Published online Aug 23, 2015. doi: 10.5494/wjh.v5.i3.107
Peer-review started: December 4, 2014
First decision: February 7, 2015
Revised: April 29, 2015
Accepted: May 16, 2015
Article in press: May 18, 2015
Published online: August 23, 2015
Processing time: 267 Days and 7.3 Hours
Transcatheter aortic valve implantation (TAVI) has been shown in improve outcome of severe aortic stenosis (AS) patients, deemed surgical high-risk or inoperable, and has grown popular in the past decade. The procedure requires accurate prior planning, and demands an integration of a “Heart Team” consisted from cardiac surgeons, interventional cardiologists, and imaging experts. The role of cardiac imaging and especially multi-slice computerized tomography (MSCT) has been a mainstay of pre-evaluation of severe AS patients that allows to accurately depict and size the cardiac and vascular structures, and has become the primary tool for procedural planning. This article is aimed to evaluate current uses of MSCT in severe AS patients undergoing TAVI, delineate the various measurements derived from this modality and review current literature regarding it’s advantages over other techniques.
Core tip: Transcatheter aortic valve implantation (TAVI) has been shown in improve outcome of severe aortic stenosis patients, deemed surgical high-risk or inoperable, and has grown popular in the past decade. The procedure requires accurate prior planning, and demands an integration of a “Heart Team” approach consisted from cardiac surgeons, interventional cardiologists, and imaging experts. The role of cardiac imaging and especially multi-slice computerized tomography (MSCT) has been a mainstay of TAVI evaluation, and allows accurate depiction and sizing of the cardiac and vascular structures. This article is aimed to review current use of MSCT in TAVI patients.
- Citation: Koifman E, Hamdan A. Multi-slice computerized tomography critical role in transcatheter aortic valve implantation plan: Review of current literature. World J Hypertens 2015; 5(3): 107-114
- URL: https://www.wjgnet.com/2220-3168/full/v5/i3/107.htm
- DOI: https://dx.doi.org/10.5494/wjh.v5.i3.107
Transcatheter aortic valve implantation (TAVI) has been shown to improve outcome of severe aortic stenosis patients deemed inoperable[1] or high risk[2], with mortality rates lower than surgical aortic valve replacement[3]. However, this procedure incurs complications such as paravalvular leak, vascular access complications, stroke, conduction defects requiring pacemaker implantation, and less commonly, annular rupture[4,5].
A “heart team” approach is recommended in any patient considered for TAVI[6,7]. This team is comprised of a multidisciplinary team including general cardiologists, cardiac surgeons, interventional cardiologists, anesthesiologists and imaging cardiologists. The assessment of each patient requires evaluation of symptoms, cardiac and valvular function[8] to determine the severity of aortic stenosis and appropriateness of intervention. Once a patient is considered for intervention, the risk of surgery should be determined according to comorbidities, patient function and frailty and technical aspects such as porcelain aorta and prior cardiac surgery[9,10]. In case of inoperability or high surgical risk, additional imaging should be performed for the evaluation of suitability for TAVI in order to determine annular size, distance between annulus and coronary artery ostium, implantation angle, and vascular access. This can be done by various methods, most commonly by multi-slice computerized tomography (MSCT). This review aim is to describe MSCT for evaluation of patients referred for TAVI.
Unlike surgery, where direct visualization and sizing of the valve is done, TAVI is performed with a 2-dimentional fluoroscopy guidance, where it is difficult to asses proper valvular size and access routes. MSCT enables extracting a large amount of data from a 3-dimentional image which include access options by measuring the diameters of the arteries and aorta in the perpendicular plane, establishing the presence of protruding atherosclerotic plaques, assessing the calcification of the arteries and aortic annulus and evaluating annular dimensions and its proximity to important anatomical landmarks such as the coronary arteries ostium (Table 1). These measurements require an accurate alignment of images in the appropriate plane done by an imaging expert and reviewed by the interventional cardiologist.
| Assessment of the aortic annulus |
| Annular shape |
| Calcification |
| Annular diameters, area and perimeter coronary artery ostia and additional |
| Aortic root dimensions |
| Coronary ostium height |
| Sinus of valsava diameter and height |
| Sinutubular junction diameter |
| Ascending aorta diameter |
| Aortic annulus plane for fluoroscopy |
| Degree of aortic angulation in relation to the annulus |
| Optimal projection angle |
| Access route evaluation |
| Pelvic and aortic minimal diameters |
| Vascular calcification |
| Vascular tortuosity |
| Presence of protruding atherosclerotic plaques and thrombi |
The acquisition of the CT data should be performed during an inspiratory breathhold while the electrocardiogram (ECG) should be recorded simultaneously to allow retrospective or prospective gating of the data. Imaging of the annulus in systole, when the aortic annulus size increases[11], may be preferable over the diastole; however, analysis of the aorta and peripheral arteries can be performed without ECG synchrony.
The aortic root is a complex anatomic structure comprised from a tri-leaflet valve inserted in a semi-lunar mode into the left ventricle and aortic root, which creates the sinuses of valsava that accommodate the coronaries origin. It lies in a close proximity to the atrioventricular node and the left bundle of the cardiac conduction system[12].
Accurate measurement of the aortic annulus is a critical step in the planning of TAVI, since it enables proper valve sizing, grades calcification of aortic annulus and measure the distance to the coronaries origin. These parameters point to possible complications including paravalvular leaks[13,14], annular rupture[15,16] and coronary arteries obstruction[17,18], all of which have adverse impact on patients outcome[19,20].
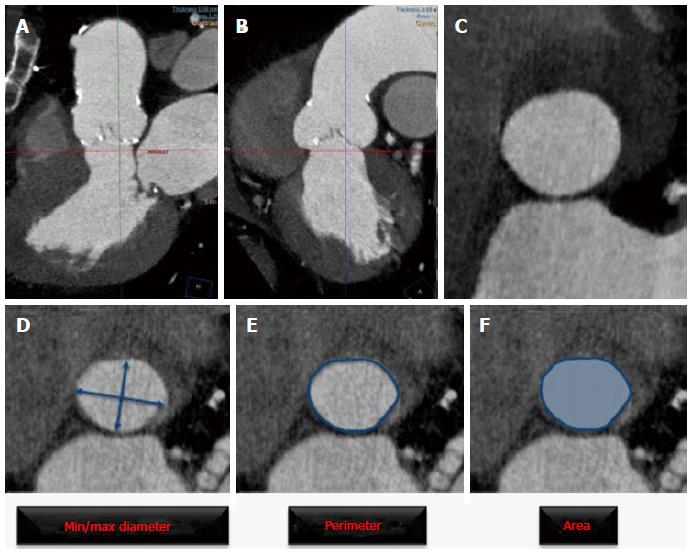
The aortic annulus has an oval shape, thus measuring its diameter in a single plane is inaccurate and misleads the operators when sizing the valve[21]. Therefore measuring annular diameter by 2D echocardiography usually provides the shorter diameter and consequently undersizes annular dimensions[22]. Precise measurement of annular dimensions requires alignment of the image in a perpendicular plane to the basal part of leaflets insertion (Figure 1). The parameters derived from annular measurements include short and long diameters, mean diameter, perimeter and area. The aortic annulus, generally elliptic, assumes a more round shape in systole, thus increasing cross sectional area without substantial change in perimeter. Perimeter changes are negligible in patients with calcified valves, because tissue properties allow very little expansion. Aortic annulus perimeter appears therefore ideally suited for accurate sizing in TAVI[11]. Inter- and Intra-observer variability of these measurement by MSCT is small and highly reproducible as shown in a recent study[23] and studies comparing different modalities for prosthesis sizing have shown reduction in paravalvular leak with MSCT measurement compared with 2D echocardiography[24,25] and therefore MSCT is currently regarded as an essential tool for accurate prosthesis sizing.
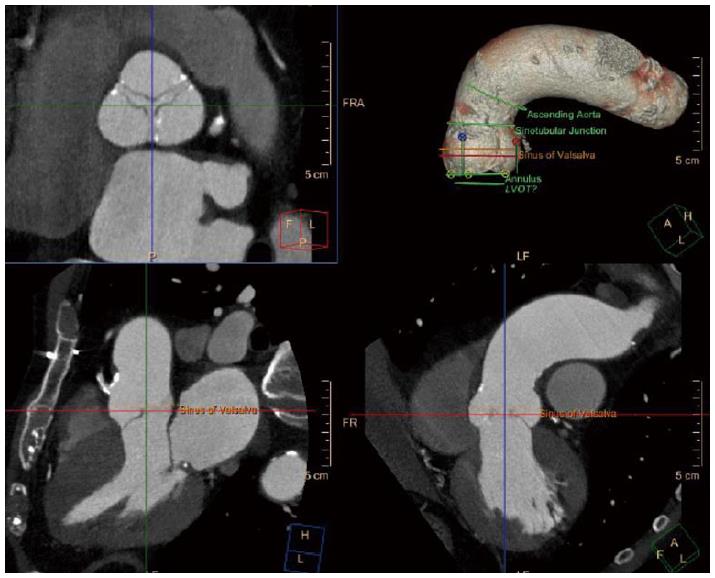
Besides aortic annular dimension, other considerations should be taken into account upon deciding valve type and size. Coronary ostia height and sinus of valsalva diameter should be measured (Figures 2 and 3), and coronary obstruction risk must be assessed since the native valve leaflets are displaced and could potentially obstruct the coronary flow. In a multicenter registry, coronary obstruction was reported in less than 1% of TAVI patients[17]. Predictors of coronary obstruction were low coronary ostia height and small sinus of valsalva diameter along with female gender, valve-in-valve procedure and balloon expandable valve[17]. The outcome of this complication is catastrophic with a 30-d mortality rate of more than 40%. Therefore, it is crucial to measure coronary ostia height, ensure adequate sinus of valsalva diameter and height according to the device requirements, as published by the manufacturer.
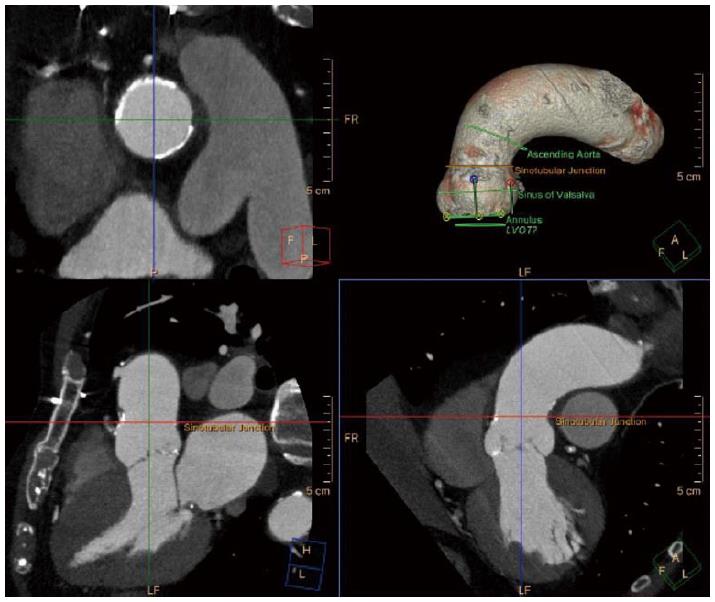
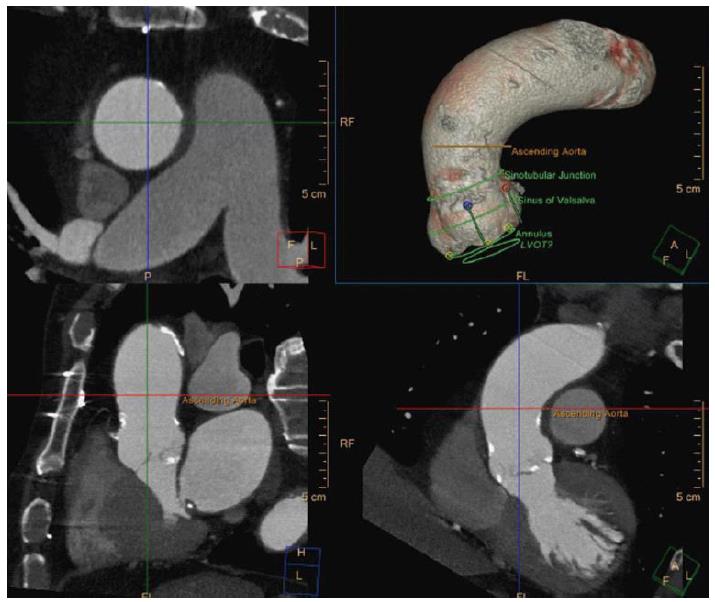
Sinotubular junction diameter (Figure 4) should also be considered since a smaller diameter than the valve implanted could pose a risk of aortic injury upon balloon inflation in balloon expandable valves. In self-expandable valves the ascending aorta (Figure 5) acts as an anchorage point, hence, large diameters as in aortic aneurysm are a contraindication for the use of this type of valve.
Positioning of the valve is a critical step in TAVI procedure, which is usually performed under angiography guidance. Since, angiography is a 2D image, precise planar projection is required for accurate centered implantation of the valve in a perpendicular angle to the native valve plane. Assessment of the proper implantation plane can be located by angiography, however, this methods has some caveats such as, additional contrast injection and radiation exposure along with the inherent requirement for interpreting a 2D image in a 3 dimensional manner. Moreover, certain anatomic features can complicate this task, like severe calcification of the aortic valve and root, which can obscure the leaflet insertion, and chest deformation, which can require extreme angles for implantation. MSCT allows a 3D image reconstruction without the need for additional contrast or radiation, and has been shown to accurately predict the valve deployment projection[26], by creating a “line of perpendicularity” which denotes the projections that can be used in order to implant the valve in an orthogonal plane to the native valve[26].
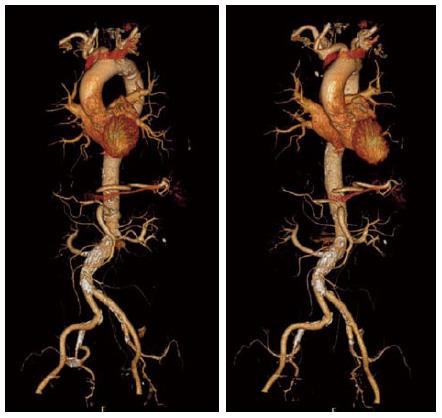
The transfemoral route is currently the default approach for TAVI procedure. The femoral arteries can accommodate a sheath with slightly higher diameter if it is not calcified; however, circumferential calcification and tortuosity especially with involvement of bifurcations are predictors of vascular complication. Thus, utilization of transfemoral approach requires precise evaluation of vascular diameters, calcification and tortuosity (Figure 6). This can be achieved by MSCT, which accurately depicts the vascular anatomy in a 3D imaging. Vascular complications have been shown to impact outcome of TAVI patients, and its incidence was above 30% in the PARTNER trial[1], where route assessment was performed by angiography. The utilization of MSCT have reduced major vascular complications rate from 8% to 1% and minor vascular complications from 24% to 8% over the 2-year study period. The vessel minimal luminal diameter being smaller than the sheath external diameter (23% vs 5%) and the presence of calcified vessels (29% vs 9%) were strong predictors for vascular complications[27].
Measurements of minimal vessel diameters should be performed after a multi-planar reconstruction along the entire course of the vessels in order to attain a perpendicular image. Vessel calcifications should be assessed according to circumferential involvement due to its limitations in accommodating the sheath, and prohibiting the safe passage of the delivery system. Vessel calcification can falsely cause underestimation of its diameter due to the “blooming” effect, in which the calcified segment appears larger than it true dimension thus reducing the size of the true lumen. Tortuosity can be evaluated after 3D reconstruction the aorta and iliofemoral vessels[28], and although severe tortuosity can be straightened. Assessment of alternative routes can be performed by reconstructing images depicting the subclavian artery diameter, calcification and course for this route, and aortic calcification for a transaortic route.
TAVI frequency is growing worldwide, and accordingly the experience with regard to planning the procedure, avoiding complications, and treating them when they occur. The utilization of advanced imaging techniques such as MSCT, with sophisticated data acquisition protocols have significantly improved our ability to assess the access site, accurately size the aortic root dimension and select the appropriate device size, and importantly estimate and avoid fatal complications. Accordingly, most of TAVI programs include an imaging specialist in the heart team. We expect that the use and experience of MSCT will grow and enhanced techniques and algorithms will allow us to further improve the outcome of patients undergoing TAVI in light of the large number of new devices that are currently available or on trial.
P- Reviewer: Castillo R, Li JD S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5086] [Cited by in RCA: 5497] [Article Influence: 366.5] [Reference Citation Analysis (1)] |
| 2. | Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4547] [Cited by in RCA: 4930] [Article Influence: 352.1] [Reference Citation Analysis (0)] |
| 3. | Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Kleiman NS. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1971] [Cited by in RCA: 2166] [Article Influence: 196.9] [Reference Citation Analysis (0)] |
| 4. | Eltchaninoff H, Prat A, Gilard M, Leguerrier A, Blanchard D, Fournial G, Iung B, Donzeau-Gouge P, Tribouilloy C, Debrux JL. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J. 2011;32:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 415] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 5. | Zahn R, Gerckens U, Grube E, Linke A, Sievert H, Eggebrecht H, Hambrecht R, Sack S, Hauptmann KE, Richardt G. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J. 2011;32:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 455] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 6. | Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1379] [Article Influence: 125.4] [Reference Citation Analysis (0)] |
| 7. | Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2682] [Cited by in RCA: 2645] [Article Influence: 203.5] [Reference Citation Analysis (0)] |
| 8. | Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3183] [Cited by in RCA: 3086] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 9. | Hattler BG, Madia C, Johnson C, Armitage JM, Hardesty RL, Kormos RL, Pham SM, Payne DN, Griffith BP. Risk stratification using the Society of Thoracic Surgeons Program. Ann Thorac Surg. 1994;58:1348-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, Cortina J, David M, Faichney A, Gabrielle F. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816-822; discussion 822-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1147] [Cited by in RCA: 1132] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 11. | Hamdan A, Guetta V, Konen E, Goitein O, Segev A, Raanani E, Spiegelstein D, Hay I, Di Segni E, Eldar M. Deformation dynamics and mechanical properties of the aortic annulus by 4-dimensional computed tomography: insights into the functional anatomy of the aortic valve complex and implications for transcatheter aortic valve therapy. J Am Coll Cardiol. 2012;59:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Piazza N, de Jaegere P, Schultz C, Becker AE, Serruys PW, Anderson RH. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ Cardiovasc Interv. 2008;1:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 427] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 13. | Hayashida K, Lefèvre T, Chevalier B, Hovasse T, Romano M, Garot P, Bouvier E, Farge A, Donzeau-Gouge P, Cormier B. Impact of post-procedural aortic regurgitation on mortality after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:1247-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Sinning JM, Hammerstingl C, Vasa-Nicotera M, Adenauer V, Lema Cachiguango SJ, Scheer AC, Hausen S, Sedaghat A, Ghanem A, Müller C. Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;59:1134-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 15. | Rezq A, Basavarajaiah S, Latib A, Takagi K, Hasegawa T, Figini F, Cioni M, Franco A, Montorfano M, Chieffo A. Incidence, management, and outcomes of cardiac tamponade during transcatheter aortic valve implantation: a single-center study. JACC Cardiovasc Interv. 2012;5:1264-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Hayashida K, Bouvier E, Lefèvre T, Hovasse T, Morice MC, Chevalier B, Romano M, Garot P, Farge A, Donzeau-Gouge P. Potential mechanism of annulus rupture during transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2013;82:E742-E746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Ribeiro HB, Webb JG, Makkar RR, Cohen MG, Kapadia SR, Kodali S, Tamburino C, Barbanti M, Chakravarty T, Jilaihawi H. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol. 2013;62:1552-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 466] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 18. | Ribeiro HB, Nombela-Franco L, Urena M, Mok M, Pasian S, Doyle D, DeLarochellière R, Côté M, Laflamme L, DeLarochellière H. Coronary obstruction following transcatheter aortic valve implantation: a systematic review. JACC Cardiovasc Interv. 2013;6:452-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 247] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 19. | Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, Klugmann S, Bedogni F, Maisano F, Marzocchi A. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 882] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 20. | Seiffert M, Conradi L, Baldus S, Schirmer J, Blankenberg S, Reichenspurner H, Diemert P, Treede H. Severe intraprocedural complications after transcatheter aortic valve implantation: calling for a heart team approach. Eur J Cardiothorac Surg. 2013;44:478-484; discussion 484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Tops LF, Wood DA, Delgado V, Schuijf JD, Mayo JR, Pasupati S, Lamers FP, van der Wall EE, Schalij MJ, Webb JG. Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2008;1:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 387] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 22. | Ng AC, Delgado V, van der Kley F, Shanks M, van de Veire NR, Bertini M, Nucifora G, van Bommel RJ, Tops LF, de Weger A. Comparison of aortic root dimensions and geometries before and after transcatheter aortic valve implantation by 2- and 3-dimensional transesophageal echocardiography and multislice computed tomography. Circ Cardiovasc Imaging. 2010;3:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 23. | Schuhbaeck A, Achenbach S, Pflederer T, Marwan M, Schmid J, Nef H, Rixe J, Hecker F, Schneider C, Lell M. Reproducibility of aortic annulus measurements by computed tomography. Eur Radiol. 2014;24:1878-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Jilaihawi H, Kashif M, Fontana G, Furugen A, Shiota T, Friede G, Makhija R, Doctor N, Leon MB, Makkar RR. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol. 2012;59:1275-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 367] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 25. | Hayashida K, Bouvier E, Lefèvre T, Hovasse T, Morice MC, Chevalier B, Romano M, Garot P, Mylotte D, Farge A. Impact of CT-guided valve sizing on post-procedural aortic regurgitation in transcatheter aortic valve implantation. EuroIntervention. 2012;8:546-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Kurra V, Kapadia SR, Tuzcu EM, Halliburton SS, Svensson L, Roselli EE, Schoenhagen P. Pre-procedural imaging of aortic root orientation and dimensions: comparison between X-ray angiographic planar imaging and 3-dimensional multidetector row computed tomography. JACC Cardiovasc Interv. 2010;3:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Toggweiler S, Gurvitch R, Leipsic J, Wood DA, Willson AB, Binder RK, Cheung A, Ye J, Webb JG. Percutaneous aortic valve replacement: vascular outcomes with a fully percutaneous procedure. J Am Coll Cardiol. 2012;59:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 28. | Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr. 2012;6:366-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 496] [Article Influence: 38.2] [Reference Citation Analysis (0)] |