INTRODUCTION
Primary aldosteronism (PA), the most common form of curable hypertension, is characterized by low plasma potassium, metabolic alkalosis, suppressed plasma renin, and non suppressible aldosterone secretion resulting from either an adrenal adenoma (aldosterone-producing adenoma) or idiopathic adrenal hyperplasia. Recent evidence clearly indicates a greater frequency of PA among patients with high blood pressure than the previously accepted prevalence of approximately 1%[1-3]. This increase in apparent prevalence is the result of widespread use of the aldosterone-to-renin ratio as a screening test that has led to a more efficient identification of this disorder[4,5].
PA was traditionally considered a relatively benign form of hypertensive disease[6], a fact that was generally ascribed to suppression of renin activity and angiotensin II generation that are the consequence of the aldosterone-induced expansion of the extracellular fluid volume[7]. More recent authors have, however, suggested that exposure to excess aldosterone levels might result in substantial cardiovascular[8] and renal damage[9]. Our current knowledge indicates that PA is associated with a variety of cardiovascular and renal sequelae[10] reflecting the capability of aldosterone to induce tissue damage beyond that induced by hypertension itself.
Aldosterone-producing adenoma and bilateral idiopathic adrenal hyperplasia are the common causes of PA, and the currently treatments are adrenalectomy or mineralocorticoid receptor antagonists respectively. Both surgical and medical treatments effectively reduce blood pressure and normalize biochemical abnormalities that characterize PA. However, normalization of blood pressure and correction of hypokalemia are not the only goals of treatment for PA and prevention of organ damage is essential in these patients to prevent major cardiovascular complications and renal events[11].
This editorial will focus on the renal aspects of PA as the kidney is a key determinant of both adaptation to aldosterone-induced sodium and water retention, thereby acting as a rescue mechanism, and of clinical outcome of PA because renal function is critical for blood pressure response to treatment[12].
ALDOSTERONE-RELATED TISSUE DAMAGE: EXPERIMENTAL EVIDENCE
Exposure to aldosterone levels inappropriate for salt status or activation of mineralocorticoid receptors can cause renal tissue injury via mechanisms that are independent of blood pressure[13]. A series of elegant experiments that were conducted by Ricardo Rocha and coworkers demonstrated that in the kidney of uninephrectomized[14] and stroke-prone spontaneously hypertensive rats[15], aldosterone produces intrarenal vascular injury, glomerular damage, and tubulointerstitial fibrosis. Because animal studies consistently indicated that aldosterone causes renal tissue damage in the context of inappropriate salt status, it was suggested that untoward effects of high-salt intake are largely dependent on activation of mineralocorticoid receptors and that this activation might reflect increased oxidative stress[16]. Infusion of aldosterone in the presence of high-salt diet increases the expression of the renal nicotinamide adenine dinucleotide phosphate [NAD(P)H]-oxidase 4 (NOX4) and the subunit “p22phox”, increasing the generation of reactive oxygen species (ROS) in the kidney. In these high-salt fed animals, administration of the mineralocorticoid receptor antagonist eplerenone, or the NAD(P)H-oxidase inhibitor apocynin, prevented aldosterone-induced increase in blood pressure, reduction of plasma nitric oxide levels, and increased urinary excretion of isoprostanes, a marker of oxidative stress[17].
Mineralocorticoid receptors with high affinity for aldosterone and cortisol have been demonstrated in epithelial and non-epithelial tissues. Under physiological conditions, most non-epithelial mineralocorticoid receptors are occupied by high concentrations of cortisol[18], whereas in epithelial tissues binding of cortisol to receptors is prevented by 11β-hydroxysteroid dehydrogenase (11β-HSD2), the enzyme that converts cortisol to the receptor-inactive hormone cortisone. In addition to the conversion of cortisol to cortisone, activity of 11β-HSD2 generates NADH from NAD and produces changes in the intracellular redox potential that might, in turn, inactivate the glucocorticoid-mineralocorticoid receptor complex[19]. It has also been demonstrated that aldosterone itself induces changes in the intracellular redox potential in many cell types[20] through an activation of the NOX1 catalytic subunit of the NAD(P)H-oxidase. Again, this aldosterone-related change in the redox potential is amplified by exposure to salt[21], leading to increased production of ROS and thereby to tissue injury. The mineralocorticoid receptor has also been demonstrated in brain, another non-epithelial tissue, particularly at sites in the anterior hypothalamus and brain stem. By acting on these sites aldosterone regulates salt appetite, sympathetic system discharge, water and electrolyte balance, and blood pressure. Sodium concentrations in the cerebrospinal fluid and the expression of the epithelial sodium channel (ENaC) in neurons are increased by administration of high-salt and aldosterone, respectively. Increase in intraneuronal sodium is mediated by increased ENaC-generated transport and stimulates generation of digitalis-like factors, thereby activating the local renin-angiotensin-aldosterone system and sympathetic outflow. Thus, aldosterone-induced sympathetic outflow resulting from the effects of the hormone on the central nervous system could contribute to maintaining high blood pressure[22]. In summary, in addition to the well-known effects of salt excess on epithelial swelling, vascular stiffening, and blood pressure increase, some effects of salt loading might depend on mineralocorticoid receptor activation and reflect, in various tissues including the kidney, increased oxidative stress.
KIDNEY IN PA
Evidence of beneficial effects of mineralocorticoid receptor antagonists was obtained in small clinical trials that were conducted in proteinuric patients with diabetic nephropathy[23] or chronic kidney disease caused by various renal condition[24]. In PA, cross-sectional evaluations have shown a high degree of variability in the prevalence of clinically relevant renal damage[25-30]. Initial kidney biopsy studies demonstrated only moderate damage in patients with PA and reported prevalence of decreased kidney function in as little as 7% of patients with this endocrine disorder[26]. Similarly, a recent single-center study has reported 24-h creatinine clearance of less than 60 mL/min per 1.73 m2 in only 7% of 56 patients with PA[30], whereas in the German Conn Registry, increased plasma creatinine concentration was found in a substantially higher percentage of patients[31]. In patients with PA, prevalence of overt proteinuria varied from 8%[26] to 24%[25], a disparity that could be explained by differences in duration and severity of disease. In a large, multicenter, Italian study[29], prevalence of microalbuminuria in patients with PA was twice that of patients with essential hypertension.
Fundamental information on the role of the kidney in PA has been obtained from two prospective studies, with short-term and long-term follow-up after treatment. Ribstein et al[32] reported a significant decrease in urinary albumin excretion after adrenalectomy in 25 patients with adrenal adenoma who were followed up for 6 mo. In a 9-year follow-up study of patients with either aldosterone-producing adrenal adenoma or idiopathic adrenal hyperplasia, we have shown that microalbuminuria is more likely to subside to normal levels after treatment than to progress to overt proteinuria[33]. In this study, restoration of normal albumin excretion was more frequent in patients with PA than in matched patients with essential hypertension and this effect appeared to be independent of blood pressure. Both these prospective studies have indicated that PA is characterized by partially reversible renal dysfunction, suggesting that albuminuria is, at least in part, a marker of a renal hemodynamic defect. In keeping with the findings of previous renal function studies[34], some of which were conducted in experimental settings[35], these two studies have demonstrated the presence of relative glomerular hyperfiltration in patients with PA as compared with appropriately matched patients with essential hypertension.
A recent analysis of 408 patients of the German Conn’s Registry[31] and the results of the Taiwan Primary Aldosteronism Investigation study[36,37] have confirmed that glomerular filtration declines soon after treatment of PA and remains relatively stable thereafter. Moreover, evaluation of intrarenal Doppler velocimetric indexes has demonstrated significantly lower intrarenal vascular resistance in patients with PA in comparison with patients with essential hypertension, and reversal of the abnormal intrarenal hemodynamic pattern 1 year after treatment[38]. Thus, findings of longitudinal studies consistently indicate that renal dysfunction in PA is characterized by reversible glomerular hyperfiltration that is associated with decreased intrarenal vascular resistance and contributes to increased urinary albumin losses. The frequency of regression of microalbuminuria in PA suggests that urinary albumin excretion is, at least in part, a marker of functional rather structural renal changes[12]. On the other hand, long-term persistence of albuminuria in a substantial proportion of patients with PA[33] is associated with detectable baseline plasma renin levels[28,30] suggesting the coexistence of structural intrarenal vascular damage, presumably due to long-standing hypertension prior to treatment.
BLOOD PRESSURE AND RENAL OUTCOMES AFTER MANAGEMENT OF PA
The current treatment for aldosterone-producing adrenal adenoma is adrenalectomy, because surgery confers a greater possibility of cure and avoids the possible side effects of mineralocorticoid receptor antagonists. Chronic administration of these agents, however, is the treatment of choice in idiopathic adrenal hyperplasia[39]. Although PA is considered correctable, in many cases, hypertension may persist after surgical or medical treatment and only approximately one-third of patients are cured, defined as having blood pressure of less than 140/90 mmHg without the use of any antihypertensive drugs[28,40,41].
Most studies on the effects of treatment of PA on blood pressure have been conducted in patients with aldosterone-producing adrenal adenoma, and a cumulative analysis of initial case series indicated a rate of hypertension cure of 59% after unilateral adrenalectomy[3]. In the majority of these series, however, cure was defined on the basis of reaching a blood pressure of less than 160/100 mmHg. More recent evidence indicates that only approximately one third of patients treated for PA achieve a blood pressure of less than 140/90 mmHg without the use of additional antihypertensive drugs[3-5]. These estimates were obtained in retrospective investigations and are in agreement with the results of a recent prospective study of PA patients with either adrenal adenoma or idiopathic adrenal hyperplasia, 39% of whom had their blood pressure normalized by adrenalectomy or spironolactone, respectively, whilst the remaining 61% showed significant improvement (decrease of blood pressure by more than 20% and/or fewer antihypertensive agents taken to normalize values)[40].
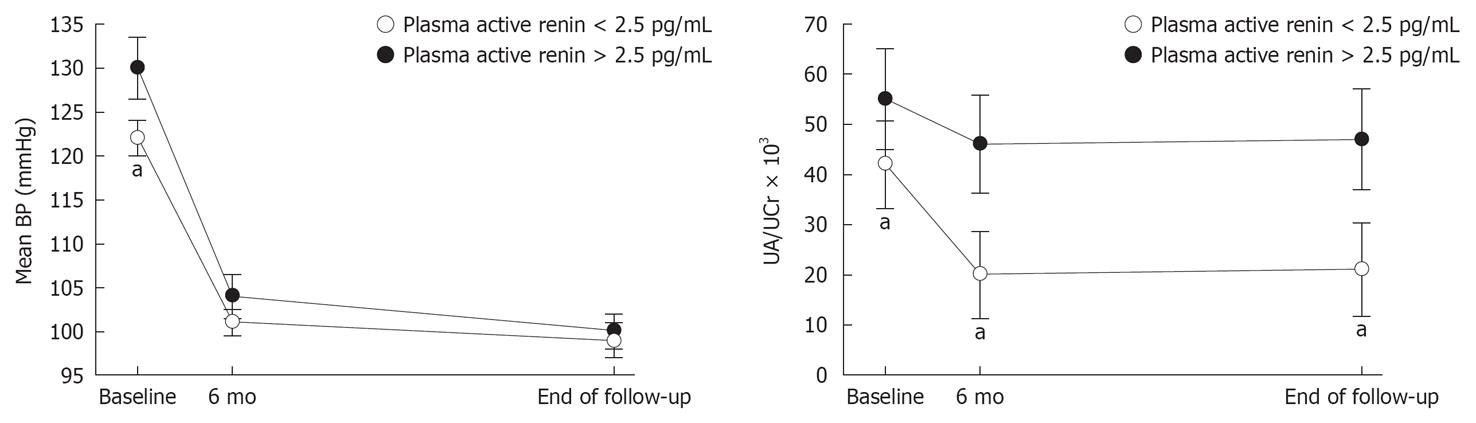
Many studies have investigated the clinical and laboratory factors associated with resolution of hypertension after treatment of PA and have identified younger age, shorter duration of hypertensive disease, lack of family history of hypertension, milder preoperative antihypertensive therapy, lower plasma potassium, greater plasma or urinary aldosterone, and lower active renin[3-5,42-50] as relevant factors. We have recently demonstrated that higher pretreatment plasma renin is associated with less frequent normalization of blood pressure, together with smaller decline in albuminuria during follow-up (Figure 1), indicating that renin escape from suppression by excess aldosterone might be a marker of more severe hypertension-related renal damage[30].
Figure 1 Values (mean ± SE) of mean blood pressure and albuminuria (urine albumin/creatinine ratio) in 56 patients who had primary aldosteronism and were categorized according to their plasma renin concentrations using the lower limit of detection for plasma active renin (2.
5 pg/mL). Variables were measured at baseline and after treatment with adrenalectomy (n = 25) or mineralocorticoid antagonists (n = 31). Short-term and long-term measurements were done after 6 mo and after an average period of 6.2 years, respectively. aP < 0.05 vs patients with plasma active renin concentrations > 2.5 pg/mL. Modified by[30].
It should be kept in mind that the key goal of treatment of all patients with high blood pressure as well as those with PA is the prevention of or recovery from organ damage, in order to decrease the risk of cardiovascular events and renal failure. In this context, evidence linking, in the long term, treatment of PA with renal prevention is recent and refers thus far, to a single-center study in which renal outcomes have been compared in 54 patients with PA and 108 patients with essential hypertension who had comparable cardiovascular risk profiles[33]. Renal outcomes were assessed by measuring the rates of change of glomerular filtration and urinary albumin excretion. After an initial fall in creatinine clearance, due to correction of the aldosterone-induced intrarenal hemodynamic adaptation[38], subsequent declines in glomerular filtration in patients with PA (-1.15 mL/min per 1.73 m2 a year) and primary hypertension (-1.06 mL/min per 1.73 m2 a year) were comparable. Urinary albumin losses did not differ between patients with PA and essential hypertension during the long-term phase of follow-up. Separate analysis of renal outcomes in patients with PA who were treated with adrenalectomy or spironolactone did not reveal significant differences. These studies clearly demonstrate that, in patients with PA, renal impairment does not differ from that seen in patients with essential hypertension when the effects of excess aldosterone are permanently removed and that, in this context, both adrenalectomy and aldosterone antagonists are of considerable therapeutic value.
CONCLUSION
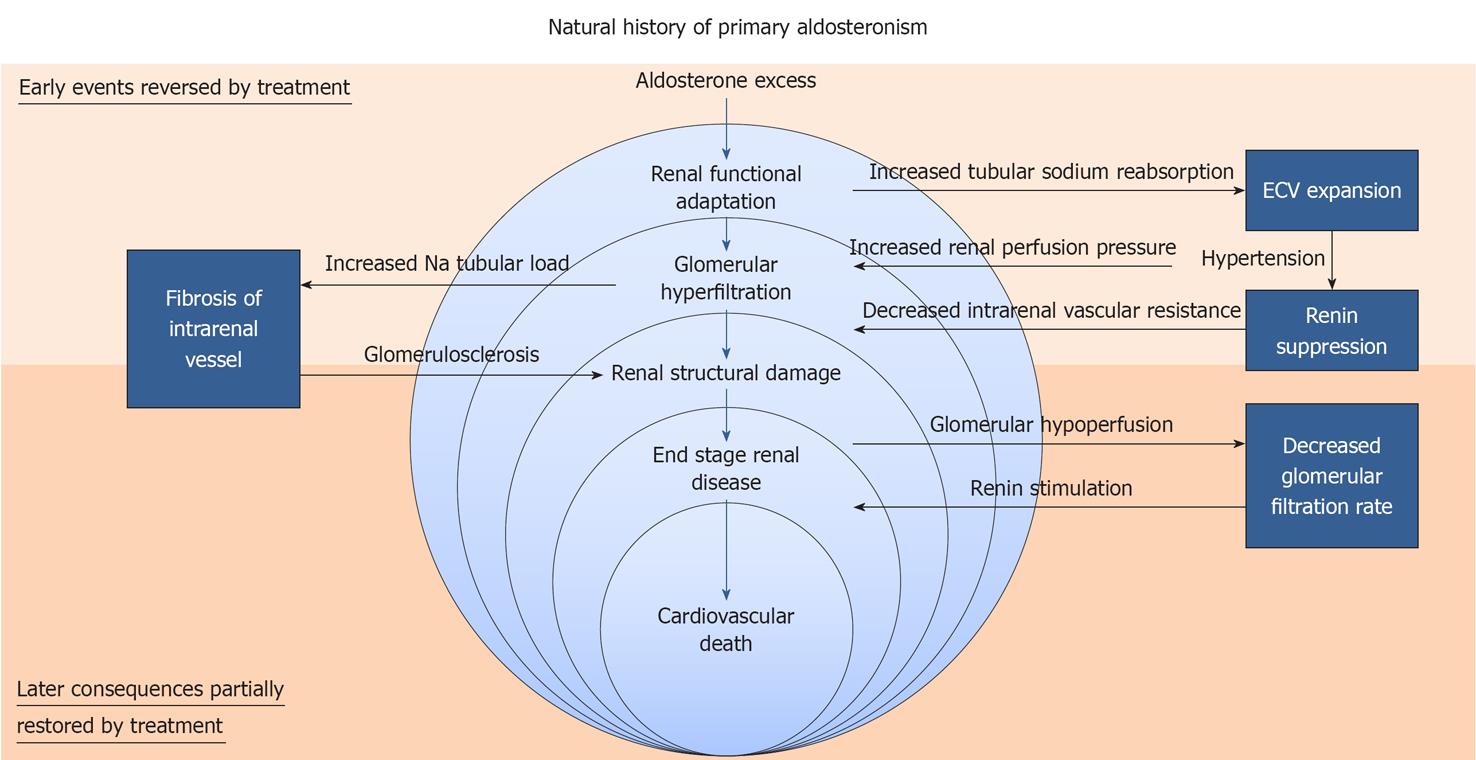
Today it is clear that PA causes a variety of cardiac, vascular, metabolic, and renal sequelae[10] that reflect the ability of inappropriately elevated aldosterone to induce tissue damage in addition to that caused by hypertension itself. However, in dealing with the role of the kidney in PA two distinct aspects need to be considered (Figure 2). On one hand, there are functional adaptations that are induced by increased tubular sodium reabsorption and lead to increase of extracellular fluid volume, hypertension, increased renal perfusion pressure, and suppression of renin with decreased intrarenal vascular resistance[38]. These intrarenal hemodynamic changes cause glomerular hyperfiltration and increase sodium excretion, with escape from the tubular effect of aldosterone[30] and recovery of a steady state. At this stage of disease, the intrarenal hemodynamic adaptation is reversible and, by preventing progressive water and sodium retention, acts as a rescue mechanism. On the other hand, there is structural damage that involves primarily the intrarenal vessels and results from both persistent hypertensive insult and the direct untoward effects of aldosterone. This damage may take several years to develop, leading to decreased glomerular perfusion and stimulation of renin release that escapes from suppression by excess plasma aldosterone and subsequent volume expansion[30]. In this context, lack of complete renin suppression in patients with PA could be the hallmark of more advanced kidney disease with specific involvement of the intrarenal vessels[12]. At this stage of disease, the kidney becomes the key determinant of the clinical outcome of PA, because kidney function is critical for blood pressure response to treatment.
Figure 2 Proposed “course of events” in aldosteronism: from renal functional adaptation to end stage renal disease, cardiovascular events, and death.
Peer reviewers: Mattias Carlstrom, Postdoc Fellow, Division of Nephrology and Hypertension, Department of Medicine, Georgetown University, Building D, Room 380, 4000 Reservoir Road, Washington, DC 20057, United States; Ruisheng Liu, MD, PhD, Associate Professor, Department of Physiology and Biophysics, University of Mississippi Medical Center, 2500 North State St., Jackson, MS 39216, United States; Eric Lazartigues, PhD, FAHA, Assistant Professor, Louisiana State University Health Sciences Center - School of Medicine, Department of Pharmacology and Experimental Therapeutics, 1901 Perdido Street, Room 5218, New Orleans, LA 70112, United States
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM