Revised: June 20, 2012
Accepted: December 1, 2012
Published online: March 9, 2013
The eye is a highly protected organ, and designing an effective therapy is often considered a challenging task. The anatomical and physiological barriers result in low ocular bioavailability of drugs. Due to these constraints, less than 5% of the administered dose is absorbed from the conventional ophthalmic dosage forms. Further, physicochemical properties such as lipophilicity, molecular weight and charge modulate the permeability of drug molecules. Vision-threatening diseases such as glaucoma, diabetic macular edema, cataract, wet and dry age-related macular degeneration, proliferative vitreoretinopathy, uveitis, and cytomegalovirus retinitis alter the pathophysiological and molecular mechanisms. Understanding these mechanisms may result in the development of novel treatment modalities. Recently, transporter/receptor targeted prodrug approach has generated significant interest in ocular drug delivery. These transporters and receptors are involved in the transport of essential nutrients, vitamins, and xenobiotics across biological membranes. Several influx transporters (peptides, amino acids, glucose, lactate and nucleosides/nucleobases) and receptors (folate and biotin) have been identified on conjunctiva, cornea, and retina. Structural and functional delineation of these transporters will enable more drugs targeting the posterior segment to be successfully delivered topically. Prodrug derivatization targeting transporters and receptors expressed on ocular tissues has been the subject of intense research. Several prodrugs have been designed to target these transporters and enhance the absorption of poorly permeating parent drug. Moreover, this approach might be used in gene delivery to modify cellular function and membrane receptors. This review provides comprehensive information on ocular drug delivery, with special emphasis on the use of transporters and receptors to improve drug bioavailability.
- Citation: Boddu SH, Nesamony J. Utility of transporter/receptor(s) in drug delivery to the eye. World J Pharmacol 2013; 2(1): 1-17
- URL: https://www.wjgnet.com/2220-3192/full/v2/i1/1.htm
- DOI: https://dx.doi.org/10.5497/wjp.v2.i1.1
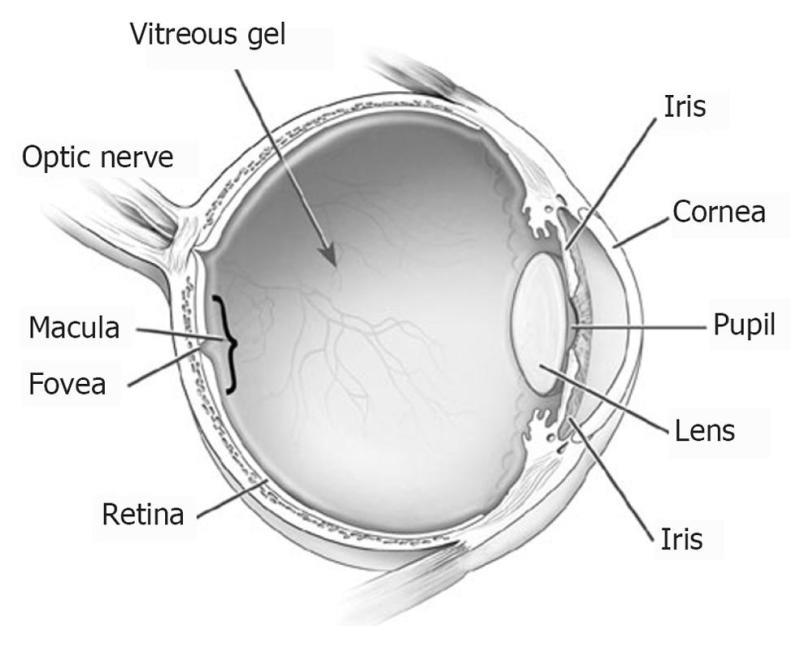
The eye is one of the most complex organs in the human body. The eye may be described as being comprised of three distinguishable regions: the outer cornea and sclera; the middle layer, which consists of the iris, ciliary body, and the choroid; and the inner region, or retina (Figure 1). Drug delivery to the eye presents unique challenges due to the complexity of this organ. Based on the route of administration, ocular drug delivery is classified into three types: (1) topical; (2) systemic; and (3) intraocular delivery. Dosage forms such as eye drops, suspensions and ointments are used for topical delivery. Eye drops account for approximately 90% of ophthalmologic market formulations[1,2] and are widely used in the delivery of anesthetics, antihistamines, β-receptor blockers, non-steroidal anti-inflammatory drugs, parasympatholytics, parasympathomimetics, prostaglandins, steroids, and sympathomimetics[3]. In some cases, eye drops devoid of medications are used for lubricating and tear-replacing solutions.
Ocular bioavailability of drugs following topical administration is significantly less (1%-5%) and hence this route is predominantly used for treatment of anterior segment disorders[4,5]. Most drugs administered topically are washed away rapidly by the nasolachrymal drainage and high tear fluid turnover[6]. Regardless of the low ocular bioavailability, eye drops are widely used because of their affordability and ease in scale up and manufacturing processes.
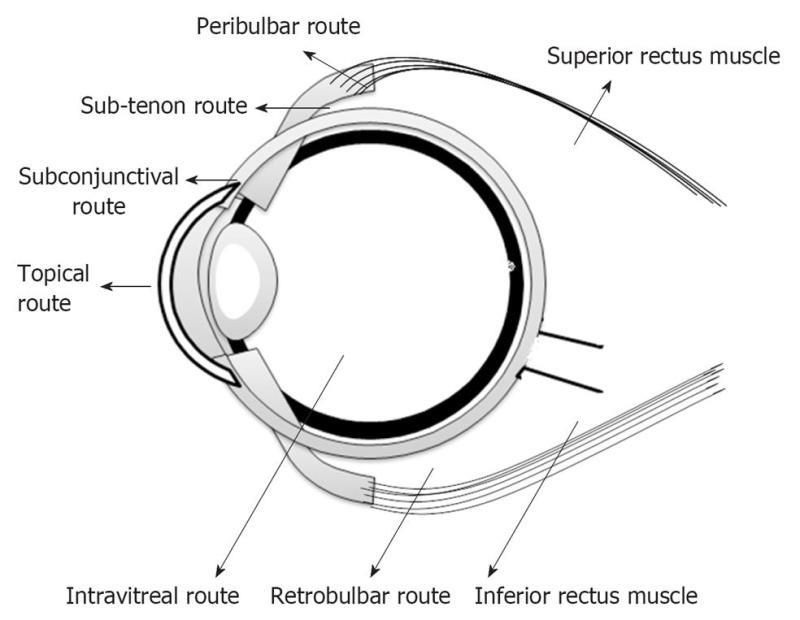
Systemic administration of drugs is preferred for posterior segment disorders affecting the retina[4]. This involves the administration of drugs as tablets, capsules or intravenous injections. The presence of the blood retinal barrier, which is selectively permeable to more lipophilic molecules, limits the entry of drug molecules into the eye and hence only 1%-2% of administered drug reaches the vitreous cavity[7]. For example, lipid-soluble drugs such as chloramphenicol and minocycline penetrate the blood retinal barrier, while aminoglycosides (amikacin) and β-lactams (cefazolin) used in the treatment of endophthalmitis, do not reach the vitreous in adequate concentrations[8]. This demands frequent administration of high doses, resulting in non-specific absorption and systemic toxicity[9,10]. Intraocular delivery in the form of intravitreal and periocular injections is becoming a popular approach for treatment of posterior segment diseases. Intravitreal administration involves the injection of drug solution/suspension directly into vitreous humor via pars plana using a 30 G needle[11]. Unlike topical and systemic routes, intravitreal injection offers high concentrations of drug in the choroid and retina. Nevertheless, intravitreal injections are very painful and are associated with several side effects such as cataract, endophthalmitis and retinal detachment[12]. Periocular injection involves administration of drug via peribulbar, posterior juxtascleral, retrobulbar, sub-tenon and subconjunctival routes (Figure 2)[13]. Periocular refers to the region surrounding the eye, and drugs that are placed close to sclera reach the posterior segment by three routes: transcleral (sclera→ choroid→ retina); transcorneal route (tear film → cornea → aqueous humor → lens → vitreous humor); and systemic circulation through the conjunctival and choroidal capillaries[13] (Figure 3). Lee et al[14] studied the permeation of radio-labeled mannitol following subconjunctival injection in rabbits. They concluded that direct penetration through the sclera is the primary pathway to the posterior segment, followed by recirculation pathway and transcorneal pathways.
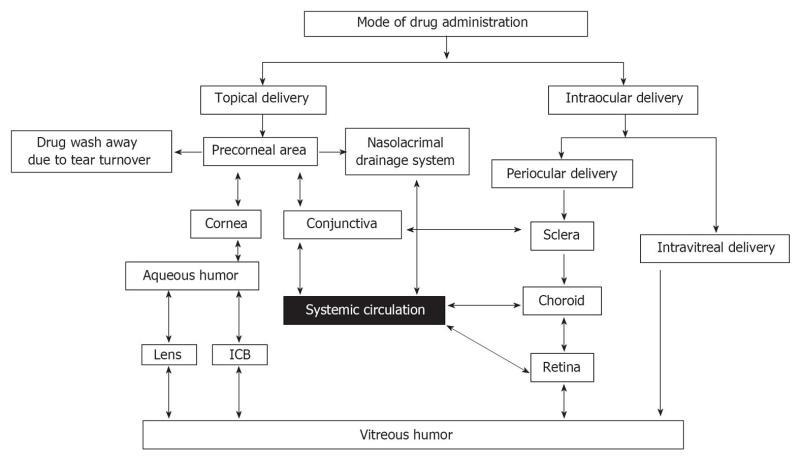
The unique anatomic and physiologic properties of the eye make it a complex organ, offering numerous challenges in developing ocular drug delivery strategies. Due to these constraints, less than 1% of the administered dose is absorbed when conventional ophthalmic forms such as solutions, suspensions, and ointments are applied to the eye[15], and up to 90% of marketed ophthalmic products may be identified as a type of conventional delivery system. This apparent disparity is quite significant and drives the translational research in the area of ocular drug delivery to overcome the unmet needs regarding the treatment of both anterior and posterior segment eye diseases[16]. Poor bioavailability of drugs from ocular dosage forms to the anterior segment is attributed to factors such as solution drainage, lacrimation, tear dilution, tear turnover, nonproductive absorption, poor residence time, and the permeability barrier of the corneal epithelial membrane[17]. Drugs applied topically to the eye can reach the intraocular tissues via the corneal and/or non-corneal (conjunctival-scleral) routes[18,19]. Tight junctions present in the apical side of the conjunctival epithelium impede the paracellular transport of hydrophilic substrates through the conjunctiva[20,21]. Thus, a healthy conjunctiva is impervious and impermeable to toxins, microbes, and allergens. However, several hydrophilic molecules have been shown to possess greater permeability through the non-corneal route than through the corneal route[22-27]. Conversely, a variety of lipophilic molecules were found to preferentially traverse the cornea rather than the non-corneal region[22,28,29]. However, the presence of hydrous stroma in the cornea may hamper permeation of highly lipophilic molecules through the cornea. The passage of molecules through the cornea depends on their lipophilicity, molecular weight, charge, and degree of ionization[18,30,31].
The blood-retinal barrier (BRB) restricts penetration of drugs into the posterior segment when administered systemically or periocularly[32]. Anatomically, two BRB’s may be differentiated: the outer BRB, presented by the retinal pigment epithelial (RPE), and the inner BRB, presented by the endothelium of the retinal vasculature[33]. Molecules with optimum membrane permeability characteristics and substrates of one of the membrane transporters can cross the BRB[33-37]. Specialized membrane transporters such as amino acid, dicarboxylate, monocarboxylic acid, nucleoside, organic ion, and peptide transporters channel nutrients, metabolites, and xenobiotics to the retina. Structural and functional delineation of these transporters will enable more drugs targeting the posterior segment to be successfully delivered topically.
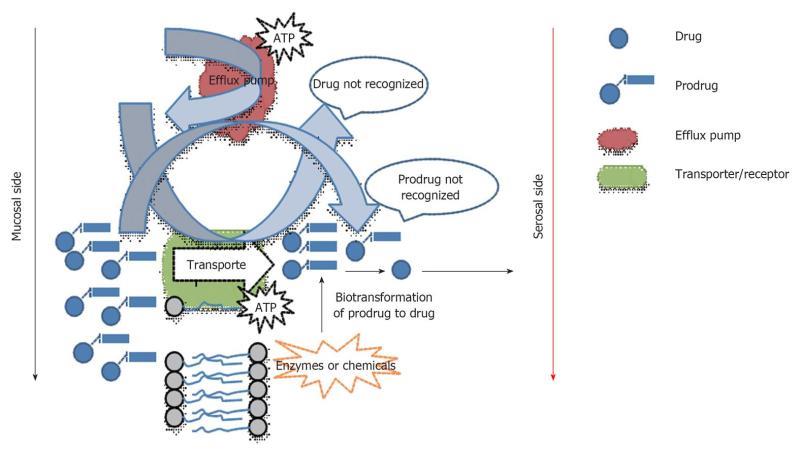
Yet another barrier that can affect the ocular bioavailability is the presence of efflux transporters such as P-glycoprotein (P-gp) and multidrug resistance-associated protein (MRP)[38-42]. Several substrates have been identified for the efflux transporters expressed in in vitro cell culture models[40,43-46]. Drug resistance mediated by efflux transporters is quite common in the area of cancer research. Efflux transporters such as P-gp and MRP are members of the ATP binding cassette (ABC) transporter family that utilizes ATP for translocation of various substrates across membranes[47]. Efflux transporters prevent the entry of toxic substances into the cells and aid in the healthy state of cells. Earlier investigation by Dano in 1973 described the evidence of efflux transporter resulting in drug resistance in Ehrlich astrocytes[48]. Later, P-gp was identified in the multidrug resistant cells and found to be responsible in the efflux of various cancer drugs like paclitaxel and doxorubicin[49]. P-gp, a transmembrane glycoprotein (approximately 170 kDa) with 10-15 kDa of N-terminal glycosylation, binds to the drug molecules and transports them out of the cell utilizing ATP hydrolysis. P-gp has wide substrate specificity for several drug classes including steroids, cardian glycosides, glucocorticoids, non-nucleoside reverse transcriptase inhibitors, protease inhibitors and immunosuppressive drugs[50]. Ocular drug resistance is a relatively new science, and presence of efflux transporters on various ocular tissues like cornea, conjunctiva, iris and retina was not known until recently. Efflux transporters have been identified extensively in major organs like the small intestine, kidney and liver, and their implication in drug delivery is well known[51]. However, the knowledge and relative expression of efflux pumps in ocular tissues is very limited, and the data published so far is limited to cell lines and lower species. These efflux transporters prevent the entry of several drug molecules into the eye (Figure 4). P-gp is expressed on the corneal epithelium[48,50], conjunctival epithelial cells[52], iris and ciliary muscle cells[53], retinal capillary endothelial cells[54], retinal pigmented epithelial cells[55,56], and ciliary non-pigmented epithelium[57]. The expression of P-gp on cornea can significantly modulate the absorption of topically administered drugs. Dey et al[58] studied the ocular absorption of [14C] erythromycin in the presence and absence of P-gp inhibitors. In the presence of P-gp inhibitors such as testosterone, verapamil, quinidine, and cyclosporine A, the ocular bioavailability of [14C] erythromycin was significantly enhanced, indicating the role of P-gp in ocular absorption of topically applied drugs. MRP is another major class of ABCC efflux transporter leading to drug resistance, and the MRP family has nine members (MRP 1-9) with varying substrate specificity[59]. MRPs are organic anion transporters and they play a vital role in the transport of anionic and neutral drugs conjugated to acidic ligands. So far, isoforms of the MRP family have been identified on ocular tissues. MRP1 expression was identified in rabbit conjunctival epithelial cells[60] and RPE cells[61], while MRP2 and MRP5 expression was identified in corneal epithelium[62,63]. In a recent study, Vellonen et al[64] compared expression of efflux proteins [MDR1 (ABCB1), MRP1-6 (ABCC1-6), and BCRP (ABCG2)] in normal human corneal epithelial tissue, primary human corneal epithelial cells (HCEpiC), and corneal epithelial cell culture model (HCE model) based on human immortal cell line. They concluded that BCRP, MRP1, and MRP5 are expressed in the corneal epithelium, while MDR1, MRP2, MRP3, MRP4, and MRP6 are not significantly expressed. Conflicting results have been observed with the expression profile of the efflux transporters in various ocular tissues, especially the human corneal epithelium. Nevertheless, a wide array of ocular drugs including antibiotics, sulfated steroids, macrolides (azithromycin and erythromycin), and quinolones (ciprofloxacin and grepafloxacin) has been proven to be substrates for these efflux pumps, which deter their ocular bioavailability[65]. To a large extent, the role of efflux pumps in ocular drug resistance remains to be explored. Although the functional significance of efflux pumps in the eye have not been elaborated completely, one may reasonably assume that they present another strategy for defending the eye from potential harm due to toxic metabolites and other external harmful molecules. P-gp and MRP have been found to be expressed on several ocular tissues[66-68]. As more research evolves in this area, formulations that contain substrates of these efflux pumps may offer opportunities to enhance the ocular bioavailability by co-administration of efflux pump inhibitors[41,69-72].
The eye is a highly compartmentalized organ with several anatomical and physiological barriers. The partial barriers that isolate the eye from the rest of the body impede the effective passage of many drugs[73]. Over the past two decades, several efforts have been made to increase the ocular bioavailability of drugs by enhancing the contact time of drugs with the target tissue, without compromising patient compliance[74,75]. Ophthalmic drug molecules should possess optimum hydrophilicity and lipophilicity for two reasons: (1) to facilitate the formulation of eye drops/injections; and (2) to allow sufficient permeability across the anatomical barriers such as corneal epithelium, choroid, retinal pigment epithelium. Drugs with an octanol/buffer distribution coefficient in the range of 100-1000 are considered to be optimum for corneal absorption[76,77]. Unfortunately, the buffer distribution coefficient of most drugs does not fall within this range, requiring the development of novel drug delivery strategies such as bioadhesive hydrogels, micro- and nanoparticles, liposomes and collagen shields. The prodrug approach is a more traditional, promising, and less expensive method for achieving the desired solubility and lipophilicity. This approach involves chemical modification of drug molecules using pro-moieties to improve their physicochemical properties[78]. The selection and linkage of pro-moieties depend on the metabolic enzymes, and after absorption, the prodrugs are subject to enzymatic hydrolysis resulting in the active parent drug. The bioreversion rate of the prodrug depends on affinity of prodrug linkage towards hydrolyzing enzyme(s), mainly esterases/peptidases and the turnover rate of the enzyme. Lipophilic chemical modification has been used successfully to improve their ocular bioavailability of various hydrophilic drugs[79,80]. For example, the bioavailability of ganciclovir (hydrophilic drug) after oral administration is 6%. This necessitates the use of high systemic doses of ganciclovir for attaining therapeutic concentrations in the eye, which gradually results in systemic toxicity. Intravitreal injections (0.2-0.4 mg) minimize systemic toxicity and increase the vitreal concentrations of ganciclovir; however, they are associated with patient non-compliance, and rapid elimination of vitreal ganciclovir (elimination t1/2: approximately 13 h in humans) requires repeated intravitreal injections, leading to side effects like retinal detachment, endophthalmitis, and vitreal hemorrhage. Short-chain carboxylic mono- and di-esters of ganciclovir, especially aminobutyrate ester of ganciclovir, exhibited maximum stability, optimum lipophilicity and sufficient solution stability at neutral or slightly acidic pH (4.0-7.0) and excellent activity against various herpes viruses such as HSV-2 and VZV[81]. This study highlights the use of the prodrug approach in enhancing the ocular bioavailability of ganciclovir without compromising the antiviral activity. More recently, the progress in molecular cloning of transporter genes led to the identification of membrane transporters/receptors that play an important role in transferring exo- and endogenous nutrients[82]. Despite the high vascularity of the retina, blood retinal barriers regulate the movement of nutrients between circulation and neural retina[83]. Therefore, most nutrients are transported into the retinal cells by specific transport/receptor systems[84]. Identification of such membrane transporters/receptors including peptide, amino acids, nucleoside and nucleobase, glucose, monocarboxylic acid, organic anion and organic cation transporters, led to the development of prodrugs for poorly permeating drug molecules[85]. Transporter-targeted prodrugs offer several advantages including: (1) improving the stability of parent drug molecule; (2) altering the physicochemical properties such as solubility and lipophilicity; (3) improving the pharmacokinetics properties; and (4) improving the permeability of drugs as the prodrugs become substrates for the influx transporters and simultaneously evade the efflux pumps (Figure 4). Table 1 presents the list of transporter/receptor(s)in the eye.
| Tissue | Transporter/receptor | Subtypes | Ref. |
| Cornea | Amino acid | LAT1, LAT2, Phenylalanine, tyrosine | [213,214] |
| Glucose | GLUT1 | [215] | |
| Nucleoside | [216] | ||
| Peptide | hPEPT1 | [90] | |
| Folate | [180] | ||
| Biotin | [175] | ||
| Conjunctiva | Acid-base | NKCC, HE1 | [217] |
| Amino acid | Bo,+ | [218] | |
| Glucose | GLUT1 | [157,219] | |
| Peptide | Dipeptide | [220] | |
| Monocarboxylate | [221] | ||
| Nucleoside | [113] | ||
| Lens | Amino acid | System A, L, Gly, Ly+, β, ASC | [222] |
| Ascorbic acid | SVCT2 | [223] | |
| Glucose | GLUT1, GLUT3 | [224] | |
| Glutathione | R-GSHT | [225] | |
| Iris-ciliary body | Glucose | GLUT1, GLUT4 | [226] |
| Nucleoside | [227] | ||
| Retina | Amino acid | Glycine, glutamine, arginie, proline, taurine | [99,228,229] |
| Glucose | GLUT1, GLUT3 | [230] | |
| Monocarboxylic acid | MCT1, MCT3 | [231,232] | |
| Nucleoside | [121,233] | ||
| Peptide | PEPT1, PEPT2, PHT1, PHT2 | [92,93,95,96] | |
| Vitamins(ascorbic acid, biotin, folic acid, riboflavin) | SVCT2, RFT, FR-α, SMVT | [85,183] |
Peptide transporters are among the most versatile membrane carrier systems with a wide range of substrate specificity. They are classified into three types: PepT1, PepT2 and peptide/histidine transporters (PHT1 and PHT2), with difference in their substrate specificity, transport capacity and affinity[86]. PepT1 belongs to solute carrier family 15 member 1 (SLC15A1) that is encoded in humans by the SLC15A1 gene. PepT1 is a low-affinity proton coupled transporter responsible for the translocation of di- and tri-peptides[87,88]. PepT2, another proton-coupled oligopeptide transporter belonging to the same family,is a high-affinity transporter that is responsible for the translocation of small peptides, β-lactam antibiotics and other peptidomimetic drugs[89]. PepT1 is predominantly expressed on the intestine and helps in the absorption of protein digestion products, while PepT2 is mainly expressed on the brain and kidney. The peptide/histidine transporters PHT1 and PHT2 are expressed on the lysosomal membrane of cells and are responsible for the efflux of histidine and small peptides from the lysosomes into the cytoplasm. The presence of an oligopeptide transport system on the corneal epithelium was identified by Anand et al[90] by studying the transport mechanism of L-valyl ester of acyclovir (L-val-ACV) across rabbit cornea in the presence of competitive inhibitors for human peptide transporter (hPepT1). Transcorneal permeation of L-val-ACV was approximately threefold higher across the intact rabbit cornea than ACV. Substrates of hPepT1 such as dipeptides, angiotensin converting enzyme inhibitors, and β-lactam antibiotics significantly inhibited the transport of L-val-ACV, indicating the presence of a carrier-mediated transport system specific for peptide. The oligopeptide transporter on the rabbit cornea opened up new avenues for the development of transporter-targeted prodrugs. Later, the same group evaluated the antiviral efficacy of val-val-ACV against herpetic epithelial and stromal keratitis. They concluded that val-val-ACV demonstrated higher water-solubility than ACV and lower cytotoxicity than trifluorothymidine. Val-val-ACV also showed excellent activity against HSV-1 in the stromal keratitis models and rabbit epithelia[91]. Peptide transporters are also expressed on the basolateral side of retina and neural retina[92,93]. The role of peptide transporters in the vitreal clearance of cephalexin, a peptide transporter substrate, was investigated using a dual probe microdialysis technique in the presence of glycyl-proline[92]. Co-administration of gly-pro increased the vitreal half-life and AUC of cephalexin, suggesting the involvement of peptide transporters in the clearance of cephalexin from the posterior chamber. Later studies performed by Majumdar et al[94] investigated the expression of peptide transporters on the retina. Ex-vivo uptake in excised rabbit retina/choroid tissues and in vivo retinal uptake using [3H] gly-sar and peptidomimetics demonstrated the functional presence of peptide transporter on the retina. Berger et al[93] studied the distribution of peptide transporter (PepT2) in the retinal Müller glial cells of the rat nervous system. Peptide transporter facing the vitreous humor can be targeted following intravitreal administration of prodrugs to achieve higher drug levels in the retina. Identification and characterization of transporters on the basolateral side of RPE is relatively difficult. Some researchers have tried to identify these transporters following systemic administration of peptide substrates and measuring the vitreous humor concentrations in the presence and absence of competitive inhibitors. For example, Dias et al[95] studied the ocular penetration of ACV and its peptide prodrugs val-ACV and val-val-ACV following systemic administration in rabbits using microdialysis. The anterior segment area under curve values of ACV, val-ACV and val-val-ACV were 53.70 (± 35.58), 139.85 (± 9.43) and 291.05 (± 88.13) min ×μmol/L, respectively. However, the drug concentration in vitreous humor was below the detection limit. The same group studied the mechanism of a dipeptide ([3H] glycylsarcosine) transport into vitreous humor, retina and aqueous humor, following systemic administration in the presence and absence of inhibitors. In the presence of inhibitors, the transport of glycylsarcosine into the aqueous, vitreous, and retina was significantly inhibited. These results indicate the expression of a peptide transporter on the blood-aqueous and blood-retinal barriers that can be exploited for the targeted delivery following systemic administration[96].
Amino acid transporters are responsible for translocation of amino acids from blood to various organs. Amino acids are responsible for protein synthesis and play a significant role in maintenance of structural and functional integrity of conjunctiva and retina/RPE. Amino acid transporters are ubiquitous in nature, with overlapping substrate specificity; hence, they are heavily exploited for targeted delivery of drugs. Amino acid transporters can be classified on the basis of sodium dependence, charge, and substrate specificity[97]. A sodium-dependent transporter binds amino acids after binding to sodium ions and undergoes a conformational change that allows the dumping of sodium ions and amino acids into the cytoplasm. System B, B0,+, IMINO, system X-(anionic), ASC (cationic, anionic, and neutral forms), and ATB0,+, belong to the sodium-dependent transporter category, while system y+ (cationic), b0,+, and system L (large) do not depend on sodium for transporting amino acids. Large amino acid transporter (system L) is expressed in two isoforms, LAT1 and LAT2, which are involved in the uptake of large aromatic or branched amino acids from extracellular fluids. LAT1 transports large neutral amino acids such as Leu, Phe, Ile, Trp, Val, Tyr, His and Met, while LAT2 transports large and small neutral amino acids[7]. Amino acid transport systems have been characterized on corneal epithelium and endothelium. The presence of various amino acid transporters such as ASCT1, LAT1 and ATB0+ has been characterized on the cornea. These transporters are involved in the transport of several amino acids such as L arginine, L-phenylalanine and L-alanine across the cornea[98]. The presence and function of amino acid transporters on human retina are heavily published in literature[99,100]. Gandhi et al[101] investigated the presence of a LAT2 on the ARPE-19 cell line. The same group also reported the presence of sodium-dependent, B0,+ amino acid transporter on rabbit corneal epithelium and human cornea and its interaction with the amino acid ester prodrugs of ACV (γ-glutamate-ACV and phenylalanine-ACV)[102]. Katragadda et al[103] studied the in vivo corneal absorption of the amino acid prodrugs ACV (L-alanine-ACV, L-serine-ACV, L-serine-succinate-ACV and L-cysteine-ACV) using a topical well model and microdialysis in rabbits. They concluded that L-serine-ACV seems to be a promising candidate for the treatment of ocular HSV infections due to its enhanced stability, comparable AUC, and high concentration at the last time point (Clast). Further studies also revealed higher antiviral activity against varicella-zoster and herpes simplex virus, and in comparison to ACV. ATB0,+ is a broad substrate-specific transporter that recognizes neutral and cationic amino acids. Studies have the shown the potential of ATB0,+ in delivery of antiviral drugs such as ACV and ganciclovir, which are covalently coupled to anionic amino acids[104]. Retinal cells have a basal requirement of amino acids for protein synthesis. Several amino acid neurotransmitters (glutamate, GABA and glycine) and neuroactive amino acids (aspartate, homocysteic acid, and taurine) have been identified in the retina[100,105-107]. High affinity, sodium-dependent glycine transporter (Glyt-1) is cloned on retinal neurons[100,108]. Glty-1 plays an important role in maintaining the glycine homeostasis in the retina of all vertebrate species. Glutamate, a major excitatory neurotransmitter, is mainly localized on the bipolar cells, retinal ganglion cells and slightly ischemic photoreceptors[109]. The vitreal levels of glutamate are mildly elevated with diabetic retinopathy and rhegmatogenous retinal detachments. This may be attributed to the high-affinity excitatory glutamate transport proteins that can be utilized in drug delivery[110]. Recently, Yamamoto et al[35] studied the gene expression level of LAT1 and LAT2 in ARPE-19 cells and concluded that both LAT1 and LAT2 are involved in L-leucine transport. These amino acid transport systems could help in the design of prodrugs that are likely to be transported across the retina for better ocular delivery and bioavailability.
Nucleosides are transported via two carrier-mediated mechanisms, namely, facilitated diffusion, also referred to as equilibrative (sodium-independent) transport system and energy-dependent transporters also referred to as concentrative (sodium-dependent) transport system[111]. These transporters have been found in the epithelium of kidneys, intestine, conjunctiva, and choroid plexus[112-114]. Two types of equilibrative (labeled hENT1 and hENT2) and five types of concentrative transporters (labeled N1 through N5) have been reported so far[114-118]. The equilibrative nucleoside transporters (ENT) are differentiated by their relative sensitivities to nitrobenzylthioinosine (NBT). hENT1 is sensitive to NBT, whereas hENT2 is not[119,120]. The differences between concentrative transporters are in their substrate specificities. The N1 transporter is specific to purines and uridine; N2 is selective to pyrimidines and adenosine; N3 has broad specificity for purines and pyrimidines; N4 is pyrimidine selective, but transports adenosine and guanosine as well; and the N5 transporter is NBT sensitive and preferentially transports guanosine[114,118,120-122]. In the eye, sodium-dependent transporters have been found in the retina[121] and conjunctiva[123]. Transport of guanosine and adenosine investigated in retinal cell cultures indicated strong temperature dependency with maximal uptake of both substrates occurring at 37 °C[121]. The transport was found to be significantly decreased when calcium and sodium ions containing electrolytes were substituted with other salts in the buffers used in the experiments. Substrate specificity testing revealed that adenosine inhibited guanosine uptake and not vice versa, indicating that separate processes exist for the uptake of each substrate. Moreover, L-N6-phenyl isopropyladenosine, N6-dimethyladenosine, 8-bromo adenosine, 5’-deoxy-5’-methylthioadenosine, and inosine significantly reduced the transport of adenosine and guanosine. The conjunctival mechanisms involved with nucleoside transport were first elucidated by Hosoya et al[113]. They reported mucosal presence of both sodium-dependent and sodium-independent hENT2 on excised rat conjunctiva. Uridine transport across the conjunctiva follows a strong mucosal to serosal directionality, temperature sensitivity, and phlorizin sensitivity. A structural feature necessary for coupling of the substrate and the transporter is the 3’-hydroxyl group of the D-ribose present in the nucleoside.
The energy for metabolic and electrochemical activity in the eye comes largely from oxidative breakdown of glucose[124]. The most prevalent and classical view regarding energy metabolism in the eye is that glucose is the primary substrate and that the highest rate of glycolysis and respiration manifests in the photoreceptor cells[125-128]. However, an entirely different hypothesis was suggested by Jones et al[129], Tsacopoulos et al[130-132] and Poitry-Yamate et al[133,134] based on their research on honeybee drone retina and guinea pig retina. Their research led to the proposal that glycolysis occurs in glial cells and that Müller cells predominate as the sole aerobic producers of lactate, serving as the primary fuel in the photoreceptors and other retinal neurons. Extensive research to establish metabolic processes occurring in the eye led to the conclusion that under normal conditions, when ambient glucose supply to the eye is adequate, glucose serves as the primary source of energy in the retina, rather than glial-generated lactate[135-137]. It has also been shown that lactate production does occur in Müller cells via aerobic metabolism of glucose[138-140]. Changes in metabolism and metabolic rate have profound implications in the progression of various ocular diseases[141-150]. Seven isoforms of the glucose transporter (GLUT1 through GLUT7) have been identified so far[151]. The facilitative glucose transporter, GLUT1, was found to be expressed in the cornea, iris-ciliary body, lens, and retina[152-156]. In addition to these glucose transporters, Na+-D-glucose transporter (SGLT1) has been found in the mucosal side of the conjunctiva[157]. Although a wealth of information is available regarding glucose transporters, their utility in ocular drug delivery still remains an elusive goal, likely due to the high substrate specificity associated with these transporters.
Ascorbic acid, also known as vitamin C, is a water soluble vitamin responsible for several metabolic and physiological functions due to its antioxidant property. Ascorbic acid protects the cornea and other intraocular tissues by absorbing the UV radiations between 280-310 nm. Higher levels of ascorbic acid in the eye prevent lens cataracts and inhibit peroxidase activity. Human ocular tissues contain significantly higher amounts of ascorbic acid due to their protective role. The concentration of ascorbic acid in tear fluid, corneal epithelium and aqueous humor are 23 ± 9.6 μmol/L, 1.33 ± 0.48 mg/g and 0.20 ± 0.1 mg/mL, respectively. The concentration of ascorbic acid in aqueous humor is approximately 20-fold higher than the plasma concentrations[158]. These figures intrigued the researcher to study the presence of ascorbic acid transporter and its role in the transport of ascorbic acid. Cellular transport of ascorbic acid is mediated by hexose transporters (GLUT) and sodium-dependent vitamin C transporters (SVCT1 and SVCT2). GLUT1 is a low affinity and high capacity transporter that facilitates the transport of the oxidized form of ascorbic acid (dehydroascorbic acid), while SVCT1 and SVCT2 are high affinity and low capacity sodium-dependent transporters that transport the reduced form, L-ascorbic acid. Interestingly, the ascorbic acid concentrations are higher in diurnal animals as compared to nocturnal animals. Neither SVCT1 nor SVCT2 was observed in the ciliary body of rat (nocturnal animal), while albino rabbit (diurnal animal) SVCT2 was expressed abundantly in pigmented epithelium of the ciliary body and expressed moderately in the deeper layers of the corneal epithelium[159]. SVCT2 is widely expressed in several ocular tissues such as ciliary body, cornea, lachrymal gland, and retina[160].
The presence of ascorbic acid on bovine corneal endothelial cells and its role in the transport of ascorbic acid to the stroma was reported by Bode et al[158]. Talluri et al[161] studied the uptake mechanism of L-ascorbic acid by rabbit corneal epithelial cells and characterized the specific transporter involved in this translocation. They concluded that SVCT2 is responsible for the uptake of L-ascorbic acid. Further, the uptake was found to be sodium-dependent and saturable at higher concentrations. Ascorbic acid transporter is utilized to some extent in drug delivery, especially in the transport of glucosamine by the facilitative glucose transporter, GLUT1. Glucosamine is an essential sugar derivative and a widely used nutraceutical agent that helps in the synthesis of glycoproteins and glycosaminoglycans[162]. Glucosamine has significant modulatory effects on insulin resistance and diabetes-associated complications[160]. Recently, SVCT2 transporter has been used in the delivery of neurotropic agents to the central nervous system (CNS). Information available in the literature supports the use of ascorbic acid-conjugated prodrugs nipecotic, kynurenic and diclofenamic acids for brain delivery[163,164]. Luo et al[165] demonstrated that amino acid-conjugated prodrugs of saquinavir improved its solubility, metabolic stability and absorptive permeability. Hence, SVCT targeted prodrug approach can be utilized as an attractive strategy to enhance the ocular absorption of drugs.
Biotin, also known as B-complex vitamin (vitamin B7), is a water soluble vitamin essential for normal cellular growth, function, and development. Biotin is a cofactor for the carboxylases that catalyze various metabolic reactions such as gluconeogenesis, fatty acid biosynthesis, and catabolism of several branched chain amino acids[166,167]. Biotin is primarily absorbed and metabolized in the intestine, liver and placenta[168-170]. The involvement of sodium-dependent multivitamin transporter (SMVT) in the uptake of biotin, pantothenate and lipoate from human placenta was first report by Grassel[171]. Studies by Said et al[169,172] and several other groups concluded that SMVT is the primary transport system responsible for the uptake of biotin uptake[170]. SMVT plays an important role in the transport of vitamins and cofactors essential for the normal functioning of the eye. Moreover, adequate biotin concentrations are required for the development of retina and correct ocular morphogenesis. So far, no study has been published relating to the biotin concentrations in mammalian retina[173]. The circulating blood is responsible for maintaining biotin concentrations in the retina. Nevertheless, the biotin transport from the circulating blood is regulated by the blood-retinal barrier, comprised of retinal capillary endothelial cells (inner BRB) and retinal pigment epithelial cells (outer BRB). Ohkura et al[174] examined the biotin transport mechanism at the inner BRB and concluded that SMVT is involved in the transport of biotin from the circulating blood to the retina, across the inner BRB.
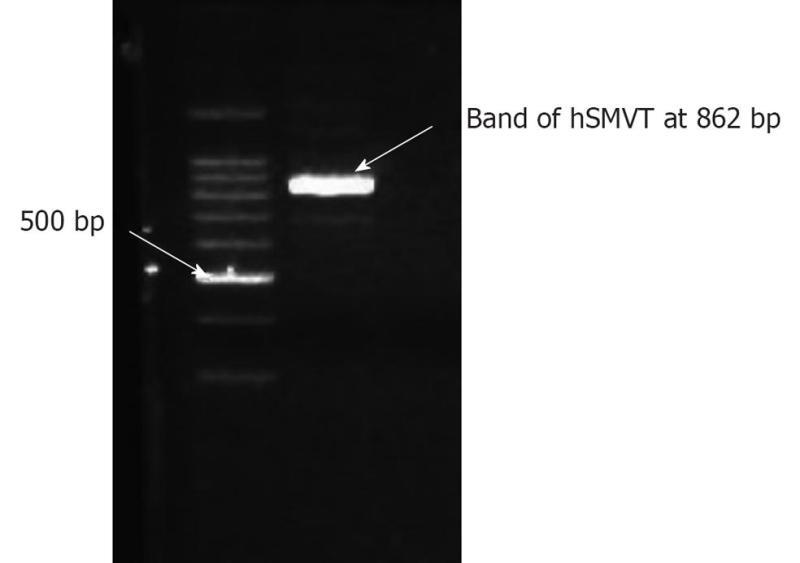
SMVT expressed on the inner BRB could be exploited in drug delivery into the retina due to its excellent capacity (Km) and broad substrate specificity. Biotin prodrugs and polymeric conjugates utilize SMVT to increase the permeability of drugs. Janoria et al[175] studied the presence of SMVT on rabbit corneal epithelial cells. From in vitro and ex vivo studies they concluded that SMVT is expressed on corneal epithelial cells and is responsible for the uptake of biotin, pantothenic acid and lipoic acid. The presence of biotin in tears further substantiates the physiological significance of this transporter. The same research group[176] characterized the presence of SMVT in human retinal pigmented epithelium cell line (ARPE-19) cells and studied the role of SMVT on the uptake of biotin-ganciclovir in both ARPE-19 and rabbit retina. Molecular identification of SMVT was conducted with reverse transcriptase polymerase chain reaction (RT-PCR) in ARPE-19 cells. The band between 800 and 900 bp in gel electrophoresis confirmed the presence of hSMVT (Figure 5). They concluded that biotin-ganciclovir prodrug is recognized by the SMVT transport system in ARPE-19 cell line and rabbit retina. Further, biotin-ganciclovir exhibited a better, therapeutically desirable pharmacological profile in the vitreous fluid, compared to ganciclovir (Table 2). These findings would be of great interest in exploring the potential of SMVT to deliver biotin conjugates.
| Parameters | GCV | (Biotin-GCV) | |
| Biotin-GCV | Regenerated GCV | ||
| AUC (mg/mL per minute) | 10.6 ± 1.27 | 17.5 ± 1.38a | 1.85 ± 0.744 |
| λz (× 10-3/min) | 2.58 ± 0.124 | 3.19 ± 0.536 | |
| T1/2 (min) | 270 ± 15.7 | 222 ± 40.5 | |
| Vss (mL) | 1.56 ± 0.100 | 1.47 ± 0.106 | |
| Cl (μL/min) | 4.39 ± 0.603 | 5.45 ± 0.673 | |
| MRT last (min) | 197 ± 22.2 | 175 ± 17.6 | 264 ± 9.26 |
| Clast (μg/mL) | 7.06 ± 1.38 | 8.28 ± 2.27 | |
| Cmax (μg/mL) | 5.37 ± 0.435 | ||
| Tmax (min) | 66.7 ± 23.1 | ||
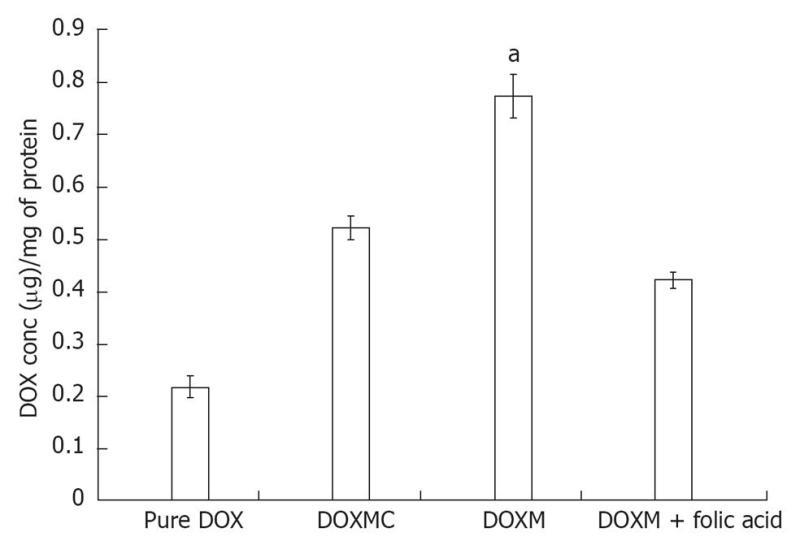
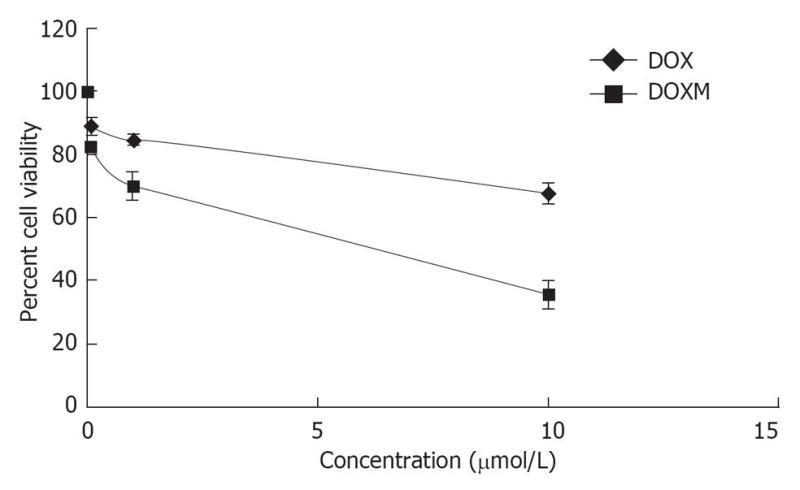
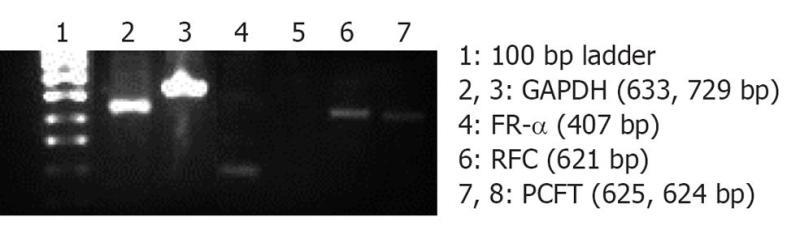
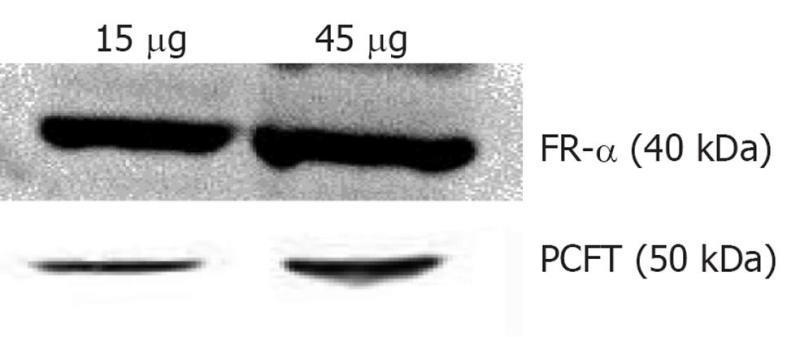
Folate, also known as vitamin B9, is a water soluble essential vitamin that enters the cells through a membrane-associated folate binding protein in addition to classical high affinity/low capacity carrier system[177,178]. Folic acid is a synthetic form of folate that plays an important role in maintaining numerous bodily function,s including the development of visual system. Folic acid deficiency results in retinal edema, retinal dysfunction, damage of photoreceptor cells, nutritional amblyopia, and optic neuropathy, leading to loss of visual function[179,180]. The hydrophilic nature of folic acid prevents it from entering the lipoidal cell membrane. Transport of folate across the cell membrane occurs predominantly via three pathways: folate receptors (FR), reduced folate carrier (RFC), and proton-coupled folate transporter (PCFT)[181]. FRs are coded by two specific genes: FR-α and FR-β, with differential tissue expression[181]. FR-α is distributed throughout the retina, including the basolateral membrane of retinal pigment epithelium[182], while RFT-1 is present only on the apical surface of retinal pigment epithelium[183]. Folate from the choroidal blood vessels is taken by the FR-α located on the basolateral side of RPE and is transferred to the apical membrane of the RPE. RFT-1, present on the apical surface, transports the folate to adjacent metabolically active photoreceptor cells[184]. Tumor cells overexpress FR, and hence folate has been widely used for targeting anti-cancer drugs in the form of prodrugs and delivery systems (folate conjugated nanoparticles and micelles)[185,186]. Kansara et al[85] investigated the expression of FR-α in human-derived retinoblastoma cell line (Y-79). These studies have also demonstrated the mechanism and intracellular regulation of folic acid uptake using various membrane transport inhibitors. Later, the same group developed and characterized folate conjugated polymeric micelles for retinoblastoma cells using doxorubicin as a model drug. Uptake of doxorubicin in Y-79 cells overexpressing FRs was approximately four times higher with folate-conjugated polymeric micelles than with pure drug (Figure 6). Moreover, folate-conjugated polymeric micelles of doxorubicin exhibited higher cytotoxicity in retinoblastoma cell line (Y-79 cells) when compared with pure doxorubicin (Figure 7)[186]. Such systems can provide sustained and targeted delivery of drugs to retinoblastoma cells following intravitreal administration. Jwala et al[180] characterized the expression of folate transport proteins in Staten’s Seruminstitut rabbit corneal (SIRC) epithelial cell line. They observed a linear increase in the uptake of [3H] Folic acid over 30 min, and the uptake process followed saturation kinetics with apparent Km of 14.2 nmol/L, Vmax of 1.5 × 10-5μmol/min per milligram protein and Kd of 2.1 × 10-6/min. Molecular evidence of FR-α and PCFT was established in SIRC epithelial cell line using RT-PCR and Western blotting analysis (Figures 8 and 9). Permeability studies have further confirmed the existence of the folate carrier-mediated system across the rabbit cornea. Drug targeting via FRs is an effective method for cell-selective drug delivery, since this process allows a satisfactory transport rate and ligand-dependent cell specificity. Targetability of various delivery systems such as liposomes, polymer conjugates, polymeric micelles and nanoparticulates has been achieved with a covalently attached folate on the surface[187].
Riboflavin, or vitamin B2, is water soluble and highly photosensitive. In its active forms, flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN) function as critical cofactors involved in the transfer of electrons during several biological redox reactions[188,189]. Since the primary source of riboflavin is dietary intake, lack of this vitamin in food, particularly during pregnancy and adolescence can lead to developmental abnormalities and other well documented clinical manifestations[189-193]. Riboflavin is found in almost all parts of the eye, including corneal epithelium and substantia propria, conjunctiva, lens, iris-ciliary body, aqueous and vitreous humors, choroid, and retina[194]. Riboflavin deficiency produces corneal vascularization, lenticular cataracts, changes in conjunctiva and lachrymal glands, and eye lesions[195-200]. Three riboflavin transporters (RFT) have been reported so far: RFT1, RFT2, and RFT3[201-203]. Structural elucidation of RFTs occurred recently, and mechanisms involving riboflavin transport via RFTs is still being researched rigorously. Kansara et al[204] investigated the uptake mechanism and intracellular transport of riboflavin in human-derived Y-79 cells, which are a model for neural retina. They were the first to establish functional evidence for the presence of a high affinity riboflavin transporter in this in vitro cell model. The carrier-mediated active transport system was found to be energy- and temperature-dependent, but sodium- and pH-independent, in nature. Several studies have been done to further the understanding of the transporter and its function in brain[205], intestine and nutrition[206,207], diseases[208,209], and microbes[210-212]. However, studies on the transporter do not seem to have caught the interest of scientists in eye research.
Drug delivery to the eye remained a major obstacle for scientists in the field. Better understating of the anatomical and physiological barriers, including the drug efflux mechanisms, is crucial to optimizing the drug delivery to the eye. Identification of nutrient transporter/receptor(s) and understanding their roles in targeted delivery of drugs to various ocular tissues has gained a lot of attention recently. This strategy can successfully evade efflux mechanism and simultaneously overcome the tight junctions that hinder the permeability of most drug molecules. Receptors can be utilized for targeted delivery of nanocarriers, which is yet another exciting and promising approach that allows sustained delivery of drugs for diseases affecting the back of the eye. On the whole, the field of ocular drug delivery holds a great future for the development of less invasive, targeted, and controlled release formulations, especially for the treatment of posterior segment diseases.
P- Reviewer Fang J S- Editor Yan JL L- Editor A E- Editor Zhang DN
| 1. | Bourlais CL, Acar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems--recent advances. Prog Retin Eye Res. 1998;17:33-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 442] [Article Influence: 16.4] [Reference Citation Analysis (2)] |
| 2. | Rupenthal ID, Alany RG. Ocular drug delivery. Pharmaceutical manufacturing handbook: Production and processes. New Jersey: John Wiley & Sons 2008; 729-767. |
| 3. | Sasaki H, Yamamura K, Nishida K, Nakamura J, Ichikawa M. Delivery of Drugs to the Eye by Topical Application. Prog Retin Eye Res. 1996;15:583-620. [RCA] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Janoria KG, Gunda S, Boddu SH, Mitra AK. Novel approaches to retinal drug delivery. Expert Opin Drug Deliv. 2007;4:371-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Shulin D. Recent Developments in Ophthalmic Drug Delivery. Pharm Sci Technol Today. 1998;1:328-335. [RCA] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 123] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Lee VH, Robinson JR. Topical ocular drug delivery: recent developments and future challenges. J Ocul Pharmacol. 1986;2:67-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 314] [Article Influence: 8.1] [Reference Citation Analysis (2)] |
| 7. | Boddu SHS. Polymeric Nanoparticles for Ophthalmic Drug Delivery: An Update on Research and Patenting Activity. Recent Patents on Nanomedicine. 2013;2:96-112. |
| 8. | Gray RH, Bates AK, Twomey JM, Claridge K. Textbook of Ocular Pharmacology Edited by Thom J. Zimmerman, Karanjit S. Kooner, Mordechai Sharir and Robert D. Fechtner. J Pharm Pharmacol. 1998;50:827. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Thrimawithana TR, Young S, Bunt CR, Green C, Alany RG. Drug delivery to the posterior segment of the eye. Drug Discov Today. 2011;16:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG. Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev. 2005;57:2010-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 276] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Marmor MF, Negi A, Maurice DM. Kinetics of macromolecules injected into the subretinal space. Exp Eye Res. 1985;40:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 12. | Boddu SH, Jwala J, Vaishya R, Earla R, Karla PK, Pal D, Mitra AK. Novel nanoparticulate gel formulations of steroids for the treatment of macular edema. J Ocul Pharmacol Ther. 2010;26:37-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert Opin Drug Deliv. 2004;1:99-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 14. | Lee TW, Robinson JR. Drug delivery to the posterior segment of the eye: some insights on the penetration pathways after subconjunctival injection. J Ocul Pharmacol Ther. 2001;17:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Mitra AK. Ophtalmic Drug Delivery Systems: Second Edition, Revised and Expanded. Drugs Pharm Sci. 2003;130:727. |
| 16. | Edelhauser HF, Rowe-Rendleman CL, Robinson MR, Dawson DG, Chader GJ, Grossniklaus HE, Rittenhouse KD, Wilson CG, Weber DA, Kuppermann BD. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci. 2010;51:5403-5420. [PubMed] |
| 17. | Boddu SH, Gunda S, Earla R, Mitra AK. Ocular microdialysis: a continuous sampling technique to study pharmacokinetics and pharmacodynamics in the eye. Bioanalysis. 2010;2:487-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Kadam RS, Cheruvu NP, Edelhauser HF, Kompella UB. Sclera-choroid-RPE transport of eight β-blockers in human, bovine, porcine, rabbit, and rat models. Invest Ophthalmol Vis Sci. 2011;52:5387-5399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Hornof M, Toropainen E, Urtti A. Cell culture models of the ocular barriers. Eur J Pharm Biopharm. 2005;60:207-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Huang AJ, Tseng SC, Kenyon KR. Paracellular permeability of corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1989;30:684-689. [PubMed] |
| 21. | Yoshida Y, Ban Y, Kinoshita S. Tight junction transmembrane protein claudin subtype expression and distribution in human corneal and conjunctival epithelium. Invest Ophthalmol Vis Sci. 2009;50:2103-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Ahmed I, Gokhale RD, Shah MV, Patton TF. Physicochemical determinants of drug diffusion across the conjunctiva, sclera, and cornea. J Pharm Sci. 1987;76:583-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 125] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Aihara M, Miyanaga M, Minami K, Miyata K, Eguchi S, Shiroma H, Sawaguchi S. A comparison of fluoroquinolone penetration into human conjunctival tissue. J Ocul Pharmacol Ther. 2008;24:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Gukasyan HJ, Kim KJ, Lee VHL. The Conjunctival Barrier in Ocular Drug Delivery. Biotechnol Pharm Aspects. 2008;7:307-320. |
| 25. | Horibe Y, Hosoya K, Kim KJ, Ogiso T, Lee VH. Polar solute transport across the pigmented rabbit conjunctiva: size dependence and the influence of 8-bromo cyclic adenosine monophosphate. Pharm Res. 1997;14:1246-1251. [PubMed] |
| 26. | Schoenwald RD, Deshpande GS, Rethwisch DG, Barfknecht CF. Penetration into the anterior chamber via the conjunctival/scleral pathway. J Ocul Pharmacol Ther. 1997;13:41-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Ranta VP, Mannermaa E, Lummepuro K, Subrizi A, Laukkanen A, Antopolsky M, Murtomäki L, Hornof M, Urtti A. Barrier analysis of periocular drug delivery to the posterior segment. J Control Release. 2010;148:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Becker U, Ehrhardt C, Schaefer UF, Gukasyan HJ, Kim KJ, Lee VH, Lehr CM. Tissue distribution of moxaverine-hydrochloride in the rabbit eye and plasma. J Ocul Pharmacol Ther. 2005;21:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Zhang W, Prausnitz MR, Edwards A. Model of transient drug diffusion across cornea. J Control Release. 2004;99:241-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Ghorbanzad’e M, Fatemi MH, Karimpour M, Andersson PL. Quantitative and qualitative prediction of corneal permeability for drug-like compounds. Talanta. 2011;85:2686-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Shen J, Deng Y, Jin X, Ping Q, Su Z, Li L. Thiolated nanostructured lipid carriers as a potential ocular drug delivery system for cyclosporine A: Improving in vivo ocular distribution. Int J Pharm. 2010;402:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Hosoya K, Tomi M, Tachikawa M. Strategies for therapy of retinal diseases using systemic drug delivery: relevance of transporters at the blood-retinal barrier. Expert Opin Drug Deliv. 2011;8:1571-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Runkle EA, Antonetti DA. The blood-retinal barrier: structure and functional significance. Methods Mol Biol. 2011;686:133-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 34. | Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12:348-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 818] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 35. | Yamamoto A, Akanuma S, Tachikawa M, Hosoya K. Involvement of LAT1 and LAT2 in the high- and low-affinity transport of L-leucine in human retinal pigment epithelial cells (ARPE-19 cells). J Pharm Sci. 2010;99:2475-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Tachikawa M, Murakami K, Martin PM, Hosoya K, Ganapathy V. Retinal transfer of nicotinate by H+ -monocarboxylate transporter at the inner blood-retinal barrier. Microvasc Res. 2011;82:385-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Tachikawa M, Okamoto M, Hirose S, Yoneyama D, Akanuma S, Terasaki T, Hosoya K. Inner blood-retinal barrier mediates l-isomer-predominant transport of serine. J Pharm Sci. 2011;100:3892-3903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Bostian K, Glinka T, Lomovskaya O, Surber M, Berkley N, Griffith D, inventors; Bacterial Efflux Pump Inhibitors for the Treatment of Ophthalmic and Otic Infections in Co-Administration with Antimicrobial Agents. United States patent US 20080132457. 2008 Jun 5. |
| 39. | Karla PK, Earla R, Boddu SH, Johnston TP, Pal D, Mitra A. Molecular expression and functional evidence of a drug efflux pump (BCRP) in human corneal epithelial cells. Curr Eye Res. 2009;34:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Karla PK, Boddu SHS, Dasari CR, Mitra AK. Outward-Directed Transport. Adler’s Physiology of the Eye. Edinburgh: Saunders Elsevier 2011; 385-393. |
| 41. | Hariharan S, Minocha M, Mishra GP, Pal D, Krishna R, Mitra AK. Interaction of ocular hypotensive agents (PGF2 alpha analogs-bimatoprost, latanoprost, and travoprost) with MDR efflux pumps on the rabbit cornea. J Ocul Pharmacol Ther. 2009;25:487-498. [PubMed] |
| 42. | Hariharan S, Thakkar NR, Mitra AK. Transporter-targeted Drug Delivery to the Retina. Retina Today. 2009;57-62. |
| 43. | Hippalgaonkar K, Srirangam R, Avula B, Khan IA, Majumdar S. Interaction between topically and systemically coadministered P-glycoprotein substrates/inhibitors: effect on vitreal kinetics. Drug Metab Dispos. 2010;38:1790-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Majumdar S, Hippalgaonkar K, Srirangam R. Vitreal kinetics of quinidine in rabbits in the presence of topically coadministered P-glycoprotein substrates/modulators. Drug Metab Dispos. 2009;37:1718-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Senthilkumari S, Velpandian T, Biswas NR, Saxena R, Ghose S. Evaluation of the modulation of P-glycoprotein (P-gp) on the intraocular disposition of its substrate in rabbits. Curr Eye Res. 2008;33:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Senthilkumari S, Velpandian T, Biswas NR, Sonali N, Ghose S. Evaluation of the impact of P-glycoprotein (P-gp) drug efflux transporter blockade on the systemic and ocular disposition of P-gp substrate. J Ocul Pharmacol Ther. 2008;24:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Davidson AL, Dassa E, Orelle C, Chen J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev. 2008;72:317-64, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1095] [Cited by in RCA: 993] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 48. | Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973;323:466-483. [PubMed] |
| 49. | Germann UA. P-glycoprotein--a mediator of multidrug resistance in tumour cells. Eur J Cancer. 1996;32A:927-944. [PubMed] |
| 50. | Colone M, Calcabrini A, Toccacieli L, Bozzuto G, Stringaro A, Gentile M, Cianfriglia M, Ciervo A, Caraglia M, Budillon A. The multidrug transporter P-glycoprotein: a mediator of melanoma invasion. J Invest Dermatol. 2008;128:957-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Beringer PM, Slaughter RL. Transporters and their impact on drug disposition. Ann Pharmacother. 2005;39:1097-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Saha P, Yang JJ, Lee VH. Existence of a p-glycoprotein drug efflux pump in cultured rabbit conjunctival epithelial cells. Invest Ophthalmol Vis Sci. 1998;39:1221-1226. [PubMed] |
| 53. | Holash JA, Stewart PA. The relationship of astrocyte-like cells to the vessels that contribute to the blood-ocular barriers. Brain Res. 1993;629:218-224. [PubMed] |
| 54. | Schlingemann RO, Hofman P, Klooster J, Blaauwgeers HG, Van der Gaag R, Vrensen GF. Ciliary muscle capillaries have blood-tissue barrier characteristics. Exp Eye Res. 1998;66:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Kennedy BG, Mangini NJ. P-glycoprotein expression in human retinal pigment epithelium. Mol Vis. 2002;8:422-430. [PubMed] |
| 56. | Juuti-Uusitalo K, Vaajasaari H, Ryhänen T, Narkilahti S, Suuronen R, Mannermaa E, Kaarniranta K, Skottman H. Efflux protein expression in human stem cell-derived retinal pigment epithelial cells. PLoS One. 2012;7:e30089. [PubMed] |
| 57. | Wu J, Zhang JJ, Koppel H, Jacob TJ. P-glycoprotein regulates a volume-activated chloride current in bovine non-pigmented ciliary epithelial cells. J Physiol. 1996;491:743-755. [PubMed] |
| 58. | Dey S, Gunda S, Mitra AK. Pharmacokinetics of erythromycin in rabbit corneas after single-dose infusion: role of P-glycoprotein as a barrier to in vivo ocular drug absorption. J Pharmacol Exp Ther. 2004;311:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Kruijtzer CM, Beijnen JH, Schellens JH. Improvement of oral drug treatment by temporary inhibition of drug transporters and/or cytochrome P450 in the gastrointestinal tract and liver: an overview. Oncologist. 2002;7:516-530. [PubMed] |
| 60. | Yang JJ, Ann DK, Kannan R, Lee VH. Multidrug resistance protein 1 (MRP1) in rabbit conjunctival epithelial cells: its effect on drug efflux and its regulation by adenoviral infection. Pharm Res. 2007;24:1490-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Aukunuru JV, Sunkara G, Bandi N, Thoreson WB, Kompella UB. Expression of multidrug resistance-associated protein (MRP) in human retinal pigment epithelial cells and its interaction with BAPSG, a novel aldose reductase inhibitor. Pharm Res. 2001;18:565-572. [PubMed] |
| 62. | Karla PK, Quinn TL, Herndon BL, Thomas P, Pal D, Mitra A. Expression of multidrug resistance associated protein 5 (MRP5) on cornea and its role in drug efflux. J Ocul Pharmacol Ther. 2009;25:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Karla PK, Pal D, Quinn T, Mitra AK. Molecular evidence and functional expression of a novel drug efflux pump (ABCC2) in human corneal epithelium and rabbit cornea and its role in ocular drug efflux. Int J Pharm. 2007;336:12-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Vellonen KS, Mannermaa E, Turner H, Häkli M, Wolosin JM, Tervo T, Honkakoski P, Urtti A. Effluxing ABC transporters in human corneal epithelium. J Pharm Sci. 2010;99:1087-1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Piddock LJ. Multidrug-resistance efflux pumps - not just for resistance. Nat Rev Microbiol. 2006;4:629-636. [PubMed] |
| 66. | Constable PA, Lawrenson JG, Dolman DE, Arden GB, Abbott NJ. P-Glycoprotein expression in human retinal pigment epithelium cell lines. Exp Eye Res. 2006;83:24-30. [PubMed] |
| 67. | Mannermaa E, Vellonen KS, Ryhänen T, Kokkonen K, Ranta VP, Kaarniranta K, Urtti A. Efflux protein expression in human retinal pigment epithelium cell lines. Pharm Res. 2009;26:1785-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Aukunuru JV, Sunkara G, Bandi N, Thoreson WB, Kompella UB. Expression of multidrug resistance-associated protein (MRP) in human retinal pigment epithelial cells and its interaction with BAPSG, a novel aldose reductase inhibitor. Pharm Res. 2001;18:565-572. [PubMed] |
| 69. | Becker U, Ehrhardt C, Daum N, Baldes C, Schaefer UF, Ruprecht KW, Kim KJ, Lehr CM. Expression of ABC-transporters in human corneal tissue and the transformed cell line, HCE-T. J Ocul Pharmacol Ther. 2007;23:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Hariharan S, Gunda S, Mishra GP, Pal D, Mitra AK. Enhanced corneal absorption of erythromycin by modulating P-glycoprotein and MRP mediated efflux with corticosteroids. Pharm Res. 2009;26:1270-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Karla PK, Pal D, Mitra AK. Molecular evidence and functional expression of multidrug resistance associated protein (MRP) in rabbit corneal epithelial cells. Exp Eye Res. 2007;84:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Werle M. Natural and synthetic polymers as inhibitors of drug efflux pumps. Pharm Res. 2008;25:500-511. [PubMed] |
| 73. | Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 634] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 74. | Weiner AL, Gilger BC. Advancements in ocular drug delivery. Vet Ophthalmol. 2010;13:395-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Yorio T, Clark AF, Wax MB. Ocular therapeutics: eye on new discoveries. 1st ed. Amsterdam: Elsevier Press 2008; 520. |
| 76. | Lee VHL. Mechanisms and facilitation of corneal drug penetration. J Control Release. 1990;11:79-90. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Schoenwald RD, Huang HS. Corneal penetration behavior of beta-blocking agents I: Physiochemical factors. J Pharm Sci. 1983;72:1266-1272. [PubMed] |
| 78. | Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Järvinen T, Savolainen J. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7:255-270. [PubMed] |
| 79. | Gao H, Mitra AK. Regioselective synthesis of various prodrugs of ganciclovir. Tetrahedron Lett. 2000;41:1131-1136. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 80. | Narurkar MM, Mitra AK. Synthesis, physicochemical properties, and cytotoxicity of a series of 5’-ester prodrugs of 5-iodo-2’-deoxyuridine. Pharm Res. 1988;5:734-737. [PubMed] |
| 81. | Dias CS, Anand BS, Mitra AK. Effect of mono- and di-acylation on the ocular disposition of ganciclovir: physicochemical properties, ocular bioreversion, and antiviral activity of short chain ester prodrugs. J Pharm Sci. 2002;91:660-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | Tsuji A. Small molecular drug transfer across the blood-brain barrier via carrier-mediated transport systems. NeuroRx. 2005;2:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 83. | Duvvuri S, Majumdar S, Mitra AK. Drug delivery to the retina: challenges and opportunities. Expert Opin Biol Th. 2003;3:45-56. [RCA] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 84. | Sirotnak FM, Tolner B. Carrier-mediated membrane transport of folates in mammalian cells. Annu Rev Nutr. 1999;19:91-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 215] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 85. | Kansara V, Paturi D, Luo S, Gaudana R, Mitra AK. Folic acid transport via high affinity carrier-mediated system in human retinoblastoma cells. Int J Pharm. 2008;355:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Terada T, Inui K. Peptide transporters: structure, function, regulation and application for drug delivery. Curr Drug Metab. 2004;5:85-94. [PubMed] |
| 87. | Nielsen CU, Brodin B. Di/tri-peptide transporters as drug delivery targets: regulation of transport under physiological and patho-physiological conditions. Curr Drug Targets. 2003;4:373-388. [PubMed] |
| 88. | Brodin B, Nielsen CU, Steffansen B, Frøkjaer S. Transport of peptidomimetic drugs by the intestinal Di/tri-peptide transporter, PepT1. Pharmacol Toxicol. 2002;90:285-296. [PubMed] |
| 89. | Ramamoorthy S, Liu W, Ma YY, Yang-Feng TL, Ganapathy V, Leibach FH. Proton/peptide cotransporter (PEPT 2) from human kidney: functional characterization and chromosomal localization. Biochim Biophys Acta. 1995;1240:1-4. [PubMed] |
| 90. | Anand BS, Mitra AK. Mechanism of corneal permeation of L-valyl ester of acyclovir: targeting the oligopeptide transporter on the rabbit cornea. Pharm Res. 2002;19:1194-1202. [PubMed] |
| 91. | Anand BS, Hill JM, Dey S, Maruyama K, Bhattacharjee PS, Myles ME, Nashed YE, Mitra AK. In vivo antiviral efficacy of a dipeptide acyclovir prodrug, val-val-acyclovir, against HSV-1 epithelial and stromal keratitis in the rabbit eye model. Invest Ophthalmol Vis Sci. 2003;44:2529-2534. [PubMed] |
| 92. | Macha S, Mitra AK. Ocular pharmacokinetics of cephalosporins using microdialysis. J Ocul Pharmacol Ther. 2001;17:485-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Berger UV, Hediger MA. Distribution of peptide transporter PEPT2 mRNA in the rat nervous system. Anat Embryol (Berl). 1999;199:439-449. [PubMed] |
| 94. | Majumdar S, Macha S, Nashed Y, Mitra AK. Expression of peptide transporters on the rabbit retina: a strategy to improve retinal delivery of ganciclovir. Lett Drug Des Discov. 2004;1:73-77. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 95. | Dias C, Nashed Y, Atluri H, Mitra A. Ocular penetration of acyclovir and its peptide prodrugs valacyclovir and val-valacyclovir following systemic administration in rabbits: An evaluation using ocular microdialysis and LC-MS. Curr Eye Res. 2002;25:243-252. [PubMed] |
| 96. | Atluri H, Anand BS, Patel J, Mitra AK. Mechanism of a model dipeptide transport across blood-ocular barriers following systemic administration. Exp Eye Res. 2004;78:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 97. | Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70:43-77. [PubMed] |
| 98. | Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch. 2004;447:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 302] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 99. | Gu S, Roderick HL, Camacho P, Jiang JX. Characterization of an N-system amino acid transporter expressed in retina and its involvement in glutamine transport. J Biol Chem. 2001;276:24137-24144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Pow DV. Amino acids and their transporters in the retina. Neurochem Int. 2001;38:463-484. [PubMed] |
| 101. | Gandhi MD, Pal D, Mitra AK. Identification and functional characterization of a Na(+)-independent large neutral amino acid transporter (LAT2) on ARPE-19 cells. Int J Pharm. 2004;275:189-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 102. | Jain-Vakkalagadda B, Pal D, Gunda S, Nashed Y, Ganapathy V, Mitra AK. Identification of a Na+-dependent cationic and neutral amino acid transporter, B(0,+), in human and rabbit cornea. Mol Pharm. 2004;1:338-346. [PubMed] |
| 103. | Katragadda S, Gunda S, Hariharan S, Mitra AK. Ocular pharmacokinetics of acyclovir amino acid ester prodrugs in the anterior chamber: evaluation of their utility in treating ocular HSV infections. Int J Pharm. 2008;359:15-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Ganapathy ME, Ganapathy V. Amino Acid Transporter ATB0,+ as a delivery system for drugs and prodrugs. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:357-364. [PubMed] |
| 105. | Massey SC, Redburn DA. Transmitter circuits in the vertebrate retina. Prog Neurobiol. 1987;28:55-96. [PubMed] |
| 106. | Mills SL, Massey SC. Labeling and distribution of AII amacrine cells in the rabbit retina. J Comp Neurol. 1991;304:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 107. | Muller JF, Marc RE. GABA-ergic and glycinergic pathways in the inner plexiform layer of the goldfish retina. J Comp Neurol. 1990;291:281-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 108. | Zafra F, Aragón C, Olivares L, Danbolt NC, Giménez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995;15:3952-3969. [PubMed] |
| 109. | Sasoh M, Ma N, Yoshida S, Semba R, Uji Y. Immunocytochemical localization of glutamate in normal and detached cat retina. Invest Ophthalmol Vis Sci. 1998;39:786-792. [PubMed] |
| 110. | Pulido JE, Pulido JS, Erie JC, Arroyo J, Bertram K, Lu MJ, Shippy SA. A role for excitatory amino acids in diabetic eye disease. Exp Diabetes Res. 2007;2007:36150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Lee VH. Membrane transporters. Eur J Pharm Sci. 2000;11 Suppl 2:S41-S50. [PubMed] |
| 112. | Cheng X, Klaassen CD. Tissue distribution, ontogeny, and hormonal regulation of xenobiotic transporters in mouse kidneys. Drug Metab Dispos. 2009;37:2178-2185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 113. | Hosoya K, Horibe Y, Kim KJ, Lee VH. Nucleoside transport mechanisms in the pigmented rabbit conjunctiva. Invest Ophthalmol Vis Sci. 1998;39:372-377. [PubMed] |
| 114. | Schaner ME, Gerstin KM, Wang J, Giacomini KM. Mechanisms of transport of nucleosides and nucleoside analogues in choroid plexus. Adv Drug Deliv Rev. 1999;39:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 115. | Majumdar S, Duvvuri S, Mitra AK. Membrane transporter/receptor-targeted prodrug design: strategies for human and veterinary drug development. Adv Drug Deliv Rev. 2004;56:1437-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 116. | Plagemann PG, Wohlhueter RM, Woffendin C. Nucleoside and nucleobase transport in animal cells. Biochim Biophys Acta. 1988;947:405-443. [PubMed] |
| 117. | Crawford CR, Cass CE, Young JD, Belt JA. Stable expression of a recombinant sodium-dependent, pyrimidine-selective nucleoside transporter (CNT1) in a transport-deficient mouse leukemia cell line. Biochem Cell Biol. 1998;76:843-851. [PubMed] |
| 118. | Lee CW, Cheeseman CI, Jarvis SM. Transport characteristics of renal brush border Na(+)- and K(+)-dependent uridine carriers. Am J Physiol. 1990;258:F1203-F1210. [PubMed] |
| 119. | Wu X, Giacomini KM. Expression of the choroid plexus sodium-nucleoside cotransporter (N3) in Xenopus laevis oocytes. Biochem Pharmacol. 1994;48:432-434. [PubMed] |
| 120. | Wu X, Gutierrez MM, Giacomini KM. Further characterization of the sodium-dependent nucleoside transporter (N3) in choroid plexus from rabbit. Biochim Biophys Acta. 1994;1191:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 121. | Schaeffer JM, Anderson SM. Nucleoside uptake by rat retina cells. Life Sci. 1981;29:939-946. [PubMed] |
| 122. | Yao SY, Ng AM, Cass CE, Baldwin SA, Young JD. Nucleobase transport by human equilibrative nucleoside transporter 1 (hENT1). J Biol Chem. 2011;286:32552-32562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 123. | Hosoya K, Lee VH, Kim KJ. Roles of the conjunctiva in ocular drug delivery: a review of conjunctival transport mechanisms and their regulation. Eur J Pharm Biopharm. 2005;60:227-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 124. | Cohen LH, Noell WK. Glucose catabolism of rabbit retina before and after development of visual function. J Neurochem. 1960;5:253-276. [PubMed] |
| 125. | Ahmed J, Braun RD, Dunn R, Linsenmeier RA. Oxygen distribution in the macaque retina. Invest Ophthalmol Vis Sci. 1993;34:516-521. [PubMed] |
| 126. | Cringle SJ, Yu DY, Alder VA. Intravitreal and intraretinal oxygen tension in the rat eye. Adv Exp Med Biol. 1992;316:113-117. [PubMed] |
| 127. | Yu DY, Cringle SJ, Alder VA, Su EN, Yu PK. Intraretinal oxygen distribution and choroidal regulation in the avascular retina of guinea pigs. Am J Physiol. 1996;270:H965-H973. [PubMed] |
| 128. | Winkler BS. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981;77:667-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 278] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 129. | Jones GJ, Tsacopoulos M. The response to monochromatic light flashes of the oxygen consumption of honeybee drone photoreceptors. J Gen Physiol. 1987;89:791-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 130. | Tsacopoulos M, Coles JA, Van de Werve G. The supply of metabolic substrate from glia to photoreceptors in the retina of the honeybee drone. J Physiol (Paris). 1987;82:279-287. [PubMed] |
| 131. | Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 490] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 132. | Tsacopoulos M, Poitry-Yamate CL, MacLeish PR, Poitry S. Trafficking of molecules and metabolic signals in the retina. Prog Retin Eye Res. 1998;17:429-442. [PubMed] |
| 133. | Poitry-Yamate CL, Poitry S, Tsacopoulos M. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci. 1995;15:5179-5191. [PubMed] |
| 134. | Poitry-Yamate CL, Tsacopoulos M. Glucose metabolism in freshly isolated Müller glial cells from a mammalian retina. J Comp Neurol. 1992;320:257-266. [PubMed] |
| 135. | Winkler BS, Pourcho RG, Starnes C, Slocum J, Slocum N. Metabolic mapping in mammalian retina: a biochemical and 3H-2-deoxyglucose autoradiographic study. Exp Eye Res. 2003;77:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 136. | Winkler BS, Sauer MW, Starnes CA. Effects of L-glutamate/D-aspartate and monensin on lactic acid production in retina and cultured retinal Müller cells. J Neurochem. 2004;89:514-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 137. | Witkovsky P, Yang CY. Uptake and localization of 3H-2 deoxy-D-glucose by retinal photoreceptors. J Comp Neurol. 1982;204:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 138. | Winkler BS, Starnes CA, Sauer MW, Firouzgan Z, Chen SC. Cultured retinal neuronal cells and Müller cells both show net production of lactate. Neurochem Int. 2004;45:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 139. | Watanabe T, Nagamatsu S, Matsushima S, Kirino T, Uchimura H. Colocalization of GLUT3 and choline acetyltransferase immunoreactivity in the rat retina. Biochem Biophys Res Commun. 1999;256:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 140. | Winkler BS, Arnold MJ, Brassell MA, Puro DG. Energy metabolism in human retinal Müller cells. Invest Ophthalmol Vis Sci. 2000;41:3183-3190. [PubMed] |
| 141. | DiMattio J, Zadunaisky JA, Altszuler N. Onset of changes in glucose transport across ocular barriers in streptozotocin-induced diabetes. Invest Ophthalmol Vis Sci. 1984;25:820-826. [PubMed] |
| 142. | Green K, Strange K, Cheeks L, Nelson E, Chapman JM, Hull DS. Effects of glucose transport inhibitors on corneal swelling rate and endothelial ion fluxes. J Toxicol Cutaneous Ocul Toxicol. 1990;9:53-66. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 143. | Kang Derwent JJ, Linsenmeier RA. Hypoglycemia increases the sensitivity of the cat electroretinogram to hypoxemia. Vis Neurosci. 2001;18:983-993. [PubMed] |
| 144. | Linsenmeier RA, Braun RD, McRipley MA, Padnick LB, Ahmed J, Hatchell DL, McLeod DS, Lutty GA. Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci. 1998;39:1647-1657. [PubMed] |
| 145. | Padnick LB, Linsenmeier RA, Goldstick TK. Oxygenation of the cat primary visual cortex. J Appl Physiol. 1999;86:1490-1496. [PubMed] |
| 146. | Poll-The BT, Maillette de Buy Wenniger-Prick CJ. The eye in metabolic diseases: clues to diagnosis. Eur J Paediatr Neurol. 2011;15:197-204. [PubMed] |
| 147. | Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783-791. [PubMed] |
| 148. | Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998;47:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 328] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 149. | Lieth E, Gardner TW, Barber AJ, Antonetti DA. Retinal neurodegeneration: early pathology in diabetes. Clin Experiment Ophthalmol. 2000;28:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 236] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 150. | Lieth E, LaNoue KF, Antonetti DA, Ratz M. Diabetes reduces glutamate oxidation and glutamine synthesis in the retina. The Penn State Retina Research Group. Exp Eye Res. 2000;70:723-730. [PubMed] |
| 151. | Gaudana R, Jwala J, Boddu SH, Mitra AK. Recent perspectives in ocular drug delivery. Pharm Res. 2009;26:1197-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 152. | Hsu SC, Molday RS. Glycolytic enzymes and a GLUT-1 glucose transporter in the outer segments of rod and cone photoreceptor cells. J Biol Chem. 1991;266:21745-21752. [PubMed] |
| 153. | Li XB, Szerencsei RT, Schnetkamp PP. The glucose transporter in the plasma membrane of the outer segments of bovine retinal rods. Exp Eye Res. 1994;59:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 154. | Lucas VA, Zigler JS. Identification of the monkey lens glucose transporter by photoaffinity labelling with cytochalasin B. Invest Ophthalmol Vis Sci. 1988;29:630-635. [PubMed] |
| 155. | Mantych GJ, Hageman GS, Devaskar SU. Characterization of glucose transporter isoforms in the adult and developing human eye. Endocrinology. 1993;133:600-607. [PubMed] [DOI] [Full Text] |
| 156. | Tsukamoto H, Mishima HK, Kurokawa T, Kiuchi Y, Sato E, Ishibashi S. Isoforms of glucose transporter in the iris-ciliary body. Jpn J Ophthalmol. 1995;39:242-247. [PubMed] |
| 157. | Turner HC, Alvarez LJ, Bildin VN, Candia OA. Immunolocalization of Na-K-ATPase, Na-K-Cl and Na-glucose cotransporters in the conjunctival epithelium. Curr Eye Res. 2000;21:843-850. [PubMed] |
| 158. | Bode AM, Vanderpool SS, Carlson EC, Meyer DA, Rose RC. Ascorbic acid uptake and metabolism by corneal endothelium. Invest Ophthalmol Vis Sci. 1991;32:2266-2271. [PubMed] |
| 159. | Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 672] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 160. | Ganapathy V, Ananth S, Smith SB, Martin PM. Vitamin C Transporters in the Retina. Ophthalmic Res. 2008;437-450. [DOI] [Full Text] |
| 161. | Talluri RS, Katragadda S, Pal D, Mitra AK. Mechanism of L-ascorbic acid uptake by rabbit corneal epithelial cells: evidence for the involvement of sodium-dependent vitamin C transporter 2. Curr Eye Res. 2006;31:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 162. | Uldry M, Ibberson M, Hosokawa M, Thorens B. GLUT2 is a high affinity glucosamine transporter. FEBS Lett. 2002;524:199-203. [PubMed] |
| 163. | Dalpiaz A, Pavan B, Scaglianti M, Vitali F, Bortolotti F, Biondi C, Scatturin A, Tanganelli S, Ferraro L, Prasad P. Transporter-mediated effects of diclofenamic acid and its ascorbyl pro-drug in the in vivo neurotropic activity of ascorbyl nipecotic acid conjugate. J Pharm Sci. 2004;93:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 164. | Manfredini S, Pavan B, Vertuani S, Scaglianti M, Compagnone D, Biondi C, Scatturin A, Tanganelli S, Ferraro L, Prasad P. Design, synthesis and activity of ascorbic acid prodrugs of nipecotic, kynurenic and diclophenamic acids, liable to increase neurotropic activity. J Med Chem. 2002;45:559-562. [PubMed] |
| 165. | Luo S, Wang Z, Patel M, Khurana V, Zhu X, Pal D, Mitra AK. Targeting SVCT for enhanced drug absorption: synthesis and in vitro evaluation of a novel vitamin C conjugated prodrug of saquinavir. Int J Pharm. 2011;414:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 166. | Luo S, Kansara VS, Zhu X, Mandava NK, Pal D, Mitra AK. Functional characterization of sodium-dependent multivitamin transporter in MDCK-MDR1 cells and its utilization as a target for drug delivery. Mol Pharm. 2006;3:329-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 167. | Dakshinamurti K, Chauhan J. Regulation of biotin enzymes. Annu Rev Nutr. 1988;8:211-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 168. | Prasad PD, Ganapathy V. Structure and function of mammalian sodium-dependent multivitamin transporter. Curr Opin Clin Nutr Metab Care. 2000;3:263-266. [PubMed] |
| 169. | Said HM. Cellular uptake of biotin: mechanisms and regulation. J Nutr. 1999;129:490S-493S. [PubMed] |
| 170. | Prasad PD, Wang H, Kekuda R, Fujita T, Fei YJ, Devoe LD, Leibach FH, Ganapathy V. Cloning and functional expression of a cDNA encoding a mammalian sodium-dependent vitamin transporter mediating the uptake of pantothenate, biotin, and lipoate. J Biol Chem. 1998;273:7501-7506. [PubMed] |
| 171. | Grassl SM. Human placental brush-border membrane Na(+)-biotin cotransport. J Biol Chem. 1992;267:17760-17765. [PubMed] |
| 172. | Said HM, Redha R, Nylander W. Biotin transport in the human intestine: site of maximum transport and effect of pH. Gastroenterology. 1988;95:1312-1317. [PubMed] |
| 173. | Spector R, Johanson CE. Vitamin transport and homeostasis in mammalian brain: focus on Vitamins B and E. J Neurochem. 2007;103:425-438. [PubMed] |
| 174. | Ohkura Y, Akanuma S, Tachikawa M, Hosoya K. Blood-to-retina transport of biotin via Na+-dependent multivitamin transporter (SMVT) at the inner blood-retinal barrier. Exp Eye Res. 2010;91:387-392. [PubMed] |
| 175. | Janoria KG, Hariharan S, Paturi D, Pal D, Mitra AK. Biotin uptake by rabbit corneal epithelial cells: role of sodium-dependent multivitamin transporter (SMVT). Curr Eye Res. 2006;31:797-809. [PubMed] |
| 176. | Janoria KG, Boddu SH, Wang Z, Paturi DK, Samanta S, Pal D, Mitra AK. Vitreal pharmacokinetics of biotinylated ganciclovir: role of sodium-dependent multivitamin transporter expressed on retina. J Ocul Pharmacol Ther. 2009;25:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 177. | Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, Kamen BA. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396-3401. [PubMed] |
| 178. | Shiokawa T, Hattori Y, Kawano K, Ohguchi Y, Kawakami H, Toma K, Maitani Y. Effect of polyethylene glycol linker chain length of folate-linked microemulsions loading aclacinomycin A on targeting ability and antitumor effect in vitro and in vivo. Clin Cancer Res. 2005;11:2018-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 179. | Golnik KC, Schaible ER. Folate-responsive optic neuropathy. J Neuroophthalmol. 1994;14:163-169. [PubMed] |
| 180. | Jwala J, Boddu SH, Paturi DK, Shah S, Smith SB, Pal D, Mitra AK. Functional characterization of folate transport proteins in Staten’s Seruminstitut rabbit corneal epithelial cell line. Curr Eye Res. 2011;36:404-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (2)] |
| 181. | Spiegelstein O, Eudy JD, Finnell RH. Identification of two putative novel folate receptor genes in humans and mouse. Gene. 2000;258:117-125. [PubMed] |
| 182. | Smith SB, Kekuda R, Gu X, Chancy C, Conway SJ, Ganapathy V. Expression of folate receptor alpha in the mammalian retinol pigmented epithelium and retina. Invest Ophthalmol Vis Sci. 1999;40:840-848. [PubMed] |
| 183. | Chancy CD, Kekuda R, Huang W, Prasad PD, Kuhnel JM, Sirotnak FM, Roon P, Ganapathy V, Smith SB. Expression and differential polarization of the reduced-folate transporter-1 and the folate receptor alpha in mammalian retinal pigment epithelium. J Biol Chem. 2000;275:20676-20684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 184. | Bridges CC, El-Sherbeny A, Ola MS, Ganapathy V, Smith SB. Transcellular transfer of folate across the retinal pigment epithelium. Curr Eye Res. 2002;24:129-138. [PubMed] |
| 185. | Shinoda T, Takagi A, Maeda A, Kagatani S, Konno Y, Hashida M. In vivo fate of folate-BSA in non-tumor- and tumor-bearing mice. J Pharm Sci. 1998;87:1521-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 186. | Boddu SH, Jwala J, Chowdhury MR, Mitra AK. In vitro evaluation of a targeted and sustained release system for retinoblastoma cells using Doxorubicin as a model drug. J Ocul Pharmacol Ther. 2010;26:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 187. | Yoo HS, Park TG. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Control Release. 2004;96:273-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 440] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 188. | Powers HJ. Current knowledge concerning optimum nutritional status of riboflavin, niacin and pyridoxine. Proc Nutr Soc. 1999;58:435-440. [PubMed] |
| 189. | Yates CA, Evans GS, Pearson T, Powers HJ. Absence of luminal riboflavin disturbs early postnatal development of the gastrointestinal tract. Dig Dis Sci. 2003;48:1159-1164. [PubMed] |
| 190. | McCormick DB. Two interconnected B vitamins: riboflavin and pyridoxine. Physiol Rev. 1989;69:1170-1198. [PubMed] |
| 191. | Bates CJ, Powers HJ, Thurnham DI. Vitamins, iron, and physical work. Lancet. 1989;2:313-314. [PubMed] |
| 192. | Fairweather-Tait SJ, Powers HJ, Minski MJ, Whitehead J, Downes R. Riboflavin deficiency and iron absorption in adult Gambian men. Ann Nutr Metab. 1992;36:34-40. [PubMed] |
| 193. | Finglas PM, de Meer K, Molloy A, Verhoef P, Pietrzik K, Powers HJ, van der Straeten D, Jägerstad M, Varela-Moreiras G, van Vliet T. Research goals for folate and related B vitamin in Europe. Eur J Clin Nutr. 2006;60:287-294. [PubMed] |
| 194. | Pirie A. THE RELATION OF RIBOFLAVIN TO THE EYE. A REVIEW ARTICLE. Br J Ophthalmol. 1943;27:291-301. [PubMed] |
| 195. | Takami Y, Gong H, Amemiya T. Riboflavin deficiency induces ocular surface damage. Ophthalmic Res. 2004;36:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 196. | Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol. 1999;14:240-248. [PubMed] |
| 198. | Heywood R, Partington H. Ocular lesions induced by vitamin B2 deficiency. Vet Rec. 1971;88:251-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 199. | Srivastava SK, Beutler E. Increased susceptibility of riboflavin deficient rats to galactose cataract. Experientia. 1970;26:250. [PubMed] |
| 200. | Verma OP. Partial degeneration of the optic nerve associated with vitamin deficiency. Indian Med Gaz. 1942;77: 646-650. |
| 201. | Yonezawa A, Masuda S, Katsura T, Inui K. Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am J Physiol Cell Physiol. 2008;295:C632-C641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 202. | Yamamoto S, Inoue K, Ohta KY, Fukatsu R, Maeda JY, Yoshida Y, Yuasa H. Identification and functional characterization of rat riboflavin transporter 2. J Biochem. 2009;145:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 203. | Yao Y, Yonezawa A, Yoshimatsu H, Masuda S, Katsura T, Inui K. Identification and comparative functional characterization of a new human riboflavin transporter hRFT3 expressed in the brain. J Nutr. 2010;140:1220-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 204. | Kansara V, Pal D, Jain R, Mitra AK. Identification and functional characterization of riboflavin transporter in human-derived retinoblastoma cell line (Y-79): mechanisms of cellular uptake and translocation. J Ocul Pharmacol Ther. 2005;21:275-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 205. | Yao Y, Yonezawa A, Yoshimatsu H, Masuda S, Katsura T, Inui K. Identification and comparative functional characterization of a new human riboflavin transporter hRFT3 expressed in the brain. J Nutr. 2010;140:1220-1226. [PubMed] |
| 206. | Subramanian VS, Rapp L, Marchant JS, Said HM. Role of cysteine residues in cell surface expression of the human riboflavin transporter-2 (hRFT2) in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2011;301:G100-G109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 207. | Subramanian VS, Subramanya SB, Rapp L, Marchant JS, Ma TY, Said HM. Differential expression of human riboflavin transporters -1, -2, and -3 in polarized epithelia: a key role for hRFT-2 in intestinal riboflavin uptake. Biochim Biophys Acta. 2011;1808:3016-3021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 208. | Bosch AM, Abeling NG, Ijlst L, Knoester H, van der Pol WL, Stroomer AE, Wanders RJ, Visser G, Wijburg FA, Duran M. Brown-Vialetto-Van Laere and Fazio Londe syndrome is associated with a riboflavin transporter defect mimicking mild MADD: a new inborn error of metabolism with potential treatment. J Inherit Metab Dis. 2011;34:159-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 209. | Ho G, Yonezawa A, Masuda S, Inui K, Sim KG, Carpenter K, Olsen RK, Mitchell JJ, Rhead WJ, Peters G. Maternal riboflavin deficiency, resulting in transient neonatal-onset glutaric aciduria Type 2, is caused by a microdeletion in the riboflavin transporter gene GPR172B. Hum Mutat. 2011;32:E1976-E1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 210. | Spitzner A, Perzlmaier AF, Geillinger KE, Reihl P, Stolz J. The proline-dependent transcription factor Put3 regulates the expression of the riboflavin transporter MCH5 in Saccharomyces cerevisiae. Genetics. 2008;180:2007-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 211. | Vogl C, Grill S, Schilling O, Stülke J, Mack M, Stolz J. Characterization of riboflavin (vitamin B2) transport proteins from Bacillus subtilis and Corynebacterium glutamicum. J Bacteriol. 2007;189:7367-7375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 212. | Zhang P, Wang J, Shi Y. Structure and mechanism of the S component of a bacterial ECF transporter. Nature. 2010;468:717-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 213. | Cooperstein DF. Amino acid transport by corneal epithelial cells from the toad, Bufo marinus. Comp Biochem Physiol A Comp Physiol. 1985;81:427-430. [PubMed] |
| 214. | Liaw J, Rojanasakul Y, Robinson JR. The effect of drug charge type and charge density on corneal transport. Int J Pharm. 1992;88:111-124. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 215. | Takahashi H, Kaminski AE, Zieske JD. Glucose transporter 1 expression is enhanced during corneal epithelial wound repair. Exp Eye Res. 1996;63:649-659. [PubMed] |
| 216. | Majumdar S, Gunda S, Mitra A. Functional expression of a sodium dependent nucleoside transporter on rabbit cornea: Role in corneal permeation of acyclovir and idoxuridine. Curr Eye Res. 2003;26:175-183. [PubMed] |
| 217. | Turner HC, Alvarez LJ, Candia OA. Identification and localization of acid-base transporters in the conjunctival epithelium. Exp Eye Res. 2001;72:519-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 218. | Hosoya K, Horibe Y, Kim KJ, Lee VH. Na(+)-dependent L-arginine transport in the pigmented rabbit conjunctiva. Exp Eye Res. 1997;65:547-553. [PubMed] |
| 219. | Horibe Y, Hosoya K, Kim KJ, Lee VH. Kinetic evidence for Na(+)-glucose co-transport in the pigmented rabbit conjunctiva. Curr Eye Res. 1997;16:1050-1055. [PubMed] |
| 220. | Sun L. Dipeptide Transport Mechanism in the Pigmented Rabbit Conjunctiva. Los Angeles: University of Southern California 1996; . |
| 221. | Horibe Y, Hosoya K, Kim KJ, Lee VH. Carrier-mediated transport of monocarboxylate drugs in the pigmented rabbit conjunctiva. Invest Ophthalmol Vis Sci. 1998;39:1436-1443. [PubMed] |
| 222. | Kinsey VE. Amino acid transport in the lens. Invest Ophthalmol. 1965;4:691-699. [PubMed] |
| 223. | DiMattio J. Active transport of ascorbic acid into lens epithelium of the rat. Exp Eye Res. 1989;49:873-885. [PubMed] |
| 224. | Merriman-Smith R, Donaldson P, Kistler J. Differential expression of facilitative glucose transporters GLUT1 and GLUT3 in the lens. Invest Ophthalmol Vis Sci. 1999;40:3224-3230. [PubMed] |
| 225. | Kannan R, Yi JR, Zlokovic BV, Kaplowitz N. Molecular characterization of a reduced glutathione transporter in the lens. Invest Ophthalmol Vis Sci. 1995;36:1785-1792. [PubMed] |
| 226. | Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H. Ultracytochemical localization of the erythrocyte/HepG2-type glucose transporter (GLUT1) in the ciliary body and iris of the rat eye. Invest Ophthalmol Vis Sci. 1991;32:1659-1666. [PubMed] |
| 227. | Williams EF, Chu TC, Socci RR, Brown LG, Walker CE, Manor EL. Comparison of nucleoside transport binding sites in rabbit iris-ciliary body and cultured rabbit nonpigmented ciliary epithelial cells. J Ocul Pharmacol Ther. 1996;12:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 228. | Tomi M, Kitade N, Hirose S, Yokota N, Akanuma S, Tachikawa M, Hosoya K. Cationic amino acid transporter 1-mediated L-arginine transport at the inner blood-retinal barrier. J Neurochem. 2009;111:716-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 229. | Tomi M, Mori M, Tachikawa M, Katayama K, Terasaki T, Hosoya K. L-type amino acid transporter 1-mediated L-leucine transport at the inner blood-retinal barrier. Invest Ophthalmol Vis Sci. 2005;46:2522-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 230. | Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H. Ultracytochemical localization of the erythrocyte/HepG2-type glucose transporter (GLUT1) in cells of the blood-retinal barrier in the rat. Invest Ophthalmol Vis Sci. 1992;33:377-383. [PubMed] |
| 231. | Hosoya K, Kondo T, Tomi M, Takanaga H, Ohtsuki S, Terasaki T. MCT1-mediated transport of L-lactic acid at the inner blood-retinal barrier: a possible route for delivery of monocarboxylic acid drugs to the retina. Pharm Res. 2001;18:1669-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 232. | Yoon H, Fanelli A, Grollman EF, Philp NJ. Identification of a unique monocarboxylate transporter (MCT3) in retinal pigment epithelium. Biochem Biophys Res Commun. 1997;234:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 233. | Williams EF, Ezeonu I, Dutt K. Nucleoside transport sites in a cultured human retinal cell line established by SV-40 T antigen gene. Curr Eye Res. 1994;13:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |