Published online Jan 28, 2022. doi: 10.5497/wjp.v11.i1.1
Peer-review started: March 25, 2021
First decision: July 27, 2021
Revised: August 11, 2021
Accepted: January 11, 2022
Article in press: January 11, 2022
Published online: January 28, 2022
Processing time: 305 Days and 22.4 Hours
Inflammatory bowel diseases (IBDs), with blurred etiology, show a rising trend and are of global concern. Of various factors involved in IBD pathogenesis and development, inflammation has been shown to play a major role. Recognition of the molecular and cellular pathways that induce IBD is an emerging subject to develop targeted therapies. Mammalian target of rapamycin (mTOR) is one the most common receptors of many inflammatory pathways, including that of IBD. To this end, we intend to overview the mTOR inhibitors for their possible efficacy in present and future approaches to treatment of IBD.
Core tip: Inflammation is the main participant in the pathogenesis and development of inflammatory bowel disease (IBD). Since the mammalian target of rapamycin (mTOR) pathways are suggested to be involved in IBD progression, inhibition of the mTOR signaling may lead to a novel treatment modality for patients with IBD. Several biologics and synthetic and natural compounds have been introduced as mTOR inhibitors, which may control or eradicate intestinal inflammatory conditions such as IBD.
- Citation: Lashgari NA, Roudsari NM, Momtaz S, Abdolghaffari AH. Mammalian target of rapamycin-novel insight for management of inflammatory bowel diseases. World J Pharmacol 2022; 11(1): 1-5
- URL: https://www.wjgnet.com/2220-3192/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.5497/wjp.v11.i1.1
Inflammatory bowel diseases (IBDs) include two major types: ulcerative colitis (UC) and Crohn’s disease (CD) that mainly progress due to abnormal dysregulation of the immune system[1]. Aberrations of the innate and adaptive immune responses and inflammatory processes play crucial roles in IBD pathogenesis[2]. Unregulated immune response stimulates Toll-like receptor-4 and the gastrointestinal enteric bacteria flora, thus activating the mucosal T cells and interferon (IFN) production and release. These events initiate the signal transduction cascades such as the nuclear factor (NF)-κB pathway; the mammalian target of rapamycin (mTOR) and transducer and activator of transcription 1 (STAT1) pathway. As a result, the activation of these pathways leads to elevation of inflammatory cytokines and induction of IBD. In the next step, leukocytes are aberrantly activated, leading to enhanced infiltration into the injured colonic site.
Therefore, modulation of inflammatory cytokines and chemokines could be important for IBD treatment. Pharmacological products or surgery are commonly used in IBD patients. Anti-inflammatory drugs (i.e. corticosteroids and aminosalicylates); immunomodulatory treatments (i.e. azathioprine, mercaptopurine, cyclosporine and methotrexate); biologic compounds [i.e. tumor necrosis factor (TNF)-α inhibitors]; and antimicrobials (i.e. ciprofloxacin and metronidazole) are current therapeutic options for IBD treatment. Among them, mTOR is a serine/threonine protein kinase of the phosphatidylinositol-3 kinase related kinase (PIKK) family, and a critical regulator of the inflammatory pathways[3,4]. mTOR has two subtypes of mTORC1 and mTORC2. Structurally, mTORC1 contains SEC13 protein 8 (mLST8)/G-protein b subunit-like protein (GbL), the regulatory-associated protein of mTOR (Raptor), DEPTOR, PRAS40, and a scaffold protein TTI1/TEL2 complex. The composition of mTOR, the mamma
The myeloid-derived suppressor cells (MDSCs) are also attributed to the etiology of IBD and have been shown to improve colitis in vivo. It was demonstrated that mTOR inhibitors could suppress MDSCs and improve IBD. MDSCs have shown superior immunosuppressive activities against IBD. mTOR inhibitors increase Tregs but reduce Th1 cells in IBD. These results indicate that some of the mTOR inhibitors attenuate IBD via Treg expansion promoted by MDSCs[6,7]. Inhibition of the mTOR pathway can improve IBD due to suppression of inflammatory processes. Hence, the factors that target the components of this pathway or the mTOR signaling proteins are of interest for drug development. Severe IBD could lead to several dangerous diseases such as colon cancer, irritable bowel syndrome, visceral hypersensitivity, neurodegenerative disease, etc. Induction of proinflammatory and inflammatory cytokines, i.e., the cytokine storm in conditions such as COVID-19 may affect IBD patients. Therefore, the mTOR inhibitors are important not only to improve IBD, but also to reduce the risk of health-threatening conditions[8,9].
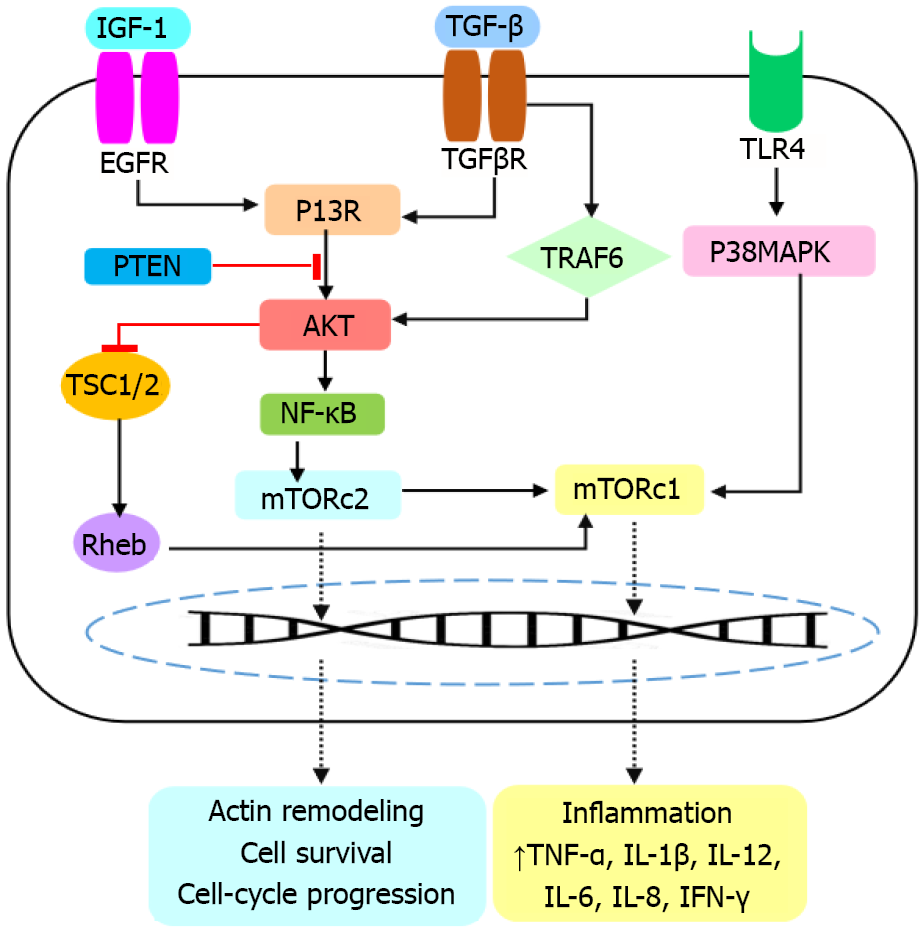
Given the high risk of inflammatory diseases and their influence on organ failure, new therapeutic triggers with fewer side effects and more specialization are needed. Clinical evidence demonstrates that inflammatory processes can increase the risk of many diseases. For example, it has been shown that inflammatory factors cause neurodegeneration and increase the risk of neurodegenerative disease such as Alzheimer’s disease, Parkinson’s disease and multiple sclerosis[10,11]. IBD could also lead to other gastrointestinal impairments such as colonic cancer. Recently, it has been proposed that IBD induces colonic angiotensin-converting enzyme 2 expression, probably due to the stimulation of cytokines storm, which finally increases susceptibility to COVID-19 and could end in death[12,13]. Although the etiopathogenesis of IBD is poorly understood, available evidence suggests that genetic susceptibility and environmental stimuli can predispose to immunological responses, and provoke IBD[14,15]. Many inflammatory signaling pathways participate in pathogenesis of IBD [16,17]. The severity of IBD relies on the location and extent of the lesions, resulting in numerous extraintestinal manifestations. The mTOR signaling pathway is one of the most important mechanisms that contributes to progression of IBD. In this context, mTOR induces the NF-κB pathway, which together participate in production of several inflammatory mediators such as IFN-γ, IL-6, IL-8, IL-1 and TNF-α[18]. The TGF-β/P13K/AKT/mTOR pathway upregulates TNF-α, IL-1β, TGF-β, IL-12 and IL-6 expression. The TLR/P38MAPK/mTOR interaction increases serum levels of IL-12, IL-6, IL-8 and TNF-α[3]. Induction of the TLR4, MAPK and NF-κB pathways stimulates autophagy by the regulation of mTOR, thus improving the gut inflammatory responses and IBD[19]. These all suggest that inhibition of mTOR and/or the mTOR-dependent downstream signaling pathways represent promising insight for IBD treatment. To assess such a hypothesis, several biologics such as everolimus, temsirolimus, deforolimus, sunitinib, bevacizumab, vedolizumab, etrolizumab, and the diverse mTOR analogs, AZD-8055, WYE-354, VS-5584, LY3023414, Ku-0063794, PI-103 and SKLB-M8 were analyzed and found to target mTOR and o block inflammatory processes[20,21]. Several natural compounds such as resveratrol, curcumin, acacetin, capsaicin, epigallocatechin-3, fisetin, harmine, panduratin A, prodigiosin, sinomenine, honokiol and isoliquiritigenin have shown the ability to inhibit mTOR. Taken together, targeting the mTOR signaling pathway could block secretion of cytokines and chemokines and not only improves IBD but also prevents the risk of other diseases, in which inflammation plays a key pathogenic role[6,22]. Future attempts should focus on planning clinical trials to evaluate the therapeutic efficacy of the mTOR inhibitors against IBD. Probable interaction of mTOR signaling with other pathways and effectors of IBD should also be considered to design targeted inhibitors with a broader action.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng Y S-Editor: Fan JR L-Editor: Kerr C P-Editor: Fan JR
| 1. | Larabi A, Barnich N, Nguyen HTT. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy. 2020;16:38-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 534] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 2. | Matsuda C, Ito T, Song J, Mizushima T, Tamagawa H, Kai Y, Hamanaka Y, Inoue M, Nishida T, Matsuda H, Sawa Y. Therapeutic effect of a new immunosuppressive agent, everolimus, on interleukin-10 gene-deficient mice with colitis. Clin Exp Immunol. 2007;148:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 3. | Lashgari NA, Roudsari NM, Momtaz S, Ghanaatian N, Kohansal P, Farzaei MH, Afshari K, Sahebkar A, Abdolghaffari AH. Targeting Mammalian Target of Rapamycin: Prospects for the Treatment of Inflammatory Bowel Diseases. Curr Med Chem. 2021;28:1605-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Feng Y, Chen X, Cassady K, Zou Z, Yang S, Wang Z, Zhang X. The Role of mTOR Inhibitors in Hematologic Disease: From Bench to Bedside. Front Oncol. 2020;10:611690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Chen X, Liu M, Tian Y, Li J, Qi Y, Zhao D, Wu Z, Huang M, Wong CCL, Wang HW, Wang J, Yang H, Xu Y. Cryo-EM structure of human mTOR complex 2. Cell Res. 2018;28:518-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Li C, Zhu F, Wang S, Wang J, Wu B. Danggui Buxue Decoction Ameliorates Inflammatory Bowel Disease by Improving Inflammation and Rebuilding Intestinal Mucosal Barrier. Evid Based Complement Alternat Med. 2021;2021:8853141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Shi G, Li D, Ren J, Li X, Wang T, Dou H, Hou Y. mTOR inhibitor INK128 attenuates dextran sodium sulfate-induced colitis by promotion of MDSCs on Treg cell expansion. J Cell Physiol. 2019;234:1618-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Murugan AK. mTOR: Role in cancer, metastasis and drug resistance. Semin Cancer Biol. 2019;59:92-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 324] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 9. | Xu F, Na L, Li Y, Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020;10:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 405] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 10. | Heras-Sandoval D, Pérez-Rojas JM, Pedraza-Chaverri J. Novel compounds for the modulation of mTOR and autophagy to treat neurodegenerative diseases. Cell Signal. 2020;65:109442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Maiese K. Targeting the core of neurodegeneration: FoxO, mTOR, and SIRT1. Neural Regen Res. 2021;16:448-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | El Ouali S, Philpott J, Vargo J, Regueiro M. COVID-19 in patients with IBD and pancreaticobiliary disorders. Cleve Clin J Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Nie K, Yang YY, Deng MZ, Wang XY. Gastrointestinal insights during the COVID-19 epidemic. World J Clin Cases. 2020;8:3934-3941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (2)] |
| 14. | Alimohammadi N, Koosha F, Rafeian-Kopaei M. Current, New and Future Therapeutic Targets in Inflammatory Bowel Disease: A Systematic Review. Curr Pharm Des. 2020;26:2668-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Guan Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J Immunol Res. 2019;2019:7247238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 615] [Article Influence: 102.5] [Reference Citation Analysis (1)] |
| 16. | Annese V. Genetics and epigenetics of IBD. Pharmacol Res. 2020;159:104892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 17. | Attauabi M, Zhao M, Bendtsen F, Burisch J. Systematic Review with Meta-analysis: The Impact of Co-occurring Immune-mediated Inflammatory Diseases on the Disease Course of Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2020;27:927-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Park S, Regmi SC, Park SY, Lee EK, Chang JH, Ku SK, Kim DH, Kim JA. Protective effect of 7-O-succinyl macrolactin A against intestinal inflammation is mediated through PI3-kinase/Akt/mTOR and NF-κB signaling pathways. Eur J Pharmacol. 2014;735:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Zhou M, Xu W, Wang J, Yan J, Shi Y, Zhang C, Ge W, Wu J, Du P, Chen Y. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine. 2018;35:345-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 252] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 20. | Tian T, Li X, Zhang J. mTOR Signaling in Cancer and mTOR Inhibitors in Solid Tumor Targeting Therapy. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 426] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 21. | Magaway C, Kim E, Jacinto E. Targeting mTOR and Metabolism in Cancer: Lessons and Innovations. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 22. | Chamcheu JC, Roy T, Uddin MB, Banang-Mbeumi S, Chamcheu RN, Walker AL, Liu YY, Huang S. Role and Therapeutic Targeting of the PI3K/Akt/mTOR Signaling Pathway in Skin Cancer: A Review of Current Status and Future Trends on Natural and Synthetic Agents Therapy. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |