Published online Feb 17, 2023. doi: 10.5497/wjp.v12.i1.1
Peer-review started: September 16, 2022
First decision: October 31, 2022
Revised: November 11, 2022
Accepted: February 7, 2023
Article in press: February 7, 2023
Published online: February 17, 2023
Processing time: 153 Days and 18.3 Hours
Itraconazole is a broad-spectrum triazole antifungal inhibiting fungal growth by inhibiting ergosterol synthesis and exhibits a nonlinear pharmacokinetic profile. Erratic absorption pattern with wide fluctuations in blood levels causes incon
To compare the oral bioavailability and bioequivalence of Fixtral SB (supra bioavailable itraconazole) with reference product R2 (supra bioavailable 2 × 50 mg itraconazole).
The study population consisted of 54 healthy volunteers, aged between 18-45 years and randomized to receive a single oral dose of either test [T; Fixtral SB (supra bioavailable itraconazole) 100 mg] or reference product (R1; Sporanox 100 mg × 2 capsules and R2; Lozanoc capsules 50 mg × 2 capsules). Blood samples were taken pre-dose and post-dose up to 96 h. The study evaluated bioequivalence by comparing the oral bioavailability of the test product with reference product R2. The pharmacodynamic characteristics of the drug were evaluated by comparing the test product with reference product R1. Pharmacokinetics (PK)-PD comparative analysis [area under the concentration-time curve (AUC)/ minimum inhibitory concentration (MIC) > 25] was performed for conventional itraconazole 100 mg and supra bioavailable itraconazole 50 mg. Adverse events (AEs) assessments were performed in each study period and post-study evaluation.
Statistical analysis of primary PK variables revealed bioequivalence, with confidence intervals being completely inside the acceptance criteria of 80%-125%. The peak concentration levels of itraconazole were achieved at 10 h (T) and 8.5 h (R2), respectively. Pharmacodynamic parameter assessment showed that AUC/MIC for R1 are comparable to Fixtral SB 100mg for MIC levels up to 16mcg/mL (P > 0.05 and observed P = 0.3196). Six AEs were observed that were mild to moderate in severity and resolved. No severe AE was reported.
Test product itraconazole Capsule 100 mg is bioequivalent with the reference product (R2) at 100 mg dose (2 capsules of Lozanoc® 50 mg) under fed conditions. Pharmacodynamics activity in terms of AUC/MIC is comparable between the test product at 100 mg dose and marketed itraconazole 200 mg. Fixtral SB is expected to have therapeutically similar efficacy at half the equivalent dose. Tested formulations were found to be safe and well tolerated.
Core Tip: Itraconazole, a triazole antifungal with a broad spectrum, has a nonlinear pharmacokinetic profile due to its variable oral bioavailability. Based on these criteria, a comparison of Fixtral SB (supra bioavailable itraconazole) 100 mg with Lozanoc capsules 50mg administered as two capsules was performed, revealing that Fixtral SB is expected to have therapeutically comparable efficacy at half the equivalent dose. In terms of area under the concentration-time curve/minimum inhibitory concentration, the pharmacodynamic activity of the test product at 100 mg dose and the marketed itraconazole 200 mg is comparable. All of the formulations tested were found to be safe and well tolerated, with manageable side effects.
- Citation: Naqvi SMH, Gala MYN, Muchhala S, Arumugam A, Panigrahi D, Patil D, Rathod R, Mane A. Pharmacokinetics/Pharmacodynamics study of Fixtral SB as compared to supra bioavailable itraconazole and conventional itraconazole. World J Pharmacol 2023; 12(1): 1-11
- URL: https://www.wjgnet.com/2220-3192/full/v12/i1/1.htm
- DOI: https://dx.doi.org/10.5497/wjp.v12.i1.1
Itraconazole is a broad-spectrum triazole antifungal with favorable pharmacokinetic profile[1]. It is an effective and well tolerated medication for superficial and invasive systemic fungal infections, including aspergillus species. Anti-fungal efficacy of its active metabolite ‘hydroxyitraconazole is double to that of parent compound[1,2]. It inhibits the fungal growth by inhibiting ergosterol synthesis, a major component of fungal cell membrane by inhibiting the enzyme 14α sterol demethylase - a microsomal cytochrome P450 enzyme, responsible for catalytic conversion of lanosterol to ergosterol[3,4].
Itraconazole exhibits a nonlinear pharmacokinetic profile due to its varied oral bioavailability[5-7]. It has prolonged terminal clearance time, biphasic elimination with a terminal half-life of roughly 24 h and reaches a steady state in 14 days[4]. Its lipophilic and weakly basic nature renders it water-insoluble, representing a rate-limiting step in its absorption in the gastrointestinal tract[4,8]. Food has an impact on the bioavailability of itraconazole as maximum absolute bioavailability (55%) is achieved in fed state[8]. It is metabolized in the liver by CYP34A enzyme to its active metabolite hydroxyitraconazole which is 99% bound to plasma proteins[4]. Metabolites are excreted through bile and urine, only 3%-18% are detected in feces[1,2].
Several studies determining the effect of food on itraconazole absorption showed that it is highly varied between subjects, and the measured area under the concentration-time curve (AUC) values in the fed and fasted states have shown significant interstudy variability[9,10]. Erratic absorption pattern with wide fluctuations in blood levels causes inconsistent and unpredictable clinical behavior of this drug in spite of its low minimum inhibitory concentration (MIC) as compared to other antifungal agents[11]. Drug interactions leading to enhanced metabolism or reduced bioavailability can also result in reduced efficacy of itraconazole[12].
Itraconazole is widely used worldwide for systemic as well as local fungal infections with different dosage regimens depending on the site of infection. Dosages from 100 mg/day to 600 mg/day of conventional itraconazole are approved for oropharyngeal esophageal candidiasis, systematic aspergillosis, candidiasis, cryptococcosis, sporotrichosis, blastomycosis and other rarely occurring systemic or tropical mycosis[13].
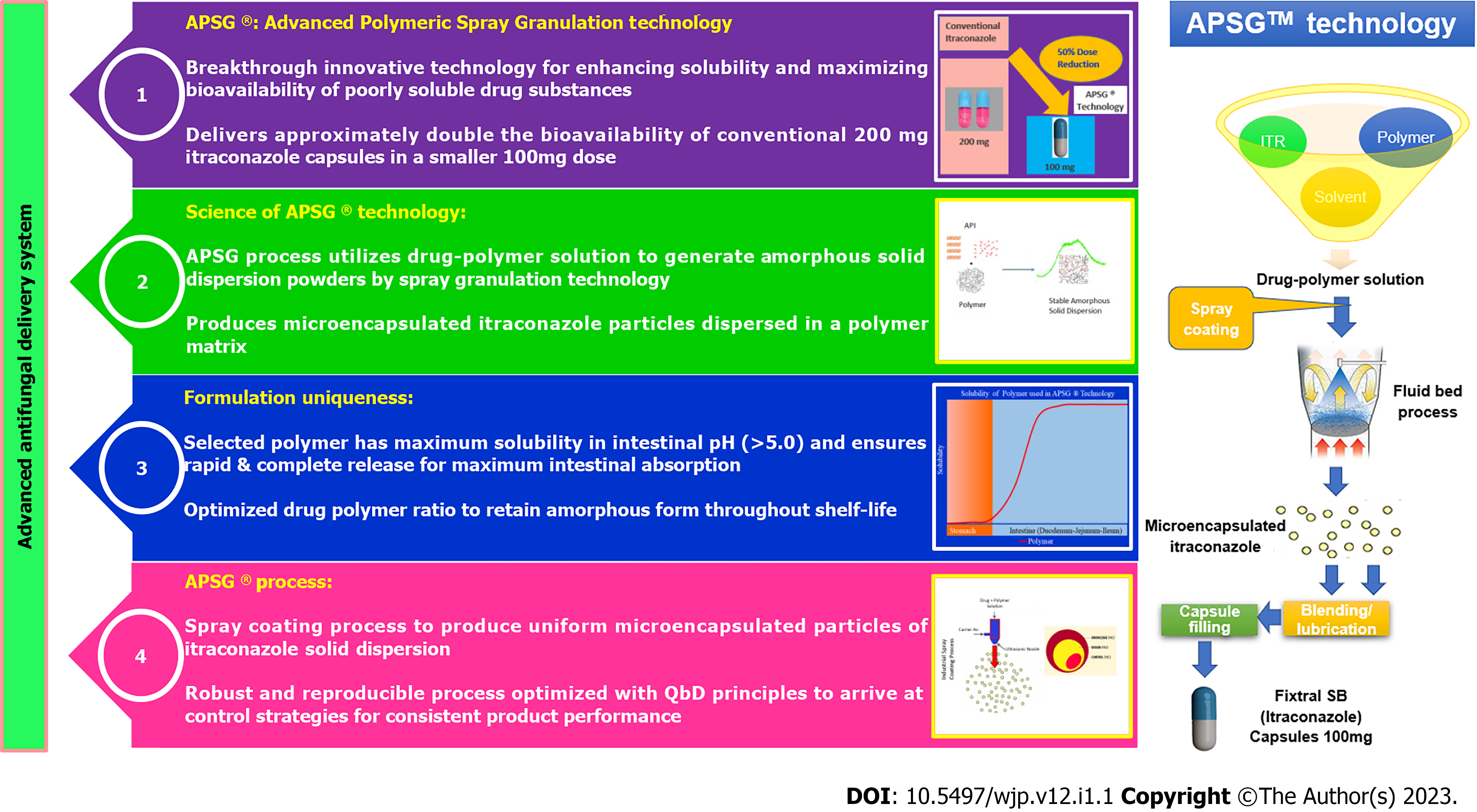
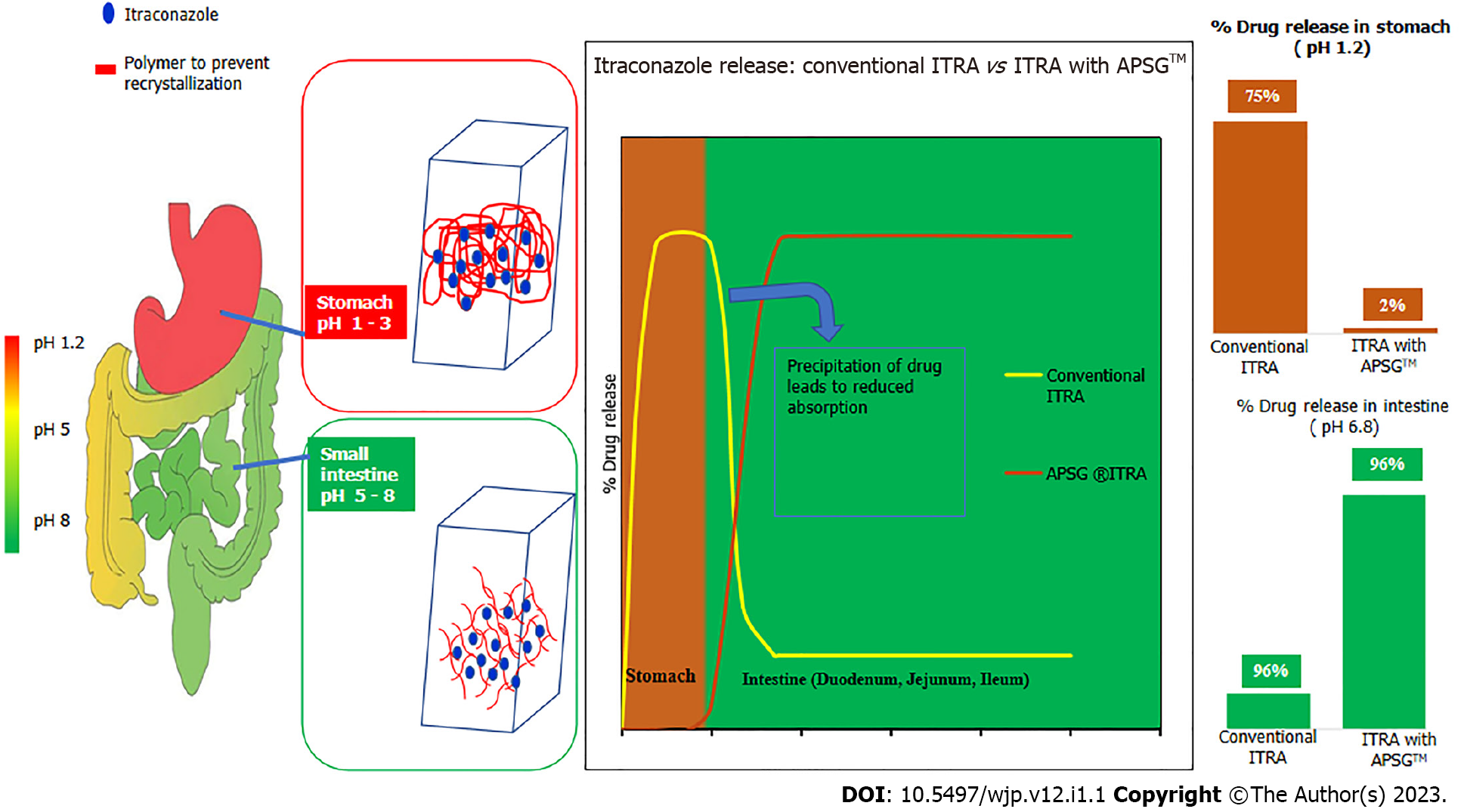
Test product Fixtral SB has been manufactured via APSG™ (Advanced Polymeric Spray Granulation), a breakthrough innovative proprietary technology for enhancing solubility and maximizing bioavailability of poorly soluble drug itraconazole as depicted in Figure 1 and Figure 2. Conventional itraconazole formulations show complete drug release in acidic pH (stomach) and minimum drug release in basic pH (Intestine). Though intestine is a good site of absorption, but as soon as itraconazole from conventional formulations enters into the small intestine, the change in pH causes the itraconazole to precipitate which was dissolved in stomach. Therefore, the change in pH due to gastric emptying and ingestion of food significantly affects the dissolution of itraconazole from conventional formulations and there by decreases bioavailability and increases variability in absorption. To overcome the limitations of conventional itraconazole formulations, APSG™ technology as depicted in Figure 1 and Figure 2 was adopted to produce amorphous solid dispersion of itraconazole by using an enteric polymer for enhancing the bioavailability. Enteric polymer is chosen over all other polymers owing to its optimum & rapid solubility in intestinal pH and ability to restrict the drug release in acidic environment which minimizes the precipitation potential of itraconazole in intestine thus maximizing bioavailability. The drug and polymer ratio has been optimized to get an optimum concentration of polymer in the formulation to provide a stable amorphous solid dispersion throughout the shelf life.
Based on this technology with improved bioavailability, itraconazole 50 mg is approved in Australia and India[4].
Pharmacokinetics (PK) parameters (Cmax, Tmax, AUC) demonstrate drug exposure in humans and pharmacodynamic parameters are derived using these conventional PK parameters. Various pharmacodynamic parameters are used to determine antifungal activity of drugs such as Cmax/MIC, T > MIC, AUC/MIC depending on concentration dependent or/and time dependent mechanism of action. For triazoles, AUC/MIC is an appropriate PD parameter to demonstrate antimicrobial activity as these have extensive post antifungal effect[14]. For efficacy with 50% maximal effect, AUC/MIC value around 25 is reported for triazole class[14].
In PK-PD comparative study of conventional itraconazole 100 mg and supra bioavailable itraconazole 50 mg, AUC/MIC > 25 was evaluated. Comparing AUC/MIC ratio for both treatment arms, it was proposed that conventional itraconaozle 100 mg is expected to have comparable efficacy at half dose i.e., 50 mg of the novel formulation and approved at half the dose[15]. Additionally target AUC/MIC 25-50 for optimal efficacy for triazole class of antifungal was reported[14,16,17].
The main objective of this study was to compare the oral bioavailability of Fixtral SB (supra bioavailable itraconazole) 100 mg (test product; Dr. Reddy’s Laboratories Limited) with Lozanoc capsules 50 mg administered as 2 capsules (reference product (R2; Mayne Pharma International pty Ltd), with equivalence for AUC parameter, in order to determine the bioequivalence of both the formulations. The secondary objective was to compare the pharmacodynamics/ characteristics of test product with Sporanox 100 mg × 2 capsules (reference product (R1; Johnson & Johnson Private Limited) and lastly to monitor the safety and tolerability of the study drugs.
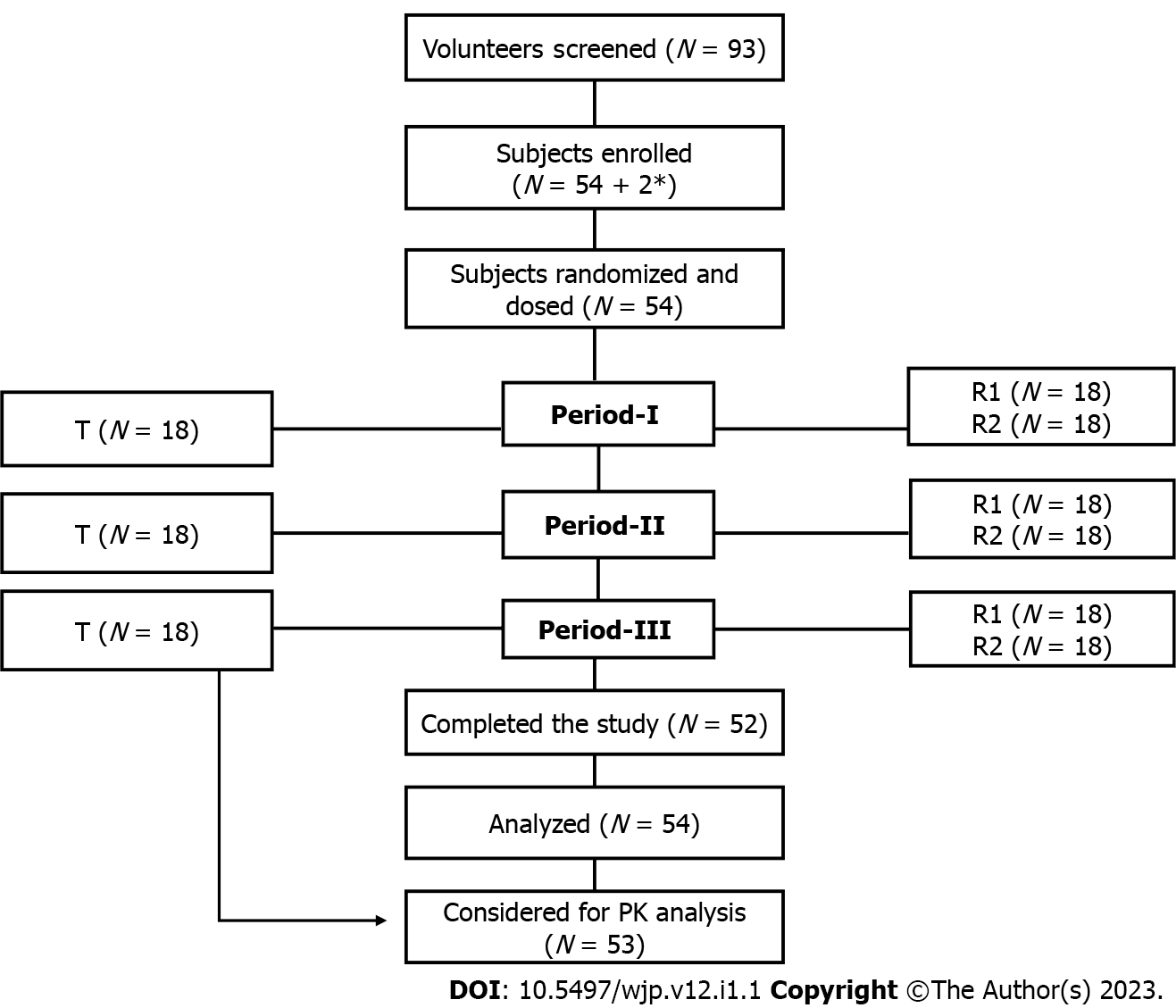
This was a single dose, randomized, three treatments, three period, three sequence, open label, balanced, crossover, oral comparative bioavailability study conducted at Accutest Research Laboratories (I) Pvt. Ltd, Maharashtra, India from 19 July 2021 to 27 August 2021. The study protocol, informed consent forms and other relevant documents (subjects’ accrual) were reviewed and approved by ‘Ethicare Ethics committee’. Pre-screening and general safety instructions in view of COVID-19 pandemic were reviewed and approved by ‘Human Care Independent Ethics Committee’. The study was performed in compliance with the principles of the Declaration of Helsinki for Medical Research involving human subjects, the Guideline for Good Clinical Practice (GCP) and the Drug Control General of India (DCGI) regulatory guidelines. All volunteers provided their written informed consent to participate before executing any study-related procedure. Study design and subjects’ disposition is presented in Figure 3.
Supra bioavailable itraconazole capsule, Fixtral SB 100 mg, (batch number CV21002) manufactured by Dr. Reddy’s Laboratories Limited, India was used as the test product. Two reference products were Sporanox® capsules 2 × 100 mg (batch number N192) manufactured by Johnson & Johnson Private Limited, Mulund, Mumbai as reference product (R1) and Lozanoc capsules® 2 × 50 mg manufactured by Mayne pharma International pty Ltd, Salisbury South, South Australia, as reference product (R2).
The study population consisted of healthy male and female volunteers, aged between 18-45 years. Participants with a body mass index (BMI) of 18.5-30 kg/m2 and a weight of ≥ 45 kg, non-pregnant, non-smokers, ex-smokers or moderate smokers with no history of drug abuse and significant alcoholism were eligible for enrolment into the study.
Subjects found to be healthy during pre-study screening/ evaluation (i.e., 21 days prior to dosing of period-I) after physical examination, vital sign assessment, ECG examination and other clinical laboratory analysis (haemogram, biochemistry, serum electrolytes, urinalysis) were included in the study.
Volunteers with clinically relevant abnormalities in medical examination/ history, or having any systemic disease (cardiovascular, renal, metabolic, gastrointestinal, haematological, etc.) medical or surgical condition interfering with the functioning of gastrointestinal tract were excluded. In addition, individuals with a known history of hypersensitivity to itraconazole and other related formulations; or requiring medication for an ailment having enzyme modifying activity (previous 28 days prior to dosing day) or using prescription medication (14 days prior to dose administration); participation in other bioequivalence study or blood donation (90 days prior to period-I dosing); positive for drug or alcohol abuse; positive for HIV, HbsAg or Hepatitis -C tests; pregnant; use of hormonal contraceptives or lactating women were not included.
A randomized, open label, balanced, three treatments, three period, three sequence, crossover, oral comparative bioavailability study was carried out in healthy male and female human volunteers under fed conditions. A total of 92 volunteers were screened, of these 54 subjects were included in the study. Subjects were housed for at least 10.50 h prior to study drug administration until 24 h post dose in each study period. Subjects were randomized to receive single oral dose of either the test product (T) or reference product (R1 and R2). Duration of treatment was 39 days including a washout period of 14 days between period I and period II and 20 days between period II and period III. After an overnight fasting of 10 h, each subject was given high fat and high calorie breakfast 30 minutes prior to the drug administration. Study subjects were given 240 mL of water at ambient temperature in sitting position. Water was restricted for 01 h pre-dose to 01 h post-dose except fluid that was taken during breakfast and 240 mL at the time of study drug administration in each study period. Subjects were asked to remain awake and in upright position up to 4 h. post dose, except for any procedural reason or in case of an adverse event. Standardized meals were given during check in night, 04, 09 and 13 h post dose in each study period. Blood samples were taken pre-dose (00 h) and post-dose up to 96 h. Samples after 24 h were collected on an ambulatory basis.
A total of 21 blood samples (5 mL/ sample) were collected in K2- ethylenediaminetetraacetic acid (EDTA) vacutainers. Approximately, 345.2 mL of total blood was withdrawn from each subject. Samples were taken pre-dose (00 h prior to dosing), and at 01, 02, 02, 03, 04, 04.50, 05, 05.50, 06, 06.50, 07, 08, 09, 10, 12, 24, 36, 48, 72 and 96 h post-dose. Following centrifugation under refrigeration at 3500 rpm for 10 minutes at 5 °C ± 3 °C, the plasma was transferred to appropriate size biological sample storage vials (previously labeled with study code and sample code), in duplicate (one aliquot as control samples and one aliquot for analysis; the aliquot for analysis was contained approximately 1.5 mL of plasma and remaining approximately 0.5 mL of plasma was transferred in control aliquot).
The amount of buffer was added according to the volume of plasma to maintain ratio. For this, 0.500 mL of 10 mmol/L Ammonium Formate pH 4.00 ± 0.05 buffer per 1 mL of plasma was added. Plasma samples were placed in biological sample storage box and the samples were then placed in deep freezer maintained at -20 °C ± 5 °C. The samples were further estimated for itraconazole and hydroxy-itraconazole by a validated LC-MS/MS analytical method.
PK parameters for each subject-formulation combination were calculated by non-compartmental model using SAS® version 9.4. The following PK parameters were calculated: Primary PK parameters included; AUC0-t - AUC - time curve measured to the last sampling time (AUC0-t), using the linear trapezoidal rule, and AUC0-inf – AUC0-t + additional area extrapolated to infinity, calculated as AUC0-t + Ct/Kel, where Ct is last measurable drug concentration and Kel is the elimination rate constant.
PK parameters AUC0-t and AUC0-inf were taken as primary efficacy variables for assessing bioequivalence between the test product (T) and reference product (R2).
Secondary PK parameters included: Cmax - maximum drug concentration (ng/mL); Tmax-time to reach maximum drug concentration; AUC0-t /AUC0-inf – ratio of AUC0-inf and AUC0-t, Kel- First-order elimination rate constant calculated from a semi-log plot of the plasma concentration vs time curve, using the method of least square regression, t1/2 - first-order terminal elimination half-life, residual area – extrapolated area (AUC0-inf – AUC0-t)/ AUC0-inf; PD parameter AUC/MIC – area under plasma concentration/ minimum inhibitory concentration.
All statistical analyses were performed using SAS® version 9.4. The plasma concentrations at each sampling time point were tabulated for each subject and product combination. Descriptive statistics for each product at each scheduled sampling time point was done.
All concentration values below the limit of quantification (BLQ) were to be set to zero for the estimation of pharmacokinetic parameters.
ANOVA on the log transformed PK parameters Cmax, AUC0-t and AUC0-inf at α level of 0.05 for itraconazole and Hydroxy itraconazole using Proc MIXED model.
Test product was concluded bioequivalent to the reference product (R2) if 90% confidence interval (CI) of geometric mean ratio of log transformed pharmacokinetic parameters AUC0-t and AUC0-inf between test (T) and reference (R2) products fall within the range of 80.00% to 125.00% for itraconazole. Additionally, supportive data on active metabolite hydroxy-itraconazole was analyzed. For each subject, AUC/MIC ratio was calculated for test as well as reference products considering three different levels of MIC, i.e., 4, 8 and 16 mcg/mL. For each MIC level, AUC/MIC > 25-50 was considered for comparison between test and reference (R1) treatment. Mean AUC/MIC is considered > 50 for optimal efficacy at these dose levels. For each MIC levels, data on the number of subjects having AUC/MIC > 25-50 and AUC/MIC > 50 was analyzed for comparison between test and reference (R1) treatments using two independent sample T Test statistical test (binomial test).
Safety parameters included, physical examination assessed at the time of screening, during study and post study. Vital signs (blood pressure, pulse rate), wellbeing assessment and 12 Lead ECG was done at the time of screening, pre and post dose at 01.00, 03.00, 05.00 and 08.00 h, in each study period, during check out and post study. Haemogram, urinalysis and biochemistry profile (lipid profile), serum electrolytes test was done during screening and post study. Adverse events (AEs) assessments were performed in each study period and post study evaluation.
A total of 54 subjects were enrolled in the study, comprising of healthy adult males (n = 44) and females (n = 10). Three subjects were withdrawn due to AEs; one subject had fever in period III during 36 h post-dose ambulatory visit, another subject had loose motion in period I pre-dose and the third subject was withdrawn from the study period I post-dose due to vomiting. Data of the subjects completing at least 2 periods of the study (provided subject has received the test product in any one of the 2 periods attended) were considered for PK and statistical analysis. Considering the estimate of T/R ratio 95 to 105 %, intra-Subject C.V (%) 34% and significance level 5%, power ≥ 80% and bioequivalence Limits 80.00 – 125.00% (Cmax and AUC), a sample size of 50 subjects was sufficient to establish bioequivalence between formulations with adequate power. However, considering the drop-out or withdrawn, a sample size of 54 ± 2 subjects was considered for the statistical analysis of test vs reference product (R2).
The mean ± standard deviation (SD) range of age, and BMI of 52 participants were: 30.74 ± 5.52 years (20-42 years) and 24.68 ± 3.40 kg/m2 (18.99 – 29.80 kg/m2), respectively.
Primary PK variables assessed for bioequivalence of test and reference products (R2) for itraconazole were compared statistically. The 90% CI value for PK parameters AUC0-t and AUC0-inf for itraconazole were 90.66 % (83.31% - 98.66%) and 88.92 % (81.58%, 96.93%), respectively. Values for both the parameters were well within the equivalence range of 80 to 125% for itraconazole. Data presented in Table 1 determine that both the test and reference product (R2) were bioequivalent under fed conditions.
| Variable | Geometric mean | T/R2 ratio (%), 90%CI | Outcome of BE result | |
| Test (T) | Reference (R2) | |||
| AUC0-t (ng × h/mL) | 2533.47 | 2794.45 | 0.9066 (83.31% - 98.65%) | Bioequivalent |
| AUC0-inf (ng × h/mL) | 2856.78 | 3212.68 | 0.8892 (81.57%– 96.92%) | Bioequivalent |
| Cmax (ng/mL) | 117.4 | 118.82 | 0.988 (88.57%-110.22%) | Bioequivalent |
The mean Cmax values for itraconazole after administration of test and reference product (R2) were 134.47ng/mL and 136.69 ng/mL respectively. Time taken to reach the maximum concentration level in plasma was 10.0 (4.5 to 24.12) h for the test product, and 8.5 (4.5 to 24.02) h for reference product (R2). The mean AUC ratio for the test and reference product (R2) was recorded as 88.04% and 87.63%, respectively.
For PD parameter AUC/MIC, the test product (T) was considered as comparable with the reference product for each MIC level if there exists no statistically significant difference (P > 0.05 and observed P = 0.3196).
In analysis of variance model of itraconazole, no significant sequence and period effect were observed for Cmax (P = 0.6848, 0.6595), AUC0-t (P = 0.5778, 0.1998) and AUC0-inf (P = 0.6430, 0.1660). In addition, no significant ‘treatment’ effect was observed for Cmax andAUC0-t. (P = 0.8548 and 0.0575). However, significant ‘treatment’ effect was observed for AUC0-inf (P = 0.0268).
During the course of study, a total of six AEs were reported. All the AEs were mild to moderate in severity. Four (7.40%) AEs were reported by two participants in the test group (feeling feverish and fever), while one (1.85%) AE was reported by a subject in the reference (R1) group (vomiting) and the other AE (1.85%) was observed in a subject in the reference (R2) group (headache). All the AEs were resolved. No severe AE (SAEs) was observed during the study.
Bioequivalence studies form an integral core in the new drug development process[18]. According to United States Food and Drug Administration (FDA), two pharmaceutically equivalent products are considered bioequivalent if the 90% CI of geometric mean ratio (GMR) of primary PK (Cmax, AUC0-t and AUC0-α) variables fall within the range of 80%-125%[19]. As per the European Medicines Agency (EMA) guidelines, a randomized, two period, two sequence, single dose, crossover design must be used for the comparison of two drug formulations. The current clinical trial was conducted in accordance with FDA guidance and EMA recommendations[19,20].
Although this was an open label study, the analyst was blinded to the sequence of test and reference products (R1 and R2) administered to the subjects. EMA recommends a washout interval after each period to ensure that drug concentrations are below the lower limit among participants, before the next period commences[20]. Similarly, in our study to avoid any residual or carry over effect, a washout period of 14 days between period I and period II and 20 days between period II and period III was given. In addition, no significant period effect was observed for Cmax, AUC0-t and AUC0-inf for itraconazole.
This study was performed under fed conditions as the absorption of itraconazole capsule is facilitated in acidic environment[4,21]. It is also stated that the oral bioavailability of itraconazole gets doubled when administered after food[10,22]. Conversely, absorption is impaired with concomitant use of antacids, or proton pump inhibitors. However, no food effect is observed in case of oral suspension with oral bioavailability is enhanced in fasting state[4,23].
In the current study, statistical analysis of primary PK variables AUC0-t and AUC0-inf revealed bioequivalence, with CIs being completely inside the acceptance criteria of 80-125% as suggested by FDA and EMA[19,20]. The statistical power was greater than 80% for itraconazole.
Following administration of test and reference product (R2), the peak concentration levels of itraconazole were achieved at 10 h and 8.5 h, respectively. Other parameters including Cmax, Kel, and t1/2 for test and reference product (R2) compared well. The mean pharmacokinetic parameters for test and Reference Product (R1 and R2) for itraconazole are summarized in Table 2. Hydroxyitraconazole data was analyzed statistically and used only as supportive data. The mean pharmacokinetic parameters for test and Reference Product (R1 and R2) for Hydroxyitraconazole are summarized in Table 3.
| Variable | mean ± SD | ||
| Test (T) | Reference (R1) | Reference (R2) | |
| Cmax (ng/mL) | 132.12 (65.31) | 308.57 (173.63) | 136.90 (67.91) |
| AUC0-t | 2738.083 (1164.121) | 4953.205 (2472.121) | 3065.766 (1295.989) |
| AUC0-inf | 3109.578 (1342.394) | 5744.760 (2917.508) | 3614.170 (1850.356) |
| Tmax | 11.45(6.05) | 6.00 (1.49) | 10.85 (6.33) |
| Kel | 0.022 (0.007) | 0.021 (0.007) | 0.023 (0.008) |
| t1/2 | 34.89 (13.18) | 37.83 (14.95) | 35.16 (20.14) |
| Residual area | 11.92 (7.41) | 12.86 (7.86) | 12.22 (9.10) |
| Variable | mean ± SD | ||
| Test | Reference (R1) | Reference (R2) | |
| Cmax (ng/mL) | 181.35 (58.30) | 310.20 (11.63) | 197.12 (56.84) |
| Tmax | 12 | 8 | 12 |
| Kel | 0.050 (0.015) | 0.043 (0.014) | 0.050 (0.019) |
| t1/2 | 15.20 (4.92) | 18.14 (7.10) | 16.01 (6.55) |
| Residual area | 2.72 (3.12) | 4.31 (5.03) | 3.37 (3.88) |
AUC/MIC correlates with efficacy of anti-fungal drug and its clinical response, i.e., microbiological cure and clinical outcome of antifungal agents. Pharmacodynamic parameter assessment showed that AUC/MIC for conventional itraconazole 200 mg are comparable to the novel improved bioavailable itraconazole 100 mg for MIC levels upto 16 µg/mL. This indicates that similar therapeutic effect is expected with half dose of supra bioavailable itraconazole as compared to conventional itraconazole[15].
Supra bioavailable itraconazole capsules (Fixtral SB) were found to be safe and well tolerated which is in line with historical data.
The test product, itraconazole capsule 100 mg is bioequivalent with the reference product (R2) at 100 mg dose (2 capsules of Lozanoc®50 mg) under fed conditions. The pharmacodynamics activity in terms of AUC/MIC is comparable between test product at 100 mg dose and marketed itraconazole 200 mg. Based on the results obtained, Fixtral SB is expected to have therapeutically similar efficacy at half the equivalent dose. All the tested formulations were found to be safe and well tolerated with resolvable side-effects.
Itraconazole is a broad-spectrum triazole antifungal. It inhibits fungal growth. It exhibits a nonlinear pharmacokinetic profile due to its varied oral bioavailability.
The test product Fixtral SB is a new formulation that has been manufactured via APSG™ (Advanced Polymeric Spray Granulation), a breakthrough innovative proprietary technology for enhancing solubility and maximizing the bioavailability of poorly soluble drug itraconazole. The new formulation is able to restrict the drug release in an acidic environment which minimizes the precipitation potential of itraconazole in the intestine thus maximizing bioavailability.
To compare the oral bioavailability and bioequivalence of Fixtral supra bioavailable itraconazole (SB) with reference product R2 (supra bioavailable 50 mg itraconazole × tablets).
The comparison of Fixtral SB (supra bioavailable itraconazole) 100 mg with Lozanoc capsules 50 mg administered as two capsules was performed, revealing that Fixtral SB is expected to have therapeutically comparable efficacy at half the equivalent dose. In terms of area under the concentration-time curve (AUC) / minimum inhibitory concentration (MIC), the pharmacodynamic activity of the test product at 100 mg dose and the marketed itraconazole 200 mg is comparable. All of the formulations tested were found to be safe and well tolerated, with manageable side effects.
Based on the results, Fixtral SB is expected to have therapeutically similar efficacy at half-equivalent doses. All the tested formulations were found to be safe and well tolerated with resolvable side effects.
The peak concentration levels of itraconazole were achieved at 10 h (T) and 8.5 h (R2), respectively. Pharmacodynamic parameter assessment shows that AUC/MIC for R1 is comparable to Fixtral SB 100 mg for MIC levels up to 16 μg /mL.
The study population consisted of 54 healthy male and female volunteers, aged between 18-45 years. Subjects were randomized to receive a single oral dose of either test product (T) or reference product (R1 and R2). Blood samples were taken pre-dose and post-dose for up to 96 h to evaluate the bioequivalence. Adverse events assessments were performed in each study period and post-study evaluation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Norman TR, Australia; Trkulja V, Croatia S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Grant SM, Clissold SP. Itraconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in superficial and systemic mycoses. Drugs. 1989;37:310-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 273] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | De Beule K, Van Gestel J. Pharmacology of itraconazole. Drugs. 2001;61 Suppl 1:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 168] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Brunton L, Hilal-Dandan R, Goodman LS. Goodman and Gilman manual of pharmacology and therapeutics: Mcgraw Hill Professional; 2013.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Lestner J, Hope WW. Itraconazole: an update on pharmacology and clinical use for treatment of invasive and allergic fungal infections. Expert Opin Drug Metab Toxicol. 2013;9:911-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Abuhelwa AY, Foster DJ, Mudge S, Hayes D, Upton RN. Population pharmacokinetic modeling of itraconazole and hydroxyitraconazole for oral SUBA-itraconazole and sporanox capsule formulations in healthy subjects in fed and fasted states. Antimicrob Agents Chemother. 2015;59:5681-5696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Hardin TC, Graybill JR, Fetchick R, Woestenborghs R, Rinaldi MG, Kuhn JG. Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrob Agents Chemother. 1988;32:1310-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 222] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Mouton JW, van Peer A, de Beule K, Van Vliet A, Donnelly JP, Soons PA. Pharmacokinetics of itraconazole and hydroxyitraconazole in healthy subjects after single and multiple doses of a novel formulation. Antimicrob Agents Chemother. 2006;50:4096-4102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Dragojević-Simić V, Kovačević A, Jaćević V, Rančić N, Djordjević S, Kilibarda V, Mikov M, Bokonjić D. Bioequivalence study of two formulations of itraconazole 100 mg capsules in healthy volunteers under fed conditions: a randomized, three-period, reference-replicated, crossover study. Expert Opin Drug Metab Toxicol. 2018;14:979-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Yun HY, Baek MS, Park IS, Choi BK, Kwon KI. Comparative analysis of the effects of rice and bread meals on bioavailability of itraconazole using NONMEM in healthy volunteers. Eur J Clin Pharmacol. 2006;62:1033-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Zimmermann T, Yeates RA, Laufen H, Pfaff G, Wildfeuer A. Influence of concomitant food intake on the oral absorption of two triazole antifungal agents, itraconazole and fluconazole. Eur J Clin Pharmacol. 1994;46:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Sardana K, Mathachan SR. Super Bioavailable Itraconazole and Its Place and Relevance in Recalcitrant Dermatophytosis: Revisiting Skin Levels of Itraconazole and Minimum Inhibitory Concentration Data. Indian Dermatol Online J. 2021;12: 1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (1)] |
| 12. | Puttick MPE, Phillips P: Itraconazole: Precautions regarding drug interactions and bioavailability. Vol. 5, Canadian Journal of Infectious Diseases. 1994. p. 179–83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | List of drugs approved from SND Division from 01.01.2020 to 31.12.2020. |
| 14. | Lepak AJ, Andes DR. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect Med. 2014;5:a019653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | MHRA. Public Assessment Report of Lozanoc, Available from: https://mhraproducts4853.blob.core.windows.net/docs/c86c25a27a87210e71b30f469ae86a729f4cb575. |
| 16. | Hope WW, Drusano GL. Antifungal pharmacokinetics and pharmacodynamics: bridging from the bench to bedside. Clin Microbiol Infect. 2009;15:602-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Pyrpasopoulou A, Iosifidis E, Antachopoulos C, Roilides E. Antifungal drug dosing adjustment in critical patients with invasive fungal infections. J Emerg Crit Care Med. 2019;3(2): 37-37. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Haidar SH, Makhlouf F, Schuirmann DJ, Hyslop T, Davit B, Conner D, Yu LX. Evaluation of a scaling approach for the bioequivalence of highly variable drugs. AAPS J. 2008;10:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Food and Drug Administration. Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products—general considerations. Food and Drug Administration, Washington, DC. 2003. |
| 20. | Ford R, Schwartz L, Dancey J, Dodd LE, Eisenhauer EA, Gwyther S, Rubinstein L, Sargent D, Shankar L, Therasse P, Verweij J. Lessons learned from independent central review. Eur J Cancer. 2009;45:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Jaruratanasirikul S, Kleepkaew A. Influence of an acidic beverage (Coca-Cola) on the absorption of itraconazole. Eur J Clin Pharmacol. 1997;52:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Barone JA, Koh JG, Bierman RH, Colaizzi JL, Swanson KA, Gaffar MC, Moskovitz BL, Mechlinski W, Van de Velde V. Food interaction and steady-state pharmacokinetics of itraconazole capsules in healthy male volunteers. Antimicrob Agents Chemother. 1993;37:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Barone JA, Moskovitz BL, Guarnieri J, Hassell AE, Colaizzi JL, Bierman RH, Jessen L. Food interaction and steady-state pharmacokinetics of itraconazole oral solution in healthy volunteers. Pharmacotherapy. 1998;18: 295-301. [PubMed] |