Published online Jun 20, 2025. doi: 10.5493/wjem.v15.i2.103481
Revised: March 10, 2025
Accepted: March 21, 2025
Published online: June 20, 2025
Processing time: 146 Days and 22.6 Hours
Type 2 diabetes mellitus (T2DM) is a metabolic disorder marked by chronic hyperglycemia and low-grade inflammation, contributing to various complications. Natural agents with immunomodulatory and antioxidant properties have gained attention as adjunct therapies. To review the effects of Allium sativum on inflammatory pathways and metabolic alterations associated with T2DM. A narrative review was performed using PubMed/MEDLINE, EMBASE, and Scielo databases. The search included terms such as “allium sativum”, “inflammation", “oxidative stress”, and “diabetes mellitus”. Studies in English and Spanish - ranging from clinical trials to meta-analyses - were selected based on relevance. Bioactive compounds such as allicin, S-allyl cysteine, and diallyl disulfide exhibit anti-inflammatory, antioxidant, hypoglycemic, and lipid-lowering actions. Preclinical studies show improved glucose metabolism, insulin sensitivity, and organ function. Moreover, clinical evidence supports reductions in fasting glucose, hemoglobin A1c, blood pressure, and oxidative stress, with good safety profiles. Allium sativum appears to be a promising adjuvant in T2DM management, offering metabolic and anti-inflammatory benefits. Nonetheless, further high-quality clinical trials are needed to confirm its long-term efficacy and standardize its therapeutic use.
Core Tip: Allium sativum (garlic) demonstrates significant potential in managing type 2 diabetes mellitus through its immunomodulatory effects, primarily due to its organosulfur compounds like allicin. Preclinical and clinical studies suggest garlic improves metabolic control by reducing inflammation, oxidative stress, glucose levels, and insulin resistance, with benefits comparable to antidiabetic drugs. It may also positively impact complications such as metabolic-associated fatty liver disease, hypertension, nephropathy, and cardiovascular disease. However, more clinical trials are needed to confirm its therapeutic efficacy and optimize its use in type 2 diabetes mellitus treatment. Garlic offers promise as a natural adjunct to conventional diabetes management.
- Citation: Mejía Delgado EM, Quiroz-Aldave JE, Durand-Vásquez MDC, Aldave-Pita LN, Fuentes-Mendoza JM, Concepción-Urteaga LA, Paz-Ibarra J, Concepción-Zavaleta MJ. Immunomodulatory effect of allium sativum in type 2 diabetes mellitus. World J Exp Med 2025; 15(2): 103481
- URL: https://www.wjgnet.com/2220-315x/full/v15/i2/103481.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i2.103481
Diabetes mellitus (DM) is a group of disorders characterized by impaired carbohydrate metabolism, wherein the body’s ability to utilize glucose as an energy source is diminished, while endogenous glucose production increases, resulting in chronic hyperglycemia[1]. In 2021, it was reported that 10.5% of the adult population was affected by DM[2]. DM contributes to a significant burden of complications, including hypertension, coronary artery disease, osteoarthritis, depression, asthma, various types of cancer, and infections, all of which reduce life expectancy in affected individuals[3]. Among the pathophysiological mechanisms underlying type 2 DM (T2DM), oxidative stress, endoplasmic reticulum stress, amyloid deposition in the pancreas, ectopic lipid accumulation in muscle, liver, and pancreas, as well as lipotoxicity and glucotoxicity, play key roles. These factors may induce an inflammatory response or be exacerbated by inflammation[4].
Various forms of metabolic stress that promote insulin resistance and T2DM also activate stress- and inflammation-induced kinases, such as IkappaB kinase and c-Jun N-terminal kinase. IkappaB kinase, in turn, activates the nuclear factor kappa B (NF-κB) transcription factor, leading to the expression of pro-inflammatory genes, such as tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and IL-1β, in the liver and adipose tissue. On the other hand, c-Jun N-terminal kinase activates transcription factors such as the Ets-like protein 1 and activating transcription factor 2[4,5]. In individuals with obesity, hypoxia in expanding adipose tissue can trigger an inflammatory response through increased secretion of chemokines such as C-C motif ligand 2, which recruits monocytes, and adipokines such as leptin and adiponectin, which have potential immunomodulatory effects[4,5].
Preclinical evidence supports the use of natural products with significant effects on various signaling pathways, including mitogen-activated protein kinases, the phosphatidylinositol 3-kinase/protein kinase B system, NF-κB, and the Janus kinase/signal transducer and activator of transcription pathway. These compounds also play a crucial role in regulating the balance between T helper 17 cells and regulatory T cells, as well as in modulating oxidative stress. Through these mechanisms, they have been shown to regulate the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-8, and IL-17, and anti-inflammatory cytokines such as IL-2 and IL-10. This knowledge forms the basis for the traditional use of medicinal plants in the treatment of immune-related diseases[6-9]. Recent research highlights allium sativum bioactive compounds, like allicin and S-allyl cysteine, which reduce oxidative stress and inflammation in T2DM, offering promising therapeutic potential[10-14]. The aim of this article is to review the immunomodulatory effects of allium sativum in the inflammatory process of patients with T2DM.
A narrative review was conducted through a bibliographic search in the PubMed/MEDLINE, EMBASE, and Scielo databases, focusing on the search terms “allium sativum”, “garlic”, “inflammation”, “anti-inflammatory”, “oxidative stress”, “antioxidative”, “diabetes mellitus”, “GLYCEM”, “dyslipidemia”, “lipid lowering”, “liver”, “MASLD”, “kidney”, “nephropathy”, and “cardiovascular disease”. Systematic reviews, meta-analyses, clinical trials, preclinical studies, narrative reviews, retrospective studies, cross-sectional studies, prospective studies, and case reports were included, all closely aligned with the objectives of this manuscript. The search was limited to documents written in Spanish or English, excluding conference abstracts. Articles were not excluded based on their publication date. A total of 122 articles were included and are cited in the references section.
Preclinical and clinical studies consistently demonstrate its efficacy in improving glycemic control, modulating oxidative stress, and attenuating systemic inflammation. Moreover, garlic supplementation has shown favorable effects on comorbid conditions, including dyslipidemia, hypertension, and metabolic dysfunction-associated steatotic liver disease (MASLD), with a significant reduction in inflammatory markers such as C-reactive protein (CRP) and TNF-α. Its bioactive compounds - particularly allicin, diallyl disulfide (DADS), and S-allyl cysteine - play a pivotal role in regulating pro- and anti-inflammatory cytokine profiles, enhancing antioxidant defenses, and ameliorating diabetes-associated complications such as nephropathy and retinopathy[11-14]. These findings support the therapeutic potential of Allium sativum as an adjunctive strategy in the holistic management of T2DM.
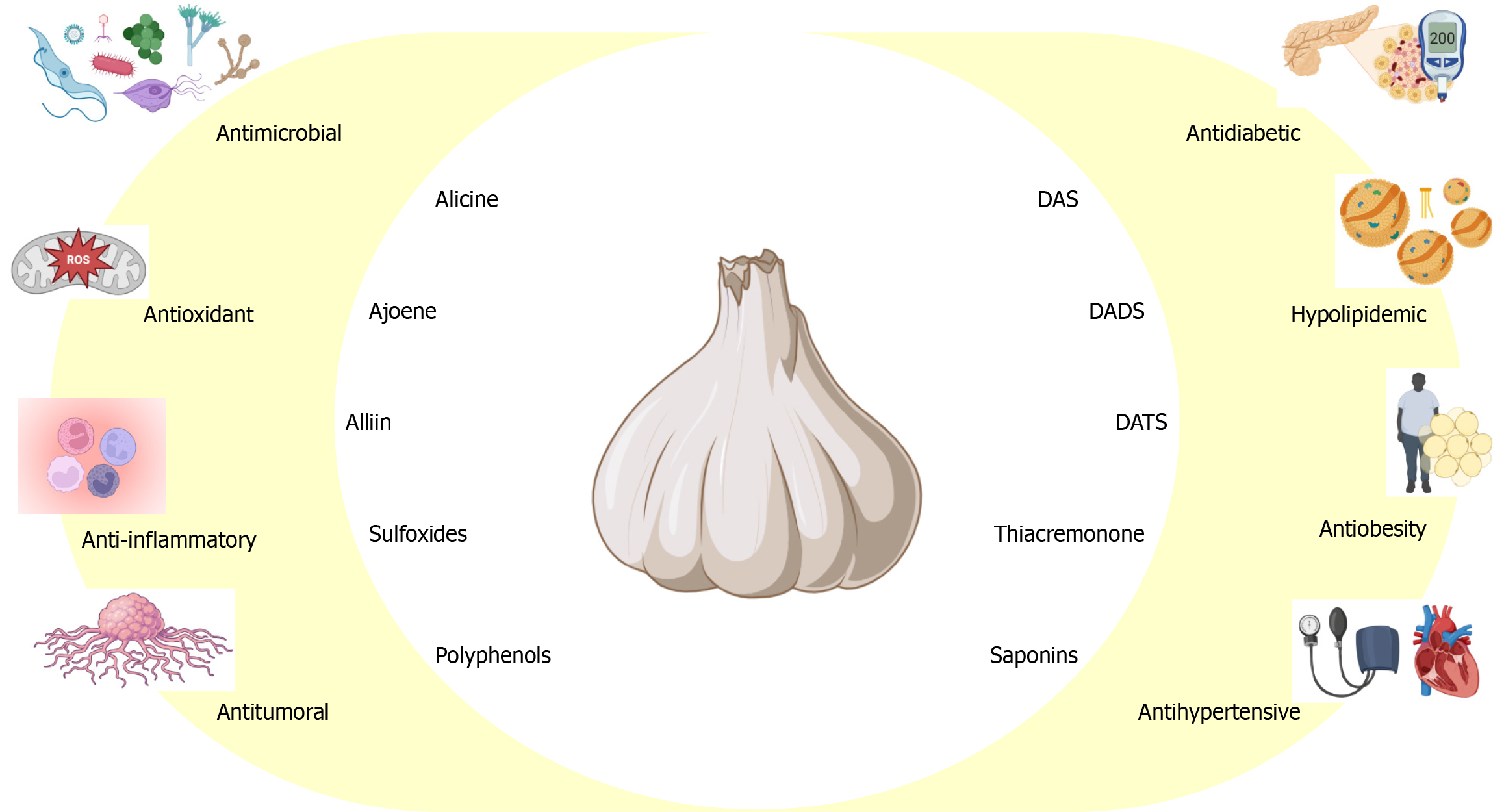
Allium sativum (garlic) is the second largest species in the Allium genus, after onion, and belongs to the subfamily Allioideae within the family Amaryllidaceae (formerly known as the Liliaceae family)[10,11]. Garlic has a long history of medicinal use as an antimicrobial, immunomodulator, antioxidant, anticancer, and anti-aging agent[11]. The primary active compounds in garlic (Figure 1) are organosulfur compounds, such as allicin, alliin, ajoene (in both E and Z forms), S-allylcysteine, diallyl sulfide (DAS), DADS, diallyl trisulfide (DATS), and vinyl dithiins, as well as saponins, phenolic compounds (e.g., β-resorcylic acid, pyrogallol, gallic acid, rutin, protocatechuic acid, quercetin), polysaccharides, and other components such as fatty acids, amino acids, vitamins, and minerals[12-16]. Allicin, the most biologically active sulfur compound in garlic, is lipophilic and responsible for its characteristic odor and taste[17]. It represents between 70% and 80% of garlic’s bioactive components[18]. Allicin is formed from alliin through the action of the enzyme alliinase, which is activated when garlic is cut or crushed[17-19]. Subsequently, allicin rapidly decomposes into other components due to heat exposure[19].
Regarding its antimicrobial effects, the antibacterial activity of garlic is mainly attributed to allicin[17,20], while its fungicidal effect is attributed to allicin, DADS, and DATS[17]. Both antiparasitic and antiviral activities are linked to allicin, ajoene, and DATS[16,17,21]. The antitumoral activity of garlic is believed to result from the enhancement of p38 expression by allicin, alliin, DADS, and DAS, inhibition of tumor vascularization, and promotion of apoptosis in cancer cells. Additionally, ajoene stimulates apoptosis, promotes peroxide production, and increases the activity of caspases-3 and caspases-8[17,18,22-24].
Allicin, DADS, and DATS are the primary antioxidant compounds in garlic, acting through the modulation of reactive oxygen species (ROS), increasing glutathione levels, and activating cellular antioxidant enzymes. Alliin, in turn, regulates ROS generation and prevents the activation of mitogen-activated protein kinases. Furthermore, DADS suppresses the enzymatic activity of cytochrome P450 2E1, reducing the production of reactive oxygen and nitrogen species[17,22,25,26]. Garlic has also been shown to protect against various natural, chemical, and industrial toxicities, as well as the adverse effects of medications, suggesting its potential as an antidote[27]. Allicin exhibits anti-inflammatory activity by inhibiting stromal cell-derived factor 1 alpha and the transendothelial migration of neutrophils. Meanwhile, DADS reduces the expression of inflammatory cytokines such as NF-κB, IL-1β, and TNF-α, as well as ROS generation, by suppressing the activity of cytochrome P450 2E1. Additionally, thiacremonone blocks NF-κB activity[17,22,28,29].
Concerning obesity, ajoene stimulates adipocyte apoptosis, reduces fat accumulation, and decreases body weight gain by activating adenosine monophosphate-activated protein kinase and increasing thermogenesis[17,30]. 1,2-vinyl dithiol decreases the expression of CCAAT/enhancer-binding protein alpha, peroxisome proliferator-activated receptor gamma 2, and lipoprotein lipase[17,31]. DAS, allicin, cysteine sulfoxide, S-allylcysteine sulfoxide, and alliin exhibit antidiabetic properties by enhancing insulin production[17,32]. Furthermore, hypolipidemic effects have been observed with various garlic preparations[17]. Allicin and DADS also reduce both systolic and diastolic blood pressure, while gamma-glutamylcysteine inhibits angiotensin-converting enzyme (ACE)[17,22,23,33].
The chemical compounds in garlic can reduce inflammatory markers through the regulation of IL-10 and NF-κB[34]. Studies have found that garlic reduces levels of CRP and TNF-α, although results regarding its effect on IL-6 Levels have been inconsistent[34-41]. Garlic does not appear to have a significant effect on leptin (pro-inflammatory) or adiponectin (anti-inflammatory) levels[35]. Furthermore, garlic enhances the proliferation and activation of immune cells, such as γδ-T cells and natural killer cells[42], while reducing the number of natural killer T cells, indicating a modulatory effect on the immune system and inflammation[39].
Garlic can improve oxidative stress through various mechanisms, including the reduction of superoxide radical production, enhancement of enzymatic and non-enzymatic antioxidant activity and expression, and the reduction of certain pro-oxidant enzymes[44]. Garlic supplementation has been shown to increase serum levels of total antioxidant capacity, reduced glutathione, and superoxide dismutase, while decreasing serum levels of malondialdehyde[43-47]. Raw garlic exhibits higher antioxidant capacity than cooked garlic. Additionally, the ethanolic extract of garlic sprouts demonstrated superior antioxidant activity compared to raw garlic extract, while aged black garlic displayed more prominent antioxidant properties than fresh garlic[48-50] This is likely due to the fact that although aged black garlic contains less allicin, it has a higher concentration of polyphenols and flavonoids[51].
In vitro, silver nanoparticles ranging from 10 nm to 30 nm, synthesized from garlic, increased glucose utilization, reduced hepatic glucose production, inhibited starch-digesting enzymes such as α-amylase and α-glucosidase, and stimulated pancreatic insulin secretion[52]. In studies conducted in rats with streptozotocin- or alloxan-induced DM, various garlic preparations were administered, including raw garlic extract (125 mg/kg/day, 250 mg/kg/day, 500 mg/kg/day, or 750 mg/kg/day, orally or intraperitoneally), ethanolic garlic extract (0.1 g/kg/day, 0.25 g/kg/day, or 0.5 g/kg/day, orally), aged garlic, garlic extract combined with “Vernonia amygdalina”, and aqueous extracts of garlic, ginger, and cayenne pepper (200 mg/kg/day or 500 mg/kg/day), over periods ranging from 1 weeks to 8 weeks. The results showed a reduction in glucose levels[43,44,53-59], glycosylated hemoglobin A1c (HbA1c)[44,55,56] and histopathological alterations[55,58], as well as an increase in serum insulin levels[44,54,57,59,60], improvement in insulin resistance[57] and body weight[43,59,60]. In some studies, the effects were dose-dependent[44,59], and were comparable to or even greater than those observed with glibenclamide or metformin[44,54,55,59]. In rabbits, similar reductions in glucose levels were also observed[61].
In patients with T2DM, garlic supplementation (300-1500 mg/day) has been associated with reductions in fasting glucose (-17 to 29 mg/dL), HbA1c (-0.4% to 0.8%) and improved insulin sensitivity. Similarly, in our trial, a polyherbal compound containing 300 mg of garlic, along with aloe vera, fenugreek, milk thistle, black seed and psyllium, reduced HbA1c by 0.5% and significantly lowered triglycerides (-40 mg/dL), total cholesterol (-35 mg/dL), and LDL (-28 mg/dL) after 12 weeks, outperforming the control group under standard therapy alone[62,63]. The results showed a reduction in fasting glucose[62,64-68], postprandial glucose[67,68], HbA1c[63,66,68] and insulin resistance[68]. In some studies, these effects were dose-dependent[66] and duration-dependent[66], with results comparable to or even superior to those observed with metformin[66,67]. No adverse effects on renal or hepatic function were reported[63,68]. On the other hand, studies that administered raw garlic (300 mg/day or 3 cloves per day for 2 weeks) or aged garlic (1200 mg/day for 4 weeks) did not show significant effects on the reduction of fasting glucose[63,69,70]. Systematic reviews and meta-analyses have demonstrated that garlic, in various forms (powder: 300 mg/day to 22400 mg/day, oil: 4000 mg/day, aged garlic extract: 1200-6000 mg/day, raw garlic: 4000 mg/day, and enteric-coated supplements: 800 mg/day), administered over periods ranging from 3 weeks to 1 year, can significantly reduce blood glucose levels, HbA1c, and fructosamine[71,72], as well as improve insulin resistance[72], without increasing the frequency of complications[73].
In rat studies with streptozotocin- or alloxan-induced DM, various garlic preparations were used: Raw garlic extract (250-500 mg/kg/day, intraperitoneally), ethanolic extract (0.1-0.5 g/kg/day, orally), aged garlic, and aqueous garlic with ginger and cayenne pepper (200-500 mg/kg/day). Treatments lasted from 1 week to 24 weeks. These showed dose-dependent reductions in total cholesterol (TC) and triglycerides (TG)[44,53,54,56,59]. Similar TC reductions were seen in rabbits[61]. In T2DM patients, raw garlic (300 mg/day), garlic tablets (300 mg three times/day), or tablets with 1.3% allicin (300 mg twice/day) were given alone or with standard treatment for 4-24 weeks. Results included reduced TC, low-density lipoprotein cholesterol (LDL), and TG[63,65,74], and increased high-density lipoprotein cholesterol (HDL)[65,74], without adverse kidney or liver effects[63].
However, aged garlic (1200 mg/day for 4 weeks) showed no significant change in TC or TG[70,74]. Systematic reviews and meta-analyses report that garlic (powder: 300-22400 mg/day, oil: 4000 mg/day, aged extract: 1200-6000 mg/day, raw: 4000 mg/day, enteric-coated: 800 mg/day) for 3 weeks to 1 year may lower TC[72,73,75-77], LDL[72,73,77], and TG[75,76], and raise HDL[72]. Still, some studies found no significant changes in LDL, HDL[75,76], apolipoprotein B[75], or TG[72]. Effects often depended on treatment duration and initial lipid levels[75]. No increase in complications was reported[73]. Yet, due to low-certainty evidence, more high-quality studies are needed. Some reviews found no significant impact of garlic on lipid profiles[78,79].
In studies conducted on rats with streptozotocin- or alloxan-induced DM, various garlic preparations were administered, including ethanolic garlic extract (0.1 g/kg/day, 0.25 g/kg/day, or 0.5 g/kg/day, orally), aged garlic, aqueous garlic extract (100 mg/kg/day or 200 mg/kg/day), and an aqueous extract of garlic, ginger, and cayenne pepper (200 mg/kg/day or 500 mg/kg/day) over periods ranging from 1 week to 8 weeks. The results showed a reduction, normalization, or prevention of elevated levels of liver enzymes such as aspartate aminotransferase, alanine aminotransferase[44,54,59,80] and lactate dehydrogenase[44], as well as a reduction in liver lipid peroxidation[56] and repair of DM-induced liver damage[59], in a dose-dependent manner[44,56].
In mice fed a high-fat diet, essential garlic oil (50 mg/kg and 100 mg/kg) and DADS (20 mg/kg) significantly reduced the release of proinflammatory cytokines in the liver and increased antioxidant capacity by inhibiting the expression of cytochrome P450 2E1, thereby protecting against the development of MASLD[81]. In adults with mild liver dysfunction, the administration of fermented garlic extracts over a 12-week period resulted in improved levels of gamma-glutamyl transferase (P = 0.066) and alanine aminotransferase (P = 0.014), with no additional adverse effects[82].
A systematic review and meta-analysis in animal models of MASLD revealed that garlic improves metabolic parameters associated with the development of the disease, such as blood glucose, insulin, and lipid levels[83]. Another systematic review, which included both animal and human studies, suggested that garlic could regulate the progression of MASLD through mechanisms such as weight reduction, modulation of lipid and glucose metabolism, and reduction of inflammation and oxidative stress[84]. A meta-analysis in humans demonstrated that the likelihood of reducing hepatic steatosis with garlic was 2.75 times higher than with placebo (P < 0.001), with improvements in liver enzymes and metabolic parameters[85]. Furthermore, another meta-analysis in humans indicated that garlic significantly reduces liver enzymes and decreases the likelihood of being diagnosed with MASLD by 46%[86].
Hypertension, which is common in patients with T2DM, is a key risk factor for cardiovascular disease (CVD) and microvascular complications[87]. Garlic, due to its content of allicin and DADS, exerts antihypertensive effects in a dose-dependent manner by inhibiting ACE, reducing the synthesis of vasoconstrictor prostanoids, increasing nitric oxide levels, and reversing arterial remodeling[33,88]. It has been shown to effectively reduce both systolic and diastolic blood pressure without increasing serious adverse effects[38,70,88-98].
In preclinical studies, garlic components have been found to inhibit ACE and reduce the concentration of angiotensin II, acting synergistically with drugs that block the renin-angiotensin system. Additionally, garlic improves the oral bioavailability of nifedipine and propranolol. When combined with carvedilol or atenolol, garlic prevents myocardial damage and reduces oxidative stress[99,100]. Based on the section discussed, Table 1 provides a brief overview of the proposed mechanisms and effects of garlic on cardiometabolic disorders.
| Condition | Mechanisms and Effects |
| Dyslipidemia | ↓ TC, TG, LDL |
| ↑ HDL | |
| Garlic forms: Raw, tablets, aged | |
| Effect varies with dose and duration | |
| MASLD | ↓ Liver enzymes (ALT, AST, GGT) |
| ↓ Lipid peroxidation | |
| ↑ Antioxidant activity | |
| Improves metabolic parameters | |
| Hypertension | ACE inhibition |
| ↓ Systolic/diastolic blood pressure | |
| ↑ NO levels | |
| Arterial remodeling | |
| Synergistic with drugs |
Studies conducted in streptozotocin- or alloxan-induced diabetic rat models have employed various garlic preparations - raw garlic extract (500 mg/kg, intraperitoneally), ethanolic garlic extract (0.1-0.5 g/kg/day, orally), aged garlic, allicin (16 mg/kg/day), aqueous garlic extract (100-2000 mg/kg/day), and mixed aqueous extracts of garlic, ginger, and cayenne pepper (200-500 mg/kg/day) - administered over 1 week to 8 weeks. These interventions consistently demonstrated reductions, normalization, or prevention of elevated serum urea and creatinine levels[54,59,80]. Garlic exhibits nephroprotective effects in diabetic models primarily through its antioxidant and anti-inflammatory properties. Aqueous garlic extract (2 g/kg/day) significantly lowered serum urea, creatinine, and uric acid concentrations, decreased renal malondialdehyde and total oxidant status, while enhancing total antioxidant capacity and reducing renal TNF-α and nitric oxide levels[101].
Similarly, allicin administration (16 mg/kg/day) improved renal function by reducing proteinuria, creatinine clearance, and urinary N-acetyl-β-D-glucosaminidase, while attenuating oxidative stress and suppressing proinflammatory cytokines, including IL-1β, IL-6, NF-κB, and transforming growth factor β1[102]. Furthermore, garlic supplementation reduced urinary protein loss[53], particularly albuminuria[103], and decreased serum uric acid levels[54]. Histopathological analyses revealed partial renal tissue repair[59,101], with efficacy in some studies exceeding that of glibenclamide[59]. Additional nephroprotective effects included reduced excretion of N-acetyl-β-D-glucosaminidase[102,103], diminished renal lipid peroxidation[56,103], and lowered expression of inflammatory mediators (IL-1β, IL-6, TNF-α, NF-κB)[101,102]. Notably, garlic reversed the diabetes-induced upregulation of angiotensin II type 1 receptors and the downregulation of type 2 receptors[103]. Many of these effects were shown to be dose-dependent[56,59]. A recent systematic review and meta-analysis reinforced garlic’s therapeutic potential in diabetic kidney disease, identifying optimal nephroprotective outcomes at garlic doses of 500 mg/kg for 8-10 weeks or garlic component doses of 45-150 mg/kg over 4-10 weeks[104].
Garlic enhances the function of brain-derived neurotrophic factor, conferring neuroprotective properties and positioning it as a complementary option in the treatment of patients with diabetic retinopathy in combination with conventional therapies. In addition, garlic suppresses the action of vascular endothelial growth factor, contributing to the reduction of angiogenesis, and inhibits the activity of hypoxia-inducible factor 1, both key factors in the development of this pathology[105]. In a study conducted in rats with streptozotocin-induced DM, raw garlic extract (0.4 g/100 g body weight) administered for 7 weeks showed favorable effects in improving morphological changes in the retina[106].
Individuals with DM are at increased risk of CVD, which is not solely attributable to the alterations caused by hyperglycemia but also to excess ectopic fat[107]. Several studies have found that garlic consumption is significantly associated with increased levels of HDL cholesterol and apolipoprotein A, as well as a reduction in TC, TG, LDL, CRP, IL-6, homocysteine, and coronary artery calcium, suggesting that garlic may reduce risk factors for CVD[38,70,71,108-116]. However, other studies have not observed any benefits in terms of reducing CVD risk[90,117-119].
Although garlic does not contain known toxic compounds and administration of garlic to rats at doses of 50 mg/kg shows no clinical adverse effects, doses exceeding 200 mg/kg may cause liver damage and impair antioxidant mechanisms[18,120,121]. Excessive consumption of raw garlic on an empty stomach may disrupt the gut microbiota. Topical application can cause painful lesions, rashes, and burns. In rodents, daily administration of 50 mg of garlic powder over extended periods inhibits spermatogenesis. Long-term exposure to high doses results in anemia and weight loss due to red blood cell lysis[18,121]. Additionally, studies have shown that allicin, one of the main active compounds of garlic, although beneficial in certain diabetic complications such as diabetic retinopathy through its antioxidative and anti-inflammatory properties mediated by activation of phosphatase and tensin homolog induced putative kinase 1/parkin-mitophagy and inhibition of the nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 inflammasome, also possesses bioactivity that could potentially affect systemic physiological processes[121]. Garlic may interact with anticoagulant and antiplatelet drugs, increasing the risk of bleeding. This effect raises concerns for surgical patients, as it can prolong bleeding time. Caution is advised when combining garlic with medications such as warfarin, aspirin, or clopidogrel[122].
Further research is needed to expand knowledge on the bioactive components of garlic, as well as its biological functions and mechanisms of action. Studies have demonstrated promising roles of compounds like allicin, particularly in oxidative stress regulation and inflammation control, as observed in experimental models of diabetic complications such as diabetic retinopathy[121]. It is also critical to study the potential impact of processing techniques on its efficacy and safety, as these may alter the concentration and activity of key compounds[120]. Moreover clinical trials in humans are required to validate the health benefits of garlic observed in preclinical studies, with a particular focus on assessing its safety and potential adverse effects. However, current evidence is still limited by factors such as short intervention periods, small sample sizes, and lack of standardized garlic preparations, which may affect the reproducibility and generalizability of the findings[120,122].
Garlic emerges as a potentially beneficial adjunct in the management of T2DM and its comorbidities or complications, demonstrating significant effects in reducing serum lipids, improving liver and kidney parameters, and regulating blood pressure and oxidative stress. Its antihypertensive, hypolipidemic, hepatoprotective, antioxidant, and anti-inflammatory properties have been evidenced in preclinical studies some clinical trials and even meta-analysis, although results vary depending on the dose, form of administration, and duration of treatment. Nevertheless, the limited quality and heterogeneity of the available evidence underscores the need for more rigorous clinical studies to confirm these findings, optimize therapeutic applications, and assess its long-term safety.
| 1. | Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Lernmark Å, Metzger BE, Nathan DM, Kirkman MS. Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Diabetes Care. 2023;46:e151-e199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 102] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 2. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4810] [Article Influence: 1603.3] [Reference Citation Analysis (36)] |
| 3. | Gregg EW, Pratt A, Owens A, Barron E, Dunbar-Rees R, Slade ET, Hafezparast N, Bakhai C, Chappell P, Cornelius V, Johnston DG, Mathews J, Pickles J, Bragan Turner E, Wainman G, Roberts K, Khunti K, Valabhji J. The burden of diabetes-associated multiple long-term conditions on years of life spent and lost. Nat Med. 2024;30:2830-2837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 4. | Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2178] [Cited by in RCA: 2626] [Article Influence: 187.6] [Reference Citation Analysis (0)] |
| 5. | Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 996] [Article Influence: 332.0] [Reference Citation Analysis (0)] |
| 6. | Gandhi GR, Mohana T, Athesh K, Hillary VE, Vasconcelos ABS, Farias de Franca MN, Montalvão MM, Ceasar SA, Jothi G, Sridharan G, Gurgel RQ, Xu B. Anti-inflammatory natural products modulate interleukins and their related signaling markers in inflammatory bowel disease: A systematic review. J Pharm Anal. 2023;13:1408-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 7. | Gandhi GR, Jothi G, Mohana T, Vasconcelos ABS, Montalvão MM, Hariharan G, Sridharan G, Kumar PM, Gurgel RQ, Li HB, Zhang J, Gan RY. Anti-inflammatory natural products as potential therapeutic agents of rheumatoid arthritis: A systematic review. Phytomedicine. 2021;93:153766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Raei Abbasabadi O, Farahpour MR. Wound Healing: A Brief Look at the Inflammatory Stage and Role of Medicinal Plants and Their Derivations on Modulating the Inflammatory Responses: A Systematic Review. Int J Low Extrem Wounds. 2023;15347346231212331. [PubMed] [DOI] [Full Text] |
| 9. | Nikiema WA, Ouédraogo M, Ouédraogo WP, Fofana S, Ouédraogo BHA, Delma TE, Amadé B, Abdoulaye GM, Sawadogo AS, Ouédraogo R, Semde R. Systematic Review of Chemical Compounds with Immunomodulatory Action Isolated from African Medicinal Plants. Molecules. 2024;29:2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Bhowmick BK, Sarkar S, Roychowdhury D, Patil SD, Lekhak MM, Ohri D, Rama Rao S, Yadav SR, Verma RC, Dhar MK, Raina SN, Jha S. Allium cytogenetics: a critical review on the Indian taxa. Comp Cytogenet. 2023;17:129-156. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Rahman K. Historical perspective on garlic and cardiovascular disease. J Nutr. 2001;131:977S-979S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Askari M, Mozaffari H, Darooghegi Mofrad M, Jafari A, Surkan PJ, Amini MR, Azadbakht L. Effects of garlic supplementation on oxidative stress and antioxidative capacity biomarkers: A systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2021;35:3032-3045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Itrat N, Nisa MU, Al-Asmari F, Ramadan MF, Zongo E. A double-blind, randomized control trial to investigate the therapeutic potential of garlic scapes for high apoprotein E levels in a high-Fat diet-induced hypercholesteremic rat model. Food Sci Nutr. 2024;12:7607-7619. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Shang A, Cao SY, Xu XY, Gan RY, Tang GY, Corke H, Mavumengwana V, Li HB. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods. 2019;8:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 15. | Kim JH, Kim JW, Yu SH, Lee J, Cho HT, Heo W, Park SJ, Lee JH, Kim YJ. Utilization of Pectinase Cocktail and High Hydrostatic Pressure for the Production of Aged Black Garlic Juice with Improved Nutritional Value. Prev Nutr Food Sci. 2019;24:357-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Rouf R, Uddin SJ, Sarker DK, Islam MT, Ali ES, Shilpi JA, Nahar L, Tiralongo E, Sarker SD. Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci Technol. 2020;104:219-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 17. | El-Saber Batiha G, Magdy Beshbishy A, G Wasef L, Elewa YHA, A Al-Sagan A, Abd El-Hack ME, Taha AE, M Abd-Elhakim Y, Prasad Devkota H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients. 2020;12:872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 345] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 18. | El-Saadony MT, Saad AM, Korma SA, Salem HM, Abd El-Mageed TA, Alkafaas SS, Elsalahaty MI, Elkafas SS, Mosa WFA, Ahmed AE, Mathew BT, Albastaki NA, Alkuwaiti AA, El-Tarabily MK, AbuQamar SF, El-Tarabily KA, Ibrahim SA. Garlic bioactive substances and their therapeutic applications for improving human health: a comprehensive review. Front Immunol. 2024;15:1277074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Rauf A, Abu-Izneid T, Thiruvengadam M, Imran M, Olatunde A, Shariati MA, Bawazeer S, Naz S, Shirooie S, Sanches-Silva A, Farooq U, Kazhybayeva G. Garlic (Allium sativum L.): Its Chemistry, Nutritional Composition, Toxicity, and Anticancer Properties. Curr Top Med Chem. 2022;22:957-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Bhatwalkar SB, Mondal R, Krishna SBN, Adam JK, Govender P, Anupam R. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Front Microbiol. 2021;12:613077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 21. | Foroutan-Rad M, Tappeh KH, Khademvatan S. Antileishmanial and Immunomodulatory Activity of Allium sativum (Garlic): A Review. J Evid Based Complementary Altern Med. 2017;22:141-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Chidike Ezeorba TP, Ezugwu AL, Chukwuma IF, Anaduaka EG, Udenigwe CC. Health-promoting properties of bioactive proteins and peptides of garlic (Allium sativum). Food Chem. 2024;435:137632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 23. | Bayan L, Koulivand PH, Gorji A. Garlic: a review of potential therapeutic effects. Avicenna J Phytomed. 2014;4:1-14. [PubMed] |
| 24. | Zhang X, Zhu Y, Duan W, Feng C, He X. Allicin induces apoptosis of the MGC-803 human gastric carcinoma cell line through the p38 mitogen-activated protein kinase/caspase-3 signaling pathway. Mol Med Rep. 2015;11:2755-2760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Chen Y, Sun J, Dou C, Li N, Kang F, Wang Y, Cao Z, Yang X, Dong S. Alliin Attenuated RANKL-Induced Osteoclastogenesis by Scavenging Reactive Oxygen Species through Inhibiting Nox1. Int J Mol Sci. 2016;17:1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Abdel-Daim MM, Shaheen HM, Abushouk AI, Toraih EA, Fawzy MS, Alansari WS, Aleya L, Bungau S. Thymoquinone and diallyl sulfide protect against fipronil-induced oxidative injury in rats. Environ Sci Pollut Res Int. 2018;25:23909-23916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Dorrigiv M, Zareiyan A, Hosseinzadeh H. Garlic (Allium sativum) as an antidote or a protective agent against natural or chemical toxicities: A comprehensive update review. Phytother Res. 2020;34:1770-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Abdel-Daim MM, Abushouk AI, Bungău SG, Bin-Jumah M, El-Kott AF, Shati AA, Aleya L, Alkahtani S. Protective effects of thymoquinone and diallyl sulphide against malathion-induced toxicity in rats. Environ Sci Pollut Res Int. 2020;27:10228-10235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Jin P, Kim JA, Choi DY, Lee YJ, Jung HS, Hong JT. Anti-inflammatory and anti-amyloidogenic effects of a small molecule, 2,4-bis(p-hydroxyphenyl)-2-butenal in Tg2576 Alzheimer's disease mice model. J Neuroinflammation. 2013;10:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Han CY, Ki SH, Kim YW, Noh K, Lee DY, Kang B, Ryu JH, Jeon R, Kim EH, Hwang SJ, Kim SG. Ajoene, a stable garlic by-product, inhibits high fat diet-induced hepatic steatosis and oxidative injury through LKB1-dependent AMPK activation. Antioxid Redox Signal. 2011;14:187-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Keophiphath M, Priem F, Jacquemond-Collet I, Clément K, Lacasa D. 1,2-vinyldithiin from garlic inhibits differentiation and inflammation of human preadipocytes. J Nutr. 2009;139:2055-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Zhai B, Zhang C, Sheng Y, Zhao C, He X, Xu W, Huang K, Luo Y. Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci Rep. 2018;8:3527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 33. | Sleiman C, Daou RM, Al Hazzouri A, Hamdan Z, Ghadieh HE, Harbieh B, Romani M. Garlic and Hypertension: Efficacy, Mechanism of Action, and Clinical Implications. Nutrients. 2024;16:2895. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Mirzavandi F, Mollahosseini M, Salehi-Abargouei A, Makiabadi E, Mozaffari-Khosravi H. Effects of garlic supplementation on serum inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr. 2020;14:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Darooghegi Mofrad M, Milajerdi A, Koohdani F, Surkan PJ, Azadbakht L. Garlic Supplementation Reduces Circulating C-reactive Protein, Tumor Necrosis Factor, and Interleukin-6 in Adults: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J Nutr. 2019;149:605-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Koushki M, Amiri-Dashatan N, Pourfarjam Y, Doustimotlagh AH. Effect of garlic intake on inflammatory mediators: a systematic review and meta-analysis of randomised controlled trials. Postgrad Med J. 2021;97:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Taghizadeh M, Hamedifard Z, Jafarnejad S. Effect of garlic supplementation on serum C-reactive protein level: A systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2019;33:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Varade S, Nadella M, Hirake A, Mungase SB, Ali A, Adela R. Effect of garlic on the components of metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. J Ethnopharmacol. 2024;318:116960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Xu C, Mathews AE, Rodrigues C, Eudy BJ, Rowe CA, O'Donoughue A, Percival SS. Aged garlic extract supplementation modifies inflammation and immunity of adults with obesity: A randomized, double-blind, placebo-controlled clinical trial. Clin Nutr ESPEN. 2018;24:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 40. | Bara-Ledesma N, Jimenez-Esteban J, Fabregate M, Fabregate-Fuente R, Cymberknop LJ, Castillo-Martinez P, Navarro-Fayos MT, Gomez Del Olmo V, Saban-Ruiz J. Effect of Encapsulated Purple Garlic Oil on Microvascular Function and the Components of Metabolic Syndrome: A Randomized Placebo-Controlled Study-The ENDOTALLIUM Study. Nutrients. 2024;16:1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 41. | Zare E, Alirezaei A, Bakhtiyari M, Mansouri A. Evaluating the effect of garlic extract on serum inflammatory markers of peritoneal dialysis patients: a randomized double-blind clinical trial study. BMC Nephrol. 2019;20:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Percival SS. Aged Garlic Extract Modifies Human Immunity. J Nutr. 2016;146:433S-436S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Barbu IA, Toma VA, Moț AC, Vlase AM, Butiuc-Keul A, Pârvu M. Chemical Composition and Antioxidant Activity of Six Allium Extracts Using Protein-Based Biomimetic Methods. Antioxidants (Basel). 2024;13:1182. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 44. | Behrouj H, Ziamajidi N, Abbasalipourkabir R, Goodarzi MT, Saidijam M. Hypoglycemic and antioxidant effects of oral administration of garlic extract in the livers of type 1 diabetic rats. J Basic Clin Physiol Pharmacol. 2018;30:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Saleh NKM, Mohamed AEA, Moussa MH, Assal Y, Lasheen NN. Garlic oil improves small intestinal motility in experimentally induced type II diabetes mellitus in female Wistar rats. PLoS One. 2024;19:e0301621. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Moosavian SP, Paknahad Z, Habibagahi Z. A randomized, double-blind, placebo-controlled clinical trial, evaluating the garlic supplement effects on some serum biomarkers of oxidative stress, and quality of life in women with rheumatoid arthritis. Int J Clin Pract. 2020;74:e13498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Sangouni AA, Mohammad Hosseini Azar MR, Alizadeh M. Effects of garlic powder supplementation on insulin resistance, oxidative stress, and body composition in patients with non-alcoholic fatty liver disease: A randomized controlled clinical trial. Complement Ther Med. 2020;51:102428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Jang HJ, Lee HJ, Yoon DK, Ji DS, Kim JH, Lee CH. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci Biotechnol. 2018;27:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 49. | Zakarova A, Seo JY, Kim HY, Kim JH, Shin JH, Cho KM, Lee CH, Kim JS. Garlic sprouting is associated with increased antioxidant activity and concomitant changes in the metabolite profile. J Agric Food Chem. 2014;62:1875-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Locatelli DA, Nazareno MA, Fusari CM, Camargo AB. Cooked garlic and antioxidant activity: Correlation with organosulfur compound composition. Food Chem. 2017;220:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 51. | Kimura S, Tung YC, Pan MH, Su NW, Lai YJ, Cheng KC. Black garlic: A critical review of its production, bioactivity, and application. J Food Drug Anal. 2017;25:62-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 52. | Jini D, Sharmila S, Anitha A, Pandian M, Rajapaksha RMH. In vitro and in silico studies of silver nanoparticles (AgNPs) from Allium sativum against diabetes. Sci Rep. 2022;12:22109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Madkor HR, Mansour SW, Ramadan G. Modulatory effects of garlic, ginger, turmeric and their mixture on hyperglycaemia, dyslipidaemia and oxidative stress in streptozotocin-nicotinamide diabetic rats. Br J Nutr. 2011;105:1210-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 54. | Liu CT, Hsu TW, Chen KM, Tan YP, Lii CK, Sheen LY. The Antidiabetic Effect of Garlic Oil is Associated with Ameliorated Oxidative Stress but Not Ameliorated Level of Pro-inflammatory Cytokines in Skeletal Muscle of Streptozotocin-induced Diabetic Rats. J Tradit Complement Med. 2012;2:135-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Kaur G, Padiya R, Adela R, Putcha UK, Reddy GS, Reddy BR, Kumar KP, Chakravarty S, Banerjee SK. Garlic and Resveratrol Attenuate Diabetic Complications, Loss of β-Cells, Pancreatic and Hepatic Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Front Pharmacol. 2016;7:360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 56. | Thomson M, Al-Qattan KK, Js D, Ali M. Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2016;16:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 57. | Kesavanarayanan KS, Priya RJ, Selvakkumar C, Kavimani S, Prathiba D. Herbal formulation, DIA-2 and Rosiglitazone ameliorates hyperglycemia and hepatic steatosis in type 2 diabetic rats. Eur Rev Med Pharmacol Sci. 2015;19:3107-3117. [PubMed] |
| 58. | El-Demerdash FM, Yousef MI, El-Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 59. | Alvarez-Jimenez L, Morales-Palomo F, Moreno-Cabañas A, Ortega JF, Mora-Rodríguez R. Effects of statin therapy on glycemic control and insulin resistance: A systematic review and meta-analysis. Eur J Pharmacol. 2023;947:175672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 60. | Kook S, Kim GH, Choi K. The antidiabetic effect of onion and garlic in experimental diabetic rats: meta-analysis. J Med Food. 2009;12:552-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Sher A, Fakhar-ul-Mahmood M, Shah SN, Bukhsh S, Murtaza G. Effect of garlic extract on blood glucose level and lipid profile in normal and alloxan diabetic rabbits. Adv Clin Exp Med. 2012;21:705-711. [PubMed] |
| 62. | Choudhary PR, Jani RD, Sharma MS. Effect of Raw Crushed Garlic (Allium sativum L.) on Components of Metabolic Syndrome. J Diet Suppl. 2018;15:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 63. | Ghorbani A, Zarvandi M, Rakhshandeh H. A Randomized Controlled Trial of a Herbal Compound for Improving Metabolic Parameters in Diabetic Patients with Uncontrolled Dyslipidemia. Endocr Metab Immune Disord Drug Targets. 2019;19:1075-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Sobenin IA, Nedosugova LV, Filatova LV, Balabolkin MI, Gorchakova TV, Orekhov AN. Metabolic effects of time-released garlic powder tablets in type 2 diabetes mellitus: the results of double-blinded placebo-controlled study. Acta Diabetol. 2008;45:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Ashraf R, Khan RA, Ashraf I. Garlic (Allium sativum) supplementation with standard antidiabetic agent provides better diabetic control in type 2 diabetes patients. Pak J Pharm Sci. 2011;24:565-570. [PubMed] |
| 66. | Zarvandi M, Rakhshandeh H, Abazari M, Shafiee-Nick R, Ghorbani A. Safety and efficacy of a polyherbal formulation for the management of dyslipidemia and hyperglycemia in patients with advanced-stage of type-2 diabetes. Biomed Pharmacother. 2017;89:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Kumar R, Chhatwal S, Arora S, Sharma S, Singh J, Singh N, Bhandari V, Khurana A. Antihyperglycemic, antihyperlipidemic, anti-inflammatory and adenosine deaminase- lowering effects of garlic in patients with type 2 diabetes mellitus with obesity. Diabetes Metab Syndr Obes. 2013;6:49-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 68. | Parham M, Bagherzadeh M, Asghari M, Akbari H, Hosseini Z, Rafiee M, Vafaeimanesh J. Evaluating the effect of a herb on the control of blood glucose and insulin-resistance in patients with advanced type 2 diabetes (a double-blind clinical trial). Caspian J Intern Med. 2020;11:12-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 69. | Alzahrani MA, Alsiary KA, Khan MA, Bushnaq A, Alzahrani B, Salama M, Alamri NS. Perception of herbs use in treating diabetes among patients attending specialized polyclinics of National Guard Health Affairs, Jeddah. J Family Med Prim Care. 2023;12:270-275. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 70. | Atkin M, Laight D, Cummings MH. The effects of garlic extract upon endothelial function, vascular inflammation, oxidative stress and insulin resistance in adults with type 2 diabetes at high cardiovascular risk. A pilot double blind randomized placebo controlled trial. J Diabetes Complications. 2016;30:723-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 71. | Shabani E, Sayemiri K, Mohammadpour M. The effect of garlic on lipid profile and glucose parameters in diabetic patients: A systematic review and meta-analysis. Prim Care Diabetes. 2019;13:28-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 72. | Zhao X, Cheng T, Xia H, Yang Y, Wang S. Effects of Garlic on Glucose Parameters and Lipid Profile: A Systematic Review and Meta-Analysis on Randomized Controlled Trials. Nutrients. 2024;16:1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 73. | Wang J, Zhang X, Lan H, Wang W. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials. Food Nutr Res. 2017;61:1377571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 74. | Ashraf R, Aamir K, Shaikh AR, Ahmed T. Effects of garlic on dyslipidemia in patients with type 2 diabetes mellitus. J Ayub Med Coll Abbottabad. 2005;17:60-64. [PubMed] |
| 75. | Zeng T, Guo FF, Zhang CL, Song FY, Zhao XL, Xie KQ. A meta-analysis of randomized, double-blind, placebo-controlled trials for the effects of garlic on serum lipid profiles. J Sci Food Agric. 2012;92:1892-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Reinhart KM, Talati R, White CM, Coleman CI. The impact of garlic on lipid parameters: a systematic review and meta-analysis. Nutr Res Rev. 2009;22:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 77. | Piragine E, Malanima MA, Ceccanti C, Guidi L, Martelli A, Lucenteforte E, Calderone V. Alliaceae versus Brassicaceae for Dyslipidemia: State of the Art and Future Perspectives. Systematic Review and Meta-Analysis of Clinical Studies. Phytother Res. 2024;38:5765-5781. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 78. | Alder R, Lookinland S, Berry JA, Williams M. A systematic review of the effectiveness of garlic as an anti-hyperlipidemic agent. J Am Acad Nurse Pract. 2003;15:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Posadzki P, AlBedah AM, Khalil MM, AlQaed MS. Complementary and alternative medicine for lowering blood lipid levels: A systematic review of systematic reviews. Complement Ther Med. 2016;29:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Ried K, Frank OR, Stocks NP. Aged garlic extract reduces blood pressure in hypertensives: a dose-response trial. Eur J Clin Nutr. 2013;67:64-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 81. | Lai YS, Chen WC, Ho CT, Lu KH, Lin SH, Tseng HC, Lin SY, Sheen LY. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J Agric Food Chem. 2014;62:5897-5906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 82. | Kim HN, Kang SG, Roh YK, Choi MK, Song SW. Efficacy and safety of fermented garlic extract on hepatic function in adults with elevated serum gamma-glutamyl transpeptidase levels: a double-blind, randomized, placebo-controlled trial. Eur J Nutr. 2017;56:1993-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 83. | Shojaei-Zarghani S, Najafi N, Fattahi MR, Safarpour AR. Influence of Garlic on the Glycemic Control and Lipid Profile in Animals with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Planta Med. 2023;89:1125-1137. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 84. | Pourreza S, Azar PS, Sanaie S, Noshadi N, Jalali S, Niazkar HR, Karimi A, Vajdi M. Therapeutic Effects and Mechanisms of Action of Garlic (Allium sativum) on Nonalcoholic Fatty Liver Disease: A Comprehensive Systematic Literature Review. Evid Based Complement Alternat Med. 2022;2022:6960211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 85. | Rastkar M, Nikniaz L, Abbasalizad Farhangi M, Nikniaz Z. Systematic review and meta-analysis of the effect of garlic in patients with non-alcoholic fatty liver disease. Indian J Gastroenterol. 2022;41:548-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Mardi P, Kargar R, Fazeli R, Qorbani M. Allium sativum: A potential natural compound for NAFLD prevention and treatment. Front Nutr. 2023;10:1059106. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 87. | de Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P, Rossing P, Zoungas S, Bakris G. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40:1273-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 418] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 88. | Xiong XJ, Wang PQ, Li SJ, Li XK, Zhang YQ, Wang J. Garlic for hypertension: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 2015;22:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 89. | Rohner A, Ried K, Sobenin IA, Bucher HC, Nordmann AJ. A systematic review and metaanalysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Stabler SN, Tejani AM, Huynh F, Fowkes C. Garlic for the prevention of cardiovascular morbidity and mortality in hypertensive patients. Cochrane Database Syst Rev. 2012;2012:CD007653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Ried K, Frank OR, Stocks NP, Fakler P, Sullivan T. Effect of garlic on blood pressure: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2008;8:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 92. | Ried K. Garlic lowers blood pressure in hypertensive subjects, improves arterial stiffness and gut microbiota: A review and meta-analysis. Exp Ther Med. 2020;19:1472-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Wang HP, Yang J, Qin LQ, Yang XJ. Effect of garlic on blood pressure: a meta-analysis. J Clin Hypertens (Greenwich). 2015;17:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 94. | Ried K. Garlic Lowers Blood Pressure in Hypertensive Individuals, Regulates Serum Cholesterol, and Stimulates Immunity: An Updated Meta-analysis and Review. J Nutr. 2016;146:389S-396S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 95. | Ried K, Travica N, Sali A. The Effect of Kyolic Aged Garlic Extract on Gut Microbiota, Inflammation, and Cardiovascular Markers in Hypertensives: The GarGIC Trial. Front Nutr. 2018;5:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 96. | Reinhart KM, Coleman CI, Teevan C, Vachhani P, White CM. Effects of garlic on blood pressure in patients with and without systolic hypertension: a meta-analysis. Ann Pharmacother. 2008;42:1766-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 97. | Meher S, Duley L. Garlic for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2006;2006:CD006065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 98. | Saadh MJ, Kariem M, Shukla M, Ballal S, Kumar A, Chahar M, Saini S, Kapila I, Hasaanzadeh S. Effects of aged garlic extract on blood pressure in hypertensive patients: A systematic review and meta-analysis of randomized controlled trials. Prostaglandins Other Lipid Mediat. 2024;175:106914. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 99. | Nyulas KI, Simon-Szabó Z, Pál S, Fodor MA, Dénes L, Cseh MJ, Barabás-Hajdu E, Csipor B, Szakács J, Preg Z, Germán-Salló M, Nemes-Nagy E. Cardiovascular Effects of Herbal Products and Their Interaction with Antihypertensive Drugs-Comprehensive Review. Int J Mol Sci. 2024;25:6388. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 100. | Gao X, Xue Z, Ma Q, Guo Q, Xing L, Santhanam RK, Zhang M, Chen H. Antioxidant and antihypertensive effects of garlic protein and its hydrolysates and the related mechanism. J Food Biochem. 2020;44:e13126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Nasiri A, Ziamajidi N, Abbasalipourkabir R, Goodarzi MT, Saidijam M, Behrouj H, Solemani Asl S. Beneficial Effect of Aqueous Garlic Extract on Inflammation and Oxidative Stress Status in the Kidneys of Type 1 Diabetic Rats. Indian J Clin Biochem. 2017;32:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Arellano Buendía AS, Tostado González M, Sánchez Reyes O, García Arroyo FE, Argüello García R, Tapia E, Sánchez Lozada LG, Osorio Alonso H. Immunomodulatory Effects of the Nutraceutical Garlic Derivative Allicin in the Progression of Diabetic Nephropathy. Int J Mol Sci. 2018;19:3107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 103. | Shiju TM, Rajesh NG, Viswanathan P. Renoprotective effect of aged garlic extract in streptozotocin-induced diabetic rats. Indian J Pharmacol. 2013;45:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 104. | Jiang Y, Li Z, Yue R, Liu G, Yang M, Long C, Yan D. Evidential support for garlic supplements against diabetic kidney disease: a preclinical meta-analysis and systematic review. Food Funct. 2024;15:12-36. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 105. | Sanie-Jahromi F, Zia Z, Afarid M. A review on the effect of garlic on diabetes, BDNF, and VEGF as a potential treatment for diabetic retinopathy. Chin Med. 2023;18:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 106. | Elosta A, Slevin M, Rahman K, Ahmed N. Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro. Sci Rep. 2017;7:39613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 107. | Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. Nat Rev Cardiol. 2023;20:685-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 165] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 108. | Gadidala SK, Johny E, Thomas C, Nadella M, Undela K, Adela R. Effect of garlic extract on markers of lipid metabolism and inflammation in coronary artery disease (CAD) patients: A systematic review and meta-analysis. Phytother Res. 2023;37:2242-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 109. | Li S, Guo W, Lau W, Zhang H, Zhan Z, Wang X, Wang H. The association of garlic intake and cardiovascular risk factors: A systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2023;63:8013-8031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 110. | Du Y, Zhou H, Zha W. Garlic consumption can reduce the risk of dyslipidemia: a meta-analysis of randomized controlled trials. J Health Popul Nutr. 2024;43:113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 111. | Fu Z, Lv J, Gao X, Zheng H, Shi S, Xu X, Zhang B, Wu H, Song Q. Effects of garlic supplementation on components of metabolic syndrome: a systematic review, meta-analysis, and meta-regression of randomized controlled trials. BMC Complement Med Ther. 2023;23:260. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 112. | Gyawali D, Vohra R, Orme-Johnson D, Ramaratnam S, Schneider RH. A Systematic Review and Meta-Analysis of Ayurvedic Herbal Preparations for Hypercholesterolemia. Medicina (Kaunas). 2021;57:546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 113. | Imaizumi VM, Laurindo LF, Manzan B, Guiguer EL, Oshiiwa M, Otoboni AMMB, Araujo AC, Tofano RJ, Barbalho SM. Garlic: A systematic review of the effects on cardiovascular diseases. Crit Rev Food Sci Nutr. 2023;63:6797-6819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 114. | Alali FQ, El-Elimat T, Khalid L, Hudaib R, Al-Shehabi TS, Eid AH. Garlic for Cardiovascular Disease: Prevention or Treatment? Curr Pharm Des. 2017;23:1028-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 115. | Ginter E, Simko V. Garlic (Allium sativum L.) and cardiovascular diseases. Bratisl Lek Listy. 2010;111:452-456. [PubMed] |
| 116. | Valls RM, Companys J, Calderón-Pérez L, Salamanca P, Pla-Pagà L, Sandoval-Ramírez BA, Bueno A, Puzo J, Crescenti A, Bas JMD, Caimari A, Salamanca A, Espinel AE, Pedret A, Arola L, Solà R. Effects of an Optimized Aged Garlic Extract on Cardiovascular Disease Risk Factors in Moderate Hypercholesterolemic Subjects: A Randomized, Crossover, Double-Blind, Sustainedand Controlled Study. Nutrients. 2022;14:405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 117. | Murali S, Smith ER, Tiong MK, Tan SJ, Toussaint ND. Interventions to Attenuate Cardiovascular Calcification Progression: A Systematic Review of Randomized Clinical Trials. J Am Heart Assoc. 2023;12:e031676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 118. | Emamat H, Tangestani H, Totmaj AS, Ghalandari H, Nasrollahzadeh J. The effect of garlic on vascular function: A systematic review of randomized clinical trials. Clin Nutr. 2020;39:3563-3570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 119. | Shekarchizadeh-Esfahani P, Hassani B, Roshanravan N, Sorraya N. Systematic review and meta-analysis of randomised, controlled trials on the effects of garlic supplementation on serum adiponectin and leptin levels. Int J Clin Pract. 2021;75:e14200. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 120. | Rana SV, Pal R, Vaiphei K, Sharma SK, Ola RP. Garlic in health and disease. Nutr Res Rev. 2011;24:60-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 121. | Xu Y, Yu J. Allicin Mitigates Diabetic Retinopathy in Rats by Activating Phosphatase and Tensin Homolog-induced Kinase 1/Parkin-mitophagy and Inhibiting Oxidative Stress-mediated NOD-like Receptor Family Pyrin Domain Containing 3 Inflammasome. J Physiol Investig. 2024;67:215-224. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 122. | Leite PM, Martins MAP, Castilho RO. Review on mechanisms and interactions in concomitant use of herbs and warfarin therapy. Biomed Pharmacother. 2016;83:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |