Published online Feb 20, 2015. doi: 10.5493/wjem.v5.i1.21
Peer-review started: August 14, 2014
First decision: September 28, 2014
Revised: October 30, 2014
Accepted: November 19, 2014
Article in press: November 19, 2014
Published online: February 20, 2015
Processing time: 160 Days and 3.7 Hours
Coronary artery disease is an event of atherosclerosis characterized by a chronic vascular inflammation. Risk factors like obesity, diabetes mellitus, hypertension, smoking, hypercholesterolemia and positive family history sometimes are not sufficiently adequate to the enhancement of cardiovascular risk assessment. In the past years numerous biomarkers, like C reactive protein, cytokines and adhesion molecules, have been observed to be related to adverse cardiovascular prognosis. Recently, several studies found an association among inflammatory biomarkers and cardiovascular diseases suggesting their utility to identify the risk of an acute ischemic event and the detection of vulnerable plaques. The emerging inflammatory markers are well divided for diagnosis and prognosis and plaque instability of coronary artery disease. Some of them, the lectin-like oxidized low density lipoprotein receptor-1 can be important both in diagnosis and in the evaluation of plaque instability, other are inserted in the above reported classification. The emerging inflammatory markers in acute-phase include amyloid A, fibrinogen and pentraxin 3 while myeloperoxidase, myeloid-related protein 8/14 and pregnancy-associated plasma protein-A are recognize markers of plaque instability. Lastly, some studies demonstrated that circulating miRNAs are involved in coronary artery disease, acute myocardial infarction and heart failure.
Core tip: In this review we want to focus the reader’s attention on the differences between inflammatory markers of cardiovascular risk already accepted by the scientific community and the emerging markers in order to encourage the healthcare services to improve laboratory techniques in early diagnosis and more precise evaluation of the risk. Is also important to use a classification according to the stage where the patient is located regarding emerging inflammatory markers for diagnosis, prognosis and plaque instability.
- Citation: Lubrano V, Balzan S. Consolidated and emerging inflammatory markers in coronary artery disease. World J Exp Med 2015; 5(1): 21-32
- URL: https://www.wjgnet.com/2220-315X/full/v5/i1/21.htm
- DOI: https://dx.doi.org/10.5493/wjem.v5.i1.21
Atherosclerosis is largely recognized as a chronic inflam-matory disorder caused by vascular and extravascular factors[1,2] and coronary artery disease (CAD) is its common manifestation. CAD could result in the development of acute coronary syndrome (ACS), which is often associated with breakage of an atherosclerotic plaque and partial or complete thrombosis of the related artery.
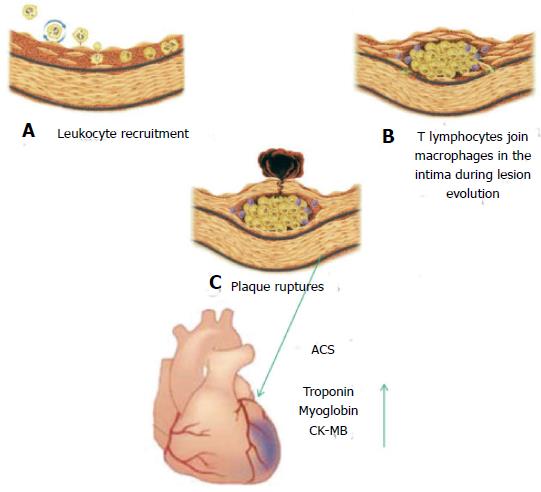
In these years a large number of studies permitted a better knowledge of the events implicated in the progression of ACS: here we summarize them (Figure 1). In these processes there is a recruitment of macrophages, that secretes lytic enzymes such as metalloproteinases. The atheroma core is constituted by foam cells and extracellular lipids shrouded by of smooth-muscle cells and collagen matrix. Plaque ruptures release adhesion molecules and soluble factors, such as D-dimers, von Willebrand factor and plasminogen activator inhibitor-1 that have an important role in thrombus formation. In a few hours after thrombus formation, but before the initiation of coronary ischemia, albumin is released. Troponin, myoglobin, and creatine kinase-MB are time-dependent release components associated with myocardial necrosis[3] (Figure 1). The extent of these events influences the circulating troponin level[4]. Therefore it is important to identify the fundamental steps leading to atherosclerotic plaque rupture.
Adequate risk assessment remains the most challenging in individuals classified into low or intermediate risk categories. Inflammation is important in the progression of atherosclerosis and in plaque rupture[1,5]. For this reason, numerous inflammatory markers have been extensively investigated as potential candidates for the enhancement of cardiovascular risk assessment.
Several recent studies have demonstrated the role of inflammation in mediating the stage of CAD, often caused by lipid accumulation.
Moreover the different part of atherogenesis could be related to inflammatory biomarkers that are important for clinical diagnosis, treatment and prognosis of patients with CAD. However, because conventional risk factors do not explain the changes in atherosclerosis, efforts have focused on developing novel biomarkers which identify vulnerable plaques and cardiovascular disease[6,7].
These new laboratory biomarkers should be standar-dized in variability, sensitivity and specificity from established risk markers. Finally, the cost of the assays has to be acceptable. In this review we analyze the inflammatory markers now considered valid in the stratification of risk for CAD and those emerging, checking if new ones can express something more than the standardized biomarkers.
C-reactive protein (CRP), a pentraxin composed of 5 subunits, is an inflammatory marker that may increase in various pathological situations, synthesized mainly in the liver, but it is also produced by leukocytes and adipocytes[8,9].
According considerable evidence, during infection or tissue necrosis circulating CRP may increase 50000 times, but it is also regarded as an independent variable of future cardiovascular events[10].
CRP fosters antigen presentation and phagocytosis attaching to phosphocholine that is usually found in cell membranes and polysaccharides in prokaryotes and fungi and binding to complement C1q complex and factor H[11,12].
Moreover it can attach low density lipoprotein (LDL), and be identify within the plaque[13] where it participates to inflammatory atherogenic processes[14]. CRP is elevated in patients with acute and chronic coronary syndromes in relation to the composition of the plaque[15,16] and is related to the complications of heart failure[17]. Low plasma levels of CRP indicate a good state of health[18], while increase when the style of life worsens. The MONICA Augsburg Study shows that low quality “Western” diet with low consumption of vegetables, fruit and fiber, extensive use of saturated fat, low physical activity and obesity, are associated with higher CRP levels[19].
Therefore, increment of CRP plasma concentration reflects not good lifestyle choices that lead to a metabolic disequilibrium and inflammation. The study of a large population has revealed that an increase in the levels of CRP (> 3 mg/L, elevated levels) was associated with mortality of 22962 subjects[20].
Ridker et al[21] showed that CRP was a better biomarker of cardiovascular diseases than LDL cholesterol. However when measured together, they give better prognostic detail than measured separately[21]. A large prospective study documented a strong association between CRP predictive power and the risks for coronary artery disease[22,23]. Moreover, the Canadian Cardiovascular Society suggested that CRP evaluation in patients at “intermediate risk”, could represent a predictive risk of a cardiovascular event from 10% to nearly 20% within the subsequent 10 years[24]. In agreement with this observation, the National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines and the American College of Cardiology Foundation-AHA Task Force on Practice Guidelines affirmed that the evaluation of CRP levels was acceptable for patients at intermediate risk[25,26].
Another study regarding people at intermediate risk for a cardiovascular event showed that the values of CRP and fibrinogen could help to prevent one additional event over a period of 10 years for every 400 to 500 people screened.
Current knowledge, however, suggests that the CRP concentration might reflect the vulnerability of the atheromatous lesion and the prospect of plaque rupture[5,27,28]. The development of high-sensitivity CRP (hs-CRP) assays has been useful to investigate its role in predicting first cardiovascular events.
Interleukin 1 (IL-1), IL-6, IL-10, monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor alpha (TNF-α) are the main investigated cytokines among those which predict cardiovascular events involved in vascular inflammation and atherosclerosis[29,30]. IL-1 and IL-6, regulate CRP production via direct stimulation of the hepatocytes[31]: IL-6 may increase plaque instability modulating the expression of TNF-alpha, and MCP-1[32]. Elevated IL-6 levels in healthy men correlated with increased risk for future MI independently from hs-CRP[33]. According to some author IL-6 seems to be a marker more sensitive and specific than CRP in vascular inflammation and CRP studies show a weaker association with cardiovascular disease than cytokines[34,35]. In the Fragmin study (FRISC-II), IL-6 increment above 5 ng/L was related with a mortality from 6- to 12-mo without a relationship with troponin and hs-CRP[36].
Therefore, IL-6 plasma concentration results as an effective independent index of increased mortality in unstable CAD and characterizes subjects who advantages of an initial invasive strategy. In addition, the intensity of plaque inflammation and its vulnerability seems to be linked with plasma IL-6 levels[36].
Some studies showed that IL-1 could have a regulatory function in the atherosclerotic development suggesting its modulation in vascular smooth muscle cell mitogenesis[37,38], in leukocyte adherence to vascular wall[39,40], in LDL metabolism[41,42], in extracellular matrix proteins[43] and in vascular permeability[44]. Moreover, IL-1 has been found to suppress vascular contractility[45] and induce pro-coagulant activity[46].
Several years ago, increased levels of IL-1α and IL-1β were detected in human atherosclerotic plaque, suggesting their local synthesis[47]. Moreover, IL-1 protein has been detected in macrophages from damaged carotid arteries[48]. In the macrophages IL-1β secretion seems to be induced by the cholesterol crystals present into the plaque[49].
The presence of increased IL-1β plasma concentration in patients affected by unstable angina indicates its important role in the acute stage[50]. However, in mouse models conflicting roles have been reported for IL1β: on the one hand the absence of IL-1β is associated with a reduction of atherosclerotic severity[51], on the other hand, IL-1β inactivation seems to be related to atherosclerotic plaque stability[52].
TNF-α plays a role in myocardial dysfunction and remodeling after acute coronary events[53]. On behalf of this effect, the CARE study showed that TNF-levels increased in recurrent coronary events after a MI compared with controls[54].
The chemokine MCP-1 recruits monocytes into the arterial wall activating these cells to induce endothelial injury[55]. In addition, a positive correlation between MCP-1 levels and the extent of coronary atherosclerosis was found in the coronary circulation of patients with unstable angina[56,57]. Moreover, MCP-1 levels have been found to correlate with older age[58], hypertension[59], hypercholesterolemia[60], and kidney failure[61], while an inverse correlation has been observed with estrogen replacement[62] and HMG-CoA reductase inhibitor therapy[60]. In several studies with small number of subjects, plasma MCP-1 levels were highest among patients with acute coronary syndromes (ACS), intermediate with stable coronary disease, and lowest among healthy control subjects.
IL-10 is an important factor for its anti-atherogenic property. In fact patients with high IL-10 levels had a reduced mortality compared with those that have only elevated CRP[63].
In 158 patients affected by stable CAD, during a 7-year follow-up period, the multivariate analysis of 10 cytokines showed IL-8 as the only independent marker for cardiovascular diseases[64]. In summary, even if the results are still controversial, in our opinion among consolidated cytokine, IL-6 represents the best prognostic biomarker in CAD.
Although very broad, adhesion molecules (CAMs) may be regarded as inflammatory markers of cardiovascular risk. Soluble CAMs (ICAM-1, VCAM, P and E selectines) are released from the surface of the cell and reflect cellular activation[65]. CAMs induce the bind between leucocytes, platelets and vascular wall[66]. After the adherence to the endothelium, the leucocytes transmigrate into the arterial wall determining the first phase of atherosclerosis[66].
Several studies reported an association between the increase of plasma CAM concentration and the risk of cardiac events[34,67,68], but their role in CAD prognosis have not been established because their finding are still quite confused.
In patients with stable CAD, CAMs plasma concen-trations were measured and informations on cardiovascular events were collected for some years. Among CAMs, only VCAM-1 resulted independently significant with future cardiovascular events[69].
In agreement with this study, other authors observed that the concentrations of sVCAM-1 > 780 ng/mL and CRP > 3 mg/L corresponded to a sensitivity > 90% for predicting future events in patients affected by acutely ACS[70].
On the contrary, other studies did not confirm these findings for sVCAM-1; instead, they suggested that CRP and sICAM-1were useful for identifying the risk of a cardiac event in patients with unstable angina who underwent coronary stenting[71]. Finally, another prospective study showed that only P-selectin and cardiac troponin I, but not the other CAMs, were significantly higher among patients who had a serious cardiac event during the subsequent 3 mo[72].
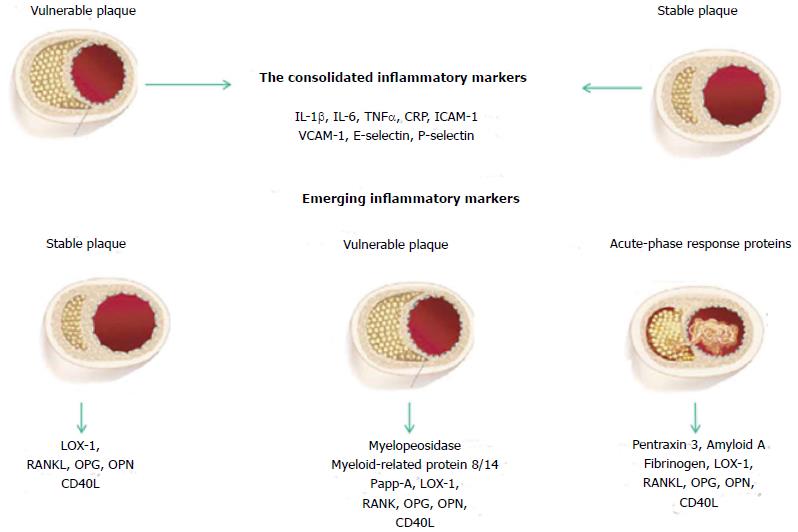
The lack of “traditional” risk factors cannot make totally free of the disease and new emerging markers of inflammation have been studied in the effort to identify biomarkers predicting the risk, and at the same time reflecting plaque instability in the early or in the acute phase. On the bases of these studies, we must point out that today is also in use a classification according to the stage where the patient is located (Figure 2).
Clinical studies have demonstrated that well-known coronary risk factors, including metabolic diseases, hypertension, obesity and smoking, are associated with oxidative stress. When the LDL are exposed to oxidative stress, they are caught in the vessel and oxidized (ox-LDL). Oxidized LDL promotes the synthesis of a large variety of cytokines and chemokines by the endothelium. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) appears to be an important receptor for ox-LDL in endothelial cells[73]. LOX-1 not only allows the passage of oxidized lipids in the cells, but as already described, may cause endothelial dysfunction/apoptosis, inflammation, and the increase smooth muscle cell number favoring the formation of atheroma[74-76].
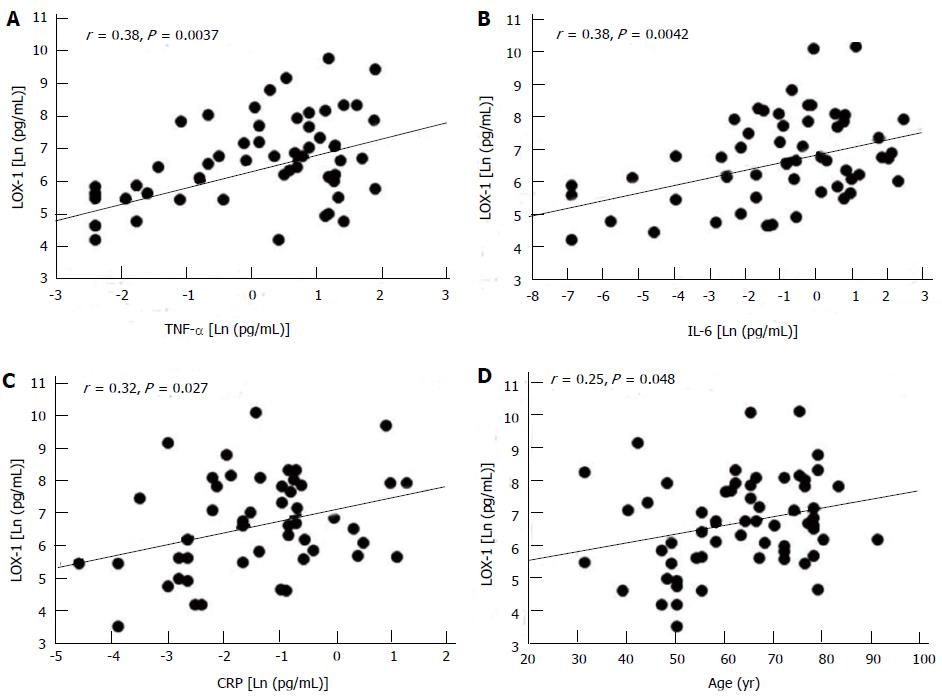
Moreover LOX-1 increment was observed to be associated with cardiovascular risk factors like hypertension and metabolic disorder. In a population of patients affected by CAD, our previous studies showed a positive relationship between circulating levels of LOX-1 and inflammatory markers: this work suggested also that LOX-1 levels increased with the severity of the disease[76] (Figure 3). Other authors underlined the importance of this novel biochemical marker for the stratification risk of the population and therapeutic strategy for CVD.
Overt cardiovascular disease is typically preceded by a long period of sub-clinical cardiovascular disease and sub-clinical atherosclerosis can be present for decades before the occurrence of a myocardial infarct event. Soluble LOX (sLOX-1) has shown to be informative either early or late in the process disease[77-79].
Recent study observed that the circulating levels of sLOX-1 are very high in acute coronary syndrome and that the plateau value is reached before troponin T, highlighting the instability of the plaque[80].
It has been reported that serum levels of sLOX-1 are also specifically elevated in acute coronary syndrome and the peak value has been reported to rise before troponin T[80]. In conclusion, sLOX-1 levels are related to the prognosis of acute coronary syndrome and reflect the instability of plaque[78].
Receptor activator of nuclear factor Kappa-B ligand (RANKL) is the ligand of the receptor inducer factor-κB (NF-κB) and belongs to the family of cytokines TNF-related. It is synthesized by T cells and stromal/osteoblastic cells and is a strong chemotactic factor for human monocytes[81]. RANKL-stimulated microvascular endothelial cells favor monocyte adhesion and trans endothelial migration thus increasing the recruitment of osteoclast- and osteoblast like cell precursor[81,82].
Osteoprotegerin (OPG) synthesized in osteoblasts is part of the TNF super-family. It binds to RANKL thereby preventing interaction with its transmembrane receptor[83].
RANKL and OPG have been shown to be potentially valuable markers for a better assessment of coronary calcification and cardiovascular risk associated with it. It has been observed that RANKL and OPG may play a key role in maturation and calcification of atherosclerotic plaque[84,85]. In fact these factors increased in serum of post-infarction of atherosclerotic animal models and of humans with unstable angina[86,87].
Osteocalcin, a protein found in bone and dentin and also synthetized in mononuclear cells, has been related with the severity of aortic calcification[88].
Osteopontin (OPN), an extracellular matrix protein and pro inflammatory cytokine, facilitates the recruitment of monocytes/macrophages through its adhesive domain[89] and promotes the inhibition of vascular calcification In fact it is increased in patients with vascular calcification resulting more like a marker than a mediator of atherosclerosis progression[90].
CD40, a member of TNF family, is also a stimulatory receptor on antigen-presenting cells of the immune system that induces inflammatory processes through the binding of the CD40 ligand (CD40L). Elevated levels of soluble CD40L (sCD40L) have been found in patients with hypercholesterolemia, ACS and cardiovascular disease. sCD40L is also associated with atherosclerosis and plaque instability[91]. In the CAPTURE trial, increased sCD40L (> 5 g/L) was related to a 6-mo mortality or nonfatal MI suggesting that sCD40L may be an independent risk marker of cardiovascular events. Statins, antihypertensive drugs, and antiplatelet agents have been shown to modulate it[92]. Moreover, sCD40L was found to be increased in smokers and positively associated with both total cholesterol and biomarkers of inflammation. However, it was not reported as an independent biomarker for the risk of MI[93].
Quantitative and qualitative changes of inflammatory markers are able to identify the acute stage of the disease. The plaque ruptures cause the consequent platelet aggregation and subsequent thrombosis, the final stage in which atherosclerosis leads to acute ischemic syndromes of AMI and sudden death[5].
The literature well documented the association between serum concentrations of acute phase proteins and the onset of coronary heart disease and myocardial infarction[94,95]. The emerging inflammatory markers in the acute-phase include pentraxin 3 (PTX3), amyloid A, and fibrinogen[96-102].
Pentraxins are a superfamily of soluble proteins with cyclic multimeric structure[103]. Among these, PTX3 a protein characterized by a long N-terminal domain, results as an important player in immunity and inflammation[104]. Dendritic cells, macrophages and endothelial cells produce PTX3 in response to IL-1 and TNF[105]. Moreover increased plasma PTX3 levels were observed in patients with cardiovascular disease and resulted also more closely related than CRP levels in acute phase of cardiac damages[106] suggesting that it could be a sensitive and specific prognostic indicator[107].
It is been also hypotheses an association of PTX3 in individuals with stable coronary artery disease and kidney dysfunction. However, an adjustment for the estimated glomerular filtration rate modestly attenuated these associations[108]. By immune histochemical staining PTX3 was strong expressed on the surface of lumen and within the atherosclerotic plaque in humans and animal models[96,97,109]. Moreover, in the same experimental models, soluble PTX3 increased in the early phase after ischemic heart events and PTX3 mRNA and protein expression enhanced in the ischemic area of the heart[110].
In a prospective study of patients with myocardial infarction and ST elevation, PTX3 predicted 3-mo mortality while other markers such as the liver-derived short pentraxin CRP or NT-proBNP, TnT, CK did not[107]. In patients with unstable angina pectoris within the six hours of the chest pain, PTX3 resulted to be more specific for ACS than neutrophil activating peptide-2 and cardiac troponin I (cTnI)[111,112].
Serum amyloid A (SAA) proteins are a family of apolipoproteins associated with high-density lipoprotein (HDL) and are now considered emerging markers of inflammation. In fact elevated SAA levels are present in coronary artery disease and indicate worse prognosis in CAD. Therefore actions involved to reduce SAA levels could improve the conditions of patients with acute CAD[113].
A study of Kosuge et al[99] reported that, in patients with ACS, increased SAA levels were associated with cardiovascular events within 30 d, without any relationship with CRP level. Therefore these data indicated SAA more useful predictor than CRP in these patients.
In the high serum SAA group the left ventricular ejection fraction, measured during follow-up, was significantly lower than in the low serum SAA group and more frequent complications, such as cardiac rupture, carcinogenic shock, subacute thrombosis, and cardiac death, were also present[100].
Furthermore SAA levels were quite well associated with coronary artery disease with a predictive risk for cardiovascular events within 3 years, while this did not happen with hs-CRP[114]. In a substudy of TIMI 11A, elevated SAA levels predicted increased risk of 14-d mortality in patients with ACS[115]. In a Women Ischemia Syndrome Evaluation study, in which women were referred for coronary angiography because of suspected ischemia, elevated SAA values were correlated with angiographic severity of CAD and 3-year risk for cardiovascular events[114]. At the same time, no relationship was observed between SAA levels and recurrent Coronary Events[116].
Several studies have indicated fibrinogen as a predictive marker in CAD[117]. Fibrinogen is involved in platelet aggregation, endothelial injury, plasma viscosity and play a central role in the formation of thrombus.
Epidemiological data have shown the important predictive role of fibrinogen in CAD, identifying it as an emerging risk factor because its measurement may improve the estimation of absolute risk obtained by conventional risk factor for CV[117].
Although it is still discussed the role of fibrinogen as inflammatory markers of risk, many studies indicated an association of hyperfibrinogenemia with atherotrhombosis.
Already in the past, some authors have demonstrated that the risk estimation for CAD could be double when fibrogenemia was also evaluated[118].
Emerging Risk Factors Collaboration showed that, the measurement of fibrinogen level in patients at risk for CAD, could prevent an additional event in the next 10 years for every 400-500 people studied[119].
However, also recent results show that the evaluation of fibrinogen during MI may be useful in identifying patients at high risks for future acute events[102].
The main cause of the acute myocardial infarction (AMI) is the plaque rupture, so that it is important to investigate new markers for early diagnosis of plaque instability.
Due to its sensitivity and specificity, troponin is commonly used in the diagnosis of ACS, even if it provides only indirect details on myocardial necrosis induced by embolization of atherothrombotic material, late event of ACS.
Inflammation is a process that is intensified in plaque instability, so that the markers of inflammation may provide indications of cellular processes related to its formation before it occurs myocardial necrosis[120].
Myeloperoxidase (MPO) is an enzyme produced by leukocytes that induces the formation of oxygen free radicals and is considered to be one major contributor in the formation and rupture of the plaque[121].
In patients with ACS, MPO produced by neutrophils, is considered a marker of plaque vulnerability as noted by several studies[122,123].
Yunoki et al[124], 2013 observed that the plasma levels of MPO have a significant inverse correlation with levels of paraoxonase-1 bound to HDL, especially, in patients with stable and unstable angina pectoris, suggesting that a mismatch between pro oxidants and anti-oxidants may contribute to the progression of coronary plaque instability[124].
Myeloid-related protein 8/14 (MRP8/14), is a heterodimer consisting of two proteins that bind calcium, calgranulin A and B, which play an important role in the signaling pathways of calcium, in cell cycle progression, cell differentiation, and in the interaction between the cytoskeleton and membrane[125]. MRP-8/14, also called calprotectin, is synthesized by activated monocytes and neutrophils, and is a pro-inflammatory protein expressed in atherosclerotic plaques.
High concentrations of MRP8/14 in the systemic circulation may reveal the presence of plaques before necrosis markers suggesting it as a good candidate for the management of ACS unstable.
PAPP-A is a high-molecular-weight zinc-binding metalloproteinase. PAPP-A was independently associated with recurrent cardiovascular events in patients with ACS. This finding supported the potential usefulness of PAPP-A as a biomarker in patients with ACS[126]. Moreover as described by Mahto et al[127] PAPP-A is the reliable marker which can discriminate the cases of MI from unstable angina and controls[127]. Another study has suggested PAPP-A to be a predictor of mortality or myocardial infarction in patients with ACS[128].
MicroRNAs (miRNAs) are short non-coding RNA molecules that regulate gene expression post-transcriptionally through suppression or degradation of target messenger RNA (mRNA).
MiRNAs were found in the circulating blood and are differently induced in patients with CAD, AMI, and heart failure[129-132].
Of interest is miR-155, which proved to be a new component of inflammatory signal transduction pathways in the pathogenesis of atherosclerosis. In fact the expression of miR-155 is considered to be a prospective marker for predicting the prognosis of CAD since it is found to be expressed mainly in patients with CAD compared to healthy subjects[133].
Inflammatory biomarkers appear to have an important prognostic value in patients with cardiovascular disease and may be useful in the diagnosis of apparently healthy subjects without known CAD who cannot be assessed with conventional risk factors.
Inflammatory biomarkers may have prognostic value for future cardiovascular risk among those at high risk or with documented cardiovascular disease. They also may be useful for identifying apparently healthy individuals, without known CAD, who may be at a higher risk than estimated by traditional risk factors.
Although recent data demonstrate that there is a close association between inflammatory biomarkers and coronary artery disease, further studies must be carried out taking into account also some important criteria typically used in the selection of a new biomarker: discrimination, calibration and reclassification, i.e. the ability of a test to discern between those that will face the disease from those that will be free, the assessment of the risk factor predicted and observed, classification in categories of low, intermediate and high risk for CAD[134].
In our opinion the best candidate for this role is LOX-1; it was observed to be associated with cardiovascular risk factors like hypertension and metabolic disorder, showing its positive relationship with inflammatory markers and its increment with the severity of the disease[76] (Figure 3). Moreover it was elevated in acute coronary syndrome and the peak value has been reported to rise before troponin T reflecting the instability of plaque[80].
In conclusion, the findings observed in a decade showed that LOX-1 could represent an important marker for clinical characterization of coronary artery disease and a target for new drugs to reduce its expression and production.
The authors are grateful to Lucrecia Mota Garcia for her English editing support and to Alison Frank for the final English revision.
P- Reviewer: Dong L, Lira FS S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 2. | Rader DJ. Inflammatory markers of coronary risk. N Engl J Med. 2000;343:1179-1182. [PubMed] |
| 3. | Morrow DA, Braunwald E. Future of biomarkers in acute coronary syndromes: moving toward a multimarker strategy. Circulation. 2003;108:250-252. [PubMed] |
| 4. | Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992;326:242-250. [PubMed] |
| 5. | Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115-126. [PubMed] |
| 6. | Panteghini M. Role and importance of biochemical markers in clinical cardiology. Eur Heart J. 2004;25:1187-1196. [PubMed] |
| 7. | Marian AJ, Nambi V. Biomarkers of cardiac disease. Expert Rev Mol Diagn. 2004;4:805-820. [PubMed] |
| 8. | Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031-H2041. [PubMed] |
| 9. | Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805-1812. [PubMed] |
| 10. | Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448-454. [PubMed] |
| 11. | Okemefuna AI, Nan R, Miller A, Gor J, Perkins SJ. Complement factor H binds at two independent sites to C-reactive protein in acute phase concentrations. J Biol Chem. 2010;285:1053-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Peisajovich A, Marnell L, Mold C, Du Clos TW. C-reactive protein at the interface between innate immunity and inflammation. Expert Rev Clin Immunol. 2008;4:379-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | de Beer FC, Soutar AK, Baltz ML, Trayner IM, Feinstein A, Pepys MB. Low density lipoprotein and very low density lipoprotein are selectively bound by aggregated C-reactive protein. J Exp Med. 1982;156:230-242. [PubMed] |
| 14. | Jin C, Lu L, Zhang RY, Zhang Q, Ding FH, Chen QJ, Shen WF. Association of serum glycated albumin, C-reactive protein and ICAM-1 levels with diffuse coronary artery disease in patients with type 2 diabetes mellitus. Clin Chim Acta. 2009;408:45-49. [PubMed] |
| 15. | Ridker PM. C-reactive protein: eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clin Chem. 2009;55:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Otake H, Shite J, Shinke T, Watanabe S, Tanino Y, Ogasawara D, Sawada T, Hirata K, Yokoyama M. Relation between plasma adiponectin, high-sensitivity C-reactive protein, and coronary plaque components in patients with acute coronary syndrome. Am J Cardiol. 2008;101:1-7. [PubMed] |
| 17. | Scirica BM, Morrow DA, Cannon CP, de Lemos JA, Murphy S, Sabatine MS, Wiviott SD, Rifai N, McCabe CH, Braunwald E. Clinical application of C-reactive protein across the spectrum of acute coronary syndromes. Clin Chem. 2007;53:1800-1807. [PubMed] |
| 18. | Kao PC, Shiesh SC, Wu TJ. Serum C-reactive protein as a marker for wellness assessment. Ann Clin Lab Sci. 2006;36:163-169. [PubMed] |
| 19. | Koenig W, Sund M, Fröhlich M, Fischer HG, Löwel H, Döring A, Hutchinson WL, Pepys MB. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237-242. [PubMed] |
| 20. | Currie CJ, Poole CD, Conway P. Evaluation of the association between the first observation and the longitudinal change in C-reactive protein, and all-cause mortality. Heart. 2008;94:457-462. [PubMed] |
| 21. | Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557-1565. [PubMed] |
| 22. | Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387-1397. [PubMed] |
| 23. | Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326-333. [PubMed] |
| 24. | Genest J, McPherson R, Frohlich J, Anderson T, Campbell N, Carpentier A, Couture P, Dufour R, Fodor G, Francis GA. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. Can J Cardiol. 2009;25:567-579. [PubMed] |
| 25. | Myers GL, Christenson RH, Cushman M, Ballantyne CM, Cooper GR, Pfeiffer CM, Grundy SM, Labarthe DR, Levy D, Rifai N. National Academy of Clinical Biochemistry Laboratory Medicine Practice guidelines: emerging biomarkers for primary prevention of cardiovascular disease. Clin Chem. 2009;55:378-384. [PubMed] |
| 26. | Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50-103. [PubMed] |
| 27. | Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844-2850. [PubMed] |
| 28. | Maseri A. Inflammation, atherosclerosis, and ischemic events -- exploring the hidden side of the moon. N Engl J Med. 1997;336:1014-1016. [PubMed] |
| 29. | Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992;6:2591-2599. [PubMed] |
| 30. | Rus HG, Vlaicu R, Niculescu F. Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis. 1996;127:263-271. [PubMed] |
| 31. | Baumann H, Gauldie J. Regulation of hepatic acute phase plasma protein genes by hepatocyte stimulating factors and other mediators of inflammation. Mol Biol Med. 1990;7:147-159. [PubMed] |
| 32. | Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, Nussberger J, Harringer W, Drexler H. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation. 2000;101:1372-1378. [PubMed] |
| 33. | Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767-1772. [PubMed] |
| 34. | Lubrano V, Cocci F, Battaglia D, Papa A, Marraccini P, Zucchelli GC. Usefulness of high-sensitivity IL-6 measurement for clinical characterization of patients with coronary artery disease. J Clin Lab Anal. 2005;19:110-114. [PubMed] |
| 35. | Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study). Am J Cardiol. 2003;92:522-528. [PubMed] |
| 36. | Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. JAMA. 2001;286:2107-2113. [PubMed] |
| 37. | Libby P, Miao P, Ordovas JM, Schaefer EJ. Lipoproteins increase growth of mitogen-stimulated arterial smooth muscle cells. J Cell Physiol. 1985;124:1-8. [PubMed] |
| 38. | Bonin PD, Fici GJ, Singh JP. Interleukin-1 promotes proliferation of vascular smooth muscle cells in coordination with PDGF or a monocyte derived growth factor. Exp Cell Res. 1989;181:475-482. [PubMed] |
| 39. | Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985;76:2003-2011. [PubMed] |
| 40. | Schleimer RP, Rutledge BK. Cultured human vascular endothelial cells acquire adhesiveness for neutrophils after stimulation with interleukin 1, endotoxin, and tumor-promoting phorbol diesters. J Immunol. 1986;136:649-654. [PubMed] |
| 41. | Rasmussen LT, Seljelid R. The modulatory effect of lipoproteins on the release of interleukin 1 by human peritoneal macrophages stimulated with beta-1,3-D-polyglucose derivatives. Scand J Immunol. 1989;29:477-484. [PubMed] |
| 42. | Haga Y, Takata K, Araki N, Sakamoto K, Akagi M, Morino Y, Horiuchi S. Intracellular accumulation of cholesteryl esters suppresses production of lipopolysaccharide-induced interleukin 1 by rat peritoneal macrophages. Biochem Biophys Res Commun. 1989;160:874-880. [PubMed] |
| 43. | Montesano R, Mossaz A, Ryser JE, Orci L, Vassalli P. Leukocyte interleukins induce cultured endothelial cells to produce a highly organized, glycosaminoglycan-rich pericellular matrix. J Cell Biol. 1984;99:1706-1715. [PubMed] |
| 44. | Martin S, Maruta K, Burkart V, Gillis S, Kolb H. IL-1 and IFN-gamma increase vascular permeability. Immunology. 1988;64:301-305. [PubMed] |
| 45. | McKenna TM, Reusch DW, Simpkins CO. Macrophage-conditioned medium and interleukin 1 suppress vascular contractility. Circ Shock. 1988;25:187-196. [PubMed] |
| 46. | Bevilacqua MP, Pober JS, Majeau GR, Fiers W, Cotran RS, Gimbrone MA. Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci USA. 1986;83:4533-4537. [PubMed] |
| 47. | Wang AM, Doyle MV, Mark DF. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci USA. 1989;86:9717-9721. [PubMed] |
| 48. | Tipping PG, Hancock WW. Production of tumor necrosis factor and interleukin-1 by macrophages from human atheromatous plaques. Am J Pathol. 1993;142:1721-1728. [PubMed] |
| 49. | Rajamäki K, Lappalainen J, Oörni K, Välimäki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. [PubMed] |
| 50. | Simon AD, Yazdani S, Wang W, Schwartz A, Rabbani LE. Circulating levels of IL-1beta, a prothrombotic cytokine, are elevated in unstable angina versus stable angina. J Thromb Thrombolysis. 2000;9:217-222. [PubMed] |
| 51. | Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656-660. [PubMed] |
| 52. | Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149-2153. [PubMed] |
| 53. | Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543-1553. [PubMed] |
| 54. | Ridker PM, Lüscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35:1782-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 452] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 55. | Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858-866. [PubMed] |
| 56. | Serrano-Martínez M, Palacios M, Lezaun R. Monocyte chemoattractant protein-1 concentration in coronary sinus blood and severity of coronary disease. Circulation. 2003;108:e75. [PubMed] |
| 57. | de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690-695. [PubMed] |
| 58. | Inadera H, Egashira K, Takemoto M, Ouchi Y, Matsushima K. Increase in circulating levels of monocyte chemoattractant protein-1 with aging. J Interferon Cytokine Res. 1999;19:1179-1182. [PubMed] |
| 59. | Parissis JT, Venetsanou KF, Kalantzi MV, Mentzikof DD, Karas SM. Serum profiles of granulocyte-macrophage colony-stimulating factor and C-C chemokines in hypertensive patients with or without significant hyperlipidemia. Am J Cardiol. 2000;85:777-79, A9. [PubMed] |
| 60. | Garlichs CD, John S, Schmeisser A, Eskafi S, Stumpf C, Karl M, Goppelt-Struebe M, Schmieder R, Daniel WG. Upregulation of CD40 and CD40 ligand (CD154) in patients with moderate hypercholesterolemia. Circulation. 2001;104:2395-2400. [PubMed] |
| 61. | Papayianni A, Alexopoulos E, Giamalis P, Gionanlis L, Belechri AM, Koukoudis P, Memmos D. Circulating levels of ICAM-1, VCAM-1, and MCP-1 are increased in haemodialysis patients: association with inflammation, dyslipidaemia, and vascular events. Nephrol Dial Transplant. 2002;17:435-441. [PubMed] |
| 62. | Störk S, Baumann K, von Schacky C, Angerer P. The effect of 17 beta-estradiol on MCP-1 serum levels in postmenopausal women. Cardiovasc Res. 2002;53:642-649. [PubMed] |
| 63. | Heeschen C, Dimmeler S, Hamm CW, Fichtlscherer S, Boersma E, Simoons ML, Zeiher AM. Serum level of the antiinflammatory cytokine interleukin-10 is an important prognostic determinant in patients with acute coronary syndromes. Circulation. 2003;107:2109-2114. [PubMed] |
| 64. | Inoue T, Komoda H, Nonaka M, Kameda M, Uchida T, Node K. Interleukin-8 as an independent predictor of long-term clinical outcome in patients with coronary artery disease. Int J Cardiol. 2008;124:319-325. [PubMed] |
| 65. | Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191-203. [PubMed] |
| 66. | Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842-851. [PubMed] |
| 67. | Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001;103:491-495. [PubMed] |
| 68. | Mulvihill NT, Foley JB, Murphy R, Crean P, Walsh M. Evidence of prolonged inflammation in unstable angina and non-Q wave myocardial infarction. J Am Coll Cardiol. 2000;36:1210-1216. [PubMed] |
| 69. | Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, Meyer J. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104:1336-1342. [PubMed] |
| 70. | Mulvihill NT, Foley JB, Murphy RT, Curtin R, Crean PA, Walsh M. Risk stratification in unstable angina and non-Q wave myocardial infarction using soluble cell adhesion molecules. Heart. 2001;85:623-627. [PubMed] |
| 71. | Doo YC, Han SJ, Park WJ, Kim SM, Choi SH, Cho GY, Hong KS, Han KR, Lee NH, Oh DJ. Associations between C-reactive protein and circulating cell adhesion molecules in patients with unstable angina undergoing coronary intervention and their clinical implication. Clin Cardiol. 2005;28:47-51. [PubMed] |
| 72. | Hillis GS, Terregino C, Taggart P, Killian A, Zhao N, Dalsey WC, Mangione A. Elevated soluble P-selectin levels are associated with an increased risk of early adverse events in patients with presumed myocardial ischemia. Am Heart J. 2002;143:235-241. [PubMed] |
| 73. | Murase T, Kume N, Kataoka H, Minami M, Sawamura T, Masaki T, Kita T. Identification of soluble forms of lectin-like oxidized LDL receptor-1. Arterioscler Thromb Vasc Biol. 2000;20:715-720. [PubMed] |
| 74. | Inoue N, Sawamura T. Lectin-like oxidized LDL receptor-1 as extracellular chaperone receptor: its versatile functions and human diseases. Methods. 2007;43:218-222. [PubMed] |
| 75. | Li D, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101:2889-2895. [PubMed] |
| 76. | Lubrano V, Del Turco S, Nicolini G, Di Cecco P, Basta G. Circulating levels of lectin-like oxidized low-density lipoprotein receptor-1 are associated with inflammatory markers. Lipids. 2008;43:945-950. [PubMed] |
| 77. | Inoue N, Okamura T, Kokubo Y, Fujita Y, Sato Y, Nakanishi M, Yanagida K, Kakino A, Iwamoto S, Watanabe M. LOX index, a novel predictive biochemical marker for coronary heart disease and stroke. Clin Chem. 2010;56:550-558. [PubMed] |
| 78. | Kume N, Mitsuoka H, Hayashida K, Tanaka M, Kita T. Soluble lectin-like oxidized low-density lipoprotein receptor-1 predicts prognosis after acute coronary syndrome--a pilot study. Circ J. 2010;74:1399-1404. [PubMed] |
| 79. | Kamezaki F, Yamashita K, Tasaki H, Kume N, Mitsuoka H, Kita T, Adachi T, Otsuji Y. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 correlates with oxidative stress markers in stable coronary artery disease. Int J Cardiol. 2009;134:285-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Hayashida K, Kume N, Murase T, Minami M, Nakagawa D, Inada T, Tanaka M, Ueda A, Kominami G, Kambara H. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome: a novel marker for early diagnosis. Circulation. 2005;112:812-818. [PubMed] |
| 81. | Mosheimer BA, Kaneider NC, Feistritzer C, Sturn DH, Wiedermann CJ. Expression and function of RANK in human monocyte chemotaxis. Arthritis Rheum. 2004;50:2309-2316. [PubMed] |
| 82. | Kindle L, Rothe L, Kriss M, Osdoby P, Collin-Osdoby P. Human microvascular endothelial cell activation by IL-1 and TNF-alpha stimulates the adhesion and transendothelial migration of circulating human CD14+ monocytes that develop with RANKL into functional osteoclasts. J Bone Miner Res. 2006;21:193-206. [PubMed] |
| 83. | Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309-319. [PubMed] |
| 84. | Hansson GK, Libby P, Schönbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281-291. [PubMed] |
| 85. | Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938-2948. [PubMed] |
| 86. | Ueland T, Yndestad A, Øie E, Florholmen G, Halvorsen B, Frøland SS, Simonsen S, Christensen G, Gullestad L, Aukrust P. Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation. 2005;111:2461-2468. [PubMed] |
| 87. | Sandberg WJ, Yndestad A, Øie E, Smith C, Ueland T, Ovchinnikova O, Robertson AK, Müller F, Semb AG, Scholz H. Enhanced T-cell expression of RANK ligand in acute coronary syndrome: possible role in plaque destabilization. Arterioscler Thromb Vasc Biol. 2006;26:857-863. [PubMed] |
| 88. | Pal SN, Rush C, Parr A, Van Campenhout A, Golledge J. Osteocalcin positive mononuclear cells are associated with the severity of aortic calcification. Atherosclerosis. 2010;210:88-93. [PubMed] |
| 89. | Smith LL, Cheung HK, Ling LE, Chen J, Sheppard D, Pytela R, Giachelli CM. Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by alpha9beta1 integrin. J Biol Chem. 1996;271:28485-28491. [PubMed] |
| 90. | Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302-2309. [PubMed] |
| 91. | Schönbeck U, Libby P. CD40 signaling and plaque instability. Circ Res. 2001;89:1092-1103. [PubMed] |
| 92. | Varo N, de Lemos JA, Libby P, Morrow DA, Murphy SA, Nuzzo R, Gibson CM, Cannon CP, Braunwald E, Schönbeck U. Soluble CD40L: risk prediction after acute coronary syndromes. Circulation. 2003;108:1049-1052. [PubMed] |
| 93. | Jefferis BJ, Whincup PH, Welsh P, Wannamethee SG, Rumley A, Lawlor DA, Ebrahim S, Lowe GD. Prospective study of circulating soluble CD40 ligand concentrations and the incidence of cardiovascular disease in a nested prospective case-control study of older men and women. J Thromb Haemost. 2011;9:1452-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 94. | Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med. 2000;343:1139-1147. [PubMed] |
| 95. | Biasucci LM, Liuzzo G, Grillo RL, Caligiuri G, Rebuzzi AG, Buffon A, Summaria F, Ginnetti F, Fadda G, Maseri A. Elevated levels of C-reactive protein at discharge in patients with unstable angina predict recurrent instability. Circulation. 1999;99:855-860. [PubMed] |
| 96. | Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson GK. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:e10-e14. [PubMed] |
| 97. | Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F, Pizzetti F, Maggioni AP, Moccetti T, Metra M. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636-641. [PubMed] |
| 98. | Lee DH, Jeon HK, You JH, Park MY, Lee SJ, Kim SS, Shim BJ, Choi YS, Shin WS, Lee JM. Pentraxin 3 as a novel marker predicting congestive heart failure in subjects with acute coronary syndrome. Korean Circ J. 2010;40:370-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Kosuge M, Ebina T, Ishikawa T, Hibi K, Tsukahara K, Okuda J, Iwahashi N, Ozaki H, Yano H, Kusama I. Serum amyloid A is a better predictor of clinical outcomes than C-reactive protein in non-ST-segment elevation acute coronary syndromes. Circ J. 2007;71:186-190. [PubMed] |
| 100. | Katayama T, Nakashima H, Takagi C, Honda Y, Suzuki S, Iwasaki Y, Yano K. Prognostic value of serum amyloid A protein in patients with acute myocardial infarction. Circ J. 2005;69:1186-1191. [PubMed] |
| 101. | Reinhart WH. Fibrinogen--marker or mediator of vascular disease? Vasc Med. 2003;8:211-216. [PubMed] |
| 102. | Coppola G, Rizzo M, Abrignani MG, Corrado E, Di Girolamo A, Braschi A, Braschi G, Novo S. Fibrinogen as a predictor of mortality after acute myocardial infarction: a forty-two-month follow-up study. Ital Heart J. 2005;6:315-322. [PubMed] |
| 103. | Introna M, Alles VV, Castellano M, Picardi G, De Gioia L, Bottazzai B, Peri G, Breviario F, Salmona M, De Gregorio L. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996;87:1862-1872. [PubMed] |
| 104. | Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337-366. [PubMed] |
| 105. | Lee GW, Lee TH, Vilcek J. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J Immunol. 1993;150:1804-1812. [PubMed] |
| 106. | Norata GD, Garlanda C, Catapano AL. The long pentraxin PTX3: a modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc Med. 2010;20:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 107. | Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, Vago L, Pasqualini F, Signorini S, Soldateschi D. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349-2354. [PubMed] |
| 108. | Dubin R, Li Y, Ix JH, Shlipak MG, Whooley M, Peralta CA. Associations of pentraxin-3 with cardiovascular events, incident heart failure, and mortality among persons with coronary heart disease: data from the Heart and Soul Study. Am Heart J. 2012;163:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 109. | Norata GD, Marchesi P, Pulakazhi Venu VK, Pasqualini F, Anselmo A, Moalli F, Pizzitola I, Garlanda C, Mantovani A, Catapano AL. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 220] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 110. | Salio M, Chimenti S, De Angelis N, Molla F, Maina V, Nebuloni M, Pasqualini F, Latini R, Garlanda C, Mantovani A. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 276] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 111. | Ustündağ M, Orak M, Güloğlu C, Sayhan MB, Alyan O, Kale E. Comparative diagnostic accuracy of serum levels of neutrophil activating peptide-2 and pentraxin-3 versus troponin-I in acute coronary syndrome. Anadolu Kardiyol Derg. 2011;11:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 112. | Soeki T, Niki T, Kusunose K, Bando S, Hirata Y, Tomita N, Yamaguchi K, Koshiba K, Yagi S, Taketani Y. Elevated concentrations of pentraxin 3 are associated with coronary plaque vulnerability. J Cardiol. 2011;58:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 113. | Filep JG, El Kebir D. Serum amyloid A as a marker and mediator of acute coronary syndromes. Future Cardiol. 2008;4:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 114. | Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, Pepine CJ, Sharaf B, Bairey Merz CN, Sopko G. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:726-732. [PubMed] |
| 115. | Morrow DA, Rifai N, Antman EM, Weiner DL, McCabe CH, Cannon CP, Braunwald E. Serum amyloid A predicts early mortality in acute coronary syndromes: A TIMI 11A substudy. J Am Coll Cardiol. 2000;35:358-362. [PubMed] |
| 116. | Harb TS, Zareba W, Moss AJ, Ridker PM, Marder VJ, Rifai N, Miller Watelet LF, Arora R, Brown MW, Case RB. Association of C-reactive protein and serum amyloid A with recurrent coronary events in stable patients after healing of acute myocardial infarction. Am J Cardiol. 2002;89:216-221. [PubMed] |
| 117. | Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499-511. [PubMed] |
| 118. | Maresca G, Di Blasio A, Marchioli R, Di Minno G. Measuring plasma fibrinogen to predict stroke and myocardial infarction: an update. Arterioscler Thromb Vasc Biol. 1999;19:1368-1377. [PubMed] |
| 119. | Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 831] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 120. | Schäfer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21:134-140. [PubMed] |
| 121. | Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102-1111. [PubMed] |
| 122. | Schaub N, Reichlin T, Meune C, Twerenbold R, Haaf P, Hochholzer W, Niederhauser N, Bosshard P, Stelzig C, Freese M. Markers of plaque instability in the early diagnosis and risk stratification of acute myocardial infarction. Clin Chem. 2012;58:246-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 123. | Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Münzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440-1445. [PubMed] |
| 124. | Yunoki K, Naruko T, Inaba M, Inoue T, Nakagawa M, Sugioka K, Ohsawa M, Iwasa Y, Komatsu R, Itoh A. Gender-specific correlation between plasma myeloperoxidase levels and serum high-density lipoprotein-associated paraoxonase-1 levels in patients with stable and unstable coronary artery disease. Atherosclerosis. 2013;231:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 125. | Ionita MG, Vink A, Dijke IE, Laman JD, Peeters W, van der Kraak PH, Moll FL, de Vries JP, Pasterkamp G, de Kleijn DP. High levels of myeloid-related protein 14 in human atherosclerotic plaques correlate with the characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol. 2009;29:1220-1227. [PubMed] |
| 126. | Bonaca MP, Scirica BM, Sabatine MS, Jarolim P, Murphy SA, Chamberlin JS, Rhodes DW, Southwick PC, Braunwald E, Morrow DA. Prospective evaluation of pregnancy-associated plasma protein-a and outcomes in patients with acute coronary syndromes. J Am Coll Cardiol. 2012;60:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 127. | Mahto S, Sharma SB, Dwivedi S, Puri D, Tripathi RL. Biomarkers for early detection of risk in female patients with coronary artery disease: pilot study. J Assoc Physicians India. 2013;61:317-319. [PubMed] |
| 128. | Iversen KK, Dalsgaard M, Teisner AS, Schoos M, Teisner B, Nielsen H, Grande P, Clemmensen P. Pregnancy-associated plasma protein-A, a marker for outcome in patients suspected for acute coronary syndrome. Clin Biochem. 2010;43:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 129. | Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 955] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 130. | Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 907] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 131. | Adachi T, Nakanishi M, Otsuka Y, Nishimura K, Hirokawa G, Goto Y, Nonogi H, Iwai N. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem. 2010;56:1183-1185. [PubMed] |
| 132. | Ai J, Zhang R, Li Y, Pu J, Lu Y, Jiao J, Li K, Yu B, Li Z, Wang R. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun. 2010;391:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 409] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 133. | Zhu J, Chen T, Yang L, Li Z, Wong MM, Zheng X, Pan X, Zhang L, Yan H. Regulation of microRNA-155 in atherosclerotic inflammatory responses by targeting MAP3K10. PLoS One. 2012;7:e46551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 134. | Wang TJ. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation. 2011;123:551-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |