Copyright
©2014 Baishideng Publishing Group Inc.
World J Pharmacol. Dec 9, 2014; 3(4): 110-119
Published online Dec 9, 2014. doi: 10.5497/wjp.v3.i4.110
Published online Dec 9, 2014. doi: 10.5497/wjp.v3.i4.110
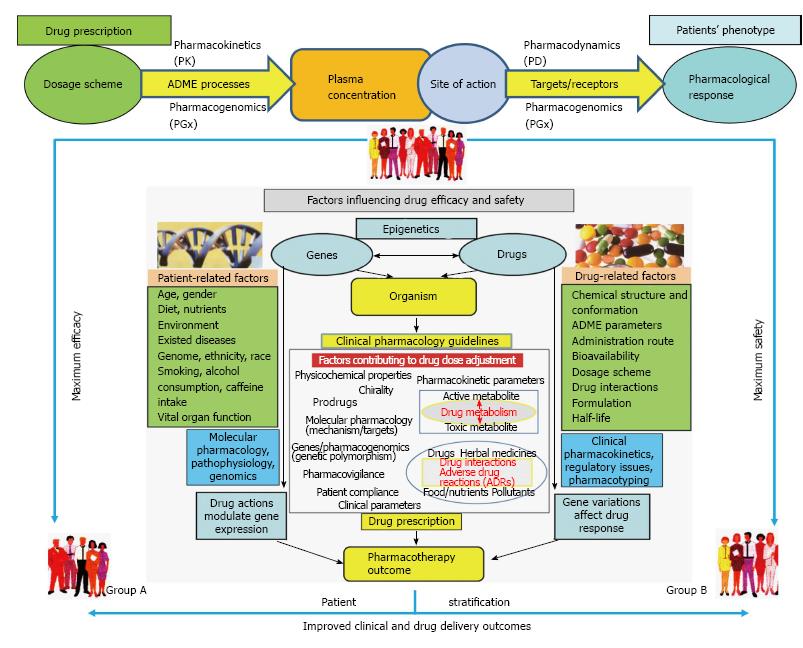
Figure 1 A roadmap of pharmacological response stages to efficiently address the pharmacotyping concepts in drug prescription.
The processes and the factors related to pharmacological effects along with the scientific environment contributing to drug delivery outcomes in terms of efficacy, safety are depicted above. The need for enriching pharmacological knowledge to advance personalized medicine decisions in the clinical setting through drug dosage scheme adjustment (i.e., PTx) is exemplified. The in vivo pharmacology experience gained thus far and it already appears in the drug regulatory environment is stressfully demanded to be empowered by pharmacogenomics knowledge in terms of PD/PK drug parameters assessment methodologies, the clinical pharmacology guidelines development and the prescription process. Complementary to this, the development of information-based workflow platforms in clinical practice incorporating algorithms to assess the efficient translation of clinical, biological, genomic and chemical information is also eagerly expected. Such a direction of major pharmacological importance permits the maximum efficacy and safety outcomes to be reached in a timely and worldwide basis for everyday healthcare (see text for more details). PTx: Pharmacotyping; PGx: Pharmacogenomics; PK: Pharmacokinetics; PD: Pharmacodynamics.
- Citation: Vizirianakis IS. Harnessing pharmacological knowledge for personalized medicine and pharmacotyping: Challenges and lessons learned. World J Pharmacol 2014; 3(4): 110-119
- URL: https://www.wjgnet.com/2220-3192/full/v3/i4/110.htm
- DOI: https://dx.doi.org/10.5497/wjp.v3.i4.110