Published online Jun 2, 2023. doi: 10.5496/wjmg.v11.i2.21
Peer-review started: February 8, 2023
First decision: April 28, 2023
Revised: May 3, 2023
Accepted: May 19, 2023
Article in press: May 19, 2023
Published online: June 2, 2023
Processing time: 115 Days and 18.7 Hours
KBG syndrome is likely underdiagnosed because of mild and non-specific features in some affected patients especially before the upper permanent central incisors eruption at about the age of 7-8 years. Somatic mosaicisms are usually recognized in the parents only after a typically affected son is diagnosed with KBG syndrome. We describe for the first time the mosaicism of a novel variant in a child with a mild KBG phenotype.
Our patient presented at 24 mo of age with short stature, hand abnormalities, facial dysmorphism and mild developmental delay. Pituitary hypoplasia and central hypothyroidism were also detected. By next generation sequencing (NGS) analysis we found a novel deletion in the ANKRD11 gene (c.4880_4893del.), that can be classified as likely pathogenic for the syndrome, with the percentage of mutated allele of 36%. We considered this finding as causative of the mild and non-specific phenotype for KBG syndrome in our patient, as previously reported in adults. A heterozygous variant in HESX1 gene, classified as variant of uncertain significance, but suspected of causing pituitary hypoplasia and hormonal deficiency, was also found. The patient started levothyroxine and growth hormone treatment.
The increased use of NGS analysis may expand the phenotypic spectrum of KBG syndrome because it allows genetic diagnosis of somatic mosaicisms also in children.
Core Tip: Somatic mosaicisms of KBG syndrome are usually recognized in the parents only after a typically affected son is diagnosed. We report for the first time the case of a somatic mosaicism for KBG syndrome diagnosed in a child with a mild and non-specific phenotype. The increased use of next generation sequencing allows a genetic diagnosis of this mosaic form in children expanding the phenotypic spectrum of the KBG syndrome.
- Citation: Franceschi R, Rivieri F, Novelli A, Ferretti D, Anesi A, Soffiati M, Porretti G, Maines E, Mucciolo M, Radetti G. Mosaicism of a novel variant in the ANKRD11 gene in a child with a mild KBG phenotype: A case report. World J Med Genet 2023; 11(2): 21-27
- URL: https://www.wjgnet.com/2220-3184/full/v11/i2/21.htm
- DOI: https://dx.doi.org/10.5496/wjmg.v11.i2.21
“KBG” represents the initials of the last name of the first three families diagnosed with the syndrome and is a rare genetic disease (OMIM 148050). Common manifestations are macrodontia (especially of the upper central incisors), typical facial features, short stature, skeletal anomalies, hearing loss, global developmental delay, and intellectual disability[1-4]. The transmission of this disease is autosomal dominant, and is caused by either heterozygous ANKRD11 point mutations (OMIM 611192) or microdeletion in chromosome 16q24.3 including the ANKRD11 gene[5] or ANKRD11 intragenic duplication[6]. The ANKRD11 gene encodes an ankyrin repeat containing protein (ANKRD11) which is indispensable in neuron proliferation and acts as a transcriptional repressor by two transcriptional repression domains (RDs: RD1, aa 318–611; and RD2, aa 2369–2663) and promoting transcription through one activation domain (AD), aa 1851–2145[1]. Since 1975, over 200 KBG patients have been described[1].
KBG syndrome is likely underdiagnosed because of mild and non-specific features in some affected patients especially before the upper permanent central incisors eruption at about the age of 7-8 years[2,7]. Macrodontia of the permanent upper incisors is a typical finding, making diagnosis prior to the eruption of these teeth a challenge[2]. According to the latest diagnostic criterion, KBG syndrome should be considered in a patient with cognitive delay/learning difficulties, speech delay or behavioral anomalies with at least two major criteria or one major and two minor criteria[2]. Major criteria are: (1) Macrodontia or phenotypic features of KBG in child with primary dentition; (2) height < 10th centile; (3) recurrent middle ear infections and/or hearing loss; and (4) 1st degree relative with KBG syndrome. Minor criteria are: Brachydactyly or relevant hand anomaly; epilepsy; cryptorchidism; feeding difficulties; palate abnormalitities; autism; large anterior fontanelle and/or delayed closure. A phenotypic variability among KBG patients has been observed intra- and interfamilial, and between patients with the 16q24.3 microdeletion compared to those harboring ANKRD11 gene mutations[1]. Somatic mosaicisms have been reported in the parents after a typically affected son was diagnosed with KBG syndrome, and exhibited a milder phenotype, suggesting that KBG phenotypes in adults might be dose-dependent[5-7].
Here we describe for the first time in a child a mosaicism of a novel variant in the ANKRD11 gene. The patient had a mild KBG phenotype and the diagnosis was performed by NGS analysis, providing insights into the spectrum of mosaic mutations.
The proband came to our attention at the age of 24 mo, owing to postnatal growth retardation (Supplementary Figure 1).
The boy was born at term (41 wk) after a pregnancy achieved with egg fertilization by intracytoplasmic sperm injection. Birth weight was 3830 g (0.84 SD), length 53 cm (1.38 SD), head circumference 34 cm (-0.76 SD). Non-consanguineous parents had a normal stature (father 179 cm, mother 182 cm, mid- parent sex-adjusted target height 187 cm).
Not informative.
Not informative.
His height was 89 cm (-2 SD), his weight was 12.6 Kg (-2 SD), and his head circumference was 50 cm (0 SD). Clinical examination revealed facial dysmorphisms, including tall forehead, widely spaced eyes, bushy eyebrows, left palpebral ptosis, prominent and anteverted ears, facial asymmetry. Skeletal anomalies included short fingers with fifth finger clinodactyly. He showed delay/cognitive impairment as assessed by Griffith’s scale.
Routine chemistry turned out as normal. ACTH was 16.7 pmol/L (normal range 5-25), cortisol 286.9 nmol/L (normal range 250-550), and prolactin 13.2 ug/L (normal range 4-15); insulin like growth factor 1 (IGF-1) 4.84 nmol/L (normal range 1.70-30.46), fT4 was repeatedly low: 9.8-10.2 pmol/L (normal range 12-22) with inappropriately normal TSH: 2.3-4.79 mU/L (normal range 0.2-4.5). Thyroid Ultrasound revealed an in situ and normal sized gland. An arginine stimulation test elicited a reduced peak of growth hormone (GH) peak 2.28 ng/mL and IGF-1 was 8.76 nmol/L (2.61-45.36). Considering growth retardation, psychomotor delay and dysmorphic features, clinical exome sequencing analysis was performed.
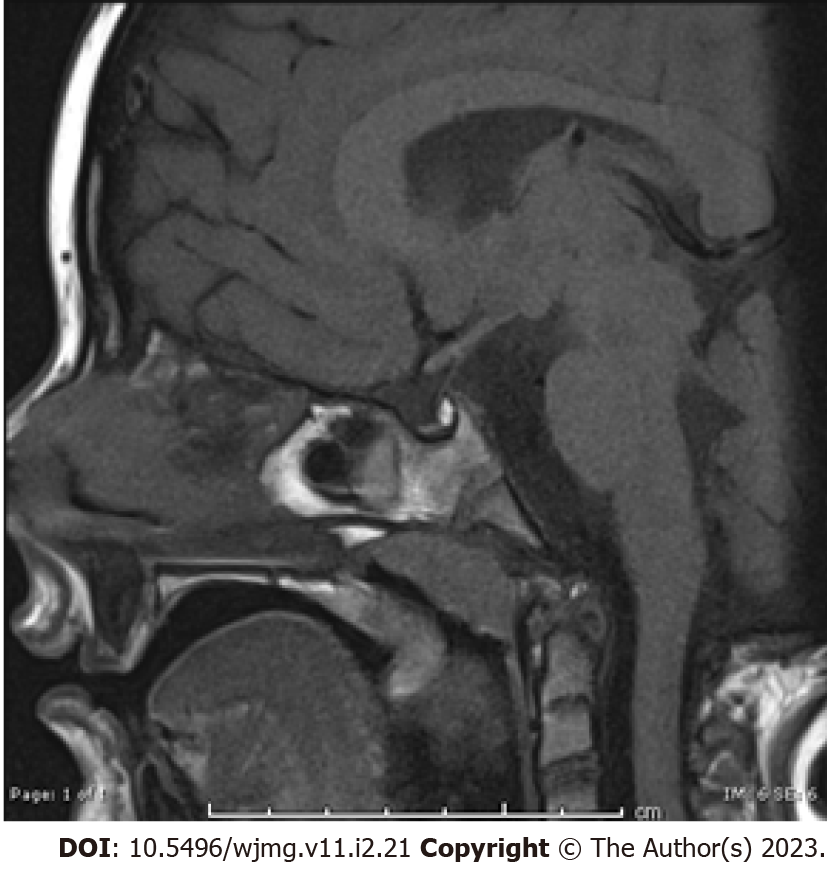
At 36 mo, bone age corresponded to 3 mo for the carpus and 12-16 mo for the phalanges, in the presence of mild malformation of the intermediate phalanx of the fifth finger (Supplementary Figure 2). He presented extra tarsal persistence of chalazion, with sub-palpebral hematomas. Pituitary magnetic resonance imaging (MRI), revealed a hypoplastic gland (Figure 1) with a normal pituitary stalk. Brain MRI excluded central nervous system abnormalities.
Exome sequencing analysis identified a deletion of 14 nucleotides in the ANKRD11 gene, c.4880_4893delCCGCCCGTCGTCTG. The deletion is a mosaic with the percentage of mutated allele of 36%. At protein level the deletion determines the introduction of a premature stop codon p.Ala1627GluTer9. The frameshift variant, not present in the father DNA, is not reported in the gnomAD database, and has never been described in literature. According to current the American College of Medical Genetics and Genomics (ACMG) guidelines[8], the variant can be classified as likely pathogenetic (class 4) for KBG syndrome.
Exome sequencing analysis also identified three variants: (1) A heterozygous variant in HESX1 gene (c.541A>G, p.Thr181Ala, NM_003865.3), inherited from the father, who presented with normal height, TSH and fT4. This variant has been reported as heterozygous and pathogenetic in a patient with isolated growth hormone deficiency and pituitary hypoplasia[9] and in a patient with combined pituitary hormone deficiency, anterior pituitary hypoplasia, and ectopic posterior lobe[10]; in silico analysis suggests that this missense variant does not affect protein structure/function and according to current ACMG guidelines[8], the variant can be classified as variant of uncertain significance (VUS); (2) a hemizygous VUS in ATRX gene (c.189G>A, p.Glu63Glu, NM_000489.5); and (3) a heterozygous VUS in NIPBL gene (p.Ile1982Leu, p.Ile1982Leu). The latest two variants are not present in the father DNA.
Once the diagnosis of central hypothyroidism was confirmed, treatment with levothyroxine was started. After GH test, we started GH treatment that changed growth trajectory (Supplementary Figure 1).
The patient is on follow up at our outpatient clinic, he is now 7 years old, and he started GH six months ago.
KBG syndrome is a rare autosomal dominant disorder characterized by short stature, delay/cognitive impairment and distinctive craniofacial characteristics. It shows a wide spectrum of clinical phenotypes and it is likely underdiagnosed because of mild and non-specific features in some affected patients especially before the upper permanent central incisors eruption at about the age of 7-8 years. Here we present, for the first time in literature, the mosaicism (36%) of a novel variant in the ANKRD11 gene that underlies a mosaic KBG phenotype in a child.
This finding led us to conclude that the variant was acquired at a postzygotic level, and is classifiable as likely pathogenetic for KBG syndrome.
Somatic mosaicism is usually recognized in the parents only after a typically affected son is diagnosed with KBG syndrome, because patients with somatic mosaicisms exhibited a milder phenotype. The phenotypic effect of mosaic ANKRD11 haploinsufficiency might be dose-dependent[4] and some experiences in the literature confirm this hypothesis (Table 1).
| Ref. | Sex and age | Molecular analysis | % mosaicism | Phenotype | Phenotype in relative with the same non-mosaic mutation | |
| Our case | 2 yr | Deletion (c.4880_4893del.) | 36 | Short stature, hand abnormalities, facial dysmorphism, mild developmental delay | - | |
| Khalifa et al[5], 2013 | Female, 31 yr (mother) | Microdeletion 16q24.3 | 38 | Round face, brachycephaly, macrodontia, abnormal dentition with malposition and extra teeth, brachydactyly, postaxial polydactyly, partial syndactyly between the 2nd and 3rd toes, short stature, learning difficulty | Female, 11 yr, Multiple congenital abnormalities including patent foramen ovale, umbilical hernia, hypospadias with chordee, penile-scrotal fusion, intestinal malrotation, chronic interstitial pulmonary disease, febrile seizure, pharyngeal dysphagia, developmental delay, dysmorphic features (round face, epicanthic folds, hypertelorism, broad arched eyebrows with synophrys, a flat nasal bridge, and a relatively small nose with a bulbous tip), brachycephaly, short neck, macrodonzia, dental malocclusion, chronic otitis media, partial syndactyly between the 2nd and 3rd toes, delayed bone age | |
| Crippa et al[6], 2015 | NA (mother) | Microduplication 16q24.3 (chr16:89,350931–89439639, hg19) | 5 | Mild facial dysmorphisms, similar to those of her children, and a nasal voice | Male, 17 yr. Short, stature, moderate intellectual disability, facial dysmorphisms including long triangular face, frontal bossing, arched and bushy eyebrows with slight synophrys, large and prominent ears, broad and high nasal bridge with bulbous nasal tip, anteverted nares, long philtrum, macrodontia of central incisors, and a nasal voice, brachymetacarpia, third-degree vesicoureteral reflux | Female, 13 yr. Short, stature, moderate intellectual disability, facial dysmorphisms including long triangular face, frontal bossing, arched and bushy eyebrows, large and prominent ears, broad and high nasal bridge with bulbous nasal tip, anteverted nares, long philtrum, macrodontia of central incisors, and a nasal voice, brachymetacarpia, ureterocele associated with duplex pelvicalyceal district |
| Guo et al[7], 2022 | Female, 30-35 yr (mother) | c.5227C>T | Only 2 out of 298 sequencing reads for this variant found in her blood | History of miscarriage, mild facial features, (e.g., synophrys, thick eyebrow, wide nasal bridge, prominent nasal tip) with speech delays and seizures in childhood | Male, 5-10 yr. More severe phenotypic features in comparison to the mother, history of seizures and concurrent speech and motor delays, mitral valve repair at around one year of age, abdominal migraines | |
| p. (Gln1743*) | ||||||
Nevertheless, recent emerging evidence also suggests that somatic mosaicism is found in apparently healthy individuals and increases with age[11].
Our patient presented with short stature (-2SD and mid-parent sex-adjusted target height of 187 cm), that is very common among patients with KBG syndrome, being found in 40%–77% of affected patients[12]. We reported typical but milder features of KBG syndrome[12]: Dysmorphic features (widely spaced eyes, bushy eyebrows, ptosis and large protruding ears), delayed bone age, hand anomalies (clinodactyly and brachydactyly), mild developmental delay and mild ocular involvement (anisotropy and left eye exodeviation). Major problems as epilepsy, intellectual disability, spinal-costal anomalies, heart defects, hearing loss, kidney abnormalities, or feeding problems, were not presented by our patient[3].
Interestingly, our patient presented with extra tarsal persistence of chalazion, with sub-palpebral hematomas; skin and hair abnormalities have been previously reported in KBG syndrome: one patient with a tendency to skin bruising, and delayed wound healing, and another with keloid scarring[3].
Primary subclinical hypothyroidism has been described in KBG syndrome[12], but our patient presented with secondary (pituitary) hypothyroidism. Our patient presented also pituitary hypoplasia, up to now reported as associated to KBG in only one patient[4] who presented with hypogonadotropic hypogonadism at 15 years, and GH deficiency.
Mutations in the transcription factor HESX1 can cause several congenital pituitary defects, ranging from isolated growth hormone deficiency[9,13] to septo-optic dysplasia (SOD) with panhypopituitarism[14]; most patients carry mutations at the heterozygous state, invariably associated with reduced penetrance, and generally show a milder phenotype than the rare homozygous patients[9,15]. According to us, in our patient pituitary hypoplasia, central hypothyroidism and GH deficit might be explained by the variant in HESX1 gene.
In conclusion, we reported for the first time in literature the case of a somatic mosaicism for KBG syndrome, diagnosed in a child with a mild and non-specific phenotype that included short stature, hand abnormalities, distinctive facial dysmorphism and mild developmental delay, in absence of macrodontia consistent with his age. A heterozygous variant in HESX gene, strongly suspected of causing pituitary hypoplasia and hormonal deficiency was also found.
KBG syndrome is likely underdiagnosed because of mild and non-specific features in some affected patients; mosaic forms are even more challenging. Our case underlines that the recognition of mosaicism is important not only for establishing a diagnosis, but also for assessing recurrence risk and for providing genetic counseling to the family. Our paper increases awareness of mild forms of KBG syndrome in children and underlines the importance of NGS analysis for an early genetic diagnosis of mosaic forms.
We appreciate the father for his collaboration.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Society for the Study of Inborn Errors of Metabolism (SSIEM); Società Italiana Malattie Metaboliche e Screening Neonatali.
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mijwil MM, Iraq; Zhang XQ, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Li Q, Sun C, Yang L, Lu W, Luo F. Comprehensive analysis of clinical spectrum and genotype associations in Chinese and literature reported KBG syndrome. Transl Pediatr. 2021;10:834-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Sirmaci A, Spiliopoulos M, Brancati F, Powell E, Duman D, Abrams A, Bademci G, Agolini E, Guo S, Konuk B, Kavaz A, Blanton S, Digilio MC, Dallapiccola B, Young J, Zuchner S, Tekin M. Mutations in ANKRD11 cause KBG syndrome, characterized by intellectual disability, skeletal malformations, and macrodontia. Am J Hum Genet. 2011;89:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 3. | Low K, Ashraf T, Canham N, Clayton-Smith J, Deshpande C, Donaldson A, Fisher R, Flinter F, Foulds N, Fryer A, Gibson K, Hayes I, Hills A, Holder S, Irving M, Joss S, Kivuva E, Lachlan K, Magee A, McConnell V, McEntagart M, Metcalfe K, Montgomery T, Newbury-Ecob R, Stewart F, Turnpenny P, Vogt J, Fitzpatrick D, Williams M; DDD Study, Smithson S. Clinical and genetic aspects of KBG syndrome. Am J Med Genet A. 2016;170:2835-2846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Gao F, Zhao X, Cao B, Fan X, Li X, Li L, Sui S, Su Z, Gong C. Genetic and Phenotypic Spectrum of KBG Syndrome: A Report of 13 New Chinese Cases and a Review of the Literature. J Pers Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Khalifa M, Stein J, Grau L, Nelson V, Meck J, Aradhya S, Duby J. Partial deletion of ANKRD11 results in the KBG phenotype distinct from the 16q24.3 microdeletion syndrome. Am J Med Genet A. 2013;161A:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Crippa M, Rusconi D, Castronovo C, Bestetti I, Russo S, Cereda A, Selicorni A, Larizza L, Finelli P. Familial intragenic duplication of ANKRD11 underlying three patients of KBG syndrome. Mol Cytogenet. 2015;8:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Guo L, Park J, Yi E, Marchi E, Hsieh TC, Kibalnyk Y, Moreno-Sáez Y, Biskup S, Puk O, Beger C, Li Q, Wang K, Voronova A, Krawitz PM, Lyon GJ. KBG syndrome: videoconferencing and use of artificial intelligence driven facial phenotyping in 25 new patients. Eur J Hum Genet. 2022;30:1244-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22536] [Article Influence: 2253.6] [Reference Citation Analysis (0)] |
| 9. | Thomas PQ, Dattani MT, Brickman JM, McNay D, Warne G, Zacharin M, Cameron F, Hurst J, Woods K, Dunger D, Stanhope R, Forrest S, Robinson IC, Beddington RS. Heterozygous HESX1 mutations associated with isolated congenital pituitary hypoplasia and septo-optic dysplasia. Hum Mol Genet. 2001;10:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Jullien N, Saveanu A, Vergier J, Marquant E, Quentien MH, Castinetti F, Galon-Faure N, Brauner R, Marrakchi Turki Z, Tauber M, El Kholy M, Linglart A, Rodien P, Fedala NS, Bergada I, Cortet-Rudelli C, Polak M, Nicolino M, Stuckens C, Barlier A, Brue T, Reynaud R; Genhypopit Network. Clinical lessons learned in constitutional hypopituitarism from two decades of experience in a large international cohort. Clin Endocrinol (Oxf). 2021;94:277-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Campbell IM, Yuan B, Robberecht C, Pfundt R, Szafranski P, McEntagart ME, Nagamani SC, Erez A, Bartnik M, Wiśniowiecka-Kowalnik B, Plunkett KS, Pursley AN, Kang SH, Bi W, Lalani SR, Bacino CA, Vast M, Marks K, Patton M, Olofsson P, Patel A, Veltman JA, Cheung SW, Shaw CA, Vissers LE, Vermeesch JR, Lupski JR, Stankiewicz P. Parental somatic mosaicism is underrecognized and influences recurrence risk of genomic disorders. Am J Hum Genet. 2014;95:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 12. | Scarano E, Tassone M, Graziano C, Gibertoni D, Tamburrino F, Perri A, Gnazzo M, Severi G, Lepri F, Mazzanti L. Novel Mutations and Unreported Clinical Features in KBG Syndrome. Mol Syndromol. 2019;10:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Vivenza D, Godi M, Faienza MF, Mellone S, Moia S, Rapa A, Petri A, Bellone S, Riccomagno S, Cavallo L, Giordano M, Bona G. A novel HESX1 splice mutation causes isolated GH deficiency by interfering with mRNA processing. Eur J Endocrinol. 2011;164:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Tajima T, Hattorri T, Nakajima T, Okuhara K, Sato K, Abe S, Nakae J, Fujieda K. Sporadic heterozygous frameshift mutation of HESX1 causing pituitary and optic nerve hypoplasia and combined pituitary hormone deficiency in a Japanese patient. J Clin Endocrinol Metab. 2003;88:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Giordano M. Genetic causes of isolated and combined pituitary hormone deficiency. Best Pract Res Clin Endocrinol Metab. 2016;30:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |