INTRODUCTION
Inhibition of the endogenous de novo synthesis of cholesterol by targeting 3-hydroxy-3 methyl CoA (HMG-CoA) reductase represents the most successful therapeutic concept of the last two decades[1-3]. HMG-CoA reductase inhibitors which are referred to as statins, resemble one of the most commonly used therapeutic approaches to treat hypercholesterolemia in cardiovascular risk patients. Importantly, statins are now also approved to exert a protective effect in primary prevention[3]. Meta-analysis of 19 trial arms and 56934 participants confirmed a statins induced reduction in the all-cause mortality (OR = 0.86, 95%CI: 0.79-0.94), combined fatal and non-fatal cardiovascular disease events (RR = 0.75, 95%CI: 0.70-0.81), combined fatal and non-fatal coronary heart disease events (RR = 0.73, 95%CI: 0.67-0.80) and combined fatal and non-fatal stroke (RR = 0.78, 95%CI: 0.68-0.89)[3]. Statins are well tolerated even in long term administrations exceeding five years, although genetic polymorphisms may exist which harbour the risk for drug-drug interactions[2-4].
Statins have long thought to act solemnly by inhibition of the endogenous production of cholesterol, mainly in the liver, which provokes a compensatory low density lipoprotein (LDL) cholesterol clearance from the plasma via an up-regulation of LDL receptors[1]. However, several additional effects of therapeutic relevance have been recognized and are now subsumed as pleiotropic effects[5].
We will shortly summarize the molecular mechanisms behind these pleiotropic effects as far as they are identified and distil a rational which supports the idea that statins may possess anti-tumour activity. Of course these ideas have been raised by others as well, so that we focus primarily on the outcome of recent clinical studies.
STATINS
Currently available statins include atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin and simvastatin[4]. They may exist as pro-drugs, containing a lactone ring which has to be opened into an acidic form like in lovastatin and simvastatin. All other members already exist in the acidic form, which is a prerequisite for potent inhibition at the catalytic site of HMG-CoA reductase[1]. Statins are substrates for heavy metabolisation and transport primarily in the liver. There is an explicit exception, pravastatin, which is water soluble and readily eliminated via the kidneys[4]. The statins also differ in their ability to reduce LDL-cholesterol. The lipid lowering efficacy is most potently seen with rosuvastatin and pitavastatin[4]. These novel statins are not well documented referring to the pleiotropic effects and anticancer effects described in the next sections.
PLEIOTROPIC EFFECTS
Most prominently statins show anti-inflammatory activity[4-6]. This is of relevance, if we understand atherosclerosis as a progressive inflammatory process. But also tumours are related to inflammation like colon, prostate, pancreas or liver cancer[7]. Statins prevent the synthesis of inflammatory cytokines, chemokines, endothelin-1 and suppress macrophage activity[5]. In particular statins inhibit lymphocyte function-associated antigen-1 (LFA-1)[8,9].
An indication for beneficial outcome due to an anti-inflammatory mechanism of action is deduced from the JUPITER study, which highlighted that patients with increased C reactive protein can benefit from rosuvastatin even in the absence of elevated LDL cholesterol levels[10]. Accordingly, the occurrence of cardiovascular events has been reduced compared to non-statin takers.
These anti-inflammatory effects are also explaining the favourable prognosis of critical cases of sepsis and the reduced mortality rate of critical ill patients taking statins[11]. Especially patients suffering from chronic kidney diseases benefit from statin usage and show reduced incidence of sepsis. On the other hand one has to account that individuals with hypercholesterolemia taking statins may have a high personal responsibility on their own health condition. Therefore, one could assume that these persons have a lowered risk to develop sepsis, and consequently, a reduced residence time at an intensive care unit[12]. Nevertheless, such a bias needs further clarification and investigation.
Statins also inhibit the cell cycle[13]. This anti-proliferative effect has been crucially linked to the plaque stabilizing activity of statins and inhibition of smooth muscle proliferation[3]. In addition, this effect has also been seen in vitro using cancer cells, like human prostate and breast cancer cells[14-16]. It is exactly this mechanism which has been attributed to be important in anti-cancer effects, which might be detectable in long term statin application.
Angiogenesis is another target of statins action. A proangiogenic action is linked to depletion of geranylgeranyl pyrophosphate with consecutive reduction of Rho and Rac GTPase activity and enhancement of endothelial nitric oxide synthase expression[17,18]. But statins exert a dualistic action on angiogenesis[19]. At pharmacological concentrations, statins favor neo-vascularization, which is of particular interest in cardiovascular diseases. Conversely, at high concentrations (above clinically relevant doses) statins are angiostatic and reduce vascularisation in tumors, also due to reduced VEGF expression[17,19].
STATINS AND CANCER
Given the fact that cholesterol is crucial for development, cell division and proper signalling statins are not recommended during pregnancy and childhood. It was also considered that statins may have a carcinogenic risk. This conjecture has been supported by preclinical data showing carcinogenic effects in rodents and one early study which found abnormal high numbers of breast cancer and prostate cancer in statin treated individuals with an given estimate odds ratio of 1.5 (95%CI: 1.0-2.3) and 1.2 (95%CI: 0.8-1.7), respectively[20]. Moreover, the hydrophilicity may also play an important role with regard to anti-tumor effects of statins.
In a systematic review including 42 studies, the overall risk of statins for cancer incidence was calculated with a risk ratio of 0.93 (95%CI: 0.77-1.1). Pravastatin, a hydrophilic statin when analysed in the same manner gave an overall carcinogenic risk of 0.98 (95%CI: 0.72-1.3), whereas that for breast cancer and prostate cancer was increased to 3.3 (95%CI: 1.7-6.3) and 1.3 (95%CI: 0.55-3.3), respectively[21]. This observation is interesting and to some extent supported by in vitro studies which for example show that lipophilic statins reduce the migration and colony formation of prostate cancer cells in human bone marrow stroma but not pravastatin[22]. Beside the pharmacokinetic characteristics of the individual statins the incidence for some tumors was elevated including the incidence of melanoma (RR = 1.6, 95%CI: 0.71-3.5) and non-melanoma skin cancer (RR = 1.3, 95%CI: 0.86-2.1).
Importantly, Kuoppala et al[21] highlight in their meta-analysis that the median follow-up time of 4.4 years is too short to provide evidence for a protective or harmful action of statins within the observational window. Recent data, however, confirm no safety risk for statins and show also a reduced risk for the development of breast or prostate cancer[23].
STATINS REDUCE CANCER
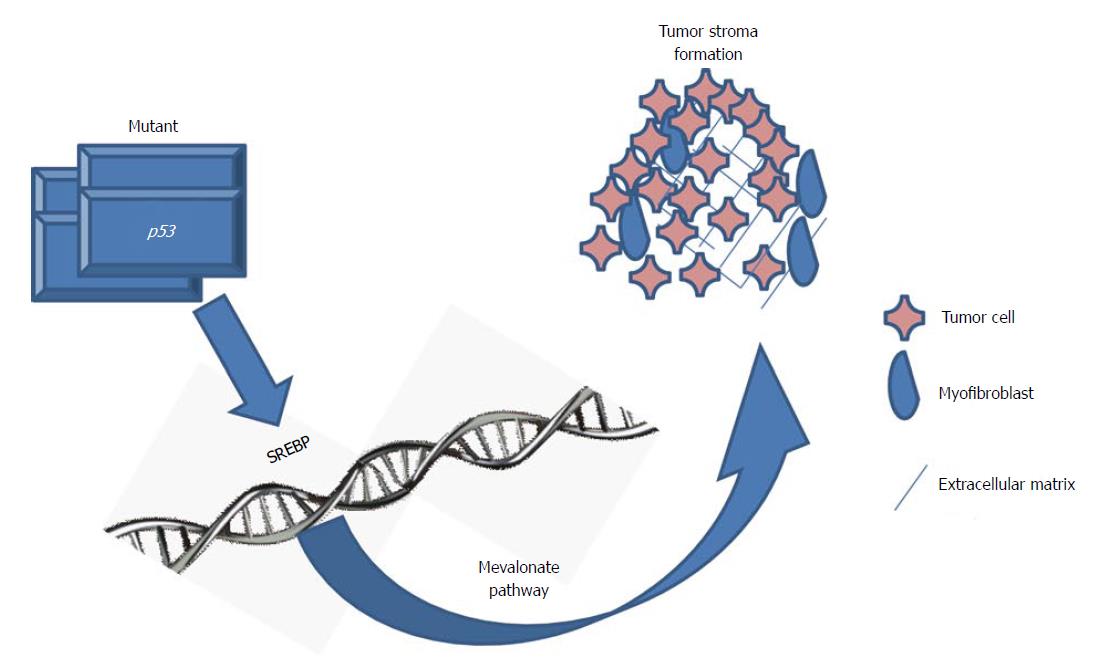
Substantional rational has been accumulated by in vitro investigations demonstrating statins impact tumor cell growth[24-27]. Very recently, in a three-dimensional cell culture model mutant p53 was investigated in tumorgenesis of breast cancer. In a genome-wide expression analysis the mevalonate pathway was significantly up-regulated by mutant p53 (Figure 1)[28]. Worth mentioning, p53 mutations correlate with highly expressed sterol biosynthesis genes in human breast tumors and are related to bad prognosis and reduced survival[28]. These findings implicate that the mevalonate pathway represents a therapeutic target for tumors bearing mutations in p53.
Figure 1 Mutated p53 and the mevalonate pathway in tumor progression.
Mutated p53 actively promotes cancer progression, particularly in breast cancer. Controlled by the sterol regulatory element-binding proteins (SREBP) mutated p53 disrupts the mammary tissue architecture by up-regulation of the mevalonate pathway[28].
Importantly, the mevalonate pathway has been targeted also on the level of isoprenoids by other drugs including farnesyl and geranylgeranyl transferase inhibitors[29]. While these compounds generally failed to show convincing efficacy in clinical trials, there are still some trials running, which focus on efficacy in acute myeloid leukemia[29].
In addition, more upstream squalene synthase inhibitors are currently under investigation for their cholesterol lowering effect[30]. Compounds like zaragozic acids, dicarboxylic acid, benzodiazepines and morpholine derivatives are potent inhibitors of the squalene synthase and reduce the cellular cholesterol content[31]. Depletion or reduction of the cellular cholesterol content has been linked to a reduction of lipid rafts and therefore reduced signalling via these receptor-dense- membrane structures. Importantly, using these compounds so far no anti-cancer effect has been established in preclinical or clinical trials[32]. Again, this is a strong indicator for causative participation of mevalonate intermediates and favours the conjecture that statins impact on ubiquinone, dolichol, and isoprenoid metabolism are important to obtain collateral effects which are responsible for anti-cancer action[24,33].
The anti-cancer potential of statins was investigated in vitro and in animal-models[25,26,34]. Statins like atorvastatin, fluvastatin, simvastatin and cerivastatin show anti-proliferative, proapoptotic, anti-invasive and X-ray sensitising effects[33,35]. A meta-analysis by the Cholesterol Treatment Trialists based on more then 90000 patients revealed a risk rate for tumour incidence of 1.0 (95%CI: 0.95-1.06) in the statin treatment group which was confirmed by later meta-analyses by Kuoppala et al[21] and Alsheikh-Ali et al[36]. Interestingly, in statin sensitive tumors the incidence of tumor development was independent of the LDL-cholesterol level[36].
Conversely, a correlation of lowered LDL-cholesterol and increased likelihood for tumor formation is prominently known as “unsuspected sickness phenomenon”. One explanation for this phenomenon is provided by the increased cholesterol turnover in subclinical disease states like during development of a carcinoma[37,38]. Regardless of all critics, Alsheikh-Al et al[39] published a meta-analysis of 15 randomized, controlled studies, where they showed both the absence of cancer-induction by statins, and confirmed the “unsuspected sickness phenomenon” model of 1980. However, a molecular explanation for the “unsuspected sickness phenomenon” is still pending.
First observed by Taylor et al[40]. in a meta-analysis-study based on 100000 patients the protective effect of statins was shown by an OR of 0.71 (95%CI: 0.56-0.89). Several case-control studies showed a reduction in cancer incidence in patients treated with statins[21,41]. For example in a prospective study the hazard ratio of breast cancer among statin users compared to nonusers was 0.91 (95%CI: 0.80-1.05), but was not statistically significant different from the control (P = 0.20)[42]. A similar result was obtained for breast cancer with an odds ratio of 0.9 (95%CI: 0.7-1.2) for statin users in a population-based, case-control study in older women[43].
One major drawback of meta-analyses and a possible source for insignificance between treatment arms may be represented by the lipophilicity of the various statins and differences in the volume of distribution, turnover-rates and particular enrichment of statins in solid tumours[4].
In a retrospective observational study hydrophobic statins revealed a dose-dependent reduction in tumor incidences[44]. Lower dosed statins gave a HR of 0.89 (95%CI: 0.75-1.07) and at high dose 0.75 (95%CI: 0.60-0.95). Accordingly, this dose-dependency allows concluding that the reduction in tumor incidences is of clinical importance.
COLON CANCER
In fact, simvastatin, atorvastatin and rosuvastatin significantly reduce colon carcinoma and were therefore suggested to be co-administrated with anti-inflammatory medications like low-dose acetylsalicylic acid[45]. Patients suffering from adenomatous polyps may benefit from long-term statin usage, as a reduction and shrinkage of polyps was observed[46]. In a recent prospective study the effect of statins on patient survival was investigated in 842 patients after curative resection of stage III colon carcinoma[47]. In all analysed end points (disease free survival, recurrent free survival and general survival) no difference was observed regardless of duration of statin intake. Thus, the application of statins during and after adjuvant chemotherapy was not associated with improved outcome, which was also independent of the KRAS mutation status. However, the combination of statins with chemotherapy was well tolerated and not associated with increased toxicity.
PROSTATE CANCER
It is considered that statins might inhibit prostate carcinogenesis by induction of apoptosis or inhibition of cell growth, angiogenesis and invasion[5]. These pleiotropic effects may also include alleviation of inflammation which may play a crucial carcinogenic role in prostate cancer. The bulk of these effects are mediated and explainable by the inhibition of the isoprenoid synthesis and thereby a substantial impact on cellular signalling via small G-proteins. However, one has to consider that in vitro experiments probing the above mechanisms of action need micromolar concentrations of statins. This is by far above the maximum peak concentrations (0.01-0.1 μmol/L) obtained during a cholesterol lowering therapy in humans[4].
Nevertheless, the existing data on the reduction of the incidence of prostate cancer by statins are not strong enough to allow a final decision whether statins are protective or not. Analysis of the California Men’s Health Study cohort revealed a 28% lower risk for prostate cancer compared to non-users, after a long term application of statins for more than 5 years[48]. Interestingly, this positive statin effect was restricted to those who took nonsteroidal anti-inflammatory drugs on a regular basis. Noteworthy, low dose acetylsalicylic acid may have a tumor protective effect per se.
In a Finnish record-linkage study investigating 4.2 million cumulated person years and an average follow-up of 8.8 years no association was found for neither beneficial nor harmful effects between the usage of statins and cancer[49]. Conversely, in a population-based case-control study from Taiwan matching 388 prostate cancer cases versus 1552 controls, a significant increase in prostate cancer (OR = 1.55, 95%CI: 1.09-2.19) was observed in the statin-ever users group. Moreover, there was a clear linear correlation between cumulative statin doses (intake) and the increase in adjusted ORs (P = 0.007)[50].
The current status on statins use as a prophylactic tool in prostate cancer is weak and impaired by lack of PSA-adjustment, and coadministrations like nonsteroidal anti-inflammatory drugs or PPARγ agonists. Prospective clinical trials are pending which would shed some light on this matter, so that we can mainly rely on observational studies and meta-analysis. In conclusion, a prophylactic usage of statins cannot be recommended for prostate cancer, due to lack of convincing data.
DISCUSSION
Whilst the growth of tumor cells is generally suppressed in the presence of lipophilic statins, clinical data on anti-cancer action are still conflicting. The reasons for that can be summarized as following.
Statins are administrated to reduce elevated LDL-cholesterol levels and to prevent cardiovascular events. Accordingly, the primary focus of any statin survey is on cardiovascular events, survival rates and LDL-cholesterol, whilst oncological parameters and diagnosis remain blurry. Therefore, the number of well documented oncological studies with statins is limited, in particular without the intention to lower LDL-cholesterol. Secondly, one has to bear in mind that cardiovascular risk factors and that for tumor development are overlapping, as these are smoking, alcohol, reduced physical activity, obesity, metabolic syndrome and diabetes mellitus.
Most of the data, also presented in this review are deduced from studies where tumor incidence, recurrence or tumor related overall survival was not defined as the primary aim. Thus it is difficult to evaluate statins possible beneficial effect on carcinogenesis in the virtual absence of prospective randomized clinical trials evaluating the above mentioned issue. Conversely, existing observational studies and meta-analyses do not address adequately the histological entity, the tumor stages, mutational status or tumor markers like for instance PSA in prostate cancer. In line with this there is also a lack of information on the use of individual statins, dosages and duration of application. Finally, insufficient documentation of coadministration or conflicting coadministration aggravates the evaluation process of an anti-cancer potential of statins. Statins have been shown to inhibit the ATP binding cassette (ABC) transporter ABCB1 (P-glycoprotein which is responsible for multidrug resistance during chemotherapy[26]. In rhabdomyosarcoma cells and neuroblastoma cells this effect is responsible for proper compartmentalization of an ABCB1 substrate like doxorubicin[26,34]. Consequently, nuclear concentrations of doxorubicin are elevated and topoisomerase II inhibition significantly augmented in the presence of simvastatin[34].
In the case that statins have an anti-cancer action, as documented in preclinical observations it is currently not clear whether this effect is a prevention of the incidence of (some) cancer(s), a reduction in tumor growth or a prevention of recurrence. Depending on the histological and molecular changes in the tumor the development of a detectable cancer takes 5-15 years or even more. Thus, this latency has a critical impact on the duration of statin intake and the observational time frame needed to obtain robust data from a clinical trial. Since prospective clinical trials have a mean duration between 3-5 years it is obvious that the onset of tumors rarely reaches significance within this limited time frame. Likewise, under these circumstances a statement or evaluation of an oncological event is difficult.
Finally, one has to consider that statins were designed to lower LDL-cholesterol. Thus it is possible that “other statins” might exist, which exert improved anti-cancer potential.
P- Reviewers: Guo H, Vetvicka V S- Editor: Zhai HH L- Editor: A E- Editor: Liu XM