumor effects with less adverse effects[8,9].
It should be noted that EPR effect is the first and necessary step for successful anticancer chemotherapy, however many factors are involved in EPR effect, by which the EPR based tumor drug delivery could be further augmented, such as angiotensin II induced hypertension, nitroglycerin/nitric oxide, carbon monoxide[6,10]. Combination of these factors with macromolecular drugs may become useful strategies for more effective antitumor nanomedicine. In addition, another important issue for satisfied nanomedicine is the fate of nano-drugs after accumulation in tumor tissues by EPR effect. The ideal condition is the active drug component in nano-drugs should be released gradually in tumor tissues, otherwise the intact nano-drugs will show less antitumor effect tough they accumulate in tumor with high concentration[10,11]. One successful strategy regarding this issue is the utilization of the acidic pH (e.g., 6.5-6.7) of tumors. Maeda et al[10] recently reported a tumor environment/pH responsive poly(N-(2-hydroxypropyl)methacrylamide) conjugated pirarubicin (P-THP), which behaves as polymeric conjugate/micelle in circulation, but liberates free THP in acidic tumor environment, resulting a remarkable antitumor effect[11]. This P-THP therapy was also translated into clinic successfully; in a patient with advanced prostate cancer with multiple lung metastasis, P-THP treatment resulted in complete remission of metastatic tumor nodules in the lung, with significantly decreased levels of prostate specific antigen (PSA, from 1472 ng/mL to 0.067 ng/mL); no severe side effects were observed and no evidence of disease relapse has been recorded for 12 mo since the administration of P-THP (unpublished data).
Another issue should be addressed is that, EPR effect is the phenomenon of blood vessels, so it may varies depending on the patient/tumor’s pathological characteristics and conditions. Namely tumors with less blood vessels, e.g., pancreatic cancer, always show less EPR effect. The EPR effect is heterogeneous even in a single tumor nodule. Thus further augmentation of EPR effect is important or necessary for treating such tumors, which could be achieved by modulating the vascular mediators in tumor such as using angiotensin II, nitric oxide/nitroglycerin, angiotensin II converting enzyme inhibitor and carbon monoxide, all of which increase EPR effect by 2-10 times and some of them (i.e., angiotensin II) were proven in clinic[6,10,12,13].
EPR effect is now becoming the “gold standard” for design and development of cancer drug, we believe EPR-based nanomedicine that is becoming a promising paradigm of anticancer strategy, will be a solution for cancer in the future.
P- Reviewer: Arsenijevic M, Masaki T, Shimada Y S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037. [PubMed] |
| 2. | Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1667] [Cited by in RCA: 1723] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 3. | Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 998] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 4. | Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5906] [Cited by in RCA: 5579] [Article Influence: 464.9] [Reference Citation Analysis (0)] |
| 5. | Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6102] [Cited by in RCA: 5942] [Article Influence: 457.1] [Reference Citation Analysis (0)] |
| 6. | Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2509] [Cited by in RCA: 2660] [Article Influence: 190.0] [Reference Citation Analysis (0)] |
| 7. | Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387-6392. [PubMed] |
| 8. | Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1410] [Cited by in RCA: 1484] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 9. | Duncan R, Vicent MJ. Polymer therapeutics-prospects for 21st century: the end of the beginning. Adv Drug Deliv Rev. 2013;65:60-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 313] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 10. | Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1622] [Cited by in RCA: 1749] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 11. | Nakamura H, Etrych T, Chytil P, Ohkubo M, Fang J, Ulbrich K, Maeda H. Two step mechanisms of tumor selective delivery of N-(2-hydroxypropyl)methacrylamide copolymer conjugated with pirarubicin via an acid-cleavable linkage. J Control Release. 2014;174:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Nagamitsu A, Greish K, Maeda H. Elevating blood pressure as a strategy to increase tumor-targeted delivery of macromolecular drug SMANCS: cases of advanced solid tumors. Jpn J Clin Oncol. 2009;39:756-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Fang J, Liao L, Yin H, Nakamura H, Shin T, Maeda H. Enhanced bacterial tumor delivery by modulating the EPR effect and therapeutic potential of Lactobacillus casei. J Pharm Sci. 2014;103:3235-3243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Nakamura H, Jun F, Maeda H. Development of next-generation macromolecular drugs based on the EPR effect: challenges and pitfalls. Expert Opin Drug Deliv. 2015;12:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |