Published online May 20, 2015. doi: 10.5493/wjem.v5.i2.50
Peer-review started: November 27, 2014
First decision: December 26, 2014
Revised: January 9, 2015
Accepted: March 30, 2015
Article in press: April 2, 2015
Published online: May 20, 2015
Processing time: 175 Days and 21 Hours
Beside many efforts to improve outcome, sepsis is still one of the most frequent causes of death in critically ill patients. It is the most common condition with high mortality in intensive care units. The complexity of the septic syndrome comprises immunological aspects - i.e., sepsis induced immunosuppression - but is not restricted to this fact in modern concepts. So far, exact mechanisms and variables determining outcome and mortality stay unclear. Since there is no typical risk profile, early diagnosis and risk stratification remain difficult, which hinders rapid and effective treatment initiation. Due to the heterogeneous nature of sepsis, potential therapy options should be adapted to the individual. Biomarkers like C-reactive protein and procalcitonin are routinely used as complementary tools in clinical decision-making. Beyond the acute phase proteins, a wide bunch of promising substances and non-laboratory tools with potential diagnostic and prognostic value is under intensive investigation. So far, clinical decision just based on biomarker assessment is not yet feasible. However, biomarkers should be considered as a complementary approach.
Core tip: Sepsis is a complex continuum of disturbed systems. Despite the presence of clinical consensus criteria, the early diagnosis especially in the perioperative setting is challenging. A magnitude of potential new biomarkers is tested for this purpose, but evidence is mounting that due to the complex nature of the syndrome, biomarkers are rather complementary tools for clinical decision making than “magic bullets”. Moreover, biomarkers are also evaluated for therapy guidance, linking diagnostic results to an individual therapeutic regime. This review summarizes the developments in the biomarker field, aiming to provide an overview about current targets and their limitations.
- Citation: Kojic D, Siegler BH, Uhle F, Lichtenstern C, Nawroth PP, Weigand MA, Hofer S, Brenner T. Are there new approaches for diagnosis, therapy guidance and outcome prediction of sepsis? World J Exp Med 2015; 5(2): 50-63
- URL: https://www.wjgnet.com/2220-315X/full/v5/i2/50.htm
- DOI: https://dx.doi.org/10.5493/wjem.v5.i2.50
The incidence of sepsis is still unreasonable high in critically ill patients and represents a major challenge in treatment. It is a common reason for admission to the intensive care unit (ICU). In European ICUs, sepsis and severe sepsis occur in 30% and 37% of the patients[1]. Gaieski and colleagues designate severe sepsis as the third most common cause of death in the United States after heart disease and malignant tumors[2]. A reason for the elevated incidence of sepsis in developed countries may be the high proportion of the elderly population[3].
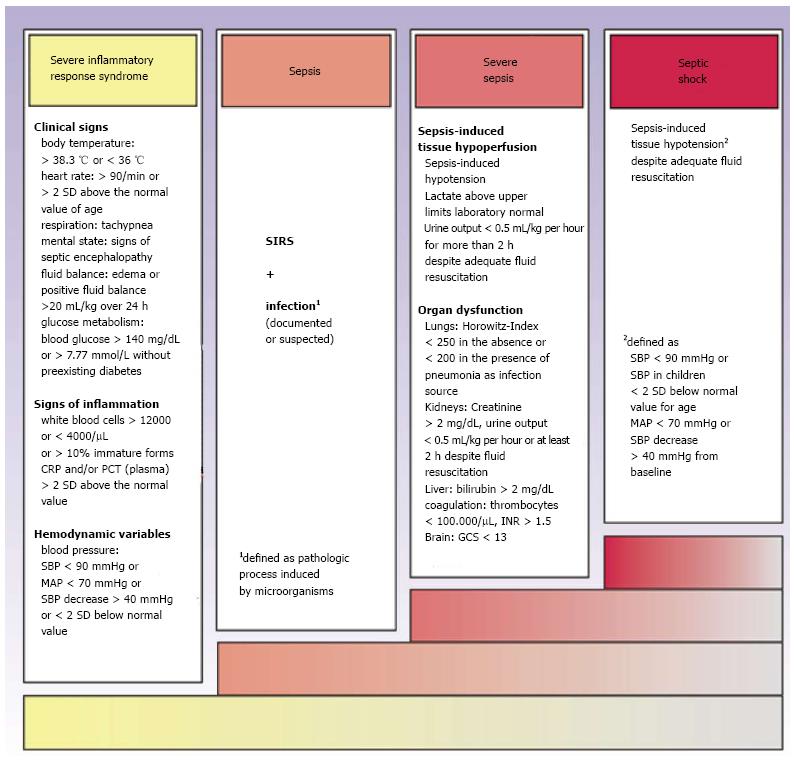
Sepsis is defined as a systemic inflammatory response syndrome (SIRS) with proven or probable infection of bacterial, fungal or viral origin[4]. Severe sepsis is characterized by additional existence of organ dysfunction, while septic shock is defined as sepsis together with the failure of the cardiovascular system to sustain adequate tissue perfusion[5] (Figure 1). Initially, the organism reacts with a proinflammatory immune response to the infectious stimulus. During the later course of disease, there is a co-existence between SIRS and compensatory mechanisms termed compensatory anti-inflammatory response syndrome (CARS, Figure 2). The resulting sepsis-induced immune suppression is characterized by a collapse of cellular immune response and an increased risk for opportunistic infections with high mortality[6]. Clinical signs of sepsis are unspecific and comprise general symptoms (i.e., aberrances of body temperature, fluid balance, glucose metabolism or mental confusion), as well as laboratory indications of inflammation or signs of hemodynamic impairment and organ dysfunction[4]. Because of the high variability of symptoms and the pathophysiological complexity, clinical recognition and severity assessment remain difficult[7].
The therapy of septic patients represents a major challenge to physicians. To improve clinical management and outcome of critically ill patients, the Surviving Sepsis Campaign guidelines were published ten years ago and have been lastly revised in 2012[8]. However, despite modern resuscitating strategies and anti-infective therapy options morbidity and mortality remain notably high in septic disease.
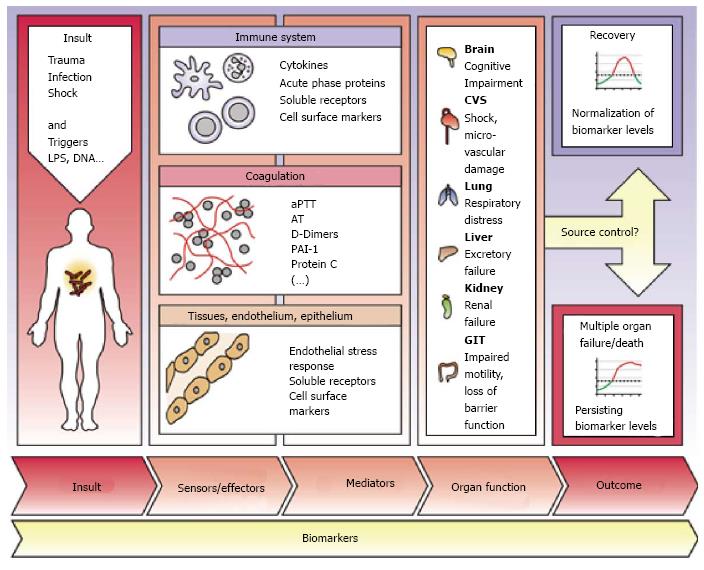
The key to successful therapy remains the early detection of septic patients. Biomarkers may provide help for clinical decision-making and predicting sepsis-related outcome[9]. Therefore, beside commonly used substances like C-reactive protein (CRP) and procalcitonin (PCT), further biomarkers are needed. Additionally, in times with increasing prevalence of multidrug-resistant pathogens and a growing consumption of anti-infective drugs biomarker-guided strategies are of enormous importance[10]. The large involvement of organs and cell systems in the inflammatory response to an infection widens the number of putative biomarker candidates[11] (Figure 3).
Triggered by inflammation, immune cells release a wide range of mediators, i.e., interleukins or tumor necrosis factor (TNF-α), into circulation. These cytokines induce the production and secretion of acute phase proteins in the liver. Many of these substances have been investigated as potential sepsis-biomarkers. The following chapter reviews important markers of this group.
The most used biomarkers in clinical settings are the acute phase proteins CRP and PCT[4,10,12]. The synthesis of CRP in the liver is triggered by interleukin-6 (IL-6) in response to tissue damages, inflammatory and/or infectious stimuli[13]. There is a notable increase of CRP level 4-6 h after stimulation. These levels double every 8 h and peak 36 to 50 h after an infection/inflammation stimulus[14]. CRP measurement is cheap and rapidly available, but increases of CRP levels are unspecific, since they can be observed, i.e., after surgery or trauma[15]. However, CRP is commonly used to screen early onset of sepsis in neonates[16]. High CRP levels correlate with disease severity and are discussed to represent the effectiveness of an antimicrobial therapy[17-19]. Although CRP assessment does not sufficiently allow to discriminate between infectious and non-infectious stimuli, a secondary rise in CRP level after 3 to 4 d after infection, surgery or trauma could be helpful for diagnosing septic complications.
PCT is a prohormone of calcitonin. The peptide precursor is released by parenchymal cells including liver, kidney and muscles cells as well as adipocytes in response to bacterial toxins. After exposition to those toxins, serum levels of PCT increase within 2 to 4 h[20] and a peak of PCT in serum can be detected after approximately 14 h[21,22]. PCT is seen as a specific biomarker for bacterial infection[23], although elevations of PCT in serum can also be observed under non-infectious conditions, such as trauma[24], major surgical procedures, pancreatitis and renal impairment[25-28]. Thus, the use of PCT as a diagnostic biomarker of sepsis is discussed in a couple of meta-analyses with conflicting results[29,30]. However, other trials investigated the potential use of PCT to guide antimicrobial therapies[31], i.e., in patients with community-acquired pneumonia (CAP), acute exacerbation of chronic bronchitis and sepsis. A recent multicentre study demonstrated, that serum PCT levels are not an accurate indicator for ventilator-associated pneumonia (VAP). Higher PCT levels were shown in CAP than in VAP[32]. Although PCT-guided antibiotic therapy is associated with a reduction in antibiotic usage and may reduce overall costs of care, an increase in hospital mortality of 7% under PCT guided conditions could not be ruled out by a meta analysis of Heyland et al[33]. Despite the association of high PCT and CRP levels in the onset of sepsis with poor outcome[34,35], the prognostic value of PCT is limited[10].
IL-6 is secreted by macrophages and T cells to stimulate immune response which occurs during infection and after trauma, especially burns or other tissue damage leading to inflammation[36,37]. Compared to CRP or PCT, IL-6 levels peak 2 h after initiation of the inflammatory cascade. Based on this rapid increase, IL-6 was introduced as a biomarker of early sepsis in emergency units[38,39]. A multicenter study showed that IL-6 can predict survival on the 28th day after sepsis onset[40]. The value of IL-6 to distinguish between SIRS and sepsis is controversially discussed[41,42], since high levels can also be detected after trauma, surgery or in patients with autoimmune diseases[43-45]. Nevertheless, the IL-6 expression correlates with sepsis severity[10]. Further prospective and multicenter studies are required to elucidate the benefit of IL-6 and acute-phase proteins like CRP and PCT in diagnosis and risk-stratification of septic patients.
The pentraxin superfamily comprises a wide range of proteins involved in the initial phase of the inflammatory response[46]. Prototypic long pentraxin 3 (PTX 3) is rapidly produced and released by various cells like mononuclear phagocytes, dendritic cells (DC), fibroblasts and endothelial cells[47-49]. It binds to specific patterns of fungi, bacteria and viruses[46,50,51] and induces the complement pathway[52]. A prospective study by Mauri and colleagues showed significantly elevated PTX3 levels in non-survivors compared to survivors over the first five days. In addition, PTX3 plasma levels were higher in the septic shock group than in patients with severe sepsis[53]. Concordantly, Bastrup-Birk and colleagues describe an association between disease severity and PTX3 levels. Furthermore, using ROC-analyses, moderate statistic values for PTX3 could be found to distinguish between patients with SIRS and healthy individuals[54]. Thus, PTX3 should be considered as a potential tool to monitor disease severity.
The expression of various proteins, i.e., cell surface receptors, is up-regulated in activated immune cells. These receptors as well as their soluble forms occur as potential biomarkers in septic patients.
The transmembrane receptor of advanced glycation end products (RAGE) belongs to the immunoglobulin superfamily. During inflammation, the receptor induces cell activation by initiating intracellular signalling cascades[10,55] resulting in rapid gene transcription[56]. RAGE is upregulated in various inflammatory diseases, such as rheumatoid arthritis[57,58], inflammatory kidney disease[59,60], arteriosclerosis[61] and inflammatory bowel disease[62]. The truncated form of the receptor, soluble RAGE (sRAGE), was demonstrated to be elevated in septic ICU patients at early onset of sepsis. There were also higher levels in nonsurvivors than survivors at day 28[63]. A multivariate analysis showed an association between sRAGE levels and mortality in acute respiratory distress syndrome (ARDS) patients, but not with severity of illness[64]. There is a relation between sRAGE and outcome of septic patients. To prove suitability of sRAGE as a prognostic marker, further clinical studies are needed.
As a member of the immunoglobulin superfamily, the triggering receptor expressed on myeloid cells-1 (TREM-1) is strongly expressed on activated phagocytes[65,66]. A soluble form is released during bacterial or fungal infections and can be detected as biomarker in distinct body fluids[67]. After an induction period of less than 2 h, plasma levels of soluble triggering receptor expresses on myeloid cells-1 (sTREM-1) peak within 24 h[68]. However, a meta-analysis of 11 studies showed only moderate diagnostic performance in differentiating sepsis from SIRS[67]. Accordingly, Bopp and colleagues could not detect significantly different plasma concentrations between healthy controls and patients with SIRS, sepsis, severe sepsis or septic shock in the early phase of disease[63]. Since some trials report elevated sTREM-1 plasma levels during non-infectious states[69,70], the role of sTREM-1 as diagnostic biomarker remains uncertain.
Bacterial infections lead to complex formation of lipopolysaccharides (LPS), LPS binding protein (LPB) and the surface receptor cluster of differentiation 14 (CD14), which is located on the cell-surface membrane of phagocytes. During inflammation, a soluble form of this complex is further cleaved and termed soluble CD14 subtype (sCD14-ST = presepsin)[71,72]. Presepsin is a promising biomarker for diagnosing sepsis, severe sepsis and septic shock compared to other biomarkers such as PCT[73-76] and seems specific for infectious diseases[71]. High plasma levels in septic patients can be detected 6 h after early onset[72]. A recent study demonstrated that measurements of presepsin levels revealed diagnostic and prognostic value in patients with severe sepsis and septic shock within the first week of ICU treatment. Presepsin showed diagnostic value for sepsis, severe sepsis and septic shock at days 1, 3 and 8 after ICU admission in comparison to PCT, IL-6, CRP, white blood cells (WBC). It also revealed prognostic effort to prospect short- and long-term mortality[77]. These findings suggest that it may be used as a prognostic marker in early risk stratification.
The urokinase-type plasminogen activator receptor (uPAR) is expressed on various cell types such as macrophages, endothelial and tumor cells. It participates in cellular immunity including migration, adhesion, angiogenesis, fibrinolysis and cell proliferation. After inflammatory stimulation, the receptor is cleaved from the cell surface by proteases and can be detected as soluble uPAR (suPAR) in urine, cerebrospinal fluid and blood[78].
Recent studies showed that high levels of suPAR are able to predict mortality in patients with diseases that are associated with inflammatory response. Additionally, the time course of suPAR seems to correlate with disease severity and the degree of organ dysfunction[79,80]. suPAR has gained growing interest because of its role as a predictor of disease severity in patients with bacteraemia[81]. In contrast to the prognostic value, the diagnostic performance of suPAR is limited[82].
Human leukocyte antigen-DR on circulating monocytes (mHLA-DR) is a major histocompatibility complex (MHC) class II cell surface receptor and was originally defined as cell surface antigen. HLA-DR molecules are upregulated in response to inflammation[83]. Monocytes with low HLA-DR expression are unable to generate a proinflammatory response to microbial challenge or properly present antigens to T cells[84,85]. Decreased mHLA-DR levels could predict poor outcome and septic complications after trauma, surgery, burn, pancreatitis and septic shock[86-89] but there is few knowledge about the underlying mechanisms[90].
A prospective randomised placebo-controlled trial used mHLA-DR to stratify the administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) in a small group of septic patients. After administration, there was an increase of monocyte release of tumor necrosis factor α (TNF-α). This biomarker-guided therapy appeared reliable and successful in reestablishing monocyte immunocompetence and shortening hospital and ICU stay[91,92]. The expression of mHLA-DR represents a valid indicator for monocyte function and should be tested in more clinical trials as it could be a reliable future marker for immunosuppression.
The multiple organ failure (MOF) is the fatal end feature in the pathophysiology of sepsis. During severe sepsis or septic shock, development of organ dysfunction dramatically increases morbidity and mortality[93]. During the acute phase of sepsis or septic shock, circulatory failure is discussed as the predominant cause of death[94,95]. However, underlying mechanisms leading to organ failure are not fully understood yet. Since the breakdown of endothelial barrier functions is estimated to play an important role in this context[96], biomarkers of endothelial integrity as well as markers of cardiac injury are reviewed in the following.
Cellular as well as metabolic alterations during organ failure result in rapidly elevated blood levels of the heart type fatty acid binding protein (hFABP)[97]. The low molecular weight protein is predominantly detectable in myocardial cytoplasm and lung tissue. It participates in the uptake and transport of long chain fatty acids and is suggested as a promising biomarker of cardiac impairment[98-100]. Recent studies indicate, that hFABP can independently predict 28-d mortality and organ failure in patients with sepsis, severe sepsis or septic shock. Additionally, a correlation between plasma concentration and sepsis-induced myocardial dysfunction has been described and elevation of hFABP is associated with an increasing mortality rate[101-103]. hFABP may thus be a promising marker to identify high risk patients in the emergency department.
Troponin T (TNT), a regulatory protein, is a highly specific marker for myocardial infarction or heart muscle cell death. Several trials accounted significantly increased TNT levels in patients with septic disease[104,105]. Contradictory, myocardial wall abnormalities, diagnosed by echocardiography, proved to be more specific in septic patients than increased troponin levels[10].
Many vasoactive hormones rise in sepsis[10]. Natriuretic peptides like atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and C-type natriuretic peptide (CNP) influence diuresis, natriuresis and vasorelaxants[106]. ANP and BNP are predictors for cardiac dysfunction and are able to predict congestive heart failure[10]. Several trials demonstrated a correlation between high BNP levels and worse morbidity and mortality in septic patients combined with low ejection fraction[107,108]. High CNP levels have been detected in sepsis. Koch et al[80] showed significantly higher N-terminal pro-CNP levels in septic than in non-septic patients.
Apoptosis represents a major mechanism of cell death in patients with sepsis and multi-organ-failure[109]. Cytokeratins are proteins of keratin-containing intermediate filaments in epithelial tissue. It is cleaved by activated caspases (caspase-cleaved cytokeratin 18, cCK-18) and can be detected with the help of the monoclonal antibody M30[110,111]. The measurement of cCK-18 concentration conduce the dimension of apoptosis in critically ill patients[112-114]. There is a correlation between persisting high concentrations of cCK-18 in the early treatment of sepsis and 28 d mortality[115]. Several trials showed higher concentration of cCK-18 in septic patients than in trauma patients or healthy individuals[116,117]. Measurements of cCK-18 could be of valuable use in detecting risk of multi-organ-failure.
Microvascular disorders contribute to the development of MOF. The integrity and function of the endothelial barrier depends on the stability of distinct receptors and their ligands, i.e., the Angiopoietin/Tie2 system. Regarding their antagonistic roles, especially the balance between Angiopoietin-1 and -2 (Ang-1 and -2) is of particular importance[118]. In a multicenter cohort study, low plasma levels of Ang-1 at admission as well as serially measured Ang-1 and -2 were associated with higher 28-d mortality in critically ill patients with severe sepsis[119]. Additionally, in patients with suspected infection, elevated Ang-2 plasma levels were detected during the first hour after admission and correlated with the development of severe sepsis and a higher mortality[120]. A number of trials investigate the value of an Ang-1/-2 ratio in predicting outcome of septic patients. This ratio was significantly elevated in children with severe sepsis and septic shock compared to control patients with or without SIRS or sepsis during the first 3 d on pediatric ICU[121]. Similarly, using Canonical Correlation Analysis, Wang et al[122] identified a strong correlation between the combination of Ang-1, Ang-2, bicarbonate and disease severity or outcome of sepsis in children. Thus, the components of the Angiopoietin/Tie2-System are promising targets of further studies to improve outcome prediction in septic patients.
Posttranslational processing of the precursor peptide preproadrenomedullin results in the generation of the vasoactive, antimicrobial and anti-inflammatory peptide adrenomedullin (ADM)[123]. Systemic inflammation and sepsis leads to an increased release of ADM into circulation[124,125], but a short half-life and other difficulties in the measurement impede the clinical use[126]. However, another fragment termed midregional-pro-adrenomedullin (MR-proADM) seems to be a promising sepsis-biomarker. When blood samples are stored at 20 °C, MR-proADM is stable up to 24 h. Thus, it seems more suitable for daily routine in the primary care setting[127]. The release of MR-proADM is increased during viral and bacterial infections. So far, the potential utility of MR-proADM to predict outcome of sepsis is controversially discussed in a number of trials. At admission to the ICU, single MR-proADM assessment may be useful to predict in-hospital mortality, although the statistic performance is not outstanding[128]. In patients with CAP, Suberviola et al[129] showed a correlation between severity of illness, outcome and the plasma levels of MR-proADM. However, this correlation could not be confirmed by another recent single-center study[130]. Further investigations are necessary to clarify this gap.
Endothelial function, i.e., the expression of surface proteins and the release of cyto- and chemokines, is crucial for migration of immune cells from circulation into tissues[131]. The proteoglycan Endocan, originally named endothelial cell-specific molecule 1, is expressed on the surface of pulmonal and renal endothelial cells. Proinflammatory cytokines like TNF-α and IL-β induce the expression and release into the bloodstream in vitro[132]. Various studies report altered levels of the proteoglycan in neoplastic diseases, since tumor-derived factors also regulate Endocan expression[133-136]. In septic patients however, elevated plasma levels are correlated with sepsis severity. Furthermore, significantly higher Endocan values can be detected in non-survivors at admission to ICU[137]. In addition, a recent study by Mihajlovic et al[138] determined a correlation between initially elevated Endocan levels and the development of sepsis induced organ failure over time[139]. Accordingly, in patients with ARDS, Endocan levels seem useful to predict mortality and multiple-organ dysfunction. Thus, Endocan may be a promising biomarker to predict disease severity and outcome of critically ill patients.
Besides numerous markers derived from plasma or other body fluids, a variety of non-laboratory tools like the assessment of body temperature, heart rate variability, blood coagulation or sidestream darkfield videomicroscopy can assist the clinician in diagnosis, outcome prediction and monitoring of septic patients. However, putative findings - although promising - are challenged by a variable user-dependency.
A non-invasive technique to identify critically ill patients is recognition of body temperature patterns. Fever as a classic symptom of septic patients has minor sensitivity and specificity in relation to diagnostic expressiveness[140,141]. Drewry et al[142] showed that abnormal body temperature curves were predictive of the diagnosis of sepsis in afebrile critically ill patients. Analysis of temperature patterns may relieve the decision to antimicrobial therapy rather than absolute temperature values.
Besides its role in the regulation of various body functions, i.e., heart rate, the autonomic nervous system participates in the complex host response to a systemic inflammation[143]. This link has been under intensive investigation. Measurement of the “heart rate variability” (HRV) is a promising technique to evaluate the autonomic cardiac regulation in patients with suspected sepsis. Although underlying mechanisms are still unclear, changes in HRV are associated with the appearance of systemic infections[144] and correlate with disease severity[145,146]. The measurement of HRV may be a promising tool to improve an early diagnosis of sepsis. In adult bone marrow transplant patients, alterations in HRV could be detected prior to the clinical diagnosis of sepsis[147]. Furthermore, in a prospective, observational study, initial detection of HRV changes in septic patients via electrocardiogram in the emergency department showed to be valuable in predicting in-hospital mortality[148]. However, since changes in cardiac function also depend on various cofactors, further research is needed to elucidate the role of HRV assessment in diagnosis and outcome prediction in patients with suspected sepsis.
Impairment of the microcirculatory blood flow (MBF) is common in patients with sepsis and designated as an important step in the development of organ failure[149]. Sidestream darkfield videomicroscopy can be used to identify alterations of microvascular parameters, i.e., the microvascular flow index, the perfused (small) vessel density or the proportion of perfused (small) vessels. Clinical studies using this technique in critically ill patients with cardiogenic or septic shock, but also in patients before and after cardiac bypass surgery, support the idea, that changes in MBF occur independent from macrocirculatory hemodynamic parameters[149-151]. In patients with sepsis, abnormalities in the sublingual MBF can be detected early in the septic progress and correlate with disease severity[152,153]. Several trials investigated beneficial effects of various interventions to optimize sublingual MBF. However, these results should be interpreted carefully due to methodological limitations[154]. Further studies about the use of sidestream darkfield videomicroscopy in the clinical setting are necessary, but should consider potential observer bias, as reported by Sallisalmi et al[155].
Thrombelastometry is a proper tool for monitoring and therapy guidance of haemostatic dysfunction[156]. The Clot-Lysis-Index (CLI) measures the mechanical properties of forming a clot in whole-blood samples in a time-dependent modality. The clot firmness is measured at 30, 45 or 60 min. Recent trails have demonstrated significantly higher CLI in septic patients than non-septic patients and control groups. These changes have been detected in patients even before sepsis was diagnosed[157,158]. The CLI could be useful as a future tool for early diagnosis of critically ill patients.
In hospitalized patients, sepsis still belongs to the most frequent causes of death[1,94,159]. Unspecific predictive signs complicate an early diagnosis, and - when sepsis is successfully diagnosed - treatment strategies are rare. So far, therapeutic approaches are limited to fluid administration, antibiotics and the attempt to sustain or restore organ function[160]. Originally, sepsis was designated as a distinct inflammatory response to an infectious stimulus[161]. Modern concepts define sepsis as a syndrome and focus on the problem, that also non-infectious stimuli result in defence mechanisms and clinical signs, which in the end do not allow a discrimination between infectious or non-infectious origin[36].
Although sepsis is a major problem for the critically ill, it is not exclusively restricted to these individuals. The exact mechanisms and variables determining outcome and mortality are not yet fully understood. As a consequence, since there is no typical risk profile, it remains hard to define patients at risk, which hinders rapid and effective treatment initiation. Nevertheless, recent studies report a reduction of acute mortality of patients with sepsis, severe sepsis or septic shock[162]. However, survivors of the acute phase are confronted with a chronic dysfunction of organ systems with high mortality, recently termed as “persistent critical illness” (PCI)[96]. Thus, early diagnosis and a rapid treatment initiation are even more of crucial importance for the prognosis. Since the role of scoring systems in initial evaluation, monitoring and outcome prediction of septic patients is controversially discussed, biomarkers should be considered as complementary tools[163]. During the last decades, a variety of promising sepsis related biomarkers has been under intensive investigation. Some, i.e., acute phase proteins, are already widely-used in clinical practice. So far, the Surviving Sepsis Campaign guidelines only recommend PCT evaluation over time to deescalate antibiotic therapies[5]. Although many efforts to find more specific biomarkers seem promising, evaluation and comparison of the results is limited by unstandardized development- and evaluation strategies.
The pathophysiology of sepsis is characterized by an impairment of various systems on cellular, tissue-specific or functional level. In contrast to the original idea of an explicit immunological dysfunction, sepsis is now seen as a more complex syndrome that is characterized by an “impaired homeostasis”. This concept combines immunological aspects with a neuroendocrine dysregulation and barrier failures[96]. The complex nature of sepsis complicates the search for new treatment targets. Furthermore, physicians are confronted with various individual comorbidities and other influencing factors[164].
Lately, Brenner et al[165] showed the involvement of alternative mediators of cellular stress in the pathophysiology of sepsis. Methylglyoxal (MG), which is one of these highly reactive carbonyl species, is produced endogenously from the spontaneous degradation of triosephosphates (glyceraldehyde-3-phosphate and dihydroxyacetone phosphate) during glycolysis. The study identified MG as a better marker for the identification of patients with sepsis in comparison to routine diagnostic markers and furthermore, MG was shown to be an early predictor for survival in patients with septic shock. Hopefully, these findings will help to improve early recognition of sepsis.
Recent research approaches try to enlighten the (epi)genetic regulation of sepsis. Hopefully, new findings in this field may once help to improve early risk stratification. In this context, it seems obvious that no single biomarker can yet feature a high diagnostic value together with an outstanding sensitivity and specificity to predict outcome and to guide (antiinfective) treatment. Thus, the combination of markers can be beneficial. However, despite some interesting trials, a promising combination is still missing.
When discussing the use of sepsis-related biomarkers, the question remains which marker should be preferred in clinical use. Due to the complexity of sepsis, adequate interpretation of laboratory results is the basis for a reasonable biomarker assessment. Here, we reviewed the state of knowledge about some key biomarkers. Although many substances and methods seem promising, most of them are not yet established in the intensive care unit. In the daily routine, the assessment of biomarkers can be a complementary tool in clinical decision making. However, it should be restricted to accurately defined problems and pursue the objective to achieve direct benefits for the critically ill patient. Together with new findings in the epigenetic field, the concept of directly linking diagnostic results to an individual therapeutic regime - also termed theragnostics[166] - may be the next step to improve the outcome of patients with sepsis. Thus, beside the need to find valuable diagnostic substances, biomarker-guided therapy approaches should gain further attention.
P- Reviewer: Puntel RL, Riccardi C, Sugawara I S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1778] [Cited by in RCA: 1903] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 2. | Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 1035] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 3. | Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 658] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 4. | Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 1676] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 5. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4031] [Cited by in RCA: 3973] [Article Influence: 331.1] [Reference Citation Analysis (0)] |
| 6. | Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 366] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 7. | Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 512] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 8. | Schorr CA, Dellinger RP. The Surviving Sepsis Campaign: past, present and future. Trends Mol Med. 2014;20:192-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Dupuy AM, Philippart F, Péan Y, Lasocki S, Charles PE, Chalumeau M, Claessens YE, Quenot JP, Guen CG, Ruiz S. Role of biomarkers in the management of antibiotic therapy: an expert panel review: I - currently available biomarkers for clinical use in acute infections. Ann Intensive Care. 2013;3:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Lichtenstern C, Brenner T, Bardenheuer HJ, Weigand MA. Predictors of survival in sepsis: what is the best inflammatory marker to measure? Curr Opin Infect Dis. 2012;25:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 813] [Cited by in RCA: 878] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 12. | Rey C, Los Arcos M, Concha A, Medina A, Prieto S, Martinez P, Prieto B. Procalcitonin and C-reactive protein as markers of systemic inflammatory response syndrome severity in critically ill children. Intensive Care Med. 2007;33:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Henriquez-Camacho C, Losa J. Biomarkers for sepsis. Biomed Res Int. 2014;2014:547818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Li HX, Liu ZM, Zhao SJ, Zhang D, Wang SJ, Wang YS. Measuring both procalcitonin and C-reactive protein for a diagnosis of sepsis in critically ill patients. J Int Med Res. 2014;42:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med. 1999;17:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 438] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 16. | Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 17. | Póvoa P, Coelho L, Almeida E, Fernandes A, Mealha R, Moreira P, Sabino H. Pilot study evaluating C-reactive protein levels in the assessment of response to treatment of severe bloodstream infection. Clin Infect Dis. 2005;40:1855-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Schmit X, Vincent JL. The time course of blood C-reactive protein concentrations in relation to the response to initial antimicrobial therapy in patients with sepsis. Infection. 2008;36:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Lobo SM, Lobo FR, Bota DP, Lopes-Ferreira F, Soliman HM, Mélot C, Vincent JL. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest. 2003;123:2043-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Gilbert DN. Use of plasma procalcitonin levels as an adjunct to clinical microbiology. J Clin Microbiol. 2010;48:2325-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24:888-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 168] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, Bohuon C. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79:1605-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 213] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1128] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 24. | Limper M, de Kruif MD, Duits AJ, Brandjes DP, van Gorp EC. The diagnostic role of procalcitonin and other biomarkers in discriminating infectious from non-infectious fever. J Infect. 2010;60:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Meisner M, Tschaikowsky K, Hutzler A, Schick C, Schüttler J. Postoperative plasma concentrations of procalcitonin after different types of surgery. Intensive Care Med. 1998;24:680-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 264] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Schneider CP, Yilmaz Y, Kleespies A, Jauch KW, Hartl WH. Accuracy of procalcitonin for outcome prediction in unselected postoperative critically ill patients. Shock. 2009;31:568-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Fritz HG, Brandes H, Bredle DL, Bitterlich A, Vollandt R, Specht M, Franke UF, Wahlers T, Meier-Hellmann A. Post-operative hypoalbuminaemia and procalcitonin elevation for prediction of outcome in cardiopulmonary bypass surgery. Acta Anaesthesiol Scand. 2003;47:1276-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Rau BM, Kemppainen EA, Gumbs AA, Büchler MW, Wegscheider K, Bassi C, Puolakkainen PA, Beger HG. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg. 2007;245:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis. 2007;7:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 594] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 30. | Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:426-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 756] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 31. | Schuetz P, Mueller B. To escalate or to de-escalte--that is the question. Crit Care Med. 2011;39:2590; author reply 2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Jung B, Embriaco N, Roux F, Forel JM, Demory D, Allardet-Servent J, Jaber S, La Scola B, Papazian L. Microbiogical data, but not procalcitonin improve the accuracy of the clinical pulmonary infection score. Intensive Care Med. 2010;36:790-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Heyland DK, Johnson AP, Reynolds SC, Muscedere J. Procalcitonin for reduced antibiotic exposure in the critical care setting: a systematic review and an economic evaluation. Crit Care Med. 2011;39:1792-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | Oberhoffer M, Vogelsang H, Russwurm S, Hartung T, Reinhart K. Outcome prediction by traditional and new markers of inflammation in patients with sepsis. Clin Chem Lab Med. 1999;37:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Viallon A, Guyomarc’h S, Marjollet O, Berger C, Carricajo A, Robert F, Laporte S, Lambert C, Page Y, Zéni F. Can emergency physicians identify a high mortality subgroup of patients with sepsis: role of procalcitonin. Eur J Emerg Med. 2008;15:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. 2013;381:774-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 492] [Article Influence: 41.0] [Reference Citation Analysis (1)] |
| 37. | van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997;176:439-444. [PubMed] |
| 38. | Bloos F, Reinhart K. Rapid diagnosis of sepsis. Virulence. 2014;5:154-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 39. | Uusitalo-Seppälä R, Koskinen P, Leino A, Peuravuori H, Vahlberg T, Rintala EM. Early detection of severe sepsis in the emergency room: diagnostic value of plasma C-reactive protein, procalcitonin, and interleukin-6. Scand J Infect Dis. 2011;43:883-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, Miller M, Barchuk WT, Fischkoff S, Kaul M. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. 2004;32:2173-2182. [PubMed] |
| 41. | Oberhoffer M, Russwurm S, Bredle D, Chatzinicolaou K, Reinhart K. Discriminative power of inflammatory markers for prediction of tumor necrosis factor-alpha and interleukin-6 in ICU patients with systemic inflammatory response syndrome (SIRS) or sepsis at arbitrary time points. Intensive Care Med. 2000;26 Suppl 2:S170-S174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Tsalik EL, Jaggers LB, Glickman SW, Langley RJ, van Velkinburgh JC, Park LP, Fowler VG, Cairns CB, Kingsmore SF, Woods CW. Discriminative value of inflammatory biomarkers for suspected sepsis. J Emerg Med. 2012;43:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 43. | Barbić J, Ivić D, Alkhamis T, Drenjancević D, Ivić J, Harsanji-Drenjancević I, Turina I, Vcev A. Kinetics of changes in serum concentrations of procalcitonin, interleukin-6, and C- reactive protein after elective abdominal surgery. Can it be used to detect postoperative complications? Coll Antropol. 2013;37:195-201. [PubMed] |
| 44. | Iking-Konert C, Bartz-Bazzanella P, Falagan D, Hofman MW, Schwarting A, Dorner T. [Interleukin-6 inhibition as a potential therapeutic target in rheumatic diseases.]. Z Rheumatol. 2013;Oct 3; Epub ahead of print. [PubMed] |
| 45. | Schlüter B, König B, Bergmann U, Müller FE, König W. Interleukin 6--a potential mediator of lethal sepsis after major thermal trauma: evidence for increased IL-6 production by peripheral blood mononuclear cells. J Trauma. 1991;31:1663-1670. [PubMed] |
| 46. | Bottazzi B, Garlanda C, Cotena A, Moalli F, Jaillon S, Deban L, Mantovani A. The long pentraxin PTX3 as a prototypic humoral pattern recognition receptor: interplay with cellular innate immunity. Immunol Rev. 2009;227:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 47. | Bottazzi B, Vouret-Craviari V, Bastone A, De Gioia L, Matteucci C, Peri G, Spreafico F, Pausa M, D’Ettorre C, Gianazza E. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem. 1997;272:32817-32823. [PubMed] |
| 48. | Diniz SN, Nomizo R, Cisalpino PS, Teixeira MM, Brown GD, Mantovani A, Gordon S, Reis LF, Dias AA. PTX3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages. J Leukoc Biol. 2004;75:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Nauta AJ, Bottazzi B, Mantovani A, Salvatori G, Kishore U, Schwaeble WJ, Gingras AR, Tzima S, Vivanco F, Egido J. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur J Immunol. 2003;33:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 273] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 50. | Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, Maccagno A, Riva F, Bottazzi B, Peri G. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 535] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 51. | Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, Doni A, Bastone A, Mantovani G, Beck Peccoz P. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 353] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 52. | Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 655] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 53. | Mauri T, Bellani G, Patroniti N, Coppadoro A, Peri G, Cuccovillo I, Cugno M, Iapichino G, Gattinoni L, Pesenti A. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med. 2010;36:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 54. | Bastrup-Birk S, Skjoedt MO, Munthe-Fog L, Strom JJ, Ma YJ, Garred P. Pentraxin-3 serum levels are associated with disease severity and mortality in patients with systemic inflammatory response syndrome. PLoS One. 2013;8:e73119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Gröne HJ, Kurschus FC, Schmidt AM. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 226] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 56. | Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting I. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792-2808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 648] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 57. | Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, De Caterina R. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 393] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 58. | Hofmann MA, Drury S, Hudson BI, Gleason MR, Qu W, Lu Y, Lalla E, Chitnis S, Monteiro J, Stickland MH. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun. 2002;3:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 296] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 59. | Greten J, Kreis I, Wiesel K, Stier E, Schmidt AM, Stern DM, Ritz E, Waldherr R, Nawroth PP. Receptors for advance glycation end-products (AGE) - expression by endothelial cells in non-diabetic uraemic patients. Nephrol Dial Transplant. 1996;11:786-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Tanji N, Markowitz GS, Fu C, Kislinger T, Taguchi A, Pischetsrieder M, Stern D, Schmidt AM, D‘Agati VD. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol. 2000;11:1656-1666. [PubMed] |
| 61. | Schmidt AM, Stern D. Atherosclerosis and diabetes: the RAGE connection. Curr Atheroscler Rep. 2000;2:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 62. | Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1392] [Cited by in RCA: 1480] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 63. | Bopp C, Hofer S, Weitz J, Bierhaus A, Nawroth PP, Martin E, Büchler MW, Weigand MA. sRAGE is elevated in septic patients and associated with patients outcome. J Surg Res. 2008;147:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 64. | Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Maeda S, Yamagishi S. Increased levels of soluble receptor for advanced glycation end products (sRAGE) and high mobility group box 1 (HMGB1) are associated with death in patients with acute respiratory distress syndrome. Clin Biochem. 2011;44:601-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 65. | Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 811] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 66. | Gibot S, Cravoisy A. Soluble form of the triggering receptor expressed on myeloid cells-1 as a marker of microbial infection. Clin Med Res. 2004;2:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 67. | Wu Y, Wang F, Fan X, Bao R, Bo L, Li J, Deng X. Accuracy of plasma sTREM-1 for sepsis diagnosis in systemic inflammatory patients: a systematic review and meta-analysis. Crit Care. 2012;16:R229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 68. | Knapp S, Gibot S, de Vos A, Versteeg HH, Colonna M, van der Poll T. Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J Immunol. 2004;173:7131-7134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 69. | Cavaillon JM. Monocyte TREM-1 membrane expression in non-infectious inflammation. Crit Care. 2009;13:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Ferat-Osorio E, Wong-Baeza I, Esquivel-Callejas N, Figueroa-Figueroa S, Duarte-Rojo A, Guzmán-Valdivia-Gómez G, Rodea-Rosas H, Torres-González R, Sánchez-Fernández P, Arriaga-Pizano L. Triggering receptor expressed on myeloid cells-1 expression on monocytes is associated with inflammation but not with infection in acute pancreatitis. Crit Care. 2009;13:R69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 71. | Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother. 2011;17:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 72. | Yaegashi Y, Shirakawa K, Sato N, Suzuki Y, Kojika M, Imai S, Takahashi G, Miyata M, Furusako S, Endo S. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J Infect Chemother. 2005;11:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 73. | Chenevier-Gobeaux C, Trabattoni E, Roelens M, Borderie D, Claessens YE. Presepsin (sCD14-ST) in emergency department: the need for adapted threshold values? Clin Chim Acta. 2014;427:34-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 74. | Endo S, Suzuki Y, Takahashi G, Shozushima T, Ishikura H, Murai A, Nishida T, Irie Y, Miura M, Iguchi H. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J Infect Chemother. 2012;18:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 75. | Liu B, Chen YX, Yin Q, Zhao YZ, Li CS. Diagnostic value and prognostic evaluation of Presepsin for sepsis in an emergency department. Crit Care. 2013;17:R244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 76. | Ulla M, Pizzolato E, Lucchiari M, Loiacono M, Soardo F, Forno D, Morello F, Lupia E, Moiraghi C, Mengozzi G. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: a multicenter prospective study. Crit Care. 2013;17:R168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 77. | Behnes M, Bertsch T, Lepiorz D, Lang S, Trinkmann F, Brueckmann M, Borggrefe M, Hoffmann U. Diagnostic and prognostic utility of soluble CD 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Crit Care. 2014;18:507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 78. | Thunø M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 212] [Reference Citation Analysis (0)] |
| 79. | Donadello K, Scolletta S, Taccone FS, Covajes C, Santonocito C, Cortes DO, Grazulyte D, Gottin L, Vincent JL. Soluble urokinase-type plasminogen activator receptor as a prognostic biomarker in critically ill patients. J Crit Care. 2014;29:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 80. | Koch A, Voigt S, Kruschinski C, Sanson E, Dückers H, Horn A, Yagmur E, Zimmermann H, Trautwein C, Tacke F. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011;15:R63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 81. | Huttunen R, Syrjänen J, Vuento R, Hurme M, Huhtala H, Laine J, Pessi T, Aittoniemi J. Plasma level of soluble urokinase-type plasminogen activator receptor as a predictor of disease severity and case fatality in patients with bacteraemia: a prospective cohort study. J Intern Med. 2011;270:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 82. | Backes Y, van der Sluijs KF, Mackie DP, Tacke F, Koch A, Tenhunen JJ, Schultz MJ. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med. 2012;38:1418-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 83. | Costantino CM, Ploegh HL, Hafler DA. Cathepsin S regulates class II MHC processing in human CD4+ HLA-DR+ T cells. J Immunol. 2009;183:945-952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med. 2008;14:64-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 85. | Wolk K, Döcke WD, von Baehr V, Volk HD, Sabat R. Impaired antigen presentation by human monocytes during endotoxin tolerance. Blood. 2000;96:218-223. [PubMed] |
| 86. | Cheron A, Floccard B, Allaouchiche B, Guignant C, Poitevin F, Malcus C, Crozon J, Faure A, Guillaume C, Marcotte G. Lack of recovery in monocyte human leukocyte antigen-DR expression is independently associated with the development of sepsis after major trauma. Crit Care. 2010;14:R208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 87. | Landelle C, Lepape A, Voirin N, Tognet E, Venet F, Bohé J, Vanhems P, Monneret G. Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med. 2010;36:1859-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 88. | Monneret G, Lepape A, Voirin N, Bohé J, Venet F, Debard AL, Thizy H, Bienvenu J, Gueyffier F, Vanhems P. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32:1175-1183. [PubMed] |
| 89. | Venet F, Tissot S, Debard AL, Faudot C, Crampé C, Pachot A, Ayala A, Monneret G. Decreased monocyte human leukocyte antigen-DR expression after severe burn injury: Correlation with severity and secondary septic shock. Crit Care Med. 2007;35:1910-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 90. | Gogos C, Kotsaki A, Pelekanou A, Giannikopoulos G, Vaki I, Maravitsa P, Adamis S, Alexiou Z, Andrianopoulos G, Antonopoulou A. Early alterations of the innate and adaptive immune statuses in sepsis according to the type of underlying infection. Crit Care. 2010;14:R96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 91. | Demaret J, Walencik A, Jacob MC, Timsit JF, Venet F, Lepape A, Monneret G. Inter-laboratory assessment of flow cytometric monocyte HLA-DR expression in clinical samples. Cytometry B Clin Cytom. 2013;84:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 92. | Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 470] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 93. | Proulx F, Joyal JS, Mariscalco MM, Leteurtre S, Leclerc F, Lacroix J. The pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2009;10:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 94. | Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244-1250. [PubMed] |
| 95. | Blanco J, Muriel-Bombín A, Sagredo V, Taboada F, Gandía F, Tamayo L, Collado J, García-Labattut A, Carriedo D, Valledor M. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care. 2008;12:R158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 357] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 96. | Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity. 2014;40:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 456] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 97. | Yan GT, Lin J, Hao XH, Xue H, Zhang K, Wang LH. Heart-type fatty acid-binding protein is a useful marker for organ dysfunction and leptin alleviates sepsis-induced organ injuries by restraining its tissue levels. Eur J Pharmacol. 2009;616:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Kakoti A, Goswami P. Heart type fatty acid binding protein: structure, function and biosensing applications for early detection of myocardial infarction. Biosens Bioelectron. 2013;43:400-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Ono T. Studies of the FABP family: a retrospective. Mol Cell Biochem. 2005;277:1-6. [PubMed] |
| 100. | Wang Q, Li H, Liu S, Wang G, Wang Y. Cloning and tissue expression of chicken heart fatty acid-binding protein and intestine fatty acid-binding protein genes. Anim Biotechnol. 2005;16:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Chen YX, Li CS. The prognostic and risk-stratified value of heart-type fatty acid-binding protein in septic patients in the emergency department. J Crit Care. 2014;29:512-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 102. | Jo YH, Kim K, Lee JH, Rhee JE, Lee JH, Kang KW, Rim KP, Hwang SS, Park HM. Heart-type fatty acid-binding protein as a prognostic factor in patients with severe sepsis and septic shock. Am J Emerg Med. 2012;30:1749-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 103. | Zhang ZC, Dai HW, Yu YH, Yang JD, Hu CB. Usefulness of heart-type fatty acid-binding protein in patients with severe sepsis. J Crit Care. 2012;27:415.e13-415.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Brivet FG, Jacobs FM, Colin P, Prat D, Grigoriu B. Cardiac troponin level is not an independent predictor of mortality in septic patients requiring medical intensive care unit admission. Crit Care. 2006;10:404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 105. | Kalla C, Raveh D, Algur N, Rudensky B, Yinnon AM, Balkin J. Incidence and significance of a positive troponin test in bacteremic patients without acute coronary syndrome. Am J Med. 2008;121:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 106. | Rubattu S, Sciarretta S, Valenti V, Stanzione R, Volpe M. Natriuretic peptides: an update on bioactivity, potential therapeutic use, and implication in cardiovascular diseases. Am J Hypertens. 2008;21:733-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 107. | Perman SM, Chang AM, Hollander JE, Gaieski DF, Trzeciak S, Birkhahn R, Otero R, Osborn TM, Moretti E, Nguyen HB. Relationship between B-type natriuretic peptide and adverse outcome in patients with clinical evidence of sepsis presenting to the emergency department. Acad Emerg Med. 2011;18:219-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 108. | Turner KL, Moore LJ, Todd SR, Sucher JF, Jones SA, McKinley BA, Valdivia A, Sailors RM, Moore FA. Identification of cardiac dysfunction in sepsis with B-type natriuretic peptide. J Am Coll Surg. 2011;213:139-146; discussion 146-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 109. | Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 954] [Cited by in RCA: 945] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 110. | Grassi A, Susca M, Ferri S, Gabusi E, D’Errico A, Farina G, Maccariello S, Zauli D, Bianchi FB, Ballardini G. Detection of the M30 neoepitope as a new tool to quantify liver apoptosis: timing and patterns of positivity on frozen and paraffin-embedded sections. Am J Clin Pathol. 2004;121:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 111. | Leers MP, Kölgen W, Björklund V, Bergman T, Tribbick G, Persson B, Björklund P, Ramaekers FC, Björklund B, Nap M. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 112. | Bantel H, Lügering A, Heidemann J, Volkmann X, Poremba C, Strassburg CP, Manns MP, Schulze-Osthoff K. Detection of apoptotic caspase activation in sera from patients with chronic HCV infection is associated with fibrotic liver injury. Hepatology. 2004;40:1078-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 113. | Kramer G, Erdal H, Mertens HJ, Nap M, Mauermann J, Steiner G, Marberger M, Bivén K, Shoshan MC, Linder S. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer Res. 2004;64:1751-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 227] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 114. | Luft T, Conzelmann M, Benner A, Rieger M, Hess M, Strohhaecker U, Görner M, Hegenbart U, Ho AD, Dreger P. Serum cytokeratin-18 fragments as quantitative markers of epithelial apoptosis in liver and intestinal graft-versus-host disease. Blood. 2007;110:4535-4542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 115. | Moore DJ, Greystoke A, Butt F, Wurthner J, Growcott J, Hughes A, Dive C. A pilot study assessing the prognostic value of CK18 and nDNA biomarkers in severe sepsis patients. Clin Drug Investig. 2012;32:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 116. | Hofer S, Brenner T, Bopp C, Steppan J, Lichtenstern C, Weitz J, Bruckner T, Martin E, Hoffmann U, Weigand MA. Cell death serum biomarkers are early predictors for survival in severe septic patients with hepatic dysfunction. Crit Care. 2009;13:R93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 117. | Roth GA, Krenn C, Brunner M, Moser B, Ploder M, Spittler A, Pelinka L, Sautner T, Wolner E, Boltz-Nitulescu G. Elevated serum levels of epithelial cell apoptosis-specific cytokeratin 18 neoepitope m30 in critically ill patients. Shock. 2004;22:218-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 118. | David S, Kümpers P, van Slyke P, Parikh SM. Mending leaky blood vessels: the angiopoietin-Tie2 pathway in sepsis. J Pharmacol Exp Ther. 2013;345:2-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 119. | Ricciuto DR, dos Santos CC, Hawkes M, Toltl LJ, Conroy AL, Rajwans N, Lafferty EI, Cook DJ, Fox-Robichaud A, Kahnamoui K. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med. 2011;39:702-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 120. | David S, Mukherjee A, Ghosh CC, Yano M, Khankin EV, Wenger JB, Karumanchi SA, Shapiro NI, Parikh SM. Angiopoietin-2 may contribute to multiple organ dysfunction and death in sepsis*. Crit Care Med. 2012;40:3034-3041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 121. | Giuliano JS, Tran K, Li FY, Shabanova V, Tala JA, Bhandari V. The temporal kinetics of circulating angiopoietin levels in children with sepsis. Pediatr Crit Care Med. 2014;15:e1-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 122. | Wang K, Bhandari V, Giuliano JS, O Hern CS, Shattuck MD, Kirby M. Angiopoietin-1, angiopoietin-2 and bicarbonate as diagnostic biomarkers in children with severe sepsis. PLoS One. 2014;9:e108461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 123. | Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51:1823-1829. [PubMed] |
| 124. | Ueda S, Nishio K, Minamino N, Kubo A, Akai Y, Kangawa K, Matsuo H, Fujimura Y, Yoshioka A, Masui K. Increased plasma levels of adrenomedullin in patients with systemic inflammatory response syndrome. Am J Respir Crit Care Med. 1999;160:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 125. | Hirata Y, Mitaka C, Sato K, Nagura T, Tsunoda Y, Amaha K, Marumo F. Increased circulating adrenomedullin, a novel vasodilatory peptide, in sepsis. J Clin Endocrinol Metab. 1996;81:1449-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 126. | Meeran K, O’Shea D, Upton PD, Small CJ, Ghatei MA, Byfield PH, Bloom SR. Circulating adrenomedullin does not regulate systemic blood pressure but increases plasma prolactin after intravenous infusion in humans: a pharmacokinetic study. J Clin Endocrinol Metab. 1997;82:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 127. | Goode KM, Nicholls R, Pellicori P, Clark AL, Cleland JG. The in vitro stability of novel cardiovascular and sepsis biomarkers at ambient temperature. Clin Chem Lab Med. 2014;52:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 128. | Suberviola B, Castellanos-Ortega A, Ruiz Ruiz A, Lopez-Hoyos M, Santibañez M. Hospital mortality prognostication in sepsis using the new biomarkers suPAR and proADM in a single determination on ICU admission. Intensive Care Med. 2013;39:1945-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 129. | Suberviola B, Castellanos-Ortega A, Llorca J, Ortiz F, Iglesias D, Prieto B. Prognostic value of proadrenomedullin in severe sepsis and septic shock patients with community-acquired pneumonia. Swiss Med Wkly. 2012;142:w13542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 130. | Akpinar S, Rollas K, Alagöz A, Seğmen F, Sipit T. Performance evaluation of MR-proadrenomedullin and other scoring systems in severe sepsis with pneumonia. J Thorac Dis. 2014;6:921-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 131. | Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765-3777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 821] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 132. | Lassalle P, Molet S, Janin A, Heyden JV, Tavernier J, Fiers W, Devos R, Tonnel AB. ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem. 1996;271:20458-20464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 308] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 133. | Abid MR, Yi X, Yano K, Shih SC, Aird WC. Vascular endocan is preferentially expressed in tumor endothelium. Microvasc Res. 2006;72:136-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 134. | Aitkenhead M, Wang SJ, Nakatsu MN, Mestas J, Heard C, Hughes CC. Identification of endothelial cell genes expressed in an in vitro model of angiogenesis: induction of ESM-1, (beta)ig-h3, and NrCAM. Microvasc Res. 2002;63:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 135. | Grigoriu BD, Depontieu F, Scherpereel A, Gourcerol D, Devos P, Ouatas T, Lafitte JJ, Copin MC, Tonnel AB, Lassalle P. Endocan expression and relationship with survival in human non-small cell lung cancer. Clin Cancer Res. 2006;12:4575-4582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 136. | Rennel E, Mellberg S, Dimberg A, Petersson L, Botling J, Ameur A, Westholm JO, Komorowski J, Lassalle P, Cross MJ. Endocan is a VEGF-A and PI3K regulated gene with increased expression in human renal cancer. Exp Cell Res. 2007;313:1285-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 137. | Scherpereel A, Gentina T, Grigoriu B, Sénéchal S, Janin A, Tsicopoulos A, Plénat F, Béchard D, Tonnel AB, Lassalle P. Overexpression of endocan induces tumor formation. Cancer Res. 2003;63:6084-6089. [PubMed] |
| 138. | Mihajlovic DM, Lendak DF, Brkic SV, Draskovic BG, Mitic GP, Novakov Mikic AS, Cebovic TN. Endocan is useful biomarker of survival and severity in sepsis. Microvasc Res. 2014;93:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 139. | Tang L, Zhao Y, Wang D, Deng W, Li C, Li Q, Huang S, Shu C. Endocan levels in peripheral blood predict outcomes of acute respiratory distress syndrome. Mediators Inflamm. 2014;2014:625180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 140. | Laupland KB, Shahpori R, Kirkpatrick AW, Ross T, Gregson DB, Stelfox HT. Occurrence and outcome of fever in critically ill adults. Crit Care Med. 2008;36:1531-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 141. | Seigel TA, Cocchi MN, Salciccioli J, Shapiro NI, Howell M, Tang A, Donnino MW. Inadequacy of temperature and white blood cell count in predicting bacteremia in patients with suspected infection. J Emerg Med. 2012;42:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 142. | Drewry AM, Fuller BM, Bailey TC, Hotchkiss RS. Body temperature patterns as a predictor of hospital-acquired sepsis in afebrile adult intensive care unit patients: a case-control study. Crit Care. 2013;17:R200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 143. | Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2722] [Cited by in RCA: 2956] [Article Influence: 118.2] [Reference Citation Analysis (0)] |
| 144. | Godin PJ, Fleisher LA, Eidsath A, Vandivier RW, Preas HL, Banks SM, Buchman TG, Suffredini AF. Experimental human endotoxemia increases cardiac regularity: results from a prospective, randomized, crossover trial. Crit Care Med. 1996;24:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 157] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 145. | Barnaby D, Ferrick K, Kaplan DT, Shah S, Bijur P, Gallagher EJ. Heart rate variability in emergency department patients with sepsis. Acad Emerg Med. 2002;9:661-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 146. | Korach M, Sharshar T, Jarrin I, Fouillot JP, Raphaël JC, Gajdos P, Annane D. Cardiac variability in critically ill adults: influence of sepsis. Crit Care Med. 2001;29:1380-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 147. | Ahmad S, Ramsay T, Huebsch L, Flanagan S, McDiarmid S, Batkin I, McIntyre L, Sundaresan SR, Maziak DE, Shamji FM. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS One. 2009;4:e6642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 148. | Chen WL, Kuo CD. Characteristics of heart rate variability can predict impending septic shock in emergency department patients with sepsis. Acad Emerg Med. 2007;14:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 149. | De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 960] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 150. | Arnold RC, Dellinger RP, Parrillo JE, Chansky ME, Lotano VE, McCoy JV, Jones AE, Shapiro NI, Hollenberg SM, Trzeciak S. Discordance between microcirculatory alterations and arterial pressure in patients with hemodynamic instability. J Crit Care. 2012;27:531.e1-531.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 151. | De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J. 2004;147:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 310] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 152. | Spanos A, Jhanji S, Vivian-Smith A, Harris T, Pearse RM. Early microvascular changes in sepsis and severe sepsis. Shock. 2010;33:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 153. | Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, Arnold RC, Colilla S, Zanotti S, Hollenberg SM. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007;49:88-98, 98.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 434] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 154. | Shapiro NI, Angus DC. A review of therapeutic attempts to recruit the microcirculation in patients with sepsis. Minerva Anestesiol. 2014;80:225-235. [PubMed] |
| 155. | Sallisalmi M, Oksala N, Pettilä V, Tenhunen J. Evaluation of sublingual microcirculatory blood flow in the critically ill. Acta Anaesthesiol Scand. 2012;56:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 156. | Ganter MT, Hofer CK. Coagulation monitoring: current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth Analg. 2008;106:1366-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 475] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 157. | Adamzik M, Eggmann M, Frey UH, Görlinger K, Bröcker-Preuss M, Marggraf G, Saner F, Eggebrecht H, Peters J, Hartmann M. Comparison of thromboelastometry with procalcitonin, interleukin 6, and C-reactive protein as diagnostic tests for severe sepsis in critically ill adults. Crit Care. 2010;14:R178. [PubMed] |
| 158. | Brenner T, Schmidt K, Delang M, Mehrabi A, Bruckner T, Lichtenstern C, Martin E, Weigand MA, Hofer S. Viscoelastic and aggregometric point-of-care testing in patients with septic shock - cross-links between inflammation and haemostasis. Acta Anaesthesiol Scand. 2012;56:1277-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 159. | Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5802] [Cited by in RCA: 5984] [Article Influence: 249.3] [Reference Citation Analysis (0)] |
| 160. | Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2315] [Cited by in RCA: 2542] [Article Influence: 211.8] [Reference Citation Analysis (0)] |
| 161. | Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644-1655. [PubMed] |
| 162. | Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1289] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 163. | Siegler BH, Weiterer S, Lichtenstern C, Stumpp D, Brenner T, Hofer S, Weigand MA, Uhle F. [Use of biomarkers in sepsis. Update and perspectives]. Anaesthesist. 2014;63:678-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 164. | Aneja R, Carcillo J. Differences between adult and pediatric septic shock. Minerva Anestesiol. 2011;77:986-992. [PubMed] |
| 165. | Brenner T, Fleming T, Uhle F, Silaff S, Schmitt F, Salgado E, Alexis U, Zimmermann S, Bruckner T, Martin E. Methylglyoxal as a new biomarker in patients with septic shock: an observational clinical study. Crit Care. 2014;18:683. [PubMed] |
| 166. | Pene F, Courtine E, Cariou A, Mira JP. Toward theragnostics. Crit Care Med. 2009;37:S50-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |