Published online Aug 20, 2016. doi: 10.5493/wjem.v6.i3.58
Peer-review started: February 16, 2016
First decision: March 24, 2016
Revised: March 30, 2016
Accepted: May 31, 2016
Article in press: June 2, 2016
Published online: August 20, 2016
Processing time: 184 Days and 14.7 Hours
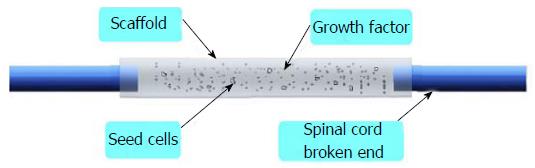
Spinal cord injury usually leads to permanent disability, which could cause a huge financial problem to the patient. Up to now there is no effective method to treat this disease. The key of the treatment is to enable the damage zone axonal regeneration and luckily it could go through the damage zone; last a connection can be established with the target neurons. This study attempts to combine stem cell, material science and genetic modification technology together, by preparing two genes modified adipose-derived stem cells and inducing them into neuron direction; then by compositing them on the silk fibroin/chitosan scaffold and implanting them into the spinal cord injury model, seed cells can have features of neuron cells. At the same time, it could stably express the brain-derived neurotrophic factor and neurotrophin-3, both of which could produce synergistic effects, which have a positive effect on the recovery of spinal cord. The spinal cord scaffold bridges the broken end of the spinal cord and isolates with the surrounding environment, which could avoid a scar effect on the nerve regeneration and provide three-dimensional space for the seed cell growth, and at last we hope to provide a new treatment for spinal cord injury with the tissue engineering technique.
Core tip: Spinal cord injury is a thorny disease to doctors. Tissue engineering including three core contents-seed cells, scaffolds and growth factors - is a promising method to cure this disease. Selecting suitable factors and maximizing the repairing effect is very important. This article is a hypothesis to choose the suitable factor which hopes to build up a promising method to treat spinal cord injury.
- Citation: Ji WC, Zhang XW, Qiu YS. Selected suitable seed cell, scaffold and growth factor could maximize the repair effect using tissue engineering method in spinal cord injury. World J Exp Med 2016; 6(3): 58-62
- URL: https://www.wjgnet.com/2220-315X/full/v6/i3/58.htm
- DOI: https://dx.doi.org/10.5493/wjem.v6.i3.58
Spinal cord injury (SCI) is a thorny issue to clinic doctors and scientists, the incidence of which increases year by year[1]. In China, SCI incidence is nearly 60 people per million and in the United States there are 12000 new patients each year, with the estimated totally number of Americans living with a SCI nearly 259000[2,3]. This disease not only causes great harm to the patient mentally and physically, but also increases the economic burden on the society, so it is very important to develop novel strategies to treat SCI.
In the basic research, scientists focus their attention on the following methods[4-6]: (1) using drugs; (2) taking advantage of stem cells; (3) making use of growth factors; (4) inhibiting inflammatory responses and hypertrophic scars; (5) promoting the axon growth; and (6) using tissue engineering technology. Among these, the most important method is tissue engineering technology, including three core factors-seed cells, scaffolds and growth factors (Figure 1). Thus how to select suitable seed cells, scaffolds and growth factors is very important, which could maximize the repair effect.
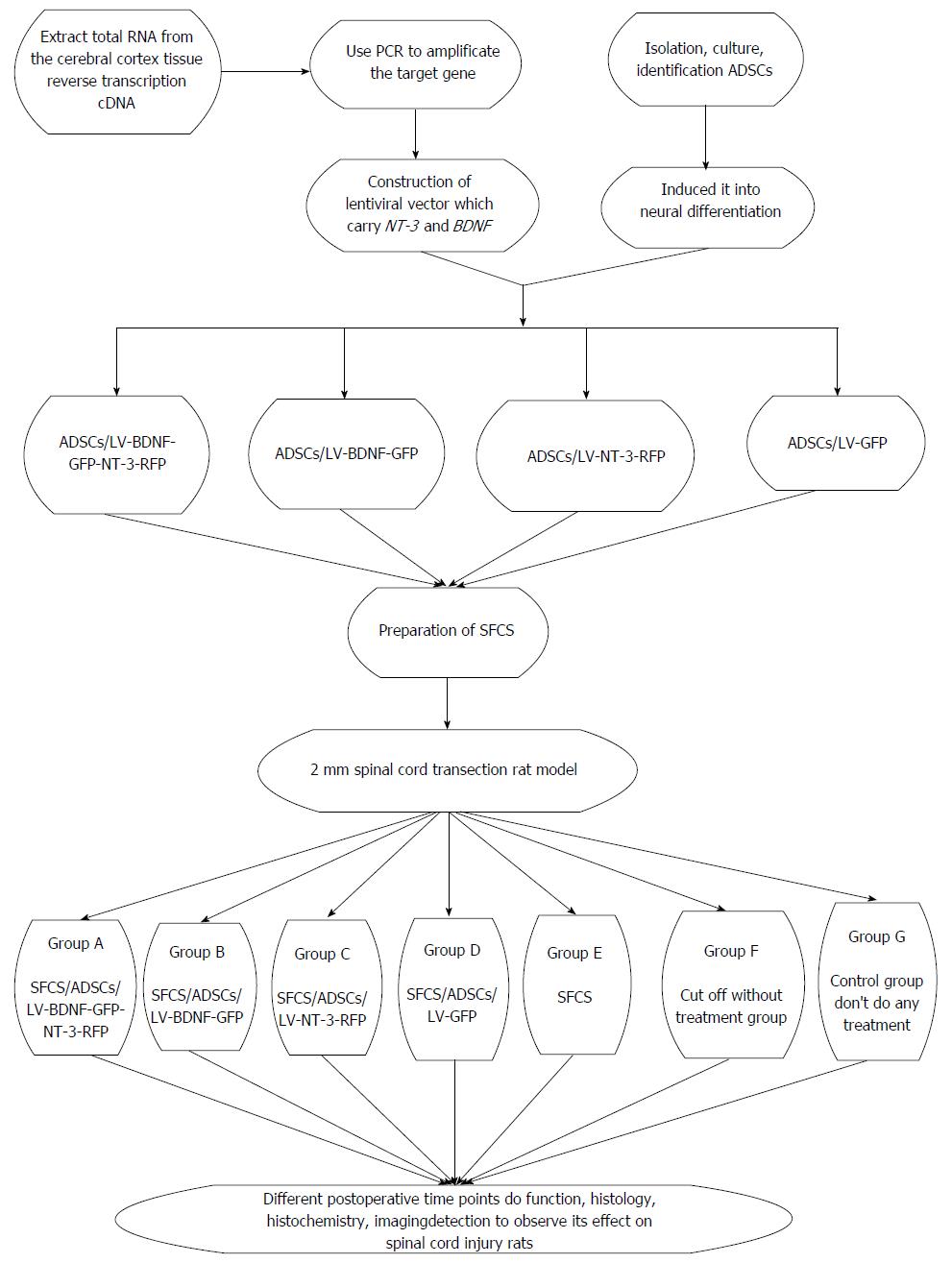
Our hypothesis is to use the tissue engineering to repair SCI in rats. Adipose-derived stem cells (ADSCs) have the characteristic of multipotential differentiation, and under certain conditions, they could differentiate into neuron cells. Constructing lentviral vectors carried brain-derived neurophic factor (BDNF) and neurotrophin-3 (NT-3) gene independently; we used it to transfect neural cells induced by the ADSCs, and thus seed cells have the properties of neurons; and at the same time, it could stably express BDNF and NT-3. Making the silk fibroin/chitosan scaffolds (SFCS), seeded transfect cells on scaffolds, implanting the composite into a 2 mm spinal cord transection rat model and taking advantage the characteristic of double gene modified neurons, they could continuously express BDNF and NT-3, and the trait of tissue engineering is shown. We are looking forward to providing a new treatment for SCI which could reduce the disability rate and mortality of SCI, and offering a solid theoretical basis for the clinical treatment of SCI (Figure 2).
Seed cell is the first core factor in tissue engineering which has also restricted the development of tissue engineering as a bottleneck[7]. Seed cell could be divided into non-stem cells and stem cells[8]. The former includes: Schwann cells, olfactory ensheathing cells, fibroblast, and endothelial cells; the latter is divided into two types: (1) adult stem cell: ADSCs and muscle derived stem cells (MPSCs); and (2) non-adult stem cell: Embryonic stem cell (ES), neural stem cells and bone mesenchymal stem cells (BMSCs). In the above seed cells, ES and neural stem cells have critically important usage in the basic research in the early period, but there exist some problems, such as ethical problem, reject reaction and limitation of source which could not be used widely in clinics[9]; BMSCs are widely used in basic research, but in clinical application, we need to extract the bone marrow from patients, which will cause trauma[10]. Thus more and more scientists focus their attention on the adult stem cells, which have all the above advantages and at the mean time avoid their shortcoming[11]. Although MPSCs are adult stem cells normally used in the tissue engineering, most of the experiments use them to cure heart diseases and urological diseases; and just a few studies use them to repair nerve injury; the reason is that the ability of neurogenic differentiation for MDSCs is lower than ADSCs[12-14]. So we think if we want to select a suitable adult stem cell to cure the SCI, ADSCs are better, which are first separated by Zuk et al[15]. Its morphology, biological characteristics and immune phenotype is similar to BMSC, and the most advanced of this stem cell is minimally invasive for the patient[16]. Thus stem cells are better than non-stem cells, and in the stem cell, adult stem cells are better than non-adult stem cells, ADSCs are the best.
Except for the seed cell, the scaffold is also very important, which could be divided into different types according to various standards. For example, according to the source of materials it could be divided into natural scaffold and synthetic scaffold; according to the ingredient, it could be divided into single ingredient scaffold and composite ingredient scaffold; according to different states, it could be divided into solid scaffold and liquid scaffold[17]. Compared to the single ingredient scaffold, the composite ingredient scaffold which includes several components is perhaps better, because it could avoid the disadvantage existing in the single ingredient scaffold and maximize the scaffold function[18]. In addition, compared to the synthetic scaffolds, the natural ingredients scaffold may be better, and the reason is that the immune reaction and inflammation reaction is lighter after being implanted in the body[19].
Silk fibroin was obtained from the silk degumming and a kind of natural structural albumen was used as a scaffold in tissue engineering in many experiments. For example, Parry in their research uses silk fibroin to cure spinal fusion and finds this scaffold has a good effect[20]. But the shortcoming of this scaffold is that it is very brittle in a dry state and difficult to handle with[21]. Chitosan is another scaffold which has been researched on its biocompatibility,biodegradability, and biotoxicity for a long time, although rapid degradation and high swelling property are its problem[22]. Thus the blending of both materials to make SFCS was suggested, and the reason is that SFCS could avoid the exclusive limitation of pure silk fibroin and chitosan, and at the mean time it has excellent mechanical properties[23]. Therefore, we think using SFCS as a scaffold to repair SCI may be a bright future direction.
From the above we know that seed cells and scaffolds are very important if we want to use tissue engineering technology to cure SCI, but besides them, growth factors could not be ignored, including nerve growth factors, BDNF, NT-3, glial cell line - derived neurotrophic factors and others, among which the most important are NT-3 and BDNF[24]. BDNF was extracted from porcine brain in 1982 and has a widespread effect on central neurons, which through the activation and binding of TrkB to expresses its biological effects, it could prevent neurons death and improve the pathological state of neurons after SCI[25]. NT-3 is found in the cloning of NGF gene, which is a multifunctional gene and has the effect on maintaining the neuron and dopaminergic neuron differentiation[26]. Up to now, NT-3 is considered to be the only gene which has a clear effect that could promote the corticospinal tract growth after SCI by binding and activating the TrkC to produce its biological effect[27]. Thus we want to know whether we could use BDNF and NT-3 at the same time, which may have a synergistic effect. If we want to implement this idea, we need to resolve the new challenge-how to use them, because injecting the growth factor directly may cause it to be lost and fail to take a long effect. Using gene modified technology could solve this problem. Morizono et al[28] did a comparison of efficiency among three kinds of viruses to transfect ADSCs and found that the highest transfection efficiency is in lentivirus. Thus lentivirus may be better, and another reason why to choose this virus carrier is that it has a main characteristic which could insert nearly 8 KB exogenous fragments and provide a possibility that connects two genes in the same carrier in the future research, and if using this transfect stem cell, gene expression may be higher than using two single genes to transfect at the same time.
If our hypothesis is confirmed, we can significantly improve the repair effect on the SCI, but we clearly know that SCI will still trouble doctors and researchers for a long time in future, and this hypothesis just provides a possibility for people in future research. If we want to successfully cure the SCI and let patients recover normally after injury, there is a long way to go. All in all, with the deepening research about tissue engineering, we think this method could be used in the clinics to cure SCI at last, which promises bright future for the patients.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kolettis TM, Lin ZY, Xavier-Elsas P, Zhao JB S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Camune BD. Challenges in the management of the pregnant woman with spinal cord injury. J Perinat Neonatal Nurs. 2013;27:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Wang M, Zhai P, Chen X, Schreyer DJ, Sun X, Cui F. Bioengineered scaffolds for spinal cord repair. Tissue Eng Part B Rev. 2011;17:177-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Whitehurst DG, Mittmann N. The value of health economics research in spinal cord injury. Spinal Cord. 2013;51:586-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Perale G, Rossi F, Santoro M, Peviani M, Papa S, Llupi D, Torriani P, Micotti E, Previdi S, Cervo L. Multiple drug delivery hydrogel system for spinal cord injury repair strategies. J Control Release. 2012;159:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Reeves A, Keirstead HS. Stem cell based strategies for spinal cord injury repair. Adv Exp Med Biol. 2012;760:16-24. [PubMed] |
| 6. | Kitamura K, Fujiyoshi K, Yamane J, Toyota F, Hikishima K, Nomura T, Funakoshi H, Nakamura T, Aoki M, Toyama Y. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PLoS One. 2011;6:e27706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Wyatt LA, Keirstead HS. Stem cell-based treatments for spinal cord injury. Prog Brain Res. 2012;201:233-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Li J, Lepski G. Cell transplantation for spinal cord injury: a systematic review. Biomed Res Int. 2013;2013:786475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | All AH, Bazley FA, Gupta S, Pashai N, Hu C, Pourmorteza A, Kerr C. Human embryonic stem cell-derived oligodendrocyte progenitors aid in functional recovery of sensory pathways following contusive spinal cord injury. PLoS One. 2012;7:e47645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Ban DX, Ning GZ, Feng SQ, Wang Y, Zhou XH, Liu Y, Chen JT. Combination of activated Schwann cells with bone mesenchymal stem cells: the best cell strategy for repair after spinal cord injury in rats. Regen Med. 2011;6:707-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Hernández J, Torres-Espín A, Navarro X. Adult stem cell transplants for spinal cord injury repair: current state in preclinical research. Curr Stem Cell Res Ther. 2011;6:273-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Woo JC, Bae WJ, Kim SJ, Kim SD, Sohn DW, Hong SH, Lee JY, Hwang TK, Sung YC, Kim SW. Transplantation of muscle-derived stem cells into the corpus cavernosum restores erectile function in a rat model of cavernous nerve injury. Korean J Urol. 2011;52:359-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Shibuya M, Miura T, Fukagawa Y, Akashi S, Oda T, Kawamura S, Ikeda Y, Matsuzaki M. Tongue muscle-derived stem cells express connexin 43 and improve cardiac remodeling and survival after myocardial infarction in mice. Circ J. 2010;74:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Kwon EB, Lee JY, Piao S, Kim IG, Ra JC, Lee JY. Comparison of human muscle-derived stem cells and human adipose-derived stem cells in neurogenic trans-differentiation. Korean J Urol. 2011;52:852-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Zuk PA. The adipose-derived stem cell: looking back and looking ahead. Mol Biol Cell. 2010;21:1783-1787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 16. | Kapur SK, Katz AJ. Review of the adipose derived stem cell secretome. Biochimie. 2013;95:2222-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Horst M, Madduri S, Milleret V, Sulser T, Gobet R, Eberli D. A bilayered hybrid microfibrous PLGA--acellular matrix scaffold for hollow organ tissue engineering. Biomaterials. 2013;34:1537-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Ji W, Hu S, Zhou J, Wang G, Wang K, Zhang Y. Tissue engineering is a promising method for the repair of spinal cord injuries (Review). Exp Ther Med. 2014;7:523-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Ji W, Zhang Y, Hu S, Zhang Y. Biocompatibility study of a silk fibroin-chitosan scaffold with adipose tissue-derived stem cells in vitro. Exp Ther Med. 2013;6:513-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Parry PV, Engh JA. High strength silk protein scaffolds: the future of spinal fusions. Neurosurgery. 2012;71:N29-N30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Garcia-Fuentes M, Meinel AJ, Hilbe M, Meinel L, Merkle HP. Silk fibroin/hyaluronan scaffolds for human mesenchymal stem cell culture in tissue engineering. Biomaterials. 2009;30:5068-5076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Lima PA, Resende CX, Soares GD, Anselme K, Almeida LE. Preparation, characterization and biological test of 3D-scaffolds based on chitosan, fibroin and hydroxyapatite for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2013;33:3389-3395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Bhardwaj N, Kundu SC. Chondrogenic differentiation of rat MSCs on porous scaffolds of silk fibroin/chitosan blends. Biomaterials. 2012;33:2848-2857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Kikuno N, Kawamoto K, Hirata H, Vejdani K, Kawakami K, Fandel T, Nunes L, Urakami S, Shiina H, Igawa M. Nerve growth factor combined with vascular endothelial growth factor enhances regeneration of bladder acellular matrix graft in spinal cord injury-induced neurogenic rat bladder. BJU Int. 2009;103:1424-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | He BL, Ba YC, Wang XY, Liu SJ, Liu GD, Ou S, Gu YL, Pan XH, Wang TH. BDNF expression with functional improvement in transected spinal cord treated with neural stem cells in adult rats. Neuropeptides. 2013;47:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Shang AJ, Hong SQ, Xu Q, Wang HY, Yang Y, Wang ZF, Xu BN, Jiang XD, Xu RX. NT-3-secreting human umbilical cord mesenchymal stromal cell transplantation for the treatment of acute spinal cord injury in rats. Brain Res. 2011;1391:102-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Guo JS, Zeng YS, Li HB, Huang WL, Liu RY, Li XB, Ding Y, Wu LZ, Cai DZ. Cotransplant of neural stem cells and NT-3 gene modified Schwann cells promote the recovery of transected spinal cord injury. Spinal Cord. 2007;45:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Morizono K, De Ugarte DA, Zhu M, Zuk P, Elbarbary A, Ashjian P, Benhaim P, Chen IS, Hedrick MH. Multilineage cells from adipose tissue as gene delivery vehicles. Hum Gene Ther. 2003;14:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |