Published online May 10, 2019. doi: 10.5494/wjh.v9.i2.17
Peer-review started: October 12, 2018
First decision: December 18, 2019
Revised: January 8, 2019
Accepted: March 12, 2019
Article in press: March 12, 2019
Published online: May 10, 2019
Processing time: 209 Days and 12.6 Hours
Pulmonary hypertension (PH) is a progressive disease with a high morbidity and mortality rate; and neointima formation leads to the irreversibility of the disease. We have previously reported that in rats, monocrotaline (MCT) injection leads to progressive disruption of endothelial cells (EC), and endothelial caveolin-1 (cav-1) loss, accompanied by the activation of pro-proliferative pathways leading to PH. Four weeks post-MCT, extensive endothelial cav-1 loss is associated with increased cav-1 expression in smooth muscle cells (SMC). Exposing the MCT-treated rats to hypoxia hastens the disease process; and at 4 wk, neointimal lesions and occlusion of the small arteries are observed.
To identify the alterations that occur during the progression of PH that lead to neointima formation.
Male Sprague-Dawley rats (150-175 g) were divided in 4 groups (n = 6-8 per group): controls (C); MCT (M, a single sc injection 40 mg/kg); Hypoxia (H, hypobaric hypoxia); MCT + hypoxia (M+H, MCT-injected rats subjected to hypobaric hypoxia starting on day1). Four weeks later, right ventricular systolic pressure (RVSP), right ventricular hypertrophy (RVH), lung histology, and cav-1 localization using immunofluorescence technique were analyzed. In addition, the expression of cav-1, tyrosine 14 phosphorylated cav-1 (p-cav-1), caveolin-2 (cav-2), cavin-1, vascular endothelial cadherin (VE-Cad) and p-ERK1/2 in the lungs were examined, and the results were compared with the controls.
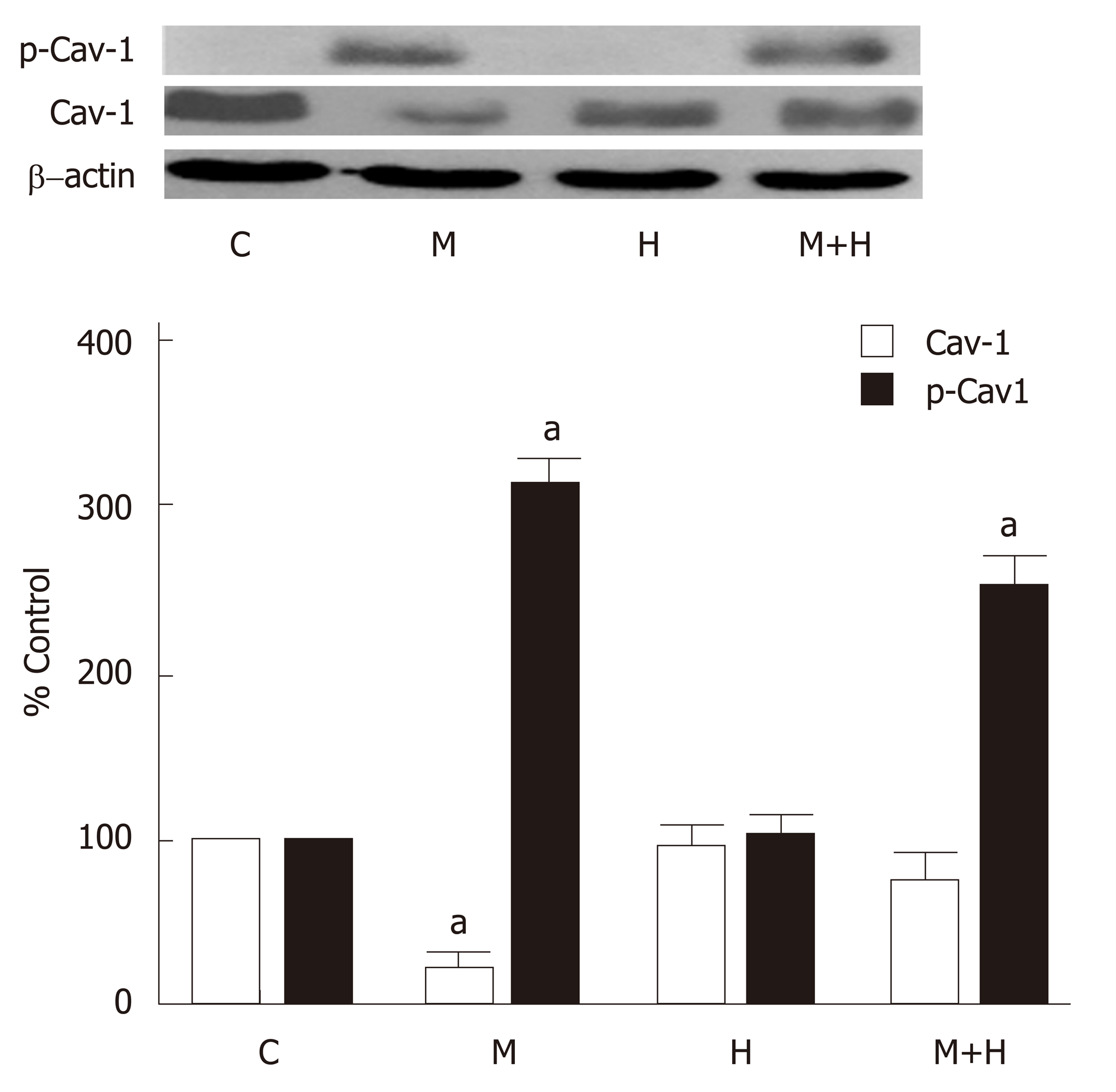
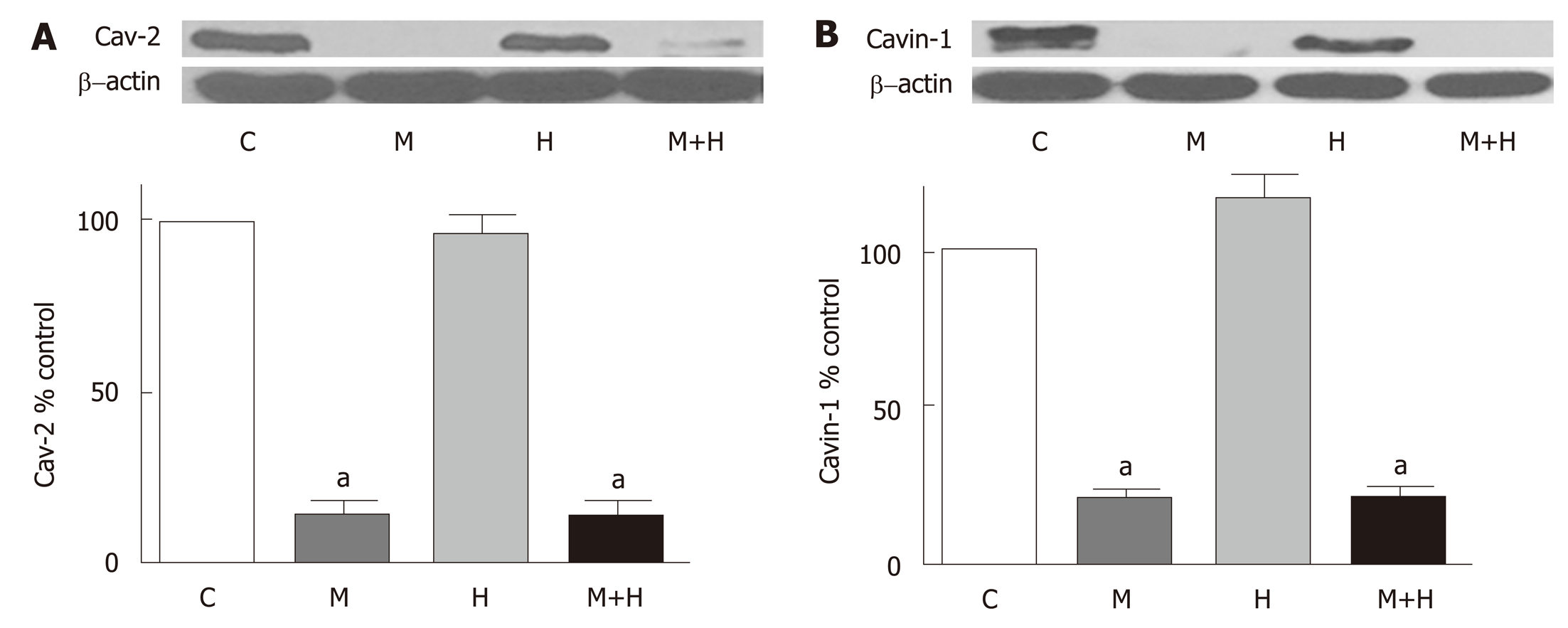
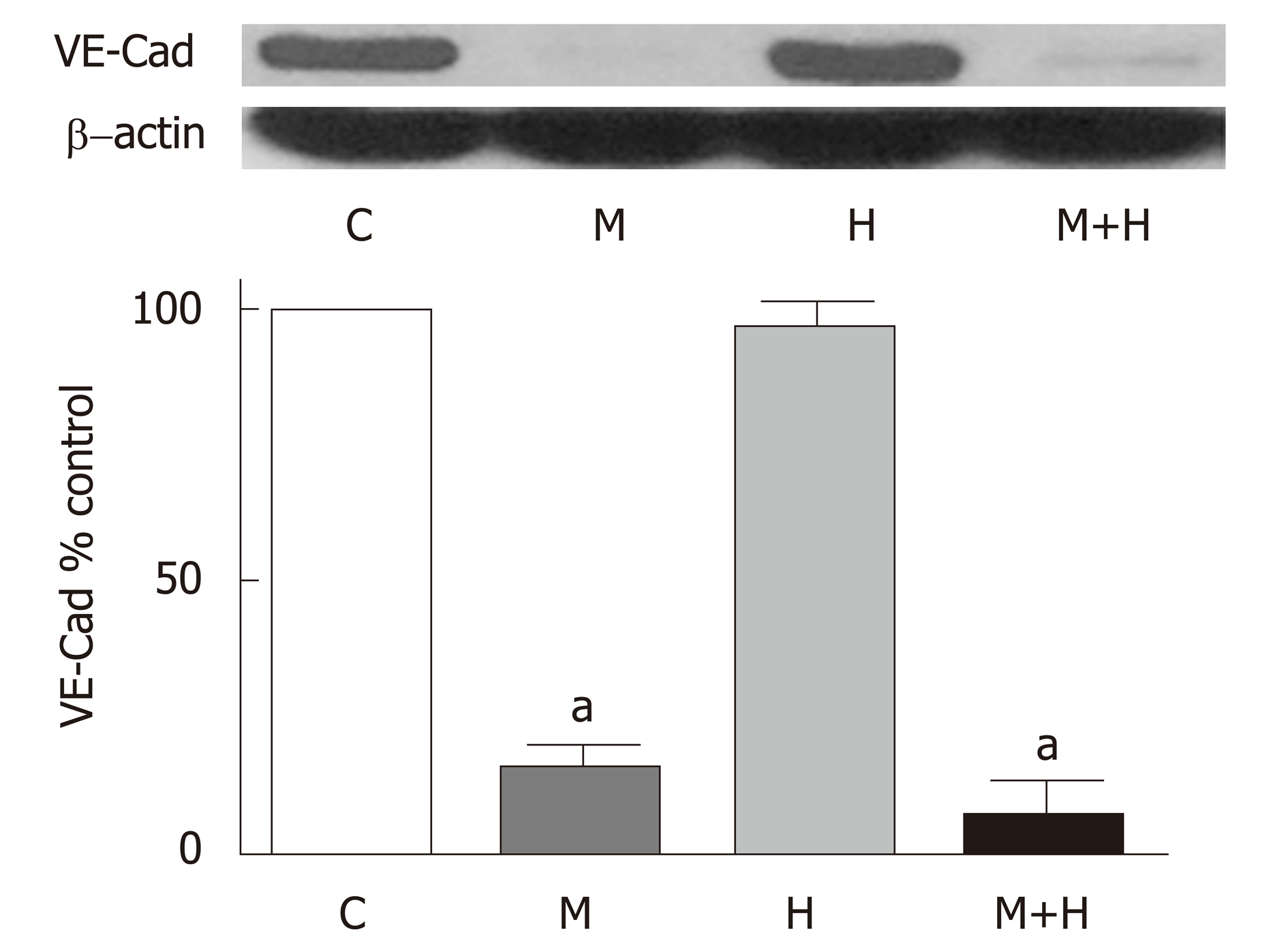
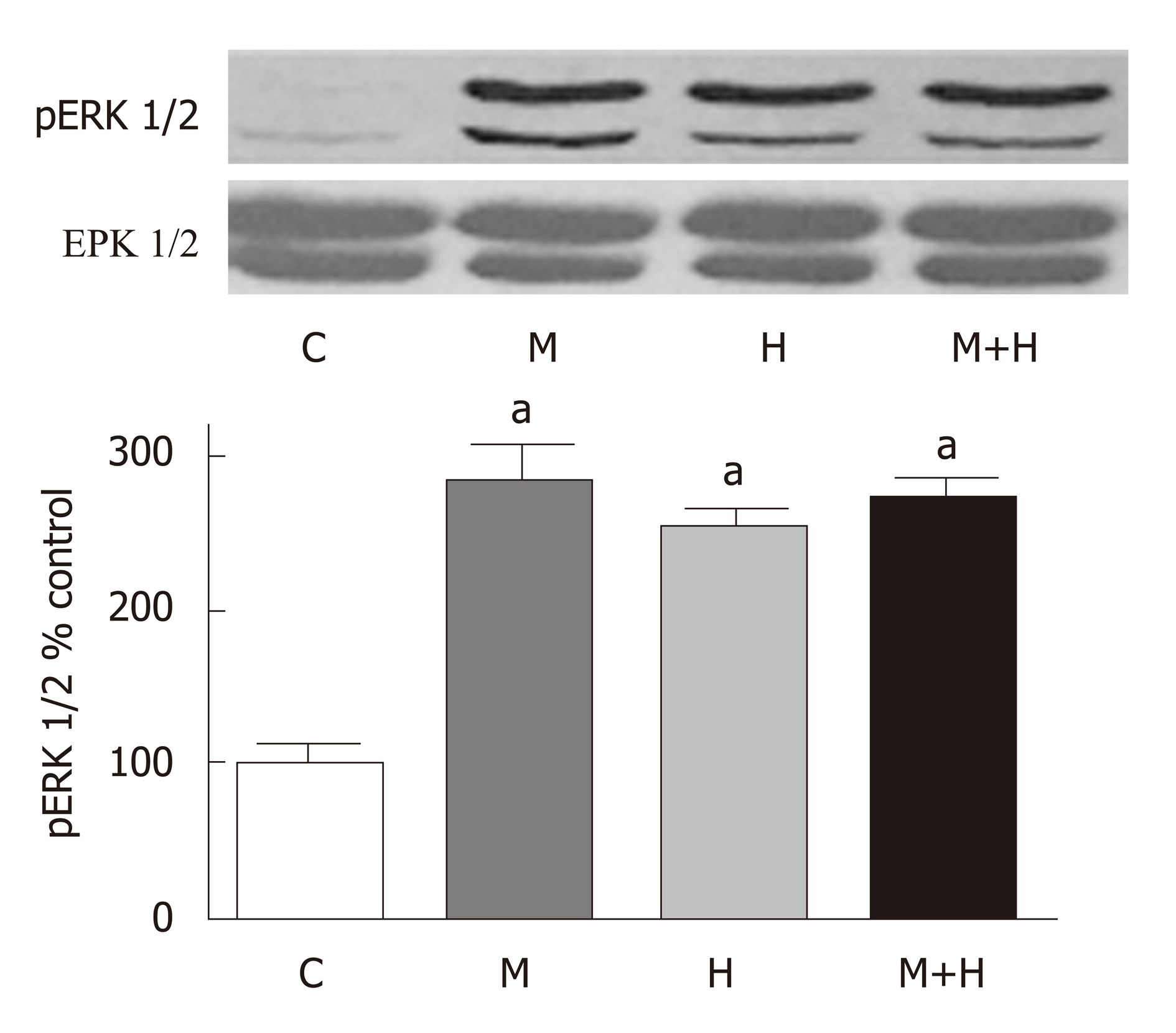
Significant PH and right ventricular hypertrophy were present in M and H groups [RVSP, mmHg, M 54±5*, H 45±2*, vs C 20±1, P < 0.05; RVH, RV/LV ratio M 0.57±0.02*, H 0.50±0.03*, vs C 0.23±0.007, P < 0.05]; with a further increase in M+H group [RVSP 69±9 mmHg, RV/LV 0.59±0.01 P < 0.05 vs M and H]. All experimental groups revealed medial hypertrophy; but only M+H group exhibited small occluded arteries and neointimal lesions. Immunofluorescence studies revealed endothelial cav-1 loss and increased cav-1 expression in SMC in M group; however, the total cav-1 level in the lungs remained low. In the M+H group, significant endothelial cav-1 loss was associated with increasing expression of cav-1 in SMC; resulting in near normalization of cav-1 levels in the lungs [cav-1, expressed as % control, C 100±0, M 22±4*, H 96±7, M+H 77±6, * = P < 0.05 vs C]. The expression of p-cav-1 was observed in M and M+H groups [M 314±4%, M+H 255±22% P < 0.05 vs C]. Significant loss of cav-2 [% control, C 100±0, M 15±1.4*, H 97±7, M+H 15±2*; M and M+H vs C, * = P < 0.05], cavin-1 [% control, C 100±0, M 20±3*, H 117±7, M+H 20±4*; M and M+H vs C, P < 0.05] and VE-Cad [% control, C 100±0, M 17±4*, H 96±9, M+H 8±3*; M and M+H vs C, P < 0.05] was present in M and M+H groups, confirming extensive disruption of EC. Hypoxia alone did not alter the expression of cav-1 or cav-1 related proteins. Expression of p-ERK1/2 was increased in all 3 PH groups [%control, C 100±0, M 284±23*, H 254±25*, M+H 270±17*; * = P < 0.05 vs C].
Both cavin-1 loss and p-cav-1 expression are known to facilitate cell migration; thus, these alterations may in part play a role in neointima formation in PH.
Core tip: Neointima leads to irreversible pulmonary hypertension (PH). Subjecting the monocrotaline (MCT)-injected rats to hypoxia accelerates the disease process; by 4 wks, neointima develops in arteries with extensive disruption of endothelial cells (EC), loss of endothelial caveolin-1 (cav-1), and enhanced cav-1 expression in smooth muscle cells. In addition, the MCT and MCT+ hypoxia groups exhibit significant loss of cavin-1 and the presence of tyrosine phosphorylated cav-1, which may facilitate cell migration and neointima formation. Hypoxia-induced PH does not exhibit EC disruption or alterations in cav-1 expression. Thus, EC integrity may determine the reversibility or irreversibility of PH.
- Citation: Huang J, Mathew R. Loss of cavin1 and expression of p-caveolin-1 in pulmonary hypertension: Possible role in neointima formation. World J Hypertens 2019; 9(2): 17-29
- URL: https://www.wjgnet.com/2220-3168/full/v9/i2/17.htm
- DOI: https://dx.doi.org/10.5494/wjh.v9.i2.17
Pulmonary hypertension (PH), a sequel of a number of unrelated systemic diseases, has a high morbidity and mortality rate. Regardless of the underlying disease, endothelial dysfunction and/or disruption plays a pivotal role in initiating vascular changes such as impaired vascular relaxation, and the activation of proliferative and anti-apoptotic pathways; subsequently leading to medial hypertrophy, elevated pulmonary artery pressure and right ventricular hypertrophy (RVH). Progressive pulmonary vascular changes lead to neointima formation resulting in the irreversibility of PH[1]. Significant advances have been made in the field of PH since it was described by Ernst von Romberg in 1891. Modern therapy has improved the quality of life; however, the vascular changes continue unabated[2,3]. One and 3-year survival rate for the patients with pulmonary arterial hypertension (PAH, Gr 1) is reported as 85%, and 58% respectively[4,5]. The mechanism of PAH, especially the neointima formation is not yet understood. The situation is compounded by the fact that the diagnosis of PH is often made after significant pathological changes have already taken place in the pulmonary vasculature.
The experimental models have significantly contributed to the understanding of the pathogenesis of PH. We have previously shown that a single subcutaneous injection of monocrotaline (MCT) results in the disruption of endothelial cells (EC), loss of endothelial cavoelin-1 (cav-1) and reciprocal activation of proliferative and antiapoptotic pathways such as PY-STAT3, Bcl-xL, and increased proliferating cell nuclear antigen (PCNA) expression before the onset of PH. The endothelial disruption and endothelial cav-1 loss are progressive; and PH is observed at 2 wk post-MCT. At 4 wk post-MCT, 85% arteries exhibited loss of endothelial cav-1; and a loss of von Willebrand factor (vWF) was observed in about 29% of pulmonary arteries. Loss of vWF was present only in the arteries with endothelial cav-1 loss. The loss of vWF, accompanied by the endothelial cav-1 loss, is indicative of extensive EC disruption and/or loss. At this stage, about 23% of the arteries, only with combined vWF and endothelial cav-1 loss exhibited enhanced expression of cav-1 in SMC[6,7]. It is worthy of note, that the increased levels of circulating vWF[8], and EC[9], have been reported in patients with irreversible PAH. Importantly, loss of endothelial cav-1 associated with enhanced expression of cav-1 in smooth muscle cells (SMC) has been observed in PAH associated with congenital heart defect[10], drug toxicity[11] and in idiopathic and heritable PAH[12,13]. Subjecting MCT-treated rats to hypobaric hypoxia accelerated the disease process; and by 4 wks, neointimal lesions were observed. Importantly, neointimal lesions were seen only in the arteries that exhibited extensive loss of endothelial cav-1 and enhanced expression of cav-1 in SMC[12]. Furthermore, SMC from idiopathic PAH in in-vitro studies have revealed enhanced expression of cav-1, increased capacitative Ca2+ entry and DNA synthesis, which can be attenuated by silencing cav-1[13], indicating that this cav-1 in SMC has become pro-proliferative.
ECs form a semi-permeable monolayer between the circulating blood and the underlying tissue. Cav-1, a 22 kD membrane protein is localized in caveolae, flask-shaped lipid rafts found on the plasma membrane. Cav-1 is expressed in most cell types such as EC, SMC, epithelial cells, fibroblasts and adipocytes. Cav-1 interacts with transducing factors that are present in or are recruited to caveolae; and it maintains them in a negative conformation. However, cav-1 has been shown to have a pro-proliferative aspect depending on the disease state and the cell context[14]. Interestingly, tyrosine 14, phosphorylated cav-1 (p-cav-1) is thought to inactivate growth inhibitory function of cav-1 scaffolding domain, and facilitate cell migration[15,16].
Polymerase 1 and transcript release factor also known as cavin-1 is an essential component of caveolae; it regulates membrane curvature by stabilizing cav-1 in caveolae. The loss of cavin-1 results in the loss of caveolae, and cav-1 release into the plasma membrane. Importantly, cav-1 is required for cavin-1 recruitment to plasma membrane[17,18]. In a carotid artery injury model, the local loss of cavin-1 is reported to promote neointima formation. Furthermore, in cultured vascular SMC, the overexpression of cavin-1 suppresses SMC proliferation and migration, whereas its inhibition promotes cell proliferation and migration[19].
The foregoing studies indicate that the loss of cavin-1 and p-cav-1 expression contribute to cell migration, and thus, facilitate neointima formation. Our aim was to elucidate whether similar changes occur in PH that might explain the mechanism of neointima formation. We analyzed the expression of cav-1, p-cav-1 and cav-1-related proteins cavin-1, caveolin-2 (cav-2), vascular endothelial cadherin (VE-Cad), and p-ERK1/2 in rats treated with MCT, hypobaric hypoxia and a group treated with MCT and exposed to hypoxia.
Protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College, and conform to the guiding principles for the use and care of laboratory animals of the American Physiological Society and the National Institutes of Health. ARRIVE guidelines were adopted.
Male Sprague-Dawley rats (age 6-8 wk, weight 150-175 g, Charles River Wilmington, MA) were allowed to acclimatize in our animal facility for 5 days before the start of the experimental protocols. They were allowed free access to laboratory chow and water. Rats were divided in 4 groups (6-8/group): Group 1; control rats (C) maintained in room air; Group 2, rats received a single subcutaneous injection of MCT (40 mg/kg, M), and kept in room air. Group 3, rats in this group were subjected to hypobaric hypoxia (atmospheric pressure 380 mmHg, H); Group 4, rats in this group received MCT 40 mg/kg and were subjected to hypobaric hypoxia starting on day 1 (M+H). We maintained 2-3 similarly treated rats in a cage. The hypoxia chamber was opened twice per week for 15 min to weigh the rats, replenish food and water, and to provide clean bedding. The rats were maintained in our Animal Facility; four weeks later, these rats were brought to the Laboratory for hemodynamic measurements and tissue collection.
All chemicals including MCT were purchased from Sigma Aldrich, St Louis, MO. Antibodies: caveolin-1 (cav-1) (sc-894, polyclonal, sc-535641, monoclonal) and VE-Cadherin (VE-Cad) (sc-64580) were bought from Santa Cruz laboratories, CA, United States; Tyr14 Phospho-Cav-1(610684) and Caveolin-2 (610684) were obtained from BD Transduction Laboratories, Palo Alto, CA; Cavin-1 (ab48824) from Abcam laboratories and p-ERK1/2 (4370) and ERK1/2 (4694) from Cell Signaling, Beverley, MA, and β-actin (A5441) from Sigma Aldrich. Secondary antibodies, Alexa 488 (donkey anti-rabbit, green color) and Alexa 594 (donkey anti-mouse, red color) were purchased from Molecular Probes, Eugene, OR.
On the day of experiment, rats were anesthetized with an intraperitoneal injection of ketamine (60 mg/kg) and xylazine (5 mg/kg), as approved by our Institution. The trachea was exposed through an incision in the neck, and cannulated with PE 240 tubing, and the rat ventilated in room air at a rate of 70-80 breaths/min. The chest was opened, PE 50 tubing inserted into the right ventricle (RV), and the pressure recorded on a Grass polygraph (model 7E). At the end of the pressure measurements, the lungs were perfused with autoclaved normal saline to remove blood. The left lung and the heart were placed in 10% buffered formalin. The right lung was quickly frozen in liquid nitrogen and stored at -80 oC for protein extraction at a later date.
A week later, the heart was removed from formalin and atria trimmed. The free wall of the RV was separated from the left ventricle and the septum (LV). The ratio of RV/LV was calculated to assess RVH.
Western blot analysis was carried out as previously described[7]. Briefly, the Lung tissue was homogenized in a buffer containing 0.1M PBS (pH 7.4), 0.5% sodium deoxycholate, 1% igepal, 0.1% SDS, and 10 μL/mL phenylmethylsulfonic fluoride, 25 μg/mL aprotinin, and 25 μg/mL leupeptin, and phosphatase inhibitor cocktail 1 (10 μL/mL) were added to homogenates placed on ice for 30 min, and centrifuged at 15000 rpm for 20 min at 4 oC. Protein concentrations in the supernatants were analyzed by Lowry’s method, using serum albumen as standard (Bio-Rad kit). Fifty or 100 μg of protein from lung supernatants were loaded and separated on a 10% sodium SDS-polyacrylamide gel (Mini Protean-II, Bio-Rad) and transferred to nitrocellulose membrane (hybond ECL, Amersham BioSciences, Piscataway, NJ, United States) using Semi Dry Transfer Cell (Bio-Rad). The membranes were blocked with 5% nonfat milk powder in Tris-Buffered-saline with Tween buffer (TBST, 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween 20) at room temperature for one hour. Membranes were then incubated with p-cav-1 (1:500), cav-1 (1:7000), caveolin-2 (1:500), cavin-1 (1: 2000), VE-Cad (1:500), or p-Erk1/2 (1:2000), overnight at 4 oC. The membranes were washed for 10 min X 3 with TBST and incubated with appropriate secondary antibody for 1 hour at room temperature, then washed for 10 minX3 with TBST buffer. The membranes were reprobed with β actin (1:10000) or Erk (1:2000) as appropriate to assess protein loading. The relative expression of the proteins was quantified using densitometric scanning and is expressed as % normal.
Formalin preserved lung tissues were processed for paraffin blocks. Five to 6 μm sections were cut and stained with H&E stain for the evaluation of pulmonary vessels. Double immunofluorescence was carried out as described previously[7,12]. Briefly, lung sections were deparaffinized in xylene (5 min X 2), rehydrated through a range of aqueous ethyl alcohol solution in H2O and immersed in PBS for 5 min. Antigen retrieval was performed by incubating the sections in 10 mM citrate buffer (pH 6), in a microwave oven for 5 min. The slides were then incubated in blocking solution (5% normal donkey serum, 0.5% Triton in PBS) for 1 hr at room temperature, followed by an overnight incubation at 4 oC with the primary antibody (cav-1α, 1:100) in blocking solution. The next day, the slides were washed with PBS for 10 minX3 and incubated in the secondary antibody, Alexa 488 (1:300, green color) at room temperature in a dark place for 1 hr followed by washing in PBS for 30 minX3. For double immunostaining, the sections were blocked again, as described earlier, and incubated overnight in the primary antibody smooth muscle α-actin (1:15); the procedure was repeated using the secondary antibody (Alexa 594, 1:200, red color). The sections were examined with a laser scanning confocal fluorescence microscope (MRC 1000, Bio-Rad).
The data are expressed as means ± SE. For statistical analysis, one-way analysis of variance followed by Scheffe’s multiple comparison tests was used. Differences were considered statistically significant at P < 0.05.
The four-week weight gain in the control group was 188±11 g. The hypoxia group gained 181 g ± 20 g (not significantly different compared with the controls). The weight gain in the M group was 130 g ± 17 g (P < 0.05 vs the control group). In the M+H group, the weight gain was significantly reduced (45 g ± 17 g, P < 0.05 vs Controls, H and M groups). Two out of 8 rats in the M + H group lost weight during the 4-wk period.
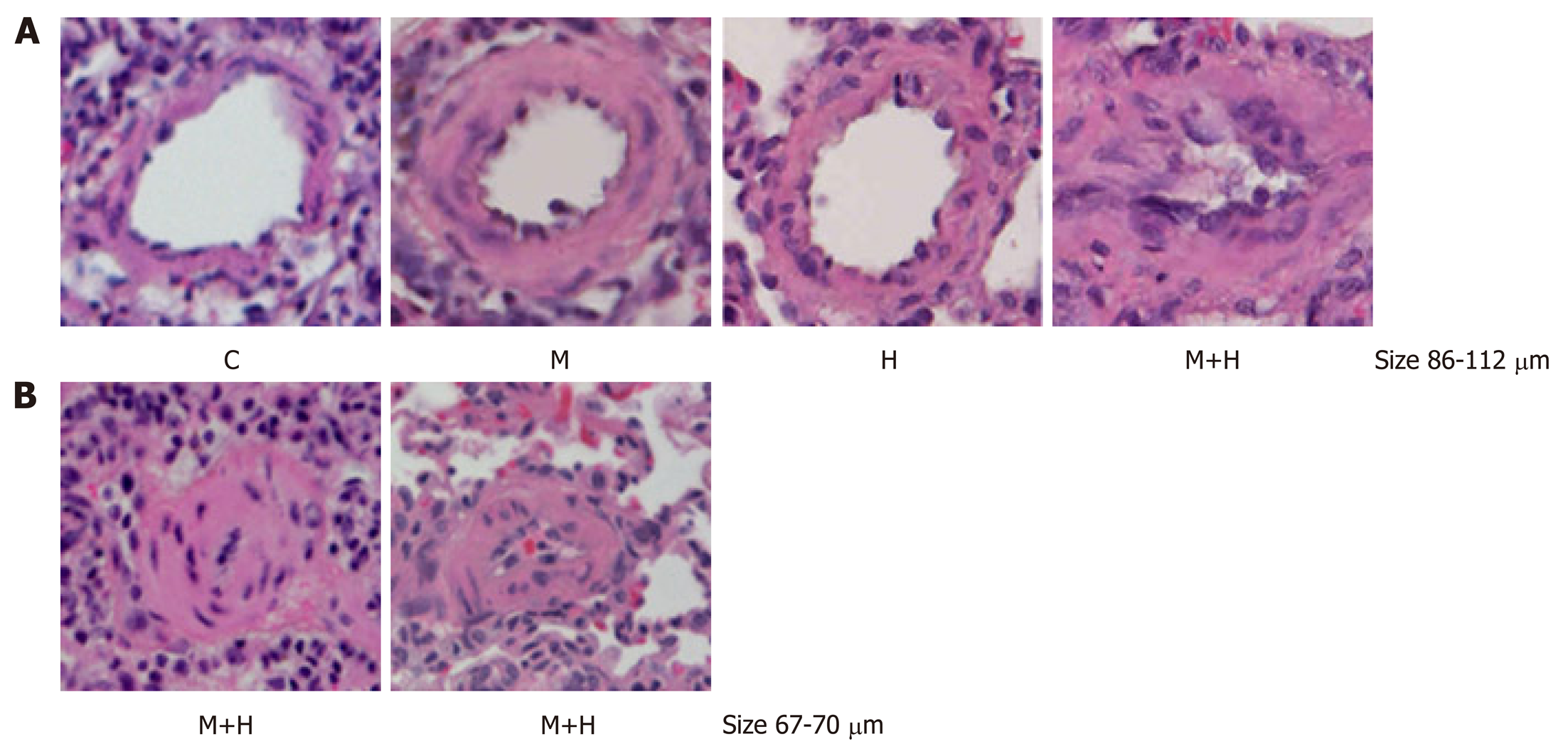
As shown in Figure 1, both M and H group revealed significantly increased right ventricular systolic pressure (RVSP) and RVH compared with the controls. RVSP and RVH were significantly higher in the M+H group compared with M and H groups. Significant medial thickness was present in the M and H group. In the M+H groups there was a further increase in medial thickening including the presence of neointimal lesion, and complete occlusion of lumens of smaller arteries (Figure 2).
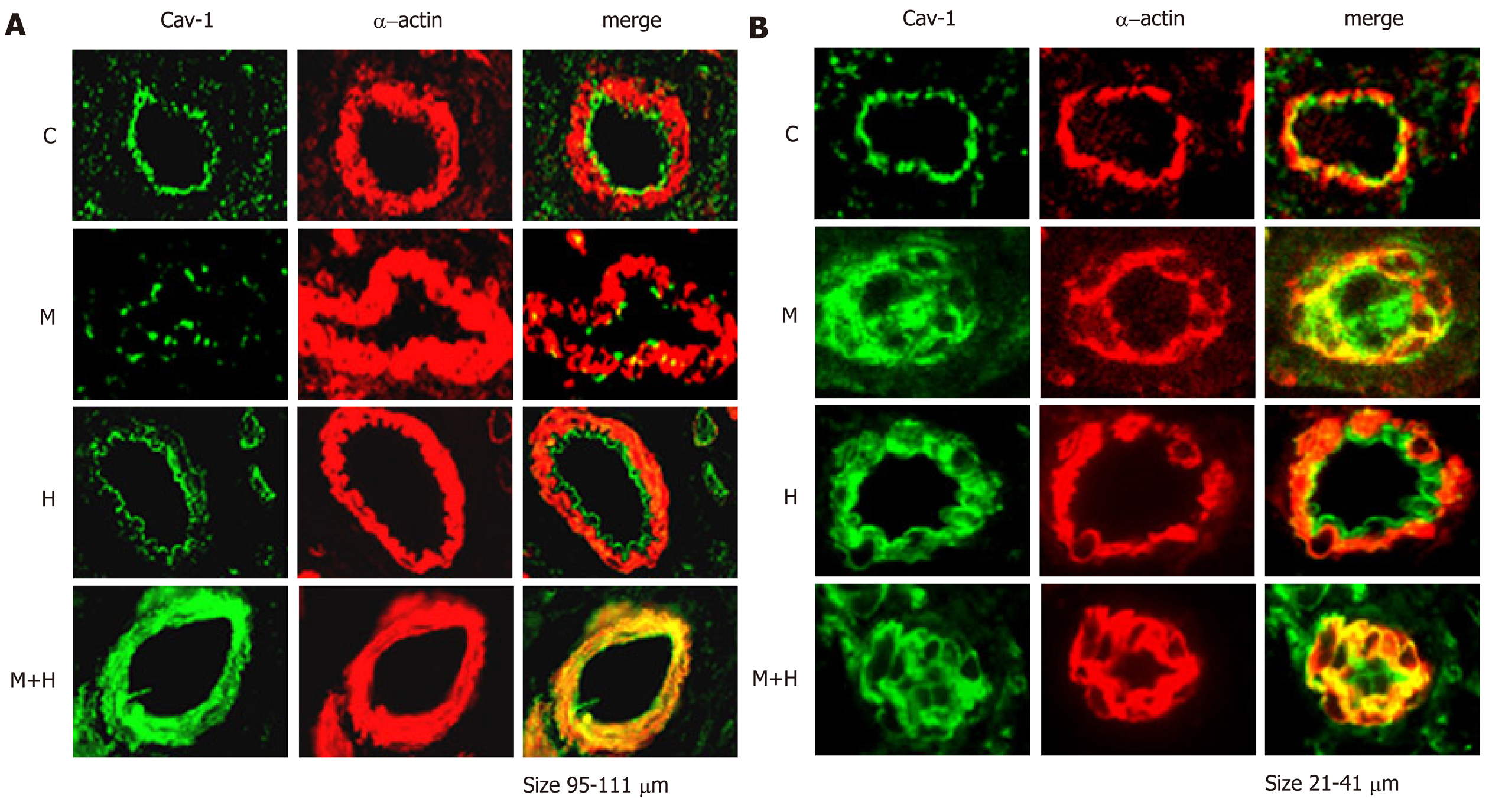
Double immunofluorescence studies were conducted to visualize the expression of cav-1 and smooth muscle α-actin. The expression of cav-1 is significantly decreased in the larger arteries (95–111 µm) from the M and M+H groups; and enhanced expression of cav-1 in SMC is observed in the M+H group (Figure 3A). Smaller arteries (21-41 µm) from M and M+H groups exhibited a significant loss of endothelial cav-1 accompanied by enhanced expression of cav-1 in SMC (Figure 3B). It is significant, that in the H group, despite increased pulmonary artery pressure, the endothelial cav-1 is not lost, nor is there enhanced expression of cav-1 in SMC.
Consistent with our previous observations[12], western blot analysis revealed a significant loss of cav-1 expression in the M group, but it is closer to normal in the M+H group (Figure 4). As shown in Figure 4, the expression of p-cav-1 is increased in the M and M+H groups. The H group, however, did not exhibit a loss of endothelial cav-1 or enhanced cav-1 expression in SMC, or the expression of p-cav-1.
In the present study, a significant loss of cav-2 is observed in the M and M+H groups (Figure 5A). Similarly, as shown in Figure 5B, there is a significant loss of cavin-1 expression in M and M+H groups. Hypoxia alone has no effect on the expression of cav-2 or cavin-1.
Since MCT-induced PH is associated with the progressive loss of endothelial cav-1; we examined the VE-Cad expression in the lungs. The expression of VE-Cad is significantly reduced in the M and M+H groups compared with controls, but it is not altered in the H group (Figure 6).
As shown in Figure 7, the expression of p-ERK1/2 is increased in the experimental groups (M, H and M+H).
The significant results of the present study are that MCT-treated rats exposed to hypoxia (M+H) develop higher RVSP and RVH compared with hypoxia (H) and MCT (M) alone groups; and furthermore, in the M+H group, neointimal lesions and occlusion of small arteries are seen. Significant loss of endothelial cav-1, cav-2, VE-Cad and cavin-1 proteins is observed in the M and M+H groups, indicating extensive EC disruption/damage. In addition, M and M+H groups exhibit enhanced expression of cav-1 in SMC accompanied by p-cav-1 expression. In all experimental groups, expression of p-ERK1/2 is increased. In the H group, there are no alterations in the expression of endothelial cav-1or cav-1-related proteins indicating that there is no EC disruption; nor is there enhanced expression of cav-1 in SMC or the presence of p-cav-1.
Progressive endothelial damage accompanied by endothelial cav-1 loss/dysfunc-tion plays a critical role in the pathogenesis of PH. Cav-1 regulates a number of signal transduction pathways that participate in cell proliferation, cell cycle and apoptosis. Modulation of signaling by cav-1 has an important implication in the development and progression of PH[20]. Cav-1 knockout mice develop PH, that is attenuated by cav-1 re-expression[21,22]. Cav-1 polymorphism has been shown to be associated with PAH[23]. As stated earlier, endothelial cav-1 loss has been observed in idiopathic PAH, heritable PAH and PAH associated with congenital heart defect and drug toxicity[10-12]. In the MCT model, we have previously shown the loss of endothelial cav-1 accompanied by a loss of several endothelial membrane proteins such as Tie2, PECAM-1 and soluble guanylate cyclase, indicative of extensive endothelial cell membrane damage[7]. PECAM-1 supports EC integrity and maintains barrier function[24]. In the present study, significant loss of VE-Cad is observed in the M and M+H groups. VE-Cad belongs to a family of calcium-dependent adhesion proteins responsible for adherence junction and barrier structure. It is tissue specific for EC and is expressed at the intercellular clefts of EC; and mediates cell-cell adhesion, maintains barrier function and contributes to the inhibition of cell growth[25-27]. VE- Cad provides EC junctional stability and controls vascular permeability. It interacts with various growth receptors and regulates endothelial proliferative signaling; and mediates contact inhibition of cell growth[28]. Furthermore, depletion of cav-1 reduces VE-Cad levels and facilitates endothelial cell permeability[29,30].
Consistent with our previous studies, a significant loss of cav-2 expression is present in M and M+H groups, but not in the H group[12]. In all cell types, cav-2 has been shown to be present associated with cav-1. It requires cav-1 for its transport from Golgi body to the plasma membrane. In the absence of cav-1, cav-2 is degraded; and its loss promotes increased cell proliferation[31,32]. Cav-2 knockout- mice have been shown to display increased proliferation of EC, hyper-cellular lung parenchyma, and cell cycle progression. Furthermore, cav-2 is thought to suppress ERK1/2 pathway[32,33]. Thus, the loss of cav-2 and VE-Cad would have negative effect on membrane permeability, and facilitate cell proliferation; thus, aggravate pulmonary vascular pathology. Interestingly, in hypoxia-induced PH, despite normal endothelial cav-1 expression, an increased activation of p-ERK1/2 is observed, suggestive of cav-1 dysfunction in hypoxia-induced PH.
Caveolae are specialized micro-domains, forming small invaginations. Caveolae act as platforms for multiple signaling activities. Cav-1 is the major protein in caveolae. Cav-1 recruits cavin-1 to the membrane; and cavin-1 is essential for caveolae formation and the stabilization of cav-1[17,18]. Loss of cav-1 is accompanied by a marked loss of cav-2 and partial reduction in cavin-1 expression in the lungs. The re-expression of cav-1 rescues cav-2 and cavin-1[34]. Cavin-1 knockout mice display lung pathological changes such as remodeled pulmonary vessels and PH. In addition, these mice have been reported to have altered metabolic phenotype, associated with, glucose intolerance, insulin resistance and dyslipidemia[35,36]. Furthermore, caveolae act as plasma membrane sensors that respond to stress[37]. Caveolae flatten in response to membrane stretch. The flattening is thought to be a protective mechanism; it buffers the membrane and prevents its rupture. However, the flattening of the membrane leads to cav-1 and cavin-1 dissociation[38]. Cavin-1 loss has been shown to promote neointima formation; and the over expression of cavin-1 suppresses vascular SMC proliferation and migration[19]. In advanced prostate cancer, cav-1 which is present in non-caveolar site because of the lack of cavin-1 is associated with progressive form of the disease; and cavin-1 re-expression neutralizes non-caveolar cav-1 in prostate cancer and reverses its effects, and decreases cell migration[39,40]. In the present study, M and M+H models of PH show loss of cavin-1, indicative of loss of caveolae. This would suggest that cav-1 in SMC is in non-caveolar site. Stretch-induced translocation of cav-1 to non-caveolar site in vascular SMC has been shown to mediate ERK activation[41]. In addition, it has recently been reported that plasma membrane stretch activates L-type voltage-dependent Ca2+ channels via tyrosine phosphorylated cav-1 and the activation of JNK in mesenteric arterial SMC[42]. This observation further supports the view that cav-1 function in non-caveolar site is distinctly different from cav-1 in caveolae[43].
Extensive endothelial cav-1 loss is accompanied by enhanced expression of cav-1 in SMC; and progressive increase in the expression and activity of matrix metalloproteinase 2 at 2 and 4 wk post-MCT[7,44]. The present study shows the loss of endothelial cav-1 accompanied by a loss of cav-2, VE-Cad and cavin-1 proteins in the M and M+H groups. These losses are indicative of extensive EC damage and/or loss, which would expose SMC to increased shear stress and strain because of the elevated pulmonary artery pressure. Vascular SMC are reported to be unresponsive to mitogens under normal tensile stress. During altered mechanical stress, protein synthesis in these cells is upregulated in response to growth factors, thus, leading to cell proliferation and neointima formation. Furthermore, during cell cyclic strain, cav-1 is thought to be involved in proliferation signaling, stretch-induced activation and cell cycle entry. Interestingly, exposure of veins to arterial pressure for 15 minutes in wild type mice results in AKT and ERK activation; the response was attenuated in cav-1 knockout mice[45,46]. Thus, it is likely that non-caveolar cav-1 in SMC in PH acts as a facilitator of cell migration.
Endothelial cav-1 loss has been shown to result in the activation of proliferative and anti-apoptotic pathways, and as the disease progresses, the continued loss of endothelial cav-1 and EC disruption results in the enhanced expression of cav-1 in SMC, which has been shown to be pro-proliferative[12,13]. Importantly, cav-1 contains a tyrosine 14 residue, a target site for phosphorylation by non-receptor tyrosine kinases; and phosphorylation at this site is associated with cell migration[16]. Cell migration is essential for neointima formation. Cav-1 has been shown to get phosphorylated at Tyr14 in response to various stimuli. It is thought to be dependent on Src kinase activity and Rho/ROCK signaling. P-cav-1 has been linked to focal adhesion stability and directional cell migration and extracellular matrix remodeling. There is evidence that tyrosine phosphorylated cav-1 is required for cell migration and metastasis; in cancer, upregulated cav-1 correlates with tumor progression. Furthermore, cav-1 phosphorylation has been reported to induce Rho activation and stabilize focal adhesion kinase and promote cell migration[47-49]. It is likely that in M and M+H models, enhanced expression of cav-1 in SMC, and increased expression of p-cav-1 coupled with cavin-1 loss may facilitate cell migration and contribute to neointima formation.
In summary, EC integrity and cav-1 play a key role in the maintenance of vascular structure and homeostasis. Our studies underscore the view that endothelial cav-1 dysfunction (in hypoxia model) or endothelial cav-1 loss (in MCT and MCT + hypoxia models) is an important initiating mechanism of PH. In the M and M+H models, progressive disruption of EC and the endothelial cav-1 loss is accompanied by the loss of cav-2, VE-Cad and cavin-1. These alterations are sufficient to activate proliferative pathways such as p-ERK1/2, and facilitate vascular remodeling and PH. Loss of endothelial cav-1 is followed by enhanced expression of cav-1 in SMC and the appearance of p-cav-1. These alterations coupled with the loss of cavin-1 would facilitate cell proliferation, migration and neointima formation, thus progress toward irreversible PH. In the hypoxia-induced PH there was no evidence of EC disruption or loss of endothelial cav-1, cavin-1, cav-2, VE-Cad or enhanced expression of cav-1 in SMC. Despite the presence of endothelial cav-1, p-ERK1/2 is activated indicating that the endothelial cav-1 is dysfunctional. However, EC integrity is maintained during hypoxia-induced PH, which may be the reason why it is reversible on normoxic exposure.
A number of cardiopulmonary and other systemic diseases are known to lead to pulmonary arterial hypertension (PAH), a serious sequel with a high mortality rate. The features of PAH are: impaired vascular relaxation, activation of proliferative pathways; smooth muscle cells (SMC) proliferation, medial hypertrophy, narrowing of the lumen, elevated pulmonary artery pressure and right ventricular hypertrophy (RVH). As the disease progresses, neointima is formed which leads to the irreversibility of PAH. Despite major advances in the field, the cure is not yet available. The diagnosis of PAH is often delayed because of the vague symptoms. This is not surprising because we have previously shown in the monocrotaline (MCT) model of pulmonary hypertension (PH), endothelial damage, progressive loss of endothelial caveolin-1 (a membrane protein) and the activation of proliferative pathways occur before the onset of PH.
By exposing the MCT-treated rats to hypoxia, the disease process is accelerated and by 4 weeks, neointimal lesions are seen, which gives us an excellent opportunity to examine the mechanism of neointima formation. If we can unravel the mechanism of neointima formation, it will allow us to expand the experimental studies to further the knowledge which will aid us in designing curative treatment.
The main objective of the study was to examine the mechanism of neointima formation which renders the diseases irreversible. Our most important observation is that the significant endothelial damage and loss of endothelial caveolin-1 is accompanied by loss of cavin-1 and caveolae, and “enhanced” expression of caveolin-1 in SMC, that is tyrosine phosphorylated. It is possible that these alterations are at least in part responsible for neointima formation.
We divided male Sprague-Dawley rats (age 6-8 wks) in 4 groups (6-8/per gr.): Gr. 1 Controls, Gr. 2 (received a single sc injection of MCT 40 mg/kg), Gr.3 (rats in the group were exposed to hypobaric hypoxia) and Gr. 4 (MCT-injected rats were exposed to hypobaric hypoxia starting on day 1). These rats were studied 4 weeks later. Hemodynamic data and RVH were assessed. In addition, we evaluated the histological changes in the pulmonary arteries and the expression of caveolin-1 using immunofluorescence technique. The expressions of caveolin-1, p-caveolin-1, caveolin-2, VE-cadherin (VE-Cad), Cavin-1 and p-Erk1/2 in the lungs were examined using western blot technique.
Significant loss of caveolin-2, VE-Cad, Cavin-1, indicative of significant endothelial damage was observed in MCT groups. In the MCT group, there was a significant loss of endothelial caveolin-1, and enhanced caveolin-1 expression in SMC in a few arteries; however, the total cav-1 expression in the lungs remained low. In MCT + hypoxia group, a significant loss of endothelial caveolin-1 was accompanied by enhanced expression of caveolin-1 in SMC in a greater number of arteries, which almost normalized the lung caveolin-1 levels. The loss of cavin-1 suggests that the caveolin-1 is not in caveolae, because cavin-1 is essential for the maintenance of caveolar curvature, and in addition, it stabilizes caveolin-1 in caveolae. Furthermore, caveolin-1 is tyrosine phosphorylated in the MCT and MCT + hypoxia groups. ERK activation is observed in all PH models including hypoxia-induced PH. Importantly, there is no loss of caveolin-1, caveolin-2, cavin-1 or VE-Cad in the hypoxia-induce PH.
Disruption of endothelial cells (EC) and progressive loss of endothelial caveolin-1 results in reciprocal activation of proliferative pathways leading to PH. Extensive damage/loss of EC exposes SMC to increased pressure and shear stress, leading to flattening of caveolae. In addition, caveolin-1 is tyrosine phosphorylated in the MCT and MCT + hypoxia groups. In cancer, loss of cavin-1 and tyrosine phosphorylation of caveolin-1 results in cell migration and metastasis. Thus, the alterations in the caveolin-1 expression in the MCT + hypoxia group may in part facilitate cell proliferation, cell migration and neointima formation. It is important to note, that hypoxia does not alter the expression of caveolin-1 and other membrane proteins; and there is no enhanced expression of caveolin-1 in SMC. However, despite the presence of endothelial caveolin-1, proliferative pathway is activated indicating that the endothelial caveolin-1 is dysfunctional in hypoxia-induced PH. There is no evidence for endothelial membrane disruption in the hypoxia model, which may explain the reported recovery of animals after removal from hypoxia.
The important observation in our study is that the disruption of EC coupled with endothelial caveolin-1 loss initiates pulmonary hypertensive changes and facilitates progression. Further studies are required to determine other factors that might facilitate the “enhanced” caveolin-1 expression in SMC in cell proliferation and migration. We will examine whether cavin-1 and caveolae can be restituted in SMC and normalize caveolin-1 expression, which may abrogate neointima formation.
This study was in part supported by Cardiovascular Medical Research & Education Fund awarded to RM.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dai HL, Karatza AA, Nurzynska D S-Editor: Ma YJ L-Editor: A E-Editor: Zhang YL
| 1. | Sakao S, Tatsumi K, Voelkel NF. Reversible or irreversible remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2010;43:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Fishman AP. Primary pulmonary arterial hypertension: a look back. J Am Coll Cardiol. 2004;43:2S-4S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Pogoriler JE, Rich S, Archer SL, Husain AN. Persistence of complex vascular lesions despite prolonged prostacyclin therapy of pulmonary arterial hypertension. Histopathology. 2012;61:597-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Thenappan T, Shah SJ, Rich S, Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982-2006. Eur Respir J. 2007;30:1103-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 5. | Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaïci A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jaïs X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 1015] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 6. | Mathew R, Huang J, Shah M, Patel K, Gewitz M, Sehgal PB. Disruption of endothelial-cell caveolin-1alpha/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation. 2004;110:1499-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Huang J, Wolk JH, Gewitz MH, Mathew R. Caveolin-1 expression during the progression of pulmonary hypertension. Exp Biol Med (Maywood). 2012;237:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Kawut SM, Horn EM, Berekashvili KK, Widlitz AC, Rosenzweig EB, Barst RJ. von Willebrand factor independently predicts long-term survival in patients with pulmonary arterial hypertension. Chest. 2005;128:2355-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Levy M, Bonnet D, Mauge L, Celermajer DS, Gaussem P, Smadja DM. Circulating endothelial cells in refractory pulmonary hypertension in children: markers of treatment efficacy and clinical worsening. PLoS One. 2013;8:e65114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Dereddy N, Huang J, Erb M, Guzel S, Wolk JH, Sett SS, Gewitz MH, Mathew R. Associated inflammation or increased flow-mediated shear stress, but not pressure alone, disrupts endothelial caveolin-1 in infants with pulmonary hypertension. Pulm Circ. 2012;2:492-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Mathew R, Huang J, Katta US, Krishnan U, Sandoval C, Gewitz MH. Immunosuppressant-induced endothelial damage and pulmonary arterial hypertension. J Pediatr Hematol Oncol. 2011;33:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Huang J, Wolk JH, Gewitz MH, Loyd JE, West J, Austin ED, Mathew R. Enhanced caveolin-1 expression in smooth muscle cells: Possible prelude to neointima formation. World J Cardiol. 2015;7:671-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, Swaney JS, Niesman IR, Schermuly RT, Pullamsetti SS, Thistlethwaite PA, Miyanohara A, Farquhar MG, Yuan JX, Insel PA. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J. 2007;21:2970-2979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Mathew R. Cell-specific dual role of caveolin-1 in pulmonary hypertension. Pulm Med. 2011;2011:573432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494-C506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 412] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 16. | Núñez-Wehinger S, Ortiz RJ, Díaz N, Díaz J, Lobos-González L, Quest AF. Caveolin-1 in cell migration and metastasis. Curr Mol Med. 2014;14:255-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Liu L, Pilch PF. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J Biol Chem. 2008;283:4314-4322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 227] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 18. | Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, Hancock JF, Parton RG. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 629] [Cited by in RCA: 579] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 19. | Zhou LJ, Chen XY, Liu SP, Zhang LL, Xu YN, Mu PW, Geng DF, Tan Z. Downregulation of Cavin-1 Expression via Increasing Caveolin-1 Degradation Prompts the Proliferation and Migration of Vascular Smooth Muscle Cells in Balloon Injury-Induced Neointimal Hyperplasia. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Mathew R. Pathogenesis of pulmonary hypertension: a case for caveolin-1 and cell membrane integrity. Am J Physiol Heart Circ Physiol. 2014;306:H15-H25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. 2002;99:11375-11380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 375] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373-2382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 23. | Austin ED, Ma L, LeDuc C, Berman Rosenzweig E, Borczuk A, Phillips JA, Palomero T, Sumazin P, Kim HR, Talati MH, West J, Loyd JE, Chung WK. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet. 2012;5:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 24. | Privratsky JR, Newman PJ. PECAM-1: regulator of endothelial junctional integrity. Cell Tissue Res. 2014;355:607-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 253] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 25. | Gavard J. Endothelial permeability and VE-cadherin: a wacky comradeship. Cell Adh Migr. 2014;8:158-164. [PubMed] |
| 26. | Kronstein R, Seebach J, Grossklaus S, Minten C, Engelhardt B, Drab M, Liebner S, Arsenijevic Y, Taha AA, Afanasieva T, Schnittler HJ. Caveolin-1 opens endothelial cell junctions by targeting catenins. Cardiovasc Res. 2012;93:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Caveda L, Martin-Padura I, Navarro P, Breviario F, Corada M, Gulino D, Lampugnani MG, Dejana E. Inhibition of cultured cell growth by vascular endothelial cadherin (cadherin-5/VE-cadherin). J Clin Invest. 1996;98:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 126] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Dejana E, Giampietro C. Vascular endothelial-cadherin and vascular stability. Curr Opin Hematol. 2012;19:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 29. | Vestweber D. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 626] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 30. | Song L, Ge S, Pachter JS. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood. 2007;109:1515-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Parolini I, Sargiacomo M, Galbiati F, Rizzo G, Grignani F, Engelman JA, Okamoto T, Ikezu T, Scherer PE, Mora R, Rodriguez-Boulan E, Peschle C, Lisanti MP. Expression of caveolin-1 is required for the transport of caveolin-2 to the plasma membrane. Retention of caveolin-2 at the level of the golgi complex. J Biol Chem. 1999;274:25718-25725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 171] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Xie L, Frank PG, Lisanti MP, Sowa G. Endothelial cells isolated from caveolin-2 knockout mice display higher proliferation rate and cell cycle progression relative to their wild-type counterparts. Am J Physiol Cell Physiol. 2010;298:C693-C701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H, Christ GJ, Edelmann W, Lisanti MP. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22:2329-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 241] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 34. | Dávalos A, Fernández-Hernando C, Sowa G, Derakhshan B, Lin MI, Lee JY, Zhao H, Luo R, Colangelo C, Sessa WC. Quantitative proteomics of caveolin-1-regulated proteins: characterization of polymerase i and transcript release factor/CAVIN-1 IN endothelial cells. Mol Cell Proteomics. 2010;9:2109-2124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Swärd K, Sadegh MK, Mori M, Erjefält JS, Rippe C. Elevated pulmonary arterial pressure and altered expression of Ddah1 and Arg1 in mice lacking cavin-1/PTRF. Physiol Rep. 2013;1:e00008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, Ravid K, Pilch PF. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 2008;8:310-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 289] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 37. | Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 689] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 38. | Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 813] [Cited by in RCA: 691] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 39. | Moon H, Lee CS, Inder KL, Sharma S, Choi E, Black DM, Lê Cao KA, Winterford C, Coward JI, Ling MT; Australian Prostate Cancer BioResource, Craik DJ, Parton RG, Russell PJ, Hill MM. PTRF/cavin-1 neutralizes non-caveolar caveolin-1 microdomains in prostate cancer. Oncogene. 2014;33:3561-3570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Aung CS, Hill MM, Bastiani M, Parton RG, Parat MO. PTRF-cavin-1 expression decreases the migration of PC3 prostate cancer cells: role of matrix metalloprotease 9. Eur J Cell Biol. 2011;90:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Kawabe J, Okumura S, Lee MC, Sadoshima J, Ishikawa Y. Translocation of caveolin regulates stretch-induced ERK activity in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;286:H1845-H1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Park SW, Shin KC, Park HJ, Yoou SK, Park JY, Kang YS, Sung DJ, Kim JG, Park SH, Kim B, Cho H, Bae YM. Caveolar remodeling is a critical mechanotransduction mechanism of the stretch-induced L-type Ca2+ channel activation in vascular myocytes. Pflugers Arch. 2017;469:829-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Sowa G, Pypaert M, Sessa WC. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci USA. 2001;98:14072-14077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 177] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Huang J, Kaminski PM, Edwards JG, Yeh A, Wolin MS, Frishman WH, Gewitz MH, Mathew R. Pyrrolidine dithiocarbamate restores endothelial cell membrane integrity and attenuates monocrotaline-induced pulmonary artery hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1250-L1259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Sedding DG, Hermsen J, Seay U, Eickelberg O, Kummer W, Schwencke C, Strasser RH, Tillmanns H, Braun-Dullaeus RC. Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ Res. 2005;96:635-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Sedding DG, Braun-Dullaeus RC. Caveolin-1: dual role for proliferation of vascular smooth muscle cells. Trends Cardiovasc Med. 2006;16:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Ortiz R, Díaz J, Díaz N, Lobos-Gonzalez L, Cárdenas A, Contreras P, Díaz MI, Otte E, Cooper-White J, Torres V, Leyton L, Quest AF. Extracellular matrix-specific Caveolin-1 phosphorylation on tyrosine 14 is linked to augmented melanoma metastasis but not tumorigenesis. Oncotarget. 2016;7:40571-40593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Joshi B, Strugnell SS, Goetz JG, Kojic LD, Cox ME, Griffith OL, Chan SK, Jones SJ, Leung SP, Masoudi H, Leung S, Wiseman SM, Nabi IR. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Res. 2008;68:8210-8220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 49. | Meng F, Saxena S, Liu Y, Joshi B, Wong TH, Shankar J, Foster LJ, Bernatchez P, Nabi IR. The phospho-caveolin-1 scaffolding domain dampens force fluctuations in focal adhesions and promotes cancer cell migration. Mol Biol Cell. 2017;28:2190-2201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |