Published online Jun 20, 2025. doi: 10.5493/wjem.v15.i2.100548
Revised: December 19, 2024
Accepted: January 2, 2025
Published online: June 20, 2025
Processing time: 238 Days and 22.1 Hours
Duchenne muscular dystrophy (DMD) is a neuromuscular disorder caused by mutations in the dystrophin gene. DMD is reported to coexist with other comor
To understand the differential expression of miRNAs in rare comorbid DMD cases.
The Sequin Form Board test, Gesell's drawing test, multiplex ligation probe amplification, and Vineland Social Maturity Scale were applied to confirm the DMD and ASD. Total RNA was isolated from samples using TRIzol. cDNA was synthesized using the Mir-X™ miRNA First-Strand Synthesis kit. qRT-PCR was performed using SYBR Advantage qPCR Premix. The results were statistically analyzed using one-way analysis of variance with Tukey's t-test.
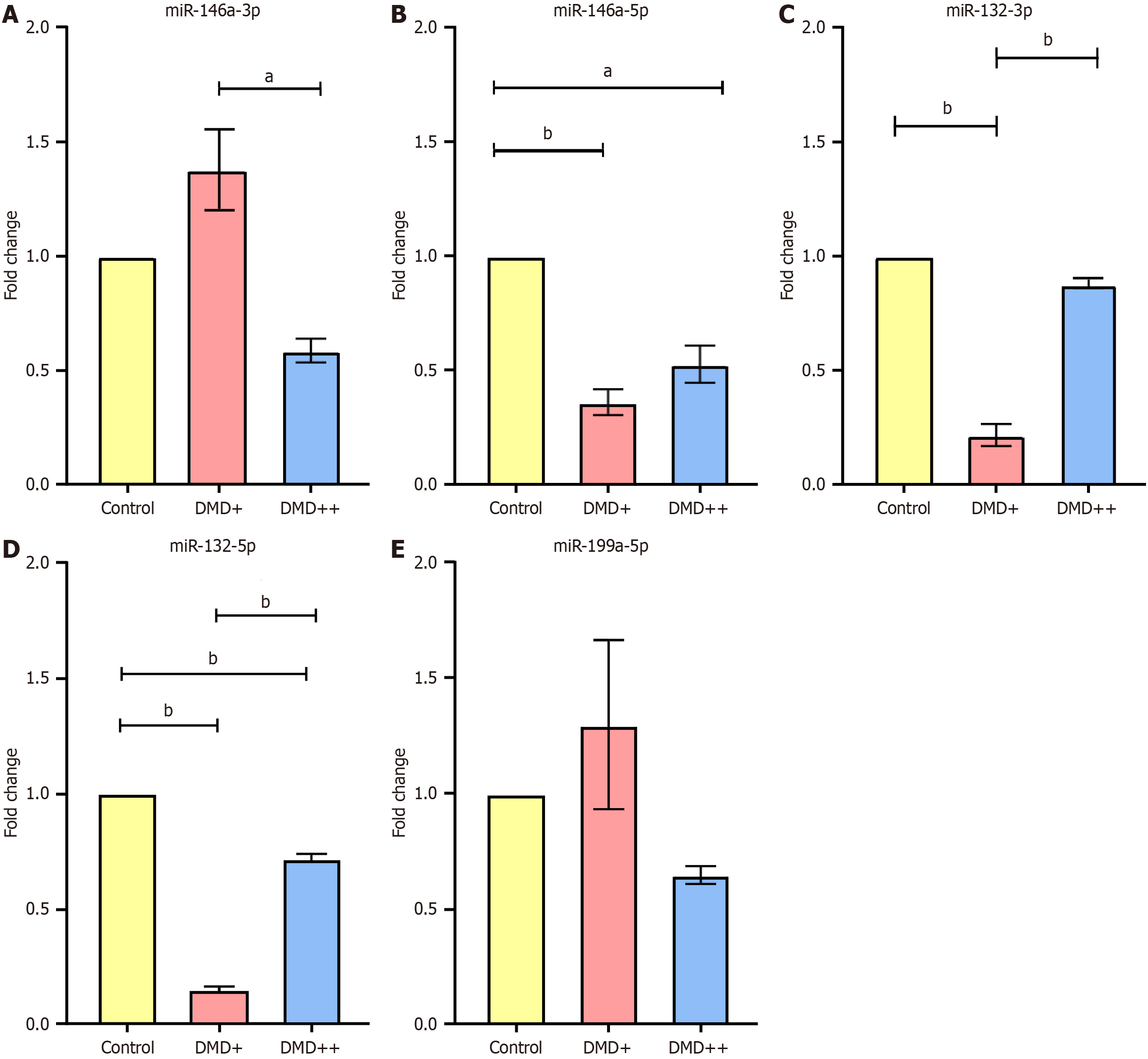
miR-146a-5p and miR-132-5p showed significant downregulation in both patient samples. miR-199a-5p and miR-146a-3p showed no change in expression between the diseased and controls. miR-132-3p showed downregulation only in the DMD+ sample (0.21 ± 0.04). The decrease in miR-132-3p can result in failed silencing of the phosphatase and tensin homolog-mediated apoptotic pathway, leading to severe skeletal muscle atrophy. Here, the downregulation of miR-132-3p in DMD+ is consistent with severe muscle loss and higher disease progression than that in DMD++. DMD++ has slower disease progression, and the expression of miRNA involved in inflammatory and apoptotic responses is more similar to that of the control.
Our study shows marked difference in miRNA expression in this rare case of DMD with autism and epilepsy. These miRNAs also serve as regulators of several muscle regeneration, apoptosis, and inflammatory pathways. This study shows the significance of studying miRNAs in such rare cases in a larger cohort to progress in several intervention treatments utilizing miRNAs.
Core Tip: This study presents a rare case of a ten-year-old boy with Duchenne muscular dystrophy (DMD) coexisting with autism spectrum disorder and epilepsy. By investigating the differential expression of miRNAs, we identified a significant downregulation of miR-132-3p in the DMD patient compared to the comorbid DMD case. This suggests that miR-132-3p downregulation may have contributed to accelerated muscle atrophy through failed silencing of the phosphatase and tensin homolog-mediated apoptotic pathway. Our findings emphasize the importance of studying such rare DMD comorbidities to enhance understanding of disease heterogeneity and improve diagnosis and treatment strategies.
- Citation: Sivakumar S, Rajavel A, Viswanathan V, Daniel EA, Gangadaran P, Natesan Sella R. miRNA dysregulation in Duchenne muscular dystrophy comorbidities. World J Exp Med 2025; 15(2): 100548
- URL: https://www.wjgnet.com/2220-315x/full/v15/i2/100548.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i2.100548
Duchenne muscular dystrophy (DMD) is characterized by progressive and severe muscle wasting. The disease manifests due to mutations in the dystrophin gene[1], which connects the cytoskeletal F-actin and dystrophin-associated glycoprotein, binding the cell to the extracellular matrix[2]. At the early age of 5, patients with DMD exhibit symptoms and lose ambulation around 10 to 12 years of age. Cardiac or respiratory failure is the most common cause of death in DMD patients between the ages of 20 to 40 years[3]. Since DMD is an X-linked recessive disease, males are more predominantly affected than females[4].
The most prevalent neural disorder accompanied by muscular dystrophy is epilepsy, which is identified at a rate of 3.1% to 12.3% in the dystrophic population[5]. About 7.5% of DMD patients with mutations between exon 31 and 62 experience epileptic seizures[6]. DMD also co-occurs with autism spectrum disorder (ASD), as dystrophin plays a significant role in brain development and function. The disruption of isoforms such as Dp140 and Dp71 especially contributes to cognitive impairment. The mechanisms through which various DMD mutations contribute to autistic features remain unknown. In a 2021 case study, a child with DMD with exons 1 to 44 deletion in dystrophin exhibited a mild intellectual disability with a history of febrile seizures; herein, deletion in the proximal region of the dystrophin gene exhibits mild intellectual disability and distal mutations beyond exon 45 exhibit severe cognitive impairment[7].
Even though neuronal disabilities and DMD are apparent mutually inclusive events, the combined occurrence of ASD, DMD, and epilepsy is a rare phenomenon. Mrazova et al[8] first reported a 4-year-old with DMD and symptoms of ASD and epilepsy. The patient possessed deletions of exons 10 and 11. However, an in-depth study detailing the molecular aspects of this rare case remains unknown.
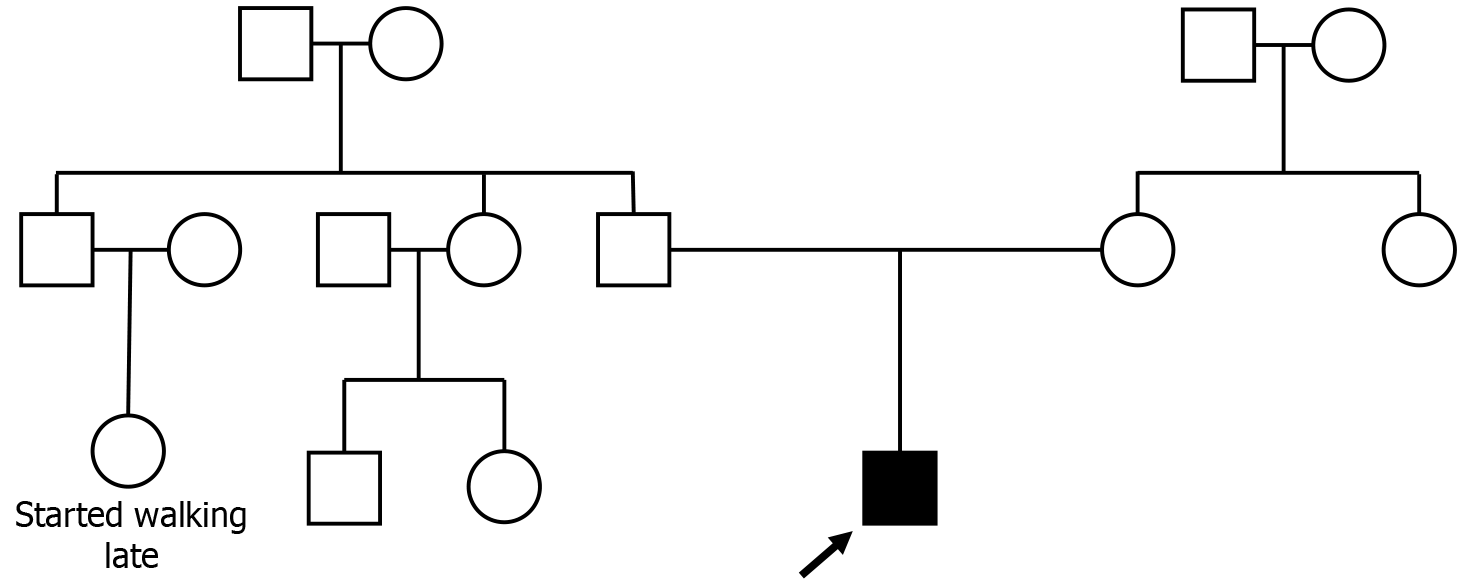
In our study, we report a similar case of DMD co-occurring with ASD and epilepsy, which, to our knowledge, is the second case reported worldwide and the first case in India. Here, a 10-year-old child who manifests symptoms of DMD, ASD, and epilepsy is presented. Multiplex ligation probe amplification analysis confirmed that the patient possessed DMD, reporting a hemizygous out-of-frame deletion of exon 44 in the dystrophin gene. Several tests to confirm autism and epilepsy were also performed. The clinical symptoms included increased creatine phosphokinase levels up to 7565 U/L (normal levels are 46 U/L to 171 U/L), underweight, walking and climbing difficulties, Gower’s sign, calf muscle hypertrophy, fat accumulation, and tip-toe walking. Figure 1 shows the stages of disease onset exhibited by the patient. The Sequin Form Board test, Gesell's drawing test, Vineland Social Maturity Scale, and Indian Scale for Assessment of Autism confirmed mild autism (60% disability) and moderate deficits in socio-adaptive behavior (75% disability).
Subsequently, epilepsy was confirmed following repeated seizures at the age of four years and by performing sleep echocardiography to analyze brain activity. Dual energy X-ray absorptiometry hip and spine scans showed osteoporosis; cardiac functioning was normal; an increased serum glutamic pyruvic transaminase level of 358 U/L was reported in the year 2021, which, in the follow-up study, was reduced to 239 U/L in 2022. However, this remains 6 times to 7 times higher than normal levels (5 U/L to 45 U/L).
The pedigree chart in Figure 2 shows the child as the proband, and the disease is suspected to be sporadic.
Contrary to the abovementioned case by Mrazova et al[8], the case represented here possessed mutations in the distal part of the gene, exon 44, showing that both proximal and distal dystrophin gene mutations can contribute to this triad. Herein, we report the clinical features of this case with additional findings of altered miRNA expressions, which provide insights into the miRNA expression and the targets of which can be studied in the future to focus on muscle regeneration or in controlling inflammation and disease progression. Studying miRNAs and their expression is essential to determine the molecular pathways they alter as DMD is inherently heterogeneous across different cases. Herein, we studied the expression of five miRNAs, broadly involved in inflammatory, cell differentiation, and proliferation pathways, across three samples: a healthy control, a patient diagnosed with DMD (represented hereafter as DMD+), and the unusual DMD case manifesting ASD and epilepsy (denoted hereafter as DMD++).
A total of 3 mL of whole blood was collected from 10-year-old subjects: (1) Healthy control; (2) DMD+; and (3) DMD++, in sodium citrate BD Vacutainer® tubes under sterile conditions. This study was approved by the ethics committee of Apollo Children’s Hospital (No. ACH-OTH-001/11-20). Informed written consent was obtained from the patient's parents before blood collection. RNA isolation was performed under sterile RNAse-free conditions using TRIzol reagent from Invitrogen, according to the manufacturer's protocol. 1 μL of GlycoBlueTM, a coprecipitant by Invitrogen, was added at the isopropanol precipitation step to increase the RNA yield and pull down existing small RNA fragments. The isolated RNA was resuspended in 20 μL of diethyl pyrocarbonate-treated water and quantified using a Nanodrop.
Mir-XTM miRNA First Strand cDNA Synthesis kit was acquired from Clontech and used for cDNA synthesis in an Agilent thermal cycler under standardized conditions. qRT-PCR was performed using the synthesized cDNA and the TB Green Advantage qPCR Premix from Clontech in a Biorad CFX96 Real-Time system. The matured miRNA sequence is used as the forward primer as recommended by the manufacturer, which is provided in Table 1. The reverse primer is the universal 3’ primer supplied along with the kit. U6 snRNA was used as the housekeeping control.
| miRNA | Forward primer | Primer length |
| miR-132-5p | 5'-ACCGTGGCTTTCGATTGTTACT-3' | 22 |
| miR-132-3p | 5'-TAACAGTCTACAGCCATGGTCG-3' | 22 |
| miR-146a-5p | 5'-TGAGAACTGAATTCCATGGGTT-3' | 22 |
| miR-146a-3p | 5'-CCTCTGAAATTCAGTTCTTCAG-3' | 22 |
| miR-199a-5p | 5'-CCCAGTGTTCAGACTACCTGTTC-3' | 23 |
All results were statistically analyzed using one-way analysis of variance with Tukey's t-test in GraphPad Prism version 8.0.1. A P value less than 0.05 was considered statistically significant.
To understand the expression of various miRNAs in dystrophic comorbidies, we performed qRT-PCR analysis for the following genes: (1) miR-146a-3p; (2) miR-146a-5p; (3) miR-132-3p; (4) miR-132-5p; and (5) miR-199a-5p. The results displayed interesting variations in several of these miRNAs distinct to each sample. The result after statistical analysis is presented in Figure 3. Figure 3A shows that miR-146a-3p is significantly downregulated in DMD++ (0.58 ± 0.05) when compared with that in DMD+ (1.37 ± 0.17). However, there is no significance in this expression when compared to that in control. miR-146a-5p showed significant downregulation in both DMD+ (0.36 ± 0.05) and DMD++ (0.52 ± 0.08) and miR-132-3p showed significant downregulation in DMD+ (0.21 ± 0.04) (Figure 3B and C). The difference in miRNA expression between DMD++ and DMD+ shows that miR-132-3p is more downregulated in DMD+ than in DMD++. miR-132-5p showed significant downregulation in both DMD+ (0.14 ± 0.01) and DMD++ (0.71 ± 0.02) in our study, and in DMD+, it was drastically downregulated. miR-199a-5p shows no significant difference between the samples [DMD+ (1.29 ± 0.36) and DMD++ (0.64 ±0.03)] and the control. All data are presented as the mean ± SEM.
In our study, we have presented a 10-year-old child exhibiting rare co-morbidities of DMD with ASD and epilepsy. Clinical evaluations confirm the pathological conditions and we further evaluated the miRNA expression patterns in this rare condition compared with DMD and healthy control. Several work on DMD, in recent trends, are focused on miRNAs, mainly towards miRNA therapeutics. Recent studies in DMD focusses on therapeutic strategies using miRNAs, that is, restoring dystrophin and improving skeletal muscle performance by intramuscular delivery of miR-25 decoys[9]. This type of research adds up to the growing list of miRNA-based therapies for dystrophy. Thus, our present rare case also warrants attention to be focused on the miRNA aspect. Although understanding the mRNA and proteins is crucial for any disease, the current trend focusses more on miRNAs as it can streamline the gene targets one can look into and also we can understand the post-transcriptional state of a gene during disease conditions. This helps us gain clarity and design future translational studies effectively.
Our work focuses on 5 miRNAs widely studied in DMD, ASD, and epilepsy and that which also closely interact with genes involved in muscle regeneration, inflammation, and apoptosis, which are important aspects to focus in DMD. The expression profiles of these 5 miRNAs, miR-146a-3p, miR-146a-5p, miR-132-3p, miR-132-5p, and miR-199a-5p, allowed us to discuss the following possibilities about disease progression. We found that miR-146a-3p (Figure 3A) was significantly downregulated in DMD++ when compared with the expression in DMD+; however, both demonstrated no relevance in expression when compared to the expression in the control. The miR-146a-5p was significantly downregulated in both DMD+ and DMD++ (Figure 3B). In a previous study, miR-146a-5p-deficient mice showed an additional increase in the expression of the fibrosis-related gene transforming growth factor beta 1 (TGF-β1) compared to that of mdx mice, and the same miRNA was upregulated in Hmox1-deficient mdx mice[10,11]. Notably, miR-146a-5p plays an important role in suppressing immune responses by binding to the 3’-UTR of tumour necrosis factor receptor-activated factor 6 (TRAF6) and interleukin-1 receptor-associated kinase 1 (IRAK1) and further blocks the activation of the nuclear factor kappa B pathway, thereby decreasing the immune response and the transcription of pri-miR-146a[12].
Apolipoprotein E (ApoE) proteins, known for their anti-inflammatory properties and involvement in lipid transport and metabolism, enhance pri-miR-146a by upregulating PU1-mediated transcription[13]. ApoE downregulation could be a reasonable explanation for decreased transcription of miR-146a and increased levels of lipid accumulation in the blood, a characteristic of DMD pathophysiology. Studies on the downregulation of ApoE in Fuchs endothelial corneal dystrophy further substantiate this claim. Moreover, ApoE knockout mdx mice showed increased plasma lipid levels and atherosclerotic plaque deposition due to the absence of lipid metabolization, compared to wild-type ApoE-deficient mice[14,15]. Therefore, miR-146a-5p downregulation could result from an impaired ApoE-dependent transcription pathway, depicting the inability to suppress immune responses by blocking IRAK1/TRAF6.
miR-132-3p was significantly downregulated in the DMD+ samples (Figure 3C). Previous studies have shown that miR-132-3p targets various genes, including phosphatase and tensin homolog (PTEN), which are important in suppressing proinflammatory responses and apoptosis and promoting neurite outgrowth. PTEN is upregulated in DMD skeletal muscles, causing muscle weakness and atrophy. miR-132-3p suppresses PTEN/forkhead box protein O3a-mediated apoptosis and blocks acetylcholinesterase, preventing acetylcholine hydrolysis, a key regulator in activating various proinflammatory responses. Thus, the downregulation of miR-132-3p in DMD+ could be related to skeletal muscle atrophy due to increased levels of PTEN. Downregulation of miR-132-3p also increases cytokine levels[16–18]. Although no significant difference was observed in the miR-132-3p levels between the control and DMD++ samples, a marked decrease was observed in the DMD+ samples. However, miR-132-3p was more downregulated in DMD+ than in DMD++, implying heterogeneity between patients and a possible variation contributing to disease progression.
miR-132-5p was significantly downregulated in our study in both DMD+ and DMD++ samples (Figure 3D). However, the fold change between DMD+ and DMD++ showed a relative difference, whereby DMD+ was drastically downregulated while DMD++ was only downregulated by 0.3-fold. Despite not being widely studied in dystrophinopathy, miR-132-5p is dysregulated in other neural disorders such as Parkinson's and Alzheimer's disease. One of the direct targets of miR-132-5p is UNC51-like kinase, which initiates autophagy. Therefore, miR-132-5p is a positive regulator of autophagy, meaning silencing this miRNA using antisense oligonucleotide was reported to improve cell survival in Parkinson's disease[19]. The miR-132-3p/5p contributes to synaptic plasticity and signaling. The level of dystroglycan, a protein bridging the dystrophin and extracellular matrix domain, decreases upon increased glucose levels, which correlates to the decline in miR-132-5p and miR-132-3p. Matrix metalloprotease-9 (MMP-9) is the key regulator of dystroglycan degradation, acting as a direct target for miR-132-3p; post-transcriptionally inhibiting MMP-9 prevents it from cleaving dystroglycan. However, the downregulation of miR-132-3p increases MMP-9 expression levels, leading to the cleavage of dystroglycan, thereby impairing the neural signaling process[20]. This scenario arises following increased glucose levels; note that glucose intolerance is also an important characteristic reported for myoclonic, Becker, and Duchenne muscular dystrophies[21,22].
Figure 3E shows that there was no significant difference in miR-199a-5p levels between the samples (DMD+ and DMD++) and the control. In a previous study, miR-199a-5p was upregulated in fibroblast-derived exosomes from DMD patients, and these increased miR-199a-5p levels reduced the expression of caveolin 1 (CAV1) and promoted fibrosis. CAV1 is a negative regulator of TGF-β1-mediated fibrosis. The effect of increased fibrosis was confirmed when control fibroblast cells were transfected with DMD exosomes, and the CAV1 transcript levels were reduced because it directly binds with miR-199a-5p. It was also confirmed that decreasing levels of CAV1 significantly upregulated fibrotic genes such as TGF-β1 and collagen, type I, alpha 1 protein[23]. However, the expression pattern we obtained in our study is statistically insignificant and shows that the miR-199a-5p expression in whole blood samples of DMD patients could differ from that of the exosomes derived from DMD fibroblast cells, thus indicating a heterogeneity between sample types.
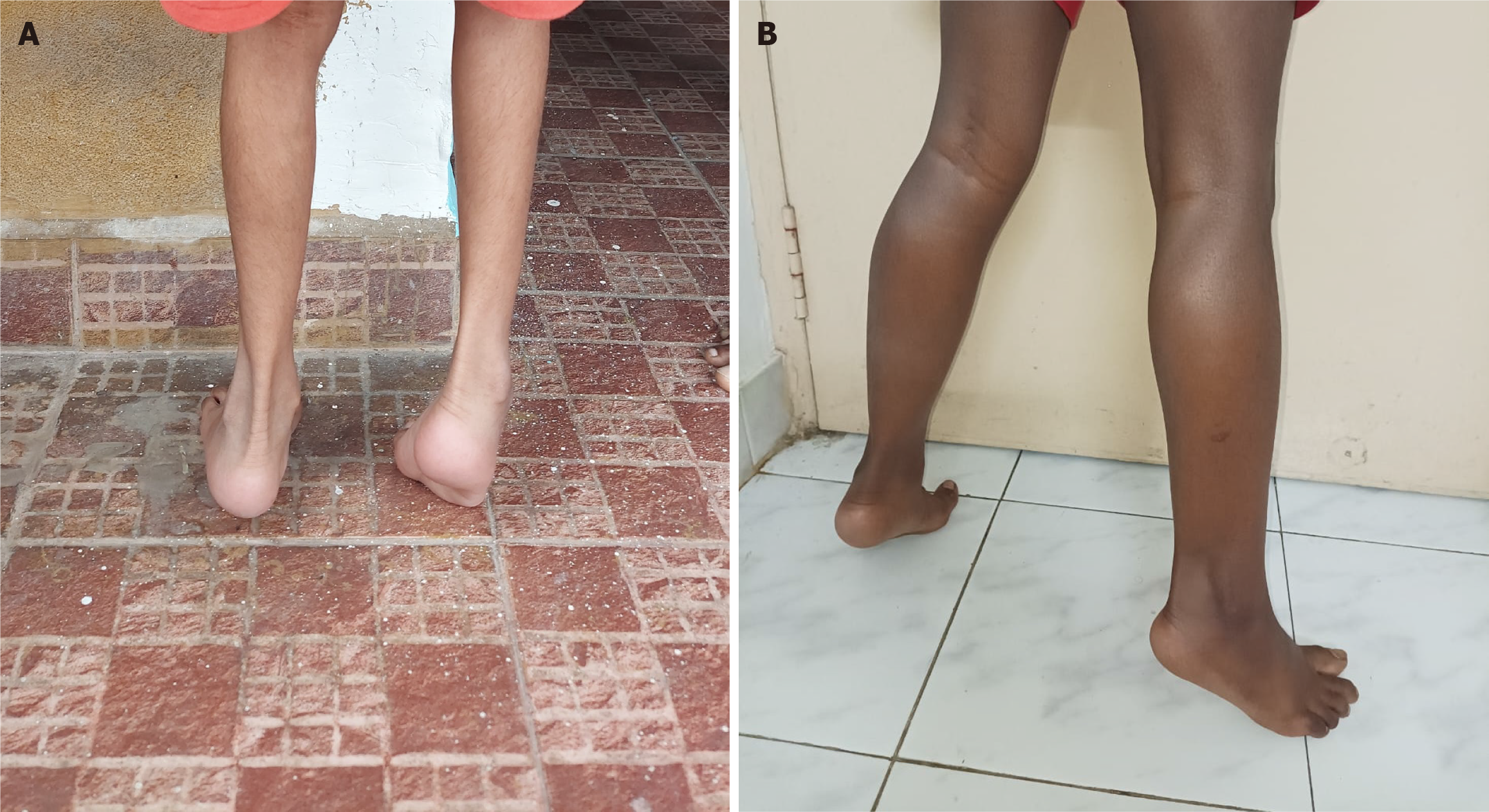
This comparative study revealed two important factors of interest: (1) Inflammatory responses; and (2) Muscle degeneration. Concerning the fold change values, miR-132-3p and miR-146a-5p show convincing results, which gives us a reason why the variations are severe in DMD+ compared to DMD++. We see a drastic downregulation of these miRNAs in DMD+, where severe progression was observed physically. It should also be noted that DMD+ is presently non-ambulant, and DMD++ is still ambulant with observed tip-toe walking. In addition, severe muscle loss was observed in DMD+ compared to DMD++, which correlates to higher disease progression due to miR-132-3p downregulation, with an increase in PTEN-mediated skeletal muscle atrophy. Figure 4 shows images of the lower limbs of two patients depicting extreme muscle loss in DMD+. It is to be noted that DMD+ has a hemizygous deletion of exons 45 to 54, eliminating most of the distal region in dystrophin, whereas DMD++ possesses a hemizygous deletion of exon 44. Deleting 10 exons in DMD+ could affect the downstream pathways, contributing to the disease severity. Although it is reported that epilepsy in dystrophinopathy occurs more frequently when mutations in dystrophin are between exons 44 and 63, our cases show a difference where DMD+ experiences no epilepsy or any neuronal disorders. Additionally, the first triad reported by Mrazova et al[8], which contained deletions in exons 10 and 11, shows that both proximal and distal mutations in DMD can cause ASD and epilepsy. The physiological changes and miRNA expression provide evidence that the disease progression is higher in DMD+. Furthermore, the miRNA expression in DMD++ is closer to the control. This observational study provides evidence that miRNA dysregulation could be a valuable marker for understanding the molecular aspect of various cases of DMD with different levels of severity. However, a large cohort study focusing on the dysregulated miRNA and its gene targets is essential to understand the complete mechanism behind neural disorders linked to dystrophy.
This study investigated a rare case manifesting DMD, ASD, and epilepsy. In addition to a clinical investigation, we compared miRNA expressions in a rare triad case against DMD+ and healthy control samples. These results reveal a stark downregulation of miR-132-3p/5p and miR-146a-5p in DMD+, whereas DMD++ showed less pronounced downregulation than the control. Correlating to the miRNA targets, we observed that the decrease in miR-132-3p can result in failed silencing of the PTEN-mediated apoptotic pathway and further lead to severe skeletal muscle atrophy. This observation is consistent with the miR-132-3p levels in DMD+, showing severe muscle loss and higher disease progression. The miRNAs also contribute to an increase in inflammatory responses due to downregulation. As per our observations, DMD+ shows more disease progression, which is also reflected in the miRNA expression. However, DMD++ has slower disease progression, and thereby, the expression of the miRNA involved in inflammation and apoptotic responses is more similar to that of the control. This study shows the significance of studying such rare cases in a larger cohort to understand the heterogeneity of the disease and pave the way for proper diagnosis and treatment in the future.
We thank Dr. Hanna LE, Dr. Scientist F, ICMR-National Institute of Research in Tuberculosis, for allowing us to access the qRT-PCR facility. We would also like to thank the Department of Genetic Engineering, SRMIST, for providing the laboratory facilities to proceed with our work. We thank the children and their parents who have agreed to participate in the study.
| 1. | Duan D, Goemans N, Takeda S, Mercuri E, Aartsma-Rus A. Duchenne muscular dystrophy. Nat Rev Dis Primers. 2021;7:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 697] [Article Influence: 174.3] [Reference Citation Analysis (0)] |
| 2. | Norwood FL, Sutherland-Smith AJ, Keep NH, Kendrick-Jones J. The structure of the N-terminal actin-binding domain of human dystrophin and how mutations in this domain may cause Duchenne or Becker muscular dystrophy. Structure. 2000;8:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Mercuri E, Bönnemann CG, Muntoni F. Muscular dystrophies. Lancet. 2019;394:2025-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 4. | Hendriksen RGF, Vles JSH, Aalbers MW, Chin RFM, Hendriksen JGM. Brain-related comorbidities in boys and men with Duchenne Muscular Dystrophy: A descriptive study. Eur J Paediatr Neurol. 2018;22:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Pane M, Messina S, Bruno C, D'Amico A, Villanova M, Brancalion B, Sivo S, Bianco F, Striano P, Battaglia D, Lettori D, Vita GL, Bertini E, Gualandi F, Ricotti V, Ferlini A, Mercuri E. Duchenne muscular dystrophy and epilepsy. Neuromuscul Disord. 2013;23:313-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Fortunato F, Rossi R, Falzarano MS, Ferlini A. Innovative Therapeutic Approaches for Duchenne Muscular Dystrophy. J Clin Med. 2021;10:820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Simone M, Margari L, Pompamea F, De Giacomo A, Gabellone A, Marzulli L, Palumbi R. Autism Spectrum Disorder and Duchenne Muscular Dystrophy: A Clinical Case on the Potential Role of the Dystrophin in Autism Neurobiology. J Clin Med. 2021;10:4370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Mrazova L, Vondracek P, Danhofer P, Pejcochova J, Jurikova Z, Honzik T, Zamecnik J, Oslejskova H. Triple Trouble: A Case Report of an Unusual Combination of Duchenne Muscular Dystrophy, Epilepsy, and Autism. Autism Open Access. 2016;6. [DOI] [Full Text] |
| 9. | Li S, Han R. Targeting miR-25 to alleviate DMD-related muscle dysfunction. Mol Ther Nucleic Acids. 2024;35:102238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 10. | Bronisz-Budzyńska I, Chwalenia K, Mucha O, Podkalicka P, Karolina-Bukowska-Strakova, Józkowicz A, Łoboda A, Kozakowska M, Dulak J. miR-146a deficiency does not aggravate muscular dystrophy in mdx mice. Skelet Muscle. 2019;9:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Pietraszek-Gremplewicz K, Kozakowska M, Bronisz-Budzynska I, Ciesla M, Mucha O, Podkalicka P, Madej M, Glowniak U, Szade K, Stepniewski J, Jez M, Andrysiak K, Bukowska-Strakova K, Kaminska A, Kostera-Pruszczyk A, Jozkowicz A, Loboda A, Dulak J. Heme Oxygenase-1 Influences Satellite Cells and Progression of Duchenne Muscular Dystrophy in Mice. Antioxid Redox Signal. 2018;29:128-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481-12486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3121] [Cited by in RCA: 3549] [Article Influence: 186.8] [Reference Citation Analysis (0)] |
| 13. | Li K, Ching D, Luk FS, Raffai RL. Apolipoprotein E enhances microRNA-146a in monocytes and macrophages to suppress nuclear factor-κB-driven inflammation and atherosclerosis. Circ Res. 2015;117:e1-e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 168] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 14. | Kuot A, Ronci M, Mills R, Klebe S, Snibson G, Wiffen S, Loh R, Corbett M, Zhou T, Chataway T, Burdon KP, Craig JE, Urbani A, Sharma S. Reduced expression of apolipoprotein E and immunoglobulin heavy constant gamma 1 proteins in Fuchs endothelial corneal dystrophy. Clin Exp Ophthalmol. 2019;47:1028-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Milad N, White Z, Tehrani AY, Sellers S, Rossi FMV, Bernatchez P. Increased plasma lipid levels exacerbate muscle pathology in the mdx mouse model of Duchenne muscular dystrophy. Skelet Muscle. 2017;7:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Parveen A, Wen Y, Roy A, Kumar A. Therapeutic Targeting of PTEN in Duchenne Muscular Dystrophy. Mol Ther. 2021;29:8-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Yue F, Song C, Huang D, Narayanan N, Qiu J, Jia Z, Yuan Z, Oprescu SN, Roseguini BT, Deng M, Kuang S. PTEN Inhibition Ameliorates Muscle Degeneration and Improves Muscle Function in a Mouse Model of Duchenne Muscular Dystrophy. Mol Ther. 2021;29:132-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Juźwik CA, S Drake S, Zhang Y, Paradis-Isler N, Sylvester A, Amar-Zifkin A, Douglas C, Morquette B, Moore CS, Fournier AE. microRNA dysregulation in neurodegenerative diseases: A systematic review. Prog Neurobiol. 2019;182:101664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 317] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 19. | Zhao J, Yang M, Li Q, Pei X, Zhu X. miR-132-5p regulates apoptosis and autophagy in MPTP model of Parkinson's disease by targeting ULK1. Neuroreport. 2020;31:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Dou Y, Tan Y, Yu T, Ma X, Zhou Y, Zhao Y, Zhao Y, Liu X. MiR-132 down-regulates high glucose-induced β-dystroglycan degradation through Matrix Metalloproteinases-9 up-regulation in primary neurons. J Cell Mol Med. 2021;25:7783-7795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Barbetti F, Guaraldi F, Ghizzoni L. Diabetes Associated with Single Gene Defects. In: S. Karger AG. Frontiers in Diabetes. Basel: S. Karger AG, 2017. [DOI] [Full Text] |
| 22. | Bostock EL, Edwards BT, Jacques MF, Pogson JTS, Reeves ND, Onambele-Pearson GL, Morse CI. Impaired Glucose Tolerance in Adults with Duchenne and Becker Muscular Dystrophy. Nutrients. 2018;10:1947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Zanotti S, Gibertini S, Blasevich F, Bragato C, Ruggieri A, Saredi S, Fabbri M, Bernasconi P, Maggi L, Mantegazza R, Mora M. Exosomes and exosomal miRNAs from muscle-derived fibroblasts promote skeletal muscle fibrosis. Matrix Biol. 2018;74:77-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |