Published online Mar 9, 2015. doi: 10.5497/wjp.v4.i1.117
Peer-review started: June 23, 2014
First decision: July 10, 2014
Revised: January 26, 2015
Accepted: February 9, 2015
Article in press: February 11, 2015
Published online: March 9, 2015
Processing time: 263 Days and 5.5 Hours
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder, affecting about 1% of the population above the age of 65. PD is characterized by a selective degeneration of the dopaminergic neurons of the substantia nigra pars compacta. This results in a marked loss of striatal dopamine and the development of the characteristic features of the disease, i.e., bradykinesia, rest tremor, rigidity, gait abnormalities and postural instability. Other types of neurons/neurotransmitters are also involved in PD, including cholinergic, serotonergic, glutamatergic, adenosine, and GABAergic neurotransmission which might have relevance to the motor, non-motor, neuropsychiatric and cognitive disturbances that occur in the course of the disease. The treatment of PD relies on replacement therapy with levodopa (L-dopa), the precursor of dopamine, in combination with a peripheral decarboxylase inhibitor (carbidopa or benserazide). The effect of L-dopa, however, declines over time together with the development of motor complications especially dyskinesia in a significant proportion of patients within 5 years of therapy. Other drugs include dopamine-receptor-agonists, catechol-O-methyltransferase inhibitors, monoamine oxidase type B (MAO-B) inhibitors, anticholinergics and adjuvant therapy with the antiviral drug and the N-methyl-D-aspartate glutamate receptor antagonist amantadine. Although, these medications can result in substantial improvements in parkinsonian symptoms, especially during the early stages of the disease, they are often not successful in advanced disease. Moreover, dopaminergic cell death continues over time, emphasizing the need for neuroprotective or neuroregenerative therapies. In recent years, research has focused on non-dopaminergic approach such as the use of A2A receptor antagonists: istradefylline and preladenant or the calcium channel antagonist isradipine. Safinamide is a selective and reversible inhibitor of MAO-B, a glutamate receptor inhibitor as well as sodium and calcium channel blocker. Minocycline and pioglitazone are other agents which have been shown to prevent dopaminergic nigral cell loss in animal models of PD. There is also an evidence to suggest a benefit from iron chelation therapy with deferiprone and from the use of antioxidants or mitochondrial function enhancers such as creatine, alpha-lipoic acid, l-carnitine, and coenzyme Q10.
Core tip: Parkinson’s disease (PD) is a neurodegenerative disorder for which currently there is no cure. The advent of many therapies such as levodopa (L-dopa), dopamine-receptor-agonists, monoamine oxidase type B inhibitors, and catechol-O-methyltransferase inhibitors helped much to ease the life and to improve health-related quality of life of PD patients. Among these drugs, L-dopa remains the most effective agent for treatment of motor symptoms in PD. These agents provide symptomatic relief for motor symptoms but there is no evidence that these could alter the natural course of the disease and prevent the progressive dopaminergic neuronal loss. There is, however, encouraging data that suggest a benefit from iron chelation therapy with deferiprone and from the use of antioxidants or mitochondrial function enhancers in preventing or delaying the progression of PD.
- Citation: Abdel-Salam OM. Drug therapy for Parkinson’s disease: An update. World J Pharmacol 2015; 4(1): 117-143
- URL: https://www.wjgnet.com/2220-3192/full/v4/i1/117.htm
- DOI: https://dx.doi.org/10.5497/wjp.v4.i1.117
Idiopathic Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by bradykinesia, tremor, rigidity and impaired postural reflexes. It is the 2nd most common neurodegenerative disorder after Alzheimer’s disease. It is estimated to affect approximately 1% of the population over 65 years of age[1,2]. The main neuropathology in PD is the progressive loss of nigrostriatal dopaminergic neurons and consequent striatal dopamine depletion[3]. When there is a loss of about 60%-70% of neurons of the substantia nigra pars compacta (SNc) and the striatal dopamine content is reduced by 70%-80%, symptoms start to appear[4,5]. The definitive diagnosis of PD is based on post-mortem histopathological findings of degeneration and loss of pigmented neurons of the SNc and the presence of intracytoplasmic eosinophilic inclusions bodies (Lewy bodies) and dystrophic neurites (Lewy neurites) present in the remaining dopaminergic neurons of the substantia nigra. The major compound of Lewy bodies is aggregated forms of the normally presynaptically located protein α-synuclein[6]. Abnormal signaling in PD is not confined to nigrostriatal dopaminergic pathwaysrestricted. Other types of neurons/neurotransmitters including cholinergic, serotonergic, glutamatergic, adenosine, and GABAergic neurotransmission are also involved in PD. Alterations in these neurotransmitter systems contributes to the development motor, non-motor, neuropsychiatric and cognitive disturbances that occur in the course of the disease and are possible targets for drug therapy [7,8].
PD is essentially a sporadic disorder, commonly referred to as idiopathic PD, while a minority of cases is familial (approximately 5%)[9]. These rare familial forms of PD are usually of early onset in contrast to the late-onset idiopathic PD. The past few years have witnessed the identification of distinct genetic loci responsible for rare Mendelian forms of PD and both autosomal dominant and recessive patterns of inheritance have been described. The rare genetic forms have helped in understanding the molecular mechanisms involved in PD including protein misfolding and aggregation, mitochondrial defects, and oxidative stress[10]. The cause of idiopathic PD is not yet fully understood, but there is accumulating evidence to support a role for environmental toxin(s) and a genetic background[11,12]. PD can also be caused by drugs especially neuroleptic agents and dopamine-blocking drugs, toxins (manganese, carbon dioxide), head trauma, tumours of basal ganglia. This is termed secondary parkinsonism[13-15].
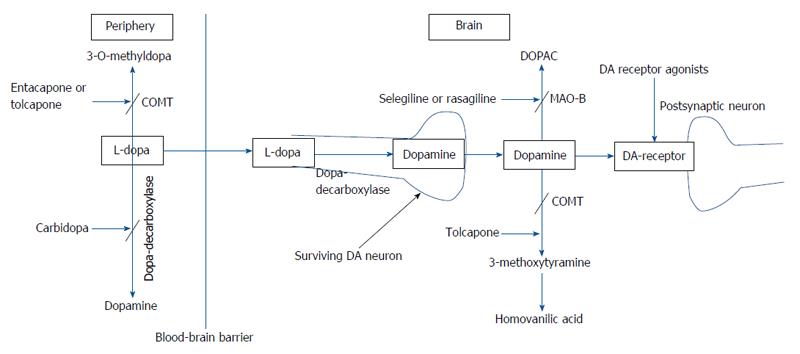
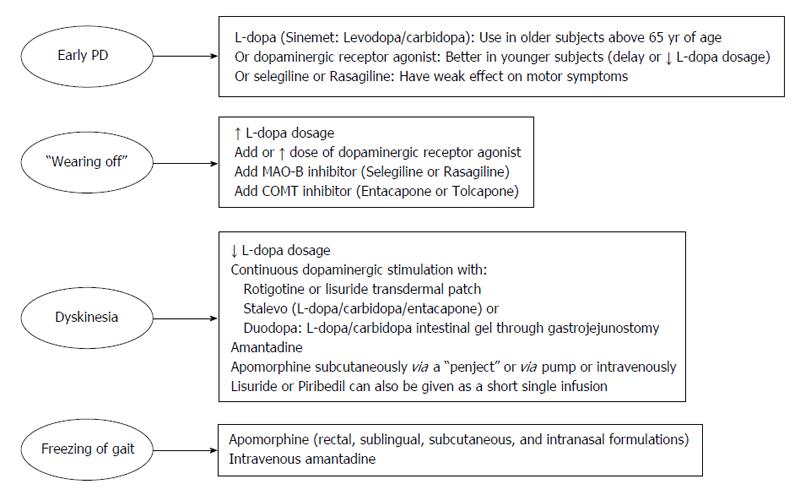
Levodopa: The main neurochemical deficit in PD is the progressive loss of dopamine producing neurons in the SNc and subsequent striatal dopamine depletion[16]. This deficit can be partially compensated for by the administration of levodopa (L-dopa) (L-3,4-dihydroxyphenylalanine), the precursor of dopamine (Figure 1). Following oral ingestion, L-dopa is actively transported from the upper small intestine into the circulation by a mechanism specific for large, neutral L-amino acids[17]. L-dopa is subject to high presystemic metabolism to dopamine in the gut by the enzyme L-amino acid decarboxylase, with only approximately 30% of an L-dopa dose reaching the systemic circulation[18]. The small fraction of the drug that reaches the brain after active transport across the blood brain barrier is rapidly converted to dopamine by aromatic L-amino acid decarboxylase. In order to inhibit conversion of L-dopa to dopamine outside the central nervous system, thus limiting systemic side effects, it is combined with a peripherally acting decarboxylase inhibitor (carbidopa or benserazide)[17]. The combined administration of L-dopa with either carbidopa (L-dopa/carbidopa at the dose ratio of 10/1 and 4/1) or benserazide (L-dopa/benserazide 4/1), have nearly tripled L-dopa oral bioavailability, markedly reducing both the required L-dopa therapeutic dose and severity of dopamine-mediated gastrointestinal and cardiovascular side-effects[18]. Ever since its introduction in 1960s, L-dopa has remained the most effective treatment in controlling the symptoms of PD and is considered the gold standard regarding the symptomatic treatment of patients with PD[19]. Compared with other available dopaminergic therapies, dopamine replacement with L-dopa is associated with the greatest improvement in motor function, as assessed by reduced scores in the Unified PD Rating Scale (UPDRS). L-dopa is the preferred therapy in patients above 65 years of age, while those below 65 are better treated with dopamine agonists[20]. When used as an initial monotherapy, L-dopa delayed the need for supplementary treatment and was well tolerated compared with dopamine agonists; 50% of the patients starting L-dopa received supplementary therapy with-in 3.6 years compared with 2.3 years in case of dopamine agonist monotherapy[21].
However, as the disease progresses, long-term use of L-dopa leads to the development of motor response complications, particularly “wearing-off”, “on-off”, dyskinesias and dystonias. These L-dopa-associated motor problems result in marked disability and decreased quality of life. End-of-dose or wearing-of phenomenon is the reappearance of motor symptoms before the next scheduled dose of L-dopa. Wearing-of usually emerges within 1-3 years of initiation of levodopa treatment[20-25]. Patients may also report a “delayed-on” that is unusual delay between a given dose of levodopa and the start of its effects. The phenomenon of “no-on” is the failure of a given dose of levodopa to elicit any response. These manifestations are related to variations in gastrointestinal transport and absorption of levodopa. On-off motor oscillations on the other hand are characterized by sudden and unpredictable shifts between functioning “on” and non-functioning “off” states[26].
Under physiological conditions, stimulation of dopamine receptors occurs in a continuous fashion. This contrasts with the effect of L-dopa which because of its short half-life of 90 min, results fluctuations in plasma levodopa levels and in pulsatile, rather than continuous stimulation of striatal dopamine receptors. It is thought that the ability of striatal neurons to take up L-dopa and store dopamine for continuous release is lost in advanced disease because of the diminished number of available neurons. It is suggested that this non-physiological pulsatile stimulation of dopamine receptors, might trigger a dysregulation of many neurotransmitter systems within the basal ganglia and is the cause for the motor fluctuations and dyskinesias[22,25,27].
Reducing “off” time can be achieved by increasing the dosage of L-dopa. Increase in L-dopa dosage, however, can be associated with hyperkinetic movements or dyskinesias. The latter are involuntary movements other than tremor and most commonly consists of chorea which represent a peak-dose effect when L-dopa brain concentration is highest and is caused by excessive levels of dopamine (“peak-dose dyskinesia”)[28]. Dyskinesia can be alleviated through reducing the dose of L-dopa, but only at the expense of worsening parkinsonism and an increase in the number of “off” episodes[22-24]. The risk of developing dyskinesia or wearing-off was found to be closely linked to L-dopa dose. Young age at onset, higher L-dopa dose, low body weight, female gender, and more severe UPDRS Part II were among factors predictive of dyskinesia[29]. The approach to manage wearing-off and dyskinesia involves the addition of drugs which would permit more continuous dopaminergic stimulation, such as dopamine agonists, catechol-O-methyl transferase inhibitors, and monoamine oxidase type B (MAO-B) inhibitors, controlled-release formulation of L-dopa, transdermal delivery (rotigotine), infusion therapies (intravenous L-dopa, subcutaneous application of apomorphine and lisuride, duodenal infusion of L-dopa)[23,24,30] (Figure 2).
One therapeutic modality involves the use of L-dopa-carbidopa intestinal gel delivered continuously through an intrajejunal percutaneous gastrostomy tube. This form of therapy has been proved successful in controlling motor symptoms in advanced disease with fluctuating symptoms unresponsive to conventional oral treatment[31,32]. This treatment reduced “off” time and increased “on” time without troublesome dyskinesia at week 12 compared to baseline[31]. Improved motor complications and improvement in quality of life, autonomy and clinical global status have been reported after long-term treatment (over 7 years) with L-dopa/carbidopa intestinal gel[32]. It has also been shown that in advanced PD patients, continuous intrajejunal L-dopa-carbidopa intestinal gel infusion maintained stable plasma L-dopa levels with minimal degree of fluctuation in L-dopa, 3-OMD, and carbidopa plasma concentrations during 2-16 h of infusion, thereby, providing continuous dopaminergic stimulation[33]. In a randomized, double-blind trial in adults aged ≥ 30 years with advanced PD and motor complications, the intestinal gel was more effective in decreasing “off” time and in increasing “on” time without troublesome dyskinesia when compared with immediate-release oral L-dopa-carbidopa[34]. L-dopa-carbidopa intestinal gel is not without complications. The technique involves an invasive procedure with attendant surgical and postsurgical complications. There are also problems related to the pump and tube, e.g., dislocation and kinking of the intestinal tube, abdominal pain. Other complications are vitamin deficiency and polyneuropathies[31,32,35]. Subacute axonal neuropathy[36] and refractory seizures and a complex partial status epilepticus[37] likely to be due to vitamin B6 and/or vitamin B12 deficiency have been described under L-dopa-carbidopa intestinal gel infusion. Studies reported increased prevalence of neuropathy in chronic L-dopa treated patients and that cumulative levodopa exposure was significantly and positively associated with the severity of peripheral neuropathy. The cause of neuropathy is not clear but elevated plasma homocysteine level methylmalonic acid and reduced vitamin B12 levels that occurs under high doses of orally administered or continuously infused levodopa/carbidopa might be involved[38,39]. Monitoring vitamin B12/B6 status before and after starting L-dopa-carbidopa intestinal gel infusion and careful observation for signs of peripheral neuropathy have been advised[40].
Dopamine-receptor-agonists: These agents exert their antiparkinsonian effects by acting directly on dopamine receptors, thereby, mimicking the endogenous neurotransmitter. Several dopamine agonists are available for the treatment of PD. These include the ergot derivatives bromocriptine, cabergoline, alpha-dihydroergocryptine, lisuride, pergolide, the non-ergot agonists pramipexole, ropinirole, rotigotine, piribedil as well as apomorphine[41]. The non-ergot group of drugs is currently the most frequently prescribed oral dopamine receptor agonists. These agents are indicated both as an initial monotherapy in early PD to delay the need for L-dopa and in combination with L-dopa in advanced disease, enabling patients to take lower doses of L-dopa, thereby reducing the frequency of L-dopa induced motor complications[42]. The addition of dopamine agonists allows around a 20%-30% reduction in the dose of L-dopa[41]. When used as an initial monotherapy, these agents are effective in controlling motor symptoms. Overtime, however, dopamine receptor agonists lose efficacy and after 3 years of treatment with agonist monotherapy, the number of patients remaining decreases to less than 50%. These patients will then require the addition of L-dopa, so as to achieve better control of their motor symptoms[43]. The rate of discontinuation of dopamine receptor agonists also appears to higher compared with L-dopa monotherapy (20% vs 1% over four years) (due to impulse control disorders, somnolence and light-headedness)[21].
The use of dopamine receptor agonists might have several benefits. First, their use early in the disease is likely to delay or reduce the incidence of dyskinesia, dystonia, and motor fluctuations resulting from long-term L-dopa therapy, possibly due to better continued rather than pulsatile stimulation of postsynaptic dopamine receptors[44,45]. One recent study showed that an initial treatment with dopamine agonists resulted in 87% lower risk for dyskinesia compared with treatment with L-dopa[46]. Second, dopamine-receptor-agonists are not metabolized to active chemicals, do not produce toxic metabolites or lead to the cytotoxic free radical formation that might be associated with metabolism of dopamine[41,47]. Third, there is also evidence that dopamine-receptor-agonists might slow the progression of disease. Clinical imaging studies targeting dopamine function with by [123I]β-CIT or [18F]Dopa imaging suggested that the rate of loss of [123I]β-CIT or [18F]Dopa uptake in early PD patients treated with dopamine agonists is slower as compared with L-dopa[48]. Table 1 lists the currently available dopaminergic receptor agonists and their side effects.
| Drug | D-receptor specificity | Dose range | Side effects |
| Ergot derivatives | |||
| Bromocryptine | D2 receptor agonist with partial dopamine D1 antagonist activity | 7.5-15 mg/d orally | Risk of developing valvular regurgitation (cumulative and dose-dependent) |
| Lisuride | D2 receptor agonist (also has a weak dopamine D1 antagonistic, serotonin 5-HT1A agonistic, and 5-HT2B receptor antagonist properties) | 0.2-3 mg/d orally | Dry mouth, nausea, weakness, postural hypotension, and headache |
| Cabergoline | D1 and D2 receptor agonist | 0.5-4 mg/d orally | Risk of developing moderate to severe valvular regurgitation |
| Non-ergot derivatives | |||
| Piribedil | D2/D3 receptor agonist with alpha(2) antagonist properties | 150-300 mg/d orally | |
| Rotigotine transdermal patch | D1, D2, and D3 receptor agonist | 2-16 mg patch/d | Patch site reactions, nausea, vomiting, dry mouth, somnolence , peripheral edema, and dyskinesia |
| Ropinirole (immediate and extended release) | D2/D3 receptor agonist | 4-24 mg/d orally | Nausea, dyspepsia, dizziness, back pain, headache, uncontrollable sleep attacks, orthostatic hypotension, leg oedema |
| Pramipexole (immediate and extended release) | D2/D3 receptor agonist | 0.25 - 4.5/d orally (Extended release pramipexole is approved as monotherapy in early PD, as well as an adjunct therapy to levodopa in advanced PD | Somnolence, cognitive adverse events, fatigue, nausea, constipation, and peripheral oedema |
| Apomorphine | D1 and D2 receptor agonist (also stimulates serotonin and α-adrenergic receptors) | 4 mg subcutaneously 20 mg continuously daily via a pump system | Local inflammation and granuloma at the subcutaneously infusion site Intravascular thrombotic complications secondary to apomorphine crystal accumulation after iv administration |
Bromocryptine: This is the earliest dopamine agonist in use which was introduced in the 1970s as adjunct therapy to L-dopa for PD patients with motor complications[49]. It is structurally related to dopamine and activates postsynaptic dopamine D2 receptors with partial dopamine D1 antagonist activity[50]. Compared with low-dose L-dopa, dyskinesia and dystonia were delayed by early use of bromocriptine, but end-of-dose failure appeared at a similar time once L-dopa was added. The rate of disease progression, however, was similar whether treatment was initiated with L-dopa or bromocryptine[51]. There was no evidence of a long-term benefit or clinically relevant disease-modifying effect on initiating treatment with bromocryptine. The initially reduced frequency in motor complications was not sustained and motor disability or mortality was not reduced over the long term. Moreover, disability scores and physical functioning were better in the L-dopa than in the bromocriptine treatment group[52]. Patients on bromocriptine have the risk of developing valvular regurgitation which occurs in a cumulative dose-dependent manner[53].
Lisuride: The drug exerts its activity primarily at postsynaptic dopamine D2 receptor sites. It has also a weak dopamine D1 antagonistic and serotonin 5-HT1A agonistic effects[50] as well as 5-HT2B receptor antagonist properties[54]. Lisuride is short-acting with a plasma half-life of 1-3 h. In contrast to bromocriptine, cabergoline, and pergolide, the drug is not associated with fibrotic cardiac valvulopathy[54,55]. Transdermal delivery using lisuride patches has been shown to improve the motor changing rate in advanced PD patients with unpredictable on-off phenomena[56].
Cabergoline: Cabergoline has selective affinity for D2-like dopamine receptors. It has a long plasma half-life of 65 h[41]. In early PD, initiating therapy with cabergoline improved motor disability and delayed the development of motor complications compared with L-dopa-treated patients[57]. A reduction in daytime sleepiness by 70% was reported following a fast switch-over from the currently used dopamine agonist to a single equivalent dose of cabergoline, administered at bedtime[58]. In patients with motor fluctuations and dyskinesia, cabergoline improved “off” or “on” hours, or both[59], improved “on” with dyskinesia’, mean dystonia intensity, time spent in severe “off’ condition, severity of “off” periods and allowed the reduction of L-dopa requirements[60]. Side effects of cabergoline include gastric upset, orthostatic hypotension, and ankle edema[59]. There were also reports of constrictive pericarditis, cardiac valvular regurgitation and pleuropulmonary disease (pleural effusion/pulmonary fibrosis)[59,61-63]. The ability of cabergoline (and bromocriptine) to cause fibrotic cardiac valvulopathies and consequent valvular regurgitation appears to be mediated through stimulation of valvular 5-HT(2B) serotonin receptors that might mediate mitogenesis and, in turn, the proliferation of fibroblasts[64,65]. Lisuride and non-ergot dopamine agonists are devoid of 5-HT(2B) agonistic activity and hence might not induce heart valve fibrosis[54,55].
Piribedil: Piribedil [1-(3,4-methylenedioxybenzyl)]-4-[(2-pyrimidinyl)]piperazine is a D2/D3 receptor agonist with alpha(2) antagonist properties. This last property of piribedil might favorably influence motor function, cognition, mood and the integrity of dopaminergic neurons[66]. In early PD, the administration of piribedil (150-300 mg/d) improved UPDRS III over a 7-mo period; the proportion of responders (UPDRS III improvement > 30%) was significantly higher for piribedil (42%) than for placebo (14%)[67]. In PD patients insufficiently controlled by L-dopa alone, early combination of piribedil (150 mg) or bromocriptine (25 mg) with L-dopa resulted in similar long-term improvement of motor symptoms (UPDRS III) over 12 mo. Piribedil-treated patients, however, required less L-dopa dose increase than those on bromocriptine[68]. In PD patients with motor fluctuations, piribedil improved motor UPDRS scores, and activities of daily living, increased the duration of effect of L-dopa and permitted the mean daily L-dopa dose to be decreased by 17%[69]. Piribedil can also be given as a short single infusion of at 2 to 16 mg to improve motor symptoms, including akinesia in PD patients with fluctuations[70]. The new sublingual formulation of piribedil at a single dose of 60 mg was superior to placebo in improving UPDRS III and aborting “off” in patients with advanced PD[71]. In PD presenting with apathy following subthalamic nucleus stimulation, piribedil improved apathy by 46.6% as well as depression[72]. Side effects of piribedil include hallucinations, dyskinesias, dizziness[69], gastrointestinal complaints[67] and sleep attacks[73].
Rotigotine: This dopamine D1, D2, and D3 receptor agonist is administered through a silicone-based transdermal patch designed for once-daily application. Steady-state plasma levels of rotigotine can be reached between 8 and 12 h, and a stable drug release is maintained throughout the 24-h patch application[74]. Rotigotine patch thus allows constant delivery of the drug and possible continuous dopaminergic stimulation. Rotigotine improved motor scores, and the activities of daily living[75]. The drug is indicated both as monotherapy for the treatment of early PD, and as adjunctive therapy to L-dopa in advanced PD with motor fluctuations, significantly reducing “off” time[76,77]. In patients with early-stage PD, rotigotine was generally well tolerated for up to approximately 6 years[76]. The majority of patients experiencing dyskinesia reported first appearance after starting L-dopa[76]. Most common adverse events reported were application site reactions, nausea, vomiting, dry mouth, somnolence, peripheral edema, and dyskinesia[75-77]. Rotigotine transdermal patch was shown to be of similar efficacy to oral pramipexole in patients with fluctuating PD over 6 mo of treatment. The absolute change in “off” time from baseline compared with placebo was -1.58 h for rotigotine and -1.94 h for pramipexole and responder rates were 67% for pramipexole, 59.7% for rotigotine[78].
Ropinirole: Immediate- and extended-release once-daily formulations of ropinirole are available. Ropinirole prolonged-release formulations has also been shown to delay the onset of dyskinesia in early PD, compared with increasing doses of L-dopa. This was achieved without significant change in UPDR Scale activities of daily living or motor scores[79]. In advanced PD patients not optimally controlled with L-dopa and who suffered troublesome nocturnal disturbance, the extended-release formulation provided 24-h symptom control and improved nocturnal symptoms. In these patients, the drug resulted in reduction in awakenings and in an increase in awake time “on”/”on” without troublesome dyskinesia during night-time and early morning[80-82]. In moderate-to-advanced PD, symptom control could be achieved 2 wk after treatment initiation[83]. Patients on pramipexole could be switched overnight to extended release ropinirole without serious adverse events[84]. Ropinirole most commonly causes nausea and sleepiness, less commonly uncontrollable sleep attacks, vertigo, dyspepsia, orthostatic hypotension, leg oedema, back pain, and headache[81,85]. Gastrointestinal complaints and sleep/fatigue were significantly higher for ropinirole than for pramipexole[86].
Pramipexole: Pramipexole shows high affinity for the D2 subfamily of dopamine receptors. The drug is effective as a symptomatic treatment in early PD, reducing UPDRS by 4-5 points relative to placebo[87]. When used as a monotherapy in early idiopathic PD, pramipexole was of comparable clinical efficacy to rasagiline[88]. It alleviated L-dopa dyskinesia when used as an “add on” therapy or in place of ergot dopamine agonist[89]. Switching patients with PD from ergot dopamine agonist, e.g., cabergoline to pramipexole, appeared to be well tolerated and effective, but adjustment of pramipexole dose is required in some patients to reduce side effects[90,91]. Significant improvement in the UPDRS was evident after 2 wk of initiating therapy with pramipexole and maintained up to 12 wk of treatment[91]. Pramipexole was associated with significantly low rates of fatigue[92] and improved depressive symptoms[93] in PD patients. There appear, however, to be no significant difference between early and delayed pramipexole initiation on UPDRS total score or striatal dopamine-transporter binding in patients with early PD[94].
Extended release pramipexole is approved as monotherapy in early PD, as well as an adjunct therapy to L-dopa in advanced PD. It has the advantage over the immediate release formulation of improved compliance because of once-daily dosing regimen and steadier plasma levels over 24 h[95]. In patients with early PD not receiving L-dopa or dopamine receptor agonists, once-daily extended-release pramipexole was of similar efficacy to the immediate release preparation (taken 3 times daily) in controlling motor symptoms, and in safety and tolerability[96,97]. In those with motor fluctuations on L-dopa therapy, the addition of either the extended-release and immediate-release preparations was of similar efficacy in improving UPDRS score and off-time compared with placebo, with similar tolerability, and safety[98]. In advanced PD, switching to one daily pramipexole formulation from thrice daily immediate-release tablet formulation was also effective in controlling motor symptoms[99].
Somnolence, fatigue, nausea, constipation, and peripheral oedema are common side effects of pramipexole[87,96]. The drug administered in a single oral dose to healthy young subjects, reduced mean sleep latency and increased total duration of sleep. These effects were not observed with L-dopa and bromocryptine[100]. In early PD, pramipexole monotherapy resulted in higher incidence of cognitive adverse events compared with ropinirole[86]. Other dopamine-receptor-agonists, e.g., rotigotine and cabergoline did not affect cognitive function in patients with early-mild disease. It has been suggested that their combined stimulation of both dopamine (D1 and D2) receptor families might account for preserving cognitive functions compared with pure D2 family stimulation that occurs with pramipexole[101].
Apomorphine: This synthetic morphine derivative exerts antiparkinsonian effects by non-selective stimulation of dopamine receptors. The drug also stimulates serotonin and α-adrenergic receptors. It is currently used in patients with advanced PD for the treatment of persistent and disabling motor fluctuations unresponsive to conventional therapy with L-dopa or dopamine receptor agonists, with or without deep brain stimulation[102]. In late stage PD, apomorphine administered via subcutaneous, intravenous routes or by inhalation, has been shown to result in long term symptomatic improvement, effectively abort “off” episodes and significantly decrease L-dopa equivalent dose[103-106]. Non-motor symptoms as hyperhidrosis, nocturia, urgency of micturition, and fatigue improved as well[106]. Switching patients with refractory motor fluctuations from subcutaneous to intravenous therapy with apomorphine resulted in 59% decrease in their additional oral anti-parkinsonian medication. Dyskinesias also significantly decreased and “off” time was virtually eliminated[104]. Continuous subcutaneous apomorphine infusion proved of symptomatic benefit in those with untreatable motor fluctuations but in whom subthalamic nucleus deep brain stimulation was contraindicated (because of L-dopa-resistant axial motor symptoms and/or cognitive decline). Daily “off” time decreased while “on” time improved together with a significant reduction in mean oral L-dopa equivalent dose[107]. In PD patients undergoing deep brain stimulation, subcutaneous apomorphine reduced the risk of neurologic and respiratory deterioration caused by perioperative withdrawal of dopaminergic medication[108].
Apomorphine causes severe nausea and vomiting. It has been suggested that the activation of human sensory transient receptor potential A1 channels by apomorphine, might contribute to adverse side effects such as nausea and painful injections[109]. The most common side effect to subcutaneous apomorphine is local inflammation at the infusion site[103,107]. Moreover, intravascular thrombotic complications, secondary to apomorphine crystal accumulation, necessitating cardiothoracic surgery, complicate intravenous therapy with apomorphine[104].
Selegiline and rasagiline: Both MAO-A and MAO-B contribute to dopamine metabolism. MAO-A is the main enzyme responsible for the metabolism of the monoamines, noradrenaline, serotonin and dopamine. MAO-B is more specific to dopamine metabolism[110]. MAO-B inhibitors are clinically being used to treat PD by blocking the degradation of dopamine and thereby providing a symptomatic relief in these patients. Selegiline (Deprenyl/Eldepryl) and rasagiline (Azilect) are irreversible selective inhibitors of the enzyme MAO-B. Selegiline, the R-optical enantiomer of deprenyl (phenyl-isopropyl-methyl-propargylamine) was approved by the Food and Drug Administration (FDA) in 1996. Selegiline is a propargyl amphetamine derivative that undergoes extensive first-pass metabolism to L-methamphetamine, L-amphetamine, and desmethyl-deprenyl. Rasagiline [N-propargyl-1-(R)-aminoindan] is a novel, highly potent irreversible MAO-B inhibitor, recently introduced in the treatment of PD. Rasagiline has received FDA approval in 2006. Rasagiline’s major metabolite is aminoindan, which has no amphetamine like properties[111,112] and thus is not likely to cause sleep disturbances compared with selegiline. In patients treated with selective MAO-B inhibitors, the risk of serotonin toxicity due to a concomitant serotonergic agent or hypertensive crisis due to dietary tyramine or sympathomimetic amines appears to be minimal and should not preclude the use of MAO-B inhibitors in treating PD[113]. There is evidence, however, that daily treatment with MAO-B inhibitor may also influence MAO-A activity. Thus in plasma samples from patients with MAO-B inhibitor therapy, there was 70% reduction of MAO-A activity compared with patients without MAO-B inhibitor treatment or healthy controls[114].
Selegiline and rasagiline are effective as initial monotherapy in early PD and as adjunctive therapy in advanced PD[115-117]. MAO-B inhibitors provide mild symptomatic benefit, compared with L-dopa and dopamine agonists. These drugs are indicated for the treatment of akinesia and motor fluctuations associated with L-dopa therapy. Both agents are safe and well tolerated at the recommended daily doses. They might delay the need start L-dopa therapy, reduce disability and reduce the rate of motor fluctuations compared with initial L-dopa therapy[96,118,119]. Rasagiline inhibits MAO-B more potently than selegiline and has the advantage of once-daily dosing and favorable tolerability[116,120]. Rasagiline was effective both as monotherapy in early PD and as adjunctive treatment in advanced PD and motor fluctuations. As monotherapy, however, rasagiline provided modest yet clinically meaningful benefit on motor symptoms (compared to other drugs)[121,122]. Early in the disease, rasagiline monotherapy at 1 mg/d improved symptoms. In advanced PD, rasagiline adjunct therapy (0.5 or 1 mg/d) to L-dopa significantly reduced the total daily “off” time[123]. Rasagiline (1 mg/d), in L-dopa-treated PD patients with motor fluctuations produced a significant improvement over placebo in UPDRS motor “off” score. Rasagiline significantly improved bradykinesia and showed trends for improvements in facial expression, speech, and axial impairment during OFF time[124]. Rasagiline has a rapid beneficial effect on PD symptoms from the first week of therapy. Objective and subjective measures of symptom severity improved at 1 wk (change from baseline in bradykinesia scores and physicians’ and patients’ global impression). The magnitude of benefit was similar in patients treated with once-daily rasagiline either as monotherapy (1.0 mg) or as adjunct therapy (0.5 mg)[125]. Rasagiline might also possess antidepressant effect. In patients with newly diagnosed PD with comorbid untreated depression, rasagiline monotherapy 1 or 2 mg/d for 8 wk, improved the activity of daily living and motor function as well as symptoms of depression. The latter effect was observed at the higher dose of 2 mg/d and appeared not to be related to the motor improvement[126]. Motor behavior, motor complications, mood and sleep improved when patients on selegiline were switched to 1 mg rasagiline[127]. Rasagiline monotherapy in early untreated disease also demonstrated better adverse events profile in the incidence of gastrointestinal symptoms and sleep disorders and less incidence of dropout rates compared with pramipexole[88].
The use of selegiline and rasagiline in the early stage of the disease might also improve long-term outcome. L-dopa-treated patients who received selegiline within 5 years from the onset of the disease exhibited significantly lower UPDRS motor scores over 7 years compared with those who received selegiline 9 to 11 years after the onset of the disease[117]. One study suggested that selegiline use (≥ 3 years) in early PD patients who were of younger age, shorter PD duration, lower UPDRS motor scores was associated with a slower progression of PD[128]. Early-start treatment with rasagiline at a dose of 1 mg/d (though not 2 mg/d) caused a smaller increase in rate of worsening in the UPDRS score between weeks 12 and 36, less worsening in the score between baseline and week 72 compared with the placebo group. The study suggested a disease-modifying effect for rasagiline[129]. In the ADAGIO study, Rascol et al[130], assessed the ability of rasagiline to modify need for additional antiparkinsonian therapy and changes in non-motor and motor changes in patients with untreated early PD. Patients received rasagiline 1 mg/d or 2 mg/d for 72 wk (early-start groups) or placebo for 36 wk followed by rasagiline 1 mg/d or 2 mg/d for 36 wk (delayed-start groups). The findings of the study suggested that rasagiline delayed the need for symptomatic antiparkinsonian drugs.
Recent interest in selegiline and rasagiline has focused on their possible neuroprotective effects that have been delineated in preclinical models of PD[131-133].
In the presence of aromatic amino acid decarboxylase inhibitors, L-dopa metabolism is predominantly shifted to the formation of 3-O-methyldopa by the enzyme catechol-O-methyltransferase (COMT), which has the highest activity in the liver and kidney[18]. The reversible COMT inhibitors tolcapone and entacapone, are being used as an adjunct to L-dopa for the symptomatic treatment of PD patients with motor fluctuations. These agents extend the elimination half-life of L-dopa by inhibiting the peripheral breakdown of L-dopa, thereby increasing L-dopa bioavailability, which will decrease “of” time and increase “on” time in fluctuating PD patients and allow the dosage of L-dopa/carbidopa to be reduced. Moreover, by stabilizing plasma L-dopa concentrations, tolcapone and entacapone permit a more continuous stimulation of dopamine receptors which in theory would reduce the risk of motor complications[25,134].
When used as an adjunct to concomitant treatment with L-dopa and a dopa decarboxylase inhibitor (DDCI), entacapone showed benefits in the quality of life and activities of daily living and was efficacious in increasing “on” time and decreasing “of” time in PD patients with wearing-off fluctuations[135-140]. Moreover, compared with L-dopa/carbidopa or L-dopa/benserazide, treatment with L-dopa/carbidopa/entacapone resulted in significantly greater improvements in non-motor domains such as depression, personal relationships, and communication[139]. In randomized, open-label study, entacapone was as effective as cabergoline in conjunction with L-dopa in decreasing the daily “off”-time and in improving the quality of life (a decrease of approximately 20% was detected in UPDRS II and III motor scores, with no differences between the groups). The effect of entacapone, however, was more quickly apparent compared with that of cabergoline[141]. In patients receiving L-dopa and a DDCI, the addition of entacapone improved UPDRS III motor scores during the first 6 mo of combined therapy, increased daily “on” time and the response duration to a single morning dose of L-dopa. The mean daily dose of L-dopa did not increase over the 5-year follow-up period, suggesting the long-term efficacy of L-dopa/DDCI and entacapone[142].
Studies suggested that early rather than delayed addition of entacapone to L-dopa/DDCI in PD patients with wearing-off provides a modest clinical benefit over L-dopa/DDCI that is maintained for up to 5 years, with an improvement in UPDR motor scale[143]. In patients with early PD, compared with L-dopa/carbidopa (Sinemet), L-dopa/carbidopa/entacapone (Stalevo) resulted in significantly greater improvement in activities of daily living and subject-reported clinical global impression without increasing motor complications[144]. Studies also suggested that switching from L-dopa/DDCI and entacapone and L-dopa/DDCI provides a significant benefit in PD patients with wearing-off[145]. Comparing immediate and delayed switch to L-dopa/carbidopa/entacapone was in favor of immediate switch in terms of greater motor improvement and quality of life[146].
The most common adverse effect of adding entacapone is the increase in dyskinesia[135,141] which would necessitate reducing the dose of L-dopa. In one study, patients with PD and with mild-to-moderate wearing-off without or with mild dyskinesias were randomly assigned to either receiving the same L-dopa-carbidopa dosage or 15%-25% less total L-dopa-carbidopa amount. The findings showed that either regimen resulted in increase in daily “on” time and a reduction in the daily time spent in “off” 4 wk after the change[147]. In a randomized, open-label trial in patients with wearing-off with conventional L-dopa/DDCI therapy, adjunct therapy with entacapone or increasing dose frequency of L-dopa without an increased total daily dose (dose fractionation) reduced the mean “off” time, and the rate of motor complications[148].
On the other hand, entacapone did not improve motor scores on the UPDR Scale when used as an adjunct to L-dopa in PD patients who do not experience motor fluctuations[149]. Moreover, initiating L-dopa therapy with L-dopa/carbidopa/entacapone was associated with a shorter time to onset and increased frequency of dyskinesia compared to L-dopa/carbidopa[150]. Entacapone was also non-efficacious in the prevention/delay of motor complications (reviewed by Fox et al[151]). In another study, entacapone (200 mg with each L-dopa dose) was ineffective in reducing the severity of motor symptoms in the “off” state in L-dopa-treated PD patients with motor fluctuations[124]. Dyskinesia is the most common adverse event of entacapone[152,153]. Entacapone was not associated with an increased risk of acute myocardial infarction, stroke, or death in elderly patients with PD[154].
Tolcapone is a longer acting and more potent COMT inhibitor compared with entacapone[155]. The agent is used in patients with severe motor fluctuations inadequately controlled with entacapone[153]. In patients with advanced PD who were switched to tolcapone because of persisting “off” periods despite treatment with entacapone, there were significant reductions in mean daily off-time duration and L-dopa dose at follow up[156]. The daytime sleepiness, global clinical impression of change, activities of daily living, and quality of life were also significantly improved after adjunctive tolcapone treatment to L-dopa/carbidopa in fluctuating PD patients[157,158]. A randomized, open-label, trial of 150 patients on a stable regimen of L-dopa/DDCI in combination with bromocriptine, lisuride, or pergolide, conducted to assess the efficacy of switching from a dopamine agonist to tolcapone, found the drug to be effective in decreasing daily “off” time, increasing “on” time (as well as other efficacy variables, e.g., UPDR scale II, III, and IVb and investigator’s global assessment scores)[159].
The most common adverse event with tolcapone is dyskinesia which might require decreasing the dose of L-dopa. Elevations of the serum liver enzymes aspartate aminotransferase and alanine aminotransferase have been reported in patients on tolcapone treatment. Therapy with tolcapone thus requires monitoring for of liver function every 2 to 4 wk for 6 mo for hepatotoxicity[156,160,161]. Severe liver injury due to tolcapone, however, appears to be a rare event[160]. In addition, studies on the safety and efficacy of the long-term use of tolcapone concluded that significant liver transaminase elevations were rare and these returned to normal in most patients[162,163]. Tolcapone causes severe diarrhea more often than entacapone[155].
Nebicapone is a new COMT inhibitor which has been found efficacious for the treatment of motor fluctuations in PD patients[164]. In randomized, double-blind, placebo-controlled study, nebicapone 75 mg and 150 mg showed greater effect in increasing “on” time and decreasing “off” time compared with entacapone 200 mg. The drug produced more sustained COMT inhibition compared with entacapone 200 mg. Nebicapone 150 mg increased L-dopa area under the plasma concentration time curve by 48.4% compared to a value of 33.3% after entacapone 200 mg[165]. Nebicapone has the risk of increasing liver transaminases[164].
Amantadine: Amantadine is an antiviral drug which was found to exert beneficial antiparkinsonian effects[166]. As an alternative to L-dopa in early PD, amantadine is associated with improvement in functional disability, and in a subset of PD patients, there is a robust symptomatic improvement[167]. The main current indication of amantadine is, however, as an adjunctive treatment for L-dopa-induced dyskinesia in late-stage PD. Amantadine, is an N-methyl-D-aspartate glutamate receptor antagonist. Increased glutamate transmission contributes to the motor symptoms in PD, and also to the progression of neurodegeneration through excitotoxic mechanisms[168]. Amantadine might also improve apathy and fatigue in PD patients[169]. The drug is well absorbed and widely distributed, little drug being present in the circulation, and is primarily eliminated through the kidneys both by glomerular filtration and tubular secretion. The dose of amantadine, therefore, requires adjustment in in patients with renal dysfunction[170]. In patients with PD on amantadine therapy, plasma amantadine concentration increased according to increasing renal dysfunction[171].
Dyskinesia can improve with amantadine and in a multi-center, double-blind, randomized, placebo-controlled trial, dyskinesia rating scale improved in 64% patients treated with amantadine compared to 16% on placebo[172]. In a randomized placebo-controlled study of 32 patients who have been on stable amantadine therapy for L-dopa-induced dyskinesia over at least one year, dyskinesia duration and intensity (assessed by UPDRS IV items) significantly increased at three-week follow-up after being switched to placebo[173]. Wash-out of amantadine in dyskinetic patients with PD significantly worsened L-dopa induced dyskinesia (with greater worsening of abnormal involuntary movement scale score)[169].
Amantadine is also effective in the treatment of freezing of gait in patients with advanced PD. In one study, freezing of gait improved by treatment with amantadine in 11 patients with advanced PD. The effect, however, decreased in a proportion (approximately 36%) of patients after 4 mo[174]. In a randomized, double-blind, placebo-controlled, multicenter trial of 42 subjects with freezing of gait, 5 d intravenous amantadine attenuated freezing severity and improved patients’ mobility[175]. Intravenous administration of amantadine has also been effective in improving parkinsonian symptoms after surgery[176]. In PD patients with subthalamic nucleus deep brain stimulation and incomplete axial benefit, gait scores significantly improved with amantadine treatment. Patients also reported subjective improvement in speech, gait or balance[177]. Side effects of amantadine includes blurred vision, visual hallucinations, peripheral edema (Malkani et al[174], 2012), reversible corneal edema after long term use[178], auditory hallucinations[179]. Myoclonus, hallucination, or delirium might develop when the plasma concentration of amantadine exceeds 3000 ng/mL[171]. Cardiac arrest, ventricular tachycardia and prolonged QTc interval have been reported following amantadine[180,181].
Studies have shown that amantadine might possess neuroprotective properties. The dug protected rat midbrain cultures from either MPP(+) or lipopolysaccharide. Amantadine possibly exerts its neuroprotective effects through the inhibition of the release of microglial pro-inflammatory factors, and/or an increase in expression of neurotrophic factors such as glial cell line-derived neurotrophic factor released from astroglia[182].
The anticholinergic drugs were the first agents to be used in the pharmacological management of PD[183]. Nowadays, however, they have limited place in the treatment of the disease. These drugs are also prescribed to ameliorate extrapyramidal symptoms caused by antipsychotic medications[184], but this also appears to be declining owing to an increase in the use of atypical anti-psychotic agents[185]. When used as monotherapy in early disease or as an adjunct to other antiparkinsonian drugs, anticholinergics are more effective than placebo in control of symptoms. Because of the high risk of cognitive, neuropsychiatric and autonomic adverse events, these agents are best avoided in the elderly[186-188].
The current place of anticholinergic agents in treatment of PD is limited to early cases and in younger patients (i.e., 60 years of age) with troublesome resting tremor because of the evidence that these agents are better than levodopa for tremor[189]. Studies, however, have shown that dopaminergic agents are as effective as anticholinergics in reducing tremor in idiopathic PD. Single-dose challenges with biperiden or apomorphine significantly reduced the amplitude of resting, postural, and action tremor. UPDRS scores for rigidity and akinesia, however, were only reduced by apomorphine[190]. Moreover, the effect of biperiden on the amplitude of the resting tremor was weaker than that of L-dopa had a good effect on the amplitude of the resting tremor[191].
Anticholinergic drugs are of little value in the treatment of rigidity, akinesia, gait dysfunction, or impaired postural reflexes[189]. It has been shown however that trihexyphenidyl might be of benefit in patients whose axial symptoms worsened after deep brain stimulation of the subthalamic nucleus. In this study UPDRS II and III decreased in response to the anticholinergic agent[192]. Studies also suggested that the use of anticholinergic drugs early in the disease progression might be of potential benefit in delaying the need for L-dopa treatment[183]. Side effects due to anticholinergic agent include dry mouth, blurred vision, tachycardia, urinary retention, constipation, impaired sweating, and central nervous system effects, e.g., memory impairment, confusion, and hallucinations, especially in older individuals[183,189].
Istradefylline: Istradefylline (KW-6002) is a selective adenosine A2A receptor antagonist which exhibit antiparkinsonian activity without worsening L-dopa induced dyskinesia. Istradefylline is not yet an FDA-approved drug. Istradefylline has been licensed as an anti-parkinsonian drug this year in Japan[193]. Istradefylline exhibits high affinity for A2A receptors, but lower affinities for the other subtypes of adenosine receptors (A1, A2B, and A3) in humans, marmosets, dogs, rats, and mice. The agent does not influence other neurotransmitter receptors, inhibit monoamine oxidases, or catechol-O-methyl transferase[194]. Unlike L-dopa, the chronic administration of istradefylline (and also of other A2A receptor antagonists: SCH 412348, vipadenant and caffeine) to rats did not result in dyskinetic activity or worsen dyskinesias when co-administered with L-dopa[195]. In non-human primates with haloperidol-induced extrapyramidal symptoms (EPS) and catalepsy, A2A receptor antagonists, SCH 412348 and KW-6002 and the A1/A2A receptor antagonist, caffeine significantly increased the time to the onset of EPS. Moreover, SCH 412348 and caffeine significantly reduced haloperidol-induced catalepsy[196]. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated marmosets with L-dopa dyskinesia, single dose acute oral administration of istradefylline enhanced and prolonged the anti-parkinsonian effects of a sub-optimal dose of L-dopa, while its chronic administration did not worsen dyskinesia[197].
When evaluated as monotherapy in patients with early PD, istradefylline 40 mg/d for 12-wk was safe and well tolerated, but failed to significantly improve motor symptoms[198]. In PD patients on L-dopa therapy with motor complications, istradefylline 10, 20 or 40 mg/d, administered as adjunctive treatment to L-dopa for 12 wk in a double-blind study did not affect “off” time duration, though at 40 mg/d it significantly improved the motor score[199]. Other studies, however, showed that istradefylline 20, 40 or 60 mg/d, given once daily for 12 wk to L-dopa-treated patients with motor complications was well tolerated and reduced daily “off” time[200-204]. The most common adverse event was dyskinesia[200-205]. Other side effects reported with istradefylline were lightheadedness, tremor, constipation, weight decrease[201], nausea, dizziness, and hallucinations[200].
Preladenant: Preladenant (SCH 420814) is an orally bioavailable selective adenosine A2A receptor antagonist in phase III development for PD treatment. In MPTP-treated primates, preladenant improved motor ability without causing any dopaminergic-mediated dyskinetic or motor complications. The drug also delayed the onset of EPS symptoms evoked by an acute haloperidol challenge in primates with previous chronic haloperidol treatment[206]. Two randomized, double-blind, placebo-controlled, ascending-dose studies, showed that the drug was generally well tolerated up to 200 mg/d. Peak plasma concentrations were reached in approximately 1 h and then declined rapidly. Preladenant caused transient mild increases in blood pressure within a few hours of administration[207]. In patients with PD and motor fluctuations who were receiving L-dopa, preladenant at 5 and 10 mg given twice daily for 12 wk reduced the mean daily “off” time relative to baseline. The most common adverse events in the L-dopa/preladenant group vs placebo were worsening of PD (11% vs 9%), somnolence (10% vs 6%), dyskinesia (9% vs 13%), nausea (9% vs 11%), constipation (8% vs 2%), and insomnia (8% vs 9%) [208]. In another study, preladenant treatment (5 mg twice a day) for 36 wk as a L-dopa adjunct in subjects with fluctuating PD, provided sustained “off” time reductions (1.4-1.9 h/d) and “on” time increases (1.2-1.5 h/d) relative to the baseline. The main side effects were dyskinesia (33%) and constipation (19%)[209].
Safinamide: Safinamide is a novel anti-parkinsonian drug currently in phase 3 clinical trials, as add-on therapy to L-dopa or a dopamine agonist in early and advanced stage PD. It is an oral alpha-aminoamide derivative, with dopaminergic and non-dopaminergic mechanisms of action involving inhibition of dopamine and noradrenaline reuptake, a selective and reversible inhibition of MAO-B, blockage of voltage-dependent sodium channels, modulation of calcium channels as well as an inhibitor of glutamate release[210-212]. Safinamide is administered once daily at doses of 50 and 100 mg[211]. In an open pilot study, safinamide (100, 150, and 200 mg once a day) improved motor performance when added to a stable dose of dopamine agonist and also decreased motor fluctuations in those treated with L-dopa[213]. Two randomized double-blind studies suggested that safinamide 100 mg/d may be effective as an “add-on” treatment to a dopaminergic agonist in early PD[214,215]. In 24-wk, double-blind study, safinamide 100 mg added to a dopamine agonist improved motor symptoms (UPDRS motor total score)[214]. Safinamide 100 mg/d for 12-mo resulted a lower rate of intervention (increase in dopamine agonist dose; addition of another dopamine agonist, L-dopa or other PD treatment) and a delay in median time to intervention of 9 d compared with placebo[215]. In both studies, there was no benefit from safinamide 200 mg. A more recent study showed that oral safinamide 50 or 100 mg/d added to L-dopa in patients with PD and motor fluctuations for 24 wk, increased time with no or non-troublesome dyskinesia, decreased “off” time, and improved UPDRS motor scores as well as clinical global impression-change[216]. In MPTP- lesioned dyskinetic macaque monkey made dyskinetic by treatment with L-dopa, pre-treatment with safinamide (3, 10, 20 and 30 mg/kg) dose-dependently reduced dyskinesia scores and prolonged the duration of the antiparkinsonian effect of L-dopa. Moreover, combined amantadine (5 mg/kg) and safinamide (20 mg/kg) exerted additional beneficial effects on L-dopa-induced dyskinesia[217].
Safinamide, also appear to exert neuroprotective effects by blocking the voltage-dependent Na+ and Ca2+ channels and the Ca2+-mediated glutamate release processes. Safinamide provided significant protection against neurological deficit and axonal degeneration in experimental autoimmune encephalomyelitis, possibly via reduction in the activation of microglia/macrophages, resulting in suppressed microglial superoxide production [218].
Zonisamide: Zonisamide (1,2-benzisoxazole-3-methanesulfonamide) is a new antiepileptic drug for treating refractory epilepsy. It is licensed in Europe and the United States for the adjunctive treatment of partial seizures (with or without secondary generalization) in adults. It is also licensed in Europe as monotherapy for adults with newly diagnosed partial epilepsy[219]. The drug inhibits voltage-gated Na+ channel, T-type voltage-sensitive Ca2+ channel, Ca2+-induced Ca2+ releasing system, and neuronal depolarization-induced glutamate release; and enhance the release of inhibitory neurotransmitters. The drug has been found by chance to exert beneficial anti-parkinsonian effects. Early studies on patients with PD demonstrated lessening of symptoms, especially wearing-off when using zonisamide (50-200 mg/d) as an “add-on” treatment[220]. When used as an adjunctive therapy in patients with insufficient response to L-dopa treatment, zonisamide (25, or 50 mg/d) resulted in significant motor improvement and reduced the duration of “off” time compared with placebo. Dyskinesia was not increased in zonisamide-treated groups[221]. In two 12-wk, randomized, double-blind trials in PD patients inadequately controlled with L-dopa, zonisamide (25 mg once daily) significantly improved motor function (UPDRS Part III total score), compared with placebo[222]. The drug appears to be generally well tolerated at doses of 25-50 mg/d[221,222]. Zonisamide also led to marked reduction in the severity of impulsive behaviors and global impulsiveness in PD patients with impulse control disorders who did not improve following a reduction of either L-dopa or dopamine agonists[223].
In experimental models of PD, zonisamide displayed antiparkinsonian and neuroprotective effects[224-228]. Several mechanisms have been proposed including (1) increased expression of astrocyte-mediated neurotrophic and anti-oxidative factors, e.g., astrocyte-derived neurotrophic factor, vascular endothelial growth factor, copper/zinc superoxide dismutase, and manganese superoxide dismutase[227]; (2) upregulating levels of manganese superoxide dismutase[225]; (3) anti-apoptotic effect[225,227-229]; (4) antioxidant effect[227-229]; (5) increased S100β-positive and glial fibrillary acidic protein-positive astrocytes and dopamine turnover[226]; (6) potent and reversible inhibition of MAO-B activity[224]; (7) delta (1) receptor mediated inhibition of the indirect pathway[230]; (8) dopamine release[231]; and (9) prevention of dopamine quinone formation[232]. Table 2 summarizes the findings of randomized double blind studies on novel antiparkinsonian drugs.
| Drug | Study objective | Outcomes | Adverse events | Ref. |
| Istradefylline | Evaluated the efficacy and safety of istradefylline, 20 and 40 mg once daily as adjunctive to L-dopa in patients with motor complications (12 wk) | ↓ daily change in "off" time vs placebo | ↑ dyskinesia | [203] |
| Istradefylline | Evaluated the efficacy and safety of istradefylline, 10, 20 and 40 mg once daily as adjunctive to L-dopa in patients with motor complications (12 wk) | No effect on "off" time duration Improved motor scores at 40 mg | - | [199] |
| Istradefylline | Evaluated the efficacy of istradefylline at an oral dose of 20 and 40 mg once daily for 12 wk in PD patients with motor complications on levodopa therapy | ↓ "off" time vs placebo | ↑ dyskinesia | [204] |
| Istradefylline | Evaluated the safety and efficacy of istradefylline 40 mg, as monotherapy in patients with PD | No significant effect in improving motor symptoms | - | [198] |
| Istradefylline | To evaluate efficacy, safety, and tolerability of istradefylline 20 mg once daily vs placebo as an adjunct to levodopa in PD subjects with motor fluctuations | ↓ "off" time | Dyskinesia, lightheadedness, tremor, constipation, and weight decrease | [201] |
| Istradefylline | To evaluate safety and efficacy of istradefylline 20 or 60 mg/d in L-dopa-treated PD subjects with motor complications | ↓ "off" time without an increase in “on” time | Dyskinesia, nausea, dizziness, and hallucinations | [200] |
| Istradefylline | To evaluate safety and efficacy of istradefylline 40 mg/d in L-dopa-treated PD subjects with prominent wearing-off motor fluctuations | ↓ "off" time without increased troublesome dyskinesia | - | [202] |
| Istradefylline | To evaluate safety and efficacy of istradefylline 20 or 40 mg/d in patients with L-dopa-motor fluctuations and peak-dose dyskinesias | ↓ "off" time | Severity of dyskinesia was unchanged, but "on" time with dyskinesia increased | [205] |
| Preladenant | To evaluate efficacy of using preladenant 5 mg twice a day as a levodopa adjunct in subjects with fluctuating PD | ↓ "off" time ↑ "on" time throughout the 36-wk treatment relative to the baseline | Dyskinesia and constipation | [209] |
| Preladenant | To evaluate safety of single and multiple rising preladenant doses compared with placebo | Preladenant was generally well tolerated up to the maximum dose tested (200 mg/d) | Transient mild increases in blood pressure within a few hours after preladenant administration | [207] |
| Preladenant | To evaluate efficacy and safety of 1, 2, 5, or 10 mg oral preladenant twice daily in patients with PD and motor fluctuations on L-dopa | 5 and 10 mg preladenant ↓ "off" time | Worsening of PD, dyskinesia, nausea, constipation, and insomnia | [208] |
| Safinamide | To evaluate efficacy and safety of safinamide 50 or 100 mg/d, as add-on to L-dopa in the treatment of PD patients with motor fluctuations | ↑ total on time with no or nontroublesome dyskinesia, ↓ decreased off time, without worsening dyskinesia | - | [216] |
| Safinamide | To evaluate efficacy of safinamide 100 or 200 mg/d as add-on treatment to single dopaminergic receptor agonist single in early PD | Safinamide 100 mg/d may be effective as add-on treatment | - | [215] |
| Safinamide | To evaluate efficacy and safety of once-daily 100 or 200 mg safinamide in patients with early PD receiving a stable dose of a single dopaminergic receptor agonist | Safinamide 100 mg/d improved motor symptoms (UPDRS part III total score) | - | [214] |
| Zonisamide | To evaluate the efficacy, safety and tolerability of daily doses of 25, 50, and 100 mg of zonisamide as an adjunctive treatment in PD | Zonisamide 25 and 50 mg/d improved motor symptoms (UPDRS part III total score) Zonisamide 50 and 100 mg ↓ "off" time without ↑ dyskinesia | - | [221] |
| Isradipine | To establish a tolerable and efficacious dosage of isradipine controlled-release in subjects with early PD not requiring dopaminergic therapy | The tolerability of 5, 10, or 20 mg of isradipine was dose dependent No difference in change in UPDRS among dosages | Peripheral oedema and dizziness | [295] |
| Isradipine | To evaluate safety and tolerability of isradipine controlled release in patients with early PD | Tolerability of isradipine CR 5, 10, 15, or 20 mg was dose dependent Isradipine had no significant effect on blood pressure or PD motor disability | Leg oedema and dizziness | [294] |
Coenzyme Q10: Coenzyme Q10 (CoQ10) or ubiquinone is a lipid-soluble molecule present in all membranes throughout the cell. It acts as an electron carrier in the mitochondrial electron transport chain, located within the inner mitochondrial membrane (transfers an electron between complexes I/II and III). CoQ10 also functions as an antioxidant, thereby protecting cellular membranes and macromolecules (e.g., proteins, lipids, DNA). CoQ10 also regenerates the pool of tocopherol[233-235]. In brains from PD patients postmortem, CoQ10 decreased in the cortex[236]. In a study involving 33 patients with PD, Jiménez-Jiménez et al[237] found no difference in serum levels of CoQ10 between patients with PD and controls. In contrast, CoQ10/cholesterol ratio inversely correlated with duration of the disease, total UPDRS score and motor examination of the UPDRS. Treatment with L-dopa or dopamine agonists had no significant effect on CoQ10/cholesterol ratio. Other studies, however, have shown elevation in oxidized form of CoQ10 in plasma[238] or decreased CoQ10 in peripheral blood lymphocytes from patients with PD[239]. Moreover, increased percentage of oxidized to total CoQ10 was detected in the cerebrospinal fluid (CSF) of patients with PD. The concentration of 8-OHdG in the CSF also increased and correlated with concentrations of oxidized to total CoQ10, thereby linking both mitochondrial oxidative damage and oxidative DNA damage in the disease process[240].
In primate model of PD induced by the nigrostriatal toxin MPTP, dopamine cell loss was prevented by treatment with coenzyme Q[241]. In the MPTP rat model of PD, both CoQ10, reduced CoQ10 (ubiquinol) exerted neuroprotective effects against MPTP induced dopamine depletion, loss of tyrosine hydroxylase neurons and the development of alpha-synuclein inclusions in SNc[242]. Orally administered CoQ10 also halted the progression of nigrostriatal degeneration induced in rats by paraquat[243] and in the MPTP mouse model of PD[244].
In patients with PD without motor fluctuations and on stable antiparkinsonian treatment, nanoparticular CoQ10 (100 mg 3 times a day) for 3 mo failed to demonstrate clinical benefit. The formulation used was associated with CoQ10 plasma levels similar to 1200 mg/d of standard formulations[245]. In another randomized, double-blind, placebo-controlled trial, treatment of PD patients with CoQ10 (300-1200 mg/d) increased plasma level in a dose-dependent manner. CoQ10 was well-tolerated and at 1200 mg/d there were significant slowing the progression of PD as measured by the total UPDRS score [246]. Improvements in the total UPDRS were also observed following 2 wk treatment with CoQ10 in 16 subjects with early idiopathic PD. Moreover, F2-isoprostanes in plasma were significantly reduced in the 400-1200 mg/d dose range (but increased at 2400 mg/d dosage). Symptomatic benefit from CoQ10 appeared to depend on initial plasma ubiquinol and F2-isoprostanes[247]. More recent phase III randomized, placebo-controlled, double-blind clinical trial, however, reported no evidence of clinical benefit (total UPDRS) from treatment with 1200 mg/d or 2400 mg/d in patients who received a diagnosis of PD within 5 years[248,249].
Creatine: Creatine is a naturally occurring amino acid consumed in meat and fish. It is also synthesized in liver, kidneys, and pancreas from glycine, arginine and methionine. The highest concentration of creatine is found in skeletal muscles (95% of body stores) with most of the remaining stores found in the heart, brain and testes. After its synthesis, creatine is released into the blood stream, from where it is taken up by cells against a concentration gradient via the creatine transporter. The active form of creatine is phosphorylcreatine (PCr). Creatine kinase is the enzyme catalyzing the reaction of the phosphorylation of creatine to PCr through the transfer of the γ-phosphate group of adenosine triphosphate to the guanidino group of creatine to yield adenosine diphosphate and high-energy PCr. In brain, the active form of creatine or phosphocreatine serves as an energy reserve being a donor of high energy phosphate molecules to adenosine diphosphate to form adenosine triphosphate (ATP). One key function of phosphocreatine is to increase ATP levels in tissue so as to stabilize neuronal membranes. Creatine and phosphocreatine are involved in the shuttle of ATP from the mitochondria, site of synthesis to the site of use in the cytosol[252,253]. Creatine is widely used as a nutritional supplement and ergogenic aid for athletes[254].
In PD patients analysis of whole brain metabolite changes using proton magnetic resonance spectroscopy, indicated higher creatine values, which might reflect greater neuronal energy expenditure early in the disease process that is compensatory[255]. Other researchers detected significantly decreased creatine (as well as N-acetylaspartate, choline, myo-inositol, glutathione and dopamine concentrations) in patients with PD. This decrease in creatine levels possibly reflected impaired energy metabolism due to mitochondrial dysfunction[256]. In the MPTP model of PD in mice, creatine protected against striatal dopamine depletions and loss of substantia nigra tyrosine hydroxylase immunoreactive neurons[257].
Creatine supplementation has been proposed as an adjunct to medication for the treatment of brain-related disorders associated with bioenergetic deficits like PD[258]. Creatine intake seems to be safe in healthy individuals and in patients with PD. in healthy males undergoing aerobic training, creatine supplementation (approximately 10 g/d) over 3 mo does not provoke renal dysfunction. Serum creatinine serum and urinary sodium and potassium were unchanged, while cystatin C levels decreased over time, suggesting an increase in glomerular filtration rate[259]. In patients with PD, creatine 10 g/d was well tolerated[260] and creatine (4 g/d) for 2 years was well tolerated, apart from gastrointestinal complaints. Despite increased serum creatinine levels, other markers of tubular or glomerular renal function, especially cystatin C, remained normal, indicating unaltered kidney function[261]. In patients with PD, a 2-year placebo-controlled randomized clinical trial showed that creatinine improved patient mood and led to a smaller dose increase of dopaminergic therapy. Creatinine, however, had no effect on overall UPDR scale scores or dopamine transporter SPECT[262]. Creatine supplementation (20 g/d for the first 5 d and 5 g/d thereafter) has been shown to enhance the benefits of resistance training in patients with PD[263].
L-Carnitine: Carnitine (3-hydroxy-4-N-trimethylammoniobutanoate) is a quaternary amine synthesized in the body from lysine and methionine mainly in liver, kidney, and muscle. Dietary carnitine from meat and dairy products provides 75% of body carnitines. Carnitine or acylcarnitines including acetyl-L-carnitine are important in the oxidation of fatty acids in mitochondria. Acetyl-L-carnitine is a constituent of the inner mitochondrial membrane[264]. In humans, plasma acetyl-L-carnitine and L-carnitine short chain esters increases following oral treatment with acetyl-L-carnitine. CSF concentrations also increases, suggesting that the agent easily crosses the blood-brain barrier[265]. The administration of acetyl L-carnitine in elderly subjects (2 g twice-a-day ) improves physical and mental fatigue as well as functional status and cognitive functions[266], Preclinical studies have shown that L-carnitine and its acetyl ester, acetyl-L-carnitine exert neuroprotective effects. Acetyl-L-carnitine act to preserve mitochondrial respiratory chain complex activity in face of inflammatory cytokine insult[267], prevent age-related oxidative mitochondrial decay[268] and maintain mitochondrial respiration and enzyme activities (NADH dehydrogenase, cytochrome C oxidase and pyruvate dehydrogenase) following contusion spinal cord injury[269]. The neuroprotective effects of acetyl-L-carnitine involves induction of heme oxygenase-1, up-regulation of heat shock protein 60, increased expression of the redox-sensitive transcription factor Nrf2[267], reduction of carbonyl formation and decreased mtDNA deletion[270]. Acetyl-L-carnitine has been reported to protect against MPTP-induced toxicity in the nonhuman primate[271].
N-acetyl cysteine: The brain in PD is exposed to inappropriately high levels of oxygen and nitrogen-derived free radicals. Post-mortem studies of PD brains have detected increased lipid peroxidation products[272] and increased protein carbonyls indicative of protein oxidation[273] as well as reduced glutathione levels[274]. One of the most important intracellular redox buffers and free radical scavengers in brain is glutathione, a tripeptide thiol that consists of glutamate, cysteine and glycine. It exists mainly in a reduced form (GSH) and the ratio of GSH to the oxidized form (GSSG) determines the oxidative status of the cell[275,276]. Therefore a decrease in glutathione bioavailability would have serious consequences on the ability of cells to withstand oxidative burden. Glutathione concentrations can be increased by N-acetyl cysteine, the N-acetyl derivative of the amino acid L-cysteine, which is rapidly hydrolyzed intracellularly to cysteine, the rate limiting substrate for glutathione synthesis[277,278].
There is accumulating evidence to suggest the usefulness of supplementation with N-acetylcysteine in neurodegenerative disorders including PD[277-279]. In transgenic mice overexpressing wild-type human alpha-synuclein, N-acetylcysteine attenuated the loss of dopaminergic terminals at 1 year and also significantly decreased the levels of human alpha-synuclein[280]. Mice lacking the excitatory amino acid transporter EAAC1 have impaired neuronal cysteine uptake and consequent reduced neuronal glutathione content. These mice exhibited age-dependent loss of dopaminergic neurons in the SNPc, nitrosative stress and neuroinflammation, which were alleviated by N-acetylcysteine treatment[281]. In rats in which GSH was depleted by treatment with 2-cyclohexene-1-one, treatment with 1.6 g/kg of N-acetylcysteine rescued the depleted levels of GSH in the brain and restored cognitive deficits[282].
In a recent clinical study, single N-acetylcysteine infusion (150 mg/kg) was able to increase blood GSH redox ratios and increase brain GSH concentrations (measured using 7-T magnetic resonance spectroscopy) in those with PD and Gaucher disease and healthy controls[283].
Alpha lipoic acid: Lipoic acid (thioctic acid, 1,2-dithiolane-3-pentanoic acid) functions as a cofactor in multienzyme complexes that catalyze the oxidative decarboxylation of pyruvate, a-ketoglutarate, and branched-chain α-keto acids. Lipoic acid and its reduced form dihydrolipoic acid are also potent antioxidants capable of scavenging a number of reactive oxygen and nitrogen species. Lipoic acid is rapidly absorbed in the gut and passed to various tissues for catabolism[284]. Alpha lipoic acid is likely to be of benefit in several brain pathologies and neurodegenerative disorders. Chronic treatment with alpha lipoic acid (and also N-acetylcysteine) was found to improve cognition In SAMP8 mice that overexpress amyloid precursor protein[285]. In rat brain glial cultures, alpha-lipoic acid decreased viral double-stranded RNA-stimulated inflammatory signaling by down-regulating interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase transcripts. It also prevented cultured glial cytotoxicity[286]. Alpha lipoic acid has been shown to protect dopaminergic neurons in vitro against apoptosis induced by the nigrostriatal toxin 1-methyl-4-phenylpyridinium (MPP+). This effect of was associated with decreased intercellular levels of reactive oxygen species and the mitochondrial transmembrane permeability[287]. It also protected dopaminergic neurons in the animal model of PD induced by stereotaxic injection of 6-hydroxydopamine in rat striatum[288]. It has been suggested that a combination of mitochondrial antioxidants/nutrients could improve mitochondrial function and/or attenuate oxidative damage implicated in PD[289]. The authors found that the combined treatment with alpha-lipoic acid and acetyl-L-carnitine was more effective than either agent alone in protecting SK-N-MC human neuroblastoma cells against rotenone-induced mitochondrial dysfunction and oxidative damage.
Isradipine: Isradipine is a dihydropyridine calcium channel antagonist, with high affinity for Cav1.3 L-type channels. L-type Ca2+ channels with a pore-forming Cav1.3 subunit underlie autonomous pacemaking in adult dopaminergic neurons in the SNPc. This poses a sustained stress on mitochondrial ATP generating oxidative phosphorylation, accelerating cellular aging and death, and rendering dopaminergic more susceptible to the effect of nigrostriatal toxins[290]. Antagonism of these channels with isradipine has been shown to exert neuroprotective effects in animal models of PD[291,292]. Isradipine afforded neuroprotection against the nigrostriatal toxin 6-OHDA injected intrastriatally, sparing dopaminergic fibers and cell bodies[291]. In human neuroblastoma SH-SY5Y cells, isradipine antagonized many effects of rotenone including production of reactive oxygen species, G1/G0 cell cycle arrest, and activation of p53/p21 signaling proteins as well as the decreased expression of the signaling proteins for cell proliferation and survival, Cyclin-dependent kinase 2, cyclin D1, and Akt[292]. Isradipine also reduced L-dopa-induced rotational behavior and abnormal involuntary movements in animal model of L-dopa-induced dyskinesia[293].
Isradipine studies are thus being conducted in humans to establish the dosage, safety and tolerability of the drug. In these studies, controlled release isradipine 10 mg daily was tolerated by 73% and 87% of patients with early PD, respectively. Peripheral edema and dizziness were most common adverse events encountered[294,295]. Isradipine displayed no significant effect on blood pressure or PD motor disability[294].
Pioglitazone: Pioglitazone is a peroxisome proliferator-activated receptor-gamma agonist of the thiazolidinedione class. The administration of this antidiabetic agent has been shown to protect dopaminergic neurons preclinical rodent models of PD[296-299]. The drug also exhibited neuroprotective properties in the non-human primate model of MPTP-induced PD. Significant improvements in clinical rating score was associated with preservation of nigrostriatal dopaminergic markers, e.g., cell counts of tyrosine hydroxylase immunoreactive- and vesicular monoamine transporter-2immunoreactive-nigral neurons[300]. Several mechanisms have been postulated to account for pioglitazone-induced neuroprotection. This included attenuation of toxin-induced glial activation and consequent suppression of pro-inflammatory cytokine (TNF-α, IL-1β, interferon-gamma) release[296,301,302]. Pioglitazone also attenuated oxidative stress[302], interfered with phosphorylation of jun N-terminal kinase and nuclear factor kappa-B, and suppressed cyclooxygenase 2 expression and the subsequent prostaglandin E(2) synthesis[298] and showed dose-dependent modulation of CD68-ir inflammatory cells[286]. Other researchers provided data that pioglitazone is effective in the MPTP mouse model through inhibition of MAO-B[299]. It has been shown that pioglitazone is a specific and reversible inhibitor of human MAO B. Other members of the glitazone class, rosiglitazone and troglitazone are weaker inhibitors of both MAO-A and MAO-B[303].
Minocycline: Minocycline is a second-generation, semi-synthetic tetracycline that received much interest for its dopaminergic neuroprotective effects observed in experimental models of PD. Minocycline inhibits microglial activation[304], exerts antioxidant and anti-inflammatory effects[305], and prevents apoptotic cell death, possibly due to attenuating endoplasmic reticulum stress and mitochondrial dysfunction[306]. Ongoing clinical trials for evaluating the effect of minocycline on disease progression, however, failed to demonstrate clinical benefit for minocycline. An 18-mo phase II trial of minocycline 200 mg/d in subjects with early PD, found no effect for the drug in slowing down the progression of disability. Symptomatic treatment of PD symptoms was required in 62% of minocycline, and 60% of placebo-treated subjects[307]. In a randomized, double-blind in patients with Multiple-System-Atrophy Parkinson-type, the progression rate over 48 wk of minocycline 200 mg/d (the change in motor function) did not differ from that of placebo. A significant deterioration in motor scores occurred in both groups. Positron emission tomography-data in two patients in the minocycline group, however, suggested that minocycline might interfere with microglial activation[308].
Neuroinflammation is a major contributing factor in the pathogenesis of PD[309]. In vitro, several non-steroidal anti-inflammatory drugs (NSAIDs) including ibuprofen, aspirin, acetaminophen, meclofenamic acid sodium salt, sulindac sulfide, ketoprofen (but not naproxen and indomethacin) inhibited the formation and stabilization of alpha-synuclein fibrils[310]. In this context, it is to be noted that the use of NSAIDs is frequent among PD patients. The chronic prescription of analgesic drugs was more prevalent in PD patients than in the general population and similar to that in osteoarthritis patients[311]. Several studies have assessed the potential for NSAIDs in lessening the progression of PD. The results of these studies were, however, inconclusive. In one study, the regular use of non-aspirin NSAIDs was associated with a lower risk of PD compared with non-regular users. A lower risk of PD (though of no statistical significance) was also observed among men and women who took 2 or more tablets of aspirin per day[312]. In their study, Wahner et al[313] found that regular use of aspirin (≥ 2 pills/wk for at least 1 mo) was associated with a decreased risk of PD; an effect seen only in women. Regular non-aspirin NSAID usage was even associated with a stronger protective effect, particularly those who reported 2 or more years of use. Other researchers provided data that ibuprofen users had a significantly lower PD risk than non-users with this effect of the drug being a dose-dependent one. The same studies found no effect for aspirin, other NSAIDs or acetaminophen in reducing the risk for PD[314,315]. Hernán et al[316], however, found that non-aspirin NSAID use was associated with a higher risk in women and a lower risk in men. Other researchers, found no evidence that NSAID use reduces the risk of PD[317-321], although one study suggested that long-term use of NSAIDs was associated with a slightly lower PD risk[321].
Studies in humans suggest the accumulation of iron in the substantia nigra of PD patients. Consequently excess iron has been implicated in the pathogenesis of PD[322-325]. One approach to halt nigrostriatal degeneration in PD might therefore involve the reduction of iron-mediated oxidative stress through the use of iron chelators. In a clinical trial in early PD patients, one such iron chelator that is deferiprone slightly improved motor signs at 6 mo and decreased motor handicap progression and iron overload at one year[326]. In a randomized clinical study on 23 patients with early stage PD (< 5 years from diagnosis), deferiprone for 6 mo elicited a non-significant small improvement in the motor UPRDS scores compared to placebo. Magnetic resonance imaging indicated significantly reduced iron content in the dentate nucleus and caudate nucleus though not in substantia nigra following deferiprone therapy[327]. In these two studies, deferiprone was well tolerated. Side effects were neutropenia or agranulocytosis[326,327]. In one study, treatment with deferiprone for 4 years in 6 patients with neurodegeneration with brain iron accumulation was associated with stabilization in motor symptoms in 5/6 patients[328].
P- Reviewer: Orlacchio A, Sanchez-Alcazar JA, Unger M S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Zhang ZX, Román GC. Worldwide occurrence of Parkinson's disease: an updated review. Neuroepidemiology. 1993;12:195-208. [PubMed] |
| 2. | de Rijk MC, Tzourio C, Breteler MM, Dartigues JF, Amaducci L, Lopez-Pousa S, Manubens-Bertran JM, Alpérovitch A, Rocca WA. Prevalence of parkinsonism and Parkinson’s disease in Europe: the EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997;62:10-15. [PubMed] |
| 3. | Hirsch EC. Biochemistry of Parkinson’s disease with special reference to the dopaminergic systems. Mol Neurobiol. 1994;9:135-142. [PubMed] |
| 4. | Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415-455. [PubMed] |
| 5. | Meissner W, Prunier C, Guilloteau D, Chalon S, Gross CE, Bezard E. Time-course of nigrostriatal degeneration in a progressive MPTP-lesioned macaque model of Parkinson’s disease. Mol Neurobiol. 2003;28:209-218. [PubMed] |
| 6. | Jellinger KA. Neuropathological spectrum of synucleinopathies. Mov Disord. 2003;18 Suppl 6:S2-12. [PubMed] |
| 7. | Schapira AH, Bezard E, Brotchie J, Calon F, Collingridge GL, Ferger B, Hengerer B, Hirsch E, Jenner P, Le Novère N. Novel pharmacological targets for the treatment of Parkinson’s disease. Nat Rev Drug Discov. 2006;5:845-854. [PubMed] |
| 8. | Brichta L, Greengard P, Flajolet M. Advances in the pharmacological treatment of Parkinson’s disease: targeting neurotransmitter systems. Trends Neurosci. 2013;36:543-554. [PubMed] |
| 10. | Riess O, Krüger R, Hochstrasser H, Soehn AS, Nuber S, Franck T, Berg D. Genetic causes of Parkinson’s disease: extending the pathway. J Neural Transm Suppl. 2006;181-189. [PubMed] |
| 11. | Di Monte DA. The environment and Parkinson’s disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003;2:531-538. [PubMed] |
| 12. | Chade AR, Kasten M, Tanner CM. Nongenetic causes of Parkinson’s disease. J Neural Transm Suppl. 2006;147-151. [PubMed] |
| 13. | Pal P-Kr, Samii A, Calne DB. Cardinal Features of Early Parkinson’s Disease. Parkinson's Disease: Diagnosis and Clinical Management, First edition. New York: Demos Medical Publishing 2002; 714. |
| 14. | Gunzler SA, Schoenberg MR, Riley DE, Walter B, Maciunas RJ. Parkinson’s Disease and Other Movement Disorders. In: Schoenberg MR, Scott JG, editors. The Little Black Book of Neuropsychology 2011; 567-644. |
| 15. | Morgan J, Sethi KD. Differential Diagnosis. Pahwa R, Lyons KE, editors. New York: Informa Healthcare USA, Inc 2007; 29-48. |
| 16. | Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181-184. [PubMed] |
| 17. | Lewitt PA. Levodopa for the treatment of Parkinson’s disease. N Engl J Med. 2008;359:2468-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 197] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 18. | Contin M, Martinelli P. Pharmacokinetics of levodopa. J Neurol. 2010;257:S253-S261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Olanow CW, Schapira AH. Therapeutic prospects for Parkinson disease. Ann Neurol. 2013;74:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Cesaro P, Defebvre L. [Drug treatment of early-stage (de novo and “honeymoon”) Parkinson disease]. Rev Neurol (Paris). 2014;170:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Nissen T, Newman EJ, Grosset KA, Daghem M, Pal G, Stewart M, Odin P, Macphee GJ, Grosset DG. Duration of L-dopa and dopamine agonist monotherapy in Parkinson’s disease. Scott Med J. 2012;57:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Bonuccelli U, Del Dotto P. New pharmacologic horizons in the treatment of Parkinson disease. Neurology. 2006;67:S30-S38. [PubMed] |
| 23. | Ondo WG. Motor complications in Parkinson’s disease. Int J Neurosci. 2011;121 Suppl 2:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Khan TS. Off spells and dyskinesias: pharmacologic management of motor complications. Cleve Clin J Med. 2012;79 Suppl 2:S8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Jankovic J, Stacy M. Medical management of levodopa-associated motor complications in patients with Parkinson’s disease. CNS Drugs. 2007;21:677-692. [PubMed] |
| 26. | Kikuchi S. Motor fluctuations in Parkinson’s disease. J Neurol. 2007;254:32-40. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Chan DKY. Adjuvant treatment to levodopa in patients with motor complications. Focus on Parkinson’s Disease. 2012;23:10-15. |
| 28. | Zesiewicz TA, Sullivan KL, Hauser RA. Levodopa-induced dyskinesia in Parkinson’s disease: epidemiology, etiology, and treatment. Curr Neurol Neurosci Rep. 2007;7:302-310. [PubMed] |
| 29. | Warren Olanow C, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, Nissinen H, Leinonen M, Stocchi F; Stalevo Reduction in Dyskinesia Evaluation in Parkinson’s Disease (STRIDE-PD) Investigators. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord. 2013;28:1064-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 357] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 30. | Pirtošek Z. Myths and realities of continuous dopaminergic stimulation. Psychiatr Danub. 2011;23:80-83. [PubMed] |
| 31. | Fernandez HH, Vanagunas A, Odin P, Espay AJ, Hauser RA, Standaert DG, Chatamra K, Benesh J, Pritchett Y, Hass SL. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease open-label study: interim results. Parkinsonism Relat Disord. 2013;19:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Zibetti M, Merola A, Artusi CA, Rizzi L, Angrisano S, Reggio D, De Angelis C, Rizzone M, Lopiano L. Levodopa/carbidopa intestinal gel infusion in advanced Parkinson’s disease: a 7-year experience. Eur J Neurol. 2014;21:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Nyholm D, Odin P, Johansson A, Chatamra K, Locke C, Dutta S, Othman AA. Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease patients. AAPS J. 2013;15:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, Vanagunas A, Othman AA, Widnell KL, Robieson WZ. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 488] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 35. | Jost WH. Unwanted effects and interaction of intrajejunal levodopa/carbidopa administration. Expert Opin Drug Saf. 2014;13:447-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Urban PP, Wellach I, Faiss S, Layer P, Rosenkranz T, Knop K, Weis J. Subacute axonal neuropathy in Parkinson’s disease with cobalamin and vitamin B6 deficiency under duodopa therapy. Mov Disord. 2010;25:1748-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Skodda S, Müller T. Refractory epileptic seizures due to vitamin B6 deficiency in a patient with Parkinson’s disease under duodopa® therapy. J Neural Transm. 2013;120:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Toth C, Breithaupt K, Ge S, Duan Y, Terris JM, Thiessen A, Wiebe S, Zochodne DW, Suchowersky O. Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Ann Neurol. 2010;68:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 39. | Rajabally YA, Martey J. Neuropathy in Parkinson disease: prevalence and determinants. Neurology. 2011;77:1947-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Müller T, van Laar T, Cornblath DR, Odin P, Klostermann F, Grandas FJ, Ebersbach G, Urban PP, Valldeoriola F, Antonini A. Peripheral neuropathy in Parkinson’s disease: levodopa exposure and implications for duodenal delivery. Parkinsonism Relat Disord. 2013;19:501-507; discussion 501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 41. | Brooks DJ. Dopamine agonists: their role in the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2000;68:685-689. [PubMed] |
| 42. | Perez-Lloret S, Rascol O. Dopamine receptor agonists for the treatment of early or advanced Parkinson’s disease. CNS Drugs. 2010;24:941-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 43. | Bonuccelli U, Pavese N. Dopamine agonists in the treatment of Parkinson’s disease. Expert Rev Neurother. 2006;6:81-89. [PubMed] |
| 44. | Stowe RL, Ives NJ, Clarke C, van Hilten J, Ferreira J, Hawker RJ, Shah L, Wheatley K, Gray R. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;CD006564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Bonuccelli U, Del Dotto P, Rascol O. Role of dopamine receptor agonists in the treatment of early Parkinson’s disease. Parkinsonism Relat Disord. 2009;15 Suppl 4:S44-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Chondrogiorgi M, Tatsioni A, Reichmann H, Konitsiotis S. Dopamine agonist monotherapy in Parkinson’s disease and potential risk factors for dyskinesia: a meta-analysis of levodopa-controlled trials. Eur J Neurol. 2014;21:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Jamrozik Z, Janik P. Role of dopaminergic receptor agonists in the treatment of Parkinson’s disease. Med Sci Monit. 1997;3:RA948-955. |
| 48. | Marek K, Jennings D, Seibyl J. Do dopamine agonists or levodopa modify Parkinson’s disease progression? Eur J Neurol. 2002;9 Suppl 3:15-22. [PubMed] |
| 49. | Calne DB, Teychenne PF, Leigh PN, Bamji AN, Greenacre JK. Treatment of parkinsonism with bromocriptine. Lancet. 1974;2:1355-1356. [PubMed] |
| 50. | Wiesbeck GA. Dopaminergic compounds: clinical data. Drugs for Relapse Prevention of Alcoholism. Birkhäuser Basel: Switzerland 2005; 155-161. |
| 51. | Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20:190-199. [PubMed] |
| 52. | Katzenschlager R, Head J, Schrag A, Ben-Shlomo Y, Evans A, Lees AJ; Parkinson’s Disease Research Group of the United Kingdom. Fourteen-year final report of the randomized PDRG-UK trial comparing three initial treatments in PD. Neurology. 2008;71:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 53. | Tan LC, Ng KK, Au WL, Lee RK, Chan YH, Tan NC. Bromocriptine use and the risk of valvular heart disease. Mov Disord. 2009;24:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Hofmann C, Penner U, Dorow R, Pertz HH, Jähnichen S, Horowski R, Latté KP, Palla D, Schurad B. Lisuride, a dopamine receptor agonist with 5-HT2B receptor antagonist properties: absence of cardiac valvulopathy adverse drug reaction reports supports the concept of a crucial role for 5-HT2B receptor agonism in cardiac valvular fibrosis. Clin Neuropharmacol. 2006;29:80-86. |
| 55. | Antonini A, Poewe W. Fibrotic heart-valve reactions to dopamine-agonist treatment in Parkinson’s disease. Lancet Neurol. 2007;6:826-829. |
| 56. | Woitalla D, Müller T, Benz S, Horowski R, Przuntek H. Transdermal lisuride delivery in the treatment of Parkinson’s disease. J Neural Transm Suppl. 2004;89-95. [PubMed] |
| 57. | Rinne UK, Bracco F, Chouza C, Dupont E, Gershanik O, Marti Masso JF, Montastruc JL, Marsden CD. Early treatment of Parkinson’s disease with cabergoline delays the onset of motor complications. Results of a double-blind levodopa controlled trial. The PKDS009 Study Group. Drugs. 1998;55 Suppl 1:23-30. [PubMed] |
| 58. | Del Dotto P, Gambaccini G, Caneparo D, Berti C, Bernardini S, Bonuccelli U. Bedtime cabergoline in Parkinson’s disease patients with excessive daytime sleepiness induced by dopamine agonists. Neurol Sci. 2003;24:170-171. [PubMed] |
| 59. | Geminiani G, Fetoni V, Genitrini S, Giovannini P, Tamma F, Caraceni T. Cabergoline in Parkinson’s disease complicated by motor fluctuations. Mov Disord. 1996;11:495-500. [PubMed] |
| 60. | Odin P, Oehlwein C, Storch A, Polzer U, Werner G, Renner R, Shing M, Ludolph A, Schüler P. Efficacy and safety of high-dose cabergoline in Parkinson’s disease. Acta Neurol Scand. 2006;113:18-24. [PubMed] |
| 61. | Ling LH, Ahlskog JE, Munger TM, Limper AH, Oh JK. Constrictive pericarditis and pleuropulmonary disease linked to ergot dopamine agonist therapy (cabergoline) for Parkinson’s disease. Mayo Clin Proc. 1999;74:371-375. [PubMed] |
| 62. | Townsend M, MacIver DH. Constrictive pericarditis and pleuropulmonary fibrosis secondary to cabergoline treatment for Parkinson’s disease. Heart. 2004;90:e47. [PubMed] |
| 63. | Pinero A, Marcos-Alberca P, Fortes J. Cabergoline-related severe restrictive mitral regurgitation. N Engl J Med. 2005;353:1976-1977. [PubMed] |
| 64. | Peralta C, Wolf E, Alber H, Seppi K, Müller S, Bösch S, Wenning GK, Pachinger O, Poewe W. Valvular heart disease in Parkinson’s disease vs. controls: An echocardiographic study. Mov Disord. 2006;21:1109-1113. [PubMed] |
| 65. | Rasmussen VG, Østergaard K, Dupont E, Poulsen SH. The risk of valvular regurgitation in patients with Parkinson’s disease treated with dopamine receptor agonists. Mov Disord. 2011;26:801-806. [PubMed] |
| 66. | Millan MJ. From the cell to the clinic: a comparative review of the partial D2/D3receptor agonist and α2-adrenoceptor antagonist, piribedil, in the treatment of Parkinson’s disease. Pharmacol Ther. 2010;128:229-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Rascol O, Dubois B, Caldas AC, Senn S, Del Signore S, Lees A; Parkinson REGAIN Study Group. Early piribedil monotherapy of Parkinson’s disease: A planned seven-month report of the REGAIN study. Mov Disord. 2006;21:2110-2115. [PubMed] |
| 68. | Castro-Caldas A, Delwaide P, Jost W, Merello M, Williams A, Lamberti P, Aguilar M, Del Signore S, Cesaro P; Parkinson-Control Study Group. The Parkinson-Control study: a 1-year randomized, double-blind trial comparing piribedil (150 mg/day) with bromocriptine (25 mg/day) in early combination with levodopa in Parkinson’s disease. Mov Disord. 2006;21:500-509. [PubMed] |
| 69. | Evidente VG, Esteban RP, Domingo FM, Carbajal LO, Parazo MA. Piribedil as an adjunct to levodopa in advanced Parkinson’s disease: the Asian experience. Parkinsonism Relat Disord. 2003;10:117-121. [PubMed] |
| 70. | Simon N, Micallef J, Reynier JC, Lesourd M, Witjas T, Alicherif A, Azulay JP, Blin O. End-of-dose akinesia after a single intravenous infusion of the dopaminergic agonist piribedil in Parkinson’s disease patients: a pharmacokinetic/pharmacodynamic, randomized, double-blind study. Mov Disord. 2005;20:803-809. [PubMed] |
| 71. | Rascol O, Azulay JP, Blin O, Bonnet AM, Brefel-Courbon C, Césaro P, Damier P, Debilly B, Durif F, Galitzky M. Orodispersible sublingual piribedil to abort OFF episodes: a single dose placebo-controlled, randomized, double-blind, cross-over study. Mov Disord. 2010;25:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Thobois S, Lhommée E, Klinger H, Ardouin C, Schmitt E, Bichon A, Kistner A, Castrioto A, Xie J, Fraix V. Parkinsonian apathy responds to dopaminergic stimulation of D2/D3 receptors with piribedil. Brain. 2013;136:1568-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 73. | Gouraud A, Millaret A, Descotes J, Vial T; French Association of Regional Pharmacovigilance Centres. Piribedil-induced sleep attacks in patients without Parkinson disease: a case series. Clin Neuropharmacol. 2011;34:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Zareba G. Rotigotine: a novel dopamine agonist for the transdermal treatment of Parkinson’s disease. Drugs Today (Barc). 2006;42:21-28. [PubMed] |
| 75. | Zhou CQ, Li SS, Chen ZM, Li FQ, Lei P, Peng GG. Rotigotine transdermal patch in Parkinson’s disease: a systematic review and meta-analysis. PLoS One. 2013;8:e69738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Giladi N, Boroojerdi B, Surmann E. The safety and tolerability of rotigotine transdermal system over a 6-year period in patients with early-stage Parkinson’s disease. J Neural Transm. 2013;120:1321-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Nicholas AP, Borgohain R, Chaná P, Surmann E, Thompson EL, Bauer L, Whitesides J, Elmer LW. A randomized study of rotigotine dose response on ‘off’ time in advanced Parkinson’s disease. J Parkinsons Dis. 2014;4:361-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Poewe WH, Rascol O, Quinn N, Tolosa E, Oertel WH, Martignoni E, Rupp M, Boroojerdi B; SP 515 Investigators. Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson’s disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol. 2007;6:513-520. [PubMed] |
| 79. | Watts RL, Lyons KE, Pahwa R, Sethi K, Stern M, Hauser RA, Olanow W, Gray AM, Adams B, Earl NL. Onset of dyskinesia with adjunct ropinirole prolonged-release or additional levodopa in early Parkinson’s disease. Mov Disord. 2010;25:858-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 80. | Reichmann H, Cooper J, Rolfe K, Martinez-Martin P. Sleep Duration and “on” Time during Different Periods of the Day and Night in Patients with Advanced Parkinson’s Disease Receiving Adjunctive Ropinirole Prolonged Release. Parkinsons Dis. 2011;2011:354760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 81. | Hauser RA, Reichmann H, Lew M, Asgharian A, Makumi C, Shulman KJ. Long-term, open-label study of once-daily ropinirole prolonged release in early Parkinson’s disease. Int J Neurosci. 2011;121:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Ray Chaudhuri K, Martinez-Martin P, Rolfe KA, Cooper J, Rockett CB, Giorgi L, Ondo WG. Improvements in nocturnal symptoms with ropinirole prolonged release in patients with advanced Parkinson’s disease. Eur J Neurol. 2012;19:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 83. | Hersh BP, Earl NL, Hauser RA, Stacy M. Early treatment benefits of ropinirole prolonged release in Parkinson’s disease patients with motor fluctuations. Mov Disord. 2010;25:927-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Lyons KE, Pahwa R. An open-label conversion study of pramipexole to ropinirole prolonged release in Parkinson’s disease. Mov Disord. 2009;24:2121-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Titlic M, Tonkic A, Jukic I, Lusic I, Dikanovic M. Side effects of ropinirole in patients with idiopathic Parkinson’s disease. Bratisl Lek Listy. 2008;109:273-275. [PubMed] |
| 86. | Zagmutt FJ, Tarrants ML. Indirect comparisons of adverse events and dropout rates in early Parkinson’s disease trials of pramipexole, ropinirole, and rasagiline. Int J Neurosci. 2012;122:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Kieburtz K; Parkinson Study Group PramiBID Investigators. Twice-daily, low-dose pramipexole in early Parkinson’s disease: a randomized, placebo-controlled trial. Mov Disord. 2011;26:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 88. | Viallet F, Pitel S, Lancrenon S, Blin O. Evaluation of the safety and tolerability of rasagiline in the treatment of the early stages of Parkinson’s disease. Curr Med Res Opin. 2013;29:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Utsumi H, Okuma Y, Kano O, Suzuki Y, Iijima M, Tomimitsu H, Hashida H, Kubo S, Suzuki M, Nanri K. Evaluation of the efficacy of pramipexole for treating levodopa-induced dyskinesia in patients with Parkinson’s disease. Intern Med. 2013;52:325-332. [PubMed] |
| 90. | Takahashi H, Nogawa S, Tachibana H, Kawamura J, Abe T, Ogino Y, Kashihara K, Hamada T, Kowa H; Pramipexole Switching Study (PraSS) Group. Pramipexole safely replaces ergot dopamine agonists with either rapid or slow switching. J Int Med Res. 2008;36:106-114. [PubMed] |
| 91. | Ohno H, Nakajima M, Fujioka S, Iwamoto K, Kawamura M. Overnight switching from ergot-derived dopamine agonists to pramipexole in patients with Parkinson’s disease: an open preliminary trial in Japan. J Clin Neurosci. 2009;16:790-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 92. | Morita A, Okuma Y, Kamei S, Yoshii F, Yamamoto T, Hashimoto S, Utsumi H, Hatano T, Hattori N, Matsumura M. Pramipexole reduces the prevalence of fatigue in patients with Parkinson’s disease. Intern Med. 2011;50:2163-2168. [PubMed] |
| 93. | Barone P, Poewe W, Albrecht S, Debieuvre C, Massey D, Rascol O, Tolosa E, Weintraub D. Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 346] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 94. | Schapira AH, McDermott MP, Barone P, Comella CL, Albrecht S, Hsu HH, Massey DH, Mizuno Y, Poewe W, Rascol O. Pramipexole in patients with early Parkinson’s disease (PROUD): a randomised delayed-start trial. Lancet Neurol. 2013;12:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 95. | Hametner EM, Seppi K, Poewe W. Pramipexole extended release in Parkinson’s disease. Expert Rev Neurother. 2011;11:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Hauser RA. Early pharmacologic treatment in Parkinson’s disease. Am J Manag Care. 2010;16 Suppl Implications:S100-S107. [PubMed] |
| 97. | Poewe W, Rascol O, Barone P, Hauser RA, Mizuno Y, Haaksma M, Salin L, Juhel N, Schapira AH; Pramipexole ER Studies Group. Extended-release pramipexole in early Parkinson disease: a 33-week randomized controlled trial. Neurology. 2011;77:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 98. | Schapira AH, Barone P, Hauser RA, Mizuno Y, Rascol O, Busse M, Salin L, Juhel N, Poewe W; Pramipexole ER Studies Group. Extended-release pramipexole in advanced Parkinson disease: a randomized controlled trial. Neurology. 2011;77:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 99. | Takanashi M, Shimo Y, Hatano T, Oyama G, Hattori N. Efficacy and safety of a once-daily extended-release formulation of pramipexole switched from an immediate-release formulation in patients with advanced Parkinson’s disease: results from an open-label study. Drug Res (Stuttg). 2013;63:639-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 100. | Micallef J, Rey M, Eusebio A, Audebert C, Rouby F, Jouve E, Tardieu S, Blin O. Antiparkinsonian drug-induced sleepiness: a double-blind placebo-controlled study of L-dopa, bromocriptine and pramipexole in healthy subjects. Br J Clin Pharmacol. 2009;67:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 101. | Brusa L, Pavino V, Massimetti MC, Bove R, Iani C, Stanzione P. The effect of dopamine agonists on cognitive functions in non-demented early-mild Parkinson’s disease patients. Funct Neurol. 2013;28:13-17. [PubMed] |
| 102. | Ribarič S. The pharmacological properties and therapeutic use of apomorphine. Molecules. 2012;17:5289-5309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 103. | Pietz K, Hagell P, Odin P. Subcutaneous apomorphine in late stage Parkinson’s disease: a long term follow up. J Neurol Neurosurg Psychiatry. 1998;65:709-716. [PubMed] |
| 104. | Manson AJ, Hanagasi H, Turner K, Patsalos PN, Carey P, Ratnaraj N, Lees AJ. Intravenous apomorphine therapy in Parkinson’s disease: clinical and pharmacokinetic observations. Brain. 2001;124:331-340. [PubMed] |
| 105. | Grosset KA, Malek N, Morgan F, Grosset DG. Inhaled apomorphine in patients with ‘on-off’ fluctuations: a randomized, double-blind, placebo-controlled, clinic and home based, parallel-group study. J Parkinsons Dis. 2013;3:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 106. | Martinez-Martin P, Reddy P, Antonini A, Henriksen T, Katzenschlager R, Odin P, Todorova A, Naidu Y, Tluk S, Chandiramani C. Chronic subcutaneous infusion therapy with apomorphine in advanced Parkinson’s disease compared to conventional therapy: a real life study of non motor effect. J Parkinsons Dis. 2011;1:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 107. | Drapier S, Gillioz AS, Leray E, Péron J, Rouaud T, Marchand A, Vérin M. Apomorphine infusion in advanced Parkinson’s patients with subthalamic stimulation contraindications. Parkinsonism Relat Disord. 2012;18:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 108. | Slotty PJ, Wille C, Kinfe TM, Vesper J. Continuous perioperative apomorphine in deep brain stimulation surgery for Parkinson’s disease. Br J Neurosurg. 2014;28:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Schulze A, Oehler B, Urban N, Schaefer M, Hill K. Apomorphine is a bimodal modulator of TRPA1 channels. Mol Pharmacol. 2013;83:542-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 110. | Victor D, Waters C. Monoamine Oxidase Inhibitors in Parkinson’s Disease. Handbook of Parkinson's Disease, Third Edition. New York: Marcel Dekker, Inc 2003; 425-436. |
| 111. | Magyar K. The pharmacology of selegiline. Int Rev Neurobiol. 2011;100:65-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 112. | Knudsen Gerber DS. Selegiline and rasagiline: twins or distant cousins? Consult Pharm. 2011;26:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 113. | Chen JJ. Pharmacologic safety concerns in Parkinson’s disease: facts and insights. Int J Neurosci. 2011;121 Suppl 2:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 114. | Bartl J, Müller T, Grünblatt E, Gerlach M, Riederer P. Chronic monoamine oxidase-B inhibitor treatment blocks monoamine oxidase-A enzyme activity. J Neural Transm. 2014;121:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 115. | Lew MF, Hauser RA, Hurtig HI, Ondo WG, Wojcieszek J, Goren T, Fitzer-Attas CJ. Long-term efficacy of rasagiline in early Parkinson’s disease. Int J Neurosci. 2010;120:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 116. | Reichmann H, Jost WH. Efficacy and tolerability of rasagiline in daily clinical use--a post-marketing observational study in patients with Parkinson’s disease. Eur J Neurol. 2010;17:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 117. | Mizuno Y, Kondo T, Kuno S, Nomoto M, Yanagisawa N. Early addition of selegiline to L-Dopa treatment is beneficial for patients with Parkinson disease. Clin Neuropharmacol. 2010;33:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 118. | Caslake R, Macleod A, Ives N, Stowe R, Counsell C. Monoamine oxidase B inhibitors versus other dopaminergic agents in early Parkinson’s disease. Cochrane Database Syst Rev. 2009;CD006661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 119. | Riederer P, Laux G. MAO-inhibitors in Parkinson’s Disease. Exp Neurobiol. 2011;20:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 120. | Leegwater-Kim J, Bortan E. The role of rasagiline in the treatment of Parkinson’s disease. Clin Interv Aging. 2010;5:149-156. [PubMed] |
| 121. | Lees A. Alternatives to levodopa in the initial treatment of early Parkinson’s disease. Drugs Aging. 2005;22:731-740. [PubMed] |
| 122. | Chen JJ, Swope DM, Dashtipour K. Comprehensive review of rasagiline, a second-generation monoamine oxidase inhibitor, for the treatment of Parkinson’s disease. Clin Ther. 2007;29:1825-1849. [PubMed] |
| 123. | Keating GM, Lyseng-Williamson KA, Hoy SM. Rasagiline: a guide to its use in Parkinson’s disease. CNS Drugs. 2012;26:781-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 124. | Stocchi F, Rabey JM. Effect of rasagiline as adjunct therapy to levodopa on severity of OFF in Parkinson’s disease. Eur J Neurol. 2011;18:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 125. | Wilson RE, Seeberger LC, Silver D, Griffith A, Conner JB, Salzman PM; LEGATO Investigators. Rasagiline: time to onset of antiparkinson effect is similar when used as a monotherapy or adjunct treatment. Neurologist. 2011;17:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 126. | Korchounov A, Winter Y, Rössy W. Combined beneficial effect of rasagiline on motor function and depression in de novo PD. Clin Neuropharmacol. 2012;35:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 127. | Müller T, Hoffmann JA, Dimpfel W, Oehlwein C. Switch from selegiline to rasagiline is beneficial in patients with Parkinson’s disease. J Neural Transm. 2013;120:761-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 128. | Zhao YJ, Wee HL, Au WL, Seah SH, Luo N, Li SC, Tan LC. Selegiline use is associated with a slower progression in early Parkinson’s disease as evaluated by Hoehn and Yahr Stage transition times. Parkinsonism Relat Disord. 2011;17:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 129. | Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, Langston W, Melamed E, Poewe W, Stocchi F. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med. 2009;361:1268-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 575] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 130. | Rascol O, Fitzer-Attas CJ, Hauser R, Jankovic J, Lang A, Langston JW, Melamed E, Poewe W, Stocchi F, Tolosa E. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease (the ADAGIO study): prespecified and post-hoc analyses of the need for additional therapies, changes in UPDRS scores, and non-motor outcomes. Lancet Neurol. 2011;10:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 131. | Jenner P, Langston JW. Explaining ADAGIO: a critical review of the biological basis for the clinical effects of rasagiline. Mov Disord. 2011;26:2316-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 132. | Binda C, Milczek EM, Bonivento D, Wang J, Mattevi A, Edmondson DE. Lights and shadows on monoamine oxidase inhibition in neuroprotective pharmacological therapies. Curr Top Med Chem. 2011;11:2788-2796. [PubMed] |
| 133. | Naoi M, Maruyama W. Monoamine oxidase inhibitors as neuroprotective agents in age-dependent neurodegenerative disorders. Curr Pharm Des. 2010;16:2799-2817. [PubMed] |
| 134. | Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K; Parkinson Study Group. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351:2498-2508. [PubMed] |
| 135. | Mizuno Y, Kanazawa I, Kuno S, Yanagisawa N, Yamamoto M, Kondo T. Placebo-controlled, double-blind dose-finding study of entacapone in fluctuating parkinsonian patients. Mov Disord. 2007;22:75-80. [PubMed] |
| 136. | Grandas F, Hernández B; PRACTICOMT Study Group. Long-term effectiveness and quality of life improvement in entacapone-treated Parkinson’s disease patients: the effects of an early therapeutic intervention. Eur J Neurol. 2007;14:282-289. [PubMed] |
| 137. | Jog M, Panisset M, Suchowersky O, Réhel B, Schecter R. Naturalistic evaluation of entacapone in patients with signs and symptoms of L-dopa wearing-off. Curr Med Res Opin. 2008;24:3207-3215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 138. | Damier P, Viallet F, Ziegler M, Bourdeix I, Rerat K. Levodopa/DDCI and entacapone is the preferred treatment for Parkinson’s disease patients with motor fluctuations in routine practice: a retrospective, observational analysis of a large French cohort. Eur J Neurol. 2008;15:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 139. | Fung VS, Herawati L, Wan Y; Movement Disorder Society of Australia Clinical Research and Trials Group; QUEST-AP Study Group. Quality of life in early Parkinson’s disease treated with levodopa/carbidopa/entacapone. Mov Disord. 2009;24:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 140. | Pellicano C, Benincasa D, Giovannelli M, Buttarelli FR, Ruggieri S, Pontieri FE. Entacapone in elderly Parkinsonian patients experiencing levodopa-related wearing-off: a pilot study. Neurol Res. 2009;31:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 141. | Deuschl G, Vaitkus A, Fox GC, Roscher T, Schremmer D, Gordin A; CAMP Study Group. Efficacy and tolerability of Entacapone versus Cabergoline in parkinsonian patients suffering from wearing-off. Mov Disord. 2007;22:1550-1555. [PubMed] |
| 142. | Brooks DJ, Leinonen M, Kuoppamäki M, Nissinen H. Five-year efficacy and safety of levodopa/DDCI and entacapone in patients with Parkinson’s disease. J Neural Transm. 2008;115:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 143. | Nissinen H, Kuoppamäki M, Leinonen M, Schapira AH. Early versus delayed initiation of entacapone in levodopa-treated patients with Parkinson’s disease: a long-term, retrospective analysis. Eur J Neurol. 2009;16:1305-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 144. | Hauser RA, Panisset M, Abbruzzese G, Mancione L, Dronamraju N, Kakarieka A; FIRST-STEP Study Group. Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/carbidopa in early Parkinson’s disease. Mov Disord. 2009;24:541-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 145. | Eggert K, Skogar O, Amar K, Luotonen L, Kuoppamäki M, Leinonen M, Nissinen H, Oertel W. Direct switch from levodopa/benserazide or levodopa/carbidopa to levodopa/carbidopa/entacapone in Parkinson’s disease patients with wearing-off: efficacy, safety and feasibility--an open-label, 6-week study. J Neural Transm. 2010;117:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 146. | Lew MF, Somogyi M, McCague K, Welsh M; Lce QoL Study Group. Immediate versus delayed switch from levodopa/carbidopa to levodopa/carbidopa/entacapone: effects on motor function and quality of life in patients with Parkinson’s disease with end-of-dose wearing off. Int J Neurosci. 2011;121:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 147. | Linazasoro G, Kulisevsky J, Hernández B; Spanish Stalevo Study Group. Should levodopa dose be reduced when switched to stalevo? Eur J Neurol. 2008;15:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 148. | Destée A, Rérat K, Bourdeix I. Is there a difference between levodopa/ dopa-decarboxylase inhibitor and entacapone and levodopa/dopa-decarboxylase inhibitor dose fractionation strategies in Parkinson’s disease patients experiencing symptom re-emergence due to wearing-off? The Honeymoon Study. Eur Neurol. 2009;61:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 149. | Olanow CW, Kieburtz K, Stern M, Watts R, Langston JW, Guarnieri M, Hubble J; US01 Study Team. Double-blind, placebo-controlled study of entacapone in levodopa-treated patients with stable Parkinson disease. Arch Neurol. 2004;61:1563-1568. [PubMed] |
| 150. | Stocchi F, Rascol O, Kieburtz K, Poewe W, Jankovic J, Tolosa E, Barone P, Lang AE, Olanow CW. Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: the STRIDE-PD study. Ann Neurol. 2010;68:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 151. | Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, Coelho M, Poewe W, Rascol O, Goetz CG, Sampaio C. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26 Suppl 3:S2-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 406] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 152. | Sampaio C, Ferreira JJ. Parkinson disease: adjunctive entacapone therapy increases risk of dyskinesia. Nat Rev Neurol. 2010;6:590-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 153. | Entacapone to Tolcapone Switch Study Investigators. Entacapone to tolcapone switch: Multicenter double-blind, randomized, active-controlled trial in advanced Parkinson’s disease. Mov Disord. 2007;22:14-19. [PubMed] |
| 154. | Graham DJ, Williams JR, Hsueh YH, Calia K, Levenson M, Pinheiro SP, Macurdy TE, Shih D, Worrall C, Kelman JA. Cardiovascular and mortality risks in Parkinson’s disease patients treated with entacapone. Mov Disord. 2013;28:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 155. | Kaakkola S. Problems with the present inhibitors and a relevance of new and improved COMT inhibitors in Parkinson’s disease. Int Rev Neurobiol. 2010;95:207-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 156. | Canesi M, Zecchinelli AL, Pezzoli G, Antonini A. Clinical experience of tolcapone in advanced Parkinson’s disease. Neurol Sci. 2008;29 Suppl 5:S380-S382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 157. | Ebersbach G, Hahn K, Lorrain M, Storch A. Tolcapone improves sleep in patients with advanced Parkinson’s disease (PD). Arch Gerontol Geriatr. 2010;51:e125-e128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 158. | Sethi K, Factor S, Watts R. Quality of life in Parkinson’s disease patients following adjunctive tolcapone therapy: results of an open-label, multicenter, community-based trial. CNS Spectr. 2010;15:27-32. [PubMed] |
| 159. | Ries V, Selzer R, Eichhorn T, Oertel WH, Eggert K; German Tolcapone Study Group. Replacing a dopamine agonist by the COMT-inhibitor tolcapone as an adjunct to L-dopa in the treatment of Parkinson’s disease: a randomized, multicenter, open-label, parallel-group study. Clin Neuropharmacol. 2010;33:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 160. | Olanow CW, Watkins PB. Tolcapone: an efficacy and safety review (2007). Clin Neuropharmacol. 2007;30:287-294. [PubMed] |
| 161. | Ebersbach G, Storch A. Tolcapone in elderly patients with Parkinson’s disease: a prospective open-label multicenter non-interventional trial. Arch Gerontol Geriatr. 2009;49:e40-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 162. | Lew MF, Kricorian G. Results from a 2-year centralized tolcapone liver enzyme monitoring program. Clin Neuropharmacol. 2007;30:281-286. [PubMed] |
| 163. | Eggert K, Oertel WH, Lees AJ; German Competence Network on Parkinson’s disease. Safety and efficacy of tolcapone in the long-term use in Parkinson disease: an observational study. Clin Neuropharmacol. 2014;37:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 164. | Ferreira JJ, Rascol O, Poewe W, Sampaio C, Rocha JF, Nunes T, Almeida L, Soares-da-Silva P; BIA-3202-202 Study Investigator. A double-blind, randomized, placebo and active-controlled study of nebicapone for the treatment of motor fluctuations in Parkinson’s disease. CNS Neurosci Ther. 2010;16:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 165. | Ferreira JJ, Almeida L, Cunha L, Ticmeanu M, Rosa MM, Januário C, Mitu CE, Coelho M, Correia-Guedes L, Morgadinho A. Effects of nebicapone on levodopa pharmacokinetics, catechol-O-methyltransferase activity, and motor fluctuations in patients with Parkinson disease. Clin Neuropharmacol. 2008;31:2-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 166. | Schwab RS, England AC, Poskanzer DC, Young RR. Amantadine in the treatment of Parkinson’s disease. JAMA. 1969;208:1168-1170. [PubMed] |
| 167. | Singer C. Managing the patient with newly diagnosed Parkinson disease. Cleve Clin J Med. 2012;79 Suppl 2:S3-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 168. | Duty S. Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson’s disease. CNS Drugs. 2012;26:1017-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 169. | Ory-Magne F, Corvol JC, Azulay JP, Bonnet AM, Brefel-Courbon C, Damier P, Dellapina E, Destée A, Durif F, Galitzky M. Withdrawing amantadine in dyskinetic patients with Parkinson disease: the AMANDYSK trial. Neurology. 2014;82:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 170. | Aoki FY, Sitar DS. Clinical pharmacokinetics of amantadine hydrochloride. Clin Pharmacokinet. 1988;14:35-51. [PubMed] |
| 171. | Nishikawa N, Nagai M, Moritoyo T, Yabe H, Nomoto M. Plasma amantadine concentrations in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:351-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 172. | Sawada H, Oeda T, Kuno S, Nomoto M, Yamamoto K, Yamamoto M, Hisanaga K, Kawamura T; Amantadine Study Group. Amantadine for dyskinesias in Parkinson’s disease: a randomized controlled trial. PLoS One. 2010;5:e15298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 173. | Wolf E, Seppi K, Katzenschlager R, Hochschorner G, Ransmayr G, Schwingenschuh P, Ott E, Kloiber I, Haubenberger D, Auff E. Long-term antidyskinetic efficacy of amantadine in Parkinson’s disease. Mov Disord. 2010;25:1357-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 174. | Malkani R, Zadikoff C, Melen O, Videnovic A, Borushko E, Simuni T. Amantadine for freezing of gait in patients with Parkinson disease. Clin Neuropharmacol. 2012;35:266-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 175. | Lee JY, Oh S, Kim JM, Kim JS, Oh E, Kim HT, Jeon BS, Cho JW. Intravenous amantadine on freezing of gait in Parkinson’s disease: a randomized controlled trial. J Neurol. 2013;260:3030-3038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 176. | Raz A, Lev N, Orbach-Zinger S, Djaldetti R. Safety of perioperative treatment with intravenous amantadine in patients with Parkinson disease. Clin Neuropharmacol. 2013;36:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 177. | Chan HF, Kukkle PL, Merello M, Lim SY, Poon YY, Moro E. Amantadine improves gait in PD patients with STN stimulation. Parkinsonism Relat Disord. 2013;19:316-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 178. | Chang KC, Kim MK, Wee WR, Lee JH. Corneal endothelial dysfunction associated with amantadine toxicity. Cornea. 2008;27:1182-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 179. | Gondim Fde A, Costa HA, Taunay TC, de Oliveira GR, Ferreira JM, Rola FH. Transient amantadine-induced musical hallucinations in a patient with Parkinson’s disease. Mov Disord. 2010;25:1505-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 180. | Manini AF, Raspberry D, Hoffman RS, Nelson LS. QT prolongation and Torsades de Pointes following overdose of ziprasidone and amantadine. J Med Toxicol. 2007;3:178-181. [PubMed] |
| 181. | Schwartz M, Patel M, Kazzi Z, Morgan B. Cardiotoxicity after massive amantadine overdose. J Med Toxicol. 2008;4:173-179. [PubMed] |
| 182. | Ossola B, Schendzielorz N, Chen SH, Bird GS, Tuominen RK, Männistö PT, Hong JS. Amantadine protects dopamine neurons by a dual action: reducing activation of microglia and inducing expression of GDNF in astroglia [corrected]. Neuropharmacology. 2011;61:574-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 183. | Brocks DR. Anticholinergic drugs used in Parkinson’s disease: An overlooked class of drugs from a pharmacokinetic perspective. J Pharm Pharm Sci. 1999;2:39-46. [PubMed] |
| 184. | Gjerden P, Slørdal L, Bramness JG. The use of antipsychotic and anticholinergic antiparkinson drugs in Norway after the withdrawal of orphenadrine. Br J Clin Pharmacol. 2009;68:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 185. | Hollingworth SA, Rush A, Hall WD, Eadie MJ. Utilization of anti-Parkinson drugs in Australia: 1995-2009. Pharmacoepidemiol Drug Saf. 2011;20:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 186. | Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;CD003735. [PubMed] |
| 187. | Chan DK. The art of treating Parkinson disease in the older patient. Aust Fam Physician. 2003;32:927-931. [PubMed] |
| 188. | Samuel M, Maidment I, Boustani M, Fox C. Clinical management of Parkinson’sdisease dementia: pitfalls and progress. Advances in Psychiatric Treatment. 2006;12:121-129. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 189. | Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology. 2009;72:S1-S136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 574] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 190. | Schrag A, Schelosky L, Scholz U, Poewe W. Reduction of Parkinsonian signs in patients with Parkinson’s disease by dopaminergic versus anticholinergic single-dose challenges. Mov Disord. 1999;14:252-255. [PubMed] |
| 191. | Milanov I. A cross-over clinical and electromyographic assessment of treatment for parkinsonian tremor. Parkinsonism Relat Disord. 2001;8:67-73. [PubMed] |
| 192. | Baba Y, Higuchi MA, Abe H, Fukuyama K, Onozawa R, Uehara Y, Inoue T, Yamada T. Anti-cholinergics for axial symptoms in Parkinson’s disease after subthalamic stimulation. Clin Neurol Neurosurg. 2012;114:1308-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 193. | Pinna A. Adenosine A2A receptor antagonists in Parkinson’s disease: progress in clinical trials from the newly approved istradefylline to drugs in early development and those already discontinued. CNS Drugs. 2014;28:455-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 194. | Saki M, Yamada K, Koshimura E, Sasaki K, Kanda T. In vitro pharmacological profile of the A2A receptor antagonist istradefylline. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:963-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 195. | Jones N, Bleickardt C, Mullins D, Parker E, Hodgson R. A2A receptor antagonists do not induce dyskinesias in drug-naive or L-dopa sensitized rats. Brain Res Bull. 2013;98:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 196. | Varty GB, Hodgson RA, Pond AJ, Grzelak ME, Parker EM, Hunter JC. The effects of adenosine A2A receptor antagonists on haloperidol-induced movement disorders in primates. Psychopharmacology (Berl). 2008;200:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 197. | Uchida S, Tashiro T, Kawai-Uchida M, Mori A, Jenner P, Kanda T. Adenosine A2A-receptor antagonist istradefylline enhances the motor response of L-DOPA without worsening dyskinesia in MPTP-treated common marmosets. J Pharmacol Sci. 2014;124:480-485. [PubMed] |
| 198. | Fernandez HH, Greeley DR, Zweig RM, Wojcieszek J, Mori A, Sussman NM; 6002-US-051 Study Group. Istradefylline as monotherapy for Parkinson disease: results of the 6002-US-051 trial. Parkinsonism Relat Disord. 2010;16:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 199. | Pourcher E, Fernandez HH, Stacy M, Mori A, Ballerini R, Chaikin P. Istradefylline for Parkinson’s disease patients experiencing motor fluctuations: results of the KW-6002-US-018 study. Parkinsonism Relat Disord. 2012;18:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 200. | Stacy M, Silver D, Mendis T, Sutton J, Mori A, Chaikin P, Sussman NM. A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology. 2008;70:2233-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 201. | Hauser RA, Shulman LM, Trugman JM, Roberts JW, Mori A, Ballerini R, Sussman NM; Istradefylline 6002-US-013 Study Group. Study of istradefylline in patients with Parkinson’s disease on levodopa with motor fluctuations. Mov Disord. 2008;23:2177-2185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 202. | LeWitt PA, Guttman M, Tetrud JW, Tuite PJ, Mori A, Chaikin P, Sussman NM; 6002-US-005 Study Group. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). Ann Neurol. 2008;63:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 272] [Article Influence: 16.0] [Reference Citation Analysis (2)] |
| 203. | Mizuno Y, Kondo T; Japanese Istradefylline Study Group. Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Mov Disord. 2013;28:1138-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 204. | Mizuno Y, Hasegawa K, Kondo T, Kuno S, Yamamoto M; Japanese Istradefylline Study Group. Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: a randomized, controlled study. Mov Disord. 2010;25:1437-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 205. | Hauser RA, Hubble JP, Truong DD; Istradefylline US-001 Study Group. Randomized trial of the adenosine A(2A) receptor antagonist istradefylline in advanced PD. Neurology. 2003;61:297-303. [PubMed] |
| 206. | Hodgson RA, Bedard PJ, Varty GB, Kazdoba TM, Di Paolo T, Grzelak ME, Pond AJ, Hadjtahar A, Belanger N, Gregoire L. Preladenant, a selective A(2A) receptor antagonist, is active in primate models of movement disorders. Exp Neurol. 2010;225:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 207. | Cutler DL, Tendolkar A, Grachev ID. Safety, tolerability and pharmacokinetics after single and multiple doses of preladenant (SCH420814) administered in healthy subjects. J Clin Pharm Ther. 2012;37:578-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 208. | Hauser RA, Cantillon M, Pourcher E, Micheli F, Mok V, Onofrj M, Huyck S, Wolski K. Preladenant in patients with Parkinson’s disease and motor fluctuations: a phase 2, double-blind, randomised trial. Lancet Neurol. 2011;10:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 209. | Factor SA, Wolski K, Togasaki DM, Huyck S, Cantillon M, Ho TW, Hauser RA, Pourcher E. Long-term safety and efficacy of preladenant in subjects with fluctuating Parkinson’s disease. Mov Disord. 2013;28:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 210. | Fariello RG. Safinamide. Neurotherapeutics. 2007;4:110-116. [PubMed] |
| 211. | Müller T. Current status of safinamide for the drug portfolio of Parkinson’s disease therapy. Expert Rev Neurother. 2013;13:969-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 212. | Richel DJ, Colly LP, Lurvink E, Willemze R. Comparison of the antileukaemic activity of 5 aza-2-deoxycytidine and arabinofuranosyl-cytosine in rats with myelocytic leukaemia. Br J Cancer. 1988;58:730-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 213. | Stocchi F, Vacca L, Grassini P, De Pandis MF, Battaglia G, Cattaneo C, Fariello RG. Symptom relief in Parkinson disease by safinamide: Biochemical and clinical evidence of efficacy beyond MAO-B inhibition. Neurology. 2006;67:S24-S29. [PubMed] |
| 214. | Stocchi F, Borgohain R, Onofrj M, Schapira AH, Bhatt M, Lucini V, Giuliani R, Anand R; Study 015 Investigators. A randomized, double-blind, placebo-controlled trial of safinamide as add-on therapy in early Parkinson's disease patients. Mov Disord. 2012;27:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 215. | Schapira AH, Stocchi F, Borgohain R, Onofrj M, Bhatt M, Lorenzana P, Lucini V, Giuliani R, Anand R; Study 017 Investigators. Long-term efficacy and safety of safinamide as add-on therapy in early Parkinson's disease. Eur J Neurol. 2013;20:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 216. | Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt M, Chirilineau D, Stocchi F, Lucini V, Giuliani R, Forrest E. Randomized trial of safinamide add-on to levodopa in Parkinson's disease with motor fluctuations. Mov Disord. 2014;29:229-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 217. | Grégoire L, Jourdain VA, Townsend M, Roach A, Di Paolo T. Safinamide reduces dyskinesias and prolongs L-DOPA antiparkinsonian effect in parkinsonian monkeys. Parkinsonism Relat Disord. 2013;19:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 218. | Morsali D, Bechtold D, Lee W, Chauhdry S, Palchaudhuri U, Hassoon P, Snell DM, Malpass K, Piers T, Pocock J. Safinamide and flecainide protect axons and reduce microglial activation in models of multiple sclerosis. Brain. 2013;136:1067-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 219. | Dupont S, Stefan H. Zonisamide in clinical practice. Acta Neurol Scand Suppl. 2012;29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 220. | Murata M, Horiuchi E, Kanazawa I. Zonisamide has beneficial effects on Parkinson’s disease patients. Neurosci Res. 2001;41:397-399. [PubMed] |
| 221. | Murata M, Hasegawa K, Kanazawa I; Japan Zonisamide on PD Study Group. Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study. Neurology. 2007;68:45-50. [PubMed] |
| 222. | Yang LP, Perry CM. Zonisamide: in Parkinson’s disease. CNS Drugs. 2009;23:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 223. | Bermejo PE, Ruiz-Huete C, Anciones B. Zonisamide in managing impulse control disorders in Parkinson’s disease. J Neurol. 2010;257:1682-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 224. | Sonsalla PK, Wong LY, Winnik B, Buckley B. The antiepileptic drug zonisamide inhibits MAO-B and attenuates MPTP toxicity in mice: clinical relevance. Exp Neurol. 2010;221:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 225. | Kawajiri S, Machida Y, Saiki S, Sato S, Hattori N. Zonisamide reduces cell death in SH-SY5Y cells via an anti-apoptotic effect and by upregulating MnSOD. Neurosci Lett. 2010;481:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 226. | Choudhury ME, Moritoyo T, Kubo M, Kyaw WT, Yabe H, Nishikawa N, Nagai M, Matsuda S, Nomoto M. Zonisamide-induced long-lasting recovery of dopaminergic neurons from MPTP-toxicity. Brain Res. 2011;1384:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 227. | Choudhury ME, Sugimoto K, Kubo M, Iwaki H, Tsujii T, Kyaw WT, Nishikawa N, Nagai M, Tanaka J, Nomoto M. Zonisamide up-regulated the mRNAs encoding astrocytic anti-oxidative and neurotrophic factors. Eur J Pharmacol. 2012;689:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 228. | Yürekli VA, Gürler S, Nazıroğlu M, Uğuz AC, Koyuncuoğlu HR. Zonisamide attenuates MPP+-induced oxidative toxicity through modulation of Ca2+ signaling and caspase-3 activity in neuronal PC12 cells. Cell Mol Neurobiol. 2013;33:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 229. | Condello S, Currò M, Ferlazzo N, Costa G, Visalli G, Caccamo D, Pisani LR, Costa C, Calabresi P, Ientile R. Protective effects of zonisamide against rotenone-induced neurotoxicity. Neurochem Res. 2013;38:2631-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 230. | Yamamura S, Ohoyama K, Nagase H, Okada M. Zonisamide enhances delta receptor-associated neurotransmitter release in striato-pallidal pathway. Neuropharmacology. 2009;57:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 231. | Gluck MR, Santana LA, Granson H, Yahr MD. Novel dopamine releasing response of an anti-convulsant agent with possible anti-Parkinson’s activity. J Neural Transm. 2004;111:713-724. [PubMed] |
| 232. | Asanuma M, Miyazaki I, Diaz-Corrales FJ, Miyoshi K, Ogawa N, Murata M. Preventing effects of a novel anti-parkinsonian agent zonisamide on dopamine quinone formation. Neurosci Res. 2008;60:106-113. [PubMed] |
| 233. | Crane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr. 2001;20:591-598. [PubMed] |
| 234. | Kagan VE, Tyurina YY, Witt E. Role of Coenzyme Q and Superoxide in Vitamin E Cycling. Fat-Soluble Vitamins. New York: Springer Science 1998; 491-507. |
| 235. | Martin SF, Burón I, Espinosa JC, Castilla J, Villalba JM, Torres JM. Coenzyme Q and protein/lipid oxidation in a BSE-infected transgenic mouse model. Free Radic Biol Med. 2007;42:1723-1729. [PubMed] |
| 236. | Hargreaves IP, Lane A, Sleiman PM. The coenzyme Q10 status of the brain regions of Parkinson’s disease patients. Neurosci Lett. 2008;447:17-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 237. | Jiménez-Jiménez FJ, Molina JA, de Bustos F, García-Redondo A, Gómez-Escalonilla C, Martínez-Salio A, Berbel A, Camacho A, Zurdo M, Barcenilla B. Serum levels of coenzyme Q10 in patients with Parkinson’s disease. J Neural Transm. 2000;107:177-181. [PubMed] |
| 238. | Sohmiya M, Tanaka M, Tak NW, Yanagisawa M, Tanino Y, Suzuki Y, Okamoto K, Yamamoto Y. Redox status of plasma coenzyme Q10 indicates elevated systemic oxidative stress in Parkinson’s disease. J Neurol Sci. 2004;223:161-166. [PubMed] |
| 239. | Mischley LK, Allen J, Bradley R. Coenzyme Q10 deficiency in patients with Parkinson’s disease. J Neurol Sci. 2012;318:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 240. | Isobe C, Abe T, Terayama Y. Levels of reduced and oxidized coenzyme Q-10 and 8-hydroxy-2’-deoxyguanosine in the cerebrospinal fluid of patients with living Parkinson’s disease demonstrate that mitochondrial oxidative damage and/or oxidative DNA damage contributes to the neurodegenerative process. Neurosci Lett. 2010;469:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 241. | Horvath TL, Diano S, Leranth C, Garcia-Segura LM, Cowley MA, Shanabrough M, Elsworth JD, Sotonyi P, Roth RH, Dietrich EH. Coenzyme Q induces nigral mitochondrial uncoupling and prevents dopamine cell loss in a primate model of Parkinson’s disease. Endocrinology. 2003;144:2757-2760. [PubMed] |
| 242. | Cleren C, Yang L, Lorenzo B, Calingasan NY, Schomer A, Sireci A, Wille EJ, Beal MF. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J Neurochem. 2008;104:1613-1621. [PubMed] |
| 243. | Muthukumaran K, Leahy S, Harrison K, Sikorska M, Sandhu JK, Cohen J, Keshan C, Lopatin D, Miller H, Borowy-Borowski H. Orally delivered water soluble Coenzyme Q10 (Ubisol-Q10) blocks on-going neurodegeneration in rats exposed to paraquat: potential for therapeutic application in Parkinson’s disease. BMC Neurosci. 2014;15:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 244. | Muroyama A. An alternative medical approach for the neuroprotective therapy to slow the progression of Parkinson’s disease. Yakugaku Zasshi. 2013;133:849-856. [PubMed] |
| 245. | Storch A, Jost WH, Vieregge P, Spiegel J, Greulich W, Durner J, Müller T, Kupsch A, Henningsen H, Oertel WH. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch Neurol. 2007;64:938-944. [PubMed] |
| 246. | Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, Juncos JL, Nutt J, Shoulson I, Carter J. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59:1541-1550. [PubMed] |
| 247. | Seet RC, Lim EC, Tan JJ, Quek AM, Chow AW, Chong WL, Ng MP, Ong CN, Halliwell B. Does high-dose coenzyme Q10 improve oxidative damage and clinical outcomes in Parkinson’s disease? Antioxid Redox Signal. 2014;21:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 248. | Beal MF. A phase III clinical trial of coenzyme Q10 (QE3) in early Parkinson’s disease: Parkinson Study Group QE3 Investigators [abstract]. Mov Disord. 2012;27:346. |
| 249. | Beal MF, Oakes D, Shoulson I, Henchcliffe C, Galpern WR, Haas R, Juncos JL, Nutt JG, Voss TS, Ravina B. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. 2014;71:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 262] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 250. | Bessman SP, Carpenter CL. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. 1985;54:831-862. [PubMed] |
| 251. | McCandless DW. Creatine Treatment. In: Epilepsy: Animal and Human Correlations 2012; 329-336. |
| 252. | Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107-1213. [PubMed] |
| 253. | Snow RJ, Murphy RM. Creatine and the creatine transporter: a review. Mol Cell Biochem. 2001;224:169-181. [PubMed] |
| 254. | Guerrero-Ontiveros ML, Wallimann T. Creatine supplementation in health and disease. Effects of chronic creatine ingestion in vivo: down-regulation of the expression of creatine transporter isoforms in skeletal muscle. Mol Cell Biochem. 1998;184:427-437. [PubMed] |
| 255. | Levin BE, Katzen HL, Maudsley A, Post J, Myerson C, Govind V, Nahab F, Scanlon B, Mittel A. Whole-brain proton MR spectroscopic imaging in Parkinson’s disease. J Neuroimaging. 2014;24:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 256. | Gröger A, Kolb R, Schäfer R, Klose U. Dopamine reduction in the substantia nigra of Parkinson’s disease patients confirmed by in vivo magnetic resonance spectroscopic imaging. PLoS One. 2014;9:e84081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 257. | Klivenyi P, Gardian G, Calingasan NY, Yang L, Beal MF. Additive neuroprotective effects of creatine and a cyclooxygenase 2 inhibitor against dopamine depletion in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. J Mol Neurosci. 2003;21:191-198. [PubMed] |
| 258. | Adhihetty PJ, Beal MF. Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases. Neuromolecular Med. 2008;10:275-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 259. | Gualano B, Ugrinowitsch C, Novaes RB, Artioli GG, Shimizu MH, Seguro AC, Harris RC, Lancha AH. Effects of creatine supplementation on renal function: a randomized, double-blind, placebo-controlled clinical trial. Eur J Appl Physiol. 2008;103:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 260. | NINDS NET-PD Investigators. A pilot clinical trial of creatine and minocycline in early Parkinson disease: 18-month results. Clin Neuropharmacol. 2008;31:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 261. | Bender A, Samtleben W, Elstner M, Klopstock T. Long-term creatine supplementation is safe in aged patients with Parkinson disease. Nutr Res. 2008;28:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 262. | Bender A, Koch W, Elstner M, Schombacher Y, Bender J, Moeschl M, Gekeler F, Müller-Myhsok B, Gasser T, Tatsch K. Creatine supplementation in Parkinson disease: a placebo-controlled randomized pilot trial. Neurology. 2006;67:1262-1264. [PubMed] |
| 263. | Hass CJ, Collins MA, Juncos JL. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: a randomized trial. Neurorehabil Neural Repair. 2007;21:107-115. [PubMed] |
| 264. | Pettegrew JW, Levine J, McClure RJ. Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer’s disease and geriatric depression. Mol Psychiatry. 2000;5:616-632. [PubMed] |
| 265. | Parnetti L, Gaiti A, Mecocci P, Cadini D, Senin U. Pharmacokinetics of IV and oral acetyl-L-carnitine in a multiple dose regimen in patients with senile dementia of Alzheimer type. Eur J Clin Pharmacol. 1992;42:89-93. [PubMed] |
| 266. | Malaguarnera M, Gargante MP, Cristaldi E, Colonna V, Messano M, Koverech A, Neri S, Vacante M, Cammalleri L, Motta M. Acetyl L-carnitine (ALC) treatment in elderly patients with fatigue. Arch Gerontol Geriatr. 2008;46:181-190. [PubMed] |
| 267. | Calabrese V, Ravagna A, Colombrita C, Scapagnini G, Guagliano E, Calvani M, Butterfield DA, Giuffrida Stella AM. Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: involvement of the transcription factor Nrf2. J Neurosci Res. 2005;79:509-521. [PubMed] |
| 268. | Long J, Gao F, Tong L, Cotman CW, Ames BN, Liu J. Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-L-carnitine. Neurochem Res. 2009;34:755-763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 269. | Patel SP, Sullivan PG, Lyttle TS, Rabchevsky AG. Acetyl-L-carnitine ameliorates mitochondrial dysfunction following contusion spinal cord injury. J Neurochem. 2010;114:291-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 270. | Alves E, Binienda Z, Carvalho F, Alves CJ, Fernandes E, de Lourdes Bastos M, Tavares MA, Summavielle T. Acetyl-L-carnitine provides effective in vivo neuroprotection over 3,4-methylenedioximethamphetamine-induced mitochondrial neurotoxicity in the adolescent rat brain. Neuroscience. 2009;158:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 271. | Bodis-Wollner I, Chung E, Ghilardi MF, Glover A, Onofrj M, Pasik P, Samson Y. Acetyl-levo-carnitine protects against MPTP-induced parkinsonism in primates. J Neural Transm Park Dis Dement Sect. 1991;3:63-72. [PubMed] |
| 272. | Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci USA. 1996;93:2696-2701. [PubMed] |
| 273. | Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J Neurochem. 1997;69:1326-1329. [PubMed] |
| 274. | Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348-355. [PubMed] |
| 275. | Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711-760. [PubMed] |
| 276. | Meister A. Glutathione biosynthesis and its inhibition. Methods Enzymol. 1995;252:26-30. [PubMed] |
| 277. | Arakawa M, Ito Y. N-acetylcysteine and neurodegenerative diseases: basic and clinical pharmacology. Cerebellum. 2007;6:308-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 278. | Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 315] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 279. | Martínez-Banaclocha MA. N-acetyl-cysteine in the treatment of Parkinson’s disease. What are we waiting for? Med Hypotheses. 2012;79:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 280. | Clark J, Clore EL, Zheng K, Adame A, Masliah E, Simon DK. Oral N-acetyl-cysteine attenuates loss of dopaminergic terminals in alpha-synuclein overexpressing mice. PLoS One. 2010;5:e12333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 281. | Berman AE, Chan WY, Brennan AM, Reyes RC, Adler BL, Suh SW, Kauppinen TM, Edling Y, Swanson RA. N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1-/- mouse. Ann Neurol. 2011;69:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 282. | Choy KH, Dean O, Berk M, Bush AI, van den Buuse M. Effects of N-acetyl-cysteine treatment on glutathione depletion and a short-term spatial memory deficit in 2-cyclohexene-1-one-treated rats. Eur J Pharmacol. 2010;649:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 283. | Holmay MJ, Terpstra M, Coles LD, Mishra U, Ahlskog M, Öz G, Cloyd JC, Tuite PJ. N-Acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin Neuropharmacol. 2013;36:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 284. | Lodge JK, Packer L. Natural sources of lipoic acid in plant and animal tissues. Antioxidant Food Supplements in Human Health. New York: Academic Press 1999; 121-134. |
| 285. | Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173-1183. [PubMed] |
| 286. | Scumpia PO, Kelly-Scumpia K, Stevens BR. Alpha-lipoic acid effects on brain glial functions accompanying double-stranded RNA antiviral and inflammatory signaling. Neurochem Int. 2014;64:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 287. | Li DW, Li GR, Lu Y, Liu ZQ, Chang M, Yao M, Cheng W, Hu LS. α-lipoic acid protects dopaminergic neurons against MPP+-induced apoptosis by attenuating reactive oxygen species formation. Int J Mol Med. 2013;32:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 288. | de Araújo DP, De Sousa CN, Araújo PV, Menezes CE, Sousa Rodrigues FT, Escudeiro SS, Lima NB, Patrocínio MC, Aguiar LM, Viana GS. Behavioral and neurochemical effects of alpha-lipoic Acid in the model of Parkinson’s disease induced by unilateral stereotaxic injection of 6-ohda in rat. Evid Based Complement Alternat Med. 2013;2013:571378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 289. | Zhang H, Jia H, Liu J, Ao N, Yan B, Shen W, Wang X, Li X, Luo C, Liu J. Combined R-alpha-lipoic acid and acetyl-L-carnitine exerts efficient preventative effects in a cellular model of Parkinson’s disease. J Cell Mol Med. 2010;14:215-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 290. | Chan CS, Gertler TS, Surmeier DJ. A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson’s disease. Mov Disord. 2010;25 Suppl 1:S63-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 291. | Ilijic E, Guzman JN, Surmeier DJ. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol Dis. 2011;43:364-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 292. | Yu X, Li X, Jiang G, Wang X, Chang HC, Hsu WH, Li Q. Isradipine prevents rotenone-induced intracellular calcium rise that accelerates senescence in human neuroblastoma SH-SY5Y cells. Neuroscience. 2013;246:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 293. | Schuster S, Doudnikoff E, Rylander D, Berthet A, Aubert I, Ittrich C, Bloch B, Cenci MA, Surmeier DJ, Hengerer B. Antagonizing L-type Ca2+ channel reduces development of abnormal involuntary movement in the rat model of L-3,4-dihydroxyphenylalanine-induced dyskinesia. Biol Psychiatry. 2009;65:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 294. | Simuni T, Borushko E, Avram MJ, Miskevics S, Martel A, Zadikoff C, Videnovic A, Weaver FM, Williams K, Surmeier DJ. Tolerability of isradipine in early Parkinson’s disease: a pilot dose escalation study. Mov Disord. 2010;25:2863-2866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 295. | Parkinson Study Group. Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD). Mov Disord. 2013;28:1823-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 296. | Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. J Neurochem. 2002;82:615-624. [PubMed] |
| 297. | Hunter RL, Choi DY, Ross SA, Bing G. Protective properties afforded by pioglitazone against intrastriatal LPS in Sprague-Dawley rats. Neurosci Lett. 2008;432:198-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 298. | Xing B, Liu M, Bing G. Neuroprotection with pioglitazone against LPS insult on dopaminergic neurons may be associated with its inhibition of NF-kappaB and JNK activation and suppression of COX-2 activity. J Neuroimmunol. 2007;192:89-98. [PubMed] |
| 299. | Quinn LP, Crook B, Hows ME, Vidgeon-Hart M, Chapman H, Upton N, Medhurst AD, Virley DJ. The PPARgamma agonist pioglitazone is effective in the MPTP mouse model of Parkinson’s disease through inhibition of monoamine oxidase B. Br J Pharmacol. 2008;154:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 300. | Swanson CR, Joers V, Bondarenko V, Brunner K, Simmons HA, Ziegler TE, Kemnitz JW, Johnson JA, Emborg ME. The PPAR-γ agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J Neuroinflammation. 2011;8:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 301. | Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:214-228. [PubMed] |
| 302. | Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem. 2007;100:1375-1386. [PubMed] |
| 303. | Binda C, Aldeco M, Geldenhuys WJ, Tortorici M, Mattevi A, Edmondson DE. Molecular Insights into Human Monoamine Oxidase B Inhibition by the Glitazone Anti-Diabetes Drugs. ACS Med Chem Lett. 2011;3:39-42. [PubMed] |
| 304. | Casarejos MJ, Menéndez J, Solano RM, Rodríguez-Navarro JA, García de Yébenes J, Mena MA. Susceptibility to rotenone is increased in neurons from parkin null mice and is reduced by minocycline. J Neurochem. 2006;97:934-946. [PubMed] |
| 305. | Faust K, Gehrke S, Yang Y, Yang L, Beal MF, Lu B. Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson’s disease. BMC Neurosci. 2009;10:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 306. | Huang CL, Lee YC, Yang YC, Kuo TY, Huang NK. Minocycline prevents paraquat-induced cell death through attenuating endoplasmic reticulum stress and mitochondrial dysfunction. Toxicol Lett. 2012;209:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 307. | NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664-671. [PubMed] |
| 308. | Dodel R, Spottke A, Gerhard A, Reuss A, Reinecker S, Schimke N, Trenkwalder C, Sixel-Döring F, Herting B, Kamm C. Minocycline 1-year therapy in multiple-system-atrophy: effect on clinical symptoms and [(11)C] (R)-PK11195 PET (MEMSA-trial). Mov Disord. 2010;25:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 309. | Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1266] [Cited by in RCA: 1452] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 310. | Hirohata M, Ono K, Morinaga A, Yamada M. Non-steroidal anti-inflammatory drugs have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. Neuropharmacology. 2008;54:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 311. | Brefel-Courbon C, Grolleau S, Thalamas C, Bourrel R, Allaria-Lapierre V, Loï R, Micallef-Roll J, Lapeyre-Mestre M. Comparison of chronic analgesic drugs prevalence in Parkinson’s disease, other chronic diseases and the general population. Pain. 2009;141:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 312. | Chen H, Zhang SM, Hernán MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60:1059-1064. [PubMed] |
| 313. | Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology. 2007;69:1836-1842. [PubMed] |
| 314. | Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann Neurol. 2005;58:963-967. [PubMed] |
| 315. | Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011;76:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 316. | Hernán MA, Logroscino G, García Rodríguez LA. Nonsteroidal anti-inflammatory drugs and the incidence of Parkinson disease. Neurology. 2006;66:1097-1099. [PubMed] |
| 317. | Hancock DB, Martin ER, Stajich JM, Jewett R, Stacy MA, Scott BL, Vance JM, Scott WK. Smoking, caffeine, and nonsteroidal anti-inflammatory drugs in families with Parkinson disease. Arch Neurol. 2007;64:576-580. [PubMed] |
| 318. | Bornebroek M, de Lau LM, Haag MD, Koudstaal PJ, Hofman A, Stricker BH, Breteler MM. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Neuroepidemiology. 2007;28:193-196. [PubMed] |
| 319. | Manthripragada AD, Schernhammer ES, Qiu J, Friis S, Wermuth L, Olsen JH, Ritz B. Non-steroidal anti-inflammatory drug use and the risk of Parkinson’s disease. Neuroepidemiology. 2011;36:155-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 320. | Driver JA, Logroscino G, Lu L, Gaziano JM, Kurth T. Use of non-steroidal anti-inflammatory drugs and risk of Parkinson’s disease: nested case-control study. BMJ. 2011;342:d198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 321. | Becker C, Jick SS, Meier CR. NSAID use and risk of Parkinson disease: a population-based case-control study. Eur J Neurol. 2011;18:1336-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 322. | Dexter DT, Wells FR, Agid F, Agid Y, Lees AJ, Jenner P, Marsden CD. Increased nigral iron content in postmortem parkinsonian brain. Lancet. 1987;2:1219-1220. [PubMed] |
| 323. | Sofic E, Riederer P, Heinsen H, Beckmann H, Reynolds GP, Hebenstreit G, Youdim MB. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm. 1988;74:199-205. [PubMed] |
| 324. | Kaur D, Andersen J. Does cellular iron dysregulation play a causative role in Parkinson’s disease? Ageing Res Rev. 2004;3:327-343. [PubMed] |
| 325. | Qureshi GA, Qureshi AA, Memon SA, Parvez SH. Impact of selenium, iron, copper and zinc in on/off Parkinson’s patients on L-dopa therapy. J Neural Transm Suppl. 2006;229-236. [PubMed] |
| 326. | Moreau C, Devos D, Kluza J, Laloux C, Petrault M, Devedjian JC, Ryckewaert G, Garcon G, Rouaix N, Jissendi P. Disease modifying strategy based upon iron chelation in Parkinson’s disease: A translational study [abstract]. Mov Disord. 2012;27:404. |
| 327. | Dexter DT, Martin-Bastida A, Kabba C, Piccini P, Sharp D, Ward R, Newbold R. A pilot 6 months efficacy and safety study utilising the iron chelator deferiprone in early stage Parkinson’s disease [abstract]. Mov Disord. 2014;29:633. |
| 328. | Cossu G, Abbruzzese G, Matta G, Murgia D, Melis M, Ricchi V, Galanello R, Barella S, Origa R, Balocco M. Efficacy and safety of deferiprone for the treatment of pantothenate kinase-associated neurodegeneration (PKAN) and neurodegeneration with brain iron accumulation (NBIA): results from a four years follow-up. Parkinsonism Relat Disord. 2014;20:651-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |