Published online May 23, 2015. doi: 10.5494/wjh.v5.i2.28
Peer-review started: September 30, 2014
First decision: November 14, 2014
Revised: December 5, 2014
Accepted: March 4, 2015
Article in press: March 6, 2015
Published online: May 23, 2015
Processing time: 234 Days and 13.7 Hours
Hypertension is currently one of the most prevalent illnesses worldwide, and is the second most common cause of heart failure, only behind ischemic cardiomyopathy. The development of novel multimodality imaging techniques in recent years has broadened the diagnostic methods, risk stratification and monitoring of treatment of cardiovascular diseases available for clinicians. Cardiovascular magnetic resonance (CMR) has a great capacity to evaluate cardiac dimensions and ventricular function, is extremely useful in ruling-out ischemic cardiomyopathy, the evaluation of the vascular system, in making the differential diagnosis for resistant hypertension and risk stratification for hypertensive cardiomyopathy and constitutes today, the method of choice to evaluate left ventricular systolic function. Computed tomography (CT) is the method of choice for the evaluation of vascular anatomy, including coronary arteries, and is also able to provide both functional and structural information. Finally, nuclear cardiology studies have been traditionally used to evaluate myocardial ischemia, along with offering the capacity to evaluate ventricular, endothelial and cardiac innervation function; information that is key in directing the treatment of the patient. In this narrative review, the most recent contributions of multimodality imaging to the patient with hypertension (CMR, CT and nuclear cardiology) will be reviewed.
Core tip: Diverse imaging modalities are playing a larger role every day in the diagnosis, treatment decisions and follow-up of patients. This is especially true in patients with hypertension. The merger of diverse imaging techniques has led to the rise of Multimodality Imaging, using tools such as cardiovascular magnetic resonance, computed tomography and nuclear cardiology that aid clinicians make the best therapeutic decisions. In this article, we will make a comprehensive review of the most novel contributions of multimodality imaging to patients with hypertension.
- Citation: Alexanderson-Rosas E, Berríos-Bárcenas E, Meave A, de la Fuente-Mancera JC, Oropeza-Aguilar M, Barrero-Mier A, Monroy-González AG, Cruz-Mendoza R, Guinto-Nishimura GY. Novel contributions of multimodality imaging in hypertension: A narrative review. World J Hypertens 2015; 5(2): 28-40
- URL: https://www.wjgnet.com/2220-3168/full/v5/i2/28.htm
- DOI: https://dx.doi.org/10.5494/wjh.v5.i2.28
Hypertension is one of the most prevalent illnesses worldwide. Data from the NHANES 2007-2010 found that approximately 6% of adults in the United States have undiagnosed hypertension and that in the adult general population; up to one-third might present this illness. It is considered that a 65% of patients presenting with heart failure have a history of Hypertension, and is currently its second most common etiology, only behind ischemic cardiomyopathy[1]. Furthermore, besides its impact on the heart, Hypertension also produces serious damage in blood vessels (including the aorta), kidneys, eyes and brain.
During the last few years, the development of multimodality imaging has contributed to a better understanding of the pathophysiology of cardiovascular diseases, also aiding in its early diagnosis and also monitoring the response to treatment. Traditionally, echocardiogram has been used as the standard imaging method for patient evaluation. However, multimodality imaging has made available a wide array of other imaging techniques [cardiovascular magnetic resonance (CMR), computed tomography (CT), positron emission tomography (PET), single-photon emission computed tomography (SPECT)] for the patient with Hypertension, which might help improve treatment and monitoring of the patients, thus contributing to control this worldwide pandemic. In this review we will touch on the most novel contributions on this subject.
CMR is an imaging method that does not use ionizing radiation, and can evaluate both cardiovascular anatomy and function with high spatial resolution and diagnostic certainty. Furthermore, with the use of gadolinium-based contrasts, it is possible to evaluate vascular anatomy. The 1.5 tesla (T) machines are the most widely used models around the world, although there have been reports of the usefulness of new 3.0 T machines. Traditionally, the most used sequences in patients with hypertensive cardiomyopathy are: tracers, Steady-state Free Precision (SSFP) cine imaging, weighted T2 STIR (short-tau inversion recovery), fast spin-echo weighted T1 and T2, first step myocardial perfusion with gadolinium, phase contrast sequences, inversion recovery sequences for late enhancement and 3D angiographies. All imaging sequences must be acquired with electrocardiographic (ECG) gating, meaning that patients presenting rhythms other than sinus might generate imaging artifacts or suboptimal images. The complete protocol has a duration of approximately 45-60 min, during which the patient must be able to withstand performing 10-20 s apneas, and must also deal with being enclosed in a tight space. Therefore, the success of the study depends greatly on the appropriate selection of the patients. In case that the patient cannot tolerate the study, the best course of action is to perform the study with the patient under general anesthesia with invasive mechanical ventilation.
The following are the most relevant contributions of CMR to patients with Hypertension.
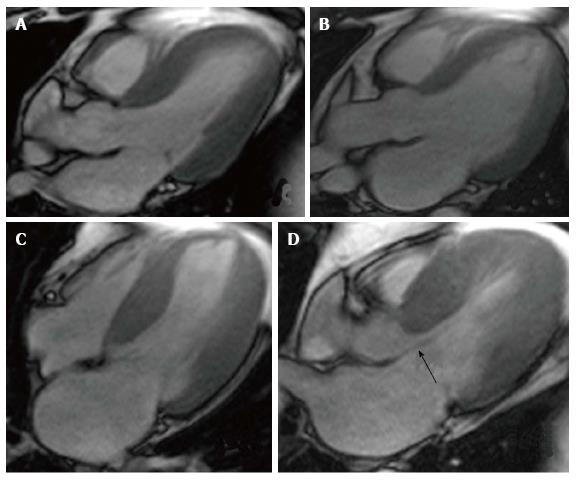
Hypertension has a direct impact on the heart, which is most pronounced in patients with poor control. The most common repercussions in cardiac anatomy are left ventricular hypertrophy and left atrial dilation (Figure 1).
The Framingham study demonstrated that left ventricular hypertrophy is associated with higher cardiovascular mortality, independently of the presence of coronary artery disease[2]. CMR is currently considered the gold standard for the quantification of cardiac dimensions, including ventricular mass, since CMR is a highly exact and reproducible method, when compared with 2D echocardiography. In a study that compared these 2 tools in a sample composed of patients with dilated cardiomyopathy, hypertrophic cardiomyopathy and healthy controls, the coefficients of intra-study variability for left ventricular mass were -1.0 ± 7.7 g for CMR and 8.7 ± 25 g for 2D echocardiography (P < 0.001)[3]. 3D echocardiography has demonstrated accuracy and reproducibility similar to CMR, however, it is still hindered by the same limitations as 2D echo: the need of a good acoustic window and experienced personnel in the usage of the device[4].
Besides the diagnosis of ventricular hypertrophy, CMR can also contribute to the monitoring of these patients. Adequate antihypertensive treatment has demonstrated to revert ventricular hypertrophy, which is associated to a better prognosis, since it prevents the progression to heart failure. These studies have used CMR as the method of choice for serial measurement of ventricular mass[5-7].
However, ventricular hypertrophy is not exclusive of hypertensive cardiomyopathy and can be observed in other illnesses, such as infiltrative diseases (Fabry’s disease, cardiac sarcoidosis, and cardiac amyloidosis), hypertrophic cardiomyopathy, aortic stenosis, exercise-induced hypertrophy, etc. CMR can readily differentiate between these different diseases and help in making the differential diagnosis in favor of hypertensive cardiomyopathy.
Left atrial dilation correlates with the severity and duration of hypertension. Traditionally, this has been measured by echocardiogram; however, CMR has proven to be a more reliable technique for measuring auricular volumes. The presence of left atrial dilation has been linked to the development of atrial fibrillation and increased mortality[8]. Furthermore, the morphology of the left atrial appendage can differentiate between patients with low and high risk of thrombus formation and subsequent embolic events[9]. Finally, left atrial dilation is related to the chronicity of ventricular diastolic dysfunction, as long as mitral valvular disease has been ruled out. Unlike echocardiography, an area of more than 20 cm2 indicates an enlarged left atrium, with the enlargement being classified as severe if the area surpasses 40 cm2[10].
The gold standard for the evaluation of diastolic dysfunction has traditionally been the echocardiogram, which evaluates the pattern of the flow across the mitral valve with Doppler technique. CMR is able to obtain very similar information, by realizing ECG-gated sequences of contrast-phase in the around the mitral valve and the pulmonary veins. This way, it is possible to obtain a time/speed curve very similar to that shown by the echo, with similar diastolic dysfunction patterns[10-12]. Also, novel indexes, such as the Normalized average sweep rate, early diastole normalized sweep peak rate and the relationship between the normalized peak sweep rate in early diastole and the normalized peak sweep rate in atrial systole with an area under the curve of 0.93, 0.88 and 0.88 respectively[13].
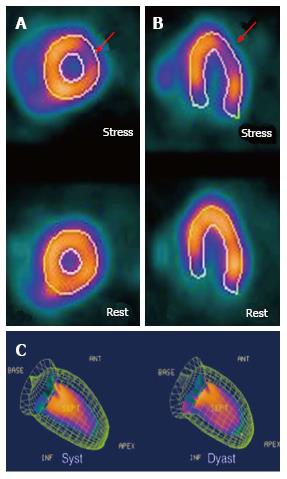
Coronary artery disease is extremely prevalent in the hypertensive population. The presence of chest pain in patients with hypertensive cardiomyopathy is complex, since most of the times it can be due to the hemodynamic changes that arise as consequence of poor blood pressure control. This is complicated even further since traditional tests, such as the cardiac stress test yields suboptimal results due to the non-ischemic electrocardiographical changes seen in hypertensive patients. CMR has proven itself as a very useful diagnostic aid to rule-out the presence of ischemic cardiomyopathy. Through the use of myocardial perfusion imaging at rest, and after pharmacological stress (Figures 2 and 3), myocardial ischemia can be diagnosed with a sensitivity of 89% and a specificity of 76%[14]. Also, in patients with hypertensive cardiomyopathy and severe systolic dysfunction, the absence of ischemic patterns (transmural or subendocardic) excludes the coexistence of coronary artery disease as the cause of heart failure.
Hypertension exerts direct damage to the great blood vessels, especially the aorta. Magnetic Resonance Angiography is a very exact method to diagnose aortic dilation, and can also be used in the presence of acute aortic syndromes. There are studies that have shown that patients with cardiovascular risk factors (including hypertension) have an increased aortic wall thickness, which directly correlates to the presence of atherosclerosis and a poor prognosis[15]. CMR is able to quantify the atherosclerotic burden and plaque composition.
Furthermore, CMR can detect the presence of vulnerable plaques, mainly by the detection of necrotic lipid cores, calcification and hemorrhage in T1 and T2 sequences, with a sensitivity ranging from 84%-100%, both in autopsy studies and in live patients[16,17]. Recent studies have demonstrated that it is possible to quantify the necrotic lipid core, which does not differ from the pathology findings (23.7% vs 20.3%, P = 0.1)[18]. Other findings that can readily be detected by CMR are: plaque fissure, endothelial denudation with platelet adhesion and fibrin deposit, late enhancement in plaques with active inflammatory activity and the severity of vascular stenosis[19].
The study of plaque composition with CMR has been used to demonstrate the beneficial effects of some therapeutic approaches. Lipid apheresis has proven to be statistically significant in diminishing the prevalence of necrotic lipid core in carotid plaques (28.1% vs 56.3%, P < 0.05)[20]. It also showed it reduced its lipid content (5.0% vs 11.6%, P < 0.05). Lipid lowering therapy has been shown to reduce the area of the lipid core of the plaques (0.7 mm2vs 10.2 mm2, P = 0.01), along with the progression of vascular stenosis[21].
As for functional evaluation, there are studies that have demonstrated an increased vascular rigidity in patients with hypertension and diabetes mellitus, measured by the speed of the aortic pulse wave and vascular compliance[22,23]. The severity of the rigidity has also been associated with an increased degree of endothelial injury. The significance of these findings is still being clarified.
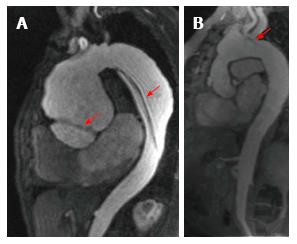
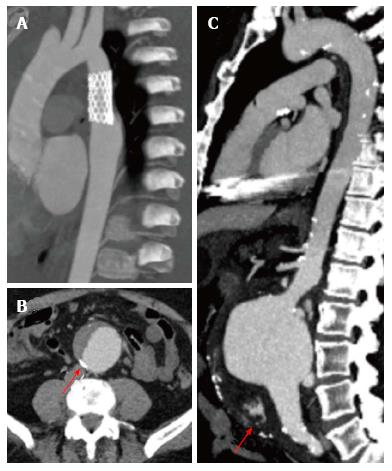
Aortic angioresonance has proven to have excellent diagnostic accuracy for the diagnosis of aortic dissection, with a sensitivity and specificity of 98% and an excellent performance compared to transesophageal echocardiography and CT (Diagnostic OR of 6.8, 6.5 and 6.1, respectively)[24]. It also offers a very high spatial resolution that allows an easy differentiation between the false and true aortic lumen and its branches. In dissections involving the aortic root, SSFP cine imaging allows the evaluation of aortic valve function, to assess the presence of regurgitation and at the same time, to measure both ventricular dimensions and function (Figure 4); all of which are vital parameters which directly affect therapeutic decisions in patients with acute aortic syndromes. The presence of liquid with an increased signal output in the pleura or pericardium are highly suggestive of aortic rupture with subsequent presence of free blood.
The fact that CMR does not emit ionizing radiation and its non-invasive nature make it a very attractive alternative to serially evaluate the diameter of aortic aneurysms. CMR has the potential to establish itself as the diagnostic tool of choice in the follow-up of patients with aortic pathology, due to the high reproducibility of its measurements.
In the case of intramural hematomas, the high spatial resolution allows to identify small hematomas that might have been overlooked even by CT. These blood collections are seen as hyperintense thickening in the aortic wall in weighted T1 sequence.
One of the drawbacks of CMR is that it is a study that requires a lot of time, and can be very uncomfortable for a patient in an acute setting.
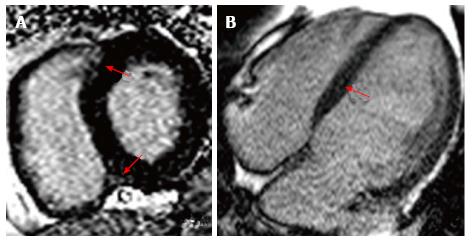
The administration of gadolinium allows the evaluation of late enhancement, which correlates with the presence of interstitial fibrosis in hypertensive cardiomyopathy. The characteristic pattern is diffuse, and found mainly in the interventricular septum[25] (Figure 5). The presence of interstitial fibrosis correlates directly with the prognosis, since it is directly associated with an increased ventricular remodeling, systolic dysfunction and malignant arrhythmias[25]. Several small studies have demonstrated that around 45% of the patients with hypertension present late enhancement after gadolinium administration, which is associated with interstitial fibrosis and coronary microangiopathy[26]. However, it is necessary to assess the direct impact these findings have regarding mortality, progression to heart failure and the development of arrhythmias. Hopefully, quantitative evaluation will shed some light on this issue, as it did with hypertrophic cardiomyopathy. At this moment, there is no available evidence that clarifies the usefulness of diagnosing interstitial fibrosis in patients with hypertensive cardiomyopathy using CMR.
Patients who present with resistant hypertension (patients without blood pressure control that are already taking 3 different drugs, one of them being a diuretic) must undergo differential diagnosis in order to rule out a secondary cause of hypertension, which are present in approximately 5% of the population[27]. The majority of secondary causes are due to endocrine dysfunction, for which the first diagnostic step are biochemical panels and hormone level tests, only after those, are imaging methods solicited. However, CMR is a very useful tool used to speed up the diagnosis of these patients. The most common causes of secondary hypertension are: primary hyperaldosteronism, renovascular disease, chronic renal insufficiency and obstructive sleep apnea. Here we will make a very brief summary of the secondary causes that can be evaluated using CMR.
The prevalence of this disease is still a matter of debate. Some studies report that it might be responsible for up to 6.1% of the cases of hypertension, and this number goes up to 18% when taking into account patients with BP of over 180/110 mmHg. It makes up 20% of the cases of secondary hypertension[28]. Clinical and laboratory findings typical of this disease include hypokalemia and hypertension; however, hypokalemia is only present in around half of the cases, and it’s only found in latter stages of the disease[28,29]. A high degree of clinical suspicion is necessary to diagnose this illness, followed by biochemical confirmation (aldosterone/renin relationship in blood serum, confirmed with oral or parenteral sodium overload). The two principal causes of primary hyperaldosteronism are: adrenal adenoma (35%) and bilateral adrenal hyperplasia (65%). In these patients, it is mandatory to realize an imaging study to rule out the presence of neoplasm, since in these cases, surgical removal of the aldosterone producing tumor can cure the patient. Adenomas are usually hypo or isointense in T1 (when compared to the liver) and slightly hyperintense in T2. CMR has demonstrated a sensitivity of 70% and a specificity of 100% for the diagnosis of these tumors[30]; these values are very similar to those offered by CT[31].
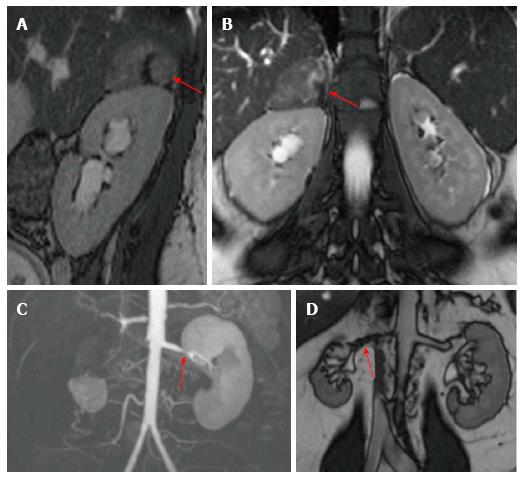
Up to 20% of patients who undergo cardiac catheterization present significant unilateral or bilateral renal artery stenosis[32] as an incidental finding; mainly in patients with extrarenal atherosclerosis[33], but it is still unclear how many of these patients have a direct repercussion on their BP. Renovascular disease is responsible for 35% of the cases of resistant hypertension[28]. CMR sequences used to study renal arteries are the same ones used during aortic angioresonance. CMR with gadolinium has shown a sensitivity of 97% and a specificity of 93% for the diagnosis of renal artery stenosis[34], with the limitation of being contraindicated in patients with a creatinine clearance of less than 30 mL/min per 1.73 m2. It must be noted that treatment using either balloon angioplasty or stenting has not shown to improve BP control or renal function[35,36] (Figure 6).
Other less frequent causes of secondary hypertension include Cushing’s syndrome and pheocromocytoma. Both these entities have very characteristic clinical presentations, so once that there is enough clinical suspicion, a biochemical confirmation must be made. Once both these criteria have been met, CMR might be used; offering a very similar diagnostic capacity to that of CT.
Over the last few decades, impressive technological advances have been made in the field of CT. The rise of machines with a very high spatial and temporal resolution coupled with ECG gating have allowed to obtain high precision coronary artery images, which is currently CT’s main use in cardiology. Among the most relevant contributions to patients with hypertension we can find the following:
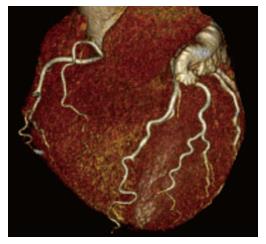
The use of CT for the evaluation of coronary artery disease (CAD) constitutes one of the most important breakthroughs in non-invasive cardiology in the last few decades. The CT machines best suited for the acquisition of these studies are those with 16 detectors or more; nowadays the most used CT scanners have 64 detectors. The acquisition must always be ECG-gated and coordinated with contrast administration. Today, thanks to the various acquisition techniques (prospective protocols, diminished voltage, high pitch, etc.) the radiation dose per study has been reduced to around 1-2 mSv.
Coronary CT has shown a great diagnostic certainty when it comes to ruling out CAD with a sensitivity of 85% (95%CI: 79%-90%) and a specificity of 90% (95%CI: 83%-94%), with an area under the curve of 0.93[37]. Furthermore, the result of the Coronary CT has a direct relationship with prognosis, having a 3-year survival of over 95%[38].
The presence of coronary artery tortuosity has been related to female gender and the presence of chronic hypertension[39]. It is believed that these changes are due to an increase in the pressure and volume load in coronary vessels, therefore these changes can be seen as a consequence of hypertension[40]. There are studies that have related coronary tortuosity with diastolic dysfunction[41]; however, this finding has not been linked with worsening in the prognosis. The relevance of the presence of coronary artery tortuosity in coronary CT studies is still being debated (Figure 7).
Coronary calcium quantification currently is made with CT machines with prospective ECG-gating capacity, which translates to reduced radiation dosage per study (1-2 mSv). CT has a sensitivity of 96% and a positive predictive value of 80% for coronary calcium, with the drawback being that it only has a specificity of 46%[42]. Patients with an Agatston score of under 100 have a very good prognosis, with a very low probability of having a positive SPECT scan[43]. The risk for coronary disease increases linearly as the Agatson score rises[44-48]; the same is seen with mortality[49].
It is known that hypertension is an important risk factor for the development of atherosclerosis. Recently, a study included 8238 asymptomatic subjects and then divided them by category of BP (according to JNC-7), demonstrating that the risk of subclinical atherosclerosis, non-calcified coronary plaques and coronary calcium score of over 100 AU increase linearly as BP levels rise[50]. Previous studies have demonstrated that the progression of coronary calcification was significantly slower in patients with adequate BP control[51]. Erbel et al[52] have shown that as BP levels rise, so does coronary calcium scores (mainly in men) as does the rate of major adverse cardiovascular events. This suggests that in patients with a stage 2 hypertension (BP > 160/100) might be candidates for a CT study to rule out subclinical atherosclerotic. However, the impact of this strategy must be evaluated in future studies.
Retrospective protocols with radiation modulation have allowed the evaluation of volumes, dimensions and mass of cardiac structures. CT offers the advantage of being able to evaluate cardiac cavities with a great temporal and spatial resolution, along with the possibility of 3D volumetric visualization. A meta-analysis comprising 27 comparative studies between CT and CMR demonstrated excellent correlations in the measurement of telesystolic, telediastolic, ejection fraction and left ventricular mass volumes (r = 0.93, 0.95, 0.93, 0.86 respectively)[53]. In hypertensive patients, the acquisition of this data has a direct impact on prognosis.
Right ventricular function has also been compared to CMR in several studies, showing good correlation between the 2 imaging modalities (r = 0.88), with an even higher reproducibility in CT[54,55]. However, the exactitude of the measurement of the right ventricle depends on the quality of the attenuation of right cavities, being necessary at least 175 HU to achieve a good measurement[56].
CT is currently the method of choice to evaluate vascular anatomy, playing a key role in the diagnosis, risk stratification and treatment of aortic disease[57]. ECG-gated studies allow the acquisition of precise aortic root images free of movement artifacts. Also, CT is a diagnostic tool that is present in most hospital centers and unlike CMR, the acquisition times are very short.
The traditional CT protocol must include non-contrast images, mainly when an acute coronary syndrome is suspected, since this sequence allows the detection of intramural hematomas. Afterwards, ECG-gated angiography is performed using contrast, allowing the evaluation of vascular anatomy. Finally, in patients with vascular implants, late images can identify vascular leaks (Figure 8). In general, CT has a great performance in the diagnosis of aortic disease (up to 92%), including its main branches[58].
In Korean hypertensive patients of over 65 years, CT studies have shown a prevalence of thoracic aortic aneurysms in 36.5% and abdominal aortic aneurysms in 6%[59]. In another study, high coronary calcium scores correlated with an increased abdominal aortic diameter and a higher incidence of aneurysms (14%) when the score was > 400 AU, especially when this coincided with other cardiovascular risk factors[60]. However, due to the scarce evidence regarding this issue, there is currently no recommendation about screening studies in these patients.
The advantages of CT for the diagnosis of renovascular disease or adrenal adenomas have been mentioned previously: good spatial resolution, short acquisition times and widely available in many hospital centers. CT has demonstrated a sensitivity of 85%-87% and a specificity of 82%-93% for the diagnosis of adrenal adenomas[31]. In the evaluation of resistant hypertension, the usage of either CT or CMR can be used interchangeably, since their diagnostic accuracy is very similar.
Nuclear cardiology is the most studied non-invasive cardiovascular imaging modality, only behind echocardiogram. Since the 1970’s, SPECT established itself as the most widely diffused imaging technique to evaluate the presence of myocardial ischemia all around the world; today, PET and hybrid imaging offer valuable information used in everyday cardiologic practice.
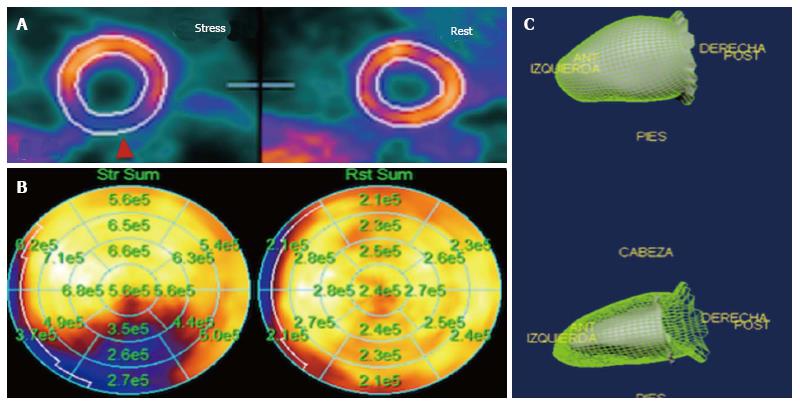
Both SPECT and PET have developed protocols for the evaluation for myocardial ischemia, which is extremely useful in hypertensive patients with angina and multiple cardiovascular risk factors. Both techniques use protocols that involve image acquisition in rest and stress, either physical or pharmacological. In a meta-analysis, the validity for SPECT to detect myocardial ischemia has shown a sensitivity of 88% (95%CI: 88%-89%) with specificity of 61% (95%CI: 59%-62%) and an area under the curve of 0.86[14]. Regarding PET, it showed a better diagnostic capability, with a sensitivity of 84% (95%CI: 81%-87%) with a specificity of 81% (95%CI: 74%-87%) and an area under the curve of 0.92[14]. When merging different techniques, a modality called hybrid imaging, SPECT/CT has shown a sensitivity of 94%-96%, specificity of 92%-95% and a negative predictive value of 97%-99%. Finally, PET/CT has shown a slightly better performance over other imaging techniques, with a sensitivity of 93% and a specificity of 99% when using 15O-H2O radioisotope[61] (Figure 9).
Patients with several risk factors can present with silent ischemia. A study of hypertensive patients without angina demonstrated that, when evaluated with pharmacologic stress-SPECT, the prevalence of reversible perfusion defects was of 27.7% and increased to 41.4% in patients with diabetes (P = 0.001), many of these being moderate to severe defects. In a sub-analysis of the same study, dyspnea and proteinuria were found to be independent predictors of silent ischemia[62].
The incorporation of cardiac-gating allowed evaluating left ventricular function at the same time as myocardial perfusion without the need of performing an additional radioisotope ventriculography, which implied a longer study time and radiation exposure. The calculation of the left ventricular ejection fraction (LVEF) is done automatically by software, without human intervention, which makes it a highly reproducible method.
Furthermore, it has shown to have a very good correlation with CMR (r = 0.82), without being statistically significant in the Bland-Altman analysis and high reproducibility[63-65]. LVEF calculation with Gated-PET has shown to have a good correlation with CMR (r = 0.76)[66], however, it has been observed that it underestimates both telesystolic and telediastolic volumes. Today, the clinical implications of this method are limited to complementing the myocardial perfusion study, with isolated LVEF measurement being reserved for investigation protocols exclusively.
Nuclear cardiology studies have been able to demonstrate the presence of endothelial lesions in patients with hypertension. A study made by our group evaluated the endothelial function using a triphasic protocol of 13N-Amonia PET (rest, cold pressor test and adenosine induced-hyperemia) in patients with recent diagnosis of hypertension, compared with a healthy control group. We found that 84% of the hypertensive patients had endothelial dysfunction measured by an ENDEVI score < 1.5 (Endothelial-dependent Vasodilation Index) and 58% presented vasomotor abnormalities measured by a CFR (coronary flow reserve) of < 2.5. These findings might be early findings of coronary artery disease[67]. Another similar study also found more significant endothelial dysfunction in patients with dyslipidemia, when compared with a healthy control group. Notably, the study group showed improvement after 8 wk of treatment with ezetimibe-simvastatin[68]. The findings of this study are very promising, especially regarding its implications in vascular risk screening, therapeutic decision-making and patient follow-up. However, more information is needed before this method demonstrates its usefulness in daily practice.
The autonomic sympathetic nervous system plays a fundamental role in the maintenance of hormonal and hemodynamic harmony in the cardiovascular system. In hypertensive patients, the increase in this system’s activity leads to the development of hypertensive cardiomyopathy. The synaptic vesicles contain adrenaline, noradrenaline and the false neurotransmitter, an analogue of noradrenaline, guanetidine. This last one can be radioactively marked, transforming itself into meta-iodinebenylguanidine (123I-mIBG) which, when found in a great enough concentration can be measured by conventional gamma-cameras[69]. There are studies that have shown altered myocardial retention of 123I-mIBG in hypertensive patients that also present left ventricular hypertrophy, mainly in the lateral and inferior left ventricular wall[70-72]. This translates to an abnormal neuroadrenergic cardiac function, which might be related to hypertension-induced myocardial damage.
This neurohormonal unbalance, diagnosed with 123I-mIBG SPECT has shown to be related with the prognosis of patients in other diseases. In patients with heart failure, a heart/mediastinum ratio (H/M) < 1.6 is related with a higher mortality, progression of disease and arrhythmias[69]. These findings have been used to evaluate the appropriateness of the therapeutic choice, mainly concerning beta-blocker therapy, although there are studies that involve ACE/ARB inhibitors and spironolactone[69]. However, the role of sympathetic innervation evaluation in hypertensive patients hasn’t been clearly defined.
In patients with hypertension of renovascualr origin, the abnormalities in sympathetic innervation diagnosed with 123I-mIBG SPECT are independent of the development of ventricular hypertrophy, which is a key aspect in which it differs from patients with essential hypertension. This might be due to the fact that myocardial injury due to hypertension of renal origin occurs earlier in the history of the disease compared with essential hypertension[73].
The use of FDG-PET in hyperaldosteronism is focused in the evaluation of adrenal masses, which show no retention of the tracer in the setting of a benign mass, unlike malignant tumors. A meta-analysis reported that FDG-PET showed an excellent diagnostic capacity to differentiate between benign and malignant adrenal masses, with a sensitivity of 97% (95%CI: 93%-98%) specificity of 91% (95%CI: 87%-94%) and an area under the curve of 0.96%[74]. When studying adrenal hyperplasia, it was seen that it did not demonstrated FDG activity.
Regarding pheochromocytoma, the vast majority showed activity when using both FDG and 123I-mIBG, though the latter was found to be unable to diagnose pheochromocytomas associated with Von-Hippel-Lindau syndrome.
Multimodality imaging studies have helped to improve the understanding of a vast number of cardiovascular illnesses, including hypertension. Among the most recent contributions we can find the evaluation of dimensions and ventricular function using CMR, CT and Gated SPECT/PET studies, the ability to exclude the presence of coronary artery disease using non-invasive methods with a high diagnostic certainty; risk stratification in hypertensive cardiomyopathy using late enhancement techniques with the aid of gadolinium contrast in CMR or the evaluation of sympathetic innervation and the evaluation of different causes of resistant hypertension. The use of these techniques is still not commonplace in everyday clinical practice, and further studies are needed before they become a standard of common clinical practice; however, the future is certainly very promising.
P- Reviewer: Gao XM, Lin GM S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 971] [Cited by in RCA: 955] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 2. | Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561-1566. [PubMed] |
| 3. | Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1075] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 4. | Kühl HP, Bücker A, Franke A, Maul S, Nolte-Ernsting C, Reineke T, Hoffmann R, Günther RW, Hanrath P. Transesophageal 3-dimensional echocardiography: in vivo determination of left ventricular mass in comparison with magnetic resonance imaging. J Am Soc Echocardiogr. 2000;13:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, Kleiman J, Krause S, Burns D, Williams GH. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108:1831-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 500] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 6. | Friedrich MG, Dahlöf B, Sechtem U, Unger T, Knecht M, Dietz R; TELMAR Investigators. Reduction (TELMAR) as assessed by magnetic resonance imaging in patients with mild-to-moderate hypertension--a prospective, randomised, double-blind comparison of telmisartan with metoprolol over a period of six months rationale and study design. J Renin Angiotensin Aldosterone Syst. 2003;4:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Solomon SD, Appelbaum E, Manning WJ, Verma A, Berglund T, Lukashevich V, Cherif Papst C, Smith BA, Dahlöf B; Aliskiren in Left Ventricular Hypertrophy (ALLAY) Trial Investigators. Effect of the direct Renin inhibitor aliskiren, the Angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009;119:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Milan A, Caserta MA, Dematteis A, Naso D, Pertusio A, Magnino C, Puglisi E, Rabbia F, Pandian NG, Mulatero P. Blood pressure levels, left ventricular mass and function are correlated with left atrial volume in mild to moderate hypertensive patients. J Hum Hypertens. 2009;23:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Yamamoto M, Seo Y, Kawamatsu N, Sato K, Sugano A, Machino-Ohtsuka T, Kawamura R, Nakajima H, Igarashi M, Sekiguchi Y. Complex left atrial appendage morphology and left atrial appendage thrombus formation in patients with atrial fibrillation. Circ Cardiovasc Imaging. 2014;7:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Fernández-Pérez GC, Duarte R, Corral de la Calle M, Calatayud J, Sánchez González J. [Analysis of left ventricular diastolic function using magnetic resonance imaging]. Radiologia. 2012;54:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Kawaji K, Codella NC, Prince MR, Chu CW, Shakoor A, LaBounty TM, Min JK, Swaminathan RV, Devereux RB, Wang Y. Automated segmentation of routine clinical cardiac magnetic resonance imaging for assessment of left ventricular diastolic dysfunction. Circ Cardiovasc Imaging. 2009;2:476-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Mendoza DD, Codella NC, Wang Y, Prince MR, Sethi S, Manoushagian SJ, Kawaji K, Min JK, LaBounty TM, Devereux RB. Impact of diastolic dysfunction severity on global left ventricular volumetric filling - assessment by automated segmentation of routine cine cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Wu V, Chyou JY, Chung S, Bhagavatula S, Axel L. Evaluation of diastolic function by three-dimensional volume tracking of the mitral annulus with cardiovascular magnetic resonance: comparison with tissue Doppler imaging. J Cardiovasc Magn Reson. 2014;16:71. [PubMed] |
| 14. | Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, Nelemans PJ, Schalla S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59:1719-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 337] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 15. | Gupta S, Berry JD, Ayers CR, Peshock RM, Khera A, de Lemos JA, Patel PC, Markham DW, Drazner MH. Left ventricular hypertrophy, aortic wall thickness, and lifetime predicted risk of cardiovascular disease: the Dallas Heart Study. JACC Cardiovasc Imaging. 2010;3:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Shinnar M, Fallon JT, Wehrli S, Levin M, Dalmacy D, Fayad ZA, Badimon JJ, Harrington M, Harrington E, Fuster V. The diagnostic accuracy of ex vivo MRI for human atherosclerotic plaque characterization. Arterioscler Thromb Vasc Biol. 1999;19:2756-2761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 227] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, Small R, Davies JW, Kerwin WS, Hatsukami TS. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104:2051-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 542] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 18. | Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, Hatsukami TS, Yuan C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 455] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 19. | Saam T, Hatsukami TS, Takaya N, Chu B, Underhill H, Kerwin WS, Cai J, Ferguson MS, Yuan C. The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology. 2007;244:64-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 20. | Grimm JM, Nikolaou K, Schindler A, Hettich R, Heigl F, Cyran CC, Schwarz F, Klingel R, Karpinska A, Yuan C. Characteristics of carotid atherosclerotic plaques of chronic lipid apheresis patients as assessed by in vivo high-resolution CMR--a comparative analysis. J Cardiovasc Magn Reson. 2012;14:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Zhao XQ, Yuan C, Hatsukami TS, Frechette EH, Kang XJ, Maravilla KR, Brown BG. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: a case-control study. Arterioscler Thromb Vasc Biol. 2001;21:1623-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Verwoert GC, Franco OH, Hoeks AP, Reneman RS, Hofman A, V Duijn CM, Sijbrands EJ, Witteman JC, Mattace-Raso FU. Arterial stiffness and hypertension in a large population of untreated individuals: the Rotterdam Study. J Hypertens. 2014;32:1606-1612; discussion 1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Shan Y, Lin J, Xu P, Zeng M, Lin H, Yan H. The combined effect of hypertension and type 2 diabetes mellitus on aortic stiffness and endothelial dysfunction: an integrated study with high-resolution MRI. Magn Reson Imaging. 2014;32:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Shiga T, Wajima Z, Apfel CC, Inoue T, Ohe Y. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med. 2006;166:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 271] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 25. | Maceira AM, Mohiaddin RH. Cardiovascular magnetic resonance in systemic hypertension. J Cardiovasc Magn Reson. 2012;14:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Andersen K, Hennersdorf M, Cohnen M, Blondin D, Mödder U, Poll LW. Myocardial delayed contrast enhancement in patients with arterial hypertension: initial results of cardiac MRI. Eur J Radiol. 2009;71:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981-2997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4118] [Cited by in RCA: 3710] [Article Influence: 161.3] [Reference Citation Analysis (0)] |
| 28. | Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1067] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 29. | Mosso L, Carvajal C, González A, Barraza A, Avila F, Montero J, Huete A, Gederlini A, Fardella CE. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 302] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 30. | Sohaib SA, Peppercorn PD, Allan C, Monson JP, Grossman AB, Besser GM, Reznek RH. Primary hyperaldosteronism (Conn syndrome): MR imaging findings. Radiology. 2000;214:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Lingam RK, Sohaib SA, Rockall AG, Isidori AM, Chew S, Monson JP, Grossman A, Besser GM, Reznek RH. Diagnostic performance of CT versus MR in detecting aldosterone-producing adenoma in primary hyperaldosteronism (Conn’s syndrome). Eur Radiol. 2004;14:1787-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Aqel RA, Zoghbi GJ, Baldwin SA, Auda WS, Calhoun DA, Coffey CS, Perry GJ, Iskandrian AE. Prevalence of renal artery stenosis in high-risk veterans referred to cardiac catheterization. J Hypertens. 2003;21:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens. 2009;27:1333-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Tan KT, van Beek EJ, Brown PW, van Delden OM, Tijssen J, Ramsay LE. Magnetic resonance angiography for the diagnosis of renal artery stenosis: a meta-analysis. Clin Radiol. 2002;57:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | van Jaarsveld BC, Krijnen P, Pieterman H, Derkx FH, Deinum J, Postma CT, Dees A, Woittiez AJ, Bartelink AK, Man in’t Veld AJ. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med. 2000;342:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 548] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 36. | Shetty R, Biondi-Zoccai GG, Abbate A, Amin MS, Jovin IS. Percutaneous renal artery intervention versus medical therapy in patients with renal artery stenosis: a meta-analysis. EuroIntervention. 2011;7:844-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1383] [Cited by in RCA: 1340] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 38. | Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 590] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 39. | Li Y, Shen C, Ji Y, Feng Y, Ma G, Liu N. Clinical implication of coronary tortuosity in patients with coronary artery disease. PLoS One. 2011;6:e24232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Xie X, Wang Y, Zhou H. Impact of coronary tortuosity on the coronary blood flow: a 3D computational study. J Biomech. 2013;46:1833-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Turgut O, Yilmaz A, Yalta K, Yilmaz BM, Ozyol A, Kendirlioglu O, Karadas F, Tandogan I. Tortuosity of coronary arteries: an indicator for impaired left ventricular relaxation? Int J Cardiovasc Imaging. 2007;23:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | He ZX, Hedrick TD, Pratt CM, Verani MS, Aquino V, Roberts R, Mahmarian JJ. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 234] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 43. | Lamont DH, Budoff MJ, Shavelle DM, Shavelle R, Brundage BH, Hagar JM. Coronary calcium scanning adds incremental value to patients with positive stress tests. Am Heart J. 2002;143:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 442] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 45. | Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 714] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 46. | Raggi P, Cooil B, Callister TQ. Use of electron beam tomography data to develop models for prediction of hard coronary events. Am Heart J. 2001;141:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 47. | Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 429] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 48. | Wayhs R, Zelinger A, Raggi P. High coronary artery calcium scores pose an extremely elevated risk for hard events. J Am Coll Cardiol. 2002;39:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 236] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 49. | Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Im TS, Chun EJ, Lee MS, Adla T, Kim JA, Choi SI. Grade-response relationship between blood pressure and severity of coronary atherosclerosis in asymptomatic adults: assessment with coronary CT angiography. Int J Cardiovasc Imaging. 2014;30 Suppl 2:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Sipahi I, Tuzcu EM, Schoenhagen P, Wolski KE, Nicholls SJ, Balog C, Crowe TD, Nissen SE. Effects of normal, pre-hypertensive, and hypertensive blood pressure levels on progression of coronary atherosclerosis. J Am Coll Cardiol. 2006;48:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 52. | Erbel R, Lehmann N, Möhlenkamp S, Churzidse S, Bauer M, Kälsch H, Schmermund A, Moebus S, Stang A, Roggenbuck U. Subclinical coronary atherosclerosis predicts cardiovascular risk in different stages of hypertension: result of the Heinz Nixdorf Recall Study. Hypertension. 2012;59:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Sharma A, Einstein AJ, Vallakati A, Arbab-Zadeh A, Mukherjee D, Lichstein E. Meta-analysis of global left ventricular function comparing multidetector computed tomography with cardiac magnetic resonance imaging. Am J Cardiol. 2014;113:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Raman SV, Shah M, McCarthy B, Garcia A, Ferketich AK. Multi-detector row cardiac computed tomography accurately quantifies right and left ventricular size and function compared with cardiac magnetic resonance. Am Heart J. 2006;151:736-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 55. | Guo YK, Gao HL, Zhang XC, Wang QL, Yang ZG, Ma ES. Accuracy and reproducibility of assessing right ventricular function with 64-section multi-detector row CT: comparison with magnetic resonance imaging. Int J Cardiol. 2010;139:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Aho MR, Gebregziabher M, Schoepf UJ, Suranyi P, Lee H, Gregg D, Costello P, Zwerner PL. Impact of right ventricular contrast attenuation on the accuracy of right ventricular function analysis at cardiac multi-detector-row CT. Eur J Radiol. 2010;73:560-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B,Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ; ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2374] [Cited by in RCA: 3103] [Article Influence: 282.1] [Reference Citation Analysis (0)] |
| 58. | Agarwal PP, Chughtai A, Matzinger FR, Kazerooni EA. Multidetector CT of thoracic aortic aneurysms. Radiographics. 2009;29:537-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 59. | Cho IJ, Jang SY, Chang HJ, Shin S, Shim CY, Hong GR, Chung N. Aortic aneurysm screening in a high-risk population: a non-contrast computed tomography study in korean males with hypertension. Korean Circ J. 2014;44:162-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Cho IJ, Heo R, Chang HJ, Shin S, Shim CY, Hong GR, Min JK, Chung N. Correlation between coronary artery calcium score and aortic diameter in a high-risk population of elderly male hypertensive patients. Coron Artery Dis. 2014;25:698-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Gaemperli O, Bengel FM, Kaufmann PA. Cardiac hybrid imaging. Eur Heart J. 2011;32:2100-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 62. | Lacourcière Y, Côté C, Lefebvre J, Dumont M. Noninvasive detection of silent coronary artery disease in patients with essential hypertension, alone or associated with type 2 diabetes mellitus, using dipyridamole stress 99mtechnetium-sestamibi myocardial perfusion imaging. Can J Cardiol. 2006;22 Suppl A:16A-21A. [PubMed] |
| 63. | Abidov A, Germano G, Hachamovitch R, Slomka P, Berman DS. Gated SPECT in assessment of regional and global left ventricular function: an update. J Nucl Cardiol. 2013;20:1118-1143; quiz 1118-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Thorley PJ, Plein S, Bloomer TN, Ridgway JP, Sivananthan UM. Comparison of 99mTc tetrofosmin gated SPECT measurements of left ventricular volumes and ejection fraction with MRI over a wide range of values. Nucl Med Commun. 2003;24:763-769. [PubMed] |
| 65. | Thorley PJ, Smith JM. Repeatability of left ventricular ejection fraction and volume measurement for 99mTc-tetrofosmin gated single photon emission computed tomography (SPECT). Nucl Med Commun. 2005;26:345-349. [PubMed] |
| 66. | Li Y, Wang L, Zhao SH, He ZX, Wang DY, Guo F, Fang W, Yang MF. Gated F-18 FDG PET for assessment of left ventricular volumes and ejection fraction using QGS and 4D-MSPECT in patients with heart failure: a comparison with cardiac MRI. PLoS One. 2014;9:e80227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Alexánderson E, Jácome R, Jiménez-Santos M, Ochoa JM, Romero E, Cabral MA, Ricalde A, Iñarra F, Meave A, Alexánderson G. Evaluation of the endothelial function in hypertensive patients with 13N-ammonia PET. J Nucl Cardiol. 2012;19:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Alexanderson E, García-Rojas L, Jiménez M, Jácome R, Calleja R, Martínez A, Ochoa JM, Meave A, Alexanderson G. Effect of ezetimibe-simvastatine over endothelial dysfunction in dyslipidemic patients: assessment by 13N-ammonia positron emission tomography. J Nucl Cardiol. 2010;17:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Chirumamilla A, Travin MI. Cardiac applications of 123I-mIBG imaging. Semin Nucl Med. 2011;41:374-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | Mitani I, Sumita S, Takahashi N, Ochiai H, Ishii M. 123I-MIBG myocardial imaging in hypertensive patients: abnormality progresses with left ventricular hypertrophy. Ann Nucl Med. 1996;10:315-21. [PubMed] |
| 71. | Kuwahara T, Hamada M, Hiwada K. Direct evidence of impaired cardiac sympathetic innervation in essential hypertensive patients with left ventricular hypertrophy. J Nucl Med. 1998;39:1486-1491. [PubMed] |
| 72. | Shimizu M, Ino H, Okeie K, Emoto Y, Yamaguchi M, Yasuda T, Fujino N, Fujii H, Fujita S, Nakajima K. Cardiac sympathetic activity in the asymmetrically hypertrophied septum in patients with hypertension or hypertrophic cardiomyopathy. Clin Cardiol. 2000;23:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Ohya Y, Sasaki M, Fujishima S, Kagiyama S, Onaka U, Kaseda S, Ohmori S, Kuwabara Y, Abe I, Fujishima M. Myocardial imaging with 123I-metaiodobenzylguanidine in essential hypertension and renovascular hypertension. Clin Exp Hypertens. 2001;23:293-304. [PubMed] |
| 74. | Boland GW, Dwamena BA, Jagtiani Sangwaiya M, Goehler AG, Blake MA, Hahn PF, Scott JA, Kalra MK. Characterization of adrenal masses by using FDG PET: a systematic review and meta-analysis of diagnostic test performance. Radiology. 2011;259:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |