Published online Nov 20, 2013. doi: 10.5493/wjem.v3.i4.56
Revised: September 29, 2013
Accepted: November 1, 2013
Published online: November 20, 2013
Processing time: 145 Days and 0.9 Hours
Polyphenol-rich fruit are believed to be healthy food for humans. Traditional Chinese Medicines (TCMs) from fruit are rich sources of polyphenols and exhibit antioxidant and anti-inflammatory activities, and have been shown experimentally to overcome some chronic diseases, including cancer. The litchi seed is one of the TCMs traditionally used for relieving pain and sweating, and has been revealed in our recent report and other studies to possess rich amounts of polyphenolic species, including flavonoids and proanthocyanidines, and exhibits strong anti-oxidant activity, and could be applied for the treatment of diabetes and cancer. Herein, we review the recent findings regarding the benefits of this TCM in the treatment of human cancer and the possible cellular and molecular mechanisms of the litchi seed.
Core tip: Litchi seeds possess rich amount of polyphenols and anti-cancer activity, which could be a potential cancer prevention or treatment agent.
- Citation: Lin CC, Chung YC, Hsu CP. Anti-cancer potential of litchi seed extract. World J Exp Med 2013; 3(4): 56-61
- URL: https://www.wjgnet.com/2220-315X/full/v3/i4/56.htm
- DOI: https://dx.doi.org/10.5493/wjem.v3.i4.56
Cancer is one of the most prevalent diseases worldwide, with high morbidity and mortality. It has been accepted that cancer is a progressive disease requiring slow and stepwise development for several years to become a life-threatening disease. Therefore, it is regarded largely as a preventable disease[1-3]. Recent advances in medical techniques have rendered some types of cancer curable, but other cancers are still difficult to cure, even under advanced treatment. Novel detection methods and treatment strategies must be developed[4]. Traditional Chinese Medicine (TCM) has been developed in China for more than two thousand years. TCMs comprise various forms of therapies, such as acupuncture, massage (Tui na), exercise (qigong), and dietary therapy, and the main part of these therapies is herbal medicines. A substantial amount of information from human, animal and cell line studies has provided evidence that consumption of certain herbal products used in TCM can exert chemopreventive effects[5]. Recent studies have revealed that some TCMs or their components exhibit anti-tumor activities towards several types of cancer, such as liver[6], lung[7], gastric[8], nasopharyngeal[9] and colorectal cancer[10]. Several clinically-used chemotherapeutic drugs are derived from TCMs, such as camptothecin, isolated from the “happy tree” (Camptotheca accuminata); etoposide, semi-synthesized from a compound of Podophyllum emodi var. chinense; vincristin and vinblastin, isolated from the Madagascar periwinkle (Catharanthus roseus); and paclitaxel, purified from Taxus chinensis[11,12]. However, severe side effects and drug resistance always lead to therapy failure when using these chemotherapeutic drugs. Other types of substances need to be discovered to overcome these problems. Phenolic compounds have been accepted to be possible chemopreventive and treatment agents for cancer[13-16]. Polyphenols are obtained mainly from plants, and some have been regarded as forming part of a healthy diet for many years, such as tea, soybean, pomegranate, and pine nuts[17]. Litchi seeds have been analyzed and were found to possess rich amounts of polyphenols and exhibit strong antioxidant and inflammatory activities[18,19]. Recently, several studies by our research group and others have further revealed that litchi seed extract exhibits anti-cancer activity towards colorectal, liver, lung, and cervical cancer[19,20]. Herein, we review the recent findings regarding the benefits of this TCM in the treatment of human cancer and the possible cellular and molecular mechanisms of this substance.
The litchi (Litchi chinensis, Sapindaceae) is a tropical fruit tree that originates from southern China and is cultivated in semi-tropical areas world-wide for the delicious taste of the fruit[21]. A TCM pharmacopoeia named the Compendium of Materia Medica revealed that litchi seeds could be used to release or loose stagnant complexion, decadent colicky and the woman angry blood pain. Another TCM pharmacopoeia named Ben-Cao-Yan-Yi also recorded the analgesic effects of litchi seeds for heartache and intestinal pain. Yet another TCM pharmacopoeia named Ben-Cao-Bei-Yao described that the pharmacologic effect of litchi seeds could affect the liver and kidney and remove the stagnant humor, pathogenic cold and the woman angry blood pain. In Chinese folk remedies, Li-Ho-San, the mixture of litchi seeds, cumin and peel, can relieve the pain of a hernia or testicular swelling. Li-Shang-San, the mixture of litchi seeds and the root powder of Aucklandia lappa Decne., can treat gastralgia, period pain and postpartum abdominal pain. In summary, litchi seeds are used in China to release stagnant humor and remove chilling, and serve as an analgesic agent that can relieve the symptoms of coughing, gastralgia, neuralgia, and testicular swelling. However, scientific studies to prove the effects of the litchi seeds are still ongoing.
In recent decades, several experimental studies have been performed in China on the pharmacologic effects of litchi seeds. Present pharmacological studies are mainly focused on the anti-hyperglycemic effect of litchi seeds. Pan and colleagues indicated that litchi seed extract or its components could repress blood sugar and liver glycogen in a rat non-insulin diabetes mellitus model[22]. Guo et al[23] reported that litchi seed water extract could increase insulin sensitivity and reduce the concentrations of blood fasting glucose, triglyceride, leptin and tumor-necrosis factor in a type-2 diabetes mellitus rat model. Li et al[24] revealed that litchi seed extract could decrease fasting blood glucose of alloxan induced diabetes mellitus rat to a level equal to that of normal rats. Indeed, many other Chinese reports have demonstrated that litchi seed extract can reduce hyperglycemia and restore the sensitivity to insulin in both type 1 or type 2 diabetes mellitus models. Litchi seeds also contain anti-hyperlipidemic agents. Pan and colleagues reviewed some Chinese studies and reported that litchi seed oil could prevent blood triglyceride and low density lipoprotein in a high-fat-fed rat model[22]. Zheng et al[25] revealed that litchi seed extract could inhibit the expression of the surface antigen of the hepatitis B virus. Zhang et al[26] found that litchi seed extract showed the protective effect in rat with nonalcoholic steatohepatitis, indicating litchi seed extract could overcome the liver damage from inflammation. In India, the seeds are powdered as an herbal medicine owing to their astringency, and after oral intake they have the reputation of relieving neuralgic pain[27]. These reports together indicated that the litchi seeds exert antihyperlipidemic, hypoglycemic and pain-relieving effects, implying multiple pharmacologic uses in TCM.
Recent studies have revealed that the litchi is a polyphenol-rich fruit. Litchi pericarp is composed of significant amounts of flavonoids and anthocyanins, including procyanidin B2, B4, epicatechin, cyanidin-3-retinoside, cyanidin-3-glucoside, quercetin-3-retinoside and quercetin-3-glucoside, etc.[27]. These components carry high free radical scavenging properties and could be used as anti-inflammation, anti-oxidation or anti-cancer agents[28,29]. Wang and colleagues showed that litchi pericarp ethanol extract inhibited the in vitro and in vivo growth of mouse hepatocellular carcinoma and both estrogen-dependent and -independent human breast carcinoma cells[30,31]. In recent reports, polyphenol compounds from litchi seeds were identified and found to be composed of a variety of proanthocyanidins and flavonoid glycoside[18,20,32]. Xu et al[32] revealed that litchi seeds contain litchitannin A1, litchitannin A2, aesculitannin A, epicatechin-(2βfOf7,4βf8)-epiafzelechin-(4Rf8)-epicatechin, proanthocyanidin A1, proanthocyanidin A2, proanthocyanidin A6, epicatechin-(7,8-bc)-4β-(4-hydroxyphenyl)-dihydro-2(3H)-pyranone, and epicatechin. All of these compounds exert strong anti-oxidant activity with ferric reducing antioxidant power values of 3.71-24.18 mmol/g and IC50 values of 5.25-20.07 μmol/L toward 2,2-diphenyl-1-picrylhydrazyl radicals. Litchitannin A2 exerts an anti-viral activity against coxsackie virus B3. Aesculitannin A and proanthocyanidin A2 exhibit anti-herpes simplex virus 1 activity[32]. The same research group also identified some flavonoid glycosides in the litchi seed, including litchioside D, (-)-pinocembrin 7-O-neohesperidoside, (-)-pinocembrin 7-O-rutinoside, taxifolin 40-O-β-D-glucopyranoside, kaempferol 7-O-neohesperidoside, tamarixetin 3-O-rutinoside, and phlorizin[20]. Some of these compounds appear to exhibit anti-neoplasm activities in lung cancer, cervical cancer and hepatocellular carcinoma cells[20]. Another report from the same group also showed the anti-neoplastic activity of a cyclopropyl-containing fatty acid glucoside from the litchi seed[33]. In our report, rich amounts of flavonoids and condensed tannins [195.3 ± 6.7 and 230.2 ± 3.6 mg catechin equivalent/g of dry mass litchi seed extract (LCSP)] in LCSP were obtained by heating litchi seeds to 70 °C followed by 70% ethanol extraction[19]. The LCSP potently inhibits colorectal carcinoma (CRC) cell proliferation. According to these results, the litchi seed could be developed as a potent anti-tumor agent.
Anti-tumor activity of litchi seed: Over the last decade, the researchers were focused on litchi seed and its active components for the anti-tumor activity [34]. Chen and colleagues treated litchi seed water extract or granules to mouse xenograft of mouse Ehrlich ascites tumor cells, sarcoma S180 tumor cells and liver tumor cells and found the reduced tumors[35]. Chen and colleagues found that litchi seed could enhance both innate and acquired immunity in S180 cell xenograft[36]. Lv et al[37] demonstrated that litchi seed extract could reduce Bcl-2/Bax ratio in tumor tissues of sarcoma S180 mouse xenograft. Xu et al[20] isolated 7 different compounds from the litchi seeds and tested their cytotoxic activity towards human lung (A549), pulmonary (LAC), liver (Hep G2), and cervical (HeLa) cancer cell lines in vitro using the MTT colorimetric assay after 72 h. They found that kaempferol 7-O-neohesperidoside represented significant cytotoxicity towards all of the test cell lines, with IC50 values of 0.53, 7.93, 0.020 and 0.051 μmol/L, respectively. Litchioside D exhibited cytotoxic activity toward LAC and Hep-G2 cells (IC50 = 0.79 and 0.030 μmol/L). Taxifolin 40-O-β-D-glucopyranoside exerted cytotocic effects towards all four cell lines, with IC50 values ranging from 1.82 to 17.58 μmol/L. Compared with adriamycin, kaempferol 7-O-neohesperidoside represented more cytotoxic effect to these four cell lines[34]. Although the active components of litchi seeds against cancer have been revealed, Weber et al[38] suggested that the treatment approaches combined with an overall treatment protocol for the tumor microenvironment and chronic systemic inflammation are likely to provide a more successful outcome than a single tactical approach. According to these findings, they concluded that kaempferol 7-O-neohesperidoside, litchioside D and Taxifolin 40-O-β-D-glucopyranoside might be involved in the antitumor activity of litchi seeds.
Our recent report revealed that LCSP exhibits inhibitory effects on two colorectal cancer cell lines, SW480 and Colo 320DM[19]. Recently, we also tested the inhibitory effect of LCSP towards human lung adenocarcinoma cell line A549, lung large cell carcinoma cell line NCI-H661, cervical carcinoma cell line C33-A, breast carcinoma cell line MDA-MB-231, oral carcinoma cell line SCC-25, and ovarian carcinoma cell line ES-2, with IC50 values as shown in Table 1. The most sensitive cell lines were A549 cells, CRC cell line Colo 320DM, SW480 and C33A cells, with IC50 values of 22.49, 23.91, 26.33 and 24.45 μg/mL, respectively. SCC-25, MDA-MB-231, ES-2 and NCI-H661 were less sensitive towards LCSP treatment, with IC50 values of 36.8, 43.7, 45.46 and 52.47 μg/mL, respectively. These results further indicate the anti-neoplastic activity of the litchi seeds. However, the exact cellular and molecular mechanisms of LCSP or its components in the inhibitory effect of cancer cell growth require further investigation. Two possible mechanisms may be the induction of cell-cycle arrest and apoptosis. We reviewed recent evidence showing that LCSP could arrest cancer cells in the G2/M phase and induce mitochondria-mediated apoptosis in CRC cells.
| Cancer type | Cell line | IC501 (μg/mL) |
| Lung adenocarcinoma | A549 | 22.49 ± 1.02 |
| Duke’C CRC | Colo 320DM | 23.91 ± 2.25 |
| Cervical carcinoma | C33A | 24.45 ± 3.36 |
| Duke’B CRC | SW480 | 26.33 ± 2.80 |
| Oral carcinoma | SCC-25 | 36.80 ± 3.03 |
| Breast carcinoma | MDA-MB-231 | 43.70 ± 2.76 |
| Ovarian carcinoma | ES-2 | 45.46 ± 4.33 |
| Lung large cell carcinoma | H661 | 52.47 ± 2.83 |
LCSP arrests CRC cells in G2/M: Our recent study revealed that LCSP-treated Colo 320DM and SW480 cell lines are partly arrested at the G2/M phase. Cyclins are the key regulatory factors controlling the cell-cycle progression in cancer cells. According to our results, LCSP may disturb cyclin expression to arrest CRC cells at the G2/M phase. Cyclin D1 is an important regulator of G1 phase progression in many different cell types, including CRC cells[39]. In our study, LCSP treatment decreased the level of cyclin D1 in Colo 320DM and SW480 cells, which was correlated with the cell cycle analysis showing G2/M phase arrest. Moreover, disruption of cyclin A, a cyclin expressed during the S phase, can block DNA replication during the S phase[40]. Cyclin B is expressed in the G2 and M phases of the cell cycle. A decrease in cyclin B blocks the cell cycle from progressing into mitosis[41]. Together with alteration of cyclin D1, these findings suggest that the effect of LCSP on the cell division cycle is mainly due to disturbance of G2/M progression. Our previous studies demonstrated that flavonoids and proanthocyanidin-rich substances such as grape seeds, longan seeds or longan flower extract could increase the numbers of G1- or S-phase cells in cancer cells[19,42-45]. LCSP-treated CRC cells exhibited significant increases in the number of G2/M-phase cells, which differed from previous reports. These findings suggested that the anti-proliferative effect induced by flavonoids and proanthocyanidin from naturally-occurring products could occur through a different cell-cycle-controlling mechanism. The different compositions of flavonoids and proanthocyanidin in each natural product might induce different expressions of cyclin proteins to control the cell cycle in CRC cells. Whether the alteration of cyclin D and A levels by LCSP treatment is the only molecular mechanism responsible for the perturbation of the M to G1 phase of the cell cycle in CRC cells needs further investigation.
LCSP induces apoptosis toward CRC cells: Apoptosis is the elimination process to remove unwanted or damaged cells during development or maintenance of tissue homeostasis in multiple cellular organisms[46,47]. Dysfunction of apoptosis has been implicated as the main mechanism causing many human chronic diseases, such as neural degeneration, autoimmune disease, AIDS and cancer[48]. Many anti-cancer drugs and chemopreventive natural products possess activity to induce cancer cells into apoptosis and concomitantly suppress cancer cell growth[47]. In our recent study, we demonstrated that LCSP could induce CRC cells to undergo apoptosis[19]. The evidence came from the phosphatidylserine translocation to the outer leaflet of the plasma membrane, which was detected using annexin V analysis and activation of the caspase pathway in treated CRC cells. Caspase 3 expression and activation plays a crucial role in polyphenolic compound-induced apoptosis in CRC cells[42,44,49-51]. In our study, the active form of caspase 3 was increased in LCSP-treated CRC cells, further indicating that LCSP-induced apoptosis is mediated by caspase 3 activation. The subsequent increase in cleavage of caspase 3 substrate PARP in LCSP-treated CRC cells confirmed the activation of caspase 3. Involvement of the Bcl-2 family of proteins may play an important role in LCSP-induced apoptosis. The Bcl-2 family members are important mediators of mitochondria-induced apoptosis in cancer cells[46,52,53]. These proteins form multimers, which act as pores in cell membranes, controlling the flow of molecules[54]. Bcl-2 proteins are important mediators of apoptosis in CRC cells[46,47]. Some family members promote apoptosis (e.g., Bax and Bad), while others inhibit it (e.g., Bcl-2 and Bcl-x)[55,56]. Bcl-2 inhibits apoptosis by inhibiting the release of cytochrome c (Apaf 2) and apoptosis inducing factor from the mitochondria to the cytoplasm, and by limiting the activation of caspase 3 by inhibiting its activator protein, Apaf 1[57]. Some studies have suggested that the ratio of Bax:Bcl-2 proteins is the determining factor in transmission of the apoptotic signal[54,58-60]. Previously, proanthocyanidine-rich grape seed extract has been found to suppress the expression of Bcl-2 protein in breast and skin carcinoma cells[61,62]. Additionally, in our previous reports, we also confirmed that longan seed extract increases the Bax:Bcl-2 ratio in CRC cells[44,63]. The Bax:Bcl-2 ratio in LCSP-treated CRC cells increased significantly, indicating the importance of the Bax:Bcl-2 ratio in cancer cell life and death[54,58]. Taken together, our results demonstrated that LCSP-induced apoptosis in CRC cells was mediated by an increasing Bax:Bcl-2 ratio, by which LCSP induced mitochondria-mediated apoptosis in CRC cells. Although the anti-cancer activity of Litchi seed extract has been revealed, the toxicity to normal cells and the possible side effect has not yet been studied. Wan and his coworkers found that oral administration of the maximum dosage of litchi seed water or ethanol extract could not cause acute toxicity to mouse[64]. However, in our recent unpublished result, litchi seed extract exhibited suppression effect on normal small intestinal cells and lung fibroblast cells at more than 50 μg/mL. These results implicated the usage of litchi seed extract at lower dose and the possible toxicity may occur in gastrointestinal and lung system.
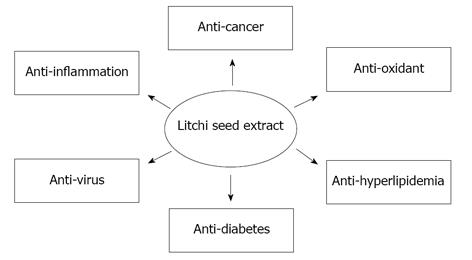
The litchi is one of the most important fruits in China, economically speaking. The seeds of the litchi were regarded as waste for a long time, and failed to be utilized. However, according to TCM pharmacopoeia, litchi seeds possess multiple pharmaceutical applications. Recent advanced biotechnology and pharmacology techniques have allowed us to gain deeper insight into the functions of this TCM using scientific methods. Litchi seed extract could overcome metabolic diseases such as diabetes mellitus, decrease triglycerides and suppress oxidation and inflammation. Some components of the litchi seed have been identified to be anti-cancer agents against lung, liver, pulmonary and cervical cancer. We further provide data to demonstrate that LCSP is also capable of inhibiting the growth of colorectal carcinoma, lung adenocarcinoma, lung large cell carcinoma, breast carcinoma, oral carcinoma, cervical carcinoma, and ovarian carcinoma cells. All of the pharmacologic effects of litchi seed extract are summarized in Figure 1. The main mechanisms of LCSP are the induction of cell-cycle arrest and apoptosis, at least in colorectal cancer cells, with the molecular mechanisms acting through decreased levels of cyclin D1, A and B1 and alteration of the Bax:Bcl-2 ratio and activation of caspase 3. However, upstream factors mediating LCSP induction of cell-cycle arrest and apoptosis need further investigation. We found that LCSP treatment could inhibit proliferation in various cancer cells and induce cell-cycle arrest and apoptosis in CRC cells, suggesting its potential as a novel chemoprevention agent for cancer in the future.
P-Reviewers: Cheng TH, Espino J, Martinez-Lostao L, Takenaga K S-Editor: Song XX L-Editor: A E-Editor: Wang CH
| 1. | Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 563] [Cited by in RCA: 611] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 2. | Tanaka T, Shnimizu M, Moriwaki H. Cancer chemoprevention by carotenoids. Molecules. 2012;17:3202-3242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 3. | Narayanan BA. Chemopreventive agents alters global gene expression pattern: predicting their mode of action and targets. Curr Cancer Drug Targets. 2006;6:711-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Ribatti D. Cancer stem cells and tumor angiogenesis. Cancer Lett. 2012;321:13-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Wang S, Penchala S, Prabhu S, Wang J, Huang Y. Molecular basis of traditional Chinese medicine in cancer chemoprevention. Curr Drug Discov Technol. 2010;7:67-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 6. | Wu P, Dugoua JJ, Eyawo O, Mills EJ. Traditional Chinese Medicines in the treatment of hepatocellular cancers: a systematic review and meta-analysis. J Exp Clin Cancer Res. 2009;28:112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Tian G, Guo L, Gao W. Use of compound Chinese medicine in the treatment of lung cancer. Curr Drug Discov Technol. 2010;7:32-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Wu M, Yao B. Advances in TCM treatment of gastric cancer and studies on the apoptosis. J Tradit Chin Med. 2002;22:303-307. [PubMed] [Cited in This Article: ] |
| 9. | Cho WC, Chen HY. Clinical efficacy of traditional Chinese medicine as a concomitant therapy for nasopharyngeal carcinoma: a systematic review and meta-analysis. Cancer Invest. 2009;27:334-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Tan KY, Liu CB, Chen AH, Ding YJ, Jin HY, Seow-Choen F. The role of traditional Chinese medicine in colorectal cancer treatment. Tech Coloproctol. 2008;12:1-6; discussion 6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Efferth T, Fu YJ, Zu YG, Schwarz G, Konkimalla VS, Wink M. Molecular target-guided tumor therapy with natural products derived from traditional Chinese medicine. Curr Med Chem. 2007;14:2024-2032. [PubMed] [Cited in This Article: ] |
| 12. | Efferth T, Li PC, Konkimalla VS, Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med. 2007;13:353-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in RCA: 366] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 13. | Brown EM, Gill CI, McDougall GJ, Stewart D. Mechanisms underlying the anti-proliferative effects of berry components in in vitro models of colon cancer. Curr Pharm Biotechnol. 2012;13:200-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Halliwell B. Antioxidants and human disease: a general introduction. Nutr Rev. 1997;55:S44-S49; discussion S49-S52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in RCA: 391] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 16. | Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2044] [Cited by in RCA: 1893] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 17. | Kaur M, Agarwal C, Agarwal R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J Nutr. 2009;139:1806S-1812S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Xu X, Xie H, Xu L, Wei X. A novel cyclopropyl-containing fatty acid glucoside from the seeds of Litchi chinensis. Fitoterapia. 2011;82:485-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Hsu CP, Lin CC, Huang CC, Lin YH, Chou JC, Tsia YT, Su JR, Chung YC. Induction of apoptosis and cell cycle arrest in human colorectal carcinoma by Litchi seed extract. J Biomed Biotechnol. 2012;2012:341479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Xu X, Xie H, Hao J, Jiang Y, Wei X. Flavonoid Glycosides from the Seeds of Litchi chinensis. J Agric Food Chem. 2011;59:1205-1209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Gontier E, Boussouel N, Terrasse C, Jannoyer M, Ménard M, Thomasset B, Bourgaud F. Litchi chinensis fatty acid diversity: occurrence of the unusual cyclopropanoic fatty acids. Biochem Soc Trans. 2000;28:578-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Pan JQ, Guo JW, Han C, Liu HC. Survey of pharmacological experimental studies on Litchi seeds. Zhonguo Xinyao Zazhi. 2000;9:14-16. [Cited in This Article: ] |
| 23. | Guo JW, Liao HF, Pan JQ, Ye BB, Jian XB, Wei DL, Dai LY. Effects of saponin of Litchi on decreasing blood glucose and controlling blood lipid in hyperlipemia-fatty liver rats fed by ligh-sugar-fat. Zhonguo Linchuang Yaolixue yu Zhiliaoxue. 2004;9:1403-1407. [Cited in This Article: ] |
| 24. | Li CG, Zhang SQ, Wang SY, Cui MM, Zhou MB, Qin Q, Wang XY, Wang J. Intervention of litchi nucleus extract fluid in the blood biochemical indexes of model mice with diabetes mellitus induced by alloxan. Zhonguo Linchuang Kangfu. 2006;10:61-63. [Cited in This Article: ] |
| 25. | Zheng M, Zheng Y. Experimental studies on the inhibition effects of 1000 Chinese medicinal herbs on the surface antigen of hepatitis B virus. J Tradit Chin Med. 1992;12:193-195. [PubMed] [Cited in This Article: ] |
| 26. | Zhang W, Gan H, Ma R, Gong Z. The protective effects of semen litchi extract on liver function in rats with nonalcoholic steatohepatitis. Shiyong Ganzangbing Zazhi. 2011;14:167-168. [Cited in This Article: ] |
| 27. | Li J, Jiang Y. Litchi flavonoids: isolation, identification and biological activity. Molecules. 2007;12:745-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Ariga T. The antioxidative function, preventive action on disease and utilization of proanthocyanidins. Biofactors. 2004;21:197-201. [PubMed] [Cited in This Article: ] |
| 29. | Kaur M, Velmurugan B, Rajamanickam S, Agarwal R, Agarwal C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm Res. 2009;26:2133-2140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Wang X, Yuan S, Wang J, Lin P, Liu G, Lu Y, Zhang J, Wang W, Wei Y. Anticancer activity of litchi fruit pericarp extract against human breast cancer in vitro and in vivo. Toxicol Appl Pharmacol. 2006;215:168-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Wang X, Wei Y, Yuan S, Liu G, Zhang YL, Wang W. Potential anticancer activity of litchi fruit pericarp extract against hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2006;239:144-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Xu X, Xie H, Wang Y, Wei X. A-type proanthocyanidins from lychee seeds and their antioxidant and antiviral activities. J Agric Food Chem. 2010;58:11667-11672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Xu XY, Xie HH, Hao J, Jiang YM, Wei XY. Eudesmane sesquiterpene glucosides from lychee seed and their cytotoxic activity. Food Chem. 2010;123:1123–1126. [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Ge R, Lu W, Zhang C. Progress of the effect and mechanism of litchi seed in antitumor activity. Guangdong Yaoxueyuan Xuebao. 2012;28:693-696. [DOI] [Cited in This Article: ] |
| 35. | Chen YH, Xioa L, Pan JQ, Lv J. Study on the anti-tumor effect of litchi seed and its containing serum. Zhonguo Yiyao Yaocai. 2010;33:1925-1929. [Cited in This Article: ] |
| 36. | Chen FY, Hu JM, Xioa L, Pan JQ. Experimental study of litchi seed on mouse tumor animal model and immune regulation. Zhonguo Yiyao Yaocai. 2009;32:774-776. [Cited in This Article: ] |
| 37. | Lv J, Shen W, Wei X, Xioa L. The influence of litchi seed extract on the expression of Bcl-2 and Bax in the tumor cells of mouse xenograft. Zhongchengyao. 2008;30:1381-1383. [Cited in This Article: ] |
| 38. | Weber DA, Wheat JM, Currie GM. Cancer stem cells and the impact of Chinese herbs,isolates and other complementary medical botanicals: a review. Zhonguo Jiehe Xixue Zazhi. 2012;10:493-503. [Cited in This Article: ] |
| 39. | Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 522] [Cited by in RCA: 631] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 40. | Pines J, Hunter T. p34cdc2: the S and M kinase? New Biol. 1990;2:389-401. [PubMed] [Cited in This Article: ] |
| 41. | Lindqvist A, Rodríguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol. 2009;185:193-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in RCA: 421] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 42. | Hsu CP, Lin YH, Chou CC, Zhou SP, Hsu YC, Liu CL, Ku FM, Chung YC. Mechanisms of grape seed procyanidin-induced apoptosis in colorectal carcinoma cells. Anticancer Res. 2009;29:283-289. [PubMed] [Cited in This Article: ] |
| 43. | Hsu CP, Lin YH, Zhou SP, Chung YC, Lin CC, Wang SC. Longan flower extract inhibits the growth of colorectal carcinoma. Nutr Cancer. 2010;62:229-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Chung YC, Lin CC, Chou CC, Hsu CP. The effect of Longan seed polyphenols on colorectal carcinoma cells. Eur J Clin Invest. 2010;40:713-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Chung YC, Huang CC, Chen CH, Chiang HC, Chen KB, Chen YJ, Liu CL, Chuang LT, Liu M, Hsu CP. Grape-seed procyanidins inhibit the in vitro growth and invasion of pancreatic carcinoma cells. Pancreas. 2012;41:447-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Park JW, Choi YJ, Jang MA, Lee YS, Jun DY, Suh SI, Baek WK, Suh MH, Jin IN, Kwon TK. Chemopreventive agent resveratrol, a natural product derived from grapes, reversibly inhibits progression through S and G2 phases of the cell cycle in U937 cells. Cancer Lett. 2001;163:43-49. [PubMed] [Cited in This Article: ] |
| 47. | Scatena R. Mitochondria and cancer: a growing role in apoptosis, cancer cell metabolism and dedifferentiation. Adv Exp Med Biol. 2012;942:287-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 48. | Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456-1462. [PubMed] [Cited in This Article: ] |
| 49. | Chen JC, Lu KW, Lee JH, Yeh CC, Chung JG. Gypenosides induced apoptosis in human colon cancer cells through the mitochondria-dependent pathways and activation of caspase-3. Anticancer Res. 2006;26:4313-4326. [PubMed] [Cited in This Article: ] |
| 50. | Roy AM, Baliga MS, Elmets CA, Katiyar SK. Grape seed proanthocyanidins induce apoptosis through p53, Bax, and caspase 3 pathways. Neoplasia. 2005;7:24-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Su CC, Lin JG, Li TM, Chung JG, Yang JS, Ip SW, Lin WC, Chen GW. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 2006;26:4379-4389. [PubMed] [Cited in This Article: ] |
| 52. | Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309-1312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6842] [Cited by in RCA: 6782] [Article Influence: 251.2] [Reference Citation Analysis (0)] |
| 53. | Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1128] [Cited by in RCA: 1097] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 54. | Reed JC. Balancing cell life and death: bax, apoptosis, and breast cancer. J Clin Invest. 1996;97:2403-2404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4399] [Cited by in RCA: 4475] [Article Influence: 139.8] [Reference Citation Analysis (0)] |
| 56. | Zhan Q, Fan S, Bae I, Guillouf C, Liebermann DA, O’Connor PM, Fornace AJ. Induction of bax by genotoxic stress in human cells correlates with normal p53 status and apoptosis. Oncogene. 1994;9:3743-3751. [PubMed] [Cited in This Article: ] |
| 57. | Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 658] [Cited by in RCA: 663] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 58. | Reed JC, Miyashita T, Takayama S, Wang HG, Sato T, Krajewski S, Aimé-Sempé C, Bodrug S, Kitada S, Hanada M. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem. 1996;60:23-32. [PubMed] [Cited in This Article: ] |
| 59. | Chresta CM, Masters JR, Hickman JA. Hypersensitivity of human testicular tumors to etoposide-induced apoptosis is associated with functional p53 and a high Bax: Bcl-2 ratio. Cancer Res. 1996;56:1834-1841. [PubMed] [Cited in This Article: ] |
| 60. | Mantena SK, Baliga MS, Katiyar SK. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis. 2006;27:1682-1691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 61. | Bandrés E, Zárate R, Ramirez N, Abajo A, Bitarte N, Garíia-Foncillas J. Pharmacogenomics in colorectal cancer: the first step for individualized-therapy. World J Gastroenterol. 2007;13:5888-5901. [PubMed] [Cited in This Article: ] |
| 62. | Meeran SM, Katiyar SK. Grape seed proanthocyanidins promote apoptosis in human epidermoid carcinoma A431 cells through alterations in Cdki-Cdk-cyclin cascade, and caspase-3 activation via loss of mitochondrial membrane potential. Exp Dermatol. 2007;16:405-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Lin CC, Chung YC, Hsu CP. Potential roles of longan flower and seed extracts for anti-cancer. World J Exp Med. 2012;2:78-85. [DOI] [Cited in This Article: ] [Cited by in CrossRef: 17] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 64. | Wan Q, Qin DL, Tian J, Xia LH, Liu J, Zhang H. Preparation of lychee nut extract and acute toxicity test. Luzhou Yixueyuan Xuebao. 2010;33:28-30. [Cited in This Article: ] |