Published online May 20, 2017. doi: 10.5493/wjem.v7.i2.49
Peer-review started: February 8, 2017
First decision: March 8, 2017
Revised: April 26, 2017
Accepted: May 3, 2017
Article in press: May 5, 2017
Published online: May 20, 2017
Processing time: 115 Days and 22.5 Hours
To characterise the role of substitutes for receptor-activator nuclear factor kappa-B ligand (RANKL) in rheumatoid arthritis (RA) joint destruction.
Synovial fluid (SF) macrophages isolated from the knee joint of RA patients were incubated with 25 ng/mL macrophage-colony stimulating factor (M-CSF) and 50 ng/mL LIGHT (lymphotoxin-like, exhibits inducible expression and competes with herpes simplex virus glycoprotein D for herpes virus entry mediator, a receptor expressed by T lymphocytes) in the presence and absence of 25 ng/mL RANKL and 100 ng/mL osteoprotegerin (OPG) on glass coverslips and dentine slices. Osteoclastogenesis was assessed by the formation of multinucleated cells (MNCs) expressing tartrate-resistant acid phosphatase (TRAP) on coverslips and the extent of lacunar resorption pit formation on dentine slices. The concentration of LIGHT in RA and osteoarthritis (OA) synovial fluid was measured by an enzyme-linked immunosorbent assay (ELISA) and the expression of LIGHT in RA and OA synovium was determined by immunohistochemistry using an indirect immunoperoxidase technique.
In cultures of RA SF macrophages treated with LIGHT and M-CSF, there was significant formation of TRAP + MNCs on coverslips and extensive lacunar resorption pit formation on dentine slices. SF-macrophage-osteoclast differentiation was not inhibited by the addition of OPG, a decoy receptor for RANKL. Resorption pits were smaller and less confluent than in RANKL-treated cultures but the overall percentage area of the dentine slice resorbed was comparable in LIGHT- and RANKL-treated cultures. LIGHT significantly stimulated RANKL-induced lacunar resorption compared with RA SF macrophages treated with either RANKL or LIGHT alone. LIGHT was strongly expressed by synovial lining cells, subintimal macrophages and endothelial cells in RA synovium and the concentration of LIGHT was much higher in RA compared with OA SF.
LIGHT is highly expressed in RA synovium and SF, stimulates RANKL-independent/dependent osteoclastogenesis from SF macrophages and may contribute to marginal erosion formation.
Core tip: Rheumatoid arthritis (RA) is an inflammatory joint condition characterised by the formation of marginal erosions due to the activity of bone resorbing osteoclasts. Osteoclasts can be formed from macrophages by both receptor-activator nuclear factor kappa-B ligand (RANKL)-dependent and RANKL-independent mechanisms. LIGHT is a potent RANKL substitute that induces significant osteoclast formation from cultures of RA synovial fluid macrophages; this results in comparable levels of resorption to that seen in RANKL-treated cultures. LIGHT also stimulated RANKL-mediated lacunar resorption. LIGHT is highly expressed in RA joints and synovial fluid and is likely to play a key role in the pathogenesis of marginal erosion formation in RA.
- Citation: Sabokbar A, Afrough S, Mahoney DJ, Uchihara Y, Swales C, Athanasou NA. Role of LIGHT in the pathogenesis of joint destruction in rheumatoid arthritis. World J Exp Med 2017; 7(2): 49-57
- URL: https://www.wjgnet.com/2220-315X/full/v7/i2/49.htm
- DOI: https://dx.doi.org/10.5493/wjem.v7.i2.49
Rheumatoid arthritis (RA) is a common inflammatory arthropathy which affects approximately 1% of the adult population[1]. RA is characterized by a heavy lymphocyte and plasma cell infiltrate in the synovium, synovial overgrowth; and extension of inflammatory tissue into bone; this leads to the formation of periarticular marginal erosions by osteoclasts, multinucleated cells which are specialised to carry out lacunar bone resorption. The osteoclast is a member of the mononuclear phagocyte system and is formed from CD14+ macrophage precursors in the presence of macrophage-colony stimulating factor (M-CSF) and receptor activator for nuclear factor κB ligand (RANKL), a tumour necrosis family (TNF) superfamily member[2-5]. RANKL is expressed by activated fibroblasts, osteoblasts and lymphocytes which also produce osteoprotegerin (OPG), a decoy receptor for RANKL that inhibits osteoclastogenesis[6,7]. The RANKL-OPG axis is key to controlling osteoclast formation and survival. A monoclonal antibody directed against RANKL, denosumab, has been shown to increase bone mineral density and to reduce pathological bone resorption in osteoporosis and RA[8-11].
Several studies have shown that osteoclasts can be formed by a RANKL-independent pathway promoted by other TNF superfamily members[12-17]. LIGHT (lymphotoxin-like, exhibits inducible expression and competes with herpes simplex virus glycoprotein D for herpes virus entry mediator, a receptor expressed by T lymphocytes) is the most potent RANKL substitute identified to date[14,18,19]. LIGHT (TNFSF14) is a type 2 transmembrane glycoprotein which is expressed on activated T lymphocytes, monocytes and dendritic cells[20-22]. LIGHT binds to two membrane-bound members of the TNFR superfamily, herpes virus entry mediator (HVEM), which is expressed by many inflammatory cells including T cells, B cells, monocytes and dendritic cells, and lymphotoxin β receptor (LTβR), which is expressed by many cell types such as fibroblasts, endothelial cells, stromal cells and monocytes but not lymphocytes[23-25]. LIGHT also binds to decoy receptor 3 (DcR3), a soluble non-signaling receptor which has been shown to modulate its function in vitro and in vivo[23,26]. LIGHT is expressed constitutively by dendritic cells and activated T cells and is mainly found in lymphoid tissues[20-22]. LIGHT has been shown to induce osteoclast formation by a RANKL-independent mechanism from monocytes[14,19].
The role LIGHT plays in inducing pathological bone resorption in RA joints is uncertain. LIGHT levels are increased in the serum of patients with RA, and blocking the action of LIGHT has been shown to reduce the severity of murine collagen-induced arthritis[14,27]. Synovial fluid (SF) macrophages are known to differentiate into osteoclasts in the presence of RANKL and M-CSF[28]. In this study we have examined the role LIGHT plays in the pathogenesis of inflammatory joint destruction by determining whether LIGHT alone can stimulate osteoclastogenesis from SF macrophages isolated from RA joints. We have also examined whether the concentration of LIGHT is higher in RA than non-inflammatory osteoarthritis (OA) SF and compared the expression of LIGHT in RA and OA synovium.
SF samples were derived from six patients (four females, one male) undergoing therapeutic arthrocentesis for inflammatory joint disease. Non-inflammatory OA control SF was derived from four patients (two females, two males) undergoing unicompartmental knee replacement. All patients diagnosed with RA were seropositive for rheumatoid factor and met the ACR/EULAR diagnostic criteria for RA[29]. RA and OA synovial tissue was derived from biopsy specimens. All patients gave informed consent and the Oxford Clinical Research Ethics Committee approved the study.
For all tissue culture incubations, α-minimum essential medium (α-MEM) (Invitrogen, United Kingdom) was supplemented with 2 mmol/L (w/v) glutamine, 10 μg/mL streptomycin, 100 IU/mL benzyl penicillin, 10% (v/v) heat-inactivated fetal bovine serum (FBS). Recombinant soluble human RANKL was purchased from Peprotech Europe (London, United Kingdom); all other cytokines were purchased from R and D Systems Europe (Abingdon, United Kingdom). Primary antibodies for immunohistochemistry were purchased from Abcam (Cambridge, United Kingdom).
SF macrophages were isolated from aspirates by centrifugation at 2500 rpm for 10 min at 4 °C; macrophages were subsequently used for osteoclastogenesis (see below) while the cell-free supernatant was frozen and stored at -80 °C for ELISA measurement of LIGHT levels. Following centrifugation of SF aspirates, the resultant cell pellet was re-suspended α-MEM/FBS and then added at 1 × 106 cells/well to 96 well tissue culture plates containing 5 mm dentine slices. After 2 h incubation, dentine slices were removed from the wells and washed vigorously in MEM/FBS to remove non-adherent cells; the cell suspension was subsequently transferred to a 24-well tissue culture plate containing 1 mL of MEM/FBS supplemented with various factors. As positive controls, SF macrophage cultures were maintained in the presence of 25 ng/mL M-CSF plus 50 ng/mL soluble RANKL. As negative controls, cultures were maintained with M-CSF alone. SF macrophages were also cultured in the presence of LIGHT (50 ng/mL) ± OPG (100 ng/mL). All SF macrophage cultures were incubated for up to 14 d, during which time the entire culture medium containing all factors was replenished every 2-3 d.
Tartrate-resistant acid phosphatase: After 14 d in culture, cells were fixed in formalin and stained histochemically for tartrate-resistant acid phosphatase (TRAP), an osteoclast marker, using naphthol AS-BI as a substrate, in the presence of 1.0 mol/L tartrate.
F-actin ring formation: Multiple rows of podosomes containing an F-actin core are often localised in the sealing zone of osteoclasts. To detect F-actin ring structure, dentine slices were fixed with 4% formaldehyde for 5 min and then permeabilised for 6 min in 0.5% Triton X-100 in phosphate buffered saline (PBS) and rinsed with PBS. The cells on dentine slices were then incubated with tetramethyl-rhodamine isothiocyanante-conjugated phalloidin (Sigma-Aldrich) for 30 min and observed using a fluorescence microscope (Olympus).
Lacunar resorption: Functional evidence of osteoclast formation was determined using a resorption assay system. Circular dentine slices (4 mm in diameter) were prepared from elephant tusk blocks, kindly supplied by the Customs and Excise, United Kingdom, and sterilised in absolute alcohol overnight. After 14-d incubation, dentine slices on which cells had been cultured, were removed from wells, rinsed in PBS, incubated in 1.0 M ammonium hydroxide for 24 h and sonicated in distilled water for 5-10 min. All cellular debris was thus removed from the dentine slice permitting examination of its surface for evidence of lacunar resorption. The slices were washed in distilled water, stained with 0.5% (w/v) toludine blue in 1.0% (w/v) boric acid pH 5.0 and examined by light microscopy. To quantify the lacunar resorption, dentine slices were photographed and the resorbed areas highlighted and measured using Adobe Photoshop CS3 and Image J (NIH, Bethesda, MD, United States).
Human LIGHT enzyme-linked immunosorbent assay (ELISA) kit (R and D Systems Europe) was used to determine the concentration of soluble LIGHT in the synovial fluid derived from RA (n = 5) and OA (n = 4) patients, as per manufacturer’s instructions. The upper and lower detection limits of the ELISA were 31 pg/mL and 2 ng/mL, respectively.
Formalin-fixed, paraffin-embedded synovial biopsies of RA and OA synovium were cut (3 μm), deparaffinized with xylene and rehydrated through a series of graded alcohols. After blocking endogenous peroxidase with 0.2% (v/v) hydrogen peroxide in 80% alcohol for 30 min, antigen retrieval was performed in 500 mL 10 mmol/L Tris + 1 mol/L EDTA (BDH, United Kingdom) buffer (pH 8.5) using a microwave for 20 min. Immunohistochemistry was performed using an indirect immunoperoxidase technique with 3,3-diaminobenzidine chromogen (EnVision™ + Dual Link System-HRP, Liquid DAB + Substrate Chromogen System, Dako, United Kingdom). Sections were incubated with an anti-LIGHT antibody (anti-hLIGHT/HVEM-L, R&D systems, United States) 1:5 overnight at room temperature followed by 30 min incubation with labeled polymer and 10 min in 3,3-diaminobenzidene. Slides were counterstained using Mayer’s haematoxylin for 3 min, blued in 2% hydrogen sodium carbonate, dehydrated, cleared in xylene and mounted using DePeX (Surgipath, United Kingdom). All sections were examined by light microscopy.
All tissue specimens and blood samples from RA and OA patients were taken after informed consent and ethical permission was obtained for participation in the study.
The statistical review of the study was performed prior and after the study was conducted and with consultation with a biomedical statistician. Data is represented as mean ± SEM. For assessment of osteoclast resorption, the area of lacunar resorption was normalized and expressed as a percentage of RANKL-induced lacunar resorption (positive control). Statistical significance was determined by Mann-Whitney test or one-way ANOVA, using GraphPad Prism (Version 6.03). P value < 0.05 were considered as statistically significant.
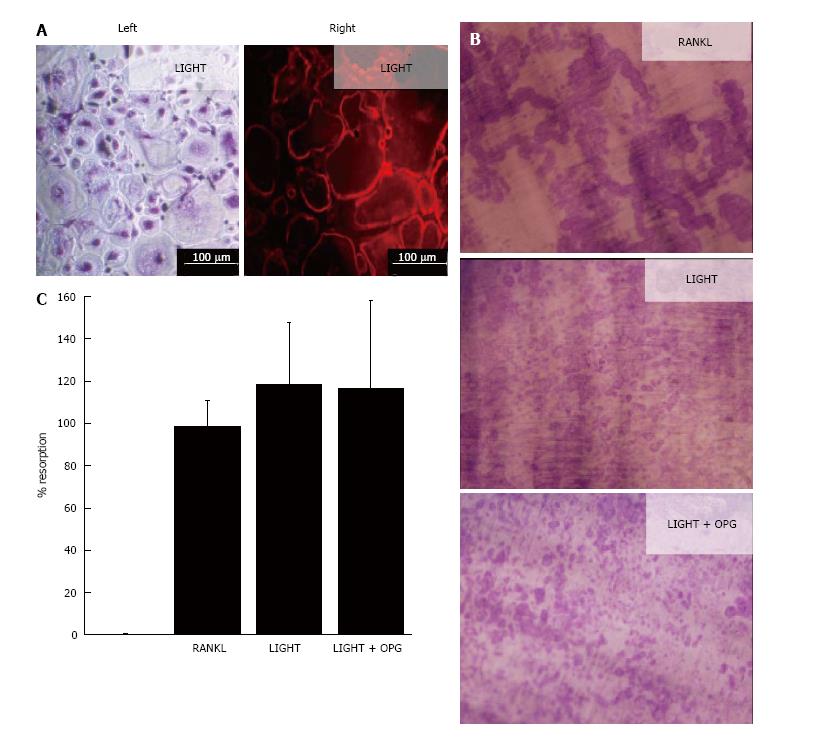
In 14-d cultures of SF macrophages incubated with M-CSF and RANKL, numerous TRAP+ and F-actin ring+ multinucleated cells were generated (Figure 1); these cells were capable of lacunar resorption when cultured on dentine slices (Figure 1). Cultures of SF macrophages with LIGHT and M-CSF under similar conditions also resulted in the formation of comparable numbers of TRAP+ and F-actin ring+ multinucleated cells (Figure 1A) and a similar level of lacunar resorption on dentine slices (Figures 1B). The addition of excess molar concentrations of OPG (100 ng/mL) did not result in a decrease in lacunar resorption pit formation compared with cultures treated with LIGHT alone (Figures 1B and C), confirming that LIGHT-induced osteoclast formation did not occur via the RANKL pathway. Resorption pits formed in LIGHT-treated SF macrophage cultures were smaller and more often single than in RANKL-treated cultures where most resorption pits were compound or confluent with many long resorption tracks being produced (Figure 1B). The overall percentage area of the dentine slice resorbed in LIGHT-treated cultures was comparable to that seen in RANKL-treated cultures (Figure 1C).
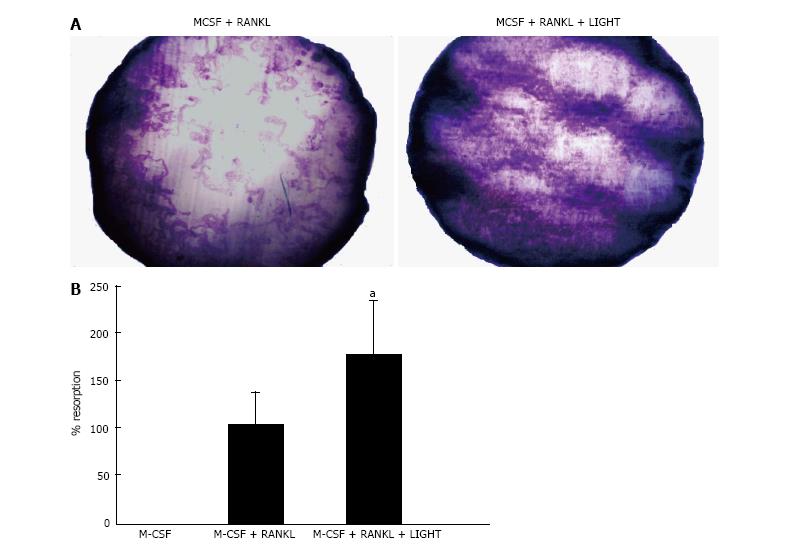
In 14-d cultures of RA SF macrophages on dentine slices incubated with M-CSF and RANKL, a significant increase in lacunar resorption was seen compared with RA SF macrophage cultures treated with RANKL alone (Figure 2).
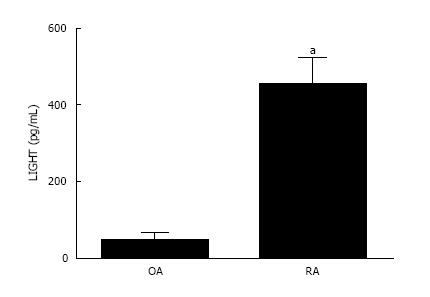
The concentration of LIGHT was significantly elevated in the synovial fluid derived from inflammatory RA compared with non-inflammatory OA joints (Figure 3). The mean level of LIGHT detected in the synovial fluid of RA patients was 452.5 ± 70.5 pg/mL. This was significantly higher (P < 0.05) compared with that found in non-inflammatory OA patients (45.43 ± 19.8 pg/mL).
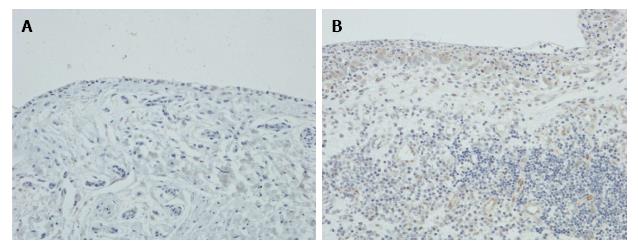
Immunohistochemistry showed that there was little or no expression of LIGHT in OA synovium, but in RA synovium LIGHT was strongly expressed by synovial lining cells and subintimal macrophages (Figure 4). Endothelial cells were also weakly stained, but lymphoid cells and fibroblasts were negative.
The canonical RANKL/OPG axis is the main pathway of osteoclastogenesis and is believed to play a major role in pathological bone resorption in RA[30,31]. RANKL expression is upregulated by inflammatory cytokines found in RA joints such as IL-1, IL-6, IL-17 and tumor necrosis factors (TNF)-α[30-32]. RANKL is a member of the TNF superfamily and it has been shown that other TNF superfamily ligands can induce RANKL-independent osteoclastogenesis. In this study we show that one of these factors, LIGHT, can substitute for RANKL and induce osteoclast formation from SF macrophages derived from RA joints. LIGHT-induced osteoclast formation was not inhibited by OPG and resulted in comparable levels of lacunar resorption to that seen in RANKL-treated cultures. LIGHT also augmented RANKL-mediated lacunar resorption and was found to be highly expressed in RA synovium and SF.
LIGHT is a potent RANKL-independent osteoclastogenic factor that has been shown in several studies to play a role in RA[13]. An increase in LIGHT levels has been noted in the serum of RA patients[14]. LIGHT is upregulated on B-lymphocytes and monocytes in RA and it has been shown to induce the expression of pro-inflammatory cytokines and metalloproteinases in macrophages and synoviocytes[33,34]. RA synovial fibroblasts express the LIGHT receptors HVEM and LTβR; LIGHT activates synovial fibroblasts, resulting in an increase in cytokines and growth factors that promote inflammation and resorption; LIGHT also induces the proliferation of RA synovial fibroblasts through LTβR[35,36]. It has also been shown that CD14+ monocytes interact with stromal cells in RA synovium to induce the formation of TRAP+ MNCs and that LIGHT enhances the generation of MNCs that release metalloproteinases including MMP9 and MMP12, both of which are found at sites of joint erosion in RA[37].
Our data indicates that LIGHT is likely to play a significant role in pathological bone resorption in RA. SF macrophages, like monocytes, are CD14+ cells that are capable of differentiating into osteoclasts[28]. These cells are present in increased numbers in RA compared with OA joints and joint fluid, as are other inflammatory cells that are known to express LIGHT. This accords with our finding that the concentration of LIGHT is increased in RA compared with non-inflammatory OA joint fluid. There was little expression of LIGHT in OA synovium whereas it was strongly expressed in RA synovium mainly in synoviocytes and subintimal macrophages. The combination of an increased number of CD14+ mononuclear phagocyte osteoclast precursors and high levels of LIGHT in the SF provides the conditions for LIGHT-induced osteoclastogenesis to exist in inflamed RA joints.
In keeping with previous studies[14], we found that LIGHT-induced osteoclast differentiation from RA SF macrophages occurred in the absence of RANKL. LIGHT-induced osteoclastogenesis was not inhibited by the RANKL inhibitor OPG and was comparable to that seen in RANKL-treated SF macrophage cultures in terms of formation of TRAP + MNC and lacunar resorption. We also found that LIGHT significantly augmented osteoclast formation in the presence of RANKL, in keeping with the findings of Ishida et al[37] who noted that LIGHT promotes RANKL-induced formation of TRAP + MNCs from CD14+ precursors. Our findings indicate that LIGHT is therefore likely to play a role in both RANKL-dependent and RANKL-independent osteoclast formation and pathological bone resorption in RA. In keeping with this conclusion, Fava et al[27] showed that prophylactic treatment with the LIGHT pathway inhibitor protein LTβR-Ig blocks induction of collagen-induced arthritis in mice and adjuvant arthritis in Lewis rats. There is likely to be a complex interplay between RANKL-independent and RANKL-dependent mechanisms in inflamed RA joints. The role that T cells and synovial fibroblasts play in RANKL/LIGHT-induced osteoclast formation is likely to be key given that these cells are known to express both RANKL and LIGHT. It has been shown the LIGHT contributes to the survival and activation of synovial fibroblasts in RA, resulting in pannus formation which promotes the generation of metalloproteinases, inflammatory cytokines and adhesion molecules[33-36].
RANKL-independent mechanisms of osteoclast formation induced by TNF superfamily members have been shown to play a role in the osteolysis associated with several neoplastic and non-neoplastic diseases of bone and joint, including giant cell tumour of bone, Ewing sarcoma, metastatic breast carcinoma, melanoma and myeloma[13,38]. LIGHT is the most potent TNF superfamily member identified to date that induces RA NKL-independent osteoclast formation. LIGHT is likely to represent a potentially useful therapeutic target in RA as inhibiting its action would not only reduce synovial inflammation but also LIGHT- and RANKL-mediated lacunar resorption, resulting in more complete healing of marginal erosions and preservation of periarticular bone in RA.
A number of studies have shown that Denosumab, a fully humanised antibody that specifically binds RANKL can be used to treat joint erosions and periarticular bone loss in RA[39-43]. Denosumab, however, unlike LIGHT, has no effect on joint inflammation[8,9,39]. It is well recognized that in the treatment of giant cell tumour of bone, withdrawal of Denosumab results in the re-emergence of osteoclasts with consequent osteolysis and regrowth of the tumour[44,45]. The use of Denosumab to treat bone loss in RA is also likely to encounter this problem as fibroblastic stromal cells that express RANKL would persist in the inflammatory environment. Following this therapy targeting LIGHT would therefore be useful in this context as it would not only inhibit the proliferation and activation of RANKL-expressing synovial fibroblasts but also reduce LIGHT- and RANKL-mediated bone resorption.
The authors would like to thank the patients and staff at Nuffield Orthopaedic Centre for assisting with conducting this research.
Rheumatoid arthritis (RA) is characterized by a heavy lymphocyte and plasma cell infiltrate in the synovium, which leads to the formation of periarticular marginal erosions by specialized multinucleated cells, osteoclasts, which carry out lacunar bone resorption. Osteoclast formation and activation is controlled by a receptor-activator nuclear factor kappa-B ligand (RANKL)-OPG axis, in RA as well as RANKL-independent pathway. One of the RANKL-independent mediators of osteoclast activation is an activated T-cell product, known as LIGHT which is also known to be expressed by dendritic cells.
Serum levels of LIGHT are increased in RA patients and blocking the action of LIGHT has been shown to reduce the severity of murine collagen-induced arthritis. It is uncertain whether LIGHT is involved in activation of RA synovial fluid macrophages to osteoclasts.
This is the first study evaluating the role of LIGHT in osteoclastogenesis of RA patients as well as demonstrating the increased LIGHT levels in synovial fluid of RA as compared to osteoarthritic patients.
The elevated levels of LIGHT in synovial fluid of RA patients and the ability of LIGHT to induce RANKL-mediated osteoclast activation indicate that this protein plays a significant role in pathogenesis of RA, which had not been previously reported.
LIGHT is an abbreviation for lymphotoxin-like, exhibits inducible expression and competes with herpes simplex virus glycoprotein D for herpes virus entry mediator, a receptor expressed by T lymphocytes) is the most potent RANKL (receptor activator for nuclear factor κB ligand) substitute identified to date, both of which are tumour necrosis family superfamily members.
The authors demonstrated the possible role of LIGHT in stimulating osteoclast resorption in synovial fluid macrophages. This type of activity was enhanced in combination of RANKL and macrophage-colony stimulating factor. Overall, the paper is logical and well-organized.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lee WH, Rothschild EM S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Symmons D, Turner G, Webb R, Asten P, Barrett E, Lunt M, Scott D, Silman A. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology (Oxford). 2002;41:793-800. [PubMed] |
| 2. | Fujikawa Y, Quinn JM, Sabokbar A, McGee JO, Athanasou NA. The human osteoclast precursor circulates in the monocyte fraction. Endocrinology. 1996;137:4058-4060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 214] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Faust J, Lacey DL, Hunt P, Burgess TL, Scully S, Van G, Eli A, Qian Y, Shalhoub V. Osteoclast markers accumulate on cells developing from human peripheral blood mononuclear precursors. J Cell Biochem. 1999;72:67-80. [PubMed] |
| 4. | Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165-176. [PubMed] |
| 5. | Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597-3602. [PubMed] |
| 6. | Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050-5055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 826] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 7. | Hofbauer LC. Osteoprotegerin ligand and osteoprotegerin: novel implications for osteoclast biology and bone metabolism. Eur J Endocrinol. 1999;141:195-210. [PubMed] |
| 8. | Rossini M, Adami G, Viapiana O, Idolazzi L, Gatti D. Denosumab, cortical bone and bone erosions in rheumatoid arthritis. Ann Rheum Dis. 2016;75:e70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | McHugh J. Rheumatoid arthritis: Bone-healing effects of denosumab in RA. Nat Rev Rheumatol. 2016;12:692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Josse R, Khan A, Ngui D, Shapiro M. Denosumab, a new pharmacotherapy option for postmenopausal osteoporosis. Curr Med Res Opin. 2013;29:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Knowles HJ, Athanasou NA. Canonical and non-canonical pathways of osteoclast formation. Histol Histopathol. 2009;24:337-346. [PubMed] |
| 13. | Sabokbar A, Mahoney DJ, Hemingway F, Athanasou NA. Non-Canonical (RANKL-Independent) Pathways of Osteoclast Differentiation and Their Role in Musculoskeletal Diseases. Clin Rev Allergy Immunol. 2016;51:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Edwards JR, Sun SG, Locklin R, Shipman CM, Adamopoulos IE, Athanasou NA, Sabokbar A. LIGHT (TNFSF14), a novel mediator of bone resorption, is elevated in rheumatoid arthritis. Arthritis Rheum. 2006;54:1451-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858-4864. [PubMed] |
| 16. | Kudo O, Fujikawa Y, Itonaga I, Sabokbar A, Torisu T, Athanasou NA. Proinflammatory cytokine (TNFalpha/IL-1alpha) induction of human osteoclast formation. J Pathol. 2002;198:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Hemingway F, Taylor R, Knowles HJ, Athanasou NA. RANKL-independent human osteoclast formation with APRIL, BAFF, NGF, IGF I and IGF II. Bone. 2011;48:938-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Mabilleau G, Pascaretti-Grizon F, Baslé MF, Chappard D. Depth and volume of resorption induced by osteoclasts generated in the presence of RANKL, TNF-alpha/IL-1 or LIGHT. Cytokine. 2012;57:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Hemingway F, Kashima TG, Knowles HJ, Athanasou NA. Investigation of osteoclastogenic signalling of the RANKL substitute LIGHT. Exp Mol Pathol. 2013;94:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Morel Y, Schiano de Colella JM, Harrop J, Deen KC, Holmes SD, Wattam TA, Khandekar SS, Truneh A, Sweet RW, Gastaut JA. Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J Immunol. 2000;165:4397-4404. [PubMed] |
| 21. | Zhai Y, Guo R, Hsu TL, Yu GL, Ni J, Kwon BS, Jiang GW, Lu J, Tan J, Ugustus M. LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest. 1998;102:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 216] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Tamada K, Shimozaki K, Chapoval AI, Zhai Y, Su J, Chen SF, Hsieh SL, Nagata S, Ni J, Chen L. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol. 2000;164:4105-4110. [PubMed] |
| 23. | Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8:21-30. [PubMed] |
| 24. | Kwon BS, Tan KB, Ni J, Oh KO, Lee ZH, Kim KK, Kim YJ, Wang S, Gentz R, Yu GL. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem. 1997;272:14272-14276. [PubMed] |
| 25. | Harrop JA, Reddy M, Dede K, Brigham-Burke M, Lyn S, Tan KB, Silverman C, Eichman C, DiPrinzio R, Spampanato J. Antibodies to TR2 (herpesvirus entry mediator), a new member of the TNF receptor superfamily, block T cell proliferation, expression of activation markers, and production of cytokines. J Immunol. 1998;161:1786-1794. [PubMed] |
| 26. | Yu KY, Kwon B, Ni J, Zhai Y, Ebner R, Kwon BS. A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J Biol Chem. 1999;274:13733-13736. [PubMed] |
| 27. | Fava RA, Notidis E, Hunt J, Szanya V, Ratcliffe N, Ngam-Ek A, De Fougerolles AR, Sprague A, Browning JL. A role for the lymphotoxin/LIGHT axis in the pathogenesis of murine collagen-induced arthritis. J Immunol. 2003;171:115-126. [PubMed] |
| 28. | Adamopoulos IE, Sabokbar A, Wordsworth BP, Carr A, Ferguson DJ, Athanasou NA. Synovial fluid macrophages are capable of osteoclast formation and resorption. J Pathol. 2006;208:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6830] [Cited by in RCA: 6174] [Article Influence: 411.6] [Reference Citation Analysis (0)] |
| 30. | Crotti TN, Dharmapatni AA, Alias E, Haynes DR. Osteoimmunology: Major and Costimulatory Pathway Expression Associated with Chronic Inflammatory Induced Bone Loss. J Immunol Res. 2015;2015:281287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 647] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 32. | Braun T, Zwerina J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res Ther. 2011;13:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 33. | Kim WJ, Kang YJ, Koh EM, Ahn KS, Cha HS, Lee WH. LIGHT is involved in the pathogenesis of rheumatoid arthritis by inducing the expression of pro-inflammatory cytokines and MMP-9 in macrophages. Immunology. 2005;114:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Kang YM, Kim SY, Kang JH, Han SW, Nam EJ, Kyung HS, Park JY, Kim IS. LIGHT up-regulated on B lymphocytes and monocytes in rheumatoid arthritis mediates cellular adhesion and metalloproteinase production by synoviocytes. Arthritis Rheum. 2007;56:1106-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Pierer M, Brentano F, Rethage J, Wagner U, Hantzschel H, Gay RE, Gay S, Kyburz D. The TNF superfamily member LIGHT contributes to survival and activation of synovial fibroblasts in rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Ishida S, Yamane S, Ochi T, Nakano S, Mori T, Juji T, Fukui N, Itoh T, Suzuki R. LIGHT induces cell proliferation and inflammatory responses of rheumatoid arthritis synovial fibroblasts via lymphotoxin beta receptor. J Rheumatol. 2008;35:960-968. [PubMed] |
| 37. | Ishida S, Yamane S, Nakano S, Yanagimoto T, Hanamoto Y, Maeda-Tanimura M, Toyosaki-Maeda T, Ishizaki J, Matsuo Y, Fukui N. The interaction of monocytes with rheumatoid synovial cells is a key step in LIGHT-mediated inflammatory bone destruction. Immunology. 2009;128:e315-e324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Brunetti G, Rizzi R, Oranger A, Gigante I, Mori G, Taurino G, Mongelli T, Colaianni G, Di Benedetto A, Tamma R. LIGHT/TNFSF14 increases osteoclastogenesis and decreases osteoblastogenesis in multiple myeloma-bone disease. Oncotarget. 2014;5:12950-12967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, van der Heijde D, Zhou L, Tsuji W, Newmark R; Denosumab Rheumatoid Arthritis Study Group. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 425] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 40. | Takeuchi T, Tanaka Y, Ishiguro N, Yamanaka H, Yoneda T, Ohira T, Okubo N, Genant HK, van der Heijde D. Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose-response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis. 2016;75:983-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 41. | Yue J, Griffith JF, Xiao F, Shi L, Wang D, Shen J, Wong P, Li EK, Li M, Li TK. Repair of bone erosion in rheumatoid arthritis by denosumab: A high-resolution peripheral quantitative computed tomography study. Arthritis Care Res (Hoboken). 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 42. | Hasegawa T, Kaneko Y, Izumi K, Takeuchi T. Efficacy of denosumab combined with bDMARDs on radiographic progression in rheumatoid arthritis. Joint Bone Spine. 2017;84:379-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Tanaka S. Regulation of bone destruction in rheumatoid arthritis through RANKL-RANK pathways. World J Orthop. 2013;4:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Gaston CL, Grimer RJ, Parry M, Stacchiotti S, Dei Tos AP, Gelderblom H, Ferrari S, Baldi GG, Jones RL, Chawla S. Current status and unanswered questions on the use of Denosumab in giant cell tumor of bone. Clin Sarcoma Res. 2016;6:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 45. | Müller DA, Beltrami G, Scoccianti G, Campanacci DA, Franchi A, Capanna R. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone-a case series. World J Surg Oncol. 2016;14:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |