Published online May 23, 2015. doi: 10.5494/wjh.v5.i2.41
Peer-review started: January 7, 2015
First decision: March 6, 2015
Revised: March 23, 2015
Accepted: April 28, 2015
Article in press: May 2, 2015
Published online: May 23, 2015
Processing time: 134 Days and 22.4 Hours
Hypertensive cardiomyopathy (HTN-CM) is a structural cardiac disorder generally accompanied by concentric left ventricular hypertrophy (LVH) associated with diastolic or systolic dysfunction in patients with persistent systemic hypertension. It occurs in the absence of other cardiac diseases capable of causing myocardial hypertrophy or cardiac dysfunction. Persistent systemic hypertension leads to structural and functional myocardial abnormalities resulting in myocardial ischemia, fibrosis, and hypertrophy. HTN-CM is predominantly a disease of impaired relaxation rather than impaired contractility, so patients are usually asymptomatic during resting conditions. However, their stiff left ventricles become incapable of handling increased blood volume and cannot produce appropriate cardiac output with the slight change of circulating volume that may occur during exercise. Importantly, the accompanying LVH is itself a risk factor for mortality and morbidity. Therefore, early detection of LVH development in patients with hypertension (referred to as HTN-CM) is critical for optimal treatment. In addition to pathological findings, echocardiography and cardiac magnetic resonance imaging are ideal tools for the diagnosis of HTN-CM. Timely diagnosis of this condition and utilization of appropriate treatment are required to improve morbidity and mortality in hypertensive patients. This review article presents an overview of the multidimensional impact of myocardial disorder in patients with hypertension. Relevant literature is highlighted and the effects of hypertension on cardiac hypertrophy and heart failure development are discussed, including possible therapeutic options.
Core tip: Hypertensive cardiomyopathy is a structural cardiac disorder generally accompanied by left ventricular hypertrophy associated with diastolic and/or systolic dysfunction in patients with persistent systemic hypertension, in the absence of other cardiac diseases. Because regression of myocardial hypertrophy is associated with a reduction in cardiovascular risk along with the improvement of cardiac function, timely diagnosis of the disease-specific pathophysiology and appropriate treatment strategy including maintaining optimal blood pressure control is very important in the care of patients with hypertension. In the present review manuscript, we have described the outline of hypertensive cardiomyopathy, pathophysiological feature of the disease, diagnosis and the treatment.
- Citation: Kuroda K, Kato TS, Amano A. Hypertensive cardiomyopathy: A clinical approach and literature review. World J Hypertens 2015; 5(2): 41-52
- URL: https://www.wjgnet.com/2220-3168/full/v5/i2/41.htm
- DOI: https://dx.doi.org/10.5494/wjh.v5.i2.41
Hypertensive cardiomyopathy (HTN-CM) is a structural cardiac disorder generally accompanied by concentric left ventricular hypertrophy (LVH) associated with diastolic or systolic dysfunction in patients with persistent systemic hypertension. HTN-CM is difficult to distinguish from other cardiac diseases that cause myocardial hypertrophy, such as hypertrophic cardiomyopathy, Fabry disease, or cardiac amyloidosis. However, when other causes are ruled out, leaving hypertension the only possible cause for LVH development, this is considered to be HTN-CM.
Hypertension (HTN) is a major global health issue, accounting for approximately 50% cases of both stroke and ischemic heart disease, and approximately 13% of the total deaths worldwide[1]. Persistent hypertension can cause structural and functional myocardial abnormalities. LVH and remodeling, frequently seen in patients with hypertension[2], is initially an adaptive response of a normal heart to an increased afterload. Hypertension leads to interstitial myocardial fibrosis[3], which has been linked to LVH development and diastolic dysfunction[4].
The renin-angiotensin-aldosterone system (RAAS) is also an important determinant of the hypertrophic response[5-7]. A relationship between angiotensin II and development of myocardial fibrosis has been described as well[8]. Importantly, the Framingham Heart Study revealed that LVH is a risk factor for cardiovascular morbidity and mortality, independent of other cardiovascular risk factors, including elevated blood pressure itself[4,9,10]. In addition, patients with persistent hypertension and LVH are susceptible to sudden death[11]. These observations emphasize the importance of early diagnosis and effective treatment of hypertension to prevent cardiac complications[12].
In this review article, we summarize the pathophysiology, mechanism, diagnostic evaluation, and management options of HTN-CM. We have focused on human studies in order to emphasize the importance of early identification and optimization of treatment in patients with hypertension.
The prevalence of LVH varies with the severity of hypertension, ranging from 20% in mild to almost 100% in severe or complicated hypertension[13]. Cuspidi et al[14], who performed a review of the echocardiographic data of 37700 individuals, reported that the prevalence rate of LVH was 19%-48% in untreated hypertensive cohorts and 58%-77% in high-risk hypertensive patients.
The development of LVH is a relatively early response to hypertension, particularly in children and adolescents[15]. Transient hypertension induced by mental stress as well as extensive elevation of blood pressure during exercise can also induce LVH[16,17]. The Framingham Heart Study showed that the left ventricular (LV) mass can be increased prior to the development of overt hypertension[18]. LVH in patients with hypertension predominantly results not only from a chronic increase in LV afterload but also a genetic component such as the DD genotype of the angiotensin-converting-enzyme (ACE) gene and B2 bradykinin receptor polymorphism[19-22].
Devereux et al[23] reported that the prevalence of LVH among hypertensive patients is influenced by gender, obesity, and possibly age. Sex-specific criteria for LV mass index identify LVH in more women than men with systemic hypertension[24].
Conventional echocardiography provides useful morphological information of LVH patterns. For example, patients with hypertrophic cardiomyopathy (HCM) frequently show asymmetrical septal hypertrophy of the LV; this is the most characteristic finding[25]. In contrast, LVH associated with hypertension or HTN-CM is characterized by symmetrical (concentric) LV hypertrophy. However, 13%-31% of patients with HCM show symmetrical hypertrophy[26,27], whereas 4%-47% of hypertensive patients manifest asymmetrical septal hypertrophy[27,28].
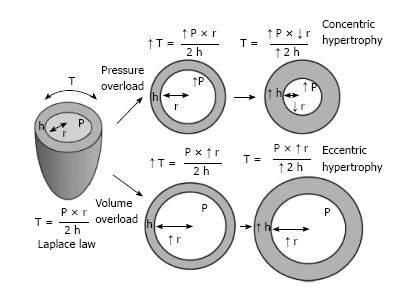
LV remodeling/hypertrophy in HTN-CM may represent an adaptive response to hemodynamic overload imposed by systemic hypertension[2]. This compensatory response can be explained by the Laplace law (Figure 1, reproduced from[2,29]). Sustained elevated blood pressure leads to an increase in LV wall stress, which is a major determinant of myocardial oxygen demand. In response to increased LV wall stress, the LV wall thickens and the LV mass increases, thereby resulting in the normalization of wall stress and development of a structural pattern known as concentric hypertrophy. Alternatively, an increase in blood volume could lead to an increase in the chamber radius, resulting in eccentric hypertrophy[2].
Ganau et al[24] investigated patterns of LVH and geometric remodeling in patients with essential hypertension. They reported that LV mass index and relative wall thickness were normal in 52% of the patients, whereas 13% had increased relative wall thickness with normal ventricular mass (concentric remodeling), 27% had increased mass with normal relative wall thickness (eccentric hypertrophy), and only 8% had “typical” hypertensive concentric hypertrophy (increase in both variables). Cuspidi et al[14] also reported that concentric LV hypertrophy is not the most frequent geometric pattern and is less commonly seen than is eccentric hypertrophy in the hypertensive subjects. Indeed, the geometric pattern of LVH affects the prognosis[30]. Patients without an increase in absolute mass, but with an increase in relative wall thickness or in the wall thickness-to-cavity diameter ratio (concentric remodeling) have the same adverse risk as those with an increase in both mass and relative wall thickness (concentric hypertrophy)[24]. Velagaleti recently reported that the data from the Framingham Heart Study revealed that heart failure risk varied by LV geometric pattern, with eccentric and concentric hypertrophy predisposing to heart failure with reduced and preserved ejection fraction, respectively, after a mean follow-up of 21 years[31].
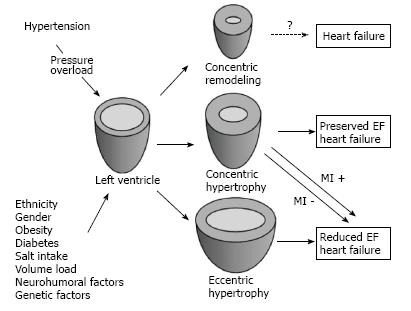
Recent reports[32,33] have described that a transition from LV concentric hypertrophy to dilation and systolic dysfunction is not a common finding, especially in the absence of coronary heart disease[2]. Observation of over one thousand patients with concentric LV hypertrophy and normal ejection fraction by Milani et al[32] revealed only 13% who progressed to systolic dysfunction by three years follow-up and this transition occurred after myocardial infarction in 42.5% of the patients. The various pathways of LV remodeling progression among hypertensive subjects are well described by Nadruz (Figure 2, reproduced from[2])
Interestingly, Khouri et al[34] recently suggested that concentric or eccentric LVH can each be subclassified into two subgroups using cardiac magnetic resonance imaging. This yields four distinct geometric patterns: eccentric non-dilated, eccentric dilated, concentric non-dilated, and concentric dilated[34]. They found that dilated type LVH was more frequently associated with low ejection fraction and elevated troponin levels. Their findings were also supported by the investigation using echocardiography performed by Bang et al[35]. This newly suggested re-classification of hypertensive patients with LVH into four groups according to the LV dilatation and increased concentricity may provide new insights into the hemodynamic and LV functional alteration in this population.
Persistent systemic hypertension induces LVH, fibrosis, diastolic dysfunction, and an increase in the activation of the RAAS, which leads to congestive heart failure[36,37]. One of the mechanisms of heart failure in patients with hypertension is LV diastolic dysfunction. LV diastolic dysfunction associated with hypertension is morphologically characterized by LV wall thickening and increased left atrial (LA) volume. In particular, LA volume is related to LV filling pressure or LA pressure, and is a prognostic marker of various cardiac diseases[38,39]. In advanced stages, hypertension induces eccentric LVH and LV systolic dysfunction[40]. Data from the Framingham Heart Study revealed that LVH is consistently identified as an independent risk factor for cardiovascular morbidity and mortality[4,9,10]. Further, hypertensive LVH or HTN-CM is associated with atrial fibrillation: the incidence increases by 40%-50% in the presence of hypertension[41]. Messerli et al[42] documented a strong correlation between hypertensive LVH or HTN-CM and an increased frequency of ventricular arrhythmias. This emphasizes the importance of understanding of the clinical manifestations of HTN-CM.
The pathophysiological mechanism by which LVH develops in patients with persistent systemic hypertension has been described in the previous sections. Both hypertension and LVH are affected by the same factors, such as angiotensin II, norepinephrine and epinephrine, and an increased peripheral and cardiac sympathetic drive[43,44]. LVH is a significant predictor for heart failure development and is associated with increased mortality[4,9,10]. Notably, patients with persistent hypertension causing HTN-CM often concomitantly have other atherosclerotic risk factors, such as obesity and diabetes. Although hypertension is the leading risk factor for LVH development, substantial evidence indicates that diabetes can also trigger this pathological remodeling response[45]. Obesity is associated with an increased risk of concentric LVH independent of elevated blood pressures[23]. Hypertensive LVH can lead to ventricular diastolic dysfunction; it is also a risk factor for myocardial infarction, which is a principal cause of LV systolic dysfunction[46,47].
In addition to LVH, diastolic dysfunction is a major factor contributing to hypertensive heart disease and the progression to “symptomatic” congestive heart failure[48]. Approximately 40% of patients with hypertensive heart disease have normal systolic function but abnormal diastolic function[48,49]. In fact, LV diastolic dysfunction is the main cause of symptomatic heart failure development in patients with hypertension[50]. LV diastolic dysfunction in HTN-CM is morphologically characterized by LV wall thickening and a persistent elevation of LV end-diastolic pressure, causing increased LA volume. The increased LA volume is the result of elevated LV filling pressure or LA pressure, which presents as exercise intolerance in patients with HTN-CM.
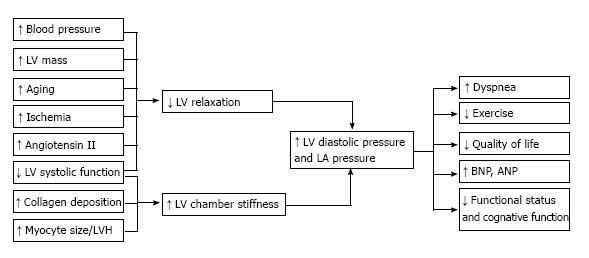
Ischemia is also an important factor leading to diastolic impairment in HTN-CM. Hypertension itself accelerates arteriosclerosis in both systemic and coronary arteries[11,51]. Furthermore, a long-standing increase in LV wall stress and workload causes LVH, and is associated with an increase in the diameter of myocardial cells without a proportional proliferation of the capillary vasculature[11]. Therefore, myocardial tissues in patients with persistent hypertension suffer from ischemia, the so-called mismatch between coronary circulation and oxygen requirement of the myocardium. This underlying myocardial ischemia and hypertrophy leads to the association of HTN-CM rather predominantly with relaxation abnormalities. The impairment of LV pressure/volume reserve means that patients with HTN-CM who have impaired relaxation are usually asymptomatic during resting conditions, but a slight change in circulating volume or an elevation of systemic vascular resistance, such as occurs during exercise, renders their stiff LV incapable of handling the increased blood volume and it cannot produce appropriate cardiac output. This can lead to a progressive decline in ventricular function and ultimately congestive heart failure. Phillips et al[48] described the mechanisms underlying LV diastolic dysfunction and the clinical consequences of this dysfunction in patients with hypertensive LVH or HTN-CM (Figure 3, reproduced from[50]).
The Framingham Heart Study reported that severe LV systolic dysfunction occurs in 3%-6% of hypertensive patients[40]. An eccentric pattern of hypertrophy is a particularly strong risk factor for LV systolic dysfunction, as shown by the Cardiovascular Health Study[52]. Severe LV systolic dysfunction [ejection fraction (EF) < 30%] occurred in 6% in the Framingham Heart Study[52]; however, hypertensive LV remodeling/hypertrophy is certainly followed by chamber dilation and heart failure if not treated appropriately. Although LV function may be initially compensatory, it is followed by progressive worsening of symptoms that ends with cardiac death[2,53]. This phenomenon was consistently reproduced in animal models of pressure overload due to aortic banding, as well as in humans with aortic stenosis and hypertrophic cardiomyopathy[53].
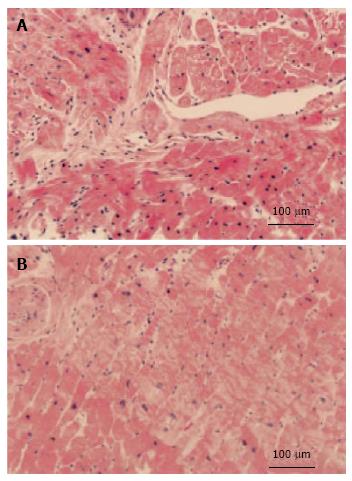
Pathological evaluation is important in the differential diagnosis of HTN-CM. Invasive endomyocardial biopsy (EMB) remains a powerful tool for obtaining a specific diagnosis in HTN-CM patients. A histopathological study revealed myocyte hypertrophy and moderate interstitial fibrosis, which was consistent with HTN-CM[54,55]. Cardiomyocyte hypertrophy in HTN-CM occurs as a result of structural remodeling of the myocardium. It is a consequence of a number of pathologic processes that are mediated by mechanical, neurohormonal, and cytokine routes and take place in the cardiomyocyte and noncardiomyocyte compartments of the heart[54]. An exaggerated accumulation of fibers within the myocardial interstitium and surrounding intramural coronary arteries and arterioles has been consistently found in postmortem human hearts and biopsy samples from patients with HTN-CM[55-57].
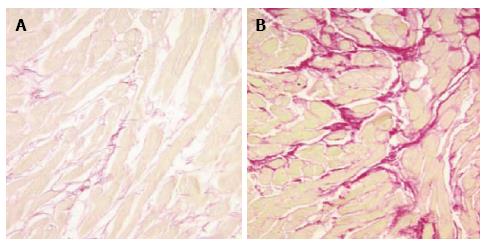
The collagen volume fraction is significantly increased in the hearts of patients with HTN-CM when compared with normotensive patients (Figures 4 and 5, reproduced from[54,56]). Several clinical observations support the possibility that fibrosis occurs by mechanical stress. Tanaka et al[58] reported that the collagen volume fraction of the LV free wall probably reflects transmural gradients of wall stress. Rossi found that the extent and severity of ventricular fibrosis paralleled the enlargement of cardiomyocytes[59]. Querejeta et al[60] reported that the collagen volume fraction correlated with systolic blood pressure and pulse pressure in the myocardium of patients with hypertension.
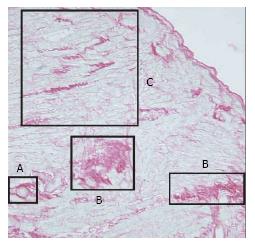
The RAAS and ACE activity may be an important determinant of the hypertrophic response[5-7]. The effect of angiotensin II may be a factor in the promotion of myocardial fibrosis[61]. Myocardial disarray (defined as bundles of myocytes oriented perpendicularly or obliquely to each other or interspersed in different directions), which is generally seen in patients with HCM, may also appear in patients in HTN-CM, although the distribution of myocardial disarray is relatively smaller in HTN-CM than in HCM. A previous study by Kato et al[62] classified patients as HCM if they showed > 33% myocyte disarray in at least one of the cross sections examined. Patients with no or < 5% myocyte disarray in all cross sections examined were classified as HTN-CM (Figure 6, reproduced from[62]).
HTN-CM arises as the result of an increase in the quantity of myocardium but it also emerges due to alterations in myocardial quality (i.e., fibrosis)[54]. Mechanical stress and hormones such as RAAS lead to fibrosis, which in turn leads to chronic heart failure.
Echocardiography is a powerful tool that provides morphological information about the LVH pattern in patients with hypertension. LVH can be detected with both electrocardiography and echocardiography[63]. The sensitivity of electrocardiography for LVH diagnosis is relatively low; therefore, echocardiography should be performed to evaluate LV morphology in patients with persistent hypertension. Levy et al[64] reviewed electrocardiographic criteria for LVH in 4684 subjects of the Framingham Heart Study and detected echocardiographic LVH in 290 men (14.2%) and 465 women (17.6%), although they found electrocardiographic features of LVH in only 2.9% of men and 1.5% of women[64]. Indeed, a prevalence of echocardiographic LVH was reported in 40% of patients with hypertension[4,65].
LV mass (LVM), LV mass index, and relative wall thickness (RWT) are the most common measurements employed in evaluation of LVH in hypertensive patients[66]. LV geometry is classified into 4 groups based on LVM and RWT: concentric LVH (increased mass and increased RWT), eccentric LVH (increased mass and normal RWT), concentric remodeling (normal mass and increased RWT), and normal geometry (normal mass and normal RWT)[4,65,66].
Several formulas are used to estimate LV mass. The original calculations from Troy were the first to be recommended as a standard for estimating LVM from M-mode measurements (Formula 1)[67].
Formula 1: LV mass = 1.05 [(LVIDD + PWTD + IVSTD)3 - (LVIDD)3] g.
Where: LVIDD = LV Internal Diameter in Diastole
PWTD = Posterior Wall Thickness in Diastole
IVSTD = Interventricular Septum Thickness in Diastole
Devereux added a slight modification by using the Penn convention as the border definition criteria (Formula 2)[68].
Formula 2: LV mass = 1.04 [(LVIDD + PWTD + IVSTD)3 - (LVIDD)3] - 13.6 g.
Subsequently, Devereux proposed a new, adjusted equation (validated on necropsy findings of 52 individuals)[69] that used the ASE convention and accounted for this discrepancy (Formula 3).
Formula 3: LV mass = 0.8 {1.04 [(LVIDD + PWTD + IVSTD)3 - (LVIDD)3]} + 0.6 g.
Relative wall thickness (RWT) is measured in clinical studies as:
RWT = (IVST + PWTD)/LVIDD
The usual reference cutoff value for increased RWT, derived from upper limits of normal samples, is 0.45[4]. The RWT provides information regarding LV geometry independent of other calculations[70], thereby precluding a requirement for most corrections. Nevertheless, significant LVH can occur without major changes in RWT, particularly when simultaneous pressure and volume overload are present; these conditions can be seen in patients with hypertension.
The American Society of Echocardiography with the European Association of Echocardiography has issued the following criteria for LVH using the modified Simpson’s rule[71]: Estimated LVM of 201-227 g (103-116 g/m2) for men and 151-171 g (89-100 g/m2) for women is mildly abnormal; Estimated LVM of 228-254 g (117-130 g/m2) for men and 172-182 g (101-112 g/m2) for women is moderately abnormal; Estimated LV mass of > 255 g (> 131 g/m2) for men and > 193 g (> 113 g/m2) for women is severely abnormal.
Assessment of diastolic dysfunction by echocardiography is also important in the management of patients with HTN-CM. Diastolic dysfunction is seen in approximately 50% of patients with hypertension[72]. The changes in conventional Doppler echocardiographic parameters, such as peak early filling velocity (E), late diastolic filling velocity (A) and its ratio, as well as deceleration time, should be monitored. Patients with long-standing hypertension and advanced stage of HTN-CM may show a pseudonormalization of E/A ratio, known as restrictive physiology.
Tissue Doppler imaging (TDI) allows quantitative assessment of ventricular function and early diastolic mitral annular velocity (E′); the ratio of E/E′, which is a parameter with correction of preload. This is a useful tool to assess the severity of diastolic dysfunction in patients with HTN-CM[73]. Kasner et al[73] performed both invasive and noninvasive assessment of diastolic dysfunction and identified the LV filling index of E/E′ (lateral) as the best index for detection of diastolic dysfunction in patients with heart failure with normal ejection fraction.
Strain and strain rate parameters derived from TDI, as well as speckle tracking echocardiography have also been reported as useful tools for detection of diastolic dysfunction, and these can aid in discriminating patients with HTN-CM from those with other causes of LVH[62,74]. The abnormalities in strain parameters may occur in a stage of subclinical diastolic dysfunction in hypertensive patients[75,76] making this a useful strategy for disease prevention[4].
Cardiac magnetic resonance imaging (CMR) offers a unique opportunity for noninvasive quantitation of both LVH with high reproducibility and myocardial fibrosis with high spatial and contrast resolution[77]. Takeda et al[78] described the power of CMR for distinguishing among cardiac amyloidosis, hypertrophic cardiomyopathy, and hypertensive heart disease, all of which present with LVH and heart failure.
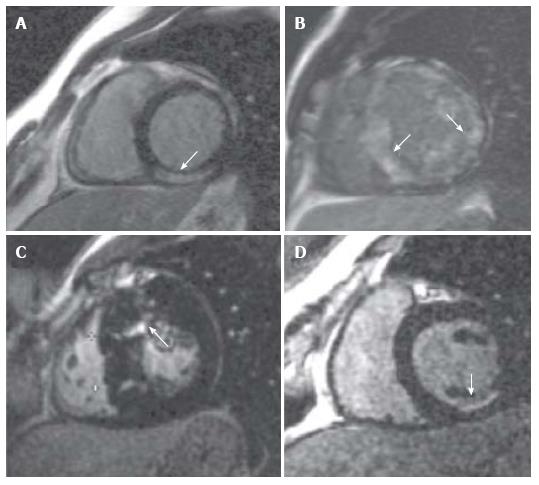
Advances in CMR provide the potential to address all these important issues in a single scan setting, thereby complementing other noninvasive tools and genetic testing[79]. CMR can provide three-dimensional data on cardiac anatomy, function, tissue characterization, coronary and microvascular perfusion and valvular disease without the use of ionizing radiation. Myocardial fibrosis or infiltration can be assessed following administration of gadolinium, an extracellular agent that accumulates in areas of interstitial expansion (i.e., due to myocardial fibrosis, edema, or infiltration). Late gadolinium enhancement (LGE) imaging detects accumulation of contrast in areas of infarction or fibrosis due to the slower contrast kinetics and greater volume of distribution in the extracellular matrix. The extent and pattern of LGE establish the correct diagnosis between HCM and HTN-CM (Figure 7, reproduced from[80]).
The use of CMR in HTN-CM diagnosis allows reproducible assessment of wall thickness and LV mass with greater accuracy when compared to echocardiography. This is particularly important for assessing small LV mass changes over time as a consequence of treatment. In addition, this capability is also of prognostic value as it represents an independent predictor of cardiac mortality[81,82]. Up to 50% of hypertensive patients display LGE[77,83]. Although no typical pattern of LGE has been described, focal nonsubendocardial distribution predominates. No correlation was found between presence of LGE and LVEF or LV end-diastolic dimensions; however, patients displaying LGE had, in general, a greater LV mass[81]. The LGE patterns in HTN-CM offer new insights into risk stratification. This modality can identify patients with HTN-CM who are at risk of diastolic heart failure as a known relationship exists between myocardial fibrosis and diastolic heart failure. This clearly can be of use in therapeutic decision making[84].
Hypertensive cardiomyopathy (HTN-CM) is a result of a complex interaction of genetic and hemodynamic factors inducing structural and functional adaptations[85]. LVH in HTN-CM is a recognized risk factor for congestive heart failure, dysrhythmia, and sudden death[4,9,10]. Better elucidation of the mechanisms producing cardiovascular end-organ damage should lead to treatment targeted at reducing the effects of hypertension on the heart and vascular system. Most antihypertensive treatments promote regression of LVH and reversal of diastolic dysfunction, which may decrease symptoms of congestive heart failure and improve survival rates[85]. LV mass regression improves survival rates in hypertensive patients[86] and is associated with reduced rate of complications of essential hypertension[87].
The RAAS is implicated in the development of cardiac hypertrophy associated with pressure overload[5-7,32,35,88]. Brilla et al[57] indicated ACE inhibition with lisinopril can regress myocardial fibrosis, regardless of LVH regression, and is accompanied by improved LV diastolic function. The Losartan Intervention for Endpoint Reduction (LIFE) study showed that the angiotensin II type 1 (AT1) receptor antagonist, Losartan, reduced LV mass and improved systolic performance, despite only a small drop in blood pressure[89]. Furthermore, in their animal study, Nagata et al[90] revealed the beneficial cardiac effects of eplerenone, which attenuates myocardial oxidative stress and coronary vascular inflammation induced by glucocorticoid-activated mineralocorticoid receptors. Gottdiener et al[91] showed that hydrochlorothiazide administration was associated with greater overall reduction of LA size when compared with other drugs used for the treatment of hypertension. In this study, an ACE inhibitor was nearly as beneficial as hydrochlorothiazide therapy (Figure 8, reproduced from[85,91]). Past studies indicated treatment with statins also reduces ACE activity in the cardiac tissue of rats.
The 3-hydroxy-3-methylgutaryl-CoA (HMG-CoA) reductase inhibitors, commonly referred to as statins, are well-known and potent lipid-lowering agents that reduce the incidence of myocardial infarction and ischemic stroke. In addition to their primary effects, the statins have pleiotropic effects on the cardiovascular system[92], including anti-inflammatory, anti-oxidative, and endothelial protective effects, and thus have been tested as therapeutic agents in heart failure[93]. Chang et al[93] showed that Rosuvastatin therapy attenuated myocardial fibrosis and LV stiffness. Saka et al[94] suggested that the effects of pitavastatin on load-induced cardiac hypertrophy and fibrosis are independent of its cholesterol lowering action and may be mediated, at least in part, through inhibition of RhoA-ERK-SRF signaling, which activates stretch-induced hypertrophy.
Considering these drug therapies, the most important issues in the treatment of HTN-CM are appropriate blood pressure control, weight loss, and dietary sodium restriction[12,13,95]. Regression of LVH and, more importantly, the prognosis of patients with HTN-CM, are both highly related to the antihypertensive response as well as the therapy used[13]. Regression of LVH continues gradually over time and may be associated with complete reversal of LVH and other abnormalities induced by hypertension, such as LA enlargement and diastolic dysfunction[96].
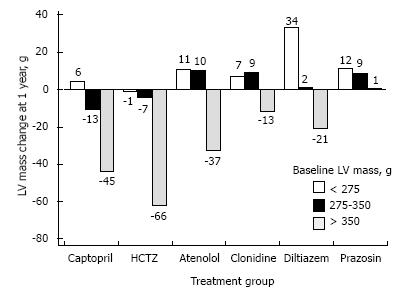
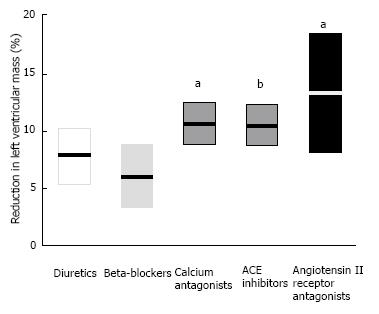
A meta-analysis published in 2003 evaluated the relative efficacy of different antihypertensive drugs for their ability to reverse LVH in patients with hypertension (Figure 9, reproduced from[97]). Notably, after statistical adjustments for duration of therapy and degree of blood pressure lowering, angiotensin II receptor blockers, calcium channel blockers, and ACE inhibitors showed more significant regression of LVH than did beta-blockers. Note that regression of LVH is associated with improvement in both systolic and diastolic function[85], as well as with a reduction in cardiovascular risk[95].
To summarize, HTN-CM is characterized by LVH and LVH-induced diastolic dysfunction rather than systolic dysfunction. This is associated with increased risk of heart failure, arrhythmias, and death. LVH itself is a risk factor for mortality and morbidity, independent of other cardiovascular risk factors, including high blood pressure. Therefore, early detection of LVH development in patients with hypertension is important in order to start effective treatment when the myocardial damage is still reversible. Echocardiography, rather than electrocardiography alone, would be an ideal tool for detection of LVH in its early stage, along with advanced measurements such as tissue Doppler and strain parameters. CMR represents another powerful tool for detection and discrimination of patients with HTN-CM from those with other LVH diseases. Because the regression of LVH is associated with a reduction in cardiovascular risk and improved cardiac function, achieving good blood pressure control is very important in the treatment of patients with HTN-CM. This can be achieved with the use of antihypertensive agents (ACE inhibitors, angiotensin receptor blockers, and aldosterone receptor antagonists), which can be effective for reverse remodeling of the myocardium, weight loss, and sodium restriction.
P- Reviewer: Pontremoli R, Tan XR, Turgut O, Zielinski T S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1604] [Cited by in RCA: 1652] [Article Influence: 97.2] [Reference Citation Analysis (4)] |
| 2. | Nadruz W. Myocardial remodeling in hypertension. J Hum Hypertens. 2015;29:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation. 1990;82:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 215] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 4. | Janardhanan R, Kramer CM. Imaging in hypertensive heart disease. Expert Rev Cardiovasc Ther. 2011;9:199-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Re RN. Intracellular renin and the nature of intracrine enzymes. Hypertension. 2003;42:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Dzau VJ. Tissue renin-angiotensin system in myocardial hypertrophy and failure. Arch Intern Med. 1993;153:937-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 143] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Cuspidi C, Ciulla M, Zanchetti A. Hypertensive myocardial fibrosis. Nephrol Dial Transplant. 2006;21:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4052] [Cited by in RCA: 3988] [Article Influence: 113.9] [Reference Citation Analysis (1)] |
| 10. | Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Santucci A, Santucci C, Reboldi G, Porcellati C. Prognostic value of left ventricular mass and geometry in systemic hypertension with left ventricular hypertrophy. Am J Cardiol. 1996;78:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Grossman E, Messerli FH. Diabetic and hypertensive heart disease. Ann Intern Med. 1996;125:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 118] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Vasan RS, Levy D. The role of hypertension in the pathogenesis of heart failure. A clinical mechanistic overview. Arch Intern Med. 1996;156:1789-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 133] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 13. | Ruilope LM, Schmieder RE. Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens. 2008;21:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 14. | Cuspidi C, Sala C, Negri F, Mancia G, Morganti A. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J Hum Hypertens. 2012;26:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 15. | Daniels SD, Meyer RA, Loggie JM. Determinants of cardiac involvement in children and adolescents with essential hypertension. Circulation. 1990;82:1243-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Schnall PL, Pieper C, Schwartz JE, Karasek RA, Schlussel Y, Devereux RB, Ganau A, Alderman M, Warren K, Pickering TG. The relationship between ‘job strain,’ workplace diastolic blood pressure, and left ventricular mass index. Results of a case-control study. JAMA. 1990;263:1929-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 181] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Devereux RB, Pickering TG, Harshfield GA, Kleinert HD, Denby L, Clark L, Pregibon D, Jason M, Kleiner B, Borer JS. Left ventricular hypertrophy in patients with hypertension: importance of blood pressure response to regularly recurring stress. Circulation. 1983;68:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 363] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Post WS, Larson MG, Levy D. Impact of left ventricular structure on the incidence of hypertension. The Framingham Heart Study. Circulation. 1994;90:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 19. | Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 331] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Ohishi M, Rakugi H, Ogihara T. Association between a deletion polymorphism of the angiotensin-converting-enzyme gene and left ventricular hypertrophy. N Engl J Med. 1994;331:1097-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Brull D, Dhamrait S, Myerson S, Erdmann J, Woods D, World M, Pennell D, Humphries S, Regitz-Zagrosek V, Montgomery H. Bradykinin B2BKR receptor polymorphism and left-ventricular growth response. Lancet. 2001;358:1155-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Kizer JR, Arnett DK, Bella JN, Paranicas M, Rao DC, Province MA, Oberman A, Kitzman DW, Hopkins PN, Liu JE. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension. 2004;43:1182-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Devereux RB, Roman MJ. Left ventricular hypertrophy in hypertension: stimuli, patterns, and consequences. Hypertens Res. 1999;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 1076] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 25. | Wigle ED, Rakowski H, Kimball BP, Williams WG. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation. 1995;92:1680-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 459] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Shapiro LM, McKenna WJ. Distribution of left ventricular hypertrophy in hypertrophic cardiomyopathy: a two-dimensional echocardiographic study. J Am Coll Cardiol. 1983;2:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 238] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Nakamura T, Sugihara H, Kinoshita N, Yoneyama S, Azuma A, Nakagawa M. Can serum carnitine levels distinguish hypertrophic cardiomyopathy from hypertensive hearts? Hypertension. 2000;36:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Dunn FG, Chandraratna P, deCarvalho JG, Basta LL, Frohlich ED. Pathophysiologic assessment of hypertensive heart disease with echocardiography. Am J Cardiol. 1977;39:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 217] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Frohlich ED, Susic D. Pressure overload. Heart Fail Clin. 2012;8:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 393] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 31. | Velagaleti RS, Gona P, Pencina MJ, Aragam J, Wang TJ, Levy D, D’Agostino RB, Lee DS, Kannel WB, Benjamin EJ. Left ventricular hypertrophy patterns and incidence of heart failure with preserved versus reduced ejection fraction. Am J Cardiol. 2014;113:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | Milani RV, Drazner MH, Lavie CJ, Morin DP, Ventura HO. Progression from concentric left ventricular hypertrophy and normal ejection fraction to left ventricular dysfunction. Am J Cardiol. 2011;108:992-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Krishnamoorthy A, Brown T, Ayers CR, Gupta S, Rame JE, Patel PC, Markham DW, Drazner MH. Progression from normal to reduced left ventricular ejection fraction in patients with concentric left ventricular hypertrophy after long-term follow-up. Am J Cardiol. 2011;108:997-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 34. | Khouri MG, Peshock RM, Ayers CR, de Lemos JA, Drazner MH. A 4-tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas heart study. Circ Cardiovasc Imaging. 2010;3:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 35. | Bang CN, Gerdts E, Aurigemma GP, Boman K, Dahlöf B, Roman MJ, Køber L, Wachtell K, Devereux RB. Systolic left ventricular function according to left ventricular concentricity and dilatation in hypertensive patients: the Losartan Intervention For Endpoint reduction in hypertension study. J Hypertens. 2013;31:2060-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | González A, López B, Díez J. Fibrosis in hypertensive heart disease: role of the renin-angiotensin-aldosterone system. Med Clin North Am. 2004;88:83-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Gardin JM, Lauer MS. Left ventricular hypertrophy: the next treatable, silent killer? JAMA. 2004;292:2396-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, Roman MJ, Devereux RB. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS). Am Heart J. 2006;151:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 295] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 39. | Rossi A, Temporelli PL, Quintana M, Dini FL, Ghio S, Hillis GS, Klein AL, Marsan NA, Prior DL, Yu CM. Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta-analysis of longitudinal data (MeRGE Heart Failure). Eur J Heart Fail. 2009;11:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Devereux RB, Bella JN, Palmieri V, Oberman A, Kitzman DW, Hopkins PN, Rao DC, Morgan D, Paranicas M, Fishman D. Left ventricular systolic dysfunction in a biracial sample of hypertensive adults: The Hypertension Genetic Epidemiology Network (HyperGEN) Study. Hypertension. 2001;38:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N-9N. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1432] [Cited by in RCA: 1450] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 42. | Messerli FH, Ventura HO, Elizardi DJ, Dunn FG, Frohlich ED. Hypertension and sudden death. Increased ventricular ectopic activity in left ventricular hypertrophy. Am J Med. 1984;77:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 315] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Greenwood JP, Scott EM, Stoker JB, Mary DA. Hypertensive left ventricular hypertrophy: relation to peripheral sympathetic drive. J Am Coll Cardiol. 2001;38:1711-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108:560-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 320] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 45. | Musilli C, Paccosi S, Pala L, Gerlini G, Ledda F, Mugelli A, Rotella CM, Parenti A. Characterization of circulating and monocyte-derived dendritic cells in obese and diabetic patients. Mol Immunol. 2011;49:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 46. | Kjeldsen SE, Devereux RB, Hille DA, Lyle PA, Dahlöf B, Julius S, Edelman JM, Snapinn SM, de Faire U, Fyhrquist F. Predictors of cardiovascular events in patients with hypertension and left ventricular hypertrophy: the Losartan Intervention for Endpoint reduction in hypertension study. Blood Press. 2009;18:348-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Diamond JA, Phillips RA. Hypertensive heart disease. Hypertens Res. 2005;28:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Phillips RA, Diamond JA. Diastolic function in hypertension. Curr Cardiol Rep. 2001;3:485-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 417] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 50. | Gandhi SK, Powers JC, Nomeir AM, Fowle K, Kitzman DW, Rankin KM, Little WC. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2001;344:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 451] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 51. | Chobanian AV, Brecher PI, Haudenschild CC. Effects of hypertension and antihypertensive therapy on atherosclerosis: state of the heart lecture. Hypertension. 1986;8:15-21. |
| 52. | Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 457] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 53. | Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 558] [Article Influence: 39.9] [Reference Citation Analysis (2)] |
| 54. | Díez J, González A, López B, Querejeta R. Mechanisms of disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med. 2005;2:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 55. | Díez J. Diagnosis and treatment of myocardial fibrosis in hypertensive heart disease. Circ J. 2008;72 Suppl A:A8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Díez J. Mechanisms of cardiac fibrosis in hypertension. J Clin Hypertens (Greenwich). 2007;9:546-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 57. | Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 443] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 58. | Tanaka M, Fujiwara H, Onodera T, Wu DJ, Hamashima Y, Kawai C. Quantitative analysis of myocardial fibrosis in normals, hypertensive hearts, and hypertrophic cardiomyopathy. Br Heart J. 1986;55:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 231] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998;16:1031-1041. [PubMed] |
| 60. | Querejeta R, Varo N, López B, Larman M, Artiñano E, Etayo JC, Martínez Ubago JL, Gutierrez-Stampa M, Emparanza JI, Gil MJ. Serum carboxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart disease. Circulation. 2000;101:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 245] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 61. | Mazzolai L, Nussberger J, Aubert JF, Brunner DB, Gabbiani G, Brunner HR, Pedrazzini T. Blood pressure-independent cardiac hypertrophy induced by locally activated renin-angiotensin system. Hypertension. 1998;31:1324-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 137] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 62. | Kato TS, Noda A, Izawa H, Yamada A, Obata K, Nagata K, Iwase M, Murohara T, Yokota M. Discrimination of nonobstructive hypertrophic cardiomyopathy from hypertensive left ventricular hypertrophy on the basis of strain rate imaging by tissue Doppler ultrasonography. Circulation. 2004;110:3808-3814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 731] [Article Influence: 29.2] [Reference Citation Analysis (1)] |
| 64. | Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 337] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 65. | Savage DD, Drayer JI, Henry WL, Mathews EC, Ware JH, Gardin JM, Cohen ER, Epstein SE, Laragh JH. Echocardiographic assessment of cardiac anatomy and function in hypertensive subjects. Circulation. 1979;59:623-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 229] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Foppa M, Duncan BB, Rohde LE. Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovasc Ultrasound. 2005;3:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 67. | Troy BL, Pombo J, Rackley CE. Measurement of left ventricular wall thickness and mass by echocardiography. Circulation. 1972;45:602-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 424] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 68. | Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3101] [Cited by in RCA: 3189] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 69. | Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4462] [Cited by in RCA: 4708] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 70. | Li L, Shigematsu Y, Hamada M, Hiwada K. Relative wall thickness is an independent predictor of left ventricular systolic and diastolic dysfunctions in essential hypertension. Hypertens Res. 2001;24:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 71. | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8282] [Cited by in RCA: 8800] [Article Influence: 463.2] [Reference Citation Analysis (0)] |
| 72. | Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2244] [Cited by in RCA: 2284] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 73. | Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 452] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 74. | Kato TS, Izawa H, Komamura K, Noda A, Asano H, Nagata K, Hashimoto S, Oda N, Kamiya C, Kanzaki H. Heterogeneity of regional systolic function detected by tissue Doppler imaging is linked to impaired global left ventricular relaxation in hypertrophic cardiomyopathy. Heart. 2008;94:1302-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circ Cardiovasc Imaging. 2009;2:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 76. | Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourcière Y, Hippler SE, Fields H, Naqvi TZ. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 248] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 77. | Rudolph A, Abdel-Aty H, Bohl S, Boyé P, Zagrosek A, Dietz R, Schulz-Menger J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009;53:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 268] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 78. | Takeda M, Amano Y, Tachi M, Tani H, Mizuno K, Kumita S. MRI differentiation of cardiomyopathy showing left ventricular hypertrophy and heart failure: differentiation between cardiac amyloidosis, hypertrophic cardiomyopathy, and hypertensive heart disease. Jpn J Radiol. 2013;31:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Assomull RG, Shakespeare C, Kalra PR, Lloyd G, Gulati A, Strange J, Bradlow WM, Lyne J, Keegan J, Poole-Wilson P. Role of cardiovascular magnetic resonance as a gatekeeper to invasive coronary angiography in patients presenting with heart failure of unknown etiology. Circulation. 2011;124:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 80. | Parsai C, O’Hanlon R, Prasad SK, Mohiaddin RH. Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J Cardiovasc Magn Reson. 2012;14:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | Myerson SG, Bellenger NG, Pennell DJ. Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension. 2002;39:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 201] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 82. | Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2389] [Article Influence: 140.5] [Reference Citation Analysis (0)] |
| 83. | Andersen K, Hennersdorf M, Cohnen M, Blondin D, Mödder U, Poll LW. Myocardial delayed contrast enhancement in patients with arterial hypertension: initial results of cardiac MRI. Eur J Radiol. 2009;71:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 84. | Martos R, Baugh J, Ledwidge M, O’Loughlin C, Conlon C, Patle A, Donnelly SC, McDonald K. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 339] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 85. | Diamond JA, Phillips RA. Regression of left ventricular hypertrophy: are there preferred drugs? Curr Hypertens Rep. 2003;5:368-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | Muiesan ML, Salvetti M, Rizzoni D, Castellano M, Donato F, Agabiti-Rosei E. Association of change in left ventricular mass with prognosis during long-term antihypertensive treatment. J Hypertens. 1995;13:1091-1095. [PubMed] |
| 87. | Koren MJ, Ulin RJ, Koren AT, Laragh JH, Devereux RB. Left ventricular mass change during treatment and outcome in patients with essential hypertension. Am J Hypertens. 2002;15:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 88. | Yamazaki T, Yazaki Y. Is there major involvement of the renin-angiotensin system in cardiac hypertrophy? Circ Res. 1997;81:639-642. [PubMed] |
| 89. | Wachtell K, Palmieri V, Olsen MH, Gerdts E, Papademetriou V, Nieminen MS, Smith G, Dahlöf B, Aurigemma GP, Devereux RB. Change in systolic left ventricular performance after 3 years of antihypertensive treatment: the Losartan Intervention for Endpoint (LIFE) Study. Circulation. 2002;106:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 90. | Nagata K, Obata K, Xu J, Ichihara S, Noda A, Kimata H, Kato T, Izawa H, Murohara T, Yokota M. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension. 2006;47:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 91. | Gottdiener JS, Reda DJ, Massie BM, Materson BJ, Williams DW, Anderson RJ. Effect of single-drug therapy on reduction of left ventricular mass in mild to moderate hypertension: comparison of six antihypertensive agents. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. Circulation. 1997;95:2007-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 161] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 92. | Ray KK, Cannon CP. Early time to benefit with intensive statin treatment: could it be the pleiotropic effects? Am J Cardiol. 2005;96:54F-60F. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Chang SA, Kim YJ, Lee HW, Kim DH, Kim HK, Chang HJ, Sohn DW, Oh BH, Park YB. Effect of rosuvastatin on cardiac remodeling, function, and progression to heart failure in hypertensive heart with established left ventricular hypertrophy. Hypertension. 2009;54:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 94. | Saka M, Obata K, Ichihara S, Cheng XW, Kimata H, Noda A, Izawa H, Nagata K, Yokota M. Attenuation of ventricular hypertrophy and fibrosis in rats by pitavastatin: potential role of the RhoA-extracellular signal-regulated kinase-serum response factor signalling pathway. Clin Exp Pharmacol Physiol. 2006;33:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | Levy D, Salomon M, D’Agostino RB, Belanger AJ, Kannel WB. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation. 1994;90:1786-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 96. | Franz IW, Tönnesmann U, Müller JF. Time course of complete normalization of left ventricular hypertrophy during long-term antihypertensive therapy with angiotensin converting enzyme inhibitors. Am J Hypertens. 1998;11:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 97. | Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003;115:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 493] [Article Influence: 22.4] [Reference Citation Analysis (0)] |