Published online Aug 20, 2014. doi: 10.5493/wjem.v4.i3.38
Revised: June 19, 2014
Accepted: July 27, 2014
Published online: August 20, 2014
Processing time: 240 Days and 17.9 Hours
AIM: To determine the plasmatic iron content and evaluate the oxidative stress (OS) markers in subjects receiving blood therapy.
METHODS: Thirty-nine individuals with unspecified anemia receiving blood transfusions and 15 healthy subjects were included in the study. Anemic subjects were divided into three subgrouP: (1) those that received up to five blood transfusions (n = 14); (2) those that received from five to ten transfusions (n = 11); and (3) those that received more than ten transfusions (n = 14). Blood samples were collected by venous arm puncture and stored in tubes containing heparin. The plasma and cells were separated by centrifugation and subsequently used for analyses. Statistical analyses were performed using Kruskal-Wallis analysis of variance followed by Dunn’s multiple comparison tests when appropriate.
RESULTS: The eletrophoretic hemoglobin profiles of the subjects included in this study indicated that no patients presented with hemoglobinopathy. Labile plasmatic iron, ferritin, protein carbonyl, thiobarbituric acid-reactive substances (TBARS) and dichlorofluorescein diacetate oxidation were significantly higher (P < 0.05), whereas total thiol levels were significantly lower (P < 0.05) in transfused subjects compared to controls. Additionally, the activity of catalase, superoxide dismutase and glutathione peroxidase were significantly lower in the transfused subjects (P < 0.05). Antioxidant enzyme activities and total thiol levels were positively correlated (P < 0.05), and negatively correlated with the levels of protein carbonyl and TBARS (P < 0.05). In contrast, protein carbonyl and TBARS were positively correlated (P < 0.05). Altogether, these data confirm the involvement of OS in patients following therapy with repeated blood transfusions.
CONCLUSION: Our data reveal that changes in OS markers are correlated with levels of labile plasmatic iron and ferritin and the number of transfusions.
Core tip: Here, the readers will find important information regarding iron accumulation and its correlation with oxidative damage markers in anemic subjects following blood therapy. This research, regarding iron accumulation and its associated toxicology is remarkable because the mechanism(s) involved in its mode of action are not fully understood. Thus, our data are extremely important for research concerning the involvement of iron overload on the development of human diseases.
- Citation: Fernandes MS, Rissi TT, Zuravski L, Mezzomo J, Vargas CR, Folmer V, Soares FAA, Manfredini V, Ahmed M, Puntel RL. Oxidative stress and labile plasmatic iron in anemic patients following blood therapy. World J Exp Med 2014; 4(3): 38-45
- URL: https://www.wjgnet.com/2220-315X/full/v4/i3/38.htm
- DOI: https://dx.doi.org/10.5493/wjem.v4.i3.38
Iron is an essential element of cells that participates in various cellular processes due to its ability to accept and donate electrons, interconverting between Fe3+ and Fe2+ forms[1]. However, this redox property renders iron potentially toxic in biologic systems. The labile plasmatic iron (LPI) component of non-transferrin-bound iron is redox-active, chelatable and capable of permeating into organs to induce tissue iron overload[2]. Thus, LPI is an accessible diagnostic marker of iron overload and cell toxicity[2]. Moreover, LPI can participate in the Fenton reaction and generate a large amount of reactive oxygen species (ROS)[3]. To prevent ROS overproduction, circulating and intracellular free iron are tightly regulated by binding to transferrin, ferritin and other proteins[4,5]. However, the iron balance can be disrupted in some situations, such as with chronic anemia, repeated blood transfusions, and following increased gastrointestinal absorption, which lead to iron overload[6]. Therefore, subjects undergoing repeated blood transfusions are at risk of iron-associated toxicity[7].
Elevated tissue iron can overwhelm protective mechanisms and lead to an increase in iron complexes with small molecules, such as nucleotides and citrate, in the serum of transfusion patients and also within cytoplasm and organelles[8,9]. Furthermore, repeated blood transfusions increase the levels of iron available to generate catalytically active complexes, free radicals and oxidative damage[9]. Thus, LPI promotes free radical formation that culminates in the oxidation of biomolecules. Accordingly, iron overload in humans and in experimental animals is associated with oxidative stress (OS)[10]. Indeed, it is known that an imbalance in the oxidant/antioxidant status of the cell is associated with OS, leading to important cellular macromolecule modifications and cell damage[11]. The cell injury observed in patients with iron overload is attributed to OS[12]. Hence, the oxidation reactions result in the formation of lipid peroxides and protein carbonyls, damaged deoxyribonucleic acid bases, and mitochondrial dysfunction[13]. Additionally, individuals with an iron overload demonstrate impaired antioxidant defenses[6]. Accordingly, the long-term consequence of chronic iron overload is organ injury, which could contribute to the initiation and development of several metabolic disorders, such as endocrinopathies, diabetes mellitus, cirrhosis, hypogonadism and heart failure[14].
In general, oxidative damage of biomolecules can be counteracted by enzymatic as well as non-enzymatic defenses. Indeed, humans have several biologic mechanisms to defend against intracellular OS. One of the most important mechanisms involves the actions of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx)[15]. In spite of a well-developed antioxidant defense system, cells can still be oxidatively damaged under some pathologic conditions[11].
Data concerning labile iron accumulation in anemic subjects receiving repeated blood transfusions and the association with oxidative damage markers are scarce in the literature. We hypothesize that OS correlates with LPI in anemic patients following therapy with repeated blood transfusions. In this study, we evaluated OS markers and the activity of enzymatic antioxidant defenses in the blood of patients receiving repeated transfusions and in control subjects (not transfused). Additionally, we determined the LPI and ferritin levels in these subjects and correlated both parameters with other evaluated markers.
1,1,3,3-tetramethoxypropane, 2-thiobarbituric acid, sodium dodecyl sulfate, 5,5’-dithiobis-(2-nitrobenzoic acid), trichloroacetic acid, 2’,7’-dichlorodihydrofluorescein diacetate (DCHF-DA), and 2,4-dinitrophenylhydrazine, were purchased from Sigma (St. Louis, MO, United States). The kit for iron determination was obtained from Bio-Systems Corp. (Beloit, WI, United States), kits for measuring SOD (RANSOD) and GPx (RANSEL) were purchased from Randox Laboratories Ltf. (Crumlin, United Kingdom), and the Total Protein kit for protein determination was obtained from BioClin (Delft, Netherlands). All the other chemicals were commercial products of the highest purity grade available.
This study was approved by the Ethics Committee in Research of Universidade Federal do Pampa. Altogether, 39 individuals with unspecified anemia receiving blood transfusions and 15 healthy subjects (blood donors) from the Banco de Sangue do Municipio de Uruguaiana were included in the study. Since most of our patients were male, the female patients were excluded from this study. Thus, both anemic and control healthy individuals were male. Anemic individuals were included in the study if they were diagnosed according to the International Classification of Diseases (anemia unspecified, ICD 10: D64.9), and were not diagnosed with other diseases, such as cancer, renal failure, hepatic disease, blood loss or others. Additionally, anemic patients had received blood therapy during the year prior to collection (i.e., no more than 12 mo from the first transfusion until sample collection). Additionally, it is important to mention here that the sample collection was done before a new transfusion, namely clinical screening. The anemic subjects were divided into three subgrouP: (1) those that received less than five blood transfusions (n = 14); (2) those that received from five to ten blood transfusions (n = 11); and (3) those that received more than ten blood transfusions (n = 14).
Blood from controls and anemic subjects was collected by venous arm puncture and stored in tubes containing heparin. The plasma and cells were separated by centrifugation at 1500 r/min for 10 min and were subsequently used for biochemical analyses. All biochemical assays were done in duplicate or triplicate, depending on availability of samples.
The electrophoretic analysis of hemoglobin was performed using a Minicap system (Sebia, Norcross, France) according to the manufacturer’s instructions, and controls were run with each test. The Minicap system uses the principle of capillary electrophoresis in free solution. Charged molecules are separated by their electrophoretic mobility in an alkaline buffer with a specific pH. Separation also occurs according to the electrolyte pH and electro-osmotic flow. Electropherograms were expressed with zones divided from Z1 to Z15 based on standardizing the location of hemoglobin as previously described[16].
LPI refers to non-heme bound, non-ferritin bound and non-transferrin-bound iron (i.e., free iron) according to the previously validated convention[17]. The LPI content was determined by its reactivity with ferrozine, in the presence of the denaturant sodium dodecyl sulfate and the reducing agents ascorbate and sodium metabisulphite, as previously described[18,19]. The results are expressed as μg/dL.
Ferritin content was determined as described by Bernard and Lauwerys[20]. Serum ferritin causes agglutination of latex particles coated with anti-human ferritin that is proportional to the concentration of ferritin and can be measured by turbidimetry. The results are expressed as μg/L ferritin.
Protein carbonyl content, which is indicative of oxidation, was determined as described by Levine et al[21]. Plasma samples were added to 0.2 mL of 10% trichloroacetic acid and placed on ice for 5 min. After centrifugation (5 min), samples were incubated for 90 min at 37 °C with 1 mL of 10 mmol/L 2,4-dinitrophenylhydrazine in 2 mol/L HCl. Finally, proteins were dissolved in 6 mol/L guanidine and interference was removed after washing with ethanol-ethyl acetate 1:1 (v/v). The extent of the damage was estimated by reading absorbance at 370 nm. The results are expressed as nmol carbonyl/mg protein.
Levels of thiobarbituric acid reactive substances (TBARS) in plasma were determined using the method described by Ohkawa et al[22]. In brief, samples were incubated in acidic medium containing 0.45% sodium dodecyl sulfate and 0.6% thiobarbituric acid at 100°C for 60 min. After centrifugation, the reaction product was determined at 532 nm using a 1,1,3,3-tetramethoxypropaneas standard and the results are expressed as nmol malondialdehyde/mg protein.
Plasmatic total thiol was determined as described by Ellman et al[23]. The colorimetric assay was carried out in 1 mol/L phosphate buffer (pH 7.4) and calculated against a standard curve constructed with glutathione. Total thiol content is expressed as nmol total thiol/mg protein.
The determination of intracellular oxidant production was based on the cleavage of DCHF-DA to DCHF, which fluoresces when oxidized by ROS according to previously described methods[24]. The plasma sample was diluted (1:10) in 10 mmol/L Tris-HCl buffer. Then, 50 μL of diluted plasma was incubated with 10 μmol/L DCHF-DA at 37 °C for 20 min. The fluorescence emission at 520 nm was measured using a Perkin-Elmer spectrofluorometer with an excitation wavelength of 488 nm and an emission wavelength of 520 nm. The results are expressed as arbitrary fluorescence units.
CAT activity was measured by the method previously described[25]. Packed erythrocytes were hemolyzed by adding 100 volumes of distilled water, then 20 μL of this hemolyzed sample was added to a cuvette and the reaction was started by the addition of 100 μL of freshly prepared 300 mmol/L H2O2 in phosphate buffer (50 mmol/L, pH 7.0) to give a final volume of 1 mL. The rate of H2O2 decomposition was measured by a spectrophotometer at 240 nm for a duration of 2 min. The CAT activity is expressed as UI/mg protein.
SOD activity was measured in erythrocytes using a RANSOD kit, which uses xanthine and xanthine oxidase to produce superoxide radicals that react with 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazol chloride to form formazan red. The SOD activity was measured by the degree of inhibition of this reaction at 505 nm and is expressed as UI/mg protein.
GPx activity was determined in erythrocytes using the RANSEL kit according to the method previously described[26]. The GPx activity is expressed as UI/mg protein.
The protein content was determined by the biuret method using the Total Protein kit with bovine serum albumin as a standard. The copper ions in an alkaline medium (biuret reagent) react with peptide, producing a purple color, whose intensity is proportional to the concentration of proteins in the samples being measured in a spectrophotometer at 545 nm.
All results are reported as median (range) and presented as box-plot graphics for the different grouP of patients. Hemoglobin, LPI and ferritin levels are presented as mean ± SD deviation. A Shapiro-Wilk test was performed to assess the normality of data distributions, and Kruskal-Wallis analysis of variance followed by Dunn’s multiple comparison tests were used when appropriate. Spearman’s correlational analyses were also performed between variables. For all analyses, we used a GraphPad Prism 5.0 software, and a P < 0.05 was considered significant.
Patient characteristics are presented in Table 1. Electrophoretic analyses indicated that all subjects had normal hemoglobin profiles (data not shown). As expected, LPI and ferritin levels were higher in the transfused subjects. Specifically, subjects receiving five or more transfusions had significantly higher LPI levels (P < 0.05), and patients receiving more than ten transfusions had significantly higher ferritin levels (P < 0.05) compared to controls. Additionally, we found that the number of transfusions was significantly correlated with LPI and ferritin levels (P < 0.05) (Table 2).
| Characteristics | Controls(n = 15) | Transfusions | ||
| < 5(n = 14) | 5-10(n = 11) | > 10(n = 14) | ||
| Age (yr) | 40.1 (20–50) | 62.8 (24–92) | 64.8 (49–84) | 57.5 (24–74) |
| Number of transfusions | 0 (0) | 3.20 (2–4) | 7.17 (5–9) | 18.78 (14–26) |
| Hemoglobin (g/dL) | 13.8 ± 0.5 | 7.5 ± 2.1 | 6.75 ± 0.5 | 4.9 ± 0.9 |
| Labile iron content (μg/dL) | 108.9 ± 13.8 | 149.2 ± 45.1 | 216.2 ± 68.3a | 366.9 ± 68.5a |
| Ferritin (μg/L) | 219.6 ± 18.2 | 190.3 ± 11.7 | 221.2 ± 16.1 | 277.5 ± 27.5a |
| LPI | Ferritin | GPx | SOD | CAT | TBARS | DCHF-DA oxidation | Carbonyl | Total thiol | |
| Transfusion number | 0.8569b | 0.7991b | -0.8796b | -0.7103b | -0.8143b | 0.5114b | 0.0111 | 0.5793b | -0.5555b |
| Total thiol | -0.4151b | -0.3354b | 0.4830b | 0.4849b | 0.5401b | -0.2790b | 0.0106 | -0.1164 | - |
| Carbonyl | 0.5583b | 0.5122b | -0.5613b | -0.3713b | -0.4862b | 0.5208b | -0.0627 | - | - |
| DCHF-DA oxidation | -0.1291 | -0.0049 | 0.0370 | -0.0446 | -0.0401 | -0.2293a | - | - | - |
| TBARS | 0.4984b | 0.4144b | -0.4638b | -0.3113b | -0.3457b | - | - | - | - |
| CAT | -0.7266b | -0.5944b | 0.8945b | 0.7251b | - | - | - | - | - |
| SOD | -0.6085b | -0.5744b | 0.7443b | - | - | - | - | - | - |
| GPx | -0.7973b | -0.7144b | - | - | - | - | - | - | - |
| Ferritin | 0.9112b | - | - | - | - | - | - | - | - |
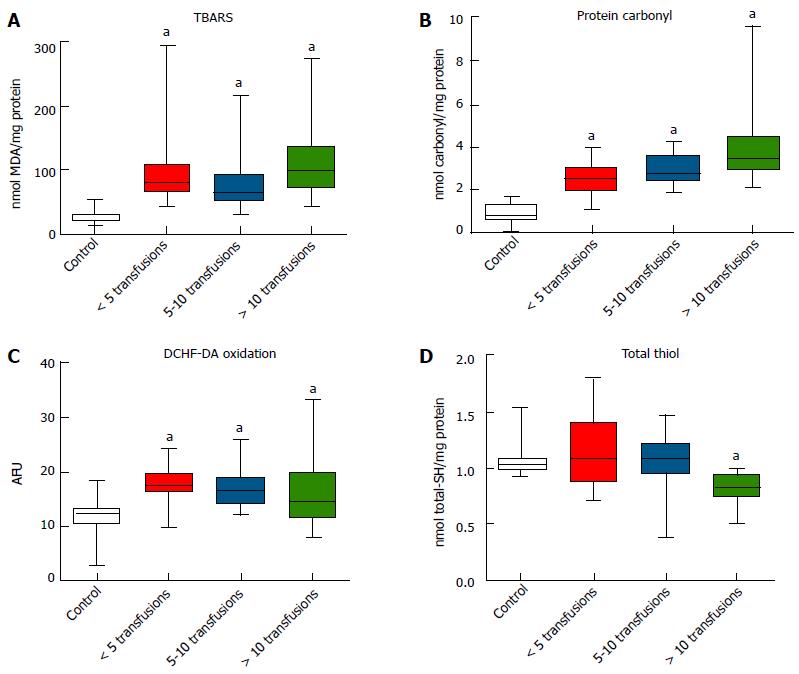
The OS markers TBARS (Figure 1A), protein carbonyl (Figure 1B) and DCFH-DA oxidation (Figure 1C) were all significantly higher in transfused subjects compared to the control group (P < 0.05). However, total thiol levels were significantly lower in subjects receiving more than ten transfusions compared to controls (P < 0.05) (Figure 1D).
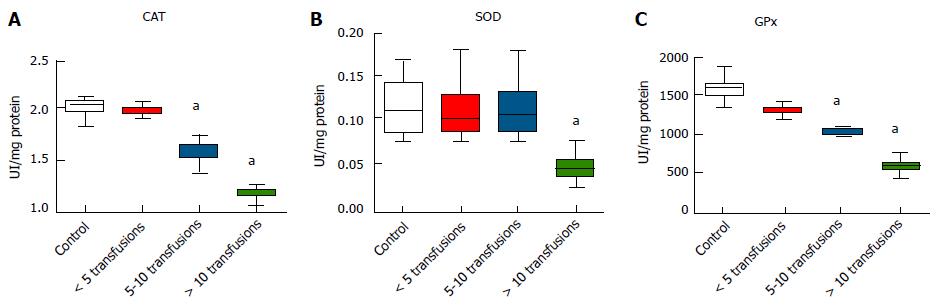
The activity CAT and GPx were significantly lower in subjects receiving five or more transfusions compared to controls (P < 0.05) (Figure 2A and C). SOD activity was significantly lower in subjects receiving more than ten transfusions compared with controls (P < 0.05) (Figure 2B). Furthermore, significant negative correlations were observed between the number of transfusions and the activity of these antioxidant enzymes (P < 0.05) (Table 2).
Additional correlations were found between LPI levels and OS markers (P < 0.05), with the exception of DCDH-DA oxidation (Table 2). LPI and ferritin were negatively correlated with antioxidant enzyme activities and total thiol, and positively correlated with carbonyl and TBARS levels (all P < 0.05). Indeed, it was found that antioxidant enzyme activities were positively correlated with total thiol levels, and negatively correlated with the levels of protein carbonyl and TBARS (P < 0.05). In contrast, protein carbonyl and TBARS levels were positively correlated (P < 0.05).
Our data are in accordance with a previous study showing that the increase in LPI content could lead to an increase in ROS generation, and consequently an increase in oxidative damage[12]. Additionally, based on data concerning hemoglobin profile, we discarded hemoglobin disorders in these individuals. These data are extremely important to avoid misinterpretations, as it was previously shown that any imbalance between α and β chains of hemoglobin (α or β-thalassemia, respectively) plays a crucial role in OS[27]. Besides, there are data linking the observed levels of the various biomarkers evaluated in this study to health outcomes, such as in renal failure[28] and breast cancer[29].
Taking into account our results and those previously found, it is plausible to assume that under blood transfusion therapy, the excess of labile (catalytically active) iron must generate free radicals via Fenton chemistry, resulting in oxidative damage to biomolecules in vivo[30]. Our assumption is further supported by a previous report showing that iron-catalyzed ROS generation leads to an increase in the genomic instability in hematopoietic progenitor cells[31]. Moreover, it was shown in animal models that iron overload causes liver damage via both oxidative and nitrosative mechanisms[32]. Indeed, we assume that under repeated blood transfusions, the iron content increases to values that overwhelm the protective mechanisms, leading to an increase in the amount of iron available to form complexes with small molecules, the “catalytically active iron complexes”. Thus, we assume that the ROS generated are responsible for the oxidation of DCHF-DA found in the transfused subjects, which is supported by a previous report showing that overload with iron (ferric nitrilotriacetate) leads to an increase in DCHF-DA oxidation in cultured rat hepatocytes[33].
Interestingly, we found some changes in the OS parameters even in the absence of significant iron accumulation, suggesting that alterations in OS markers could precede iron accumulation in patients following blood therapy. Accordingly, it seems logical that the differences in other parameters, such as hemoglobin and ferritin levels, could potentially contribute to the different oxidative state among patients. Thus, it is difficult to affirm that iron alone is the primary factor responsible for these differences. This point is extremely relevant and deserves further attention in future investigations.
The results of this study also show that levels of TBARS significantly increased in subjects receiving blood transfusions, which was positively correlated to LPI content, ferritin content and the number of transfusions. These findings are in accordance to previous reports showing that the levels of lipid peroxidation products were increased in β-thalassaemic patients receiving blood transfusions[19] and in subjects with hepatic iron overload[6]. Moreover, we found a significant increase in the protein carbonyl in the subjects receiving repeated blood transfusions, which was correlated with LPI content. Additionally, our data are in accordance to a previous paper showing a significant increase in the protein carbonyl content associated with iron overload[33].
A significant reduction in total thiol levels was found in the subjects receiving repeated blood transfusions, which is consistent with a report showing a decrease in thiol content in the liver of rats treated with iron[34]. Albeit not completely understood, we believe that thiols are oxidized (consumed/used) in these subjects due to OS status following iron overload. Another possibility is that the iron could react non-enzymatically with thiols in plasma to generate ROS, which directly leads to reduction of antioxidant capacity in plasma and the increased susceptibility of blood components to oxidation[35]. Thus, this thiol-dependent free radical generation by iron overload might be a potential contributing factor for the changes in the oxidative markers reported here. Our assumptions are supported by a study showing that oxygen radicals can be produced by iron-catalyzed auto-oxidation of cysteine or glutathione[36]. Therefore, the generated ROS (either by Fenton chemistry or by iron-catalyzed auto-oxidation of thiols) may be responsible for the oxidation of other biomolecules reported here, such as lipids and proteins.
The results of the present study demonstrate a marked decrease in antioxidant enzyme activity in the subjects with iron overload, which is consistent with previous reports[30,33]. Moreover, enzyme activities were negatively correlated with LPI, TBARS and protein carbonyl levels. In line with this, we presume that the decrease in the enzymatic activity of antioxidants further contributed to the OS condition. Indeed, Chakraborty et al[19] showed that the decrease in antioxidant enzymes strongly contributes to an increase in OS markers (TBARS, protein carbonyl and ROS). Although our data do not support this supposition, we hypothesize that a decrease in the antioxidant enzymes reported here could, at least in part, be due to a decrease in their expression. Indeed, it was previously shown that both CAT and GPx were downregulated under OS conditions in human cells[37]. However, the mechanisms regulating the expression of antioxidant enzymes under iron overload remain to be explored in more detail.
Our data confirms the involvement of OS in patients following therapy with repeated blood transfusions. Additionally, we found that the changes in the OS markers are correlated with iron content, ferritin and the number of transfusions. Thus, iron chelators that efficiently decrease the levels of labile iron are candidates to counteract the iron-induced ROS generation[38]. However, more studies are necessary to better understand the mechanism(s) associated with iron-induced oxidative changes, to minimize the side effects associated to blood transfusion therapy, and to provide some clinical benefits. As antioxidant supplementation is not entirely safe and may cause unfavorable effects to different patients, more discussion on its potential benefits is warranted[39].
In conclusion, our data confirm the involvement of OS and its correlation with LPI and ferritin in unspecified anemic patients following therapy with repeated blood transfusions. However, we found some alterations of OS markers even in the absence of significant iron accumulation, which encourages us to further explore the changes in the OS parameters that occur before iron overload in subjects receiving blood therapy.
Iron is an essential element that participates in several metabolic activities of cells. However, in excess, iron can be a cause of oxidative stress (OS) in subjects undergoing blood transfusion therapy. Despite this, the relationship between plasmatic iron content, OS markers and the activity of antioxidant enzymes in anemic subjects receiving repeated blood transfusions remains to be better characterized.
Blood therapy has been used in medical practice to treat anemic patients. However, the increase in the iron level in patients following blood therapy must be considered. Thus, the purpose of this research was to better understand the changes associated with OS markers in patients undergoing blood therapy in order to prevent iron-supported oxidative damage in anemic subjects.
Previous data have shown that blood therapy is associated with iron overload, and consequently, with oxidative changes in various tissues. However, efficient therapies to prevent the side effects associated with repeated blood transfusions are not known. Thus, elucidative studies regarding the plasmatic oxidative changes associated with iron overload are necessary. Here, the authors found that anemic subjects undergoing transfusions show increased levels of plasmatic labile iron, protein carbonyl, thiobarbituric acid reactive substances, and 2’,7’-dichlorodihydrofluorescein diacetate oxidation, as well as decreased total thiol levels. Additionally, the activities of superoxide dismutase, catalase, and glutathione peroxidase were significantly lower in the transfused subjects. Significant correlations were found between the number of transfusions, plasmatic iron content, OS markers and the activity of the antioxidant enzymes.
The results of this study suggest that antioxidants could be associated with blood therapy. Additionally, iron chelators that efficiently decrease the levels of labile iron could be used to counteract the iron-induced generation of reactive oxygen species. However, more studies are necessary to better understand the mechanism(s) associated with iron-induced oxidative changes in order to minimize the side effects associated with blood transfusion therapy and to provide clinical benefits.
This is a study that contains important information regarding iron accumulation in anemic subjects receiving repeated blood transfusions and its correlation with the plasmatic oxidative damage markers in these subjects.
P- Reviewer: Erikson KM, Koch TR, Naito Y S- Editor: Wen LL L- Editor: AmEditor E- Editor: Wu HL
| 1. | Premkumar K, Min K, Alkan Z, Hawkes WC, Ebeler S, Bowlus CL. The potentiating and protective effects of ascorbate on oxidative stress depend upon the concentration of dietary iron fed C3H mice. J Nutr Biochem. 2007;18:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Orino K, Lehman L, Tsuji Y, Ayaki H, Torti SV, Torti FM. Ferritin and the response to oxidative stress. Biochem J. 2001;357:241-247. [PubMed] [Cited in This Article: ] |
| 4. | Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54:176-186. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1028] [Cited by in RCA: 947] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 5. | Hershko C, Graham G, Bates GW, Rachmilewitz EA. Non-specific serum iron in thalassaemia: an abnormal serum iron fraction of potential toxicity. Br J Haematol. 1978;40:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in RCA: 322] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, Porter J, Evans P, Vichinsky E, Harmatz P. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254-263. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Lambing A, Kachalsky E, Mueller ML. The dangers of iron overload: bring in the iron police. J Am Acad Nurse Pract. 2012;24:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Zanninelli G, Loréal O, Brissot P, Konijn AM, Slotki IN, Hider RC, Ioav Cabantchik Z. The labile iron pool of hepatocytes in chronic and acute iron overload and chelator-induced iron deprivation. J Hepatol. 2002;36:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Gutteridge JM, Rowley DA, Griffiths E, Halliwell B. Low-molecular-weight iron complexes and oxygen radical reactions in idiopathic haemochromatosis. Clin Sci (Lond). 1985;68:463-467. [PubMed] [Cited in This Article: ] |
| 10. | Sinha S, Saxena R. Effect of iron on lipid peroxidation, and enzymatic and non-enzymatic antioxidants and bacoside-A content in medicinal plant Bacopa monnieri L. Chemosphere. 2006;62:1340-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Domanski AV, LaPhina EA, Zavodnik IB. Oxidative processes induced by tert-butyl hydroperoxide in human red blood cells: chemiluminescence studies. Biochemistry (Mosc). 2005;70:761-769. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Gattermann N, Rachmilewitz EA. Iron overload in MDS-pathophysiology, diagnosis, and complications. Ann Hematol. 2011;90:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Welch KD, Davis TZ, Van Eden ME, Aust SD. Deleterious iron-mediated oxidation of biomolecules. Free Radic Biol Med. 2002;32:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Cunningham MJ, Macklin EA, Neufeld EJ, Cohen AR. Complications of beta-thalassemia major in North America. Blood. 2004;104:34-39. [PubMed] [Cited in This Article: ] |
| 15. | Scott MD. H2O2 injury in beta thalassemic erythrocytes: protective role of catalase and the prooxidant effects of GSH. Free Radic Biol Med. 2006;40:1264-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Kim JE, Kim BR, Woo KS, Kim JM, Park JI, Han JY. Comparison of capillary electrophoresis with cellulose acetate electrophoresis for the screening of hemoglobinopathies. Korean J Lab Med. 2011;31:238-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Kuross SA, Hebbel RP. Nonheme iron in sickle erythrocyte membranes: association with phospholipids and potential role in lipid peroxidation. Blood. 1988;72:1278-1285. [PubMed] [Cited in This Article: ] |
| 18. | Repka T, Shalev O, Reddy R, Yuan J, Abrahamov A, Rachmilewitz EA, Low P, Hebbel RP. Nonrandom association of free iron with membranes of sickle and beta-thalassemic erythrocytes. Blood. 1993;82:3204-3210. [PubMed] [Cited in This Article: ] |
| 19. | Chakraborty D, Bhattacharyya M. Antioxidant defense status of red blood cells of patients with beta-thalassemia and Ebeta-thalassemia. Clin Chim Acta. 2001;305:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Bernard A, Lauwerys R. Turbidimetric latex immunoassay for serum ferritin. J Immunol Methods. 1984;71:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464-478. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 4061] [Cited by in RCA: 4133] [Article Influence: 118.1] [Reference Citation Analysis (0)] |
| 22. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 17627] [Cited by in RCA: 18572] [Article Influence: 403.7] [Reference Citation Analysis (0)] |
| 23. | Ellman G, Lysko H. A precise method for the determination of whole blood and plasma sulfhydryl grouP. Anal Biochem. 1979;93:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in RCA: 180] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Karja NW, Kikuchi K, Fahrudin M, Ozawa M, Somfai T, Ohnuma K, Noguchi J, Kaneko H, Nagai T. Development to the blastocyst stage, the oxidative state, and the quality of early developmental stage of porcine embryos cultured in alteration of glucose concentrations in vitro under different oxygen tensions. Reprod Biol Endocrinol. 2006;4:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 15211] [Cited by in RCA: 14950] [Article Influence: 364.6] [Reference Citation Analysis (0)] |
| 26. | Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158-169. [PubMed] [Cited in This Article: ] |
| 27. | Manca L, Masala B. Disorders of the synthesis of human fetal hemoglobin. IUBMB Life. 2008;60:94-111. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Bartnicki P, Fijałkowski P, Majczyk M, Błaszczyk J, Banach M, Rysz J. Effect of methoxy polyethylene glycol-epoetin beta on oxidative stress in predialysis patients with chronic kidney disease. Med Sci Monit. 2013;19:954-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Panis C, Herrera AC, Victorino VJ, Campos FC, Freitas LF, De Rossi T, Colado Simão AN, Cecchini AL, Cecchini R. Oxidative stress and hematological profiles of advanced breast cancer patients subjected to paclitaxel or doxorubicin chemotherapy. Breast Cancer Res Treat. 2012;133:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Zhang Y, Huang Y, Deng X, Xu Y, Gao Z, Li H. Iron overload-induced rat liver injury: Involvement of protein tyrosine nitration and the effect of baicalin. Eur J Pharmacol. 2012;680:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Naka K, Muraguchi T, Hoshii T, Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal. 2008;10:1883-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 32. | Toyokuni S. Iron as a target of chemoprevention for longevity in humans. Free Radic Res. 2011;45:906-917. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Ye SF, Hou ZQ, Zhang QQ. Protective effects of Phellinus linteus extract against iron overload-mediated oxidative stress in cultured rat hepatocytes. Phytother Res. 2007;21:948-953. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Devi SL, Anuradha CV. Oxidative and nitrosative stress in experimental rat liver fibrosis: Protective effect of taurine. Environ Toxicol Pharmacol. 2010;29:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Chung KY, Lee SJ, Chung SM, Lee MY, Bae ON, Chung JH. Generation of free radical by interaction of iron with thiols in human plasma and its possible significance. Thromb Res. 2005;116:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Dabbagh AJ, Mannion T, Lynch SM, Frei B. The effect of iron overload on rat plasma and liver oxidant status in vivo. Biochem J. 1994;300:799-803. [PubMed] [Cited in This Article: ] |
| 37. | Yoshida H, Sasaki K, Hirowatari Y, Kurosawa H, Sato N, Furutani N, Tada N. Increased serum iron may contribute to enhanced oxidation of low-density lipoprotein in smokers in part through changes in lipoxygenase and catalase. Clin Chim Acta. 2004;345:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Thephinlap C, Ounjaijean S, Khansuwan U, Fucharoen S, Porter JB, Srichairatanakool S. Epigallocatechin-3-gallate and epicatechin-3-gallate from green tea decrease plasma non-transferrin bound iron and erythrocyte oxidative stress. Med Chem. 2007;3:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Arruda MM, Mecabo G, Rodrigues CA, Matsuda SS, Rabelo IB, Figueiredo MS. Antioxidant vitamins C and E supplementation increases markers of haemolysis in sickle cell anaemia patients: a randomized, double-blind, placebo-controlled trial. Br J Haematol. 2013;160:688-700. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |