Published online Nov 23, 2013. doi: 10.5494/wjh.v3.i4.27
Revised: September 27, 2013
Accepted: October 17, 2013
Published online: November 23, 2013
Processing time: 144 Days and 23.6 Hours
Variability is an aspect of blood pressure (BP) relatively unknown and poorly evaluated systematically in clinical practice. Although the introduction of intensive BP measurement methods, such as ambulatory blood pressure monitoring provided evidence of the importance of BP variability in the short-term, more recently, however, emphasis has been placed on the relevance of variability of BP in the medium- and long-term. The adverse cardiovascular consequences of high BP not only depend on absolute BP values, but also on BP variability. Independently of mean BP levels, BP variations in the short- and long-term are associated an increased risk of cardiovascular events and mortality. Also, it has been suggested that modulation of such variability may explain the different level protection exerted by different antihypertensive-drug classes.
Core tip: A recent focus of interest has been the relationship between variability of blood pressure (BP) and cardiovascular events. It has been documented that the impact of hypertension on the risk of cardiovascular diseases including cardiovascular-related death not only depends on absolute BP values, but also on BP variations in the short- and long-term. For this reason, besides to reducing absolute BP levels, control of BP variability is highly desirable and an important target of antihypertensive treatment.
- Citation: Pelegrí A, Arboix A. Blood pressure variability and cerebrovascular disease. World J Hypertens 2013; 3(4): 27-31
- URL: https://www.wjgnet.com/2220-3168/full/v3/i4/27.htm
- DOI: https://dx.doi.org/10.5494/wjh.v3.i4.27
It has been extensively recognized that hypertension is one the main risk factors for acute stroke. Some particular aspects of elevated blood pressure (BP), such as its systolic and diastolic components, pulse wave, the circadian pattern or the pharmacological control have been progressively defined as aspects with more or less impact on the global vascular risk associated with high BP.
The scientific interest in the role of BP variability was initially originated from technical advances allowing monitoring of BP during short periods of time, obtaining multiple measurements with the possibility of evaluating variations in the short term. Ambulatory blood pressure monitoring (ABPM) allowed assessing, in a reproducible manner, BP changes at different times of the day as well as between measurements, and although not consistently, it was shown that this short-term BP variability was an independent risk factor of cardiovascular morbidity and mortality[1]. Both morning surges and pronounced BP falls at night have been reported as predictors of silent or clinically manifested cerebrovascular disease.
However, and in general, most clinical trials and clinicians involved in the management of hypertension try to assess the real clinical condition of the patients by obtaining median BP levels, excluding distorting extreme and difficult to interpret values. The majority of clinical practice guidelines for hypertension emphasize to target BP levels below a certain cut-point according to the availability of scientific evidence for different patients’ conditions, but no recommendations are made regarding the importance of measuring and targeting BP variability.
On the other hand, BP variability involves different concepts, which in turn adds difficulty in the definition and assessment of its relevance from a clinical perspective[2]. This brief review is focused on a description of the scarce and recent evidences regarding the significance of the different aspects of BP variability as a risk factor of acute cerebrovascular disease.
In the subgroup of patients of the Syst-Eur trial[3] in which ABPM was performed on admission to the study, only night-time systolic BP calculated as the standard deviation (SD) and only in the placebo group (but not in the active treatment group) was associated with an increase risk of stroke during the trial. In a reduced group of hypertensive subjects in which visit-to-visit BP variability and ABPM was assessed, Eguchi et al[4] also reported that only sleep systolic BP was an independent predictor of hard cardiovascular events defined as stroke, myocardial infarction and sudden death.
On the other hand, population-based studies, such as the Ohasama study, demonstrated that circadian BP variations (e.g., excessive nocturnal dipping of BP) may cause cerebrovascular lesions but also increased differences between diurnal and nocturnal BP may be associated with an increase in the relative risk of cardiovascular-related mortality and stroke[5,6] but correlated poorly with mean BP[7]. Therefore, there are evidences that alterations in the circadian BP behavior especially nocturnal systolic BP variations may increase the risk of cardiovascular events. In some cases, it cannot be excluded, that these alterations may be related with sleep-disordered breathing, particularly obstructive sleep apnea[8,9], which in turn may cause BP fluctuations through multiple pathogenetic mechanisms. The excessive nocturnal dipping of BP and a nocturnal rise of BP due to obstructive sleep apnea have been both considered important factors of the association between short-term BP variability and cardiovascular events but, in our opinion, it cannot be currently established which of the two factors is related to a higher cardiovascular risk.
However, not all evidences converge on the same line. Variability of BP measured by ABPM has been recently studied in a meta-analysis using data of the International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome, which included prospective studies of 11 populations and health outcomes in 8938 subjects, concluding that in a large population cohort which provided sufficient statistical power, BP variability assessed from 24-h ambulatory recordings did not contribute significantly to cardiovascular risk stratification obtained by absolute BP values[10]. The authors of this interesting meta-analysis, however, recognized the limitations of the study, which referred to the general applicability of results (particularly to Africans of black ancestry and African Americans), the fact that intermittent techniques of ABPM are less precise to capture short-term BP variability than continuous BP recording, and the low power to detect variability among strata (e.g. considering a tw-sided α-level of 0.05, the power to detect a 0.24 difference between normotensive and hypertensive subjects in the log-transformed hazard ratio of all cardiovascular events was only 46%)[10].
Day-by-day BP variability defined as within-subject SDs of home measurements was also studied in the Ohasama cohort. An increase in systolic BP variability of +1 between-subject SD was associated with increased hazard ratios for cardiovascular (1.2, P = 0.002) and stroke mortality (1.41, P = 0.0009) over a median follow-up of 11.9 years[11]. A similar assessment in an ethnically different population has also confirmed the importance of medium-term BP variability[12].
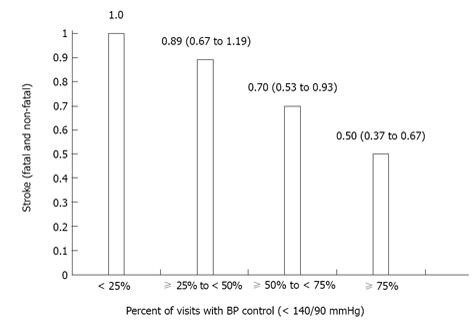
Variability of BP assessed in a more prolonged period of time was evaluated in the International Verapamil SR-Trandolapril (INVEST) Study, in which consistency of BP control, defined as the proportion of visits in which BP was in control, was related to outcome. As proportion of visits with BP control increased, there was an associated steep reduction in cardiovascular morbidity and mortality, particularly in case of stroke[13]. Figure 1 shows a decrease in hazard ratio (95% confidence interval) for fatal and non-fatal stroke as the percentage of visits with BP control increases from < 25% (reference) to ≥ 75% in the INVEST study[13]. Also, sporadic increases in BP even in patients with an acceptable level of BP control have been shown to increase the cardiovascular risk[7,14].
It seems clear that visit-to-visit inter-individual variation of BP readings occurs in routine practice and that within-subject SDs of systolic BP increases as the interval between successive visits also increases. A frequent finding, even with ABPM, is to observe that only a small percentage of patients maintain an adequate control of BP in several consecutive visits. Following data from small observational studies with inconclusive results, the analysis of four cohorts of patients with history of transient ischemic attack or in patients on BP-lowering drugs (Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm) have provided more consistent results[14]. Visit-to-visit residual variability in systolic BP during antihypertensive treatment was a strong predictor of stroke and coronary events, independent of mean systolic BP in clinic or on ABPM[14]. Although an inverse causality by a higher variability in women, older age, diabetics, smokers, and patients with peripheral vascular disease or atrial fibrillation cannot be excluded, the pharmacological treatment received was the most determinant factor of this variability. The finding of the association between BP variability and antihypertensive pharmacological treatment prompted further analyses, such as a recently published report by Muntner et al[15] using data of the Third National Health and Nutrition Examination Survey. The characteristics of this registry based on data of United States adults ≥ 20 years of age, the collection of which was probably not performed in optimal conditions to assess BP variability, concluded that the risk of all-cause mortality was 50% higher in subjects with a SD of systolic BP across visits of 4.80 mmHg[15]. This relationship between BP variability and risk of mortality for all causes was also demonstrated in the subset of the study population with strictly normal BP[15]. Similar conclusions were reported by Shimbo et al[16] in the analysis of the association between visit-to-visit variability of BP and stroke (ischemic and hemorrhagic) in postmenopausal women from the Women’s Health Initiative over a median follow-up of 5.4 years. In this study, there was a significant association between increased visit-to-visit variability and increased risk of stroke, particularly with systolic BP below 120 mmHg[16].
On the other hand, re-analyses of older trials, such as the Medical Research Council’s Study or the more recent antihypertensive and lipid-lowering treatment to prevent heart attack trial suggest an association between the greater and lower BP variability during the study determined by the type of antihypertensive treatment and stroke protection. In fact, a meta-analysis of randomized controlled trials in which the effect of different BP-lowering treatments on the within-subject variance of BP, expressed as ratio of the variances, was assessed confirmed a relationship between interindividual BP variability and the results obtained[17].
In more specific populations, such as elderly people in the prospective study of pravastatin in the elderly at risk (PROSPER), visit-to-visit variability determined by the SD of a minimum of five BP measurements over 1 year was an independent predictor of all-cause and vascular-related mortality, although in this study visit-to-visit variability was not a predictor of stroke[18]. Since the reliability of BP variability increases with the number of measurements, authors of the PROSPER study suggested the possibility that measures of BP variability every 3 mo during the randomized phase of the trial (mean follow-up 3.2 years) may still have underestimated the true effect of variability on clinical outcomes[18]. These results are consistent with recently published data of the Cardiovascular Health Study, a longitudinal cohort study of vascular risk factors and disease in 3852 elderly subjects in whom long-term visit-to-visit systolic BP variability was independently associated with a higher risk of subsequent mortality and myocardial infarction but not stroke[19]. In a population of hemodialysis patients, in which visit-to-visit variability was extremely high compared with other populations, visit-to-visit variability of BP was a major determinant of cardiovascular events[20].
When subclinical or small-vessel cerebral lesions detected by radio imaging techniques and their relationship with different parameters of BP variability has been assessed, patients with progression of this type of cerebral lesions showed a higher SD and coefficient of variation of systolic BP as an indication of higher BP fluctuation over time besides a greater mean BP level[21,22]. In experimental studies, BP-lowering treatments that reduced BP variability in a model of spontaneously hypertensive rats also showed a higher protection for target organ damage[23].
A particular case unfortunately not frequently being taken into account refers to acute variations of BP after an acute episode of cerebral ischemia. The importance of this hemodynamic alteration and its management has been a matter of controversy for years and event at the present time. Although different clinical guidelines suggest treatment strategies, which not always are the same, recommendations are based on insufficiently solid evidences[24,25]. The variability of BP during either the acute or subacute phase of stroke has been assessed in a limited number of studies. An increase in post-stroke BP variability has been demonstrated as well as its relationship with upper airway obstruction in this type of patients[26]. It has been shown that sequelae, neurological impairment or mortality[27] but also worsening of radiological lesions[28] increased with a higher BP variability in the acute stroke phase. A retrospective analysis of BP variability in the subacute period of an ischemic stroke also revealed a linear and independent association with functional outcome at 3 mo in large study sample[29].
In relation to the consideration of BP variability in daily practice, different questions emerge, such as the minimum number of visits that should be established, the optimal interval between visits, or the most adequate parameters to measure variability of BP, including within-subject SD, coefficient of variation, residual SD, successive variation, or average real variability[14,30].
Moreover, it is necessary to select therapeutic strategies associated with a higher stability of BP both in the short- and long-term. In a systematic review of randomized controlled trials comparing different types of β-blocker with placebo or other agents, pooled estimates of the effect of treatment on group variability in BP (ratio of the variances) and on the risk of stroke vs myocardial infarction during follow-up were determined[31]. Compared with other antihypertensives, variability in systolic BP was increased more by non-selective β-blockers, Also, the increase in stroke risk with non-selective β-blockers was significantly more marked than with β1-selective agents[31]. It has been shown that drug-class effects on interindividual variation in BP can account for differences in effects of antihypertensive drugs on the risk of stroke independently of effects on mean systolic BP[32]. To prevent stroke most effectively, BP-lowering drugs should reduce mean BP without increasing variability; ideally they should reduce both. Lower BP variability achieved by calcium-channel blockers and thiazide diuretics as compared with other antihypertensive-drug classes correlates with a more effective protection of the risk of stroke[33].
Although the effect of BP variability on the risk of cardiovascular events especially stroke has been the focus of attention in recent years, it should be noted, firstly, that not all observations have consistently confirmed the relationship between variability of BP and cardiovascular events, and secondly, that most evidences are based on data from reviews and/or post hoc analyses of clinical trials, which have been primarily designed with other objectives. Therefore, prospective studies aimed to assess the significance of the different aspects of BP variability as unequivocal cardiovascular risk factor are warranted.
There now is evidence that BP variability (expressed as average day and nighttime values, day-to-day or visit-to-visit) if augmented, increases the cardiovascular risk independent of the average of conventionally acquired BP readings. Also, antihypertensive-drug classes differ in their effects on visit-to-visit BP variability and associated risk of stroke. Antihypertensive treatment should ideally target alteration in BP variability, in addition to reducing absolute BP levels. However, to determine the causes of increased visit-to-visit BP variability, its best estimate and whether or not treatments that reduce blood pressure variability (and to what extent/target) improve clinical outcomes are open questions for which definitive answers are still pending.
The authors thank Marta Pulido, MD, for editing the manuscript and editorial assistance.
P- Reviewers: Efstathiou S, Lazartigues E, Uehara Y S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Mancia G, Bombelli M, Facchetti R, Madotto F, Corrao G, Trevano FQ, Grassi G, Sega R. Long-term prognostic value of blood pressure variability in the general population: results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension. 2007;49:1265-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 280] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 2. | Mancia G. Prognostic value of long-term blood pressure variability: the evidence is growing. Hypertension. 2011;57:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PW, Jaaskivi M, Nachev C, Parati G, O’Brien ET. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251-2257. [PubMed] |
| 4. | Eguchi S. [A study of articulatory motor function and its variability at the neuro-muscular level (author’s transl)]. Nihon Jibiinkoka Gakkai Kaiho. 1978;81:926-931. [PubMed] |
| 5. | Imai Y, Hozawa A, Ohkubo T, Tsuji I, Yamaguchi J, Matsubara M, Michimata M, Hashimoto J, Fujiwara T, Nagai K. Predictive values of automated blood pressure measurement: what can we learn from the Japanese population - the Ohasama study. Blood Press Monit. 2001;6:335-339. [PubMed] |
| 6. | Kario K, Shimada K, Pickering TG. Clinical implication of morning blood pressure surge in hypertension. J Cardiovasc Pharmacol. 2003;42 Suppl 1:S87-S91. [PubMed] |
| 7. | Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1303] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 8. | Kario K. Obstructive sleep apnea syndrome and hypertension: ambulatory blood pressure. Hypertens Res. 2009;32:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Johansson JK, Kronholm E, Jula AM. Variability in home-measured blood pressure and heart rate: associations with self-reported insomnia and sleep duration. J Hypertens. 2011;29:1897-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 374] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 11. | Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, Inoue R, Hoshi H, Hashimoto J, Totsune K. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 314] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 12. | Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Prognostic value of the variability in home-measured blood pressure and heart rate: the Finn-Home Study. Hypertension. 2012;59:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 13. | Mancia G, Messerli F, Bakris G, Zhou Q, Champion A, Pepine CJ. Blood pressure control and improved cardiovascular outcomes in the International Verapamil SR-Trandolapril Study. Hypertension. 2007;50:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 542] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 15. | Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 355] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 16. | Shimbo D, Newman JD, Aragaki AK, LaMonte MJ, Bavry AA, Allison M, Manson JE, Wassertheil-Smoller S. Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: data from the Women’s Health Initiative. Hypertension. 2012;60:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Rothwell PM. Does blood pressure variability modulate cardiovascular risk? Curr Hypertens Rep. 2011;13:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Poortvliet RK, Ford I, Lloyd SM, Sattar N, Mooijaart SP, de Craen AJ, Westendorp RG, Jukema JW, Packard CJ, Gussekloo J. Blood pressure variability and cardiovascular risk in the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). PLoS One. 2012;7:e52438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Suchy-Dicey AM, Wallace ER, Mitchell SV, Aguilar M, Gottesman RF, Rice K, Kronmal R, Psaty BM, Longstreth WT. Blood pressure variability and the risk of all-cause mortality, incident myocardial infarction, and incident stroke in the cardiovascular health study. Am J Hypertens. 2013;26:1210-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Rossignol P, Cridlig J, Lehert P, Kessler M, Zannad F. Visit-to-visit blood pressure variability is a strong predictor of cardiovascular events in hemodialysis: insights from FOSIDIAL. Hypertension. 2012;60:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Liu W, Liu R, Sun W, Peng Q, Zhang W, Xu E, Cheng Y, Ding M, Li Y, Hong Z. Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke. 2012;43:2916-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Yang YL, Yu LT, Wu ZT, Yu JG, Zhang JM, Chen QH, Bao YC, Liu JG. Synergic effects of levamlodipine and bisoprolol on blood pressure reduction and organ protection in spontaneously hypertensive rats. CNS Neurosci Ther. 2012;18:471-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Schrader J, Lüders S, Kulschewski A, Berger J, Zidek W, Treib J, Einhäupl K, Diener HC, Dominiak P. The ACCESS Study: evaluation of Acute Candesartan Cilexetil Therapy in Stroke Survivors. Stroke. 2003;34:1699-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 441] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 25. | Sandset EC, Bath PM, Boysen G, Jatuzis D, Kõrv J, Lüders S, Murray GD, Richter PS, Roine RO, Terént A. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 362] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 26. | Turkington PM, Bamford J, Wanklyn P, Elliott MW. Effect of upper airway obstruction on blood pressure variability after stroke. Clin Sci (Lond). 2004;107:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Stead LG, Gilmore RM, Vedula KC, Weaver AL, Decker WW, Brown RD. Impact of acute blood pressure variability on ischemic stroke outcome. Neurology. 2006;66:1878-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Delgado-Mederos R, Ribo M, Rovira A, Rubiera M, Munuera J, Santamarina E, Delgado P, Maisterra O, Alvarez-Sabin J, Molina CA. Prognostic significance of blood pressure variability after thrombolysis in acute stroke. Neurology. 2008;71:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Kang J, Ko Y, Park JH, Kim WJ, Jang MS, Yang MH, Lee J, Lee J, Han MK, Gorelick PB. Effect of blood pressure on 3-mo functional outcome in the subacute stage of ischemic stroke. Neurology. 2012;79:2018-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Pierdomenico SD. Indices of blood pressure variability and cardiovascular risk. Hypertension. 2010;56:e21; author reply e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Webb AJ, Fischer U, Rothwell PM. Effects of β-blocker selectivity on blood pressure variability and stroke: a systematic review. Neurology. 2011;77:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 565] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 33. | Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Poulter NR, Sever PS. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 520] [Article Influence: 34.7] [Reference Citation Analysis (0)] |