Published online Mar 9, 2015. doi: 10.5497/wjp.v4.i1.160
Peer-review started: November 13, 2014
First decision: December 26, 2014
Revised: January 3, 2015
Accepted: January 18, 2015
Article in press: Janurary 20, 2015
Published online: March 9, 2015
Processing time: 120 Days and 5.8 Hours
Gastrointestinal cancers are the most common human cancers in both men and women worldwide. Several epidemiological and experimental studies suggest a relationship between gastrointestinal cancers risk and dietary factors. Natural honey has been widely used in traditional medicine for many centuries to treat a wide range of ailments and complaints. Honey contains various components that exhibit wide activities including antibacterial, anti-inflammatory, antioxidant and anticancer properties. The anticancer effects of honey are mediated via diverse mechanisms, including inhibition of proliferation, induction of apoptosis, suppression of free radicals and modulation of inflammatory signalling pathways. The present review assesses the chemopreventive effects of natural honey and its components in the modulation of gastrointestinal cancers and its modes of action in the prevention of the development of gastrointestinal tumors. Honey can be an approach as a cancer-preventive strategy which merits further experimental and clinical research in the near future.
Core tip: Natural honey has been widely used in traditional medicine to treat a wide range of ailments and complaints. Honey contains various components that exhibit wide activities including antibacterial, anti-inflammatory, antioxidant and anticancer properties. The present review assesses the chemopreventive effects of natural honey and its components in the modulation and prevention of gastrointestinal cancers.
- Citation: Abdel-Latif MM. Chemoprevention of gastrointestinal cancers by natural honey. World J Pharmacol 2015; 4(1): 160-167
- URL: https://www.wjgnet.com/2220-3192/full/v4/i1/160.htm
- DOI: https://dx.doi.org/10.5497/wjp.v4.i1.160
Cancer is a leading cause of death worldwide. It strikes more than one third of the world population and it’s the cause of more than 20% of all deaths[1]. Gastrointestinal cancers (cancers of the digestive system) include cancers of the esophagus, gallbladder, liver, pancreas, stomach, small intestine, large intestine (colon) and rectum. The risk of gastrointestinal cancers varies greatly by individual ethnic group, lifestyles and risk factors[2]. Considering the magnitude of the problem of cancer and the failure of conventional therapy of affects greatly the mortality rates for many types of cancers, new approaches to control of cancer and discovery of new agents is of great importance.
In recent years, with rising prevalence of gastrointestinal cancers by several factors such as changing lifestyle and improved screening and diagnosis, besides the obstacles of the current cancer therapy (chemotherapy and radiotherapy), there has been a great trend towards the use of dietary factors and natural products among cancer patients[3,4]. The incidence of gastrointestinal cancers varies considerably from place to place and from time to time. It is clear that environmental factors play an important part in the development of these cancers and that many of these factors may be preventible. Oesophageal adenocarcinoma has been dramatically rising over the past decades[5], and this rise in this cancer has been associated with an increased prevalence of gastroesophageal reflux disease and Barrett’s esophagus. Fruit and vegetable consumption has been reported to have a protective effect and associated with a lower risk of oesophageal cancer. Gastric adenocarcinoma is the second leading cause of cancer worldwide[5]. Foods that are smoked, dried, or pickled have been associated with an increased risk of gastric cancer[6,7]. Vegetable and fruit intake has consistently been associated with a decreased incidence of gastric cancer[8]. Pancreatic cancer is also one of the most devastating cancers, with a 5-year survival of only 6%[9]. High dietary fiber intake, vegetable and fruit intake reduces pancreatic cancer risk[10]. Colorectal cancer is also one of the leading cancers in both men and women in several countries[11], and several epidemiological and experimental studies suggest a link between colon cancer risk and dietary factors[12,13].
Carcinogenesis is a multistep process induced by molecular and genetic changes that disrupt signaling pathways regulating proliferation, apoptosis and differentiation[14]. The search for anticancer agents from natural sources for prevention and treatment of cancer is of a considerable interest in recent years. Several approaches are explored for the prevention and treatment of cancer including chemoprevention in animal models and clinical trials[15]. Dietary supplements and natural compounds are one approach is used to reverse or prevent the development of cancer by modulating the molecular processes of initiation, promotion, and progression stages. It has been reported that diet components such as turmeric, garlic, ginger, cruciferous vegetables and green tea play an important role in cancer prevention[16,17]. The role of diet and prevention of gastrointestinal cancers is evolving and much data from basic science and animal models. Natural honey has been recently the focus of basic research and clinical studies for its several therapeutic benefits including cancer.
Since ancient times, natural honey has been widely used as a conventional medicine, and is extensively used for its therapeutic effects in recent years. Ancient Egyptians, Chinese, Greeks and Romans employed honey for wounds and diseases of the intestine[18]. In the Holy Quran, Almighty Allah mentioned the special ability of honey to heal and cure disease. Scientific research has proven the therapeutic benefits of honey in treating several human diseases. The physical properties of natural honey depend on water content, temperature, the type of flora and the proportion of its sugars. The color of the honey varies according to the floral source and its mineral content, which usually ranges from water white to dark amber (Figure 1).
A large number of natural honeys are available worldwide and are either locally produced or imported from other countries. The type of natural honey depends on which species of plants were flowering when the bees were producing the honey. Honey from a single floral source greatly varies from honey of the same floral source that obtained from different locations or seasons. The percentage of fructose, glucose, amount and type of amino acids and the organic acids vary by floral source. The floral source affects not only the physicochemical properties of the honey, but also the antimicrobial activity of honey[19-21]. Different types of honey differ in their color, flavor and density. The antibacterial quality of honey varies among different types of honey depending on geographical locations, seasonal source, harvesting, purity and storage conditions[22-24]. A survey of the antibacterial activity of 52 samples of 24 types of honey of Saudi and some international honeys revealed that the antibacterial activity of the majority of the investigated honeys did not show large variations[25]. The equivalent phenol percent concentrations for the majority of honeys ranged between 5.5% and 7.9%. It was also noticed in this study that there was no relationship between color and antibacterial activity of honey. The antimicrobial activity of honey could be attributed to several factors like the osmotic effect of honey, acidity, the presence of hydrogen peroxide, the presence of antibacterial phytochemical components and the in-vivo antibacterial activity of honey[22,26-29]. In-vitro antioxidant activity of Saudi Sidr honey “monofloral type of honey” revealed a strong antioxidant activity[30]. Furthermore, pretreatment with Sidr honey prior to the administration of CCl4 significantly prevented the increase of the serum levels of enzyme markers and reduced oxidative stress in rats.
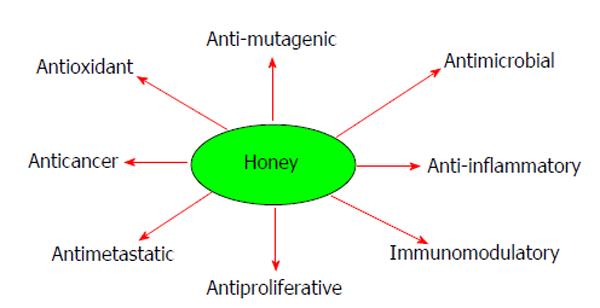
The composition of natural honey varies, depending on many factors such as the geographical areas, source of honeybee food, climate, environmental conditions and the processing it undergoes[31-35]. Honey contains about 200 substances including fructose, glucose, amino acids, vitamins, minerals, water and enzymes[18,31], as shown in Table 1. Natural honey uses a combination of components, including hydrogen peroxide, acidity, osmotic effect, high sugar concentration and polyphenols to prevent diseases and fight infection. All natural honey contains flavonoides, phenolic acids, ascorbic acid, tocopherols, catalase, superoxide dismutase, reduced glutathione[18,36]. Some of these flavonoids and phenolic compounds include chrysin, kaempferol, quercetin, pinobanksin, pinocembrin, luteolin, apigenin, genistein, naringenin, hesperetin, P-coumaric acid, gallic acid, ellagic acid, ferulic acid, syringic acid, caffeic acid and vanillic acid. Natural honey and its components have been shown to possess a wide range of medicinal properties such as anti-inflammatory, gastroprotective, antioxidant, antitumor and anticancer effects[37-41]. Figure 2 depicts some of the actions of natural honey.
| Component | Average (value per 100 g) |
| Carbohydrates | 82.4 g |
| Fructose | 38.5 g |
| Glucose | 31 g |
| Sucrose | 1 g |
| Other sugars | 11.7 g |
| Dietary fiber | 0.2 g |
| Fat | 0 g |
| Protein | 0.3 g |
| Water | 17.1 g |
| Riboflavin (Vit. B2) | 0.038 mg |
| Niacin (Vit. B3) | 0.121 mg |
| Pantothenic acid (Vit. B5) | 0.068 mg |
| Pyridoxine (Vit. B6) | 0.024 mg |
| Folate (Vit. B9) | 0.002 mg |
| Vitamin C | 0.5 mg |
| Calcium | 6 mg |
| Iron | 0.42 mg |
| Magnesium | 2 mg |
| Phosphorus | 4 mg |
| Potassium | 52 mg |
| Sodium | 4 mg |
| Zinc | 0.22 mg |
Potential mechanisms of honey actions were found to include regulation of cell cycle, induction of apoptosis, activation of mitochondrial pathway, inhibition of angiogenesis and modulation of oxidative stress. Honey can inhibit the development of cancer by blocking the three stages of carcinogenesis (initiation, promotion and progression). The inhibitory effect of honey on carcinogenesis can be attributed to the presence of its active components, especially flavonoids and phenolic constituents. Honey plays an important role in preventing inflammatory tissues from producing free radicals[42]. Caffeic acid phenethyl ester (CAPE), an active component of propolis, has many biological and pharmacological activities including antioxidant, antiinflammatory, antiviral action, anti-proliferative effect, apoptosis-inducing effect and anticancer effect[43-46]. Caffeic acid esters have been shown to have an inhibitory effect on tumor cell proliferation and transformation by the down regulation of many cellular enzymatic pathways including protein tyrosine kinase, cycloxygenase and ornithine decarboxylase pathways[47-50].
Inflammation plays an important role in the development of several diseases including gastrointestinal diseases and cancer[51,52]. Inflammation is recognized as a risk factor for gastric inflammation and Helicobacter pylori (H. pylori) infection and clinical intervention by natural products such as honey may provide an approach for reducing inflammation and H. pylori-associated diseases, particularly gastric cancer. Honey contains many phenolic compounds such as ellagic acid, gallic acid, caffeic acid, quercetin and chrysin, which correlated to its antioxidant and anti-inflammatory activities[36,52,53]. It was suggested that honey and its components can inhibit inflammation via inactivation of nuclear factor kappa-B (NF-κB) and inhibition of transcription of genes for pro-inflammatory mediators such as COX-2, tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6) and inducible nitric oxide synthase[54,55]. Gelam honey has shown to have anti-inflammatory effects by reducing the edema size and inhibiting the production of the pro-inflammatory mediators nitric oxide, prostaglandin E2, TNF-α, and IL-6 in rats[56]. Honey has been reported to potentiate the gastric protection effects of sucralfate against ammonia-induced gastric lesions in rats[57]. Alagwu has reported that honey intake caused cytoprotection on the gastric mucosa of albino rats[58]. Oral administration of honey has been reported to protect against gastrointestinal infection such as gastritis, duodenitis and gastric ulcer caused by bacteria[59-61]. Nasutia et al[62] demonstrated that oral pretreatment of honey prevented indomethacin-induced gastric lesions in rats. Perfusion of the stomach with isotonic honey resulted in a marked reduction of the lesions caused by ethanol and indomethacin in rats[38,63].
H. pylori infection represents the most common risk factor underlying chronic inflammation and gastric cancer[64,65]. H. pylori can lead to mucosal inflammation and cancer development through several mechanisms including H. pylori virulence factors such as CagA and VacA genotypes and inflammatory mediators that induce cellular signalling alterations in gastric cells[65]. The intake of honey also helps treat H. pylori infection[66]. Natural honey from New Zealand and Saudi Arabia at concentrations 20% (v/v) inhibited the growth of H. pylori in vitro[67,68]. Honey had an inhibitory effect on H. pylori in vitro at solutions of both 10% and 20% honey[67]. al Somal et al[59] also found that Manuka honey from New Zealand, at concentrations as low as 5% v/v, completely inhibit the growth of H. pylori, and that 2.5% v/v partially inhibits the growth of H. pylori[68]. Osmotic effects were shown to be the most important parameter for killing H. pylori as all carbohydrate solutions > or = 15% (v/v) inhibited 100% of the H. pylori. The therapeutic effect of honey was attributed to the antibacterial properties[68,69]. Osato et al[70] also reported that commercial honeys and the artificial solution were effective as Manuka honey in inhibiting growth of all H. pylori isolates at concentrations 15% v/v. It has also been reported that the use of honey with triple therapy regimen may help shorten the time required to eliminate H. pylori from stomach lining of patients with gastritis or duodenal ulcer caused by H. pylori infection[71].
H. pylori has been shown to activate mitogen-activated protein kinases and transcription factors such as AP-1 and NF-κB that regulate cell proliferation and differentiation in gastric epithelial cells using several different bacterial components and host signaling pathways[72,73]. NF-κB and activator protein-1 (AP-1) are key regulators of inflammation and signaling cascades that lead to carcinogenesis. There are numerous agents including honey have been reported to suppress NF-κB activation and act as potential chemopreventive agents for inflammation and cancer[74]. CAPE blocked H. pylori-induced NF-κB and AP-1 expression in gastric cancer cells, and CAPE also suppressed H. pylori-induced cell proliferation and production of the cytokines TNF-α and IL-8 and COX-2 expression[55]. Therefore, the inhibition of these molecules by CAPE could result in suppression of many genes during H. pylori-induced inflammation. Wu et al[75] demonstrated that the activity of NF-κB and the expression of matrix metalloproteinase-9, IL-1beta, and IL-8 in gastric cancer cells by H. pylori significantly reversed by CAPE treatment, which suggested that CAPE could be promising adjuvant agent against gastric cancer. In Monogolian gerbils, CAPE treatment elicited anti-inflammatory effects on H. pylori-induced chronic gastritis. CAPE significantly inhibited H. pylori-stimulated NF-κB activation and mRNA expression of several inflammatory factors in a dose-dependent manner, and prevented degradation of IκB-alpha and phosphorylation of p65 in gastric cancer cells[76].
There are many research studies support the use of natural honey for cancer prevention and treatment, especially cancers of the gastrointestinal tract. Nutritional studies have indicated that consumption of honey modulates the risk of the development of gastric cancer, and also honey induced apoptosis in gastric mucosa[77]. It was postulated that CAPE may be a promising adjuvant treatment in gastric cancer[78]. The chemopreventive actions of honey and its components have been also studied in various colon cancer models. Gelam and Nenas honeys suppressed the growth of HT 29 colon cancer cells by inducing DNA damage and apoptosis and suppressing inflammation[79]. Jaganathan also demonstrated the anti-proliferative effect of Caffeic acid, one of the phenolic constituents of honey, inhibited in the colon cancer cells HCT15 and HT29[80]. Honey induced apoptosis by causing the depletion of intracellular non-protein thiols and reduced the mitochondrial membrane potential and increased generation of reactive oxygen species. Furthermore, honey constituents induced apoptosis in colon cancer cells[81]. Orsolić et al[82] showed that honey exerted anti-metastatic effect in a murine tumor model with colon carcinoma. Supplementation of diet with honey and Nigella sativa had a protective effect against methylnitrosourea-induced oxidative stress, inflammatory response and carcinogenesis in Sprague Dawely rats[83]. Caffeic acid esters derivatives inhibited azoxymethane-induced colonic colonie preneoplastic lesions, ornithine decarboxylase, tyrosine protein kinase, and lipoxygenase activities and aberrant crypt foci formation, which are relevant to colon carcinogenesis in rat colon[50]. Caffeic acid and its ester are potent inhibitors of human colon adenocarcinoma cell growth[84]. Dietary administration of phenylethyl-3-methylcaffeate significantly inhibited the incidence and multiplicity of invasive, noninvasive adenocarcinomas of the colon, and also suppressed the colon tumor volume by 43% compared to the control diet, and also inhibited the formation in colonic tumors by 15%-30% in the animals[48]. Gribel’ et al[85] indicated that honey possessed moderate antitumor effect and pronounced antitumor activity of 5-flurouracil and cyclophosphamide against five different strains of rat and mouse tumors. Furthermore, honey potentiated the antitumor activity of the chemotherapeutic drugs 5-fluorouracil and cyclophosphamide in colorectal cancer cells[86,87].
The anticancer effects of natural honey and its components on liver cancer cells have been investigated in a number of studies[88-90]. Treatment of hepatocellular carcinoma HepG2 cells with bee honey and Nigella sativa led to a significant decrease in both the number of viable HepG2 cells and the levels of nitric oxide and improved the total antioxidant status and caspase-3 activity, especially in HepG2 cells treated with higher doses of bee honey Nigella sativa (20% and 5000 μg/mL)[88]. It has been reported that Spanish honeys were most effective in protecting against food mutagen-induced DNA damage in HepG2 cells, which was attributed to its antioxidant and free radical scavenging properties[89]. Gelam honey was selectively cytotoxic to liver cancer cells and found that the IC50 value of gelam honey towards HepG2 was 25% whereas it was 70% for normal human hepatocytes (WRL-68)[90]. Abdel Aziz et al[91] reported that honey extracts exerted cytotoxic, antimetastatic and anti-angiogenic effects in HepG2 cells. Treatment with diethylnitrosamine induced hepatic cancer in rats and the neoplastic hepatic cells were reduced in the liver of honey-treated DEN-induced rats[92]. These studies indicate that honey has an anticancer effect on various types of cancer cells and exerts its protective effect against the development of cancer by modulating the molecular and cellular mechanisms of carcinogenesis stages. Some of the mechanisms by which honey may exert its anticancer effects are cell cycle arrest, activation of mitochondrial pathway, induction of mitochondrial outer membrane permeabilization, induction of apoptosis, modulation of oxidative stress, amelioration of inflammation, modulation of insulin signaling, and inhibition of angiogenesis[37].
The effect of honey was also investigated in pancreatic cancer. Caffeic acid phenethyl ester induced apoptosis in human pancreatic cancer cells by activation of caspase-3/caspase-7 and mitochondrial dysfunction[93]. Treatment with CAPE slightly restored the expression of E-cadherin and markedly reversed the transforming growth factor-β-induced overexpression of vimentin at 24 h in Human pancreatic cancer cells (PANC-1) cells. CAPE suppressed the expression of Twist 2 and growth of PANC-1 xenografts without significant toxicity in an orthotopic pancreatic cancer model. These data suggest that CAPE could suppress the epithelial-mesenchymal transition in pancreatic cancer[94].
Natural honey has many therapeutic benefits and medical uses. The different effects of natural honey including anti-inflammatory antioxidants and anticancer effects highlight its importance in the prevention of gastrointestinal cancers and improvement of cancer therapies. Some evidence of the anticancer effects of honey has been reported from in-vitro and in-vivo studies in gastrointestinal cancers, however, further investigation of anticancer effects of honey in animal and clinical studies are required to prove its therapeutic efficacy in chemoprevention strategies.
P- Reviewer: Ukleja A S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25545] [Article Influence: 1824.6] [Reference Citation Analysis (7)] |
| 2. | Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1754] [Cited by in RCA: 1882] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 3. | Oh B, Butow P, Mullan B, Beale P, Pavlakis N, Rosenthal D, Clarke S. The use and perceived benefits resulting from the use of complementary and alternative medicine by cancer patients in Australia. Asia Pac J Clin Oncol. 2010;6:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Zanini S, Marzotto M, Giovinazzo F, Bassi C, Bellavite P. Effects of Dietary Components on Cancer of the Digestive System. Crit Rev Food Sci Nutr. 2014;Epub ahead of print. [PubMed] |
| 5. | Blot WJ, Devesa SS, Kneller RW, Fraumeni JF. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289. [PubMed] |
| 6. | Coggon D, Barker DJ, Cole RB, Nelson M. Stomach cancer and food storage. J Natl Cancer Inst. 1989;81:1178-1182. [PubMed] |
| 7. | Graham S, Haughey B, Marshall J, Brasure J, Zielezny M, Freudenheim J, West D, Nolan J, Wilkinson G. Diet in the epidemiology of gastric cancer. Nutr Cancer. 1990;13:19-34. [PubMed] |
| 8. | Kono S, Hirohata T. Nutrition and stomach cancer. Cancer Causes Control. 1996;7:41-55. [PubMed] |
| 9. | Yeo TP, Hruban RH, Leach SD, Wilentz RE, Sohn TA, Kern SE, Iacobuzio-Donahue CA, Maitra A, Goggins M, Canto MI. Pancreatic cancer. Curr Probl Cancer. 2002;26:176-275. [PubMed] |
| 10. | Howe GR, Burch JD. Nutrition and pancreatic cancer. Cancer Causes Control. 1996;7:69-82. [PubMed] |
| 11. | Wingo PA, Tong T, Bolden S. Cancer statistics, 1995. CA Cancer J Clin. 1995;45:8-30. [PubMed] |
| 12. | Wynder EL, Shigematsu T. Environmental factors of cancer of the colon and rectum. Cancer. 1967;20:1520-1561. [PubMed] |
| 13. | Reddy BS. Diet and colon cancer: evidence from human and animal model studies. Diet, Nutrition and Cancer: A Critical Evaluation. Boca Raton, FL: CRC Press 1986; 47-65. |
| 14. | López-Lázaro M. A new view of carcinogenesis and an alternative approach to cancer therapy. Mol Med. 2010;16:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Boone CW, Kelloff GJ, Malone WE. Identification of candidate cancer chemopreventive agents and their evaluation in animal models and human clinical trials: a review. Cancer Res. 1990;50:2-9. [PubMed] |
| 16. | Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768-780. [PubMed] |
| 17. | Chun KS, Kim EH, Lee S, Hahm KB. Chemoprevention of gastrointestinal cancer: the reality and the dream. Gut Liver. 2013;7:137-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health: a review. J Am Coll Nutr. 2008;27:677-689. [PubMed] |
| 19. | Liu JR, Ye YL, Lin TY, Wang YW, Peng CC. Effect of floral sources on the antioxidant, antimicrobial, and anti-inflammatory activities of honeys in Taiwan. Food Chem. 2013;139:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Gorjanović SZ, Alvarez-Suarez JM, Novaković MM, Pastor FT, Pezo L, Battino M, Sužnjević DŽ. Comparative analysis of antioxidant activity of honey of different floral sources using recently developed polarographic and various spectrophotometric assays. J Food Comp Anal. 2013;30:13-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Alvarez-Suarez JM, Giampieri F, Battino M. Honey as a source of dietary antioxidants: structures, bioavailability and evidence of protective effects against human chronic diseases. Curr Med Chem. 2013;20:621-638. [PubMed] |
| 22. | Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011;1:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 356] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 23. | Wilkinson JM, Cavanagh HM. Antibacterial activity of 13 honeys against Escherichia coli and Pseudomonas aeruginosa. J Med Food. 2005;8:100-103. [PubMed] |
| 24. | Irish J, Blair S, Carter DA. The antibacterial activity of honey derived from Australian flora. PLoS One. 2011;6:e18229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | Halawani E, Shohayeb M. Survey of the antibacterial activity of Saudi and some international honeys. J Microbiol Antimicrob. 2011;3:94-101. |
| 26. | Kwakman PH, te Velde AA, de Boer L, Speijer D, Vandenbroucke-Grauls CM, Zaat SA. How honey kills bacteria. FASEB J. 2010;24:2576-2582. [PubMed] |
| 27. | Kwakman PH, Zaat SA. Antibacterial components of honey. IUBMB Life. 2012;64:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 29. | Allen KL, Molan PC, Reid GM. A survey of the antibacterial activity of some New Zealand honeys. J Pharm Pharmacol. 1991;43:817-822. [PubMed] |
| 30. | Al-Yahya M, Mothana R, Al-Said M, Al-Dosari M, Al-Musayeib N, Al-Sohaibani M, Parvez MK, Rafatullah S. Attenuation of CCl4-Induced Oxidative Stress and Hepatonephrotoxicity by Saudi Sidr Honey in Rats. Evid Based Complement Alternat Med. 2013;2013:569037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | White JW. Composition of honey. Honey: A Comprehensive Survey. London: Heinemann 1979; 157-192. |
| 32. | Viuda-Martos M, Ruiz-Navajas Y, Fernandez-Lopez J, Perez-Alvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008;73:R117-124. |
| 33. | Gheldof N, Wang XH, Engeseth NJ. Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem. 2002;50:5870-5877. [PubMed] |
| 34. | Azeredo L da C, Azeredo MAA, De Souza SR, Dutra VML. Protein contents and physicochemical properties in honey samples of Apis mellifera of different floral origins. Food Chem. 2003;80:249-254. [RCA] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Aljadi AM, Kamaruddin MY. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004;85:513-518. [RCA] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 36. | Eteraf-Oskouei T, Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran J Basic Med Sci. 2013;16:731-742. [PubMed] |
| 37. | Erejuwa OO, Sulaiman SA, Wahab MS. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules. 2014;19:2497-2522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 38. | Gharzouli K, Amira S, Gharzouli A, Khennouf S. Gastroprotective effects of honey and glucose-fructose-sucrose-maltose mixture against ethanol-, indomethacin-, and acidified aspirin-induced lesions in the rat. Exp Toxicol Pathol. 2002;54:217-221. [PubMed] |
| 39. | Al-Waili NS, Saloom KY, Al-Waili TN, Al-Waili AN, Akmal M, Al-Waili FS, Al-Waili HN. Influence of various diet regimens on deterioration of hepatic function and hematological parameters following carbon tetrachloride: a potential protective role of natural honey. Nat Prod Res. 2006;20:1258-1264. [PubMed] |
| 40. | Kassim M, Achoui M, Mustafa MR, Mohd MA, Yusoff KM. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr Res. 2010;30:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 41. | Ahmed S, Othman NH. Honey as a potential natural anticancer agent: a review of its mechanisms. Evid Based Complement Alternat Med. 2013;2013:829070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Bilsel Y, Bugra D, Yamaner S, Bulut T, Cevikbas U, Turkoglu U. Could honey have a place in colitis therapy? Effects of honey, prednisolone, and disulfiram on inflammation, nitric oxide, and free radical formation. Dig Surg. 2002;19:306-311; discussion 311-312. [PubMed] |
| 43. | Chen YJ, Shiao MS, Wang SY. The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anticancer Drugs. 2001;12:143-149. [PubMed] |
| 44. | Lee YJ, Liao PH, Chen WK, Yang CY. Preferential cytotoxicity of caffeic acid phenethyl ester analogues on oral cancer cells. Cancer Lett. 2000;153:51-56. [PubMed] |
| 45. | Usia T, Banskota AH, Tezuka Y, Midorikawa K, Matsushige K, Kadota S. Constituents of Chinese propolis and their antiproliferative activities. J Nat Prod. 2002;65:673-676. [PubMed] |
| 46. | Nomura M, Kaji A, Ma W, Miyamoto K, Dong Z. Suppression of cell transformation and induction of apoptosis by caffeic acid phenethyl ester. Mol Carcinog. 2001;31:83-89. [PubMed] |
| 47. | Nagaoka T, Banskota AH, Tezuka Y, Saiki I, Kadota S. Selective antiproliferative activity of caffeic acid phenethyl ester analogues on highly liver-metastatic murine colon 26-L5 carcinoma cell line. Bioorg Med Chem. 2002;10:3351-3359. [PubMed] |
| 48. | Rao CV, Desai D, Rivenson A, Simi B, Amin S, Reddy BS. Chemoprevention of colon carcinogenesis by phenylethyl-3-methylcaffeate. Cancer Res. 1995;55:2310-2315. [PubMed] |
| 49. | Borrelli F, Izzo AA, Di Carlo G, Maffia P, Russo A, Maiello FM, Capasso F, Mascolo N. Effect of a propolis extract and caffeic acid phenethyl ester on formation of aberrant crypt foci and tumors in the rat colon. Fitoterapia. 2002;73 Suppl 1:S38-S43. [PubMed] |
| 50. | Rao CV, Desai D, Simi B, Kulkarni N, Amin S, Reddy BS. Inhibitory effect of caffeic acid esters on azoxymethane-induced biochemical changes and aberrant crypt foci formation in rat colon. Cancer Res. 1993;53:4182-4188. [PubMed] |
| 51. | Sun B, Karin M. The therapeutic value of targeting inflammation in gastrointestinal cancers. Trends Pharmacol Sci. 2014;35:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Khuda-Bukhsh AR, Das S, Saha SK. Molecular approaches toward targeted cancer prevention with some food plants and their products: inflammatory and other signal pathways. Nutr Cancer. 2014;66:194-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Hussein SZ, Yusoff KM, Makpol S, Yusof YA. Antioxidant capacities and total phenolic contents increase with gamma irradiation in two types of Malaysian honey. Molecules. 2011;16:6378-6395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Hussein SZ, Mohd Yusoff K, Makpol S, Mohd Yusof YA. Gelam honey attenuates carrageenan-induced rat paw inflammation via NF-κB pathway. PLoS One. 2013;8:e72365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 55. | Abdel-Latif MM, Windle HJ, Homasany BS, Sabra K, Kelleher D. Caffeic acid phenethyl ester modulates Helicobacter pylori-induced nuclear factor-kappa B and activator protein-1 expression in gastric epithelial cells. Br J Pharmacol. 2005;146:1139-1147. [PubMed] |
| 56. | Hussein SZ, Mohd Yusoff K, Makpol S, Mohd Yusof YA. Gelam Honey Inhibits the Production of Proinflammatory, Mediators NO, PGE(2), TNF-α, and IL-6 in Carrageenan-Induced Acute Paw Edema in Rats. Evid Based Complement Alternat Med. 2012;2012:109636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 57. | Ali AT, Al Swayeh OA. Honey potentiates the gastric protection effects of sucralfate against ammonia-induced gastric lesions in rats. Saudi J Gastroenterol. 2003;9:117-123. [PubMed] |
| 58. | Alagwu EA, Nneli RO, Egwurugwu JN, Osim EE. Gastric cytoprotection and honey intake in albino rats. Niger J Physiol Sci. 2011;26:39-42. [PubMed] |
| 59. | al Somal N, Coley KE, Molan PC, Hancock BM. Susceptibility of Helicobacter pylori to the antibacterial activity of manuka honey. J R Soc Med. 1994;87:9-12. [PubMed] |
| 60. | Haffejee IE, Moosa A. Honey in the treatment of infantile gastroenteritis. Br Med J (Clin Res Ed). 1985;290:1866-1867. [PubMed] |
| 61. | Alnaqdy A, Al-Jabri A, Al Mahrooqi Z, Nzeako B, Nsanze H. Inhibition effect of honey on the adherence of Salmonella to intestinal epithelial cells in vitro. Int J Food Microbiol. 2005;103:347-351. [PubMed] |
| 62. | Nasutia C, Gabbianellib R, Falcionib G, Cantalamessa F. Antioxidative and gastroprotective activities of anti-inflammatory formulations derived from chestnut honey in rats. Nut Res. 2006;26:130-137. [RCA] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | Ali AT. Prevention of ethanol-induced gastric lesions in rats by natural honey, and its possible mechanism of action. Scand J Gastroenterol. 1991;26:281-288. [PubMed] |
| 64. | Ali ATM. Natural honey accelerates healing of indomethacin-induced antral ulcers in rats. Saudi Med J. 1995;16:161-166. |
| 65. | Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979-2990. [PubMed] |
| 66. | Lamb A, Chen LF. Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer. J Cell Biochem. 2013;114:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 67. | Drouin E. Helicobacter pylori: novel therapies. Can J Gastroenterol. 1999;13:581-583. [PubMed] |
| 68. | Ali AT, Chowdhury MN, al Humayyd MS. Inhibitory effect of natural honey on Helicobacter pylori. Trop Gastroenterol. 1991;12:139-143. [PubMed] |
| 69. | Ndip RN, Malange Takang AE, Echakachi CM, Malongue A, Akoachere JF, Ndip LM, Luma HN. In-vitro antimicrobial activity of selected honeys on clinical isolates of Helicobacter pylori. Afr Health Sci. 2007;7:228-232. [PubMed] |
| 70. | Osato MS, Reddy SG, Graham DY. Osmotic effect of honey on growth and viability of Helicobacter pylori. Dig Dis Sci. 1999;44:462-464. [PubMed] |
| 71. | Nzeako BC, Al-Namaani F. The antibacterial activity of honey on helicobacter pylori. Sultan Qaboos Univ Med J. 2006;6:71-76. [PubMed] |
| 72. | Lamb A, Chen LF. The many roads traveled by Helicobacter pylori to NFκB activation. Gut Microbes. 2010;1:109-113. [PubMed] |
| 73. | Seo JH, Lim JW, Kim H, Kim KH. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest. 2004;84:49-62. [PubMed] |
| 74. | Ralhan R, Pandey MK, Aggarwal BB. Nuclear factor-kappa B links carcinogenic and chemopreventive agents. Front Biosci (Schol Ed). 2009;1:45-60. [PubMed] |
| 75. | Wu CS, Chen MF, Lee IL, Tung SY. Predictive role of nuclear factor-kappaB activity in gastric cancer: a promising adjuvant approach with caffeic acid phenethyl ester. J Clin Gastroenterol. 2007;41:894-900. [PubMed] |
| 76. | Toyoda T, Tsukamoto T, Takasu S, Shi L, Hirano N, Ban H, Kumagai T, Tatematsu M. Anti-inflammatory effects of caffeic acid phenethyl ester (CAPE), a nuclear factor-kappaB inhibitor, on Helicobacter pylori-induced gastritis in Mongolian gerbils. Int J Cancer. 2009;125:1786-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 77. | Ghaffari A, Somi MH, Safaiyan A, Modaresi J, Ostadrahimi A. Honey and apoptosis in human gastric mucosa. Health Promot Perspect. 2012;2:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 78. | Ribeiro U, Safatle-Ribeiro AV. Caffeic acid phenethyl ester (CAPE) may be a promising adjuvant treatment in gastric cancer. J Clin Gastroenterol. 2007;41:871-873. [PubMed] |
| 79. | Wen CT, Hussein SZ, Abdullah S, Karim NA, Makpol S, Mohd Yusof YA. Gelam and Nenas honeys inhibit proliferation of HT 29 colon cancer cells by inducing DNA damage and apoptosis while suppressing inflammation. Asian Pac J Cancer Prev. 2012;13:1605-1610. [PubMed] |
| 80. | Jaganathan SK. Growth inhibition by caffeic acid, one of the phenolic constituents of honey, in HCT 15 colon cancer cells. ScientificWorldJournal. 2012;2012:372345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 81. | Jaganathan SK, Mandal M. Involvement of non-protein thiols, mitochondrial dysfunction, reactive oxygen species and p53 in honey-induced apoptosis. Invest New Drugs. 2010;28:624-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 82. | Orsolić N, Knezević A, Sver L, Terzić S, Hackenberger BK, Basić I. Influence of honey bee products on transplantable murine tumours. Vet Comp Oncol. 2003;1:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Mabrouk GM, Moselhy SS, Zohny SF, Ali EM, Helal TE, Amin AA, Khalifa AA. Inhibition of methylnitrosourea (MNU) induced oxidative stress and carcinogenesis by orally administered bee honey and Nigella grains in Sprague Dawely rats. J Exp Clin Cancer Res. 2002;21:341-346. [PubMed] |
| 84. | Rao CV, Desai D, Kaul B, Amin S, Reddy BS. Effect of caffeic acid esters on carcinogen-induced mutagenicity and human colon adenocarcinoma cell growth. Chem Biol Interact. 1992;84:277-290. [PubMed] |
| 85. | Gribel’ NV, Pashinskiĭ VG. [The antitumor properties of honey]. Vopr Onkol. 1990;36:704-709. [PubMed] |
| 86. | Wattenberg LW. Chemoprevention of cancer by naturally occurring and synthetic compounds. Cancer Chemoprevention. Boca Ranton: CRC Press 1986; 19-40. |
| 87. | Hakim L, Alias E, Makpol S, Ngah WZ, Morad NA, Yusof YA. Gelam honey and ginger potentiate the anti cancer effect of 5-FU against HCT 116 colorectal cancer cells. Asian Pac J Cancer Prev. 2014;15:4651-4657. [PubMed] |
| 88. | Hassan MI, Mabrouk GM, Shehata HH, Aboelhussein MM. Antineoplastic effects of bee honey and Nigella sativa on hepatocellular carcinoma cells. Integr Cancer Ther. 2012;11:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 89. | Haza AI, Morales P. Spanish honeys protect against food mutagen-induced DNA damage. J Sci Food Agric. 2013;93:2995-3000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Jubri Z, Narayanan NNN, Karim NA, Ngah WZW. Antiproliferative activity and apoptosis induction by gelam honey on liver cancer cell line. Int J Appl Sci Technol. 2012;2:135-141. |
| 91. | Abdel Aziz A, Rady H, Amer M, Kiwan H. Effect of some honey bee extracts on the proliferation, proteolytic and gelatinolytic activities of the hepatocellular carcinoma Hepg2 cell line. Aust J Basic Appl Sci. 2009;3:2754-2769. |
| 92. | El-kott AF, Kandeel AA, El-Aziz SFA, Ribea HM. Anti-tumor effects of bee honey on PCNA and P53 expression in the rat hepatocarcinogenesis. Int J Cancer Res. 2012;8:130-139. |
| 93. | Chen MJ, Chang WH, Lin CC, Liu CY, Wang TE, Chu CH, Shih SC, Chen YJ. Caffeic acid phenethyl ester induces apoptosis of human pancreatic cancer cells involving caspase and mitochondrial dysfunction. Pancreatology. 2008;8:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 94. | Chen MJ, Shih SC, Wang HY, Lin CC, Liu CY, Wang TE, Chu CH, Chen YJ. Caffeic Acid phenethyl ester inhibits epithelial-mesenchymal transition of human pancreatic cancer cells. Evid Based Complement Alternat Med. 2013;2013:270906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |