Peer-review started: September 3, 2014
First decision: October 14, 2014
Revised: November 18, 2014
Accepted: January 15, 2015
Article in press: January 19, 2015
Published online: March 9, 2015
Processing time: 193 Days and 16.5 Hours
The majority of cancer drugs entering clinical trials fail to reach the market due to poor efficacy. Preclinical efficacy has been traditionally tested using subcutaneous xenograft models that are cheap, fast and easy to perform. However, these models lack the correct tumor microenvironment, leading to poor clinical predictivity. Selecting compounds for clinical trials based on efficacy results obtained from subcutaneous xenograft models may therefore be one important reason for the high failure rates. In this review we concentrate in describing the role and importance of the tumor microenvironment in progression of breast and prostate cancer, and describe some breast and prostate cancer cell lines that are widely used in preclinical studies. We go through different preclinical efficacy models that incorporate the tissue microenvironment and should therefore be clinically more predictive than subcutaneous xenografts. These include three-dimensional cell culture models, orthotopic and metastasis models, humanized and transgenic mouse models, and patient-derived xenografts. Different endpoint measurements and applicable imaging techniques are also discussed. We conclude that models that incorporate the tissue microenvironment should be increasingly used in preclinical efficacy studies to reduce the current high attrition rates of cancer drugs in clinical trials.
Core tip: It is today a recognized major problem in cancer drug development that the vast majority of drugs entering clinical trials fail to reach the market due to poor efficacy. One important reason for this is the wide use of subcutaneous xenograft models that are cheap, fast and easy to perform, but lack tumor microenvironment. Concentrating on breast and prostate cancer, we explain why the presence of tumor microenvironment is important, and describe different types of preclinical efficacy models that incorporate tumor microenvironment. We state the importance of using these models to reduce the high failure rates in clinical trials.
- Citation: Valta M, Fagerlund K, Suominen M, Halleen J, Tuomela J. Importance of microenvironment in preclinical models of breast and prostate cancer. World J Pharmacol 2015; 4(1): 47-57
- URL: https://www.wjgnet.com/2220-3192/full/v4/i1/47.htm
- DOI: https://dx.doi.org/10.5497/wjp.v4.i1.47
During the course of multistep tumorigenesis of breast and prostate carcinomas, neoplastic epithelial cells are in a continuous interplay with mesenchymal cells that form the tumor-associated stroma. This tumor microenvironment is constituted by endothelial cells, pericytes, myoepithelial cells, osteoblasts, immune cells, fibroblasts, cancer stem cells, and many other cells that secrete growth factors and cytokines[1]. While complex interactions between these different cell types reshape the surrounding extracellular matrix (ECM) as cancer progresses, also neoplastic and stromal cells undergo constant changes. Endpoint of this extreme plasticity is that a tumor almost never contains two completely identical cells[2]. While tumor heterogeneity remains a major obstacle to effective cancer treatment and personalized medicine, it can also be used as a biomarker to predict the risk of progression and therapeutic resistance[3].
An optimal preclinical model mimics these plastic genetic and phenotypic changes that occur within human disease, is heterogenic, and results in appropriate tumor growth and spread[4]. Mouse (Mus musculus) has emerged as the main species of in vivo tumor biology due to its basic physiology and genome size that are similar to human[5]. Other advantages for using mice include the ease of genetic manipulation, low maintenance cost, and short gestation period[6]. Here we rationalize how mouse models of breast and prostate cancer can help us to understand the interaction between microenvironment and cancer cells in neoplastic progression. Major differences between human and mouse tissue architecture and different research models will be discussed.
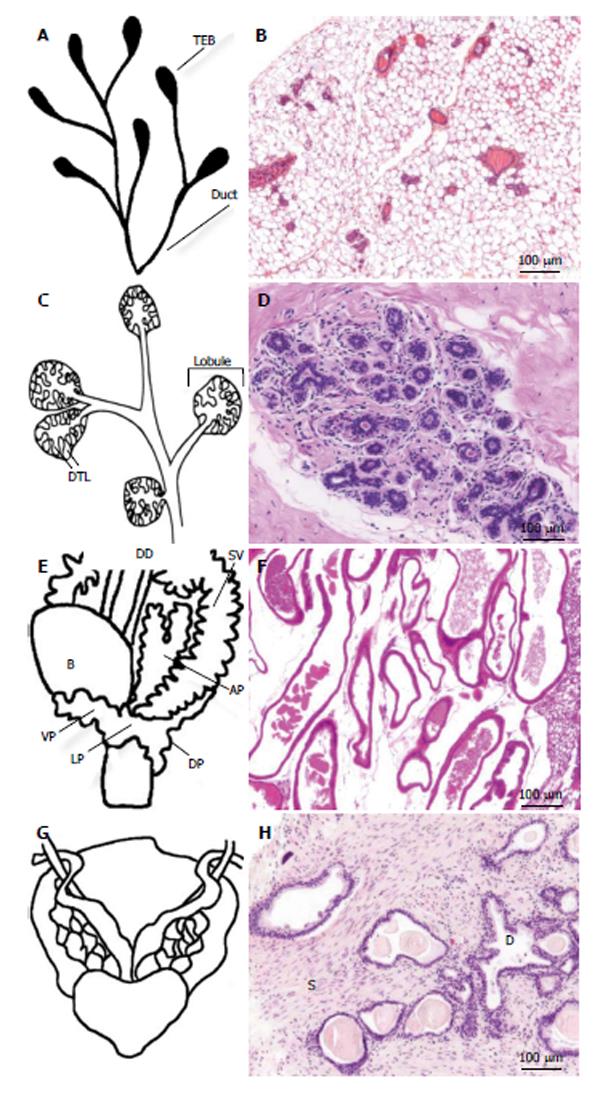
In mammals, the morphology of mammary gland changes throughout the entire reproductive life. Ductal morphogenesis, as well as carcinogenesis, are regulated by steroid and polypeptide hormones and growth factors that act as local epithelial-mesenchymal inductive signals. The glandular part of the human and murine mammary tissue is composed of major lactiferous ducts that arise inside the nipple, branch into terminal ducts, and end up in acini that are embedded in the intralobular stroma[7,8]. The acini are composed of a bilayer of inner milk producing luminal cells and outer myoepithelial cells[9]. The human acini with the surrounding intralobular stroma are termed terminal ductal lobular unit. It is comprised of a small group of lobules, resembling a cluster of grapes at the end of a stem[10]. The murine mammary tissue is organized differently. The corresponding functional units are termed lobuloalveolar units. Unlike in human, the individual ducts branch minimally and end in single bulbous terminal end-buds (Figure 1)[11].
Breast cancer usually originates from the epithelium, but the stroma has a profound effect on tumor growth, invasion, metastasis, and drug resistance[12]. The mouse mammary stroma is histologically different from the human stroma[13,14]. Human mammary epithelium is surrounded by fibrous connective tissue, whereas mouse tissue consists of larger number of adipose cells and smaller proportion of connective tissue (Figure 1). Also, the human breast contains fat, but it is not in contact with the epithelium[11].
Both human and murine prostates are muscular glands that surround urethra. The prostate is covered with a capsule, and it is in close contact with accessory sexual glands such as coagulating gland in mice, bulbourethral gland in humans, and seminal vesicles in both. The obvious difference in gross anatomy between human and murine prostates is that murine prostate is composed of separate ventral, dorsal, and lateral lobes, whereas human prostate is a single nut-shaped gland that is divided to lobes or zones according to their location and function. In humans, there are two lateral lobes in the anterior end of the gland. The anterior lobe is located behind the lateral lobe, anterior to urethra. It is constructed of fibromuscular tissue, and activates during ejaculation. On the posterior to the urethra there is an area called median lobe, and on the posterior to the median lobe a very thin area called posterior lobe. The human prostate can also be divided into an anteriorly located central zone, an urethra surrounding transition zone, and a peripheral zone, which is the largest zone and the most common location of a tumor[4,15].
The prostatic tissue is composed of exocrine glands, ducts, and fibromuscular stroma. The human and mouse prostates contain similar cell types, but the proportion of stroma is larger in the human prostate (Figure 1). Of the mouse prostatic lobes, the dorsolateral lobe resembles most the human prostate histologically and biochemically[4,16]. Therefore, the dorsolateral prostate is an appropriate inoculation or implantation site in xenograft models.
During carcinogenesis, the stroma undergoes extensive changes in gene expression, and often proliferates actively[17]. The stroma co-evolves with its tumor and adapts to the needs of the tumor[18]. For example the amount of collagens increases in tumor ECM, which makes it thicker and may act as a physical or cell attachment - based barrier to drugs. Despite the differences in organization of the stroma between humans and mice, similar gene activation as in patients is seen in the stroma of transgenic and xenograft-bearing mice[19,20].
BT-20 was the first commercial breast cancer cell line. It was established in 1958, followed by the still very popular MD Anderson series (MDA), and MCF-7 cell lines 20 years later[21-23]. Breast cancer, as well as prostate cancer, is a very heterogenous disease, and until today there are no comprehensive models available to study them. However, human breast cancer cell lines (summarized in Table 1) are available that represent the main categories of breast cancer[24].
| Name | Histopathologicalclassification | Immunohistochemicalclassification |
| MCF-7 | Luminal A | ER+, PR+, Her2- |
| SUM185 | Luminal A | ER+, PR-, Her2- |
| T47D | Luminal A | ER+, PR+, Her2- |
| BT-474 | Luminal B | ER+, PR+, Her2+ |
| ZR-75 | Luminal B | ER+, PR-, Her2+ |
| SKBR3 | Her2-positive | ER-, PR-, Her2+ |
| MDA-MB-453 | Her2-positive | ER-, PR-, Her2+ |
| MDA-MB-468 | Basal | ER-, PR-, Her2- |
| SUM190 | Basal | ER-, PR-, Her2+ |
| BT-20 | Basal | ER-, PR-, Her2- |
| MDA-MB-231 | Claudin-low | ER-, PR-, Her2- |
| HS-578T | Claudin-low | ER-, PR-, Her2- |
| Cal-51 | Claudin-low | ER-, PR-, Her2- |
Table 2 summarizes the most commonly used human prostate cancer cell lines. PC-3 and DU-145 cells were originally cloned from bone and brain metastases of prostate cancer, respectively[25,26]. Their tumorigenicity is high and they form metastases when inoculated into immunodeficient mice[27], and they can thus be considered as models of advanced disease. However, these very popularly used cell lines lack expression of androgen receptor (AR) and prostate specific antigen (PSA), which are both characteristic for hormone-responsive prostate cancer. LNCaP cells express AR and secrete PSA, but they have limited tumorigenicity and respond aberrantly to androgen therapy because of a mutated AR, and they are also sensitive to other sex steroids[28]. Some newer prostate cancer cell lines respond to androgens and secrete PSA, including VCaP cells[29-31], 22Rv1 cells[32] and PC-346 cells[33]. A panel of transplantable human-derived xenografts (CWR, MDA Pca, LuCaP, and LAPC series) have interesting characteristics that mimic human disease[26]. Their benefit is the relevant tissue architecture with stromal support, which improves tumor growth and metastasis.
| Name | Site of origin | Hormonal status | PSA expression |
| PC-3 | Bone | AR- | No |
| DU-145 | Brain | AR- | No |
| LNCaP | Lymph node | AS | Yes |
| C4-2B | Subline of LNCaP | AI | Yes |
| VCaP | Bone | AS | Yes |
| CWR22 | Prostate | AS | Yes |
| 22Rv1 | Subline of 22Rv1 | AI | Yes |
| PC-346 | Prostate | AS | Yes |
Currently, in vitro drug testing is mostly based on traditional two-dimensional (2D) monoculture models that utilize immortalized cancer cell lines in systems that cannot incorporate the tissue microenvironment. However, 3D cell cultures have raised considerable attention in recent years because of their potential to deliver higher quality and more accurate information that is more representative and predictive of drug responses in vivo. Currently, the main applications of 3D cell cultures include cancer therapy and studies of cell-to-cell and cell-to-matrix interactions. It is known that both cancer cells and normal cells cultured in 3D in the presence of ECM components show differences in gene expression, differentiation and proliferation when compared to cells cultured as monolayer in 2D. The importance of the microenvironment was highlighted by Mina Bissell’s research group, who were the first to recognize that normal mammary epithelial cells grown in monolayers divided exponentially through several passages, but when the cells were grown in 3D Matrigel culture, they responded to microenvironmental signals by reducing proliferation and differentiating into nearly normal-sized mammary acinar structures[34]. An interesting finding was also that when cultured in the presence of a matrix that contained a combination of reconstituted basement membrane proteins, including type I collagen and normal breast fibroblasts, MCF-7 cancer cells were induced to near-complete tumor phenotype reversion[35].
The most widely used 3D culture structures are spheroids that can be formed by multiple different approaches, including scaffolds such as hydrogels, and as floating structures formed either by hanging drop method or by low attachment coatings. The spheroid systems allow co-culturing of different cell populations for studying the role of cell-to-cell or cell-to-ECM interactions, and therefore provide an improved approximate of the in vivo tissue architecture. Multiple cell types, such as stromal fibroblasts, nerve ganglia or endothelial cells, have been seeded within a matrix gel to influence spheroid growth and define specific roles or interactions with prostate cancer cells, including DU-145, LNCaP and PC-3 cells[36]. Also, co-culture of bone stromal derived HS5 cells and PC-3 cells in Matrigel scaffold displays up-regulated invasion and proliferation, along with altered expression of epithelial-to-mesenchymal and chemokine protein constituents involved in metastatic progression[37]. Additionally, multiple cells, including PC-3, osteoblasts and endothelial cells, have been seeded into hanging drops to form heterogeneous aggregates recapitulating the in vivo growth behavior of cancer cells within the bone metastatic prostate cancer microenvironment[38]. In breast cancer, the surrounding microenvironment, including stromal fibroblasts, is believed to promote the progression of ductal carcinoma in situ (DCIS) to invasive ductal carcinoma[39-43]. Indeed, human mammary fibroblasts cultured in a 3D matrix have been shown to secrete more paracrine signaling molecules than in 2D monolayer cultures, increasing the invasive progression in MCF10-DCIS.com cells[44]. Even though the role of the matrix in regulating fibroblast behavior has been studied, the consequences of modified fibroblast behavior with cancer cells remains poorly understood.
The term xenograft implies transplantation of material between species. Most commonly, human cells or tissue implants are grafted into immunodeficient mice. If the transplanted material is from genetically nearly identical individuals, it can be transplanted into immunocompetent mice to produce syngeneic tumors. Syngeneic models allow to study the role of adaptive immunity in tumor progression, which is a benefit compared with xenografts. However, the fact that the cells are from murine origin and very rarely respond to hormonal therapy may hamper the results. There are several good syngeneic models for breast cancer, such as Balb/cC3H-originated 4T1 subline grafted into Balb/c mice[45], S115 cells grafted into DD/Sio mice[46], and Py8119 cells grafted into C57BL mice[47]. Until now, there are only few syngeneic models for prostate cancer such as RM1 cells or TRAMP-C2 cells in C57BL mice[48,49].
Subcutaneous inoculation of tumor cells is a popular and inexpensive way to perform xenograft models. However, these models can be used only in studies of primary tumor growth because of restricted spread and formation of metastases due to incomplete blood and lymphatic vasculature[50,51]. This, and the fact that these models lack the correct microenvironment for the tumor cells, leads to poor clinical predictivity. The correct tumor microenvironment is important not only for the processes of tumorigenesis, invasion and metastasis, but also for its potential effects on efficacy of tested drug candidates. The correct microenvironment can either improve the efficacy of tissue-specific targeted therapies, or protect the cancer cells from the therapy[12]. The wide use of subcutaneous xenografts and relying on the obtained results is probably one important reason why a very high number of cancer drug candidates fail in clinical trials due to poor efficacy[52]. However, many other reasons such as non-enhanced patient groups, tumor heterogeneity, and low number of clinically relevant events also contribute to the high failure rates.
Clinically much more relevant xenograft models are orthotopic models, where breast cancer cells are inoculated into the mammary fat pad, and prostate cancer cells into the prostate. In these models the cancer cells form primary tumors in the relevant tumor microenvironment and interact with the mouse stromal cells[53-55]. Orthotopic models can also include formation of metastases, depending on the characteristics of the used cell line[56,57]. Typically, orthotopic breast and prostate tumors metastasize into local (inguinal or iliac and sacral, respectively) lymph nodes, liver and lungs[58,59]. Bone metastasis is a common and deadly complication of both breast and prostate cancer. Some breast and prostate cancer models produce bone metastases, but macroscopic bone tumors are rarely, if ever, observed using orthotopic models[56]. By inoculating tumor cells into the bone marrow cavity of the mouse tibia, tumor cell-bone interactions can be studied. Although several steps of the metastasis cascade remain unstudied in this model, the intratibial tumors provide valuable information about the tumor-bone interaction.
Tumor cells can also be inoculated directly into the tail vein or the left cardiac ventricle in order to mimic metastatic disease[60,61]. These models are clinically highly relevant, since at the time of diagnosis of breast and prostate cancer, dormant tumor cells can be found in bone marrow cavity[62]. The models are based on Paget’s seed and soil-hypothesis, where a small number of tumor cells have evolved towards metastatic phenotype after a series of somatic mutations[63]. Some laboratories have succeeded in enrichment of bone- or lung-seeking tumor cell populations, and created sublines of some commonly used cell lines. Examples of such breast cancer sublines are bone-seeking MDA-MB-231(SA) and MDA-MB-231(B02) cells[64,65], and MDA-MB-231(LM) cells that form tumors in lungs when inoculated into the blood stream[59].
The major limitation of using xenograft models with immunocompromised mice is the lack of immune cells in the tumor microenvironment. The use of human stroma may be a solution to this problem. Kuperwasser et al[66] injected human mammary stromal and epithelial cells into cleared murine mammary fat pads. This chimeric mouse ‘‘humanized mammary fat pad’’ was found to be similar to that of humans and allowed genetic manipulation of the human stroma. Currently, there are no xenograft models where bone metastases are formed from orthotopic tumors with a relevant rate. Several laboratories have introduced a humanized mouse, where human bone tissue is first grafted into immunodeficient mice and after inoculation of the human breast or prostate tumor cells, metastases have been formed into human bone instead of mouse bone[67-69], underlining the importance of species-specificity of the microenvironment in metastasis formation. However, the effect of possible differences in bone metabolism of the transplant vs normal bone cannot be ruled out, since there is clear evidence of higher bone metabolism connected to higher metastasis rate[70]. Challenges of the model include the availability of human bone, donor-related variance, immune reactions, and difficulties in implant functionality and viability[71,72].
Genetically engineered mouse models are physiologically relevant models to study tumor progression, because they include natural microenvironment and immune competence. However, most transgenic breast and prostate cancer models are hormone-independent and do not respond to hormone therapy[73,74]. Also, mouse tumors are often mesenchymal instead of epithelial origin[75], and none of the transgenic models include the entire heterogeneity and plasticity of human carcinogenesis.
When an oncogene is overexpressed in mammary gland or prostate epithelium, the most commonly used promoter elements are the mouse mammary tumor virus (MMTV) long terminal repeat, human cytomegalovirus and ubiquitin promoters, the rat probasin gene, the rat C3 prostate steroid-binding protein gene, the human PSA gene, and the mouse cryptic gene[76-79]. Hruska et al[80] created an estrogen receptor overexpressing conditional mouse line that developed mammary adenocarcinomas, which responded to estrogen and had similarities to human breast cancer histology. The transgenic adenocarcinoma mouse prostate (TRAMP) model was established in 1995, and TRAMP mice have been widely used in oncology[78,81]. In the TRAMP model, SV40 small and large T-antigens inactivate tumor-suppressor proteins and enhance the development of neoplasia[78,82]. TRAMP mice develop prostate adenocarcinoma and metastasize into para-aortic lymph nodes and lungs, and occasionally to distant sites[78]. Disadvantage of the model is that metastases develop at a relatively low frequency[4]. In addition, Chiaverotti et al[83] have shown that the background of TRAMP mice (FVB instead of C57/BL) influenced the tumor type. FVB mice frequently developed neuroendocrine-type prostate tumors, while C57/BL mice developed adenocarcinomas. In addition to TRAMP mice, a popular transgenic model is c-Myc overexpression[84]. A structural variation of the c-Myc gene is common in cancer, and accordingly the increased copy number of c-Myc results in a homologous gene-expression profile with human c-Myc-overexpressing cancer, such as disappearance of NKX3.1 during tumorigenesis[85].
Alternatively, the role of specific genes in breast and prostate tumorigenesis can be studied using knockout mice. Since ablation of important genes often leads to embryonic or early fatality, genetically modified mice with conditional knockouts have been developed. Germ-line mutations in oncogenes BRCA1 and BRCA2, in which DNA repair function is interrupted, account for the majority of familial breast cancers. In order to study the role of BRCA1 in breast cancer, MMTV-cre mice have been created, and used to produce conditional mammary BRCA1 knockout mice[86].
Inactivation of the tumor suppressor gene PTEN is associated in approximately 70% of advanced human prostate cancers[87]. PTEN+/-, PTEN hypomorph, and PTEN conditional knock-out models have been established to study prostate cancer progression[87-89]. Conditional PTEN knock-out leads to prostate cancer with lymph node and lung metastases[88,89]. In addition to the cre-loxP system, tissue-specific, conditional knock-out models have been created using the tetracycline promoter system under the regulation of tet operator promoter. In this model, the specific gene is expressed only under doxycycline supplementation[80].
While cell line based models have provided invaluable knowledge of cancer progression, the utility of these systems is diminished in the light of the findings that patient derived tumor cell lines have significantly different gene expression patterns when compared to the original cell lines or the xenografted tumors[90-92]. Patient-derived xenografts (PDXs) are recent advances in personalized medicine. These models use mouse avatars, where fresh tumor tissue from the patient is grafted in order to study which therapies are most effective for an individual cancer patient. A large number of drugs or drug combinations can then be screened in the mice, which increases the likelihood that a given treatment will benefit the patient. In addition to clinics, PDX models are used increasingly as tumor models in drug development. An obvious benefit of PDX models vs traditional cell line - based subcutaneous xenografts is that they possess the natural tissue architecture and composition[93].
However, PDX models have many challenges. The success rate for implanting human tumors in mice is low and depending on the tumor type, engraftment efficiencies vary a lot. In clinical use, it takes more than six months to generate PDXs and screen potential therapies, and many patients die before they can benefit from the results. Although the patient tumor is engrafted along with human stromal components and is sustained during several passages[94], murine stroma may gradually replace the human stroma and lead to confounding results. High cost of PDX technology also limits their use. However, increased use of PDX systems with modern molecular biology techniques will continue to improve the methodology and may help more patients in the future.
There are several companies that offer breast cancer PDX models, but none that offer prostate cancer PDX models. Human prostate cancer xenografts have been implanted in immunodeficient mice subcutaneously or under the renal capsule to study, maintain, or even expand the tumor tissue[95]. This technique has been particularly tested for the propagation of the tumor tissue from castration resistant prostate cancer, which is available for research only in very limited amounts from biopsy samples.
Experimental tumors are evaluated using immunohistochemical markers and histomorphometry that are already established in clinic. The major obstacle of comparing experimental tumors with clinical specimens is the mouse background, which may hamper immunohistochemical stainings. Also the need for an experienced disease model pathologist may be an obstacle.
The classical endpoint in subcutaneous xenograft models is tumor dimension measurement by caliper, where tumor volume can be calculated using the formula V = a × b2/2, “a” being the biggest dimension of the tumor and “b” the perpendicular dimension[96]. If the tumors are dissected the formula of three dimensions can be used, where V = π/6 (a × b × c)[97]. Naturally, caliper measurements can only be used if the tumors are palpable. The rapid evaluation of novel drugs in animal models requires developing clinically translatable noninvasive imaging strategies, which are discussed below.
Optical imaging is based on a signal produced by a reporter protein. The signal can be produced by constitutive expression of a fluorescent protein[98], or by enzymatic activation of an inactive substrate[99]. In both options, tumor-producing cell lines need to be transfected with a reporter molecule. A popular method of transfection is the use of genome-integrated viruses. However, they contain a risk of genotoxicity and unpredicted effects due to random integration, which may directly affect the expression levels of not only surrounding but also distant genes. Also, both plasmid and virus based methods can modify the cell behavior indirectly because they typically contain unmethylated or hypomethylated CpG sequences that act as ligands for Toll-like receptor 9, and therefore activate the immune system[100,101]. The third obstacle is that cells may spit out the redundant reporter material during the course of the experiment[102]. In a recent study, these problems were avoided by transfecting cells using non-integrated, episomal CpG-depleted lentivector with a scaffold/matrix-attachment region that acts as an initiation point of replication during mitosis, and enables efficient and stable production of labelled cell lines[103,104].
In addition to optical imaging, bone metastases can be imaged and quantitatively analysed using radiography, micro-computed tomography (CT)[105,106], or micro-magnetic resonance imaging (MRI)[107]. Multimodality functional imaging approach effectively combines the advantages of optical imaging, CT and MRI to analyze breast or prostate cancer bone lesions. Soft tissue metastases can be detected using ultrasound imaging[108], MRI[107], or ex vivo by histology and quantitative polymerase chain reaction[56]. Micro-ultrasound imaging can be used to image the surrounding tissue at 3 cm depth, which is usually sufficient for detecting tumors in mice, but difficult for detecting metastases due to their small size. Micro-MRI combined with a contrast agent that specifically attaches to prostate specific membrane antigen receptor, a marker implicated in prostate tumor progression and metastasis, may prove to be a sensitive technique[109].
Today, popular methods of functional imaging are single-photon emission CT and positron emission tomography combined either with CT or MRI. Although clinical use of these techniques is increasing in oncology for diagnosis and image guided radiotherapy planning, their use in preclinical studies is still limited due to their poor resolution and because they are very expensive[110].
There are several types of xenograft models available for breast and prostate cancer research (summarized in Table 3). Subcutaneous models are most widely used because they are cheap, fast and easy to perform, but they lack the correct tumor microenvironment. The presence of tumor microenvironment is very important and necessary for obtaining results that are clinically predictive. It would be important to use preclinical efficacy models that incorporate tumor microenvironment instead of or in addition to subcutaneous models to decrease the very high number of cancer drugs that fail in clinical trials due to poor efficacy.
| Type | Relevant ME | Metastases | Costs | Ease | Ref. |
| Subcutaneous | No | No | Low | Easy | [51] |
| Orthotopic BrCa | Yes | Yes | Low | Easy | [45] |
| Orthotopic PCa | Yes | Yes | Medium | Difficult | [55] |
| Intratibial | Yes | Yes | Medium | Difficult | [56] |
| Intravenous/cardiac | Yes | Yes | Medium | Medium | [60] |
| Humanized | Yes | Yes | High | Difficult | [67] |
| PDX | Yes | No | High | Difficult | [94] |
Dr. Natalija Eigéliené is warmly thanked for providing a representative photomicrograph of normal human mammary tissue.
P- Reviewer: Lo Nigro C, Spahn M S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19516] [Article Influence: 780.6] [Reference Citation Analysis (0)] |
| 2. | Varga J, De Oliveira T, Greten FR. The architect who never sleeps: tumor-induced plasticity. FEBS Lett. 2014;588:2422-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Polyak K. Tumor heterogeneity confounds and illuminates: a case for Darwinian tumor evolution. Nat Med. 2014;20:344-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Roy-Burman P, Wu H, Powell WC, Hagenkord J, Cohen MB. Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocr Relat Cancer. 2004;11:225-254. [PubMed] |
| 5. | Guénet JL. The mouse genome. Genome Res. 2005;15:1729-1740. [PubMed] |
| 6. | Cheon DJ, Orsulic S. Mouse models of cancer. Annu Rev Pathol. 2011;6:95-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 258] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 7. | Sakakura T, Suzuki Y, Shiurba R. Mammary stroma in development and carcinogenesis. J Mammary Gland Biol Neoplasia. 2013;18:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Pollard JW. Tumour-stromal interactions. Transforming growth factor-beta isoforms and hepatocyte growth factor/scatter factor in mammary gland ductal morphogenesis. Breast Cancer Res. 2001;3:230-237. [PubMed] |
| 9. | Sims AH, Howell A, Howell SJ, Clarke RB. Origins of breast cancer subtypes and therapeutic implications. Nat Clin Pract Oncol. 2007;4:516-525. [PubMed] |
| 10. | Russo J, Lynch H, Russo IH. Mammary gland architecture as a determining factor in the susceptibility of the human breast to cancer. Breast J. 2001;7:278-291. [PubMed] |
| 11. | Parmar H, Cunha GR. Epithelial-stromal interactions in the mouse and human mammary gland in vivo. Endocr Relat Cancer. 2004;11:437-458. [PubMed] |
| 12. | Dittmer J, Leyh B. The impact of tumor stroma on drug response in breast cancer. Semin Cancer Biol. 2015;31C:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Haagensen CD. The physiology of the breast as it concerns the clinician. Am J Obstet Gynecol. 1971;109:206-209. [PubMed] |
| 14. | Topper YJ, Freeman CS. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980;60:1049-1106. [PubMed] |
| 15. | Lee CH, Akin-Olugbade O, Kirschenbaum A. Overview of prostate anatomy, histology, and pathology. Endocrinol Metab Clin North Am. 2011;40:565-575, viii-ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Sugimura K. [Staging and tissue characterization of prostate carcinoma: role of endorectal MR imaging and MR spectroscopy]. Hinyokika Kiyo. 2000;46:855-859. [PubMed] |
| 17. | Sadlonova A, Bowe DB, Novak Z, Mukherjee S, Duncan VE, Page GP, Frost AR. Identification of molecular distinctions between normal breast-associated fibroblasts and breast cancer-associated fibroblasts. Cancer Microenviron. 2009;2:9-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18:884-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 987] [Cited by in RCA: 897] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 19. | Werbeck JL, Thudi NK, Martin CK, Premanandan C, Yu L, Ostrowksi MC, Rosol TJ. Tumor Microenvironment Regulates Metastasis and Metastasis Genes of Mouse MMTV-PymT Mammary Cancer Cells In Vivo. Vet Pathol. 2014;51:868-881. [PubMed] |
| 20. | Loeffler M, Krüger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955-1962. [PubMed] |
| 21. | Lasfargues EY, Ozzello L. Cultivation of human breast carcinomas. J Natl Cancer Inst. 1958;21:1131-1147. [PubMed] |
| 22. | Cailleau R, Olivé M, Cruciger QV. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro. 1978;14:911-915. [PubMed] |
| 23. | Soule HD, Vazguez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409-1416. [PubMed] |
| 24. | Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13:215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1089] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 25. | van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205-225. [PubMed] |
| 26. | van Weerden WM, Bangma C, de Wit R. Human xenograft models as useful tools to assess the potential of novel therapeutics in prostate cancer. Br J Cancer. 2009;100:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Stephenson RA, Dinney CP, Gohji K, Ordóñez NG, Killion JJ, Fidler IJ. Metastatic model for human prostate cancer using orthotopic implantation in nude mice. J Natl Cancer Inst. 1992;84:951-957. [PubMed] |
| 28. | Veldscholte J, Berrevoets CA, Brinkmann AO, Grootegoed JA, Mulder E. Anti-androgens and the mutated androgen receptor of LNCaP cells: differential effects on binding affinity, heat-shock protein interaction, and transcription activation. Biochemistry. 1992;31:2393-2399. [PubMed] |
| 29. | Loberg RD, St John LN, Day LL, Neeley CK, Pienta KJ. Development of the VCaP androgen-independent model of prostate cancer. Urol Oncol. 2006;24:161-168. [PubMed] |
| 30. | Korenchuk S, Lehr JE, MClean L, Lee YG, Whitney S, Vessella R, Lin DL, Pienta KJ. VCaP, a cell-based model system of human prostate cancer. In Vivo. 2001;15:163-168. [PubMed] |
| 31. | Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644-648. [PubMed] |
| 32. | Sramkoski RM, Pretlow TG, Giaconia JM, Pretlow TP, Schwartz S, Sy MS, Marengo SR, Rhim JS, Zhang D, Jacobberger JW. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim. 1999;35:403-409. [PubMed] |
| 33. | van Weerden WM, de Ridder CM, Verdaasdonk CL, Romijn JC, van der Kwast TH, Schröder FH, van Steenbrugge GJ. Development of seven new human prostate tumor xenograft models and their histopathological characterization. Am J Pathol. 1996;149:1055-1062. [PubMed] |
| 34. | Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064-9068. [PubMed] |
| 35. | Krause S, Maffini MV, Soto AM, Sonnenschein C. The microenvironment determines the breast cancer cells’ phenotype: organization of MCF7 cells in 3D cultures. BMC Cancer. 2010;10:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, Chakraborty S, Rowley D. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate. 2001;49:213-223. [PubMed] |
| 37. | Windus LC, Glover TT, Avery VM. Bone-stromal cells up-regulate tumourigenic markers in a tumour-stromal 3D model of prostate cancer. Mol Cancer. 2013;12:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Hsiao AY, Torisawa YS, Tung YC, Sud S, Taichman RS, Pienta KJ, Takayama S. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials. 2009;30:3020-3027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 39. | Hu M, Peluffo G, Chen H, Gelman R, Schnitt S, Polyak K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci USA. 2009;106:3372-3377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 192] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 40. | Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, Richardson A, Violette S, Nikolskaya T, Nikolsky Y. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 389] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 41. | Sharma M, Beck AH, Webster JA, Espinosa I, Montgomery K, Varma S, van de Rijn M, Jensen KC, West RB. Analysis of stromal signatures in the tumor microenvironment of ductal carcinoma in situ. Breast Cancer Res Treat. 2010;123:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Espina V, Mariani BD, Gallagher RI, Tran K, Banks S, Wiedemann J, Huryk H, Mueller C, Adamo L, Deng J. Malignant precursor cells pre-exist in human breast DCIS and require autophagy for survival. PLoS One. 2010;5:e10240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430-1441. [PubMed] |
| 44. | Sung KE, Su X, Berthier E, Pehlke C, Friedl A, Beebe DJ. Understanding the impact of 2D and 3D fibroblast cultures on in vitro breast cancer models. PLoS One. 2013;8:e76373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 45. | Säfholm A, Tuomela J, Rosenkvist J, Dejmek J, Härkönen P, Andersson T. The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility. Clin Cancer Res. 2008;14:6556-6563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Tarkkonen KM, Nilsson EM, Kähkönen TE, Dey JH, Heikkilä JE, Tuomela JM, Liu Q, Hynes NE, Härkönen PL. Differential roles of fibroblast growth factor receptors (FGFR) 1, 2 and 3 in the regulation of S115 breast cancer cell growth. PLoS One. 2012;7:e49970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Biswas T, Gu X, Yang J, Ellies LG, Sun LZ. Attenuation of TGF-β signaling supports tumor progression of a mesenchymal-like mammary tumor cell line in a syngeneic murine model. Cancer Lett. 2014;346:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Tse BW, Russell PJ, Lochner M, Förster I, Power CA. IL-18 inhibits growth of murine orthotopic prostate carcinomas via both adaptive and innate immune mechanisms. PLoS One. 2011;6:e24241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Zhang M, Ju W, Yao Z, Yu P, Wei BR, Simpson RM, Waitz R, Fassò M, Allison JP, Waldmann TA. Augmented IL-15Rα expression by CD40 activation is critical in synergistic CD8 T cell-mediated antitumor activity of anti-CD40 antibody with IL-15 in TRAMP-C2 tumors in mice. J Immunol. 2012;188:6156-6164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Sharkey FE, Fogh J. Metastasis of human tumors in athymic nude mice. Int J Cancer. 1979;24:733-738. [PubMed] |
| 51. | Tuomela J, Grönroos TJ, Valta MP, Sandholm J, Schrey A, Seppänen J, Marjamäki P, Forsback S, Kinnunen I, Solin O. Fast growth associated with aberrant vasculature and hypoxia in fibroblast growth factor 8b (FGF8b) over-expressing PC-3 prostate tumour xenografts. BMC Cancer. 2010;10:596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Wartha K, Herting F, Hasmann M. Fit-for purpose use of mouse models to improve predictivity of cancer therapeutics evaluation. Pharmacol Ther. 2014;142:351-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Rembrink K, Romijn JC, van der Kwast TH, Rübben H, Schröder FH. Orthotopic implantation of human prostate cancer cell lines: a clinically relevant animal model for metastatic prostate cancer. Prostate. 1997;31:168-174. [PubMed] |
| 54. | Tuomela J, Sandholm J, Karihtala P, Ilvesaro J, Vuopala KS, Kauppila JH, Kauppila S, Chen D, Pressey C, Härkönen P. Low TLR9 expression defines an aggressive subtype of triple-negative breast cancer. Breast Cancer Res Treat. 2012;135:481-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Tuomela JM, Valta MP, Väänänen K, Härkönen PL. Alendronate decreases orthotopic PC-3 prostate tumor growth and metastasis to prostate-draining lymph nodes in nude mice. BMC Cancer. 2008;8:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Valta MP, Tuomela J, Vuorikoski H, Loponen N, Väänänen RM, Pettersson K, Väänänen HK, Härkönen PL. FGF-8b induces growth and rich vascularization in an orthotopic PC-3 model of prostate cancer. J Cell Biochem. 2009;107:769-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Tuomela J, Valta M, Seppänen J, Tarkkonen K, Väänänen HK, Härkönen P. Overexpression of vascular endothelial growth factor C increases growth and alters the metastatic pattern of orthotopic PC-3 prostate tumors. BMC Cancer. 2009;9:362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Hafeez BB, Zhong W, Fischer JW, Mustafa A, Shi X, Meske L, Hong H, Cai W, Havighurst T, Kim K. Plumbagin, a medicinal plant (Plumbago zeylanica)-derived 1,4-naphthoquinone, inhibits growth and metastasis of human prostate cancer PC-3M-luciferase cells in an orthotopic xenograft mouse model. Mol Oncol. 2013;7:428-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518-524. [PubMed] |
| 60. | Shevrin DH, Gorny KI, Kukreja SC. Patterns of metastasis by the human prostate cancer cell line PC-3 in athymic nude mice. Prostate. 1989;15:187-194. [PubMed] |
| 61. | Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, Yang H, Zhau HE, Balian G, Chung LW. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998;77:887-894. [PubMed] |
| 62. | Yu C, Shiozawa Y, Taichman RS, McCauley LK, Pienta K, Keller E. Prostate cancer and parasitism of the bone hematopoietic stem cell niche. Crit Rev Eukaryot Gene Expr. 2012;22:131-148. [PubMed] |
| 64. | Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res. 2001;16:1486-1495. [PubMed] |
| 65. | Peyruchaud O, Winding B, Pécheur I, Serre CM, Delmas P, Clézardin P. Early detection of bone metastases in a murine model using fluorescent human breast cancer cells: application to the use of the bisphosphonate zoledronic acid in the treatment of osteolytic lesions. J Bone Miner Res. 2001;16:2027-2034. [PubMed] |
| 66. | Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004;101:4966-4971. [PubMed] |
| 67. | Nemeth JA, Harb JF, Barroso U, He Z, Grignon DJ, Cher ML. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res. 1999;59:1987-1993. [PubMed] |
| 68. | Yonou H, Yokose T, Kamijo T, Kanomata N, Hasebe T, Nagai K, Hatano T, Ogawa Y, Ochiai A. Establishment of a novel species- and tissue-specific metastasis model of human prostate cancer in humanized non-obese diabetic/severe combined immunodeficient mice engrafted with human adult lung and bone. Cancer Res. 2001;61:2177-2182. [PubMed] |
| 69. | Kuperwasser C, Dessain S, Bierbaum BE, Garnet D, Sperandio K, Gauvin GP, Naber SP, Weinberg RA, Rosenblatt M. A mouse model of human breast cancer metastasis to human bone. Cancer Res. 2005;65:6130-6138. [PubMed] |
| 70. | Ottewell PD, Wang N, Meek J, Fowles CA, Croucher PI, Eaton CL, Holen I. Castration-induced bone loss triggers growth of disseminated prostate cancer cells in bone. Endocr Relat Cancer. 2014;21:769-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 71. | Reichert JC, Saifzadeh S, Wullschleger ME, Epari DR, Schütz MA, Duda GN, Schell H, van Griensven M, Redl H, Hutmacher DW. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials. 2009;30:2149-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 285] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 72. | Holzapfel BM, Thibaudeau L, Hesami P, Taubenberger A, Holzapfel NP, Mayer-Wagner S, Power C, Clements J, Russell P, Hutmacher DW. Humanised xenograft models of bone metastasis revisited: novel insights into species-specific mechanisms of cancer cell osteotropism. Cancer Metastasis Rev. 2013;32:129-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Kirma NB, Tekmal RR. Transgenic mouse models of hormonal mammary carcinogenesis: advantages and limitations. J Steroid Biochem Mol Biol. 2012;131:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Cardiff RD. Validity of mouse mammary tumour models for human breast cancer: comparative pathology. Microsc Res Tech. 2001;52:224-230. [PubMed] |
| 76. | Bosland MC. Use of animal models in defining efficacy of chemoprevention agents against prostate cancer. Eur Urol. 1999;35:459-463. [PubMed] |
| 77. | Green JE, Greenberg NM, Ashendel CL, Barrett JC, Boone C, Getzenberg RH, Henkin J, Matusik R, Janus TJ, Scher HI. Workgroup 3: transgenic and reconstitution models of prostate cancer. Prostate. 1998;36:59-63. [PubMed] |
| 78. | Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM, Greenberg NM. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096-4102. [PubMed] |
| 79. | Marconett CN, Morgenstern TJ, San Roman AK, Sundar SN, Singhal AK, Firestone GL. BZL101, a phytochemical extract from the Scutellaria barbata plant, disrupts proliferation of human breast and prostate cancer cells through distinct mechanisms dependent on the cancer cell phenotype. Cancer Biol Ther. 2010;10:397-405. [PubMed] |
| 80. | Hruska KS, Tilli MT, Ren S, Cotarla I, Kwong T, Li M, Fondell JD, Hewitt JA, Koos RD, Furth PA. Conditional over-expression of estrogen receptor alpha in a transgenic mouse model. Transgenic Res. 2002;11:361-372. [PubMed] |
| 81. | Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439-3443. [PubMed] |
| 82. | Kasper S, Sheppard PC, Yan Y, Pettigrew N, Borowsky AD, Prins GS, Dodd JG, Duckworth ML, Matusik RJ. Development, progression, and androgen-dependence of prostate tumors in probasin-large T antigen transgenic mice: a model for prostate cancer. Lab Invest. 1998;78:319-333. [PubMed] |
| 83. | Chiaverotti T, Couto SS, Donjacour A, Mao JH, Nagase H, Cardiff RD, Cunha GR, Balmain A. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol. 2008;172:236-246. [PubMed] |
| 84. | Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223-238. [PubMed] |
| 85. | He WW, Sciavolino PJ, Wing J, Augustus M, Hudson P, Meissner PS, Curtis RT, Shell BK, Bostwick DG, Tindall DJ. A novel human prostate-specific, androgen-regulated homeobox gene (NKX3.1) that maps to 8p21, a region frequently deleted in prostate cancer. Genomics. 1997;43:69-77. [PubMed] |
| 86. | Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng CX. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22:37-43. [PubMed] |
| 87. | Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356-362. [PubMed] |
| 88. | Simpson L, Parsons R. PTEN: life as a tumor suppressor. Exp Cell Res. 2001;264:29-41. [PubMed] |
| 89. | Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van Dyke T. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. [PubMed] |
| 90. | Jin K, Teng L, Shen Y, He K, Xu Z, Li G. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin Transl Oncol. 2010;12:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 91. | Daniel VC, Marchionni L, Hierman JS, Rhodes JT, Devereux WL, Rudin CM, Yung R, Parmigiani G, Dorsch M, Peacock CD. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69:3364-3373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 92. | Kopetz S, Lemos R, Powis G. The promise of patient-derived xenografts: the best laid plans of mice and men. Clin Cancer Res. 2012;18:5160-5162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 93. | Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 434] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 94. | Thong AE, Zhao H, Ingels A, Valta MP, Nolley R, Santos J, Young SR, Peehl DM. Tissue slice grafts of human renal cell carcinoma: an authentic preclinical model with high engraftment rate and metastatic potential. Urol Oncol. 2014;32:43.e23-43.e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 95. | Toivanen R, Taylor RA, Pook DW, Ellem SJ, Risbridger GP. Breaking through a roadblock in prostate cancer research: an update on human model systems. J Steroid Biochem Mol Biol. 2012;131:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 96. | Carlsson G, Gullberg B, Hafström L. Estimation of liver tumor volume using different formulas - an experimental study in rats. J Cancer Res Clin Oncol. 1983;105:20-23. [PubMed] |
| 97. | Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148-154. [PubMed] |
| 98. | Bobek V, Hoffman RM, Kolostova K. Site-specific cytomorphology of disseminated PC-3 prostate cancer cells visualized in vivo with fluorescent proteins. Diagn Cytopathol. 2013;41:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 99. | Quintana E, Piskounova E, Shackleton M, Weinberg D, Eskiocak U, Fullen DR, Johnson TM, Morrison SJ. Human melanoma metastasis in NSG mice correlates with clinical outcome in patients. Sci Transl Med. 2012;4:159ra149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 100. | Bauer S, Kirschning CJ, Häcker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237-9242. [PubMed] |
| 101. | Hyde SC, Pringle IA, Abdullah S, Lawton AE, Davies LA, Varathalingam A, Nunez-Alonso G, Green AM, Bazzani RP, Sumner-Jones SG. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat Biotechnol. 2008;26:549-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 102. | Haase R, Argyros O, Wong SP, Harbottle RP, Lipps HJ, Ogris M, Magnusson T, Vizoso Pinto MG, Haas J, Baiker A. pEPito: a significantly improved non-viral episomal expression vector for mammalian cells. BMC Biotechnol. 2010;10:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 103. | Argyros O, Wong SP, Fedonidis C, Tolmachov O, Waddington SN, Howe SJ, Niceta M, Coutelle C, Harbottle RP. Development of S/MAR minicircles for enhanced and persistent transgene expression in the mouse liver. J Mol Med (Berl). 2011;89:515-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Wong SP, Harbottle RP. Genetic modification of dividing cells using episomally maintained S/MAR DNA vectors. Mol Ther Nucleic Acids. 2013;2:e115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 105. | Valta MP, Tuomela J, Bjartell A, Valve E, Väänänen HK, Härkönen P. FGF-8 is involved in bone metastasis of prostate cancer. Int J Cancer. 2008;123:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 106. | Clark DP, Badea CT. Micro-CT of rodents: state-of-the-art and future perspectives. Phys Med. 2014;30:619-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 107. | Jennbacken K, Gustavsson H, Tesan T, Horn M, Vallbo C, Welén K, Damber JE. The prostatic environment suppresses growth of androgen-independent prostate cancer xenografts: an effect influenced by testosterone. Prostate. 2009;69:1164-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 108. | Kraaij R, van Weerden WM, de Ridder CM, Gussenhoven EJ, Honkoop J, Nasu Y, Bangma CH. Validation of transrectal ultrasonographic volumetry for orthotopic prostate tumours in mice. Lab Anim. 2002;36:165-172. [PubMed] |
| 109. | Bates D, Abraham S, Campbell M, Zehbe I, Curiel L. Development and characterization of an antibody-labeled super-paramagnetic iron oxide contrast agent targeting prostate cancer cells for magnetic resonance imaging. PLoS One. 2014;9:e97220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 110. | Minn H. PET and SPECT in low-grade glioma. Eur J Radiol. 2005;56:171-178. [PubMed] |