Published online Aug 20, 2017. doi: 10.5493/wjem.v7.i3.58
Peer-review started: March 28, 2017
First decision: May 9, 2017
Revised: June 11, 2017
Accepted: June 30, 2017
Article in press: July 3, 2017
Published online: August 20, 2017
Processing time: 143 Days and 21.5 Hours
Bone marrow, the vital organ which maintains lifelong hemopoiesis, currently receives considerable attention, as a source of multiple cell types which may play important roles in repair at distant sites. This emerging function, distinct from, but closely related to, bone marrow roles in innate immunity and inflammation, has been characterized through a number of strategies. However, the use of surgical models in this endeavour has hitherto been limited. Surgical strategies allow the experimenter to predetermine the site, timing, severity and invasiveness of injury; to add or remove aggravating factors (such as infection and defects in immunity) in controlled ways; and to manipulate the context of repair, including reconstitution with selected immune cell subpopulations. This endows surgical models overall with great potential for exploring bone marrow responses to injury, inflammation and infection, and its roles in repair and regeneration. We review three different murine surgical models, which variously combine trauma with infection, antigenic stimulation, or immune reconstitution, thereby illuminating different aspects of the bone marrow response to systemic injury in sepsis, trauma and allergy. They are: (1) cecal ligation and puncture, a versatile model of polymicrobial sepsis; (2) egg white implant, an intriguing model of eosinophilia induced by a combination of trauma and sensitization to insoluble allergen; and (3) ectopic lung tissue transplantation, which allows us to dissect afferent and efferent mechanisms leading to accumulation of hemopoietic cells in the lungs. These models highlight the gain in analytical power provided by the association of surgical and immunological strategies.
Core tip: Bone-marrow generates multiple cell types which may play important roles in repair at distant sites. The use of surgical models in the characterization of this emerging function has hitherto been limited. Surgical strategies allow nevertheless the experimenter to predetermine the site, timing, severity and invasiveness of injury; to add or remove aggravating factors (such as infection and defects in immunity) in controlled ways; and to manipulate the context of repair, including reconstitution with selected immune cell subpopulations. Here we review surgical models with great potential for exploring bone-marrow responses to injury, inflammation and infection.
- Citation: Xavier-Elsas P, Ferreira RN, Gaspar-Elsas MIC. Surgical and immune reconstitution murine models in bone marrow research: Potential for exploring mechanisms in sepsis, trauma and allergy. World J Exp Med 2017; 7(3): 58-77
- URL: https://www.wjgnet.com/2220-315X/full/v7/i3/58.htm
- DOI: https://dx.doi.org/10.5493/wjem.v7.i3.58
Bone marrow is a vital organ, primarily because of its central role in adult hemopoiesis; accordingly, its failure requires lifesaving correction by hemopoietic cell transplantation[1-3]. In addition to maintaining steady-state hemopoiesis in healthy subjects, bone marrow supports emergency, or stress, hemopoiesis, i.e., the lineage-selective expansion of hemopoietic cells to meet the exceptional demands of hemorrhage[4,5] or infection[6-9]. The cumulative evidence, however, highlights a third important function of bone marrow in whole-body homeostasis, namely its role in repair following injury of distant sites, especially the skin[10-13], Central Nervous System[14-22], eye[23-26], heart[27-32], lungs[33,34], liver[35-37], gastric mucosa[38,39], chronic wounds associated with diabetes and vasculopathy[40-46], oral mucosa and teeth[47-51], bone and cartilage[52-54] and skeletal muscle[55-59] among other structures.
This emerging role of bone marrow is discussed here as related, but not identical, to its better-known role in maintaining steady-state and emergency counts of blood cells and corpuscles. The following independent, but complementary, lines of evidence support a role for bone marrow in repair/regeneration of damaged extramedullary structures.
Developmental evidence: Bone marrow cells are capable of giving rise to a wide range of cell types which reconstitute parenchima of other organs, especially the brain and spinal cord[60-66], skeletal muscle[67,68], the heart[59-64], the liver[35,36] and skin[37-39].
Therapeutic evidence: Bone marrow cells have a beneficial effect on damaged organs in humans and in animal studies, including brain and spinal cord[14-17], heart[27-32] and skin[10-13]. The magnitude and duration of this benefit and the underlying cellular mechanisms, however, have shown important variations between studies, injured sites and experimental models, fueling sometimes long-standing controversies, such as that concerning the cellular mechanisms of therapeutic action in myocardial infarction[68-72].
Correlative evidence: Treatments which mobilize bone marrow cells in the context of bone marrow transplantation and emergency hemopoiesis[1-8], such as infusion of granulocyte colony-stimulating factor (G-CSF), have consistently beneficial effects on models of CNS[18-22], cardiovascular[73-78], and skin[79] injury, to name but a few, suggesting that the repair function of the bone marrow is integrated with its better understood roles in steady-state and emergency hemopoiesis.
Furthermore, sophisticated protocols have been developed to optimize the healing potential of bone marrow cells in some major incapacitating clinical conditions such as stroke[80] and diabetic chronic ulcers[40,46], lending further support to the view that bone marrow is indeed a source of highly diversified cell types for repair, capable of functional reconstitution (i.e., of regenerating the injured tissue). Despite considerable advances made in this effort to improve over Nature, it remains unclear why, in the absence of these interventions, the reparative function of the bone marrow response to injury has such a limited impact on the functional recovery from stroke, myocardial infarction or chronic ulcers, all known to entail chronic disabilities to a variable degree.
An apparently less ambitious, but important, function of repair is to keep the host alive, even if total functional recovery through regeneration cannot be attained. This is particularly visible when a wound anywhere in the skin or mucosae creates an access into internal organs that poses a clear and present threat to survival, since blood can get out and germs can get in. Wound healing in previously healthy skin, such as typically is the case in surgery, begins with the vital process of blood clotting[81-86], and the clot is the primary organizing structure for wound healing[83,86]. Activation of the coagulation cascade is paralleled, when exposure to microbes or other triggers occurs, by activation of the complement cascade through the alternative pathway[87].
The neutrophils that clear the wound from invading bacteria[88] and the monocytes/macrophages that progressively transform that matrix into granulation tissue[89-91] are themselves bone marrow-derived. A great amount of evidence, however, further ascribes an important role in fibrotic (i.e., permanent, as distinct from granulation tissue, which is transient) healing of wounds to cells that ultimately share a bone marrow origin: Blood-borne fibrocytes[92,93] and myofibroblasts[94-96]. While this reinforces the view of bone marrow as exporter of vital parts for repair (and hopefully for regeneration), we understand but little of these complex processes. For instance, fibrocytes and myofibroblasts can also differentiate from resident cells[93,97], so the additional benefit provided by their circulating counterparts is not always obvious.
In addition to fibrosis, angiogenesis from bone marrow-derived endothelial progenitors in damaged tissues also contributes to nonregenerative repair in many contexts[98-100]. This is of conceptual interest because hemopoietic and angiogenic stem cells stem from an immediate common ancestor[101].
Indeed, angiogenesis may be intimately related to other events dependent on the bone marrow: Recent studies in humans and mice suggest that endothelial changes indicative of angiogenesis are among the earliest signs of immune damage to the lungs in the context of asthma, and even precede the arrival of eosinophils, which is one of the hallmarks of allergic inflammation; in addition, several lines of evidence suggest that production of the eosinophil-selective chemoattratant eotaxins by proangiogenic hematopoietic progenitor cells plays a major role in the subsequent development of TH2 polarization as well as in the accumulation of bone marrow-derived eosinophils in the lungs[102-105].
This hypothesis portrays angiogenesis and eosinophilia as distinct steps in the same sequence; furthermore, it suggests a close relationship between angiogenesis and extramedullary hemopoiesis, since the cell type which promotes angiogenesis is a specialized hemopoietic progenitor; finally, it deviates from the commonly held view that hemopoietic progenitor accumulation follows inflammation, advancing instead the view that hemopoietic progenitor accumulation promotes eosinophilic infiltration, which is part of allergic inflammation. Consistent with this view, colonization of the lungs by hemopoietic progenitors has also been shown in allergic disease models[106,107], suggesting that in situ production of some hemopoietic cell types from mobilized bone marrow progenitors participates in the systemic response to injury. This phenomenon, which accompanies immune-mediated local inflammatory responses, does not match the classical presentation of extramedullary hemopoiesis[108,109].
Although its biological significance remains incompletely understood (just as the role of proangiogenic hemopoietic progenitors, mentioned above, in the colonization) this phenomenon highlights the diversity of bone marrow reparative functions. Some studies attribute unique functions to these colonizing progenitors, including the maintenance of chronic inflammation in some experimental conditions[110]; it remains to be established, however, whether this duplicates the behaviour described above for proangiogenic hemopoietic progenitor cells[102-105], which encompasses the production of eosinophil-selective chemoattractants (eotaxins).
As discussed below, in the context of surgical models associating surgery and allergic sensitization, hemopoietic progenitor colonization requires specific immune responses because these provide the required hemopoietic cytokines. This dependence of a chronic inflammatory process involving nonspecific mediators on a preexisting specific immune response is reminiscent of hypersensitivity granuloma formation, a type of cellular immune reaction that is long-lived in the tissues, and variably associated with angiogenesis, fibrosis and eosinophilia[111].
These distinct local processes (injury, blood clotting, activation of the complement cascade, acute inflammation with neutrophil infiltration, clearing of debris and apoptotic bodies by macrophages, granulation tissue formation and organization, fibrosis and epithelial regeneration) are often thought of as following each other smoothly in the ideal case of a sterile surgical wound. In these conditions, inflammatory mechanisms operate very effectively and for just as long as needed, so a surgical wound healing “by first intention” does not look very much like the textbook picture of inflammation as an unpleasant combination of redness, heat, swelling and pain, most often aggravated by some functional impairment.
This illustrates the paradox that if you notice inflammation, it is because it has not properly done its job of containing damage, preventing infection and preparing the regeneration of normal structure. Nevertheless, the cleanest of surgical wounds must still be handled with care, because it might reopen, bleed or get infected following mild mechanical trauma. Granulation tissue is well-known for its propensity for bleeding, which is at least in part accountable for by ongoing local angiogenesis[112]. Surgical wounds may also remain more sensitive to pain than normal tissue, long after surgery, which demonstrates the persistence of hyperalgesic mechanisms associated with long-term effects of transient exposure to inflammatory mediators such as prostaglandins, bradykinin and numerous cytokines[113-115].
All of this shows that, even in the absence of significant infection, trauma and damage inflicted to the tissue trigger low-grade (subclinical) inflammation, which eventually resolves, and ushers in epithelial and connective tissue repair. Bone marrow is an active participant throughout this long sequence, but its contribution varies over time, since it begins by supporting inflammatory mechanisms, and ends by helping repair and regenerative mechanisms. We will here focus on repair and regenerative mechanisms, discussing the inflammatory mechanisms only as factors impairing or promoting repair and regeneration, and highlighting the usefulness of surgical models as experimental approaches to the reparative function of bone marrow in systemic injury.
Only a minority of the experimental approaches taken to probe the relationship of the bone marrow to repair at distant sites are surgical approaches, although it is virtually impossible to carry out surgery without causing some degree of injury, which in turn elicits repair. We define a surgical model, for the purposes of this review, as a systematic procedure using any combination of surgical techniques to study the contributions of bone marrow in the repair and/or regeneration of tissues distant from the bone marrow (i.e., not contiguous to bone marrow, nor including any of it). Such definition therefore intentionally excludes healing processes to which bone marrow may contribute as part of a local response, such as may occur during repair of fractures in hemopoietically active bones.
An examination of the concrete example of bone healing sheds light on the reasons why these alternative scenarios are respectively converted or excluded by our definition. Bone marrow housed in axial skeleton of the adult[116], may contribute to the repair of distant bones which have no hemopoietically active marrow themselves, a description that fits most remaining bones.
This situation is covered by the definition, because it is assumed that no direct damage to the hemopoietically active bone marrow has occurred, and it must therefore have been called into action by some long-distance signal originating elsewhere. By contrast, in the situation where the hemopoietically active bone itself is damaged and its marrow has been involved to some extent, we no longer can distinguish between the effects of local factors that promote the adaptation and recovery from local injury, on the one hand, and the effects of systemically generated signals, on the other hand. This does not imply that no systemic signals or factors operate when a hemopoietically active bone is damaged and its marrow is involved; it implies, however, that this situation does not provide a useful surgical model for the role of bone marrow in systemic injury, since the model’s value is linked to its ability to unambiguously dissect mechanisms.
Hence, in a useful surgical model afferent signals can be defined and controlled by the experimenter independently of the central organ (bone marrow) that responds to them; such signals originate in widely different structures, but uniformly elicit adaptations at the central organ which ultimately result in an output that is biologically meaningful (repair-promoting cells, for instance) and presumably delivered at the source of the signal. In the exploration of a surgical model, the location of the source of the afferent signal is less relevant than the nature and properties of this afferent signal, and the reactions of the central organ to it.
By placing an emphasis on afferent signals to which the central organ adapts by generating a biologically meaningful output (support at distance for repair), and by closing a loop in which the target of this beneficial response is the same bodily structure (the source of afferent signals) that conveyed the need for bone marrow help in the first place, the definition highlights the problem-solving value of surgical models for dissecting general mechanisms.
This value not only stems from the ability of the experimenter to unambiguously determine the site of injury which acts as a source of afferent signals; it is also reinforced by the experimenter’s ability to collect and examine the output of the bone marrow on its way to this very site (which is, by definition, always known and always accessible). The latter feature allows the experimenter to examine the composition, properties and migration patterns of these multiple bone marrow-derived cell populations, which may shed light on their possible roles in repair and/or regeneration.
Below we will outline a variety of modifications of preexisting surgical protocols from our own and from other groups, aiming at the separate study of these aspects. In all but one of these models, the object of interest is bone marrow itself, not any the solid organs that can appeal to the bone marrow and benefit from its response.
Because bone marrow is easily studied in laboratory mice[117], this overcomes one of the usual limitations of experimental surgery, which is the need to work with experimental animals large enough to allow for handling of live solid organs in vivo. Mice offer undisputed advantages for immunological studies, including the availability of numerous conventional inbred strains and genetically modified or mutant strains, as well as of reagents which can be used to probe the roles of cytokines, receptors, mediators and leukocyte populations in bone marrow responses[118,119]. Furthermore, mice can be housed in small units, making experiments with many distinct treatment groups of genetically homogeneous animals routinely feasible in standard animal facilities at an affordable cost. All of the models discussed below are surprisingly simple, and do not require above-average surgical skills, which makes them accessible to most laboratories.
The conceptual sequence of afferent signal - central adaptation - output matched to demand, has a number of aspects which have been insufficiently explored. For instance: Are the afferent signal and the export process totally unrelated, or, to the contrary, are the exported cells guided by the same kind of afferent signals that elicited their production in the first place? This is an apparently simple question, and the answer might be important, because matching the efferent product (the output) to the afferent stimulus would provide a simple and attractive mechanism of delivery.
Inflammation and repair are different phases in the same continuum. While neither has a predetermined duration, inflammation precedes repair. Since injury is followed by inflammation (either sterile or compounded by infection), and inflammatory mechanisms, when successful, clear infection, remove debris and prepare the setting for repair, one might advance a simple hypothesis: Bone marrow continuously produces leukocytes, both polymorphonuclear and mononuclear, which find their way into injured sites very effectively[88]; other bone marrow-derived cell populations, with reparative or regenerative potential, just follow at latter times the trail of leukocytes into injured sites (for instance, by responding to the same chemoattractant signals). The prediction of this hypothesis would be that where there is inflammation, bone marrow will naturally deliver cells useful in repair.
However, inflammation and infection severely impair healing, as shown in many clinical and experimental settings[120,121]; in addition, clinical and surgical observations show that inflammation is usually on the way out before repair steps in[88,90,91,122-124]. This paradox prompts us to reject the hypothesis as originally formulated, and to revise it as follows: Ongoing inflammation and established infection severely impair healing, so that resolution of inflammation and elimination of pathogens must precede healing. The prediction of the revised hypothesis is that where inflammation has resolved, bone marrow can deliver cells useful in repair. Accordingly, signals originating in resolving inflammation, as distinct from signals originating in ongoing inflammation, should be relevant to bone marrow function in repair.
One example of systemic signal known to be associated with resolution of inflammation that has a strong effect on bone marrow is G-CSF, which is believed to couple the rate of neutrophil death in inflammatory sites to the rate of neutrophil production in bone marrow[125]. G-CSF is one of the most effective mobilizers of cells in a variety of in vivo models of repair[18-22,73-79].
In addition, other nonspecific factors may provide afferent signals to request bone marrow support following systemic injury. Although they do not necessarily convey information that uniquely identifies the location of the source of the afferent signal, they might be proportionate to the magnitude or severity of injury (such as the area of a skin burn, or the volume of infarcted tissue, or to the degree of invasiveness, as defined by the rupture of internal barriers, and involvement of internal structures).
In addition to cytokines[125-130], adrenal glucocorticoids which promote hyperglycemia and insulin resistance[131-135], small polypeptide fragments of the activation of the complement cascade[87], and soluble intracellular molecules released during cell death, which are capable of activating a variety of receptors for damage-associated molecular patterns, or DAMPs[136,137] might play such roles. In the case of wounds exposed to a contaminated environment, in the skin or mucosae, chemical signals generated by receptors for pathogen-associated molecular patterns, or PAMPs[126,138], would possibly compound those arising in damage unrelated to infection. Chemokines are especially interesting because they attract many different cell types with high selectivity[88,123,139,140]. Chemokine gradients ensure a diffusible afferent signal that also identifies, as long as the gradient is maintained, the source of this signal, enabling the biologically relevant cell types exported by the bone marrow to reach this source and provide some benefit. Much information exists already about a sophisticated chemokine axis, which is known to control migration of stem cells in different physiological contexts, independently of tissue injury[139,140]. Whether a comparable mechanism underlies the reparative function of bone marrow is, therefore, an important issue for which there is no definitive answer yet, as discussed below.
Nonspecific signals are usually thought of as unrelated to adaptive (acquired) immune responses, and therefore lacking specificity and memory in the immunological sense. However, specific immune responses triggered by antigen or allergen involve release of cytokines[141,142]; while the stimulus is highly specific and amplified by memory, the output lacks both specificity and memory. The effects of specific immune responses are seldom discussed in the context of bone marrow function in surgical injury and wound repair. Nevertheless, specific immune responses may profoundly and durably imprint granulocyte production in bone marrow[143], suggesting the possibility of immunoregulatory influences on wound healing through bone marrow effects.
The many studies mentioned[10-59], concerning bone marrow contribution to repair and regeneration at distant sites, may suggest all important questions have already been answered, and there is nothing left to investigate. However, several basic aspects remain incompletely understood: (1) afferent signaling: How does the bone marrow detect damage outside the bone marrow? (2) selectivity: How does bone marrow adapt to meet specific demands related to the particular time, location, severity and type of injury by exporting the right cell type(s)? (3) delivery: How do the right cell types exported by the bone marrow get to the right place? (4) usefulness: How do these cell types help in repair and regeneration at the injured sites in a natural situation? (5) redundance vs complementarity: To what extent the bone marrow response duplicates or complements the repair and regeneration mechanisms that are intrinsic to each injured site? And (6) limits: What are the natural limits of bone marrow response in repair and regeneration and how can complex strategies help it overcome these limitations?
Most of the above issues (1-5) can be addressed experimentally, while the last one (6) is admittedly of a more philosophical nature. The three first issues (1-3) are discussed below in detail, because they can be effectively analyzed using in surgical models.
The term “model” is used here because we believe it reproduces in the laboratory a situation existing in real life. Cutting and suturing the skin of a mouse is, in this sense, a “model” of a moderate-severity surgical intervention in the skin, as often occurs in a variety of real-life situations. In this case, however, the focus in the model is on how this cutting and suturing, which is a basic common feature shared by all these real-life situations, affects the bone marrow and benefits from its help. This focus on an common denominator makes it irrelevant, for the experimental reasoning, where in the skin the wound was made (i.e., the location or origin of the signal), while how much tissue was injured and to which depth (i.e., the intensity or magnitude of the signal) remain clearly relevant.
One good illustration of these differences is provided by the immunoneuroendocrine response to trauma, a major factor intrinsic to surgical wounds at all sites. This response is not only stereotyped across a variety of sites, but is very similar to those elicited by a wide variety of physical and psychological stressors. This is characterized by increased circulating levels of adrenal glucocorticoids[131,133]. While much of the current literature on glucocorticoids tends to emphasize their anti-inflammatory and immunosuppressive effects, there is evidence that the stress response is an adaptive physiological response and that both it boosts immunity and stimulates repair processes[144-147]. Importantly, glucocorticoids have been for a long time discussed as having a deleterious effect on wound healing and fibrosis[148-150], although this effect is highly dependent on the clinical context and the timing of exposures[150,151]; an important correlate of this effect on wound healing is the strong evidence that glucocorticoids can trigger ulceration of the digestive mucosa, and probably contribute to the pathogenesis of stress ulcers[152]. If taken at face value, this would suggest the paradox that injury elicits an immunoneuroendocrine response which through glucocorticoid-dependent or glucocorticoid-mediated effects makes healing more, not less, difficult[153,154].
Even though models begin as experimental systems designed to mimic a real-life situation, they soon become enriched by secondary aspects that no longer aim to reproduce anything in real life, but to help dissection of the mechanisms involved. A surgical “model” in which the skin is cut and sutured, but in which drugs or cells are injected, is no longer the simple imitation of dermatological surgery, but a controlled setting for testing hypotheses on the mechanisms mobilized by dermatological surgery, through the observations of the superimposed effects of pharmacological intervention or of selected regulatory cell subpopulations. Because this response is independent of the location and even of the type of injury, it is possible to evaluate it, in a separate set of experiments (which we term a thematic module) in a surgical model. Within this module, the possibility that at least some of the actions of glucocorticoid hormones promote repair and/or regeneration, thereby modifying the negative effects that have been classically identified, presents an interesting opportunity for research.
Modules help us organize the thinking about a surgical model, as shown in the following situations: The first open issue in our list, for example, concerns the nature of one or more afferent signals that trigger an adaptive response in bone marrow. These signals are likely to be generated as a consequence of injury, and play a role in alerting the organism as a whole about the damage inflicted on one of its parts. Many molecules with this general alarm function have been described, including several cytokines[125-131], along with products of the activation of the coagulation and complement cascades[87]. The issue is therefore not whether diffusible alarm signals connect injured sites to systemic responses, but rather whether one of the known molecules endowed with this function connects injured sites to stimulation of a bone marrow response promoting repair, in addition to inducing other well-characterized, coordinate effects on the central and peripheral nervous system, endocrine glands, liver and adipose tissue[125-131]. One important aspect of afferent signaling is that it represents an adaptation to injury, and is likely to cease once injury has been compensated by the local and systemic mechanisms it mobilizes. As such, the duration of afferent signals are a major (but not the only) determinant of the duration of the systemic response. In this respect, very little is known about the duration of bone marrow responses to injury at distant sites.
The second point in our list of open issues, that of the selectivity of response, is more complex, because it involves several distinct aspects of the response: Diversity, proportionality, context and invasiveness. Unlike the liver, which has a coordinate but stereotyped acute phase response to inflammatory cytokines, especially IL-6[155], bone marrow has a variety of ways to provide for the needs of injured sites at distance, so diversity of response is a central issue. To illustrate this issue with one concrete example out of many possibilities, it is unclear to what extent bone marrow responses to brain injury, on the one hand, resemble those elicited by damage to the skin, on the other hand. Along the same lines, it is also unclear whether distinct types of skin injury (exemplified by the clinically relevant cases and clearly distinct cases of sterile surgical wounds vs contaminated burns) elicit comparable responses from bone marrow[10-13,155]. In addition, even within a single type of skin damage, the invasiveness of the lesion may differ greatly, raising the germane issue of proportionality of the response to the severity of injury. Surgery, of course, offers a major experimental approach to the issue of proportionality, because the timing, location, size and depth of a surgical wound can be precisely controlled by the experimenter. The issue of context relates not to the bone marrow response per se, but to the background to which this response will be directed. Surgical wounds of comparable invasiveness can be inflicted to different interfaces of the organism with the environment, as exemplified by skin and oral mucosa. These have different structures, compositions, functions and immunological defenses, but share the features of being colonized by potentially harmful microorganisms and being subject to frequent mechanical injury. Most injuries to the skin and to the oral mucosa in subjects without an underlying disease heal within a short time, which testifies to the effectiveness of innate immunity as well as repair mechanisms at both locations, but tells us little about the relationship between immunity and repair at either site. Does the bone marrow response discriminate between surgical injuries inflicted upon the skin and the oral mucosa, to give a concrete example[47]? Simple as the question may seem, it has no clear-cut answer at this time, although it certainly deserves attention.
Surgery further provides an excellent approach to the issue of invasiveness within a single context (for instance, surgical access from the skin into underlying structures) since this involves qualitative as well as quantitative shifts. Sterile surgical opening of the skin can be the first step in invasion of internal spaces, such as the peritoneal cavity. Roughly speaking, this progression is a matter of quantitative increase in damage, only up to the point where the internal barrier presented by the peritoneal membrane is violated, thereby marking a quantal leap in periculosity as access to vital organs is obtained. In this case, invasiveness per se, i.e., in the absence of infection, is the variable of interest. Does a deeper surgical wound, which provides access to the viscera, elicit a bone marrow response qualitatively distinct from that observed with a deep cut to the skin alone, or is it just a quantitative change? Even though this is a very straightforward issue, we do not have a clear-cut answer on that.
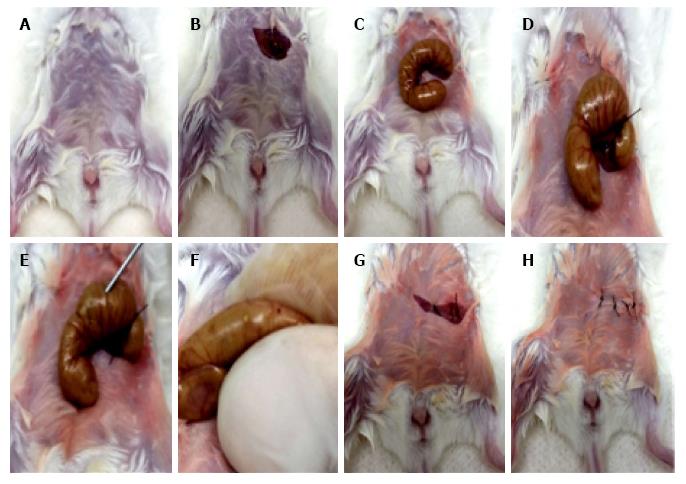
By taking this reasoning a little bit deeper, surgical injuries in this internal space - the peritoneal cavity - may compound the issue of invasiveness with that of life-threatening infection. Indeed, one of the most widely used models for studying sepsis in animals is cecal ligation and puncture (CLP)[156,157], a combination of invasive surgery exposing abdominal viscera, on the one hand, and direct mechanical attack on the intestinal containment structures (which are punctured at specific sites after cecal ligation), on the other hand (Figure 1 for a graphic summary of the procedure).
Interestingly enough, even this brutal invasion of a central space in the organism (which despite its crudity accurately reflects critical phases in the real-life situation of polymicrobial peritonitis resulting from a perforating wound to the abdomen) admits of degrees of severity, allowing us to distinguish between a sublethal procedure with a high rate of spontaneous recovery, and a so-called lethal procedure, which involves a higher microbial load in the peritoneal cavity but can nevertheless be successfully treated with aggressive antimicrobial therapy (Figure 1). By varying the number of puncture holes (Figure 1), the gauge of the needles used, and by providing antibiotic therapy, one generates distinct outcomes, ranging from full recovery to a uniformly lethal sepsis. Even more interestingly, the traumatic and the infectious components of the CLP procedure can be distinguished by injecting a controlled amount of cecal slurry in the peritoneal cavity, thereby bypassing the trauma of invasive surgery[158]. Although this modified protocol is proposed as a better alternative to CLP, it actually provides a very convenient alternative for the study of responses to trauma as opposed to responses to infection, which can itself be included as part of the modular structure of the CLP model.
CLP, therefore, is a versatile surgical procedure which provides many opportunities to study the impact of each these variables - anesthesia, external trauma, invasion of the cavity, manipulation of the intestine, perforation of the intestines, polymicrobial peritonitis and antibiotic treatment. Thanks to moderate severity and/or antibiotic treatment, CLP even provides a window on the “day after” when infection has apparently been eliminated and the organism is expected to go back to business as usual.
Interestingly, many studies suggest that a protracted immunosuppressed state overshadows subjects surviving sepsis[158-160]. To what extent this immunosuppression may reflect long-term adaptations in bone marrow function - which is essential for appropriate defenses against infection - remains to be established, but is undoubtedly a relevant, open issue. It is clear, however, that bone marrow-derived cells, especially neutrophils, play a key role in the immunological deficits associated with sepsis[161-163]. Here, again, it is important to distinguish between the effects of sepsis as a whole[161] and the effects of the trauma component[162], even though the cellular target is the same (neutrophil). Of course, the observation of long-term immunosuppression in the sepsis-survivors, and the known fact that neutrophils are short-lived in the circulation and thereby replaced through fast and intense neutropoiesis in the bone marrow[8,125,163,164] prompts the hypothesis that a bone marrow adaptation to the context of sepsis might contribute to this vulnerable state. Strategies of immune reconstitution, involving, for instance, myeloablation followed by bone marrow transplantation or adoptive transfer of neutrophils from normal syngeneic donor mice might therefore enrich what is already a very interesting surgical model.
Therefore, infection and the associated immunological dysfunction that overshadows the aftermath of sepsis play privileged parts in this surgical model (CLP). It is important to point out, in this respect, that infection and immunity are known to affect the bone marrow in many respects, but very little is known about how either affects the ability of bone marrow to support a systemic response to injury (as distinct from a systemic response to infection).
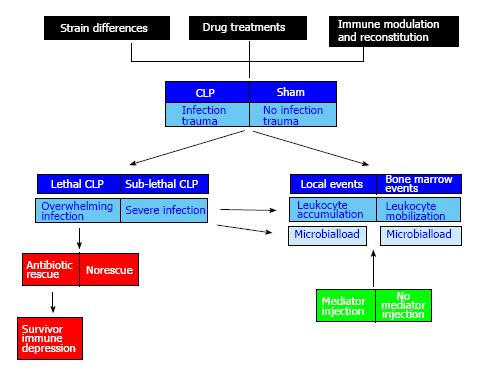
This highlights the importance of adapting current surgical models such as CLP to separately focus on each of these aspects - local injury, systemic trauma, invasion, infection, immunity - through a more elaborate design. The multiplicity of variables to be studied can only be managed rationally by isolating each one of them in a thematic module, which is embodied in the appropriate experimental and control groups. A surgical sepsis model, such as CLP, can therefore unfold as a large modular construct (Figure 2). Due to the number of groups involved and the amount of work it brings, this may present a formidable challenge to the experimenter; all the same, it remains a fascinating challenge. Although we use CLP here as particularly suitable example of a complex surgical model that allows us to separately dissect important variables in discrete modules, this reasoning can easily be adapted to other surgical models, such as those discussed at later sections.
Recent observations in our group have shown how versatile and interesting the CLP model is for the study of bone marrow function in systemic injury. While CLP is one of the most intensively studied models of sepsis worldwide, little of the research focuses on the bone marrow events. We have been able to detect three major events in sepsis (Xavier-Elsas et al, manuscript in preparation) by looking at murine bone marrow in sham-operated (trauma and invasion of the peritoneal cavity, but no perforation) and CLP mice (all of the preceding, plus perforation and polimicrobial peritonitis): (1) A decrease in bone marrow neutrophil counts, which is accompanied by an increase in peritoneal exudate neutrophil counts, during the first 24 h following surgery; (2) The lack of a significant decrease in bone marrow neutrophil counts, and a significant decrease in peritoneal exudate neutrophil counts, in the same period of observation, when the CLP mice lack functional 5-lipoxygenase (5-LO), the indispensable enzyme in the synthesis of leukotrienes[165-167]; (3) The correction of the defective response of 5-LO-deficient mice, both with respect to decrease in bone marrow neutrophils and increase in peritoneal neutrophils, by i.p. administration of leukotriene B4, a powerful neutrophil chemoattractant generated through the 5-LO pathway; and (4) The presence of bacteria in bone marrow of 5-LO-deficient mice 24 h after CLP, but not in bone marrow of wild-type CLP controls.
Bone marrow is therefore intensely involved and deeply affected in surgical sepsis models. Much of what we see is a response to infection, not to trauma, because the appropriate (sham-operated) controls, contained in a separate module that isolates on the surgical trauma component (Figure 2), show no significant decrease in bone marrow neutrophil counts, and only a minor neutrophil accumulation in the peritoneal exudate.
These observations suggest that the decrease in neutrophil counts in bone marrow is due to a rapid mobilization of mature neutrophils to blood and ultimately to infected sites, especially the primary focus of infection, inside the peritoneal cavity. The main argument for this hypothesis is that neutrophils in peritoneal exudate are increased over the same period, although the numbers of neutrophils lost from bone marrow are somewhat higher than the numbers of neutrophils acquired by the peritoneal exudate. An additional argument is that both events are prevented by a single change in the system, namely the inactivation of 5-LO. Finally, this is reinforced by restoration of both events by a single procedure, namely the administration of exogenous LTB4 to 5-LO-deficient mice, which lack endogenous production of LTB4. Overall, the evidence is that bone marrow releases neutrophils in large numbers during the initial 24 h of CLP-induced sepsis, which for the most part enter the initial focus of infection and successfully fight it (the observations were done with a sublethal CLP protocol, in which survival is the rule). Importantly, this critical mobilization function is highly dependent on 5-LO, and the key 5-LO product was shown to be LTB4.
In addition to the mobilizing cytokines such as G-CSF, a wide variety of neutrophil chemoattractants exist, which are expected to be generated in the context of sepsis, including C5a from activation of the Complement system through the alternative pathway[168,169], cytokines such as TNF-α[170,171], and chemokines, including MIP-1α and MCP-1[172,173]. In addition, the CXCL12 (SDF-1)-CXCR4 chemokine axis, which plays an essential role in homeostatic maintenance of the hemopoietic niche in bone marrow[174], and in the phenomena of stem cell/progenitor mobilization and homing to injured tissues[175], may also be important for the large-scale mobilization of neutrophils from the bone marrow reserve pool into peripheral blood, in experimental sepsis[176]. With so many apparently redundant systems, it is rather unexpected that a specialized, nonredundant, role is played by 5-LO in mobilization of neutrophils from bone marrow in the CLP model.
An equally unexpected finding is the detection of bacteria inside bone marrow in vivo, in mice lacking appropriate mobilization, since this shows that timely mobilization effectively protects this vital structure in an early phase of sepsis. It also raises the issue of whether bacterial invasion of bone marrow is more than a biomarker of severity - is it a factor that prevents further mobilization, and possibly further dysregulates host defenses? The thoughtful exploration of the CLP model and its multiple variants might shed some light on this important problem.
Intense and chronic eosinophilia induced by subcutaneous heat-coagulated egg white implants (EWI, for short) was first described by Professor Mario Mariano and his associates[177-179]. It remains a most interesting phenomenon, although much of the underlying mechanisms remains incompletely understood. EWI proved very effective as a means of sensitizing to ovalbumin, as shown by vigorous eosinophilia in lung interstitium and in bronchoalveolar lavage fluid of mice receiving EWI in the dorsum and challenged with purified ovalbumin by the respiratory route[178]. These morphological changes were accompanied by the functional abnormalities common to murine asthma models, including airway hyperreactivity[178]. The cellular composition and kinetics of the inflammatory infiltrates at the ovalbumin challenge site resemble those of the late phase in type I hypersensitivity reactions, so the authors proposed it would be a good experimental model for the late phase reaction[177]. EWI induces ovalbumin-specific cytophilic IgG1 and IgE antibodies, but the latter become detectable only after ovalbumin challenge[177,178]; to our knowledge, a role for mast cells in the development of the eosinophilia has not been established. Eosinophilia at the challenge site (lungs) and in the bone marrow was shown, paralleled by measurements of eosinophil peroxidase activity in the tissues[177,178]. By contrast, a role of cellular immunity in the phenomenon has been demonstrated by adoptive transfer of lymph node lymphocytes, which induce eosinophilia following ovalbumin challenge in the recipients[177]. Whether these are IL-5-secreting TH2 lymphocytes has not been formally established, to the best of our knowledge. Importantly, the eosinophilia in the lungs and bone marrow was sensitive to oral tolerance induction[179]. This procedure targets T and B cells and decreases specific antibody titers in the EWI model, especially IgE titers[179]; by contrast, in conventional sensitization/challenge protocols, the effect of oral tolerance induction on specific IgE and IgE titers was modest, while adoptive transfer protocols showed a major impact on cell-mediated specific immunity[180].
Further characterization of the EWI model might nevertheless prove informative, since two distinct variables are relevant here: (1) the nature of the allergen (ovalbumin); and (2) the physical state of the allergen (an insoluble pellet with heat-denatured protein). Recognition of allergen epitopes during peritoneal (or airway) challenge with native ovalbumin by cells sensitized by heat-denatured allergen following EWI points to T cells as the critical factor promoting eosinophilia, as T cell epitopes, unlike those recognized by serum antibody, are preserved even after partial proteolysis and heat denaturation of protein antigens[141].
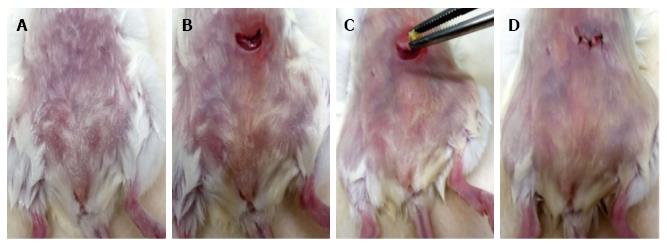
EWI is also of interest in a discussion of surgical models because it necessarily involves moderate-severity surgical trauma in the absence of infection (Figure 3)[181]. Even in this comparatively simple context, distinct modules allow us to dissect the role of trauma and the role of allergen. Several independent lines of evidence support the view that adrenal glucocorticoid hormones surge in the first 24 h after surgery, both in sham-implanted controls (full surgery but no allergen) and EWI recipients (full surgery and allergen implant)[181]. This is accompanied by significant bone marrow eosinophilia, showing that increased glucocorticoids, rather than killing eosinophils inside bone marrow, stimulate their production. Glucocorticoid surge (but not baseline) levels, are required for the eosinophilia of bone marrow in this model, both in sham-implanted controls and EWI-recipients, as shown by three independent approaches. However, as specific sensitization to the allergen pellet progresses over the first two weeks, eosinophilia subsides in the sham-implanted controls but persists and increases in the EWI-recipients, showing that, in the latter, it is driven by specific immunity[181].
Interestingly, the nonspecific bone marrow eosinophilic response is demonstrable in the sham-implanted controls up to two weeks after surgery; such a period is considerably longer than the duration of the glucocorticoid surge. This raises the possibility that transient rises in glucocorticoid levels due to the surgical trauma induce persistent effects on bone marrow cells, through reversible modifications in chromatin structure (“epigenetic” effects), similar to those described by other studies of trauma and stress[182,183].
Immune reconstitution strategies might also enrich the multiple possibilities of the EWI model. The effects of glucocorticoids on bone marrow eosinophilia in vivo (which are directly relevant to the EWI model) are variable among strains. A systematic screening showed that while wild-type C57BL/6 controls (B6) respond to glucocorticoid administration with bone marrow eosinophilia (thereby mimicking the effect of surgical trauma alone in the EWI model), perforin-deficient knockout mice from the same background lack this response. Reconstitution of the glucocorticoid-induced eosinophilic response is achieved through transfer of splenic T lymphocytes from wild-type (but not from perforin-deficient) donors to perforin-deficient recipients[184]. Other strains, not restricted to the B6 background, were also shown to lack an eosinophilic response to exogenous and/or endogenous glucocorticoid exposure in the bone marrow (manuscript in preparation). To our knowledge, none of these unresponsive strains has been studied using the EWI model, but it is of obvious interest to study a response to insoluble allergen pellets, previously shown (in wild-type mice) to be driven by an acute glucocorticoid surge, in mutant mice lacking this eosinophilic response to glucocorticoids, especially if reconstitution of both the bone marrow response and the eosinophilia at the implant site can be achieved by adoptive transfer of immunoregulatory lymphocyte populations.
Another open issue is whether eosinophilia in the EWI model is accompanied by fibrosis. Studies from many groups suggest a relationship between eosinophils and eosinophilia, on the one hand, and fibrosis resulting from a wide variety of pathological processes[185-192], on the other hand. This relationship has been proposed for eosinophilic esophagitis[188], toxoplasmosis[191], schistosomiasis[111], and the extensive remodelling of the airways associated with the chronic phase of asthma[189,190]. Airway remodelling, not easily reversed, involves many different pathobiological components, including angiogenesis, thickening of basal membrane, hyperplasia of mucus-secreting (“goblet”) cells, smooth muscle cell proliferation, increased collagen deposition, among others[191,193]. Hence, its fibrotic component, which is part of a much more complex scenario, is consistent with the view of airway remodelling as a misguided repair process. Because it develops in the presence of chronically infiltrating eosinophils, but is abolished by eosinophil depletion[191], eosinophils would appear to promote fibrosis, at least in these experimental conditions. It should be noted that eotaxin, the eosinophil-selective chemoattractant[194] and enhancer of eosinopoiesis in the bone marrow[195], is strongly involved in fibroblast-eosinophil interactions[192], although its main contribution may lie in recruiting eosinophils, rather than in activating fibroblasts. Eotaxin promotes eosinophil production in the bone marrow indirectly, through secondary production of cysteinyl-leukotrienes, a potent proallergic series of 5-lipoxygenase derivatives[195]. These lipid mediators were shown to induce the gp130-signaling cytokines[190,193,196], IL-6 and IL-11, which have strong profibrotic actions of their own[188]. So far, the evidence that eosinophils are associated with a tissue composition that evolves into fibrosis is strong; by contrast, definitive evidence that they are a major driving force in fibrotic processes is lacking.
Addressing the cellular mechanisms of eosinophilia and fibrosis in the original version of the EWI model would be difficult because it involves introduction of the allergen under the skin, which leads to accumulation of infiltrating eosinophils in solid tissue; as a consequence, laborious and expensive tissue excision/dissociation and cell separation techniques are required to isolate the eosinophils from the lesion and to study their properties, as well as their relationship to fibroblasts, fibrocytes and myofibroblasts isolated from the same site.
A minor modification of the original EWI protocol - namely introducing the allergen pellet in the peritoneal cavity (Figure 2) - has recently allowed us to recover eosinophils and other infiltrating cell types from the site of the implant by a simple peritoneal lavage, which is fast and quantitative. This modification allows us to study the properties of these eosinophils and their ability to promote fibrosis. It also facilitates the characterization of other cell types present at the same site. In this respect, a further modification of the EWI model has allowed us to precisely define the specificity of the T cells responding to the insoluble allergen, since EWI induces eosinophilia in DO11 transgenic mice of the BALB/c background, which have an essentially monoclonal T cell response to a peptide of ovalbumin associated with an autologous Class II molecule (I-Ad)[197]. It is very convenient that in wild-type mice as well as in DO11 transgenic mice the eosinophilia induced by ovalbumin sensitization is abolished by oral tolerance induction, thus providing a further control for the specificity of the eosinophilia in the modified EWI models. A third minor modification of the original protocol - keeping the original implant site (subcutaneous) and attracting eosinophils to the peritoneal cavity by local challenge with ovalbumin - has already allowed us to study the mechanisms of their accumulation[198] in response to allergen, and provides an obvious alternative setting for comparison, which should be informative on the issue how much the physical state of the allergen influences the outcome.
In principle, EWI can be studied in the absence of eosinophilia as well. In this case, dblGATA-1 mutant mice, which lack eosinophils, can be studied following EWI, since eosinophilia is not expected, but inflammation of other sorts is likely to the develop as a result of ovalbumin sensitization. Immune reconstitution of dblGATA-1 mice with purified eosinophils from normal donors can help us understand which features of the model are dependent on eosinophilic inflammation.
The colonization of the lungs by hemopoietic progenitors committed to the eosinophil lineage, following allergen exposure of sensitized subjects, which parallels the accumulation of mature eosinophils in the same organ, has been described in human and animal studies[106,107,110,198,199]. It is a less conspicuous result of the allergic reaction, not only because progenitors in the challenged lungs are largely outnumbered by mature infiltrating eosinophils, but because progenitors are defined by their developmental potential, rather than by a unique morphology or surface phenotype. A progenitor, independently of its hemopoietic lineage, is a relatively rare cell type in bone marrow or peripheral blood, phenotypically distinct from a stem cell[200], which in the presence of the appropriate hemopoietic cytokine environment gives rise to a clonal growth in semisolid media[143,195]; this amplification potential was, for a long time the main reason why progenitor colonization of the lungs has received so much attention[110,201]. More recently, however, this was reinforced by evidence that these progenitors may be important in ways unrelated to proliferation, such as a strong proinflammatory activity due to secretion of cytokines and other mediators[202].
We next summarize what has been learned about the underlying mechanisms using a specific surgical model. This model - ectopic tissue transplantation in the peritoneal cavity[199] - is somewhat more challenging than CLP or EWI, not because it requires greater surgical ability, but because it involves tissue transplantation, hence a particular donor-recipient combination, established through a surgical procedure. Of course, clinical lung transplantation substitutes presumably healthy whole lungs for diseased ones, in the anatomically correct (orthotopic) site, and this is a challenge for the surgeon in many respects, as the ultimate goal is to restore as much as possible normal respiratory function and correct the secondary cardiovascular and hematological abnormalities, such as pulmonary hypertension and polycytemia. In this surgical model, however, none of these complexities is involved, because the recipient’s lungs remain untouched; instead, a piece of lung tissue is placed into an anatomically incorrect (ectopic) cavity (peritoneal rather than thoracic) and no effort is made to make it function as a respiratory organ. So, it this “model” does not mimic a meaningul situation in clinical lung transplantation, why should we even mention it?
The answer is that the use of ectopic lung tissue transplantation to explore bone marrow roles in systemic injury is not intended to reproduce clinical lung transplantation; instead, it provides important insights of little-understood allergic processes. Allergic processes associated with transplantation have consistently been reported in humans, both in the context of bone marrow and hemopoietic cell transplantation and of solid organ transplantation, especially of liver, but also of heart, pancreas and lungs[203-208]. Such observations suggest that transmission of an asthma-like experimental disease of the lungs through lung tissue transplantation can be achieved. Ectopic transplantation of lung tissue was conceived as a rather crude, but effective, experimental approach to the hypothesis that lung releases some “asthma-inducing” mediator(s). Similar strategies were successfully used in the functional characterization of thymus (which is transplantable under the kidney capsule) as well as various endocrine glands; this success reflects the fact that these structures export cells or molecules to the general circulation, not necessarily restricted by a precise anatomical connection to a particular outlet.
Ectopic lung tissue transplantation is easy to perform because the lower lobe of the right lung is anatomically accessible and can be handled individually; the lung lobe remains viable for the duration of the experiment and releases a number of mediators, including the cytokines, IL-5 and eotaxin, in the peritoneal lavage fluid[199]. In many respects the transplanted tissue behaves as a sponge imbibed into a soup of mediators; of course, the procedure does not mimic a meaningful situation in lung transplantation, because we are implanting damaged tissue into a recipient which has perfectly healthy lungs in the right place.
Despite its artificialy, the ectopic lung tissue transplantation model allows us to analyze the entire procedure as a sequence in which separate modules address variables which: (1) operate in the donor alone; (2) operate in the recipient alone; and (3) originate in the surgical procedure. The outcome of interest (accumulation of eosinophil progenitors in the recipient’s own lungs) is dependent on both donor-related and recipient-related variables, but can only be detected through the surgical procedure. It is observed only when lung tissue from sensitized and airway-challenged donor mice is surgically implanted into the peritoneal cavity of histocompatible recipient mice which have been sensitized but not challenged[199]. Hence the outcome requires events of all three classes: (1) those operating in the donor alone (sensitization and challenge); (2) those operating in the recipient alone (sensitization without challenge); and (3) those that bring together the two preceding contexts, through surgery, thereby adding trauma, anesthesia and other factors to an already complex scenario.
This outcome is very unexpected, and prompts us to reexamine a number of assumptions. The issue here is how matching of output to demand in the responses of bone marrow to systemic injury is achieved; in other words, which mechanisms underlie an effective delivery of bone marrow-derived cells at the injured site among many uninjured sites in the same tissue or organ. The ectopic lung tissue transplantation model is therefore concerned with location of the source of afferent signals and with its relationship to the output from bone-marrow.
There is published evidence of repair of lung by bone marrow-derived cells[209,210]; logically, this demonstration requires that the target organ has been somehow damaged. By contrast, the entry of bone marrow cells (eosinophil progenitors) into uninjured lungs, as evidenced in the ectopic lung tissue transplantation model is not expected, and is likely to be missed by the experimenter, if the experimental design does not address this possibility. The observation that an injured piece of lung tissue, placed inside the peritoneal cavity, somehow promotes the colonization of healthy lung tissue by eosinophil progenitors suggests that signals emanating from injured lung tissue promote the mobilization of eosinophil progenitors from bone marrow, but that these colonize lung tissue that is untouched by both surgery and allergy. Perhaps this is made possible by a constitutive process of lung colonization by progenitors that occurs in the absence of damage[211]; if so, these progenitors are unlikely to call anyone’s attention by their proinflammatory actions[202]. At any rate, the observation suggests that matching delivery of bone marrow cells to the exact site that was injured is only one of several possibilities, and that some bone marrow cells may be mobilized and ultimately recruited into healthy tissues as well, provided injured tissue releases an afferent signal.
Surgical models have just arrived at an intersection of many exciting aspects of immunology, experimental pathology and pharmacology, and they contribute something that has received comparatively little attention in these highly competitive fields of research - namely, a focus on simple experiments on living animals, with the goal of dissecting variables that affect the entire body.
Surgical models combine the advantage of little competition with the thrill of creativity in experimentation. A surgical model can be rich enough in itself, as is the case of CLP; or look more like a curiosity, as is the case of EWI; or even appear as something exotic, bordering on the esoteric, as ectopic lung tissue transplantation. What makes these all three surgical models interesting and potentially useful is their power of adaptation to research in immunology, experimental pathology and pharmacology.
This adaptation is accomplished by expanding each model through the inclusion of novel variables (such as, to name but a few, sensitization and challenge; drug administration; transfer of immunologically relevant cell subpopulations; mutations affecting the immune response), which can be studied separately as the subjects of experiments-within-the-experiment (our, as we prefer to call them, thematic modules). Because our group, coming from a long-term commitment to bone-marrow research, has been pleasantly surprised by the convenience of these three models to approach complex issues in a simple way, we hope this summary of our experience will encourage others to pursue the exploration of surgical models in their own specialized fields of interest.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Goebel WS, Kravtsov VY, Wozniak GE S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Chinen J, Buckley RH. Transplantation immunology: solid organ and bone marrow. J Allergy Clin Immunol. 2010;125:S324-S335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Dalle JH, Peffault de Latour R. Allogeneic hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Int J Hematol. 2016;103:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Fabricius WA, Ramanathan M. Review on Haploidentical Hematopoietic Cell Transplantation in Patients with Hematologic Malignancies. Adv Hematol. 2016;2016:5726132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Napolitano LM. Anemia and Red Blood Cell Transfusion: Advances in Critical Care. Crit Care Clin. 2017;33:345-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Kiang JG, Smith JT, Anderson MN, Swift JM, Christensen CL, Gupta P, Balakathiresan N, Maheshwari RK. Hemorrhage Exacerbates Radiation Effects on Survival, Leukocytopenia, Thrombopenia, Erythropenia, Bone Marrow Cell Depletion and Hematopoiesis, and Inflammation-Associated microRNAs Expression in Kidney. PLoS One. 2015;10:e0139271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14:302-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 602] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 7. | Furusawa J, Mizoguchi I, Chiba Y, Hisada M, Kobayashi F, Yoshida H, Nakae S, Tsuchida A, Matsumoto T, Ema H. Promotion of Expansion and Differentiation of Hematopoietic Stem Cells by Interleukin-27 into Myeloid Progenitors to Control Infection in Emergency Myelopoiesis. PLoS Pathog. 2016;12:e1005507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14:3-8. [PubMed] |
| 9. | Espinoza JL, Kotecha R, Nakao S. Microbe-Induced Inflammatory Signals Triggering Acquired Bone Marrow Failure Syndromes. Front Immunol. 2017;8:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Borue X, Lee S, Grove J, Herzog EL, Harris R, Diflo T, Glusac E, Hyman K, Theise ND, Krause DS. Bone marrow-derived cells contribute to epithelial engraftment during wound healing. Am J Pathol. 2004;165:1767-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 279] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Rea S, Giles NL, Webb S, Adcroft KF, Evill LM, Strickland DH, Wood FM, Fear MW. Bone marrow-derived cells in the healing burn wound--more than just inflammation. Burns. 2009;35:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Zheng K, Wu W, Yang S, Huang L, Chen J, Gong C, Fu Z, Zhang L, Tan J. Bone marrow mesenchymal stem cell implantation for the treatment of radioactivityinduced acute skin damage in rats. Mol Med Rep. 2015;12:7065-7071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95:213-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 15. | Kakabadze Z, Kipshidze N, Mardaleishvili K, Chutkerashvili G, Chelishvili I, Harders A, Loladze G, Shatirishvili G, Kipshidze N, Chakhunashvili D. Phase 1 Trial of Autologous Bone Marrow Stem Cell Transplantation in Patients with Spinal Cord Injury. Stem Cells Int. 2016;2016:6768274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1351] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 17. | Hess DC, Hill WD, Martin-Studdard A, Carroll J, Brailer J, Carothers J. Bone marrow as a source of endothelial cells and NeuN-expressing cells After stroke. Stroke. 2002;33:1362-1368. [PubMed] |
| 18. | Nishio Y, Koda M, Kamada T, Someya Y, Kadota R, Mannoji C, Miyashita T, Okada S, Okawa A, Moriya H. Granulocyte colony-stimulating factor attenuates neuronal death and promotes functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol. 2007;66:724-731. [PubMed] |
| 19. | Six I, Gasan G, Mura E, Bordet R. Beneficial effect of pharmacological mobilization of bone marrow in experimental cerebral ischemia. Eur J Pharmacol. 2003;458:327-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Kawada H, Takizawa S, Takanashi T, Morita Y, Fujita J, Fukuda K, Takagi S, Okano H, Ando K, Hotta T. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation. 2006;113:701-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Koda M, Nishio Y, Kamada T, Someya Y, Okawa A, Mori C, Yoshinaga K, Okada S, Moriya H, Yamazaki M. Granulocyte colony-stimulating factor (G-CSF) mobilizes bone marrow-derived cells into injured spinal cord and promotes functional recovery after compression-induced spinal cord injury in mice. Brain Res. 2007;1149:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Park HK, Chu K, Lee ST, Jung KH, Kim EH, Lee KB, Song YM, Jeong SW, Kim M, Roh JK. Granulocyte colony-stimulating factor induces sensorimotor recovery in intracerebral hemorrhage. Brain Res. 2005;1041:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Li Y, Atmaca-Sonmez P, Schanie CL, Ildstad ST, Kaplan HJ, Enzmann V. Endogenous bone marrow derived cells express retinal pigment epithelium cell markers and migrate to focal areas of RPE damage. Invest Ophthalmol Vis Sci. 2007;48:4321-4327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Harris JR, Brown GA, Jorgensen M, Kaushal S, Ellis EA, Grant MB, Scott EW. Bone marrow-derived cells home to and regenerate retinal pigment epithelium after injury. Invest Ophthalmol Vis Sci. 2006;47:2108-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Demirayak B, Yüksel N, Çelik OS, Subaşı C, Duruksu G, Unal ZS, Yıldız DK, Karaöz E. Effect of bone marrow and adipose tissue-derived mesenchymal stem cells on the natural course of corneal scarring after penetrating injury. Exp Eye Res. 2016;151:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Atmaca-Sonmez P, Li Y, Yamauchi Y, Schanie CL, Ildstad ST, Kaplan HJ, Enzmann V. Systemically transferred hematopoietic stem cells home to the subretinal space and express RPE-65 in a mouse model of retinal pigment epithelium damage. Exp Eye Res. 2006;83:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Hattan N, Kawaguchi H, Ando K, Kuwabara E, Fujita J, Murata M, Suematsu M, Mori H, Fukuda K. Purified cardiomyocytes from bone marrow mesenchymal stem cells produce stable intracardiac grafts in mice. Cardiovasc Res. 2005;65:334-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, Muguruma Y, Tsuboi K, Itabashi Y, Ikeda Y. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581-3587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 405] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 29. | Misao Y, Takemura G, Arai M, Sato S, Suzuki K, Miyata S, Kosai K, Minatoguchi S, Fujiwara T, Fujiwara H. Bone marrow-derived myocyte-like cells and regulation of repair-related cytokines after bone marrow cell transplantation. Cardiovasc Res. 2006;69:476-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3891] [Cited by in RCA: 3540] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 31. | Behbahan IS, Keating A, Gale RP. Bone Marrow Therapies for Chronic Heart Disease. Stem Cells. 2015;33:3212-3227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Xu M, Wani M, Dai YS, Wang J, Yan M, Ayub A, Ashraf M. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110:2658-2665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Dupuis J, Préfontaine A, Villeneuve L, Ruel N, Lefebvre F, Calderone A. Bone marrow-derived progenitor cells contribute to lung remodelling after myocardial infarction. Cardiovasc Pathol. 2007;16:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Spees JL, Whitney MJ, Sullivan DE, Lasky JA, Laboy M, Ylostalo J, Prockop DJ. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. 2008;22:1226-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Baba S, Fujii H, Hirose T, Yasuchika K, Azuma H, Hoppo T, Naito M, Machimoto T, Ikai I. Commitment of bone marrow cells to hepatic stellate cells in mouse. J Hepatol. 2004;40:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Cho KA, Ju SY, Cho SJ, Jung YJ, Woo SY, Seoh JY, Han HS, Ryu KH. Mesenchymal stem cells showed the highest potential for the regeneration of injured liver tissue compared with other subpopulations of the bone marrow. Cell Biol Int. 2009;33:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | El-Akabawy G, El-Mehi A. Mobilization of endogenous bone marrow-derived stem cells in a thioacetamide-induced mouse model of liver fibrosis. Tissue Cell. 2015;47:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Komori M, Tsuji S, Tsujii M, Murata H, Iijima H, Yasumaru M, Nishida T, Irie T, Kawano S, Hori M. Efficiency of bone marrow-derived cells in regeneration of the stomach after induction of ethanol-induced ulcers in rats. J Gastroenterol. 2005;40:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Nishida T, Tsuji S, Tsujii M, Ishii S, Yoshio T, Shinzaki S, Egawa S, Irie T, Kakiuchi Y, Yasumaru M. Cultured bone marrow cell local implantation accelerates healing of ulcers in mice. J Gastroenterol. 2008;43:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Yamaguchi Y, Yoshida S, Sumikawa Y, Kubo T, Hosokawa K, Ozawa K, Hearing VJ, Yoshikawa K, Itami S. Rapid healing of intractable diabetic foot ulcers with exposed bones following a novel therapy of exposing bone marrow cells and then grafting epidermal sheets. Br J Dermatol. 2004;151:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Papayannopoulos V. Sweet NETs, Bitter Wounds. Immunity. 2015;43:223-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Rogers LC, Bevilacqua NJ, Armstrong DG. The use of marrow-derived stem cells to accelerate healing in chronic wounds. Int Wound J. 2008;5:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Simka M. Delayed healing of chronic leg ulcers can result from impaired trafficking of bone marrow-derived precursors of keratinocytes to the skin. Med Hypotheses. 2007;69:637-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Guo WY, Wang GJ, Wang P, Chen Q, Tan Y, Cai L. Acceleration of diabetic wound healing by low-dose radiation is associated with peripheral mobilization of bone marrow stem cells. Radiat Res. 2010;174:467-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Rodriguez-Menocal L, Shareef S, Salgado M, Shabbir A, Van Badiavas E. Role of whole bone marrow, whole bone marrow cultured cells, and mesenchymal stem cells in chronic wound healing. Stem Cell Res Ther. 2015;6:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 46. | Tong C, Hao H, Xia L, Liu J, Ti D, Dong L, Hou Q, Song H, Liu H, Zhao Y. Hypoxia pretreatment of bone marrow-derived mesenchymal stem cells seeded in a collagen-chitosan sponge scaffold promotes skin wound healing in diabetic rats with hindlimb ischemia. Wound Repair Regen. 2016;24:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 47. | Verstappen J, van Rheden RE, Katsaros C, Torensma R, Von den Hoff JW. Preferential recruitment of bone marrow-derived cells to rat palatal wounds but not to skin wounds. Arch Oral Biol. 2012;57:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Zhou LL, Liu HW, Wen XX, Xie H. Involvement of bone marrow stem cells in periodontal wound healing. Chin J Dent Res. 2014;17:105-110. [PubMed] [DOI] [Full Text] |
| 49. | Kawai T, Katagiri W, Osugi M, Sugimura Y, Hibi H, Ueda M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy. 2015;17:369-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 50. | Wang Y, Zhou L, Li C, Xie H, Lu Y, Wu Y, Liu H. Bone marrow-derived cells homing for self-repair of periodontal tissues: a histological characterization and expression analysis. Int J Clin Exp Pathol. 2015;8:12379-12389. [PubMed] |
| 51. | Verstappen J, Katsaros C, Torensma R, Von den Hoff JW. Bone marrow-derived cells in palatal wound healing. Oral Dis. 2010;16:788-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Mehrotra M, Williams CR, Ogawa M, LaRue AC. Hematopoietic stem cells give rise to osteo-chondrogenic cells. Blood Cells Mol Dis. 2013;50:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Tsujigiwa H, Hirata Y, Katase N, Buery RR, Tamamura R, Ito S, Takagi S, Iida S, Nagatsuka H. The role of bone marrow-derived cells during the bone healing process in the GFP mouse bone marrow transplantation model. Calcif Tissue Int. 2013;92:296-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Che X, Guo J, Li X, Wang L, Wei S. Intramuscular injection of bone marrow mononuclear cells contributes to bone repair following midpalatal expansion in rats. Mol Med Rep. 2016;13:681-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Ojima K, Uezumi A, Miyoshi H, Masuda S, Morita Y, Fukase A, Hattori A, Nakauchi H, Miyagoe-Suzuki Y, Takeda S. Mac-1(low) early myeloid cells in the bone marrow-derived SP fraction migrate into injured skeletal muscle and participate in muscle regeneration. Biochem Biophys Res Commun. 2004;321:1050-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Abedi M, Foster BM, Wood KD, Colvin GA, McLean SD, Johnson KW, Greer DA. Haematopoietic stem cells participate in muscle regeneration. Br J Haematol. 2007;138:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Abedi M, Greer DA, Colvin GA, Demers DA, Dooner MS, Harpel JA, Weier HU, Lambert JF, Quesenberry PJ. Robust conversion of marrow cells to skeletal muscle with formation of marrow-derived muscle cell colonies: a multifactorial process. Exp Hematol. 2004;32:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Fukada S, Miyagoe-Suzuki Y, Tsukihara H, Yuasa K, Higuchi S, Ono S, Tsujikawa K, Takeda S, Yamamoto H. Muscle regeneration by reconstitution with bone marrow or fetal liver cells from green fluorescent protein-gene transgenic mice. J Cell Sci. 2002;115:1285-1293. [PubMed] |
| 59. | Sherwood RI, Christensen JL, Weissman IL, Wagers AJ. Determinants of skeletal muscle contributions from circulating cells, bone marrow cells, and hematopoietic stem cells. Stem Cells. 2004;22:1292-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Jin K, Mao XO, Batteur S, Sun Y, Greenberg DA. Induction of neuronal markers in bone marrow cells: differential effects of growth factors and patterns of intracellular expression. Exp Neurol. 2003;184:78-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 61. | Kabos P, Ehtesham M, Kabosova A, Black KL, Yu JS. Generation of neural progenitor cells from whole adult bone marrow. Exp Neurol. 2002;178:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 62. | Movaghar B, Tiraihi T, Mesbah-Namin SA. Transdifferentiation of bone marrow stromal cells into Schwann cell phenotype using progesterone as inducer. Brain Res. 2008;1208:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Park JE, Seo YK, Yoon HH, Kim CW, Park JK, Jeon S. Electromagnetic fields induce neural differentiation of human bone marrow derived mesenchymal stem cells via ROS mediated EGFR activation. Neurochem Int. 2013;62:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 64. | Tang Y, Cui YC, Wang XJ, Wu AL, Hu GF, Luo FL, Sun JK, Sun J, Wu LK. Neural progenitor cells derived from adult bone marrow mesenchymal stem cells promote neuronal regeneration. Life Sci. 2012;91:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 65. | Kataoka K, Medina RJ, Kageyama T, Miyazaki M, Yoshino T, Makino T, Huh NH. Participation of adult mouse bone marrow cells in reconstitution of skin. Am J Pathol. 2003;163:1227-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Medina RJ, Kataoka K, Miyazaki M, Huh NH. Efficient differentiation into skin cells of bone marrow cells recovered in a pellet after density gradient fractionation. Int J Mol Med. 2006;17:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 67. | Ji KH, Xiong J, Fan LX, Hu KM, Liu HQ. Rat marrow-derived multipotent adult progenitor cells differentiate into skin epidermal cells in vivo. J Dermatol. 2009;36:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Andrade J, Lam JT, Zamora M, Huang C, Franco D, Sevilla N, Gruber PJ, Lu JT, Ruiz-Lozano P. Predominant fusion of bone marrow-derived cardiomyocytes. Cardiovasc Res. 2005;68:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafé M. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 384] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 70. | Nygren JM, Jovinge S, Breitbach M, Säwén P, Röll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 714] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 71. | Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1499] [Cited by in RCA: 1373] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 72. | Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1658] [Cited by in RCA: 1477] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 73. | Kanellakis P, Slater NJ, Du XJ, Bobik A, Curtis DJ. Granulocyte colony-stimulating factor and stem cell factor improve endogenous repair after myocardial infarction. Cardiovasc Res. 2006;70:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Tatsumi K, Otani H, Sato D, Enoki C, Iwasaka T, Imamura H, Taniuchi S, Kaneko K, Adachi Y, Ikehara S. Granulocyte-colony stimulating factor increases donor mesenchymal stem cells in bone marrow and their mobilization into peripheral circulation but does not repair dystrophic heart after bone marrow transplantation. Circ J. 2008;72:1351-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Bittira B, Shum-Tim D, Al-Khaldi A, Chiu RC. Mobilization and homing of bone marrow stromal cells in myocardial infarction. Eur J Cardiothorac Surg. 2003;24:393-398. [PubMed] |
| 76. | Brunner S, Huber BC, Fischer R, Groebner M, Hacker M, David R, Zaruba MM, Vallaster M, Rischpler C, Wilke A. G-CSF treatment after myocardial infarction: impact on bone marrow-derived vs cardiac progenitor cells. Exp Hematol. 2008;36:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Fujita J, Mori M, Kawada H, Ieda Y, Tsuma M, Matsuzaki Y, Kawaguchi H, Yagi T, Yuasa S, Endo J. Administration of granulocyte colony-stimulating factor after myocardial infarction enhances the recruitment of hematopoietic stem cell-derived myofibroblasts and contributes to cardiac repair. Stem Cells. 2007;25:2750-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 78. | Akihama S, Sato K, Satoh S, Tsuchiya N, Kato T, Komatsuda A, Hirokawa M, Sawada K, Nanjo H, Habuchi T. Bone marrow-derived cells mobilized by granulocyte-colony stimulating factor facilitate vascular regeneration in mouse kidney after ischemia/reperfusion injury. Tohoku J Exp Med. 2007;213:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Fine JD, Manes B, Frangoul H. Systemic granulocyte colony-stimulating factor (G-CSF) enhances wound healing in dystrophic epidermolysis bullosa (DEB): Results of a pilot trial. J Am Acad Dermatol. 2015;73:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Sun J, Wei ZZ, Gu X, Zhang JY, Zhang Y, Li J, Wei L. Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Exp Neurol. 2015;272:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 81. | Zuliani-Alvarez L, Midwood KS. Fibrinogen-Related Proteins in Tissue Repair: How a Unique Domain with a Common Structure Controls Diverse Aspects of Wound Healing. Adv Wound Care (New Rochelle). 2015;4:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 82. | Boral BM, Williams DJ, Boral LI. Disseminated Intravascular Coagulation. Am J Clin Pathol. 2016;146:670-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 83. | Gando S, Otomo Y. Local hemostasis, immunothrombosis, and systemic disseminated intravascular coagulation in trauma and traumatic shock. Crit Care. 2015;19:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 84. | Cohen MJ, Christie SA. Coagulopathy of Trauma. Crit Care Clin. 2017;33:101-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 85. | Hayakawa M. Pathophysiology of trauma-induced coagulopathy: disseminated intravascular coagulation with the fibrinolytic phenotype. J Intensive Care. 2017;5:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 86. | O’Keefe RJ. Fibrinolysis as a Target to Enhance Fracture Healing. N Engl J Med. 2015;373:1776-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Lupu F, Keshari RS, Lambris JD, Coggeshall KM. Crosstalk between the coagulation and complement systems in sepsis. Thromb Res. 2014;133 Suppl 1:S28-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 88. | Su Y, Richmond A. Chemokine Regulation of Neutrophil Infiltration of Skin Wounds. Adv Wound Care (New Rochelle). 2015;4:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 89. | Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, Roy S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185:2596-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 579] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 90. | Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2865] [Cited by in RCA: 2823] [Article Influence: 313.7] [Reference Citation Analysis (0)] |
| 91. | Minutti CM, Knipper JA, Allen JE, Zaiss DM. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol. 2017;61:3-11. [PubMed] [DOI] [Full Text] |
| 92. | Preston SL, Alison MR, Forbes SJ, Direkze NC, Poulsom R, Wright NA. The new stem cell biology: something for everyone. Mol Pathol. 2003;56:86-96. [PubMed] |
| 93. | Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 337] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 94. | Brittan M, Chance V, Elia G, Poulsom R, Alison MR, MacDonald TT, Wright NA. A regenerative role for bone marrow following experimental colitis: contribution to neovasculogenesis and myofibroblasts. Gastroenterology. 2005;128:1984-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 95. | Yamaguchi Y, Kubo T, Murakami T, Takahashi M, Hakamata Y, Kobayashi E, Yoshida S, Hosokawa K, Yoshikawa K, Itami S. Bone marrow cells differentiate into wound myofibroblasts and accelerate the healing of wounds with exposed bones when combined with an occlusive dressing. Br J Dermatol. 2005;152:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 97. | Yano T, Miura T, Ikeda Y, Matsuda E, Saito K, Miki T, Kobayashi H, Nishino Y, Ohtani S, Shimamoto K. Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovasc Pathol. 2005;14:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 98. | Bluff JE, Ferguson MW, O’Kane S, Ireland G. Bone marrow-derived endothelial progenitor cells do not contribute significantly to new vessels during incisional wound healing. Exp Hematol. 2007;35:500-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Eming SA, Brachvogel B, Odorisio T, Koch M. Regulation of angiogenesis: wound healing as a model. Prog Histochem Cytochem. 2007;42:115-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 243] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 100. | Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 306] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 101. | Pascual-Anaya J, Albuixech-Crespo B, Somorjai IM, Carmona R, Oisi Y, Alvarez S, Kuratani S, Muñoz-Chápuli R, Garcia-Fernàndez J. The evolutionary origins of chordate hematopoiesis and vertebrate endothelia. Dev Biol. 2013;375:182-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 102. | Duong HT, Erzurum SC, Asosingh K. Pro-angiogenic hematopoietic progenitor cells and endothelial colony-forming cells in pathological angiogenesis of bronchial and pulmonary circulation. Angiogenesis. 2011;14:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 103. | Rose JA, Erzurum S, Asosingh K. Biology and flow cytometry of proangiogenic hematopoietic progenitors cells. Cytometry A. 2015;87:5-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 104. | Asosingh K, Cheng G, Xu W, Savasky BM, Aronica MA, Li X, Erzurum SC. Nascent endothelium initiates Th2 polarization of asthma. J Immunol. 2013;190:3458-3465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 105. | Asosingh K, Hanson JD, Cheng G, Aronica MA, Erzurum SC. Allergen-induced, eotaxin-rich, proangiogenic bone marrow progenitors: a blood-borne cellular envoy for lung eosinophilia. J Allergy Clin Immunol. 2010;125:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 106. | Southam DS, Widmer N, Ellis R, Hirota JA, Inman MD, Sehmi R. Increased eosinophil-lineage committed progenitors in the lung of allergen-challenged mice. J Allergy Clin Immunol. 2005;115:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 107. | Gaspar Elsas MI, Maximiano ES, Joseph D, Bonomo A, Vargaftig BB, Xavier Elsas P. Isolation and characterization of hemopoietic cells from lungs of allergic mice. Chest. 2003;123:345S-348S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 108. | Yamamoto K, Miwa Y, Abe-Suzuki S, Abe S, Kirimura S, Onishi I, Kitagawa M, Kurata M. Extramedullary hematopoiesis: Elucidating the function of the hematopoietic stem cell niche (Review). Mol Med Rep. 2016;13:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 109. | Johns JL, Christopher MM. Extramedullary hematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Vet Pathol. 2012;49:508-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 110. | Denburg JA, van Eeden SF. Bone marrow progenitors in inflammation and repair: new vistas in respiratory biology and pathophysiology. Eur Respir J. 2006;27:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 111. | Elbaz T, Esmat G. Hepatic and intestinal schistosomiasis: review. J Adv Res. 2013;4:445-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 112. | Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 937] [Cited by in RCA: 1170] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 113. | Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2363] [Cited by in RCA: 2754] [Article Influence: 172.1] [Reference Citation Analysis (0)] |
| 114. | Schaible HG. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther. 2014;16:470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 208] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 115. | Petho G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92:1699-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 116. | Le Ster C, Lasbleiz J, Kannengiesser S, Guillin R, Gambarota G, Saint-Jalmes H. A fast method for the quantification of fat fraction and relaxation times: Comparison of five sites of bone marrow. Magn Reson Imaging. 2017;39:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Ezeh PC, Xu H, Wang SC, Medina S, Burchiel SW. Evaluation of Toxicity in Mouse Bone Marrow Progenitor Cells. Curr Protoc Toxicol. 2016;67:18.9.1-18.9.12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 118. | Onodera T, Sakudo A, Tsubone H, Itohara S. Review of studies that have used knockout mice to assess normal function of prion protein under immunological or pathophysiological stress. Microbiol Immunol. 2014;58:361-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 119. | Viney M, Lazarou L, Abolins S. The laboratory mouse and wild immunology. Parasite Immunol. 2015;37:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 120. | Loi F, Córdova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 791] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 121. | LeBert DC, Huttenlocher A. Inflammation and wound repair. Semin Immunol. 2014;26:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 122. | Lech M, Gröbmayr R, Weidenbusch M, Anders HJ. Tissues use resident dendritic cells and macrophages to maintain homeostasis and to regain homeostasis upon tissue injury: the immunoregulatory role of changing tissue environments. Mediators Inflamm. 2012;2012:951390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 123. | Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41:694-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 800] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 124. | Alessandri AL, Sousa LP, Lucas CD, Rossi AG, Pinho V, Teixeira MM. Resolution of inflammation: mechanisms and opportunity for drug development. Pharmacol Ther. 2013;139:189-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 125. | Weigand MA, Hörner C, Bardenheuer HJ, Bouchon A. The systemic inflammatory response syndrome. Best Pract Res Clin Anaesthesiol. 2004;18:455-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 126. | Adib-Conquy M, Cavaillon JM. Stress molecules in sepsis and systemic inflammatory response syndrome. FEBS Lett. 2007;581:3723-3733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 127. | Sriskandan S, Altmann DM. The immunology of sepsis. J Pathol. 2008;214:211-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 128. | Surbatovic M, Veljovic M, Jevdjic J, Popovic N, Djordjevic D, Radakovic S. Immunoinflammatory response in critically ill patients: severe sepsis and/or trauma. Mediators Inflamm. 2013;2013:362793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 129. | Robertson CM, Coopersmith CM. The systemic inflammatory response syndrome. Microbes Infect. 2006;8:1382-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 130. | Pallister I. An update on the systemic response to trauma. Orthop Trauma. 2010;24:24-28. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 131. | Kanczkowski W, Sue M, Zacharowski K, Reincke M, Bornstein SR. The role of adrenal gland microenvironment in the HPA axis function and dysfunction during sepsis. Mol Cell Endocrinol. 2015;408:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 132. | Vanhorebeek I, Langouche L. Molecular mechanisms behind clinical benefits of intensive insulin therapy during critical illness: glucose versus insulin. Best Pract Res Clin Anaesthesiol. 2009;23:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 133. | Offner PJ, Moore EE, Ciesla D. The adrenal response after severe trauma. Am J Surg. 2002;184:649-653; discussion 653-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 134. | Li L, Messina JL. Acute insulin resistance following injury. Trends Endocrinol Metab. 2009;20:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 135. | Cree MG, Fram RY, Barr D, Chinkes D, Wolfe RR, Herndon DN. Insulin resistance, secretion and breakdown are increased 9 months following severe burn injury. Burns. 2009;35:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 136. | Krysko O, Løve Aaes T, Bachert C, Vandenabeele P, Krysko DV. Many faces of DAMPs in cancer therapy. Cell Death Dis. 2013;4:e631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 137. | Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 1148] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 138. | Delgado M, Singh S, De Haro S, Master S, Ponpuak M, Dinkins C, Ornatowski W, Vergne I, Deretic V. Autophagy and pattern recognition receptors in innate immunity. Immunol Rev. 2009;227:189-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 139. | Rankin SM. Chemokines and adult bone marrow stem cells. Immunol Lett. 2012;145:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 140. | Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 233] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 141. | Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125:S3-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 1156] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 142. | Huang W, August A. The signaling symphony: T cell receptor tunes cytokine-mediated T cell differentiation. J Leukoc Biol. 2015;97:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 143. | Gaspar Elsas MI, Joseph D, Elsas PX, Vargaftig BB. Rapid increase in bone-marrow eosinophil production and responses to eosinopoietic interleukins triggered by intranasal allergen challenge. Am J Respir Cell Mol Biol. 1997;17:404-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 144. | Guyre PM, Yeager MP, Munck A. Glucocorticoid Effects on Immune Responses. Neuroimmune Biol. 2007;7:147-167. |
| 145. | Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem. 2011;286:38703-38713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 146. | Xie X, Yan X, Lin Z, Jin X. Differential effects of low- and high-dose glucocorticoids on the innate immunity of corneal epithelium in vitro. Ocul Immunol Inflamm. 2011;19:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 147. | van de Garde MD, Martinez FO, Melgert BN, Hylkema MN, Jonkers RE, Hamann J. Chronic exposure to glucocorticoids shapes gene expression and modulates innate and adaptive activation pathways in macrophages with distinct changes in leukocyte attraction. J Immunol. 2014;192:1196-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 148. | Brangham AD. The effect of cortisone on wound healing. Br J Exp Pathol. 1951;32:77-84. [PubMed] |
| 149. | Annotations. Cortisone and wound healing. The Lancet. 1953;733-734. |
| 150. | Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 267] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 151. | Bitar MS, Farook T, Wahid S, Francis IM. Glucocorticoid-dependent impairment of wound healing in experimental diabetes: amelioration by adrenalectomy and RU 486. J Surg Res. 1999;82:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 152. | Maruyama S, Minagawa M, Shimizu T, Oya H, Yamamoto S, Musha N, Abo W, Weerasinghe A, Hatakeyama K, Abo T. Administration of glucocorticoids markedly increases the numbers of granulocytes and extrathymic T cells in the bone marrow. Cell Immunol. 1999;194:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 153. | Kahan V, Andersen ML, Tomimori J, Tufik S. Stress, immunity and skin collagen integrity: evidence from animal models and clinical conditions. Brain Behav Immun. 2009;23:1089-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 154. | Ebrecht M, Hextall J, Kirtley LG, Taylor A, Dyson M, Weinman J. Perceived stress and cortisol levels predict speed of wound healing in healthy male adults. Psychoneuroendocrinology. 2004;29:798-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 155. | Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 501] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 156. | Efron PA, Mohr AM, Moore FA, Moldawer LL. The future of murine sepsis and trauma research models. J Leukoc Biol. 2015;98:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 157. | Starr ME, Steele AM, Saito M, Hacker BJ, Evers BM, Saito H. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS One. 2014;9:e115705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 158. | Hu SB, Zider A, Deng JC. When host defense goes awry: Modeling sepsis-induced immunosuppression. Drug Discov Today Dis Models. 2012;9:e33-e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 159. | Bermejo-Martin JF, Andaluz-Ojeda D, Almansa R, Gandía F, Gómez-Herreras JI, Gomez-Sanchez E, Heredia-Rodríguez M, Eiros JM, Kelvin DJ, Tamayo E. Defining immunological dysfunction in sepsis: A requisite tool for precision medicine. J Infect. 2016;72:525-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 160. | Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 1041] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 161. | Zonneveld R, Molema G, Plötz FB. Measurement of functional and morphodynamic neutrophil phenotypes in systemic inflammation and sepsis. Crit Care. 2016;20:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 162. | Hazeldine J, Hampson P, Lord JM. The impact of trauma on neutrophil function. Injury. 2014;45:1824-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 163. | Leliefeld PH, Wessels CM, Leenen LP, Koenderman L, Pillay J. The role of neutrophils in immune dysfunction during severe inflammation. Crit Care. 2016;20:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 164. | Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 903] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 165. | Fullerton JN, O’Brien AJ, Gilroy DW. Lipid mediators in immune dysfunction after severe inflammation. Trends Immunol. 2014;35:12-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 166. | Rådmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta. 2015;1851:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 348] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 167. | Mashima R, Okuyama T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015;6:297-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 168. | Markiewski MM, DeAngelis RA, Lambris JD. Complexity of complement activation in sepsis. J Cell Mol Med. 2008;12:2245-2254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 169. | Huber-Lang M, Kovtun A, Ignatius A. The role of complement in trauma and fracture healing. Semin Immunol. 2013;25:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 170. | Nakae S, Suto H, Berry GJ, Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;109:3640-3648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 171. | Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J Immunol. 2010;184:6891-6900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 172. | Cascieri MA, Springer MS. The chemokine/chemokine-receptor family: potential and progress for therapeutic intervention. Curr Opin Chem Biol. 2000;4:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 173. | Zisman DA, Kunkel SL, Strieter RM, Tsai WC, Bucknell K, Wilkowski J, Standiford TJ. MCP-1 protects mice in lethal endotoxemia. J Clin Invest. 1997;99:2832-2836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 194] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 174. | Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 175. | Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 176. | Delano MJ, Kelly-Scumpia KM, Thayer TC, Winfield RD, Scumpia PO, Cuenca AG, Harrington PB, O’Malley KA, Warner E, Gabrilovich S. Neutrophil mobilization from the bone marrow during polymicrobial sepsis is dependent on CXCL12 signaling. J Immunol. 2011;187:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 177. | Facincone S, De Siqueira AL, Jancar S, Russo M, Barbuto JA, Mariano M. A novel murine model of late-phase reaction of immediate hypersensitivity. Mediators Inflamm. 1997;6:127-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 178. | de Siqueira AL, Russo M, Steil AA, Facincone S, Mariano M, Jancar S. A new murine model of pulmonary eosinophilic hypersensitivity: contribution to experimental asthma. J Allergy Clin Immunol. 1997;100:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 179. | Russo M, Jancar S, Pereira de Siqueira AL, Mengel J, Gomes E, Ficker SM, Caetano de Faria AM. Prevention of lung eosinophilic inflammation by oral tolerance. Immunol Lett. 1998;61:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 180. | Xavier-Elsas P, Silva CL, Pinto L, Queto T, Vieira BM, Aranha MG, De Luca B, Masid-de-Brito D, Luz RA, Lopes RS. Modulation of the effects of lung immune response on bone marrow by oral antigen exposure. Biomed Res Int. 2013;2013:474132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 181. | Elsas PX, Neto HA, Cheraim AB, Magalhães ES, Accioly MT, Carvalho VF, e Silva PM, Vargaftig BB, Cunha FQ, Gaspar Elsas MI. Induction of bone-marrow eosinophilia in mice submitted to surgery is dependent on stress-induced secretion of glucocorticoids. Br J Pharmacol. 2004;143:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 182. | Lirk P, Fiegl H, Weber NC, Hollmann MW. Epigenetics in the perioperative period. Br J Pharmacol. 2015;172:2748-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 183. | Provençal N, Binder EB. The effects of early life stress on the epigenome: From the womb to adulthood and even before. Exp Neurol. 2015;268:10-20. [PubMed] [DOI] [Full Text] |
| 184. | Xavier-Elsas P, da Silva CL, Vieira BM, Masid-de-Brito D, Queto T, de Luca B, Vieira TS, Gaspar-Elsas MI. The In Vivo Granulopoietic Response to Dexamethasone Injection Is Abolished in Perforin-Deficient Mutant Mice and Corrected by Lymphocyte Transfer from Nonsensitized Wild-Type Donors. Mediators Inflamm. 2015;2015:495430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 185. | Lee YM, Kim SS, Kim HA, Suh YJ, Lee SK, Nahm DH, Park HS. Eosinophil inflammation of nasal polyp tissue: relationships with matrix metalloproteinases, tissue inhibitor of metalloproteinase-1, and transforming growth factor-beta1. J Korean Med Sci. 2003;18:97-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 186. | Gomes I, Mathur SK, Espenshade BM, Mori Y, Varga J, Ackerman SJ. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immunol. 2005;116:796-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 187. | Fransén-Pettersson N, Duarte N, Nilsson J, Lundholm M, Mayans S, Larefalk Å, Hannibal TD, Hansen L, Schmidt-Christensen A, Ivars F, Cardell S, Palmqvist R, Rozell B, Holmberg D. A New Mouse Model That Spontaneously Develops Chronic Liver Inflammation and Fibrosis. PLoS One. 2016;11:e0159850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 188. | Cheng E, Souza RF, Spechler SJ. Tissue remodeling in eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1175-G1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 189. | Kariyawasam HH, Robinson DS. The role of eosinophils in airway tissue remodelling in asthma. Curr Opin Immunol. 2007;19:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 190. | Knight DA, Ernst M, Anderson GP, Moodley YP, Mutsaers SE. The role of gp130/IL-6 cytokines in the development of pulmonary fibrosis: critical determinants of disease susceptibility and progression? Pharmacol Ther. 2003;99:327-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 191. | McConnell JF, Sparkes AH, Blunden AS, Neath PJ, Sansom J. Eosinophilic fibrosing gastritis and toxoplasmosis in a cat. J Feline Med Surg. 2007;9:82-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 192. | Puxeddu I, Bader R, Piliponsky AM, Reich R, Levi-Schaffer F, Berkman N. The CC chemokine eotaxin/CCL11 has a selective profibrogenic effect on human lung fibroblasts. J Allergy Clin Immunol. 2006;117:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 193. | Lee KS, Kim SR, Park HS, Park SJ, Min KH, Lee KY, Jin SM, Lee YC. Cysteinyl leukotriene upregulates IL-11 expression in allergic airway disease of mice. J Allergy Clin Immunol. 2007;119:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 194. | Luz RA, Xavier-Elsas P, de Luca B, Masid-de-Brito D, Cauduro PS, Arcanjo LC, dos Santos AC, de Oliveira IC, Gaspar-Elsas MI. 5-lipoxygenase-dependent recruitment of neutrophils and macrophages by eotaxin-stimulated murine eosinophils. Mediators Inflamm. 2014;2014:102160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 195. | Queto T, Gaspar-Elsas MI, Masid-de-Brito D, Vasconcelos ZF, Ferraris FK, Penido C, Cunha FQ, Kanaoka Y, Lam BK, Xavier-Elsas P. Cysteinyl-leukotriene type 1 receptors transduce a critical signal for the up-regulation of eosinophilopoiesis by interleukin-13 and eotaxin in murine bone marrow. J Leukoc Biol. 2010;87:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 196. | Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720-1723. [PubMed] |
| 197. | Cheraim AB, Xavier-Elsas P, de Oliveira SH, Batistella T, Russo M, Gaspar-Elsas MI, Cunha FQ. Leukotriene B4 is essential for selective eosinophil recruitment following allergen challenge of CD4+ cells in a model of chronic eosinophilic inflammation. Life Sci. 2008;83:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 198. | Maximiano ES, Elsas PX, de Mendonça Sales SC, Jones CP, Joseph D, Vargaftig BB, Gaspar Elsas MI. Cells isolated from bone-marrow and lungs of allergic BALB/C mice and cultured in the presence of IL-5 are respectively resistant and susceptible to apoptosis induced by dexamethasone. Int Immunopharmacol. 2005;5:857-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 199. | Xavier-Elsas P, Santos-Maximiano E, Queto T, Mendonça-Sales S, Joseph D, Gaspar-Elsas MI, Vargaftig BB. Ectopic lung transplantation induces the accumulation of eosinophil progenitors in the recipients’ lungs through an allergen- and interleukin-5-dependent mechanism. Clin Exp Allergy. 2007;37:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 200. | Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2338] [Cited by in RCA: 2359] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 201. | Rådinger M, Lötvall J. Eosinophil progenitors in allergy and asthma - do they matter? Pharmacol Ther. 2009;121:174-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 202. | Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, Liu YJ, Howie KJ, Denburg JA, Gauvreau GM. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 203. | Desai S, Walker SA, Shaw PJ, Riches PG, Hobbs JR, Wild G, Harper JI. Expression of donor allergic response patterns by bone marrow transplant recipients. Lancet. 1984;2:1148. [PubMed] |
| 204. | Khan F, Hallstrand TS, Geddes MN, Henderson WR, Storek J. Is allergic disease curable or transferable with allogeneic hematopoietic cell transplantation? Blood. 2009;113:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 205. | Dewachter P, Vézinet C, Nicaise-Roland P, Chollet-Martin S, Eyraud D, Creusvaux H, Vaillant JC, Mouton-Faivre C. Passive transient transfer of peanut allergy by liver transplantation. Am J Transplant. 2011;11:1531-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 206. | Hallstrand TS, Sprenger JD, Agosti JM, Longton GM, Witherspoon RP, Henderson WR. Long-term acquisition of allergen-specific IgE and asthma following allogeneic bone marrow transplantation from allergic donors. Blood. 2004;104:3086-3090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 207. | Ozdemir O. New developments in transplant-acquired allergies. World J Transplant. 2013;3:30-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 208. | Khalid I, Zoratti E, Stagner L, Betensley AD, Nemeh H, Allenspach L. Transfer of peanut allergy from the donor to a lung transplant recipient. J Heart Lung Transplant. 2008;27:1162-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 209. | Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181-5188. [PubMed] |
| 210. | Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 620] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 211. | Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, Janss T, Starkl P, Ramery E, Henket M. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126:3279-3295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 391] [Article Influence: 43.4] [Reference Citation Analysis (0)] |