Published online Dec 20, 2024. doi: 10.5493/wjem.v14.i4.99235
Revised: September 8, 2024
Accepted: September 20, 2024
Published online: December 20, 2024
Processing time: 105 Days and 18 Hours
Diabetic retinopathy (DR) is a serious microvascular complication of diabetes mellitus and may result in irreversible visual loss. Laser treatment has been the gold standard treatment for diabetic macular edema and proliferative diabetic retinopathy for many years. Of late, intravitreal therapy has emerged as a cornerstone in the management of DR. Among the diverse pharmacotherapeutic options, anti-vascular endothelial growth factor agents have demonstrated remarkable efficacy by attenuating neovascularization and reducing macular edema, thus preserving visual acuity in DR patients.
Core Tip: Intravitreal therapy has revolutionized the treatment for diabetic retinopathy (DR). The various treatment options include intravitreal anti-vascular endothelial growth factor injections, triamcinolone acetonide and steroid implants. Intravitreal therapy can be used for the management of Diabetic Macular Edema and Proliferative Diabetic Retinopathy. Additionally, corticosteroids have shown promising results by exerting anti-inflammatory effects and stabilizing blood-retinal barrier integrity. Recent advancements have introduced novel agents targeting various pathways implicated in DR pathogenesis, such as angiopoietin-2 and integrins, offering potential avenues for tailored therapeutic interventions. This review aims to comprehensively examine diverse facets of intravitreal therapy concerning the management of DR and its associated complications.
- Citation: Arora A, Morya AK, Gupta PC, Menia NK, Nishant P, Gupta V. Intravitreal therapy for the management of diabetic retinopathy: A concise review. World J Exp Med 2024; 14(4): 99235
- URL: https://www.wjgnet.com/2220-315x/full/v14/i4/99235.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i4.99235
Diabetes mellitus (DM) has emerged as a major public health problem[1]. Diabetic retinopathy (DR) ranks as one of the most prevalent complications of DM and is the primary cause of visual impairment and blindness in working age groups (15–64 years)[1-5]. Visual impairment may occur due to diabetic macular edema (DME) or as a result of complications of proliferative DR (PDR) like vitreous hemorrhage, neovascular glaucoma and tractional retinal detachment[5-7].
In DR, prolonged high blood sugar levels are believed to harm endothelial cells and basement membrane proteins within the retinal blood vessels. This damage, along with increased vascular permeability and the production of proinflammatory cytokines, can cause retinal ischemia, which may trigger an excessive increase in vascular endothelial growth factor (VEGF) expression. VEGF along with proinflammatory cytokines increase vascular permeability and promote the breakdown of the retinal-blood barrier, exacerbating the disease and leading to vision loss[7-9].
This understanding has led physicians to adopt intravitreal therapy to address DR-related complications and improve vision in patients. By administering the drug directly into the vitreous cavity, therapeutic doses can be obtained for a longer duration without systemic adverse effects. Intravitreal drugs that have been used in the management of DR can be broadly divided into two groups: Anti-VEGF agents and corticosteroids. VEGF inhibitors that have been evaluated in prospective, randomized phase 2 and phase 3 clinical trials, and demonstrated favourable results for patients with DME and DR include bevacizumab, ranibizumab, aflibercept, brolucizumab and faricimab. Intravitreal corticosteroids include Triamcinolone and dexamethasone implant (Ozurdex®; Allergan, Inc., Irvine, CA, United States).
This review seeks to offer a thorough overview of intravitreal therapies used in the management of DR and DME. Additionally, we have discussed newer emerging agents that are being developed for intravitreal use in patients with DR.
A thorough search on PubMed, Embase, Reference Citation Analysis, Embase and Google Scholar was performed of keywords – Intravitreal therapy in Diabetic Retinopathy. Latest and highly cited articles written in English were included.
Intravitreal therapy has revolutionized the treatment for DR. The various treatment options include intravitreal anti-VEGF injections, triamcinolone acetonide and steroid implants. Intravitreal therapy can be used for the management of DME and PDR.
Intravitreal agents used in the management of DME can be grouped into: (1) Intravitreal anti-VEGF agents; (2) Intravitreal corticosteroids; and (3) Intravitreal anti-VEGF agents.
VEGF plays a central role in the pathogenesis of sight-threatening retinal changes in DR. VEGF drives ischemic changes at the level of the retina and stimulates the growth of new vessels. These vessels are fragile, and highly permeable and leakage from them can lead to exudation in the macular area. Also, new vessels can easily rupture leading to pre-retinal and vitreous hemorrhage. Anti-VEGF agents, therefore, remain the cornerstone for the treatment of DR and DME.
Anti-VEGF agents include: (1) Bevacizumab; (2) Ranibizumab; (3) Aflibercept; (4) Brolucizumab; and (5) Faricimab (Table 1).
| Drug | Active molecule | Molecular weight | Dosage | Mechanism of action | Year of FDA approval for use in DR/DME |
| Bevacizumab | Recombinant humanized monoclonal antibody targeting VEGF | 145 kDa | 1.25 mg/0.05 mL | Non-specific blockade of VEGF on endothelial surface | 2004 |
| Ranibizumab | Recombinant humanized, monoclonal antibody fragment | 45 kDa | 0.5 mg/0.05 mL | Binds VEGF-A, VEGF-B, and placental growth factor | 2012 |
| Aflibercept | Recombinant protein and attaches to the VEGF receptor 1 and 2 | 115 kDa | 2 mg/0.05 mL | Blocks VEGF-A, VEGF-B, Placental growth factor-1 and 2 | 2014 |
| Brolucizumab | Humanized single-chain variable fragment | 26 kDa | 6 mg/0.05 mL | Inhibits VEGF A and VEGF 165 | 2022 |
| Faricimab | Bispecific antibody | 149 kDa | 6 mg/0.05 mL | Targets both VEGF-A and angiopoietin-2 | 2022 |
Bevacizumab is a recombinant humanized monoclonal antibody targeting VEGF. It is structurally composed of human immunoglobulin G1 with regions derived from murine antibodies. Its molecular weight is 145 kDa. Initially developed for cancer therapy, bevacizumab was first approved by the Food and Drug Administration (FDA) in 2004 for metastatic colorectal cancer. Its off-label use in ophthalmology began with the treatment of neovascular age-related macular degeneration (AMD), showcasing significant efficacy in reducing macular edema and improving visual acuity. Despite the lack of FDA approval for ocular use, cost-effectiveness makes bevacizumab an important tool in the armamentarium of DME management.
The recommended intravitreal dosage of bevacizumab (off-label use) for DME is 1.25 mg (0.05 mL). Typically, the injections are administered monthly. The treatment frequency may be adjusted based on the patient's response and visual acuity improvement.
The BOLT (Bevacizumab or Laser Therapy) study, a two-year, randomized controlled trial compared bevacizumab to laser therapy in patients with DME. Patients who received intravitreal bevacizumab had significant improvement in visual acuity and central macular thickness compared to laser treatment. Specifically, at 12 months, patients treated with bevacizumab had a mean gain of 8.6 letters in visual acuity compared to a loss of 0.5 letters in the laser group. At 24 months, the bevacizumab group maintained a mean gain of 8.6 letters, while the laser group had a mean gain of only 0.9 letters[9-11].
The studies conducted by the Diabetic Retinopathy Clinical Research Network (DRCR.net) have been instrumental in establishing the role of bevacizumab in DME. The Protocol T study, a head-to-head comparison of aflibercept, ranibizumab, and bevacizumab, revealed that aflibercept and ranibizumab offered superior visual outcomes at one year for patients with baseline visual acuity of 20/50 or worse, but bevacizumab was comparable in those with better baseline vision and more cost-effective[11,12]. Post hoc analyses of DRCR Protocol T further confirmed bevacizumab's efficacy in various subgroups of DME patients[12].
Cochrane review has consistently supported the use of bevacizumab in DME. A comprehensive review of multiple clinical trials concluded that intravitreal bevacizumab is effective in improving visual acuity and reducing central macular thickness in DME patients. The review emphasized that while aflibercept and ranibizumab might offer slightly better outcomes in specific scenarios, bevacizumab remains a valuable option, especially in settings where cost is a significant concern[12].
Currently, bevacizumab's off-label use in DME is widespread and endorsed by numerous clinical guidelines, recognizing its balance of efficacy, safety, and cost. Its indication extends to patients with central-involved DME, offering an accessible alternative to more expensive anti-VEGF therapies. Despite the absence of FDA approval for ocular indications, bevacizumab continues to be an important treatment modality, supported by robust clinical evidence and extensive real-world application.
Ranibizumab is a recombinant, humanized, monoclonal antibody fragment with a molecular weight of 48 kDa. The fragment is derived from a bevacizumab antibody and needs recombinant processing to produce the desired Fab-Y0317 molecule. The small size of the molecule increases the ocular penetration. The FDA approved Ranibizumab for DME in 2012.
The commonly used dosages of ranibizumab (0.1 mg/0.01 mL) are 0.5 mg (0.05 mL) and 0.3 mg (0.03 mL). The standard dosing regimen comprises an initial series of three four-weekly (q4) injections. Thereafter, treatment is often individualized based on the patient's response, with the potential for adjustments in the injection frequency.
Phase III trials, such as RISE and RIDE[12-16] used a fixed dosing regimen, either monthly or bimonthly treatment after a loading phase. However, this regimen is difficult to follow in real-world clinical practice. To optimize the treatment effects and cost-effectiveness, different treatment protocols have been developed in recent years. Of these, the two most commonly used protocols are: (1) Pro re nata (PRN); and (2) Treat-and-extend (T&E) regimen; Pro re nata (PRN).
In the pro re nata (PRN) approach, also known as "as needed," the frequency of injections is minimized while adhering to a fixed follow-up schedule. This allows for close monitoring of the patient's response to treatment[17-19].
The treat-and-extend (T&E) regimen involves incrementally increasing the interval between follow-up visits after the patient has demonstrated a sufficient response to treatment. During each visit, an injection is administered to maintain therapeutic efficacy[18,19].
Pivotal phase 3 trials for the use of intravitreal Ranibizumab in patients with DME, such as RISE and RIDE, demonstrated significant visual acuity improvement with Ranibizumab treatment compared to sham injections. In these studies, approximately 39% and 45% of patients, respectively, achieved a ≥ 15-letter gain in best-corrected visual acuity (BCVA) at 24 months[13-16].
Further validation of Ranibizumab's efficacy came from the DRCR Protocol I study, which showed that Ranibizumab, combined with prompt or deferred laser, resulted in superior vision outcomes over laser treatment alone. At 2 years, 50% of Ranibizumab-treated patients gained ≥ 10 letters in BCVA, compared to 28% in the laser group[20-23].
Post hoc analyses of these trials provided additional insights, revealing sustained benefits in visual acuity and anatomical improvements over extended periods. For instance, patients initially treated with Ranibizumab maintained their visual gains and macular thickness reductions through extended follow-up[21-24].
The Cochrane systematic review analyzed 15 randomized controlled trials involving over 4000 participants to evaluate the efficacy of ranibizumab in DME. The findings revealed that ranibizumab significantly improves BCVA with an average gain of +6.6 letters at one year, compared to a minimal change of -0.5 letters in control groups receiving sham or laser treatment. Additionally, ranibizumab markedly reduced central retinal thickness (CRT), demonstrating its effectiveness in resolving macular edema. The review also affirmed ranibizumab's favorable safety profile, with a low incidence of adverse events[13-23].
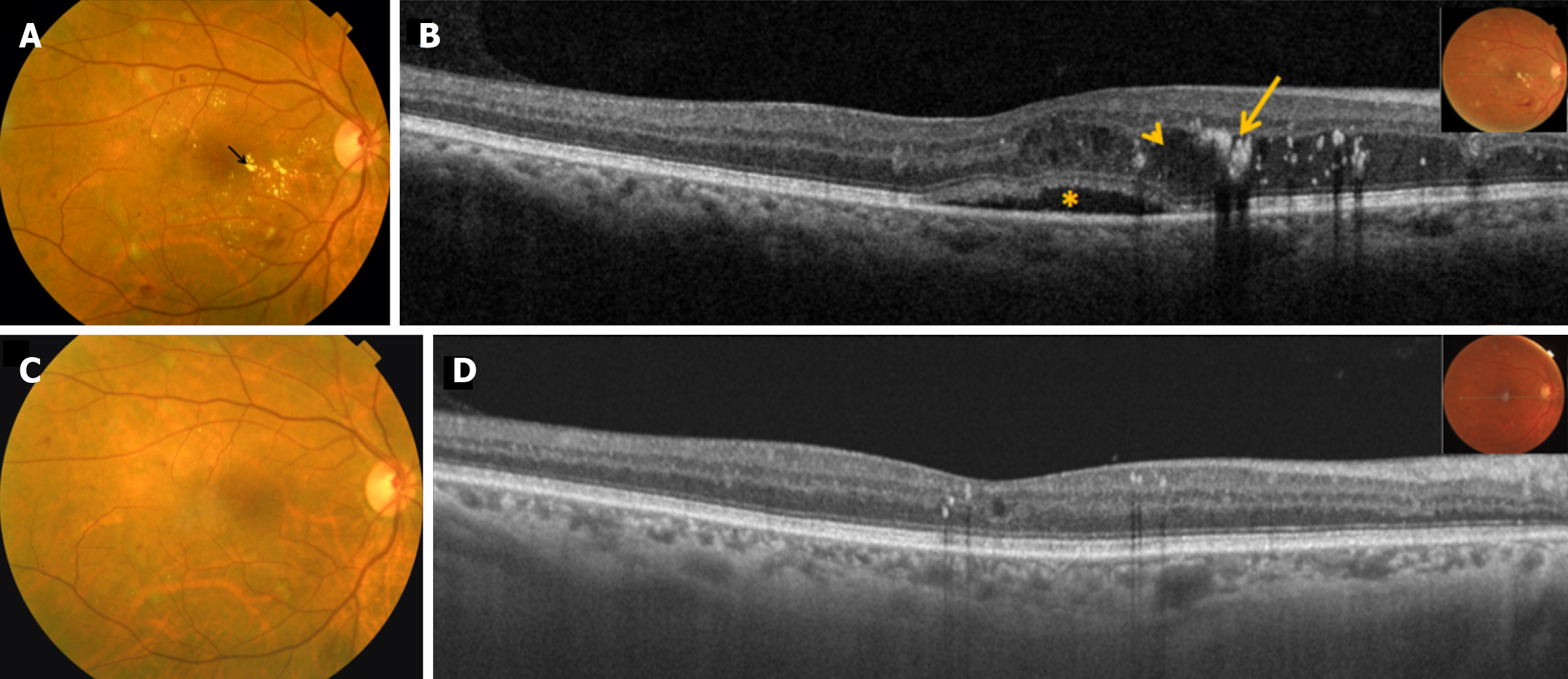
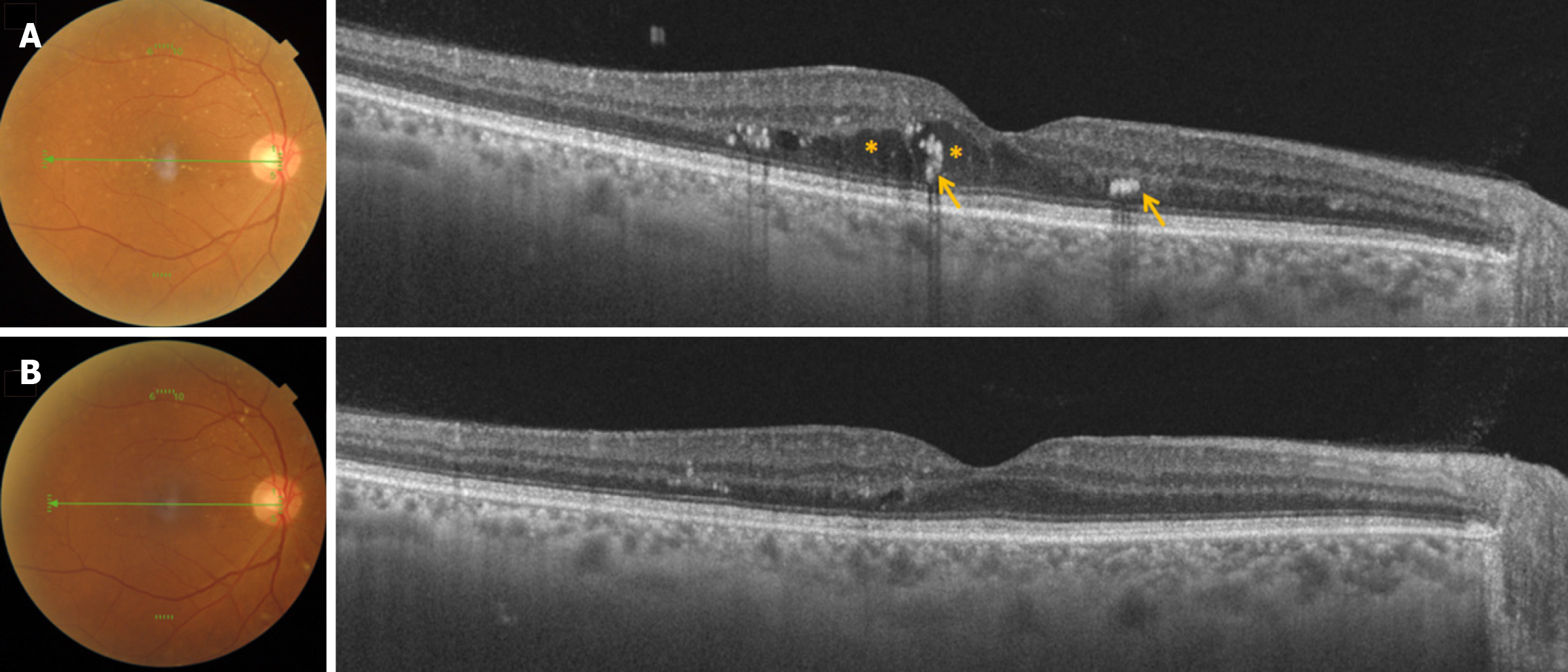
Ranibizumab, therefore, remains a cornerstone in the therapeutic arsenal against DME, supported by extensive clinical trial data, post hoc analyses, and real-world evidence. Its ability to significantly improve and sustain visual outcomes while maintaining a favorable safety profile underscores its vital role in preserving vision in diabetic patients. Because of this, Ranibizumab remains the first-line agent and the standard of care in the management of DME (Figures 1 and 2).
Aflibercept is a recombinant protein and attaches to the VEGF receptor 1 and 2. Its molecular weight is 115 kDa. The molecule blocks VEGF-A, VEGF-B, and Placental growth factor-1 and 2. Aflibercept was first developed as a treatment for wet AMD and received FDA approval for this indication in 2011. Its application for DME was approved by the FDA in 2014. Ever since, aflibercept has become a crucial component in the management of DR, providing an efficacious option for patients with DME.
The standard dosing regimen for aflibercept in DME is an initial series of five monthly injections (2 mg/0.05 mL), followed by injections every two months. In clinical practice, many physicians adopt a treat-and-extend approach, adjusting the interval between doses based on individual patient responses.
Numerous clinical trials have established the efficacy of aflibercept in DME, providing a robust foundation of evidence for its use. The VIVID and VISTA trials were pivotal in this context, offering detailed data on their therapeutic impact[21-24]. In the VIVID trial, aflibercept administered at 2 mg every four weeks or every eight weeks resulted in a mean improvement in BCVA of 10.5 and 10.7 letters, respectively, at 52 weeks, compared to a 1.2-letter improvement in the laser group. Similarly, the VISTA trial reported mean BCVA gains of 12.5 and 10.7 letters for the same dosing regimens, vs a 0.2-letter improvement with laser treatment at 52 weeks. Furthermore, aflibercept-treated patients exhibited a substantial decrease in central retinal thickness, with reductions of 185.9 μm and 183.1 μm in the VIVID study and 191.1 μm and 189.3 μm in the VISTA study, corresponding to the two dosing schedules[20-24].
The DRCR Protocol V examined the outcomes of initial aflibercept treatment against laser photocoagulation and observation in patients with good baseline visual acuity. Immediate aflibercept treatment did not significantly prevent vision loss compared to the other strategies. This suggests the importance of personalized treatment, taking into account initial visual acuity and disease severity to optimize outcomes for each patient[20-24].
Similarly, in another study by DRCR (Protocol T), patients with poorer visual acuity, when treated with aflibercept showed significantly greater improvements in BCVA compared to those receiving bevacizumab or ranibizumab[11-25].
Long-term studies have reinforced the sustained benefits of aflibercept. Five-year follow-up of patients in Protocol T confirmed that the visual acuity gain achieved with aflibercept could be maintained over an extended period, with a manageable safety profile[21-25]. Additionally, the extended results of the VISTA and VIVID studies at 148 weeks demonstrated that aflibercept continued to provide significant visual and anatomical improvement[21-26].
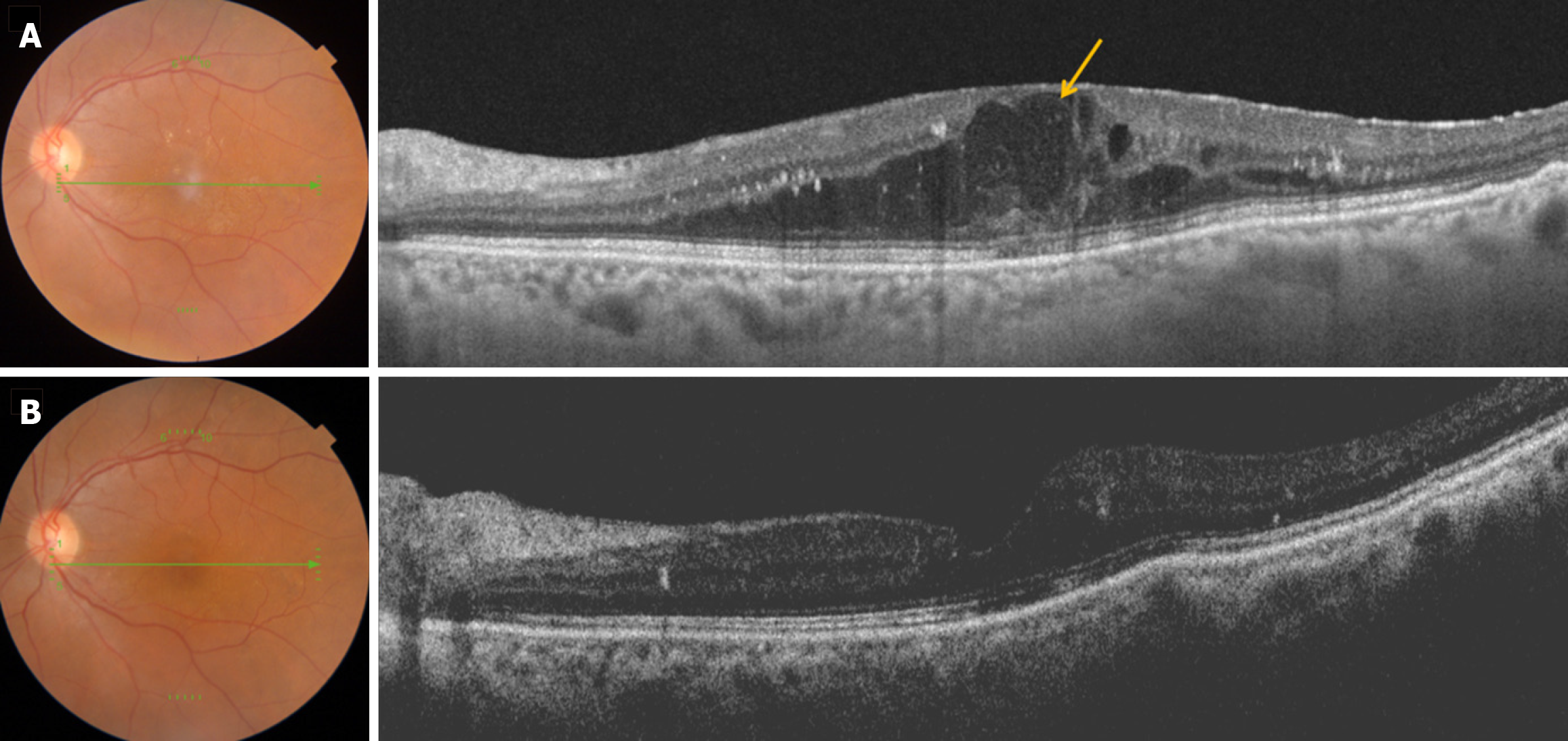
Thus, aflibercept remains a first-line treatment for DME, particularly in patients with severe visual impairment at baselinegiven its efficacy and manageable safety profile (Figure 3).
Brolucizumab is a humanized single-chain variable fragment (scFv) antibody that functions by binding with high specificity to VEGF(VEGF). It has a low molecular weight of 26 kDa. The structural design of brolucizumab enhances its specificity and potentially extends its therapeutic duration compared to conventional antibodies targeting VEGF. Brolucizumab was initially approved by the FDA for the treatment of neovascular AMD in October 2019. Its approval for DME followed in June 2022 based on the results of the pivotal KESTREL and KITE Phase 3 trials.
Brolucizumab is initially administered with a loading phase, involving injections (6 mg/0.05 mL) every six weeks for the first five doses. Subsequently, the maintenance phase adjusts to injections every 8 to 12 weeks based on individual response and disease activity.
The KESTREL and KITE trials were randomized, double-masked studies that evaluated the efficacy and safety of brolucizumab compared to aflibercept in patients with DME[21-29]. 926 patients from 36 countries were enrolled. Both trials demonstrated the non-inferiority of brolucizumab to aflibercept in terms of improvement in BCVA. Patients treated with brolucizumab experienced a mean gain of 12.2 letters, compared to 11.0 letters in the aflibercept group. Also, patients who received brolucizumab showed superior anatomical outcomes, with a higher proportion of patients achieving a significant reduction in CRT and resolution of retinal fluid compared to aflibercept. More than half of brolucizumab 6 mg subjects could be maintained on q12w dosing after loading, thereby potentially reducing treatment burden for patients[21-29].
A study by Rübsam et al[25] comparing intravitreal brolucizumab and aflibercept in patients with DME revealed significant improvement in BCVA and reduction in CRT. The study evaluated 35 eyes from 24 patients treated with brolucizumab and 40 eyes from 31 patients treated with aflibercept. At week 36, treatment-naïve DME eyes treated with brolucizumab showed an improvement of +6.4 letters (P = 0.03), while those treated with aflibercept gained +9.5 letters (P = 0.001). Additionally, recalcitrant DME eyes exhibited BCVA improvement of +5 letters with brolucizumab (P = 0.006) and +5.5 letters with aflibercept (P = 0.02). Both treatments also led to significant CRT reduction. Notably, the mean treatment interval for brolucizumab was longer at 11.3 weeks, compared to 6.5 weeks for aflibercept in treatment-naïve eyes, and 9.3 weeks vs 5.3 weeks for pretreated eyes, suggesting that brolucizumab may offer the benefit of reduced injection frequency[22-29].
However, while brolucizumab shows promising efficacy, the incidence of intraocular inflammation (IOI) and retinal vasculitis in real-world settings remains a concern. Reports indicate that IOI rates range from 2% to 4%, with some studies citing higher rates in specific patient groups or dosages[22-29]. Even in KITE and KESTREL trials, rates of IOI were higher in the brolucizumab group compared to aflibercept, with incidence reported in 4.7% and 3.7% of patients receiving brolucizumab 3 mg and 6 mg, respectively[22-29].
Thus, brolucizumab offers superior anatomical and visual outcomes with the potential for extended dosing intervals. However, recently, serious side effects like retinal vasculitis and severe intraocular inflammation have been reported with the use of brolucizumab. As it is a relatively newer agent with limited availability of long-term safety data, continued monitoring through real-world studies is required to ascertain its long-term safety and efficacy in DME management.
Faricimab is a novel bispecific antibody targeting both VEGF-A and angiopoietin-2 (Ang-2) designed specifically for ocular use.
Faricimab was developed to overcome the limitations of current anti-VEGF therapies by concurrently targeting Ang-2, which plays a role in vascular maturation and stability.
Faricimab's recommended dosage regimen involves intravitreal injections (6 mg/0.05 mL) administered initially every 8 weeks during the loading phase, followed by an extended dosing interval based on disease activity assessment.
YOSEMITE and RHINE trials by Wykoff et al[27] confirmed faricimab's efficacy and safety in DME. These double-masked, randomized phase 3 trials included over 1800 patients globally. A longer dosing regimen at 12 weeks and 16 weeks was evaluated. Both trials demonstrated that faricimab was non-inferior to aflibercept in improving BCVA, with patients gaining an average of 11.8 letters in the YOSEMITE trial and 10.7 letters in the RHINE trial. These gains were comparable to aflibercept, which showed gains of 10.3 and 10.2 letters, respectively. Additionally, faricimab led to a significant reduction in CRT, with a reduction of 187.1 µm in YOSEMITE and 195.8 µm in RHINE, compared to 170.3 µm and 169.1 µm for aflibercept. Notably, 52% of patients in YOSEMITE and 51% in RHINE achieved and maintained a 16-week dosing interval by the end of one year, indicating faricimab's potential for extended treatment intervals. The safety profile was comparable to aflibercept, though careful monitoring for intraocular inflammation and rare cases of retinal vasculitis is necessary[23-32].
Real-world studies and subgroup analyses have similarly supported the efficacy of faricimab across diverse patient demographics[23-33]. Thus, Faricimab represents a significant advancement in the management of DME, supported by robust clinical data demonstrating efficacy, durability, and manageable safety profile. Ongoing research continues to explore its long-term benefits and optimize treatment protocols, reaffirming its pivotal role in improving visual outcomes and quality of life for patients with DME. However, despite its promising efficacy, faricimab is associated with notable side effects, including IOI and rare instances of retinal vasculitis. Vigilance and prompt management of these adverse events are crucial for optimizing treatment outcomes[24-32].
The DRCR Protocol T study conducted a randomized clinical trial comparing the efficacy of three anti-vascular endothelial growth factor (anti-VEGF) agents-aflibercept, bevacizumab, and ranibizumab—in treating DME associated with central retinal involvement and visual acuity loss. The trial revealed that all three agents improved visual acuity, with aflibercept showing superior results, especially in patients with worse baseline visual acuity. Specifically, aflibercept led to an average improvement of 18.9 letters, whereas ranibizumab and bevacizumab resulted in gains of 14.2 and 11.8 letters, respectively, after one year.
The study highlighted the potential for greater treatment benefits with aflibercept in patients starting with poorer vision. However, in cases of better initial visual acuity, the differences among the three agents were minimal, making bevacizumab a cost-effective option despite its slightly lower efficacy[10-25].
Cheng et al[29] performed a systemic review to compare the efficacy of glucocorticoids and various anti-VEGF, in the treatment of DME, and evaluated various clinical treatment regimens consisting of different therapeutic measures.
The study examined 39 randomized controlled trials and found that at a 3-month follow-up, the combination of intravitreal bevacizumab (IVB) and triamcinolone acetonide (TA) was the most effective in improving best-corrected visual acuity (BCVA) and reducing central macular thickness. However, at the 6-month follow-up, intravitreal dexamethasone (DEX) implants showed superior efficacy, particularly in patients with severe macular oedema and impaired vision. This again highlights the importance of tailoring treatment strategies based on the severity and duration of the condition.
Inflammation plays an important role in the pathogenesis of DME. Hyperglycemia results in elevated levels of inflammatory cytokines, such as interleukin-1β (IL-1β), nuclear factor-κB (NF-κB), Vascular endothelial growth factor (VEGF), tumor necrosis factor α (TNFα), transforming growth factor-beta, and Intercellular Adhesion Molecule-1 in retinal pericytes and Müller cells[26-34]. Chronic hyperglycemia leads to the loss of pericytes and the disruption of tight junctions in retinal endothelial cells, which promotes the release of VEGF and other inflammatory mediators, including IL-1, IL-6, IL-8, TNFα, matrix metalloproteinases, kallikrein-kinin, and monocyte chemoattractant protein-1[29-35]. These mediators, along with VEGF, further compromise tight junction integrity, increasing vascular permeability and contributing to DME. Intravitreal corticosteroid injections can suppress these inflammatory pathways, thereby playing an important role in the treatment of DR and DME.
Several corticosteroids are used in intravitreal injections, including: (1) Triamcinolone Acetonide: One of the earliest corticosteroids used for DME, known for its anti-inflammatory properties; (2) Dexamethasone Implant (Ozurdex): A biodegradable implant that provides sustained release of the drug over several months; and (3) Fluocinolone Acetonide Implant (IluvienTM): Another long-acting implant that releases corticosteroid for up to three years.
TA is a potent corticosteroid with anti-inflammatory properties. Its application in ophthalmology, particularly for DME, gained attention in the early 2000s as an alternative to laser photocoagulation.
TA can be administered via intravitreal injection in different doses, typically ranging from 1 mg to 4 mg. The choice of dose may depend on the severity of DME, the patient's response to treatment, and the occurrence of side effects. A commonly used dose in clinical studies is 4 mg.
The DRCR-Protocol B is a landmark study that compared the efficacy of intravitreal TA with focal/grid laser photocoagulation for DME. Laser photocoagulation was more effective than TA in improving visual acuity over two years. TA provided more rapid short-term improvement in visual acuity compared to laser treatment. However, the patients treated with TA showed a higher incidence of adverse events, including elevated IOP and cataract progression[30-38].
Similarly in a Cochrane review by Rittiphairoj et al[33], TA was found to be effective in improving visual acuity and reducing central macular thickness in patients with DME. However, its use was also associated with significant adverse events, particularly increased intraocular pressure and cataract formation, making it less favourable compared to anti-VEGF agents for the long-term management of DME.
Abdel-Maboud et al[34] performed a meta-analysis to compare the efficacy and safety of intravitreal TA vs intravitreal Bevacizumab alone or combined IVB+IVT in the treatment of DME. The authors found no significant difference in the long-term outcomes between the two treatments regarding visual acuity and central macular thickness. However, IVT was associated with higher rates of increased intraocular pressure (IOP).
To sum up, TA has been a valuable addition to the therapeutic arsenal for DME. Its anti-inflammatory and anti-permeability properties can provide significant short-term improvement in visual acuity. Its low cost makes TA a useful option in DME management in resource-starved populations. However, the risk of side effects such as elevated IOP and cataract formation limits its long-term use. Thus, the choice of TA should be individualized, taking into account the patient's specific condition, response to previous treatments, and potential risks.
Dexamethasone is a more potent anti-inflammatory agent compared to TA and is associated with fewer side effects. Ozurdex® (Allergan, Inc., Irvine, CA, United States) is a sustained-release dexamethasone implant and provides long-acting anti-inflammatory action. The frequency of intravitreal injections needed is markedly reduced resulting in better patient compliance and fewer hospital visits. The implant is made from sustained release biodegradable copolymer that gradually degrades into lactic acid and glycolic acid, which are further metabolized into carbon dioxide and water. Ozurdex was FDA-approved in September 2014 for the treatment of DME.
The drug delivery system is a single-use device which uses a 22 G needle to insert the implant intravitreally leaving a self-sealing suture-less wound. The implant contains 0.7mg of dexamethasone and provides long-lasting effects up to 6 months.
The MEAD trials[33-43] were two large, randomized, multicenter phase 3 studies with identical protocols involving 1048 patients with center-involving DME. Based on the results of these very trials, the FDA approval of Ozurdex was granted for DME. The participants were divided into three groups: Ozurdex 0.7 mg, Ozurdex 0.35 mg, and a sham group. Upon three-year follow-ups, significant improvement in BCVA was observed with both doses of Ozurdex compared to sham, along with a notable reduction in central retinal thickness. Cataract formation and elevated IOP were significant side effects. 65% of phakic eyes receiving the DEX implant developed cataracts compared to 20% in the sham group. About 40% of patients in the Ozurdex group required IOP-lowering medications, vs only 10% in the sham group[33-44].
Following FDA approval, numerous studies have evaluated the efficacy and safety of the DEX implant. Special conditions such as vitrectomized eyes and DME refractory to anti-VEGF treatments have also been explored.
According to the EURETINA guidelines, dexamethasone implant can be considered a second-line treatment for patients who do not respond to anti-VEGF injections after 3 to 6 doses. It can also be considered as primary option for those with contraindications to anti-VEGF agents, such as a recent history of major cardiovascular events, pregnancy, or an unwillingness to adhere to monthly injections[34-44]. The PLACID trial was the first randomized multicenter study evaluating the use of Ozurdex in DME[38-44]. It compared two groups: One receiving Ozurdex plus laser at one month, and the other receiving laser monotherapy. Results showed a significantly higher proportion of patients achieving a 10-letter or more improvement in visual acuity in the Ozurdex plus laser group at nine months. The combination also proved superior in improving central retinal thickness in patients with diffuse macular edema. Elevated intraocular pressure was managed with medications in 16% of patients, and 20% of phakic eyes experienced cataract-related adverse events.
The MOZART study, a small retrospective analysis, assessed the 0.7 mg DEX implant's effectiveness and safety in DME patients with visual impairment. It demonstrated favorable anatomical and functional outcomes with a single injection and manageable side effects[38-45].
The CHROME study was a retrospective study and included patients with macular edema secondary to various retinal disease, including DME. For the DME cohort, while central retinal thickness reduction was achieved, significant visual acuity improvement was more noticeable in pseudophakic eyes[39-45].
The CHAMPLAIN study focused on the DEX implant's safety and efficacy in vitrectomized eyes, addressing the faster clearance of anti-VEGF agents in these eyes. This phase 2 clinical study found a 6-letter gain in BCVA at eight weeks and maintained a 3-letter gain at 26 weeks. Additionally, 30% of patients experienced a 10-letter gain in BCVA two months post-injection[40-44]. The recent ARTES study group conducted an extensive evaluation of dexamethasone in real-life settings for DME, considering previous treatment history, diabetes duration, and control levels. It concluded that treatment-naive eyes had better baseline and follow-up BCVA, while late DME and uncontrolled diabetes were associated with poorer outcomes. It also suggested shorter treatment intervals than the officially recommended six months[41-45].
DEX implant has been shown to be useful in in previously treated DME patients[42-47]. The DRCR.net Protocol U trial compared combination therapy of ranibizumab and a dexamethasone implant to ranibizumab alone in persistent DME, showing a significant reduction in central foveal thickness with the combination therapy[43-49].
A meta-analysis on single-dose dexamethasone implantation for persistent DME refractory to anti-VEGF therapy indicated significant anatomical and functional improvements[44-51]. The BEVORDEX trial, comparing bevacizumab and dexamethasone implant, found similar functional outcomes but greater CRT reduction with fewer injections in the dexamethasone group[45-51]. A consensus among Spanish experts recommended DEX implants as first-line therapy for specific DME patient groups, such as pseudophakic, poor-adherents, vitrectomized, candidates for cataract surgery, and those with a history of cardiovascular events. They also suggested using DEX implants after inadequate anti-VEGF response, typically after three injections[46-52].
Despite clinical trials suggesting the therapeutic effect of Ozurdex lasts at least six months, real-life practice indicates that more frequent dosing may be required for some patients. A study by Bucolo et al[49] on the long-term use of DEX implants in DME noted that about one-third of eyes needed retreatment before six months.
DEX implant has also been shown to be useful in patients with DME who undergo cataract surgery. Gupta et al[50] evaluated role of intraoperative intravitreal dexamethasone implant in patients with diabetic retinopathy with/without macula edema undergoing phacoemulsification in a two-arm, randomized, assessor-blinded trial. 151 patients with type-2 diabetes mellitus and cataract were divided into two groups: DEX group vs standard of care (SOC) group, i.e. phacoemulsification and intraocular lens implantation without injection DEX implant. The patients in DEX group had reduced central macular thickness and required lesser number of rescue interventions at the end of 12 weeks follow up compared to SOC group. The authors concluded that patients undergoing cataract surgery with DR with/without macular edema benefit from DEX implant with effects lasting for at least three months[48-53].
In summary, DEX implants have demonstrated favorable anatomical and functional outcomes in randomized controlled trials for DME. However, they exhibit a relatively higher incidence of ocular side effects, including an increased risk of cataract formation and elevated intraocular pressure, compared to the gold standard anti-VEGF agents. Consequently, DEX implants are typically considered a second-line treatment for DME (Figure 3). Nevertheless, they may be preferred as a first-line therapy in cases where anti-VEGF agents are contraindicated, such as during pregnancy or in patients with a recent history of major cardiovascular or cerebrovascular events. Additionally, DEX implants can be considered a primary treatment option for vitrectomized and pseudophakic eyes. They are also beneficial for patients with refractory or recalcitrant macular edema. The advantages of DEX implants over anti-VEGF agents include ease of administration, comparable cost, extended periods of remission, reduced frequency of injections, and consequently, fewer hospital visits.
The fluocinolone acetonide (FAc) implant, marketed as IluvienTM was developed to address the need for sustained drug delivery to the retina, offering a steady release of the corticosteroid over an extended period, thereby providing sustained drug delivery to the retina. IluvienTM received FDA approval in September 2014 for the treatment of DME in patients previously treated with corticosteroids without a significant rise in intraocular pressure.
The IluvienTM implant contains 0.19 mg of fluocinolone acetonide. It is administered intravitreally, typically through a 25-gauge needle, and designed to continuously release the drug over three years. The implant releases fluocinolone acetonide at an initial rate of 0.25 µg/day, averaging 0.2 µg/day, and is effective for up to 36 months.
Several studies have evaluated the efficacy and safety of Iluvien in DME.
The FAME (Fluocinolone Acetonide for Diabetic Macular Edema) phase 3 trials[49-55] were instrumental in validating the efficacy of the FAc implant, Iluvien, for the treatment of DME. These multicenter, randomized, double-masked trials included over 950 patients who were followed for 36 months. The primary endpoint was the proportion of patients achieving a ≥ 15-letter improvement in BCVA from baseline, a critical measure of clinical significance in visual function improvement. Patients receiving the FAc implant showed significant and sustained improvement in visual acuity compared to the control group, which received a sham injection. Specifically, at 24 months, 28.7% of patients in the low-dose FAc group (0.2 µg/day) achieved a ≥ 15-letter gain in BCVA, compared to 16.2% in the sham group. This benefit was maintained at the 36-month mark, with 28.7% in the FAc group vs 18.9% in the control group showing similar gains. In addition to visual acuity improvement, the FAME trials also reported a significant reduction in central retinal thickness, a key indicator of DME severity. Patients treated with the FAc implant exhibited a mean reduction in CRT from baseline, highlighting the implant's ability to mitigate retinal swelling effectively.
The safety profile of the FAc implant was also thoroughly assessed. The commonly identified side effects, such as increased intraocular pressure and cataract formation, were managed with standard ophthalmic interventions[49-55].
PALADIN, a phase 4 study, further confirmed the long-term safety and efficacy of the 0.19 mg FAc implant. It focused on patients with DME who had previously received corticosteroid treatment without significant IOP increase. Over a three-year follow-up, the study demonstrated sustained visual acuity improvement, with an average BCVA gain of 4.5 letters from baseline. The implant significantly reduced the treatment burden, with 76% of eyes not requiring additional therapy during the study period. Side effects were consistent with earlier trials, primarily increased IOP and cataract formation, manageable with standard ophthalmic care[51-56].
A systematic review of real-world studies by Kodjikian et al[54] involving 1880 eyes demonstrated that the FAc implant for DME resulted in a mean peak visual gain of +8.7 letters and a mean central retinal thickness reduction of 34.3%[52-57]. The review highlighted that patients with lower baseline BCVA and more recent DME experienced better outcomes. However, those with chronic DME often required more frequent rescue therapies. Additionally, ocular hypertension was reported in 20.1% of cases, but only 0.6% needed surgical intervention, and 43.2% of phakic patients underwent cataract extraction.
Thus, Iluvien has emerged as a useful option in managing chronic DME. It is currently positioned as a second or third-line treatment for DME, particularly beneficial for patients unresponsive to anti-VEGF therapies. Its long-term efficacy in reducing treatment frequency and improving visual outcomes has shown promising results, even though ocular hypertension and cataracts remain important adverse effects.
PDR is a severe and advanced stage of diabetic retinopathy characterized by the neovascularization of the retina and optic disc. This pathological angiogenesis occurs in response to retinal ischemia, driven by upregulated VEGF. Newly formed vessels are fragile, leading to recurrent vitreous hemorrhage, tractional retinal detachment, and potential vision loss. Pan-retinal photocoagulation (PRP) is the standard of care for the management of PDR. However, PRP may result in reduced peripheral vision and worsening of DME. Recently, intravitreal anti-VEGF agents have been used in the management of PDR.
Ranibizumab, an anti-VEGF agent, was approved by the FDA for the treatment of PDR in 2017. It works by inhibiting VEGF, a key molecule in angiogenesis, thereby preventing the formation of abnormal blood vessels in the retina and reducing vascular permeability.
DRCR network has conducted extensive research on ranibizumab for PDR. The landmark Protocol S study compared ranibizumab with PRP, demonstrating that intravitreal ranibizumab was non-inferior to PRP in visual acuity outcomes at two years[53-56]. Mean visual acuity improvement was 2.8 letters in the ranibizumab group vs 0.2 letters in the PRP group (P < 0.001). A follow-up study evaluating five-year outcomes confirmed these findings, with 53% of the ranibizumab group maintaining stable or improved vision compared to 46% in the PRP group. Additionally, rani
Lang et al[58] in the PRIDE study's second-year follow-up found that combination therapy of Ranibizumab and PRP could offer enhanced outcomes in some patients[54-61]. Ranibizumab was found to be more effective than PRP, particularly in reducing central retinal thickness and preventing progression to advanced DR stages. Additionally, combination therapy involving ranibizumab and PRP has been shown to enhance overall efficacy, providing a comprehensive approach to managing PDR. Another study by Gross et al[56] highlighted the sequence effect in combined Ranibizumab and PRP therapy, emphasizing the timing and order of treatments for optimal efficacy.
Thus, Ranibizumab represents a significant advancement in the treatment of PDR, offering comparable, if not superior, outcomes to traditional PRP with added benefits in terms of patient quality of life and reduced procedural complications. Ongoing research and trials will continue to refine its application, potentially establishing it as the first-line therapy for PDR.
Ranibizumab has also shown promise as a preoperative adjunct in vitrectomy for PDR with vitreous hemorrhage. Studies report that preoperative administration of Ranibizumab reduces intraoperative bleeding and postoperative complications, facilitating easier and safer surgical interventions.
A study by Li et al[59] demonstrated that patients pre-treated with Ranibizumab had significantly better surgical outcomes and faster recovery[58-63]. In this study, 50 patients with PDR and vitreous hemorrhage were divided into two groups: One group received a preoperative intravitreal injection of Ranibizumab, while the other group did not. The results indicated that the Ranibizumab pre-treatment group had a significantly lower incidence of intraoperative bleeding (12% vs 36%, P < 0.05) and postoperative complications such as recurrent vitreous hemorrhage and retinal detachment (8% vs 24%, P < 0.05). Additionally, patients in the Ranibizumab group had a shorter mean duration of surgery (45 minutes vs 60 minutes, P < 0.05) and a faster postoperative visual recovery, with a higher percentage achieving a visual acuity of 20/40 or better at three months post-surgery (68% vs 44%, P < 0.05).
These findings are consistent with other studies in the field. For instance, a meta-analysis conducted by Beaulieu et al[57] reviewed several randomized controlled trials and concluded that preoperative Ranibizumab significantly reduces intraoperative complications and improves surgical outcomes in patients undergoing vitrectomy for PDR. The pooled data showed a reduction in intraoperative bleeding by 50% and a 30% reduction in the incidence of postoperative complications.
Furthermore, a study by Li et al[59] supported these results, demonstrating that preoperative Ranibizumab administration not only facilitates vitrectomy but also enhances the overall anatomical and functional outcomes. Patients who received Ranibizumab had a higher rate of complete vitreous clearance and fewer required additional surgical interventions compared to the control group.
The mechanism behind these benefits is thought to involve Ranibizumab’s ability to inhibit VEGF, thereby reducing neovascularization and vessel permeability. This leads to decreased intraoperative bleeding and a more stable intraocular environment during surgery, which contributes to better postoperative outcomes and faster recovery times.
Thus, preoperative use of Ranibizumab in vitrectomy for PDR with vitreous hemorrhage is associated with significant improvements in surgical safety and efficacy. These findings highlight the potential of Ranibizumab as a valuable adjunctive therapy in the management of complex diabetic retinal diseases.
Aflibercept has emerged as a useful treatment option for PDR. FDA approved aflibercept for the treatment of PDR in 2019.
Several studies have explored the efficacy of Aflibercept in PDR. The DRCR Protocol W is one of the most notable, examining the long-term visual outcomes of Aflibercept in preventing vision-threatening complications in DR. This four-year randomized trial demonstrated that patients receiving Aflibercept had significantly better visual outcomes compared to those undergoing standard treatment, with an average visual acuity improvement of 7.5 letters (P < 0.001)[64]. In another DRCR study[64], a randomized clinical trial, 205 adults with vitreous hemorrhage from proliferative DR were randomized to intravitreous aflibercept (n = 100) or vitrectomy with panretinal photocoagulation (n = 105). The primary outcome, mean visual acuity over 24 weeks, showed no significant difference between aflibercept (59.3) and vitrectomy group. At 4 weeks, aflibercept group had lower visual acuity compared to vitrectomy, but differences at 2 years were not significant. The study suggested that while initial treatment choice did not significantly affect visual outcomes over 24 weeks, larger studies may be needed to discern the potential long-term benefits of initial vitrectomy with panretinal photocoagulation.
Xie et al[62] conducted a comprehensive meta-analysis of Aflibercept for the long-term treatment of DME and PDR[64-70]. The analysis included multiple randomized controlled trials and found that Aflibercept significantly reduced central retinal thickness and improved visual acuity. Specifically, Aflibercept-treated eyes showed an average reduction in central retinal thickness of 137.5 microns and an improvement in visual acuity of 10.3 letters (P < 0.01). The CLARITY study[64-70], another significant trial, compared intravitreal Aflibercept with PRP for PDR. This non-inferiority trial concluded that Aflibercept was as effective as PRP in maintaining or improving BCVA at 52 weeks. Aflibercept-treated eyes had a mean BCVA improvement of 3.9 letters, while the PRP group showed a mean change of 3.3 letters (P < 0.001).
The ability of Aflibercept to reduce retinal neovascularization and stabilize or improve vision makes it a valuable tool in the management of PDR. However, some limitations and considerations must be addressed. The long-term sustainability of Aflibercept's benefits and the optimal frequency of injections remain areas requiring further research. Additionally, combination therapies involving Aflibercept and PRP or other treatment modalities are being explored to enhance therapeutic outcomes. For instance, a study by Tao et al[64] found that combining Aflibercept with PRP resulted in better control of high-risk PDR compared to Aflibercept alone, suggesting that combination therapy might be more effective in certain patient populations[65].
In conclusion, both Ranibizumab and Aflibercept potentially offer comparable or superior outcomes to traditional PRP. Ranibizumab has demonstrated efficacy in visual acuity improvement and reduction of vision-impairing complications while Aflibercept has shown promising visual outcomes and central retinal thickness reduction. The perioperative use of Ranibizumab in vitrectomy further underscores its utility in enhancing surgical safety and outcomes. Ongoing research and clinical trials continue to refine the applications and optimize the treatment protocols of these anti-VEGF agents.
Biosimilars are biologic medical products that are highly similar to already approved reference biologics. Biosimilars are usually developed once the patent on the original biologic expires. They offer significant potential in reducing treatment costs and increasing accessibility. The introduction of biosimilars into clinical practice represents a transformative advancement in the management of DR and DME, providing more affordable therapeutic options without compromising efficacy or safety.
Ranibizumab (Lucentis) biosimilars have been developed and approved in various regions. Razumab (Intas Pharmaceuticals Ltd.) was the first biosimilar of ranibizumab to be developed. Sufficient literature evidence is available on the clinical efficacy and safety of Razumab in real-world practice. The CESAR study[66] was a multicenter, prospective clinical trial that evaluated the efficacy and safety of Razumab in 324 patients with DME. The study demonstrated significant improvement in BCVA and reduction in Central macular thickness at 6 months follow-up. Importantly, the safety profile of Razumab was found to be comparable to the reference ranibizumab, with adverse events being mild to moderate and consistent with those reported for the innovator drug. In another multicenter retrospective study by Chakraborty et al[66] compared innovator ranibizumab with its biosimilar. The study included 250 patients, evenly split between those receiving the biosimilar and those receiving the reference product. Over a follow-up period of 12 months, both groups exhibited comparable improvement in BCVA and reduction in central macular thickness. The incidence of adverse events was similar across both cohorts, further supporting the equivalence of the biosimilar to its reference product in both efficacy and safety.
RanizuRel™ is another Ranibizumab biosimilar developed by Reliance Life Sciences. Sharma et al[67] assessed the safety of Ranizurel in clinical practice in Ranizurel Safety Evaluation in Real-World (RaSER) study. They concluded that Ranizurel has a favourable safety profile, with a low incidence of adverse effects reported among patients.
Aflibercept biosimilars are emerging as promising alternatives for treating DME. One notable biosimilar, Yesafili (aflibercept-jbvf), has recently been approved by the FDA in May 2024 as the first interchangeable biosimilar to Eylea (aflibercept) offering a cost-effective option for DME.
The advent of biosimilars for ranibizumab and aflibercept marks a significant milestone in the management of diabetic retinopathy and diabetic macular edema. These biosimilars offer efficacy and safety profiles comparable to their reference biologics, thus providing additional treatment options that can potentially reduce healthcare costs. Continuous research and post-marketing surveillance will be essential to further establish the long-term efficacy and safety of these biosimilars, ensuring their sustained integration into clinical practice.
In recent times, newer drugs are being developed to effectively control systemic disease as well as to act locally. Previously, trials like the United Kingdom Prospective Diabetes Study and Diabetes Control and Complications Trial have established the role of strict control of DM in reducing systemic complications of DM. Novel Drugs like Ruboxistaurin which modulate VEGF expression have been tested in clinical trials. The drug inhibits the beta isoform of protein Kinase C and downregulates VEGF expression. A reduction in the occurrence of sight-threatening complications after the use of Ruboxistaurin has been highlighted in the study conducted by PKC- DRS group. The group also highlighted the improvement in vision in some patients with DR. A decrease in the progression of DME and limited requirement of laser treatment was also noted in the study[69]. Other newer drugs being studied include octreotide, fiderestat and ranirestat. Increased levels of growth hormone and Insulin-like Growth factor can worsen DR. Octreotide is a synthetic somatostatin analogue which acts as a growth hormone-inhibitor. It has been used to prevent neovascularization and protect against the development of proliferative DR[70]. Fiderestat and ranirestat are aldose reductase inhibitors which lead to strict control of glycemia and have shown a reduction in retinal thickness secondary to their use in patients with DME[71]. Previously, long-term use of fiderestat has shown the suppression of the development of DR changes in animal models[72].
The major drawback of intravitreal therapy is the temporary and short-lived effect of the agents used. The sight-threatening complications and ischemic changes may develop despite therapy. The risk for re-bleeding, progression and reappearance of pathological changes may persist after the time-bound effect of the drug wanes off. Also, once the severe ischemic changes have set in, the reversal of the effects is difficult even after therapy. Other side effects include cataract formation, glaucoma and endophthalmitis[72]. Diabetes mellitus is fast becoming a pandemic involving both the anterior as well as the posterior segment of the human eye. Artificial – intelligence will play a major role in a customized treatment for the ophthalmic – patients[73-75]. This futuristic modality will help in identifying the best suited anti – VEGF therapy for a particular patient of diabetic-retinopathy.
Upcoming novel therapies like stem cell therapy, the use of nanotechnology, and vesicular systems may revolutionize the therapy of DR in the near future. There have been serious concerns about the serious side effects of anti-VEGF therapy like cardiovascular risk factors and stroke. However, a recent meta-analysis did not highlight any significant risk after anti-VEGF therapy.
| 1. | Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL 3rd, Klein R; American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27 Suppl 1:S84-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 639] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 2. | Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1251] [Article Influence: 96.2] [Reference Citation Analysis (0)] |
| 3. | Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 2286] [Article Influence: 152.4] [Reference Citation Analysis (0)] |
| 4. | Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 368] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, Gardiner TA, Lyons TJ, Hammes HP, Simó R, Lois N. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016;51:156-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 718] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 6. | Calderon GD, Juarez OH, Hernandez GE, Punzo SM, De la Cruz ZD. Oxidative stress and diabetic retinopathy: development and treatment. Eye (Lond). 2017;31:1122-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 7. | Stewart MW. Anti-vascular endothelial growth factor drug treatment of diabetic macular edema: the evolution continues. Curr Diabetes Rev. 2012;8:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Boyer DS, Hopkins JJ, Sorof J, Ehrlich JS. Anti-vascular endothelial growth factor therapy for diabetic macular edema. Ther Adv Endocrinol Metab. 2013;4:151-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 9. | Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, Peto T, Egan C, Bunce C, Leslie RD, Hykin PG. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012;130:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 289] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 10. | Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ, Elman MJ, Ferris FL, Friedman SM, Melia M, Pieramici DJ, Sun JK, Beck RW. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1174] [Cited by in RCA: 1132] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 11. | Bressler NM, Beaulieu WT, Maguire MG, Glassman AR, Blinder KJ, Bressler SB, Gonzalez VH, Jampol LM, Melia M, Sun JK, Wells JA 3rd; Diabetic Retinopathy Clinical Research Network. Early Response to Anti-Vascular Endothelial Growth Factor and Two-Year Outcomes Among Eyes With Diabetic Macular Edema in Protocol T. Am J Ophthalmol. 2018;195:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Virgili G, Parravano M, Evans JR, Gordon I, Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev. 2018;10:CD007419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 13. | Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, Ehrlich JS; RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1226] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 14. | Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang J, Rubio RG, Adamis AP, Ehrlich JS, Hopkins JJ; RIDE and RISE Research Group. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 635] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 15. | Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M, Weichselberger A, Wolf S. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399-2405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 529] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 16. | Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A; RESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 991] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 17. | Freund KB, Korobelnik JF, Devenyi R, Framme C, Galic J, Herbert E, Hoerauf H, Lanzetta P, Michels S, Mitchell P, Monés J, Regillo C, Tadayoni R, Talks J, Wolf S. Treat-and-extend Regimens with Anti-vegf Agents in Retinal Diseases: A Literature Review and Consensus Recommendations. Retina. 2015;35:1489-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 18. | Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL 3rd, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064-1077.e35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1061] [Cited by in RCA: 1027] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 19. | Sun JK, Wang PW, Taylor S, Haskova Z. Durability of Diabetic Retinopathy Improvement with As-Needed Ranibizumab: Open-Label Extension of RIDE and RISE Studies. Ophthalmology. 2019;126:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, Midena E, Kaiser PK, Terasaki H, Marcus DM, Nguyen QD, Jaffe GJ, Slakter JS, Simader C, Soo Y, Schmelter T, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Zeitz O, Metzig C, Brown DM. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 593] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 21. | Glassman AR, Baker CW, Beaulieu WT, Bressler NM, Punjabi OS, Stockdale CR, Wykoff CC, Jampol LM, Sun JK; DRCR Retina Network. Assessment of the DRCR Retina Network Approach to Management With Initial Observation for Eyes With Center-Involved Diabetic Macular Edema and Good Visual Acuity: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2020;138:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Glassman AR, Wells JA 3rd, Josic K, Maguire MG, Antoszyk AN, Baker C, Beaulieu WT, Elman MJ, Jampol LM, Sun JK. Five-Year Outcomes after Initial Aflibercept, Bevacizumab, or Ranibizumab Treatment for Diabetic Macular Edema (Protocol T Extension Study). Ophthalmology. 2020;127:1201-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 23. | Brown DM, Emanuelli A, Bandello F, Barranco JJE, Figueira J, Souied E, Wolf S, Gupta V, Ngah NF, Liew G, Tuli R, Tadayoni R, Dhoot D, Wang L, Bouillaud E, Wang Y, Kovacic L, Guerard N, Garweg JG. KESTREL and KITE: 52-Week Results From Two Phase III Pivotal Trials of Brolucizumab for Diabetic Macular Edema. Am J Ophthalmol. 2022;238:157-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 24. | Wykoff CC, Garweg JG, Regillo C, Souied E, Wolf S, Dhoot DS, Agostini HT, Chang A, Laude A, Wachtlin J, Kovacic L, Wang L, Wang Y, Bouillaud E, Brown DM. KESTREL and KITE Phase 3 Studies: 100-Week Results With Brolucizumab in Patients With Diabetic Macular Edema. Am J Ophthalmol. 2024;260:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 25. | Rübsam A, Hössl L, Rau S, Böker A, Zeitz O, Joussen AM. Real-World Experience with Brolucizumab Compared to Aflibercept in Treatment-Naïve and Therapy-Refractory Patients with Diabetic Macular Edema. J Clin Med. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Garweg JG, Keiper J, Pfister IB, Schild C. Functional Outcomes of Brolucizumab-Induced Intraocular Inflammation Involving the Posterior Segment-A Meta-Analysis and Systematic Review. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Wykoff CC, Abreu F, Adamis AP, Basu K, Eichenbaum DA, Haskova Z, Lin H, Loewenstein A, Mohan S, Pearce IA, Sakamoto T, Schlottmann PG, Silverman D, Sun JK, Wells JA, Willis JR, Tadayoni R; YOSEMITE and RHINE Investigators. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022;399:741-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 258] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 28. | Penha FM, Masud M, Khanani ZA, Thomas M, Fong RD, Smith K, Chand A, Khan M, Gahn G, Melo GB, Khanani AM. Review of real-world evidence of dual inhibition of VEGF-A and ANG-2 with faricimab in NAMD and DME. Int J Retina Vitreous. 2024;10:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 29. | Cheng Z, Liu X. Comparing the efficacy of glucocorticoids and anti-VEGF in treating diabetic macular edema: systematic review and comprehensive analysis. Front Endocrinol (Lausanne). 2024;15:1342530. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Kowluru RA, Zhong Q, Kanwar M. Metabolic memory and diabetic retinopathy: role of inflammatory mediators in retinal pericytes. Exp Eye Res. 2010;90:617-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol. 1999;14:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Diabetic Retinopathy Clinical Research Network (DRCR.net), Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, Hartnett E, Ip MS, Kim JE, Kollman C. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 33. | Rittiphairoj T, Mir TA, Li T, Virgili G. Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev. 2020;11:CD005656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Abdel-Maboud M, Menshawy E, Bahbah EI, Outani O, Menshawy A. Intravitreal bevacizumab versus intravitreal triamcinolone for diabetic macular edema-Systematic review, meta-analysis and meta-regression. PLoS One. 2021;16:e0245010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Boyer DS, Yoon YH, Belfort R Jr, Bandello F, Maturi RK, Augustin AJ, Li XY, Cui H, Hashad Y, Whitcup SM; Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 763] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 36. | Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, Jonas J, Larsen M, Tadayoni R, Loewenstein A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237:185-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 438] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 37. | Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD, Liu CC, Li XY, Hollander DA, Schiffman RM, Whitcup SM; Ozurdex PLACID Study Group. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013;120:1843-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 38. | Guigou S, Hajjar C, Parrat E, Merite PY, Pommier S, Matonti F, Prost-Magnin O, Meyer F. [Multicenter Ozurdex® assessment for diabetic macular edema: MOZART study]. J Fr Ophtalmol. 2014;37:480-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Lam WC, Albiani DA, Yoganathan P, Chen JC, Kherani A, Maberley DA, Oliver A, Rabinovitch T, Sheidow TG, Tourville E, Wittenberg LA, Sigouin C, Baptiste DC. Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: the CHROME study. Clin Ophthalmol. 2015;9:1255-1268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Boyer DS, Faber D, Gupta S, Patel SS, Tabandeh H, Li XY, Liu CC, Lou J, Whitcup SM; Ozurdex CHAMPLAIN Study Group. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31:915-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 266] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 41. | Rosenblatt A, Udaondo P, Cunha-Vaz J, Sivaprasad S, Bandello F, Lanzetta P, Kodjikian L, Goldstein M, Habot-Wilner Z, Loewenstein A; ARTES Study Group. A Collaborative Retrospective Study on the Efficacy and Safety of Intravitreal Dexamethasone Implant (Ozurdex) in Patients with Diabetic Macular Edema: The European DME Registry Study. Ophthalmology. 2020;127:377-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Escobar-Barranco JJ, Pina-Marín B, Fernández-Bonet M. Dexamethasone Implants in Patients with Naïve or Refractory Diffuse Diabetic Macular Edema. Ophthalmologica. 2015;233:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Malclès A, Dot C, Voirin N, Agard É, Vié AL, Bellocq D, Denis P, Kodjikian L. Real-Life Study in Diabetic Macular Edema Treated with Dexamethasone Implant: The Reldex Study. Retina. 2017;37:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 44. | Iglicki M, Busch C, Zur D, Okada M, Mariussi M, Chhablani JK, Cebeci Z, Fraser-Bell S, Chaikitmongkol V, Couturier A, Giancipoli E, Lupidi M, Rodríguez-Valdés PJ, Rehak M, Fung AT, Goldstein M, Loewenstein A. Dexamethasone Implant for Diabetic Macular Edema in Naive Compared with Refractory Eyes: The International Retina Group Real-Life 24-Month Multicenter Study. The IRGREL-DEX Study. Retina. 2019;39:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 45. | Maturi RK, Glassman AR, Liu D, Beck RW, Bhavsar AR, Bressler NM, Jampol LM, Melia M, Punjabi OS, Salehi-Had H, Sun JK; Diabetic Retinopathy Clinical Research Network. Effect of Adding Dexamethasone to Continued Ranibizumab Treatment in Patients With Persistent Diabetic Macular Edema: A DRCR Network Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2018;136:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 46. | Yuan Q, Liu Y, Xu H, Gao Y, Qin L, Gou Y, Tao M, Zhang M. Efficacy and safety of single-dose dexamethasone implantation for patients with persistent diabetic macular edema: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2022;260:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, Goodwin S, Aroney C, McAllister IL, Fraser-Bell S. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014;121:2473-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 229] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 48. | García Layana A, Adán A, Ascaso FJ, Cabrera F, Donate J, Escobar Barranco JJ, Peralta G, Reyes García R, Rodríguez Maqueda M, Ruiz-Moreno JM, Vinagre I; MOMENTUM-D Study Group. Use of intravitreal dexamethasone implants in the treatment of diabetic macular edema: Expert recommendations using a Delphi approach. Eur J Ophthalmol. 2020;30:1042-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Bucolo C, Gozzo L, Longo L, Mansueto S, Vitale DC, Drago F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: A systematic review of real-world studies. J Pharmacol Sci. 2018;138:219-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 50. | Gupta PC, Ram J, Kumar MP, Agarwal A, Gupta V, Singh R, Bansal R, Katoch D, Dogra MR, Gupta A. Effect of sustained-release long-acting intravitreal dexamethasone implant in patients of non-proliferative diabetic retinopathy undergoing phacoemulsification: A randomized controlled trial. Indian J Ophthalmol. 2021;69:3263-3272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 51. | Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, Tolentino M, Gupta A, Duarte L, Madreperla S, Gonder J, Kapik B, Billman K, Kane FE; FAME Study Group. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626-635.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 52. | Yang Y, Bailey C, Holz FG, Eter N, Weber M, Baker C, Kiss S, Menchini U, Ruiz Moreno JM, Dugel P, Lotery A; FAME study group. Long-term outcomes of phakic patients with diabetic macular oedema treated with intravitreal fluocinolone acetonide (FAc) implants. Eye (Lond). 2015;29:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Singer MA, Sheth V, Mansour SE, Coughlin B, Gonzalez VH. Three-Year Safety and Efficacy of the 0.19-mg Fluocinolone Acetonide Intravitreal Implant for Diabetic Macular Edema: The PALADIN Study. Ophthalmology. 2022;129:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 54. | Kodjikian L, Baillif S, Creuzot-Garcher C, Delyfer MN, Matonti F, Weber M, Mathis T. Real-World Efficacy and Safety of Fluocinolone Acetonide Implant for Diabetic Macular Edema: A Systematic Review. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Writing Committee for the Diabetic Retinopathy Clinical Research Network; Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB, Bressler NM, Browning D, Elman MJ, Ferris FL 3rd, Friedman SM, Marcus DM, Melia M, Stockdale CR, Sun JK, Beck RW. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA. 2015;314:2137-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 556] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 56. | Gross JG, Glassman AR, Liu D, Sun JK, Antoszyk AN, Baker CW, Bressler NM, Elman MJ, Ferris FL 3rd, Gardner TW, Jampol LM, Martin DF, Melia M, Stockdale CR, Beck RW; Diabetic Retinopathy Clinical Research Network. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018;136:1138-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 57. | Beaulieu WT, Bressler NM, Melia M, Owsley C, Mein CE, Gross JG, Jampol LM, Glassman AR; Diabetic Retinopathy Clinical Research Network. Panretinal Photocoagulation Versus Ranibizumab for Proliferative Diabetic Retinopathy: Patient-Centered Outcomes From a Randomized Clinical Trial. Am J Ophthalmol. 2016;170:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Lang GE, Stahl A, Voegeler J, Quiering C, Zaremba L, Lorenz K, Spital G, Liakopoulos S. Observational outcomes in proliferative diabetic retinopathy patients following treatment with ranibizumab, panretinal laser photocoagulation or combination therapy - The non-interventional second year follow-up to the PRIDE study. Acta Ophthalmol. 2022;100:e578-e587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Li S, Yang Y, Zou J, Zeng J, Ding C. The efficacy and safety of intravitreal injection of Ranibizumab as pre-treatment for vitrectomy in proliferative diabetic retinopathy with vitreous hemorrhage. BMC Ophthalmol. 2022;22:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Maturi RK, Glassman AR, Josic K, Baker CW, Gerstenblith AT, Jampol LM, Meleth A, Martin DF, Melia M, Punjabi OS, Rofagha S, Salehi-Had H, Stockdale CR, Sun JK; DRCR Retina Network. Four-Year Visual Outcomes in the Protocol W Randomized Trial of Intravitreous Aflibercept for Prevention of Vision-Threatening Complications of Diabetic Retinopathy. JAMA. 2023;329:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 61. | Antoszyk AN, Glassman AR, Beaulieu WT, Jampol LM, Jhaveri CD, Punjabi OS, Salehi-Had H, Wells JA 3rd, Maguire MG, Stockdale CR, Martin DF, Sun JK; DRCR Retina Network. Effect of Intravitreous Aflibercept vs Vitrectomy With Panretinal Photocoagulation on Visual Acuity in Patients With Vitreous Hemorrhage From Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA. 2020;324:2383-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 62. | Xie X, Lian C, Zhang Z, Feng M, Wang W, Yuan X, Shi Y, Liu T. Aflibercept for long-term treatment of diabetic macular edema and proliferative diabetic retinopathy: a meta-analysis. Front Endocrinol (Lausanne). 2023;14:1144422. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 63. | Intravitreal aflibercept compared with panretinal photocoagulation for proliferative diabetic retinopathy: the CLARITY non-inferiority RCT. Southampton (UK): NIHR Journals Library; 2018 . [PubMed] |
| 64. | Tao Y, Jiang P, Zhao Y, Song L, Ma Y, Li Y, Wang H. Retrospective study of aflibercept in combination therapy for high-risk proliferative diabetic retinopathy and diabetic maculopathy. Int Ophthalmol. 2021;41:2157-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Verma L, Thulasidas M, Purohit A, Gupta A, Narula R, Talwar D. Clinical efficacy and safety of Razumab® (CESAR) study: Our experience with the world's first biosimilar Ranibizumab. Indian J Ophthalmol. 2021;69:347-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 66. | Chakraborty D, Sengupta S, Mondal S, Boral S, Das A, Sinha TK, Bhattacharya R, Maitra R. Comparison of Innovator vs. Biosimilar Ranibizumab in Treating Diabetic Macular Edema: A Multicenter Retrospective Study. Ophthalmol Ther. 2022;11:629-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 67. | Sharma S, Sharma T, Prasad S, Gopalakrishnan M, Chaturvedi A. Treatment Landscape of Macular Disorders in Indian Patients with the Advent of Razumab™ (World's First Biosimilar Ranibizumab): A Comprehensive Review. Ophthalmol Ther. 2021;10:431-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 68. | PKC-DRS2 Group; Aiello LP, Davis MD, Girach A, Kles KA, Milton RC, Sheetz MJ, Vignati L, Zhi XE. Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113:2221-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 69. | Grant MB, Mames RN, Fitzgerald C, Hazariwala KM, Cooper-DeHoff R, Caballero S, Estes KS. The efficacy of octreotide in the therapy of severe nonproliferative and early proliferative diabetic retinopathy: a randomized controlled study. Diabetes Care. 2000;23:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Kutlutürk Karagöz I, Allahverdiyev A, Bağırova M, Abamor EŞ, Dinparvar S. Current Approaches in Treatment of Diabetic Retinopathy and Future Perspectives. J Ocul Pharmacol Ther. 2020;36:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Kato N, Yashima S, Suzuki T, Nakayama Y, Jomori T. Long-term treatment with fidarestat suppresses the development of diabetic retinopathy in STZ-induced diabetic rats. J Diabetes Complications. 2003;17:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Kodjikian L, Bellocq D, Mathis T. Pharmacological Management of Diabetic Macular Edema in Real-Life Observational Studies. Biomed Res Int. 2018;2018:8289253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 73. | Morya AK, Gowdar J, Kaushal A, Makwana N, Biswas S, Raj P, Singh S, Hegde S, Vaishnav R, Shetty S, S P V, Shah V, Paul S, Muralidhar S, Velis G, Padua W, Waghule T, Nazm N, Jeganathan S, Reddy Mallidi A, Susan John D, Sen S, Choudhary S, Parashar N, Sharma B, Raghav P, Udawat R, Ram S, Salodia UP. Evaluating the Viability of a Smartphone-Based Annotation Tool for Faster and Accurate Image Labelling for Artificial Intelligence in Diabetic Retinopathy. Clin Ophthalmol. 2021;15:1023-1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Morya AK, Ramesh PV, Kaur K, Gurnani B, Heda A, Bhatia K, Sinha A. Diabetes more than retinopathy, it's effect on the anterior segment of eye. World J Clin Cases. 2023;11:3736-3749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Reference Citation Analysis (0)] |
| 75. | Morya AK, Janti SS, Sisodiya P, Tejaswini A, Prasad R, Mali KR, Gurnani B. Everything real about unreal artificial intelligence in diabetic retinopathy and in ocular pathologies. World J Diabetes. 2022;13:822-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |