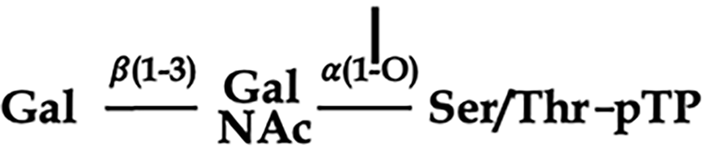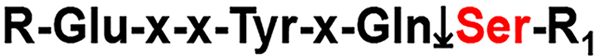Published online Nov 29, 2024. doi: 10.5497/wjp.v13.i2.97723
Revised: October 3, 2024
Accepted: November 12, 2024
Published online: November 29, 2024
Processing time: 168 Days and 6.6 Hours
Adenoviruses pose a serious health risk particularly in the absence of any clinically approved treatment. As adenoviral infections are quite frequent and recent outbreaks manifest more virulent variant strains, the need to develop an effective treatment remains a priority. The adenoviral protein, preterminal protein (pTP), is one of the key common products of the viral lifecycle as it is necessary to initiate viral replication and hence the infection process. This makes pTP a potential chemotherapeutic target in the search for and development of an effective treatment for adenoviral induced infections. Here we report, for the first time, that glycosylation of pTP in situ prevents binding to ssDNA in vitro.
To explore whether specific structural tailoring of the adenoviral protein pTP, imparts the potential to scupper the viral replication process.
All chemicals used were of reagent grade. Overexpression of pTP was achieved using the ‘BAC to BAC’ expression system. The presence and relative concentration of the protein was determined throughout the incubation period by the Bradford assay. The pTP was identified by MALDI-TOFF and sodium dodecyl sulphate polyacrylamide gel electrophoresis. For the removal of the aminosugar, a deglycosylase enzyme kit from PROZYME was used. Purification of cloned pTP (6xHis) was done with a ssDNA cellulose column followed by a Ni-NTA column. His-tags were excised with the Tobacco etch virus protease. Protein fractionation was performed with a fraction collector coupled to a UV detector (280 nm) from Pharmacia.
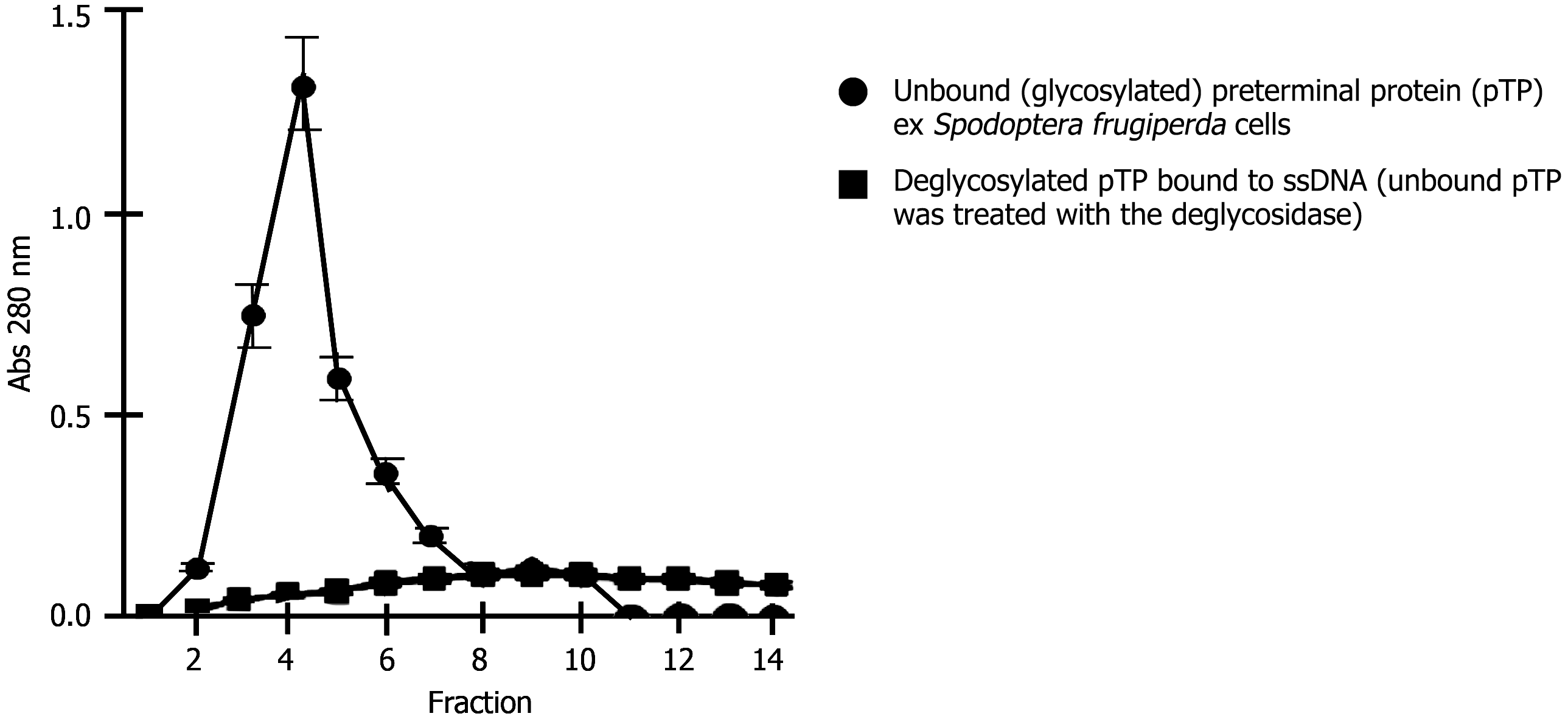
The pTP overexpressed in insect cells (Spodoptera frugiperda) (> 96 hours), is unable to bind to ssDNA in vitro. Treatment of this unbound protein with a deglycosidase enzyme that is specific for the removal of truncated unsubstituted O-linked Galβ(1-3)GalNAc-α1 disaccharides bound to Thr or Ser in a glycoprotein, restores binding to ssDNA. Data is presented as a linegraph for both the glycosylated and the deglycosylated proteins. Each point represents the mean of triplicate experiments (from different batches). Means and standard deviation were calculated and plotted on a line graph (with error bars).
The finding that glycosylation of cloned pTP in situ prevents binding to ssDNA in vitro could aid in the deve
Core Tip: A key objective in antiviral research is to recognize appropriate targets within the viral life cycle that are inde
- Citation: Walsh HA. Preterminal protein, the achilles heel of adenoviridae: Implications for adenoviral infections. World J Pharmacol 2024; 13(2): 97723
- URL: https://www.wjgnet.com/2220-3192/full/v13/i2/97723.htm
- DOI: https://dx.doi.org/10.5497/wjp.v13.i2.97723
Human adenoviruses (HAd), serotypes A to F, represent pervasive and clinically significant human pathogens that are responsible for many respiratory diseases (common cold, pharyngitis, and pneumonia), tonsillitis, cystitis, conjunctivitis (pink eyes) and gastroenteritis. They also infect the urinary tract, lymph system and heart. These infections (serotype dependent) can range from mild to severe and in the case of respiratory infections (they infect the mucous membranes), affect individuals with typical flu-like symptoms such as coughing, wheezing, fever and runny nose[1-3].
Characteristically, human adenoviral infections are unresponsive to most medications currently available on the market and treatment is mainly supportive and focused on relieving the symptoms. Whilst HAd infections are largely subclinical in the immunocompetent, there is significant morbidity and mortality linked to HAd infections in immunocompromised patients [cancer, organ transplants and human immunodeficiency virus (HIV) infected individuals], pediatrics, individuals from overpopulated areas and underdeveloped communities. Suffice it to say that the availability of effective antiviral therapies would have significant clinical benefits for humans. To date there is no Food and Drug Administration (FDA) approved antiviral for the treatment of HAd infections and attempts at overcoming this hurdle have met with limited success thus far, in the search for an effective therapeutic approach. The broad-spectrum anti-viral, Cidofovir, a nucleoside analogue, clinically approved by the FDA for treating cytomegalovirus induced retinitis in Acquired Immune Deficiency Syndrome (AIDS) patients, are efficacious against HAd serotypes tested in clinical trials but with varying results including reports of nephrotoxicity[1,3,4]. The phospholipid conjugate of Cidofovir, Brindofovir, has been shown, in phase 1 clinical trials, to have increased oral bio-availability and an improved side effect profile (reduced levels in kidney) when compared to the parent molecule. Disappointingly, phase 2 clinical trials were statistically no different to the placebo[5].
Alternative therapeutic approaches such as immunotherapy are promising but are labour intensive, not cost effective and limited with respect to efficacy against the different HAd strains. Cidofovir and intravenous immunoglobulin have been used clinically as a combination therapy to treat adenoviral hepatitis in a liver transplant patient with some success[6].
However, it remains a major challenge to develop antivirals and other treatment regimens that are effective against a range of HAd serotypes. It stands to reason that knowledge of key aspects of the life cycle of adenoviridae could prove invaluable in facilitating development of novel HAdenoviral therapies with high specificity and/or efficacy[7]. A specific area of the adenoviral infectious process that has been receiving a lot of attention over the years in pursuit of this elusive goal, is the adenoviral DNA replication cycle. The present study presents a brief overview of the relevant salient features of the adenoviral DNA replication cycle and the pertinent experimental procedures (and results) involved in acquiring preterminal protein (pTP), our molecule of interest. It concludes with a discussion on the findings and potential impact a specific post-translational modification, such as glycosylation, would have on the in vivo DNA binding properties of pTP and ultimately on the viral DNA infection process.
Here we present an abridged version of the adenoviral DNA replication process, emphasising the pivotal role played by the viral polypeptide, pTP.
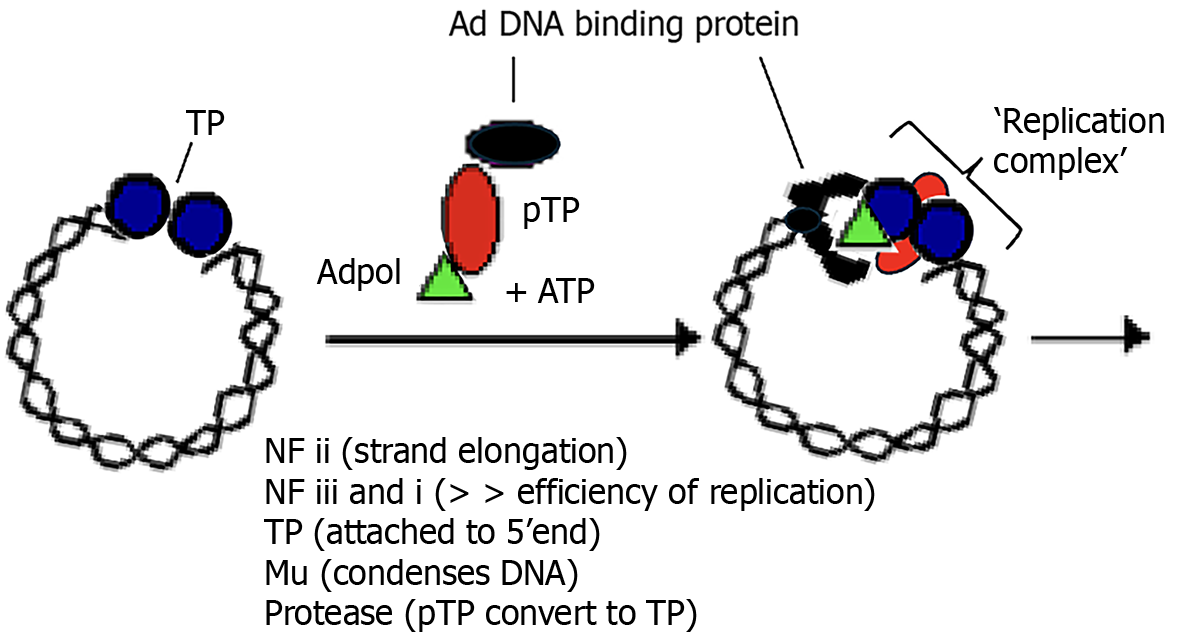
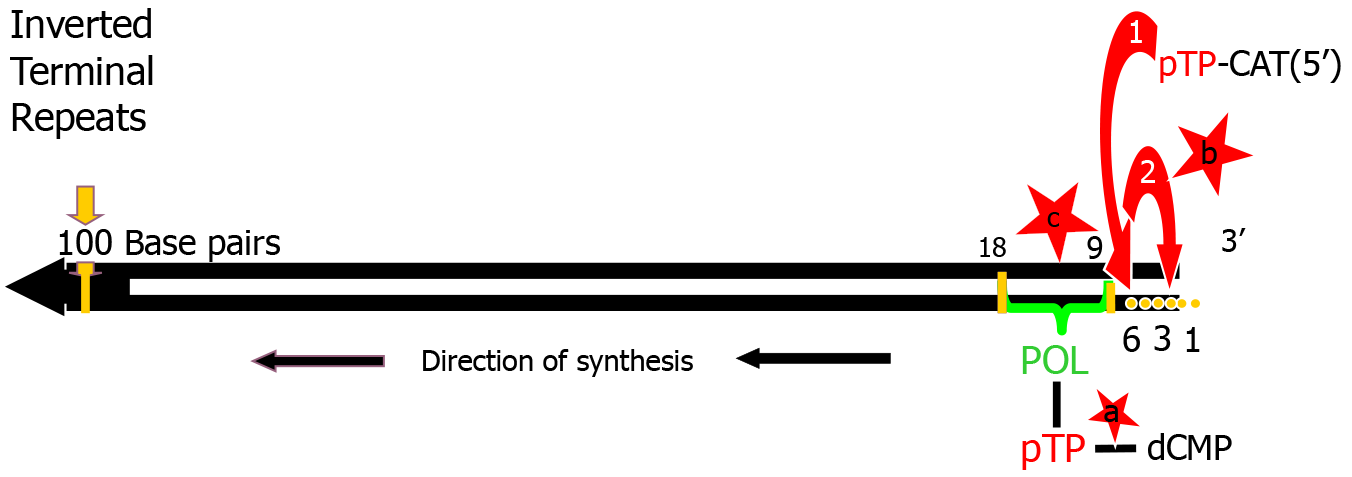
Adenoviral replication machinery: The HAd genome is a linear double-stranded DNA molecule of approximately 25-36 kilo-base pairs with inverted terminal repeats (ITRs) [e.g. adenoviruses (Ad)5 = 103, Ad2 = 102] (Figure 1). Ad replication origins[2] are located at the ends of the genome within the ITRs. Terminal protein (TP) is covalently attached to the 5’ end of the mature virion. This 55 kDa polypeptide is cleaved from a 80 kDa precursor, pTP, by an adenoviral protease at the end of the replication cycle[7]. TP protects the viral genome from nuclease degradation. TP-DNA, in cell culture, has also been shown to have a higher infectivity titre than the ‘naked’ viral genome[7].
The C-terminal end of precursor pTP forms a duplex with dCMP, that serves as a primer for initiation of viral repli
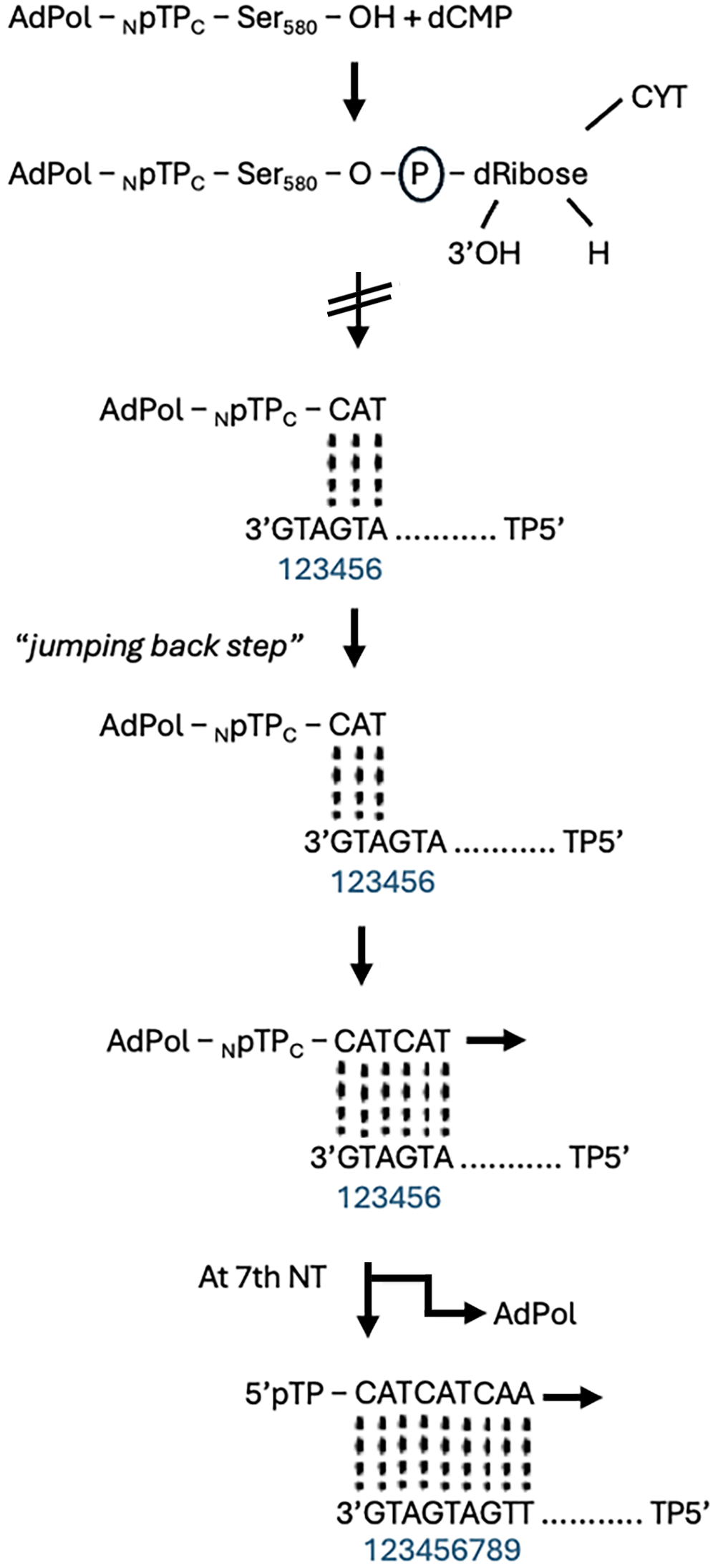
Mechanism of replication: There is general consensus that Ad deploy a protein priming mechanism to activate DNA replication (Figure 2). The aim of this study is to explore whether the removal of specific endogenously added amino sugar residues from cloned viral pTP, expressed in insect Spodoptera frugiperda (Sf-9) cells, restores binding to single stranded DNA in vitro[11-13]. The polypeptide, pTP, is a key component of the infection process and is covalently bound via the hydroxyl (OH) group of a specific Serine580 residue, to the α phosphoryl group of the terminal dCMP of the fledgling nascent strand to form the heterodimer primer, Adpol-NpTPC-dCMP, a reaction catalysed by Adpol[14]. The 3’OH of dRibose of this primer serves as a starting point for the synthesis of the nascent strand.
The linking of the second nucleotide adenosine monophosphate and third thiamine monophosphate, is also catalysed by Adpol[14-16]. Base pairing with the 2nd 3’G4T5A6 (at positions 4, 5, and 6) trinucleotide on the template strand guides the synthesis of the first CAT trinucleotide. This CAT triplet then jumps back to positions 1, 2, and 3 on the template strand, base pairing with the 3’G1T2A3 trinucleotide[16]. The synthesis of the 2nd CAT triplet of the viral nascent strand by Adpol, then proceeds through the 2nd 3’G4T5A6 (TP) triplet. By the time the 7th nucleotide of the nascent strand is synthesised, Adpol has completely separated from pTP[16,17], a separation process which began at the synthesis of the 1st trinucleotide. Displacement of the extant complementary strand by the replication fork, from the template strand, occurs as Adpol continues to process the nascent strand[14,18].
Figure 2 is a schematic representation of the initiation of viral DNA replication that illustrates how the 4-6bp template G4T5A6 trinucleotide guides the synthesis of the 5’pTPC-CAT trinucleotide which then jumps/slides back to pair with the template 3’G1T2A3 at positions 1, 2 and 3, after which chain elongation by AdPol proceeds[19-23].
For a more detailed account of the preparatory materials and protocols used, refer to Addendum A.
Cloned pTP (6xHis-tag), recombinant baculovirus, Sf-9 insect cells (as described previously[9,10]), MALDI–TOF MS (ABSciex 4800), ssDNA cellulose column, nickel column, peristaltic pump and fraction collector (Pharmacia), Bradford reagent, Invitrogen NuPAGE precast gels and arecombinant endo-O-deglycosidase kit[24]. All chemicals were of reagent grade and purchased from Sigma-Aldrich, unless otherwise indicated. GlycoProTM endo-O-glycosidase [recombinant from Streptococcus pneumonia (S.pneumonia)] obtained from PROZYME Inc., (Addendum C).
Adenoviral replication provides a convenient alternative model to study human DNA replication in addition to providing insight into key areas of the adenoviral life cycle. An absolute requirement for such an investigation is the need for homogeneous protein of sufficient quantities. Fulfilling this criteria for pTP, our protein of interest, involved cloning and overexpression of the viral polypeptide in Sf-9 insect cells using the ‘BAC to BAC’ expression system, as described previously[9,10] followed by extraction and purification. Infected cells were harvested after 96 hours and collected by centrifugation, washed twice with phosphate-buffered saline (10 mL) and stored at -70 °C until required. The purification of pTP was performed using a ssDNA cellulose column (regions within the N-terminus are known to bind to HAd DNA) followed by a Ni-NTA (binds histidine with high affinity) column as described previously[10]. The presence and concentration of protein were determined throughout the incubation period using the Bradford assay. During the purification, pTP was identified by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) for both column (ssDNA and Ni-NTA) steps.
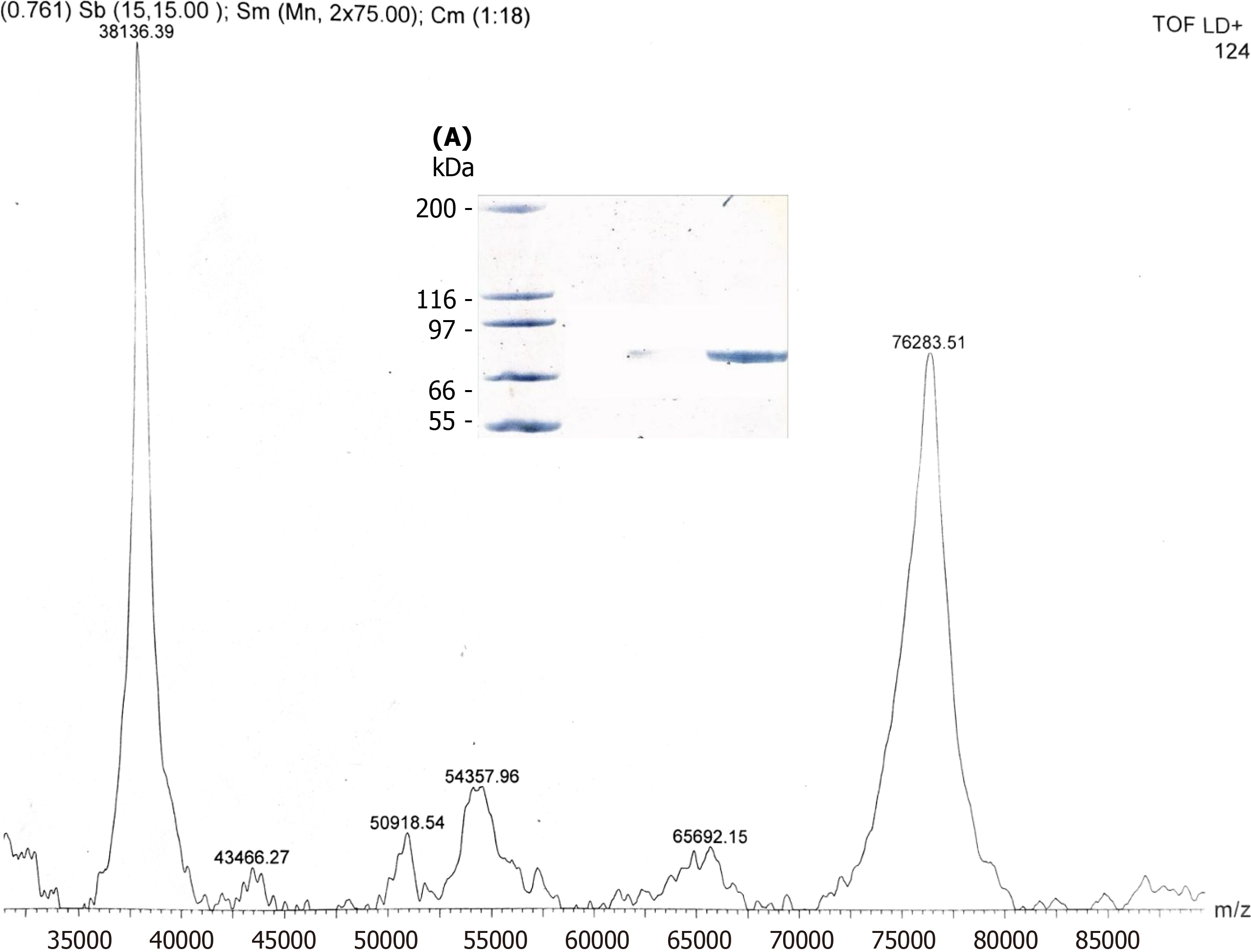
Optimal production of ‘active’ pTP by Sf-9 occurred at 72 hours of incubation. The overall yield of ‘active’ recombinant pTP becomes progressively less beyond this point. At 96 hours, the inactive (inability to bind ssDNA) portion is nearly 95% of the total rec-pTP and appears in the flow through as most of the protein did not bind to the DNA column. When this unbound pTP was passed through the Ni-NTA column there was nearly complete retention > 95% (graph not shown), confirming that the ssDNA flow through protein was indeed the cloned his-tagged pTP. Repeating the DNA binding step after elution with imidazole from the Ni-NTA column (this was done twice and with different batches) again resulted in no adherence to the ssDNA column. The identity of ‘unbound’ pTP from these purification steps was also confirmed by MALDI-TOFF MS and SDS-PAGE in every instance (Figure 3).
Suspecting that the protein was post-translationally modified by the host, it was decided to test whether the viral polypeptide was glycosylated. A recombinant endo-O-deglycosidase kit was purchased and unbound fractions pooled and treated with the enzyme from S.pneumonia (Figure 4). The protocol supplied was used with minor modifications[24]. This enzyme is specific for unsubstituted Galβ(1-3)GalNAc-α1 disaccharides O-linked to Ser or Thr residues of glyco
Results depict that the binding activity of unbound pTP can be successfully restored upon treatment with the deglycosidase (Figure 5).
It would thus appear that pTP is post translationally modified by the insect cells through glycosylation with Gal(β1-3)GalNAc-α1. This fact is not surprising given that the Sf-9 insect cell system is chosen as an expression system for recombinant human proteins precisely because of the presence of similar post translational modification systems such as glycosylation and phosphorylation. Gal(β1-3)GalNAc-α1, also known as the Thomsen-Friedenreich antigen (TF-antigen), is a tumour associated carbohydrate antigen (TACA) and known recognition marker associated with a number of metastasizing organotropic cancers (e.g. prostate cancer) and infections caused by some bacteria and viruses (including HAd) across the eukaryotic kingdom. This molecule serves an important role as mediator of cellular communication between affected cells in such instances[25].
Though it has been shown that aberrant glycosylation can promote metastasis and invasiveness in some human cancers[25], TACAs do also have roles that are beneficial to the host (not only in cancers) as is shown here. The fact that Sf-9 cells link the TF-antigen to a toxic viral protein to prevent it from binding to its genome, emphasises the importance this molecule plays in the natural defense systems of many living organisms. Potentially glycosylation could also account for the extremely low yields (± 15%) of ‘free’ pTP [even after increasing the Tobacco etch virus (TEV) protease incubation period from 4 hours to 8 hours] for the His-tag removal reaction. Yields for both 72 hours and 96 hours incubations were consistently below 20%. The TEV protease cleavage site is identified as having a serine residue[26]. It is possible that this Ser residue at the cleavage site is glycosylated and would consequently impede catalysis (Figure 6).
Based on these findings it appears that His-tagged recombinant proteins, incorporating a Ser residue at the active site, are best not expressed in Sf-9 cells.
Selecting glycosylation as a strategy to modify the structure of pTP has a number of potential benefits for the invaded host: It allows for identification (tagging) of pTP. The protein is in this way targeted for destruction. It also maintains pTP in a soluble state so as to prevent precipitation (most likely due to protein-protein interactions) at relatively high concentrations (deglycosylation resulted in precipitation in vitro) which would cause cell death. The protein is also prevented from binding to host and viral DNA, a host cell directed counter activity that is beneficial to the ‘host’.
Traversing the nuclear envelope in moving from the rough endoplasmic reticulum (RER) to the nucleus is bound to be hampered or prevented. The natural product, rotenone, has recently been shown, using plaque assays, to significantly reduce adenoviral replication (IC50 = 3.9 nM). This drug acts by preventing movement of the virion from the endosomes to the nucleus[27].
Glycosylation thus appears to be a specific response to an event that threatens the continued existence of the insect cell host and as such can be viewed as a natural survival strategy. It is thus entirely feasible that glycosylation, as a defence mechanism to invasion by HAd in vivo, could effectively combat the viral onslaught by targeting three susceptible areas for direct inhibition of viral replication.
The meaning represented by ‘a’ in Figure 7: Undermining the protein priming mechanism through glycosylation of serine580 and as a consequence inhibit formation of the pTPC-dCMP duplex primer (pTP-Ser-OH+dCMP) that is so vital for viral replication and propagation.
The meaning represented by ‘b’ in Figure 7: Causing steric hindrance at the ‘jumping back’ step of the pTP-CAT trinucleotide given that a highly regulated interaction between NpTPC and vDNA would be an absolute requirement for correct positioning of template (TP-GTA) and nascent (pTP-CAT) strand base pairs.
The meaning represented by ‘c’ in Figure 7: Preventing the association between pTPN and adenoviral DNA at the ORI (bp 9-18) on the template strand during the formation of the pre-initiation complex.
The results of this study provide evidence that an in vivo glycosylated pTP has differential affinity for ssDNA (in vitro). Furthermore, by implication, translocation from the site of synthesis in the cytosol (RER) into the nucleus is likely to be hindered. It will be these factors which could ensure that viral replication does not progress (in vivo). Nonetheless, a glycosylated pTP (there are other potential Ser and Thr glycosylation sites on pTP[28]) will have a negative impact on base pairing between template, TP-GTA and newly synthesised nascent pTP-CAT. Interaction of Adpol with viral DNA (also with pTPN) is required for vDNA replication, particularly at initiation[14]. It thus stands to reason that the stability and alignment of the interaction between pTP and AdPol, if altered by an event such as glycosylation, are likely to have a detrimental effect on initiation and progression of vDNA replication. This will likely reduce the efficiency of Adpol-catalysed viral DNA synthesis and/or result in abortive virion assembly, preventing viral propagation.
Agents that control the activities of glycosidases have been found to have therapeutic effects not only against many carbohydrate-related diseases but as shown, potentially also against adenoviral infections. In fact, glycosidase inhibitors have been used chemotherapeutically, in a clinical setting, for a few decades already, to treat a range of unrelated diseases such as diabetes, influenza, HIV infections, tumor metastasis, and glycosyl sphingolipid storage diseases[29,30].
The fact that adenoviral infections are generally localised, facilitates tissue specific targeting in the form of a reaction cocktail, constituted of ingredients that would include the endo-O-glycopeptide, in addition to agents that would inhibit ‘on site’ glycosidases. It follows then that a potential regimen to treat, for example, HAd induced conjunctivitis, could include a mixture of molecules as part of an eye drop formulation (localised chemotherapy) consisting of: (1) N-Acetylgalactosaminyltransferase (to add the sugar to pTP)[31]; (2) The substrate Gal(β1-3)GalNAc-α1[30] (can also be used in isolation); (3) An endo-O-glycosidase inhibitor such as galactostatin or deoxynojirimycin to prevent removal of the sugar from pTP[29]; and (4) Cidofovir (a deoxycytidine analogue) to inhibit the viral polymerase AdPol[7].
Inhibition of viral glycosidases and/or stimulation of glycosyl transferases may be viewed as complementary to other antiviral therapeutic strategies (adjunctive), bearing in mind that different viruses require different treatment regimes. One only has to consider the chemotherapeutic approach used in the treatment of HIV/AIDS (e.g. dolutegravir the integrase inhibitor), to appreciate the feasibility of such an approach.
The finding that glycosylation by Sf-9 cells of cloned pTP in situ prevents binding to ssDNA in vitro could thus aid in the development of an effective treatment, in isolation or as an adjunct together with another anti-viral therapy, for adenoviral infections. Glycosylation could potentially be afforded multiple roles in the preservation of a living organism. In this particular instance, a known TACA, Gal(β1-3)GalNAc-α1, the TF-antigen, is harnessed by Sf-9 larval cells as an integral part of a natural survival strategy to safeguard against the toxic effects of pTP. Firstly, the 3-dimensional structure of pTP is altered, which impedes subsequent binding to host DNA. Secondly, this would most likely also hamper movement across the nuclear envelope from the site of synthesis on the rough endoplasmic reticulum. Thirdly, the fact that Sf-9 cells tag an alien protein, such as pTP, with a known ubiquitous mammalian recognition element[27] provides corroborative evidence for the exploitation of the TF-antigen as a target in the development of an antigen-specific therapeutic vaccine. This could be directed at a range of organotropic cancers and other pertinent viral and bacterial infections. It is envisioned that our laboratory, in the near future, will pursue the testing of various formulations of the cocktail ingredients (as outlined above), on virus induced localised infections, such as keratoconjunctivitis, in animal models such as the rat.
| 1. | Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev. 2014;27:441-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 587] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 2. | American Academy of Pediatrics Adenovirus infections. 26th edition of the Red Book: Report of the Committee on Infectious Diseases. Elk Grove Village: American Academy of Pediatrics, 2003: 190-192. |
| 3. | MacNeil KM, Dodge MJ, Evans AM, Tessier TM, Weinberg JB, Mymryk JS. Adenoviruses in medicine: innocuous pathogen, predator, or partner. Trends Mol Med. 2023;29:4-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | Kinchington PR, Romanowski EG, Jerold Gordon Y. Prospects for adenovirus antivirals. J Antimicrob Chemother. 2005;55:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Li L, Xie Z, Xu L. Current antiviral agents against human adenoviruses associated with respiratory infections. Front Pediatr. 2024;12:1456250. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Haq A, Gregston A, Elwir S, Spak CW. Treatment of Viral Hepatitis Due to Adenovirus in a Liver Transplantation Recipient: The Clinical Use of Cidofovir and Intravenous Immunoglobulin. Liver Transpl. 2022;28:505-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Van der Vliet, PC, Hoeben, RC. Adenovirus. In: De Pamphilis ML. DNA replication and human disease. New York: Cold Spring Harbor Laboratory Press, 2006: 645-661. |
| 8. | Doerfler W, Böhm P. The Molecular Repertoire of Adenoviruses II. Current Topics in Microbiology and Immunology. Berlin: Springer, 1995. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Webster A, Leith IR, Hay RT. Domain organization of the adenovirus preterminal protein. J Virol. 1997;71:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Liu H, Naismith JH, Hay RT. Identification of conserved residues contributing to the activities of adenovirus DNA polymerase. J Virol. 2000;74:11681-11689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Pronk R, Stuiver MH, van der Vliet PC. Adenovirus DNA replication: the function of the covalently bound terminal protein. Chromosoma. 1992;102:S39-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | De Jong RN, Meijer LA, van der Vliet PC. DNA binding properties of the adenovirus DNA replication priming protein pTP. Nucleic Acids Res. 2003;31:3274-3286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | De Jong RN, van der Vliet PC, Brenkman AB. Adenovirus DNA replication: Protein priming, jumping back and the role of the DNA binding protein DBP. Adenoviruses: Model and vectors in virus-host interactions. Berlin: Springer, 2003: 272. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Lichy JH, Field J, Horwitz MS, Hurwitz J. Separation of the adenovirus terminal protein precursor from its associated DNA polymerase: role of both proteins in the initiation of adenovirus DNA replication. Proc Natl Acad Sci USA. 1982;79:5225-5229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Kulanayake S, Tikoo SK. Adenovirus Core Proteins: Structure and Function. Viruses. 2021;13:388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Hoeben RC, Uil TG. Adenovirus DNA replication. Cold Spring Harb Perspect Biol. 2013;5:a013003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Lichy JH, Horwitz MS, Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci USA. 1981;78:2678-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 113] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Bosher J, Robinson EC, Hay RT. Interactions between the adenovirus type 2 DNA polymerase and the DNA binding domain of nuclear factor I. New Biol. 1990;2:1083-1090. [PubMed] |
| 19. | Challberg MD, Kelly TJ Jr. Adenovirus DNA replication in vitro. Proc Natl Acad Sci USA. 1979;76:655-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 204] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | King AJ, van der Vliet PC. A precursor terminal protein-trinucleotide intermediate during initiation of adenovirus DNA replication: regeneration of molecular ends in vitro by a jumping back mechanism. EMBO J. 1994;13:5786-5792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | King AJ, Teertstra WR, van der Vliet PC. Dissociation of the protein primer and DNA polymerase after initiation of adenovirus DNA replication. J Biol Chem. 1997;272:24617-24623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | van Leeuwen HC, Rensen M, van der Vliet PC. The Oct-1 POU homeodomain stabilizes the adenovirus preinitiation complex via a direct interaction with the priming protein and is displaced when the replication fork passes. J Biol Chem. 1997;272:3398-3405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Temperley SM, Hay RT. Recognition of the adenovirus type 2 origin of DNA replication by the virally encoded DNA polymerase and preterminal proteins. EMBO J. 1992;11:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | ProZyme. O-GLYCANASE™ (Endo-Į-N-Acetylgalactosaminidase). Available from: https://www.agilent.com/cs/library/usermanuals/public/TDP-GK80090_O-Glycanase_Technical_Data_Sheet_022814AF.pdf. |
| 25. | Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. In: Wu AM. The Molecular Immunology of Complex Carbohydrates. Berlin: Springer, 2001: 369-402. [RCA] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 341] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 26. | Carrington JC, Dougherty WG. A viral cleavage site cassette: identification of amino acid sequences required for tobacco etch virus polyprotein processing. Proc Natl Acad Sci U S A. 1988;85:3391-3395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 154] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Balsera-Manzanero M, Ghirga F, Ruiz-Molina A, Mori M, Pachón J, Botta B, Cordero E, Quaglio D, Sánchez-Céspedes J. Inhibition of adenovirus transport from the endosome to the cell nucleus by rotenone. Front Pharmacol. 2023;14:1293296. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Swiss Institute of Bioinformatics, ExPASy, SWISS-PROT. Available from: https://www.expasy.org/resources/uniprotkb-swiss-prot. |
| 29. | Asano N. Glycosidase inhibitors: update and perspectives on practical use. Glycobiology. 2003;13:93R-104R. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 585] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 30. | Kajimoto T, Node M. Inhibitors against glycosidases as medicines. Curr Top Med Chem. 2009;9:13-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |