Published online Dec 20, 2024. doi: 10.5493/wjem.v14.i4.96412
Revised: September 23, 2024
Accepted: October 22, 2024
Published online: December 20, 2024
Processing time: 177 Days and 21.2 Hours
Autologous blood therapy has emerged as a promising modality in managing ocular surface disorders. This review provides a comprehensive overview of the current literature regarding the use of autologous blood in ocular surface dis
Core Tip: There are several reviews in the literature about autologous tissue therapy and its usefulness in eye care including stem cells and tissue transplants. Blood derivatives are playing a progressively significant role in management of ocular surface disorders. This paper reviews studies on autologous blood use in ocular surface disorders, its preparation and administration, patient selection and potential side effects. Recent clinical trials are also reviewed and future considerations are discussed.
- Citation: Suleman A, Aluyi-Osa G, Ashipa F, Spadea L, Gagliano C, D’Esposito F, Zeppieri M, Musa M. Autologous blood in the management of ocular surface disorders. World J Exp Med 2024; 14(4): 96412
- URL: https://www.wjgnet.com/2220-315x/full/v14/i4/96412.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i4.96412
Ocular surface diseases (OSD) are disorders plaguing the surface structures of the eyes; namely the conjunctiva, cornea, and tear glands[1]. The most common manifestations of OSDs are dry eyes and blepharoconjunctivitis. OSDs usually present with gritty sensations, redness, mucous or stringy discharge, occasional blurry vision, and excessive tearing. OSDs often lead to visual impairment and consequently reduced quality of living[2]. Although autologous blood medicine has been known for centuries, its use in the care of OSD is relatively novel and has not been extensively reviewed. The authors sought to review all publications on autologous blood used in ocular surface disorders.
The ocular surface constitutes the boundary between the most anterior ocular tissues and the external surroundings. It is most anteriorly lined by squamous epithelial cells and protected from foreign objects and desiccation by the reflex shutting of the lids. The eyelids are a door-like structure whose action dictates what enters the eye. The lids cover the anterior eye and divide into the superior and inferior lids, which are continuous with each other and with the skin and muscles of the face at the nasal and lateral canthi. The eyelids are like a hinged double door entrance regulator with hair follicles for eyelashes arranged in two to three rows at the point on the eyelid margin just anterior to the mucocutaneous junction. The mucocutaneous junction can be described as the transition point where the stratified squamous epithelial of the skin is replaced by the cuboidal epithelial cells of the conjunctival[3]. The opening and shutting of the eyelids are enabled by the levator palpebral superioris, and the orbicularis oculi respectively. The orbicularis oculi inserts posterior to the subcutaneous tissue of the eyelid and anterior to the tarsal plate. The meibomian gland has its origin in the tarsal plate. It has its excretory ducts running through the tarsal plate in an imperfect straight line-like manner and end up as orifices at the lid margin via which its secretions (meibum) are discharged. The meibomian gland secretes the outermost layer of the tear film, and there are approximately 40 to 50 meibomian glands in the upper lid and 20 to 30 meibomian glands in the lower lid[4]. The glands of Moll and Zeis are both exocrine glands that maintain the health of the lids and lashes. The Zeis gland is a unilobar sebaceous gland, located at the anterior aspect of the eyelash base in the lid margin, while the Moll gland is a modified apocrine sweat gland located at the posterior aspect of the base of the cilia[5].
The conjunctiva is a translucent, well-vascularised mucous membrane that extends from the mucocutaneous junction at the lid margins and is continuous throughout the ocular surface until its epithelium becomes continuous with the cornea epithelium at the corneoscleral junction. As it extends from the mucocutaneous junction, it is named according to the part of the ocular surface it covers namely; the palpebral, fornix, and bulbar conjunctiva. The palpebral conjunctiva is firmly attached to the underlying tarsal plate while the bulbar conjunctival is loosely connected to the tenons capsule which links the conjunctival to the underlying sclera[5]. At the nasal canthus, the bulbar conjunctival makes a small crescent fold known as the plica semilunaris, and just nasal to it, is a small, mildly elevated, pink-yellowish nodule that consists of a mixture of tissues called the caruncle or caruncular lacrimalis. Histologically, the conjunctival epithelium is three to five cells thick and its basal cells are made up of cuboidal cells which subsequently become polyhedral flattened cells as they ascend more superficially[5]. The conjunctival epithelium consists of non-keratinized stratified squamous epithelia and stratified columnar cells. Goblet cells are scattered throughout the epithelium, having their greatest density in the inferonasal aspect of the conjunctival epithelium[6]. The conjunctival stromal or subtantia propria which is the deeper part of the conjunctiva consists of a more superficial adenoid layer and a deep fibrous layer, and the accessory lacrimal glands of Krause and Wolfring are located in the deeper fibrous layer. Numerous immune cells such as mo
The conjunctival epithelium becomes continuous with cornea epithelial cells at the limbus. Stellate symmetric ridges found at the limbus form the palisades of Vogt, which is considered a reservoir for cornea stem cells[7]. The cornea is a transparent, absolute avascular, curved refractive tissue. The avascularity of the cornea is maintained by several factors including the absence of blood vessels, the manner of stromal fibrillar arrangement, and the cornea endothelium pump action. This ‘no-blood’ vessel status is necessary to maintain its transparency and refractive status. The cornea can further be described as the refractive powerhouse of the eye. It possesses an average refractive power of 43.00 DS, constituting about 75% of the total refractive power of the eye[8]. The cornea is divided into five layers namely; the epithelium, bowman’s membrane, stromal, descemet membrane, and endothelium.
The cornea epithelium is about 50 μm thick and consists of five to seven layers of cells. The outermost layer of the cornea epithelium is made up of non-keratinized squamous epithelial cells. The cells of the cornea epithelial surface regulate the movement of substances into the cornea through the tears. These cells are attached at their lateral walls, close to the surface of the cell facing the lumen by tight junctions also known as zonular occludens[9]. This barrier complex forms an effective semi-permeable membrane that allows certain substrates through into the cornea. Furthermore, the surface epithelial cells produce a thick glue-like transcellular barrier known as glycocalyx; which helps to adjoin the mucin layer of the tears to the cornea epithelial surface[10]. Moreover, the surface epithelial cells possess finger-like (microvilli) and ridge-like (microplicae) projections which aid in fastening the precorneal tear film to the cornea epithelial surface. The middle layer of the cornea epithelium consists of polyhedral-shaped wing cells which are attached each to other by gap junctions and desmosomes, and to the underlying germinal basal layer by desmosomes. The basal layer of the corneal epithelium consists of a single layer of columnar cells. The basal layer is where mitosis occurs, thus, older basal cells move up to the middle layer to become rhombic-like wing cells and subsequently to the epithelial surface where they are finally sloughed off due to aging or epithelial erosion.
The pre-cornea tear film is three-layered namely; the lipid layer, the aqueous layer, and the mucin layer. The tear film is coated anteriorly by the secretion from the meibomian gland. This layer of the tear film ensures that the integrity of the precorneal tear film remains intact. The aqueous aspect of the tear film is produced chiefly by the lacrimal gland with additions from the accessory lacrimal glands. The innermost layer which adjoins the precorneal tear film to the cornea surface epithelium is produced by the goblet cells scattered throughout the conjunctival epithelium.
The anatomical orientation of the ocular surface structures is designed in such a way that confers on the globe the ability to protect, regulate entrance, lubricate, and refract light rays. The eyelids shut against invading foreign objects consequently, fortifying the eye from foreign entities which could pose significant injury and health risks to the globe. The precorneal tear film is a mixture consisting of epitheliotrophic factors[11]. In patients with ocular surface disorders, the keratinocyte morphology and function are affected, making the use of tear substitutes inadequate in addressing the problem of OSDs[12,13].
Blood is a multifaceted fluid composed of several elements that collaborate to maintain homeostasis and support various physiological functions. The main components of blood are red blood cells, white blood cells, platelets, and plasma. Red blood cells, or erythrocytes, are the most prevalent cellular element, responsible for carrying oxygen from the lungs to body tissues and aiding in the removal of carbon dioxide. White blood cells, or leukocytes, are essential for the immune response, protecting the body against pathogens and foreign invaders. Platelets, or thrombocytes, are small cell fragments that facilitate blood clotting and wound healing. Plasma, the liquid part of blood, acts as a medium for transporting nutrients, hormones, and waste products, and helps maintain electrolyte balance and pH levels[14]. Each component of blood is essential for overall health and well-being, contributing to the body’s ability to function properly and respond to various challenges. Numerous studies have extensively explored the functions and characteristics of these blood com
The various pathways through which the diseases of the ocular surface structures exert their pathological changes on the ocular surface are dependent on the type and nature of ocular morbidity, severity, and the ocular surface structure involved. However, these pathogenic processes are often initiated secondary to the breakdown of the ocular surface defense system offered by the tear film. The breakdown of the precorneal tear film integrity can result from cellular infiltration of the serous glands[18], fibrosis of the serous glands[19], prolonged and subsequently auto-sustained inflammatory reaction of the ocular surface from activated acquired immunity as well as conjunctival epithelia metaplasia[20,21]. Other etiologic factors include iatrogenic factors[22], graft versus host disease, chronic meibomian dysfunction, trauma, hormonal and nutritional deficiencies, and drugs.
This review investigates the mechanisms that cause ocular surface abnormalities and explores the current autologous blood-based therapies used to treat these problems. The present study provides a concise overview of ongoing clinical trials, aiming to compare the effectiveness of autologous blood therapy with traditional eyedrops. The text provides a comprehensive explanation of the safe processes and protocols used in autologous blood medicine. It also examines the potential adverse effects and future prospects of this innovative therapy.
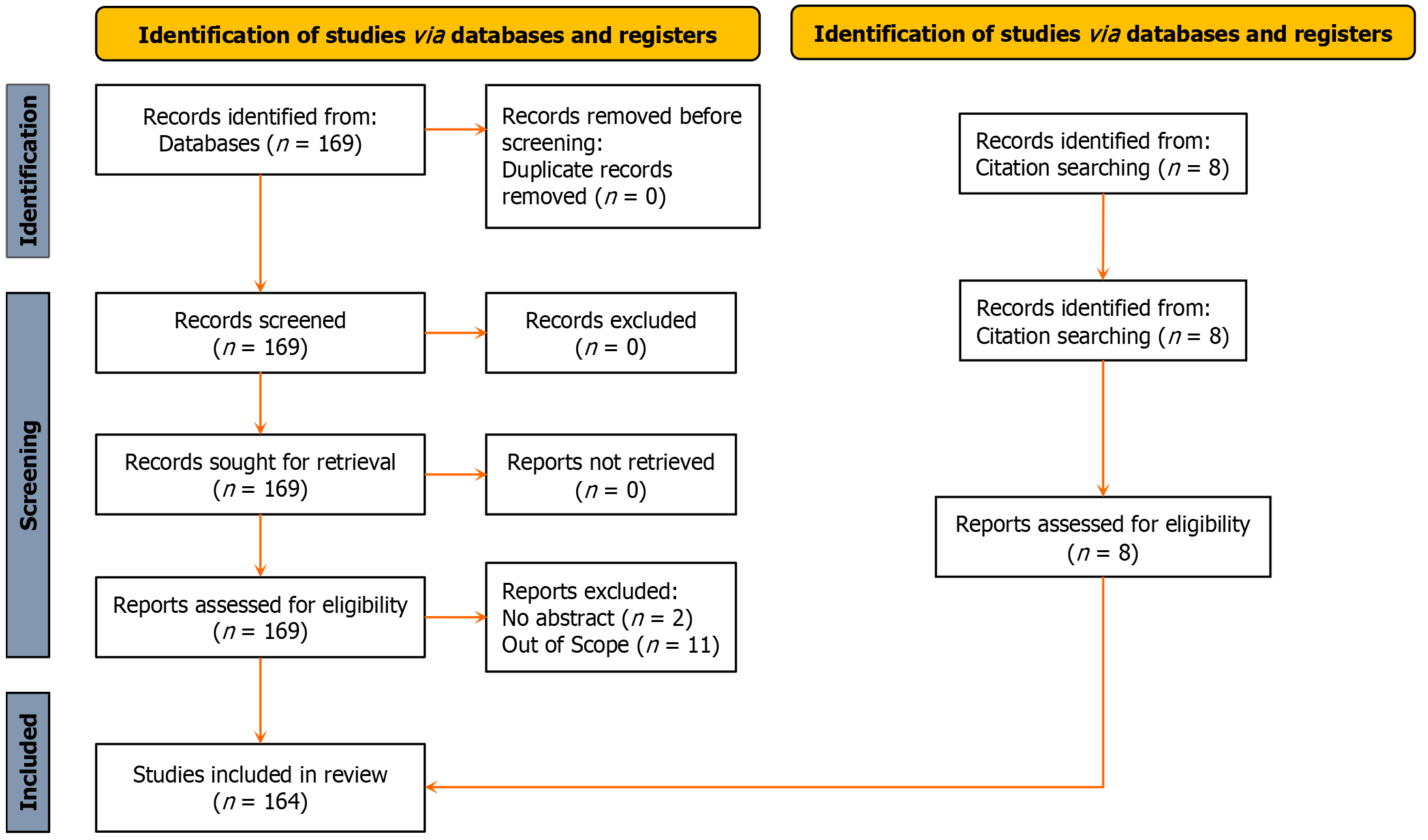
Only full-length papers published in English language were used for this review. The PubMed (https://pubmed.ncbi.nlm.nih.gov) database was queried using the keywords “autologous blood ocular surface disease”, generating the following search algorithm; (“autolog”[All Fields] OR “autologeous”[All Fields] OR “autologic”[All Fields] OR “autological”[All Fields] OR “autologous”[All Fields] OR “autologously”[All Fields]) AND (“blood”[MeSH Subheading] OR “blood”[All Fields] OR “blood”[MeSH Terms] OR “bloods”[All Fields] OR “haematology”[All Fields] OR “hematology”[MeSH Terms] OR “hematology”[All Fields] OR “haematoma”[All Fields] OR “hematoma”[MeSH Terms] OR “hematoma”[All Fields] OR “haemorrhage”[All Fields] OR “hemorrhage”[MeSH Terms] OR “hemorrhage”[All Fields] OR “haemorrhages”[All Fields] OR “hemorrhages”[All Fields] OR “haemorrhagic”[All Fields] OR “haemorrhaging”[All Fields] OR “hematologies”[All Fields] OR “haematomas”[All Fields] OR “hematomas”[All Fields] OR “hematoma s”[All Fields] OR “hematomae”[All Fields] OR “hemorrhaged”[All Fields] OR “hemorrhagic”[All Fields] OR “hemorrhagical”[All Fields] OR “hemorrhaging”[All Fields]) AND (“ocul surf”[Journal] OR (“ocular”[All Fields] AND “surface”[All Fields]) OR “ocular surface”[All Fields]) AND (“disease”[MeSH Terms] OR “disease”[All Fields] OR “diseases”[All Fields] OR “disease s”[All Fields] OR “diseased”[All Fields]). All publications retrieved were screened for relevance and eligibility by three authors (Suleman A, Aluyi-Osa G, and Musa M). A total of 169 publications were retrieved. 2 records were excluded for not having abstracts while 11 were out of scope.
Three authors examined the extracted results for uniqueness, relevance to the topic, language, and content. Where one of the authors flagged a study as out of scope, the other reviewer was allowed to re-scrutinize before discarding the pub
The mechanism of action of autologous blood is the promotion of cellular differentiation, proliferation, and migration of these cells due to the presence of very metabolically active substances such as growth factors, immunoglobulins, fibronectin, vitamins A, and anti-proteases[24-27]. Autologous blood therapy also supplies cytokines and growth factors that are found lacking in natural[28]. Autologous serum has been used in the development of cornea and oral epithelial cells making the case for its role in the proliferation of epithelial cells[29,30].
Autologous blood is usually used to treat persistent cornea defects and dry eyes. Still, it has also been shown to be effective in the management of superior limbic keratoconjunctivitis, recurrent erosion syndrome, aniridic keratopathy neurotrophic keratopathy, graft versus host disease, ocular cicatricial pemphigoid, Mooren’s ulcer, filtering bleb post trabeculotomy and post-keratorefractive surgery[31,32]. In dry eyes the epitheliotrophic factors (fibronectin, growth factors, and vitamins) are reduced hence leaving the ocular surface at risk of infections, reduced optical properties, and persistent epithelial defect.
Shtein et al[33] in their review of 10 studies of the effect of autologous serum-based eyedrops on dry eyes and 4 studies of the effect on persistent epithelial defect showed that the majority of the studies reported a great improvement in symptoms, signs, or both. Unpreserved blood serum has shown similar properties to the tears hence the use of auto
Higuchi[42] showed a new serum protein selenoprotein which proved to be more efficacious in the treatment of dry eye by showing a better cornea fluorescein score and reduced cornea oxidative stress in eyes when it was applied compared to phosphate-buffered saline. Studies showed that when autologous blood is used as a therapy for dry eyes, there is a resulting improvement in tear stability, as seen by better staining scores under the slip lamp corneal examina
López-García et al[49] in a prospective study, showed improvement in ocular symptoms in patients with keratopathy associated with aniridia when autologous serum eyedrops were used. Autologous blood serum has also been found effective in treating neurotrophic keratopathy, resulting in epithelial healing. This led to the belief that autologous blood contains neurotrophic properties[50]. In vivo confocal microscopy has revealed early evidence that autologous plasma therapy can improve cornea nerve regeneration, this is beneficial in patients with neurotrophic keratopathy[51]. Mucin serum and retinoic acid are important components in the maintenance of healthy ocular surface[52]. In their work, Alio et al[22] found out that autologous serum eyedrops are effective management plan for post-lasik associated keratopathy. Patients who have undergone a corneal transplant are at risk of significant ocular morbidity in the healing phase[53-55]. They reported an amelioration of symptoms with this novel therapy. In patients with systemic autoimmune diseases that present with dry eye diseases, autologous serum therapy has been shown to improve both subjective and objective findings[56-59]. In patients with recurrent epithelial defects, autologous blood serum has been found to be useful when combined with contact lenses[60].
Merayo-Lloves et al[61] and Anitua et al[62] in their retrospective cohort study, showed that PRGFs could be a safe and effective treatment of refractory OSD. The safety profile of autologous blood therapy in a hospital setting was proven true under strict protocol of storage and preparation[63]. In cases of ocular burn, autologous serum tears derived from the umbilical cord are more effective than artificial tears, as seen in the double-blind prospective randomized clinical control research by Kumar et al[64] and similar studies[65,66]. Edelmann et al[67] in a clinical trial carried out amongst dogs with spontaneous chronic cornea epithelial defects post epithelial debridement with diamond-burr showed no significant statistical difference in epithelization when using platelet-rich plasma or artificial tears. Mircheff et al[68] and Fea et al[69] in their clinical studies using in vivo confocal microscopy, confirmed that autologous platelet lysate eyedrops improved the ocular surface disease indices in patients with primary Sjogren syndrome. Other researchers have gone as far as recommending the autologous blood is adopted as a standard of care therapy for ocular surface wound healing[70]. Studies have been carried out to ascertain the effectiveness of platelet-rich fibrin derivative as compared to the human amniotic membrane, they found out that platelet-rich fibrin had more efficacy, probably due to its anatomical and chemical makeup[71-74]. Noble et al[75] in their randomized controlled trial, showed that autologous blood therapy (ABT) proved beneficial to patients with severe ocular surface disorders, as most patients reported improvement in symptoms.
Urzua et al[76] in a randomized double-blind clinical trial, proved that autologous serum in the short term provided better symptomatic relief in comparison to conventional artificial tears. Comparison of the autologous serum and plasma-rich protein in terms of epitheliotrophic properties showed negligible difference in baseline conditions. Platelet rich plasma is usually easier to prepare hence it is a viable substitute for autologous serum[77,78]. Rawat et al[79] showed that autologous platelet rich plasma was more effective in the management of moderate to severe OSDs. Platelet rich plasma lysate have also been indicated in the management of OSDs[80,81] In comparison with artificial tear substitutes, there is low evidence of their efficacy in managing dry eye diseases[82]. Randomized controlled trials have shown that there is a better improvement in OSDI scores when autologous blood therapy is used compared to artificial tears[83,84]. The effect of amniotic membrane suspension and autologous serum varies from individual to individual, depending on individual growth factor concentration[85-88]. Semeraro et al[89] in their clinical trial, showed that 50% blood serum improved the signs and symptoms of patients formally treated by conventional therapy. Zheng and Zhu[90] also suggested autologous serum eyedrops to be more effective than artificial tears in treating complaints associated with moderate to very severe dry eyes, and might therefore be a better option.
Factors influencing patient selection include: (1) The severity of the ocular surface disorder: Patients with moderate to severe OSDs who didn’t respond well to conventional therapy could benefit from autologous blood therapy; (2) Certain types of dry eye disease that other treatments may not have helped such as dry eyes secondary to meibomian gland dysfunction or aqueous deficiency; (3) Cornea epithelial damage, recurrent erosion of the cornea, or persistent defect of the epithelial despite the use of standard therapy; (4) Patients with an inflammatory component to OSD could benefit from ABT due to the anti-inflammatory properties present in autologous blood; (5) Patients with blood diseases who have contraindications to blood collection are not recommended for ABT; (6) The patient’s willingness to undergo the procedure involved in ABT[91]; (7) Techniques and protocols for autologous blood preparation and administration; (8) Blood component differentiation and separation was pioneered in 1960[92]. While there are several ways blood can be collected for ocular therapy, two major routes are; (9) Fingerprick method; here blood is collected by pricking the fingers and the blood collected is applied on the lower fornix[93]. This method has been advocated by several authors to be efficacious especially in the absence of serum[94-96]; and (10) Blood collection via the arm; here the blood is collected directly from the arm of the patient usually consisting of plasma and platelet concentrates after preparation from the initial peripheral blood[97].
Generally, the route of administration of ABT is topical, applying the serum directly on the ocular surface is usually done frequently to improve discomfort experienced by the patient[98]. The amount of times it would be applied depends on the degree of discomfort and symptoms experienced by patients[99,100]. In some studies, they confirmed that the sub-conjunctival surface can also be a route of application of autologous blood concentrate in the treatment of ocular burn, and this might even be a more economical and practical technique[101-103].
Success has been reported in most of the cases in published literature, with a few rare complications[104]. The limitations of ABT are the cost and inconvenience of obtaining blood-derived products[105,106], and the paucity of data with strong evidence from randomized double-masked clinical studies supporting its use[107-110]. Transmission of blood-borne diseases is possible if autologous serum is not aseptically acquired[111]. The lack of standardized protocol for blood collection, centrifugation, and other things needed makes it difficult to attain clinical use of autologous blood serum[112,113]. One major challenge faced during outpatient therapy, is usually based on good manufacturing and delivery medium from the point of production to centers these autologous blood serums are finally utilized[114-116]. In line with this challenge of storage of autologous serum, studies suggest that autologous serum can be stored for about 6 months in freezer conditions, without alteration in potency, and still be used to attain re-epithelization in cases of ocular surface anomaly[117-120]. More studies have to be done on the effect of different levels of immunosuppressives on ocular surface integrity and symptomatic relief[121].
There seems to be no significant difference in tear osmolality in patients with dry eye disease and those without dry eye disease who underwent autologous serum therapy[122]. Mesenchymal stem cell and limbal stem cell-derived autologous serum have been shown to have similar potency in treating OSD, as seen in the work carried out by Campbell et al[123]. Amniotic assisted conjunctival epithelial redirection, proves to be viable in reducing complications associated with the sequential sector conjunctiva epitheliopathy[124]. In some retrospective comparative case series, epithelial cells obtained from oral mucosa, are capable of staying on the cornea following transplantation, and maintain good corneal surface integrity[125]. Autologous serum eyedrops and allogenic serum eyedrops are almost equally effective in ameliorating symptoms and improving quality of life[126-129].
Umbilical cord blood or serum has been shown to contain a lot of biologically active chemicals that can nourish the ocular surface thus treating OSDs[130]. Hassan et al[94] in their feasibility studies, showed that autologous blood obtained from pricking the finger can be used as an adjunct to conventional medical therapy to improve the OSDI score.
Allogeneic blood therapy has also been shown to improve epitheliotrophic properties of the ocular surface in patients with OSDs similar to autologous blood therapy[131]. Calf blood extract gel has been shown to relieve dry eye symptoms in patients with chronic graft versus host disease due to its ability to promote healing and regeneration of epithelial cells[131,132]. Autologous blood therapy when used with punctal plugs has been shown to improve symptoms and signs in patients with chronic graft versus host disease refractory to artificial tears[133]. Autologous serum eyedrops obtained post-plasmapheresis and IV immunoglobulin infusion have been said to help manage patients with ocular complications secondary to toxic epidermal necrolysis[134].
Studies have isolated tumor necrosis factor as an inflammatory marker for dry eyes[135]. Tumor necrosis factor-inhibiting protein (a component of autologous blood lysate) has been found to help suppress signs related to Sjogren syndrome, by affecting histopathology of the lacrimal gland but having no effect on the conjunctiva tissues[136-139]. De-proteinized calf blood extract has also been reported to reduce the recovery time of patients who underwent pterygium excision, even when compared to the use of non-steroidal anti-inflammatory drugs, or even ocular lubricant[140]. The use of autologous serum in cases of severe reduction in limbal stem cells secondary to contact lens used has been found to substitute for the need for surgical intervention, especially if attended to on time and aggressively[141,142]. It is important to note that prompt ophthalmic evaluation and an interdisciplinary approach to care are very important, especially in cases where transplantation of tissue is involved, in other to prevent progressive and subsequent vision deterioration[19].
The use of autologous blood in the management of OSDs is affected by financial and logistic barriers[143]. The lack of proper regulation on the use of autologous blood serum in the eye care world makes its widespread use in the mana
Autologous serum-impregnated soft bandage contact lens shows promising results in the treatment of dye eyes stemming from autoimmune conditions like Sjogren syndrome[150]. Ang et al[151] showed that autologous serum-derived oral epithelial cells can be used for treating severe OSDs. Studies have also confirmed a dose-dependent effect of autologous serum on outcomes in dry eye diseases[152,153]. The use of isolated stem cells, through clonal analysis, has been found to also be effective in the treatment of corneal defects due to its ability to regenerate corneal tissue[154]. Some evidence also points to the effectiveness of complete autologous submandibular gland replacement, as it has been shown to have a good outcome, relieve symptoms, and improve quality of life[155-157]. Autologous tear serum has been found to affect the corneal nerve plexus as seen using in-vivo confocal microscopy[158,159]. Chiang et al[160] reported on a case series of patients treated with allogenic tear serum after suffering graft versus host disease. The use of corneal sheets mounted on platelet-poor plasma is an emerging trend in cases of limbal stem cell deficiency, and it helps in the treatment of bilateral ocular surface disorders[161-163]. Recent research has also been directed toward hemoderivatives including the platelets; associated with the platelets are the growth factors that are involved in the wound-healing process of the cornea and the conjunctiva[164].
The use of Xeno-feeder-free limbal stem cells in the treatment of ocular surface disorders is on its way, as it might be a potential substitute imminently, so more studies are being carried out in this regard[165]. Multiple research publications have found that a novel treatment (platelet-rich plasma) could ameliorate persistent epithelial defects and attributed it to the fact that it contains a lot of epidermal growth factor[166,167]. By the process of inducing the presence of anti-inflammatory mediators, dry eyes stemming from autoimmune conditions can be alleviated and this is a possible area of interest in the near future[168,169].
Wiącek et al[170] examined the long-term effects of autologous blood therapy in the management of retinitis pigmentosa by monitoring key indices at one month and then every quarter leading up to a year. They reported the therapy to be safe and efficacious over the one-year period. Lee and Chen[171] also evaluated the safety profile of long-term autologous blood therapy in managing chronic dry eye. They reported no significant complications even up to 17 months of constant use among a section of the cohort. Platelet-lysate drops, an autologous blood derivative, have also been reported to have long-term usefulness in the management of ocular graft versus host disease[172].
While some smaller-scale data exists, the authors could not find studies on large-scale or multicenter experimental studies comparing autologous blood therapy to conventional drugs.
Autologous blood therapy in ocular surface disorder management presents an interesting proposition to the eye care industry. Autologous blood serum has successfully been used to manage many ocular surface disorders arising primarily secondary to other conditions and surgeries. The results of this review underscore the potential of autologous blood therapy to enhance patient outcomes in ocular surface disorders. Implementing this therapy in clinical practice could enhance healing, reduce inflammation, and improve the overall management of these disorders. It combines well with conventional medications and compares favorably with them. While it’s widespread use is still hampered by regulations and the absence of a unanimous guideline for preparation and prescription.
| 1. | Khanna RC. Ocular surface disorders. Community Eye Health. 2017;30:S1-S2. [PubMed] |
| 2. | Jongkhajornpong P, Anothaisintawee T, Lekhanont K, Numthavaj P, McKay G, Attia J, Thakkinstian A. Short-term Efficacy and Safety of Biological Tear Substitutes and Topical Secretagogues for Dry Eye Disease: A Systematic Review and Network Meta-analysis. Cornea. 2022;41:1137-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Knop E, Knop N, Zhivov A, Kraak R, Korb DR, Blackie C, Greiner JV, Guthoff R. The lid wiper and muco-cutaneous junction anatomy of the human eyelid margins: an in vivo confocal and histological study. J Anat. 2011;218:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Kaur K, Stokkermans TJ. Meibomian Gland Disease. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024. [PubMed] |
| 5. | Takahashi Y, Watanabe A, Matsuda H, Nakamura Y, Nakano T, Asamoto K, Ikeda H, Kakizaki H. Anatomy of secretory glands in the eyelid and conjunctiva: a photographic review. Ophthalmic Plast Reconstr Surg. 2013;29:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Shumway CL, Motlagh M, Wade M. Anatomy, Head and Neck, Eye Conjunctiva. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024. [PubMed] |
| 7. | Moshirfar M, Masud M, Harvey DH, Payne C, Bruce E, Ronquillo YC, Hoopes PC. The Multifold Etiologies of Limbal Stem Cell Deficiency: A Comprehensive Review on the Etiologies and Additional Treatment Options for Limbal Stem Cell Deficiency. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Musa MJ, Zeppieri M. Spectacle Correction of Ametropias. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024. [PubMed] |
| 9. | Erickson KK, Sundstrom JM, Antonetti DA. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007;10:103-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Weng J, Trinh S, Lee R, Metwale R, Sharma A. Impact of High Glucose on Ocular Surface Glycocalyx Components: Implications for Diabetes-Associated Ocular Surface Damage. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | García-Martín E, Pernía-López S, Romero Jiménez RM, García-Valcárcel B, Martínez-Ortega PA, Sanjurjo-Saez M. The use of autologous serum eye drops for the treatment of ocular surface disorders. Eur J Hosp Pharm. 2019;26:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Katsakoulas I, Lougovoi C, Paraskevopoulou P, Vougioukas N. Protocol of Blood Serum Eye Drops. Int J Pharm Compd. 2015;19:252-260. [PubMed] |
| 13. | Geerling G, Hartwig D. [Autologous serum-eye-drops for ocular surface disorders. A literature review and recommendations for their application]. Ophthalmologe. 2002;99:949-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Farley A, Hendry C, McLafferty E. Blood components. Nurs Stand. 2012;27:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Keric D, Myers G, Stafford J. Health halo or genuine product development: Are better-for-you alcohol products actually healthier? Health Promot J Austr. 2022;33:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Savage T, Davis A, Fischhoff B, Morgan MG. A strategy to improve expert technology forecasts. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Minami A, Kurebayashi Y, Takahashi T, Otsubo T, Ikeda K, Suzuki T. The Function of Sialidase Revealed by Sialidase Activity Imaging Probe. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Zhu Z, Stevenson D, Schechter JE, Mircheff AK, Atkinson R, Trousdale MD. Lacrimal histopathology and ocular surface disease in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Tappeiner C, Heiligenhaus A, Halter JP, Miserocchi E, Bandello F, Goldblum D. Challenges and concepts in the diagnosis and management of ocular graft-versus-host disease. Front Med (Lausanne). 2023;10:1133381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Perez VL, Mah FS, Willcox M, Pflugfelder S. Anti-Inflammatories in the Treatment of Dry Eye Disease: A Review. J Ocul Pharmacol Ther. 2023;39:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 21. | Jirsova K, Seidler Stangova P, Palos M, Mahelkova G, Kalasova S, Rybickova I, Utheim TP, Vesela V. Aberrant HLA-DR expression in the conjunctival epithelium after autologous serum treatment in patients with graft-versus-host disease or Sjögren's syndrome. PLoS One. 2020;15:e0231473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Alio JL, Pastor S, Ruiz-Colecha J, Rodriguez A, Artola A. Treatment of ocular surface syndrome after LASIK with autologous platelet-rich plasma. J Refract Surg. 2007;23:617-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Maniaci A, Lavalle S, Masiello E, Lechien JR, Vaira L, Boscolo-Rizzo P, Musa M, Gagliano C, Zeppieri M. Platelet-Rich Plasma (PRP) in the Treatment of Long COVID Olfactory Disorders: A Comprehensive Review. Biomedicines. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 24. | Hayat H, Masarwa D. [Autologous serum as a treatment for the ocular surface disease]. Harefuah. 2023;162:187-188. [PubMed] |
| 25. | Bernabei F, Roda M, Buzzi M, Pellegrini M, Giannaccare G, Versura P. Blood-Based Treatments for Severe Dry Eye Disease: The Need of a Consensus. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Freire V, Andollo N, Etxebarria J, Durán JA, Morales MC. In vitro effects of three blood derivatives on human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2012;53:5571-5578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | López-García JS, García-Lozano I, Rivas L, Martínez-Garchitorena J. [Use of autologous serum in ophthalmic practice]. Arch Soc Esp Oftalmol. 2007;82:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Giannaccare G, Versura P, Buzzi M, Primavera L, Pellegrini M, Campos EC. Blood derived eye drops for the treatment of cornea and ocular surface diseases. Transfus Apher Sci. 2017;56:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 29. | Nakamura T, Inatomi T, Sotozono C, Ang LP, Koizumi N, Yokoi N, Kinoshita S. Transplantation of autologous serum-derived cultivated corneal epithelial equivalents for the treatment of severe ocular surface disease. Ophthalmology. 2006;113:1765-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Nakamura T, Ang LP, Rigby H, Sekiyama E, Inatomi T, Sotozono C, Fullwood NJ, Kinoshita S. The use of autologous serum in the development of corneal and oral epithelial equivalents in patients with Stevens-Johnson syndrome. Invest Ophthalmol Vis Sci. 2006;47:909-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Jeng BH. Use of autologous serum in the treatment of ocular surface disorders. Arch Ophthalmol. 2011;129:1610-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Quinto GG, Campos M, Behrens A. Autologous serum for ocular surface diseases. Arq Bras Oftalmol. 2008;71:47-54. [PubMed] |
| 33. | Shtein RM, Shen JF, Kuo AN, Hammersmith KM, Li JY, Weikert MP. Autologous Serum-Based Eye Drops for Treatment of Ocular Surface Disease: A Report by the American Academy of Ophthalmology. Ophthalmology. 2020;127:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | Anitua E, Muruzabal F, Pino A, Prado R, Azkargorta M, Elortza F, Merayo-Lloves J. Proteomic Characterization of Plasma Rich in Growth Factors and Undiluted Autologous Serum. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004;88:1467-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 289] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 36. | Balal S, Udoh A, Pappas Y, Cook E, Barton G, Hassan A, Hayden K, Bourne RRA, Ahmad S, Pardhan S, Harrison M, Sharma B, Wasil M, Sharma A. The feasibility of finger prick autologous blood (FAB) as a novel treatment for severe dry eye disease (DED): protocol for a randomised controlled trial. BMJ Open. 2018;8:e026770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Dang DH, Riaz KM, Karamichos D. Treatment of Non-Infectious Corneal Injury: Review of Diagnostic Agents, Therapeutic Medications, and Future Targets. Drugs. 2022;82:145-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 38. | Anitua E, de la Sen-Corcuera B, Orive G, Sánchez-Ávila RM, Heredia P, Muruzabal F, Merayo-Lloves J. Progress in the use of plasma rich in growth factors in ophthalmology: from ocular surface to ocular fundus. Expert Opin Biol Ther. 2022;22:31-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Mrugacz M, Zywalewska N. [HLA-DR antigen expression on conjunctival epithelial cells in patients with dry eye]. Klin Oczna. 2005;107:278-279. [PubMed] |
| 40. | Semeraro F, Forbice E, Nascimbeni G, Taglietti M, Romano V, Guerra G, Costagliola C. Effect of Autologous Serum Eye Drops in Patients with Sjögren Syndrome-related Dry Eye: Clinical and In Vivo Confocal Microscopy Evaluation of the Ocular Surface. In Vivo. 2016;30:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Anitua E, Muruzabal F, de la Fuente M, Riestra A, Merayo-Lloves J, Orive G. PRGF exerts more potent proliferative and anti-inflammatory effects than autologous serum on a cell culture inflammatory model. Exp Eye Res. 2016;151:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Higuchi A. Autologous Serum and Serum Components. Invest Ophthalmol Vis Sci. 2018;59:DES121-DES129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Celebi AR, Ulusoy C, Mirza GE. The efficacy of autologous serum eye drops for severe dry eye syndrome: a randomized double-blind crossover study. Graefes Arch Clin Exp Ophthalmol. 2014;252:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Kojima T, Ishida R, Dogru M, Goto E, Matsumoto Y, Kaido M, Tsubota K. The effect of autologous serum eyedrops in the treatment of severe dry eye disease: a prospective randomized case-control study. Am J Ophthalmol. 2005;139:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 45. | Vazirani J, Sridhar U, Gokhale N, Doddigarla VR, Sharma S, Basu S. Autologous serum eye drops in dry eye disease: Preferred practice pattern guidelines. Indian J Ophthalmol. 2023;71:1357-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 46. | Yoon CH, Lee HJ, Park HY, Kim H, Kim MK, Jeoung JW, Oh JY. Effects of topical autologous serum on the ocular surface in patients with toxic corneal epitheliopathy induced by anti-glaucoma drugs. Int Ophthalmol. 2020;40:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Anitua E, de la Fuente M, Sánchez-Ávila RM, de la Sen-Corcuera B, Merayo-Lloves J, Muruzábal F. Beneficial Effects of Plasma Rich in Growth Factors (PRGF) Versus Autologous Serum and Topical Insulin in Ocular Surface Cells. Curr Eye Res. 2023;48:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 48. | López-García JS, García-Lozano I, Rivas L, Giménez C, Suárez-Cortés T, Acera A. Changes in Corneal Expression of MUC5AC after Autologous Serum Eyedrop Treatment in Patients with Limbal Stem Cell Deficiency. Curr Eye Res. 2019;44:934-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 49. | López-García JS, Rivas L, García-Lozano I, Murube J. Autologous serum eyedrops in the treatment of aniridic keratopathy. Ophthalmology. 2008;115:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Matsumoto Y, Dogru M, Goto E, Ohashi Y, Kojima T, Ishida R, Tsubota K. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111:1115-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 232] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 51. | Rao K, Leveque C, Pflugfelder SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol. 2010;94:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Hori Y, Spurr-Michaud S, Russo CL, Argüeso P, Gipson IK. Differential regulation of membrane-associated mucins in the human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2004;45:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | López-García JS, García-Lozano I, Rivas L, Giménez C, Acera A, Suárez-Cortés T. Effects of Autologous Serum Eye Drops on Conjunctival Expression of MUC5AC in Patients With Ocular Surface Disorders. Cornea. 2016;35:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Battat L, Macri A, Dursun D, Pflugfelder SC. Effects of laser in situ keratomileusis on tear production, clearance, and the ocular surface. Ophthalmology. 2001;108:1230-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 55. | Musa M, Zeppieri M, Enaholo ES, Chukwuyem E, Salati C. An Overview of Corneal Transplantation in the Past Decade. Clin Pract. 2023;13:264-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 56. | Ali TK, Gibbons A, Cartes C, Zarei-Ghanavati S, Gomaa M, Gonzalez I, Gonzalez AE, Ozturk HE, Betancurt C, Perez VL. Use of Autologous Serum Tears for the Treatment of Ocular Surface Disease From Patients With Systemic Autoimmune Diseases. Am J Ophthalmol. 2018;189:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Hussain M, Shtein RM, Sugar A, Soong HK, Woodward MA, DeLoss K, Mian SI. Long-term use of autologous serum 50% eye drops for the treatment of dry eye disease. Cornea. 2014;33:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 58. | Mukhopadhyay S, Sen S, Datta H. Comparative role of 20% cord blood serum and 20% autologous serum in dry eye associated with Hansen's disease: a tear proteomic study. Br J Ophthalmol. 2015;99:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Harloff S, Hartwig D, Kasper K, Wedel T, Müller M, Geerling G. [Epitheliotrophic capacity of serum eye drops from healthy donors versus serum from immunosuppressed patients with rheumatoid arthritis]. Klin Monbl Augenheilkd. 2008;225:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Choi JA, Chung SH. Combined application of autologous serum eye drops and silicone hydrogel lenses for the treatment of persistent epithelial defects. Eye Contact Lens. 2011;37:370-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Merayo-Lloves J, Sanchez RM, Riestra AC, Anitua E, Begoña L, Orive G, Fernandez-Vega L. Autologous Plasma Rich in Growth Factors Eyedrops in Refractory Cases of Ocular Surface Disorders. Ophthalmic Res. 2015;55:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Anitua E, Muruzabal F, Tayebba A, Riestra A, Perez VL, Merayo-Lloves J, Orive G. Autologous serum and plasma rich in growth factors in ophthalmology: preclinical and clinical studies. Acta Ophthalmol. 2015;93:e605-e614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 63. | Lagnado R, King AJ, Donald F, Dua HS. A protocol for low contamination risk of autologous serum drops in the management of ocular surface disorders. Br J Ophthalmol. 2004;88:464-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Kumar A, Chaurasiya D, Sultan S, Soni D, Kubrey S, Singh P, Verma S, Mohan RR, Sharma B. Therapeutic Profile of Human Umbilical Cord Blood Serum and Autologous Serum Therapies in Treatment of Ocular Surface Disorders: A Pilot Study. J Ocul Pharmacol Ther. 2023;39:36-47. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 65. | Tovar AA, White IA, Sabater AL. Use of Acellular Umbilical Cord-Derived Tissues in Corneal and Ocular Surface Diseases. Medicines (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Sharma N, Goel M, Velpandian T, Titiyal JS, Tandon R, Vajpayee RB. Evaluation of umbilical cord serum therapy in acute ocular chemical burns. Invest Ophthalmol Vis Sci. 2011;52:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 67. | Edelmann ML, Mohammed HO, Wakshlag JJ, Ledbetter EC. Clinical trial of adjunctive autologous platelet-rich plasma treatment following diamond-burr debridement for spontaneous chronic corneal epithelial defects in dogs. J Am Vet Med Assoc. 2018;253:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Mircheff AK, Wang Y, Schechter JE, Li M, Tong W, Attar M, Chengalvala M, Harmuth J, Prusakiewicz JJ. Multiple Natural and Experimental Inflammatory Rabbit Lacrimal Gland Phenotypes. Ocul Surf. 2016;14:460-483.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 69. | Fea AM, Aragno V, Testa V, Machetta F, Parisi S, D'Antico S, Spinetta R, Fusaro E, Grignolo FM. The Effect of Autologous Platelet Lysate Eye Drops: An In Vivo Confocal Microscopy Study. Biomed Res Int. 2016;2016:8406832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 70. | Kasper K, Godenschweger L, Hartwig D, Unterlauft JD, Seitz B, Geerling G. [On the use of autologous serum eyedrops in Germany: results of a survey among members of the Cornea Section of the German Ophthalmological Society (DOG)]. Ophthalmologe. 2008;105:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Dereli Can G, Akcan G, Can ME, Akdere ÖE, Çaylı S, Şimşek G, Gümüşderelioğlu M. Surgical and Immunohistochemical Outcomes of Scleral Reconstruction with Autogenic, Allogenic and Xenogenic Grafts: An Experimental Rabbit Model. Curr Eye Res. 2020;45:1572-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Levy N, Wang Yin GH, Noharet R, Ghazouane R, Grimaud F, Aboudou H, Darque A, Delmotte N, Veran J, Hoffart L, Denis D, Sabatier F, Magalon J. A retrospective analysis of characteristic features of responder patients to autologous serum eye drops in routine care. Ocul Surf. 2019;17:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 73. | Merayo-Lloves J, Sanchez-Avila RM, Riestra AC, Anitua E, Begoña L, Orive G, Fernandez-Vega L. Safety and Efficacy of Autologous Plasma Rich in Growth Factors Eye Drops for the Treatment of Evaporative Dry Eye. Ophthalmic Res. 2016;56:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Panda A, Jain M, Vanathi M, Velpandian T, Khokhar S, Dada T. Topical autologous platelet-rich plasma eyedrops for acute corneal chemical injury. Cornea. 2012;31:989-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 75. | Noble BA, Loh RS, MacLennan S, Pesudovs K, Reynolds A, Bridges LR, Burr J, Stewart O, Quereshi S. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004;88:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 76. | Urzua CA, Vasquez DH, Huidobro A, Hernandez H, Alfaro J. Randomized double-blind clinical trial of autologous serum versus artificial tears in dry eye syndrome. Curr Eye Res. 2012;37:684-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 77. | Sharun K, Chandran D, Manjusha KM, Mankuzhy PD, Kumar R, Pawde AM, Dhama K, El-Husseiny HM, Amarpal. Advances and prospects of platelet-rich plasma therapy in veterinary ophthalmology. Vet Res Commun. 2023;47:1031-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 78. | Metheetrairut C, Ngowyutagon P, Tunganuntarat A, Khowawisetsut L, Kittisares K, Prabhasawat P. Comparison of epitheliotrophic factors in platelet-rich plasma versus autologous serum and their treatment efficacy in dry eye disease. Sci Rep. 2022;12:8906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 79. | Rawat P, Agrawal R, Bhaisare V, Walia S, Kori N, Gupta R. Autologous platelet-rich plasma eye drop versus artificial tear eye drop for symptomatic dry eye disease: A prospective comparative interventional study. Indian J Ophthalmol. 2022;70:1549-1553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Wróbel-Dudzińska D, Przekora A, Kazimierczak P, Ćwiklińska-Haszcz A, Kosior-Jarecka E, Żarnowski T. The Comparison between the Composition of 100% Autologous Serum and 100% Platelet-Rich Plasma Eye Drops and Their Impact on the Treatment Effectiveness of Dry Eye Disease in Primary Sjogren Syndrome. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 81. | Merolle L, Iotti B, Berni P, Bedeschi E, Boito K, Maurizi E, Gavioli G, Razzoli A, Baricchi R, Marraccini C, Schiroli D. Platelet-rich Plasma Lysate for Treatment of Eye Surface Diseases. J Vis Exp. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 82. | Manzur Yarur F, Ordenes G, Cruzat A. Autologous serum compared to artificial tear drops for dry eye disease. Medwave. 2021;21:e8213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Wang L, Cao K, Wei Z, Baudouin C, Labbé A, Liang Q. Autologous Serum Eye Drops versus Artificial Tear Drops for Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ophthalmic Res. 2020;63:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 84. | Beylerian M, Lazaro M, Magalon J, Veran J, Darque A, Grimaud F, Stolowy N, Beylerian H, Sabatier F, Hoffart L. [Autologous serum tears: Long-term treatment in dry eye syndrome]. J Fr Ophtalmol. 2018;41:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 85. | Zhang J, Crimmins D, Faed JM, Flanagan P, McGhee CNJ, Patel DV. Characteristics of Platelet Lysate Compared to Autologous and Allogeneic Serum Eye Drops. Transl Vis Sci Technol. 2020;9:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 86. | Stachon T, Wu MF, Bischoff M, Huber M, Langenbucher A, Seitz B, Szentmáry N. [Amniotic Membrane Suspension and Autologous Serum - Are they Important for Wound Healing?]. Klin Monbl Augenheilkd. 2017;234:1015-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Pınarlı FA, Okten G, Beden U, Fışgın T, Kefeli M, Kara N, Duru F, Tomak L. Keratinocyte growth factor-2 and autologous serum potentiate the regenerative effect of mesenchymal stem cells in cornea damage in rats. Int J Ophthalmol. 2014;7:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 88. | Bradley JC, Bradley RH, McCartney DL, Mannis MJ. Serum growth factor analysis in dry eye syndrome. Clin Exp Ophthalmol. 2008;36:717-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 89. | Semeraro F, Forbice E, Braga O, Bova A, Di Salvatore A, Azzolini C. Evaluation of the efficacy of 50% autologous serum eye drops in different ocular surface pathologies. Biomed Res Int. 2014;2014:826970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 90. | Zheng N, Zhu SQ. Randomized controlled trial on the efficacy and safety of autologous serum eye drops in dry eye syndrome. World J Clin Cases. 2023;11:6774-6781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 91. | Murtaza F, Toameh D, Chiu HH, Tam ES, Somani S. Autologous Platelet-Rich Plasma Drops for Evaporative Dry Eye Disease from Meibomian Gland Dysfunction: A Pilot Study. Clin Ophthalmol. 2022;16:2199-2208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 92. | Basu D, Kulkarni R. Overview of blood components and their preparation. Indian J Anaesth. 2014;58:529-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 93. | Erikitola OO, Williams O, Fern A, Lyall D. Fingerprick Autologous Blood in the Treatment of Severe Dry Eyes and Ocular Surface Disease. Cornea. 2021;40:1104-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Hassan A, Balal S, Cook E, Dehbi HM, Pardhan S, Bourne R, Ahmad S, Sharma A. Finger-Prick Autologous Blood (FAB) Eye Drops for Dry Eye Disease: Single Masked Multi-Centre Randomised Controlled Trial. Clin Ophthalmol. 2022;16:3973-3979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 95. | Balal S, Nitiahpapand R, Hassan A, Than J, Patel A, Kumar B, Sharma A. Finger-Prick Autologous Blood in the Treatment of Persistent Corneal Epithelial Defects. Cornea. 2020;39:594-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Than J, Balal S, Wawrzynski J, Nesaratnam N, Saleh GM, Moore J, Patel A, Shah S, Sharma B, Kumar B, Smith J, Sharma A. Fingerprick autologous blood: a novel treatment for dry eye syndrome. Eye (Lond). 2017;31:1655-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 97. | Vaidakis D, Papapanou M, Siristatidis CS. Autologous platelet-rich plasma for assisted reproduction. Cochrane Database Syst Rev. 2024;4:CD013875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 98. | Cui D, Li G, Akpek EK. Autologous serum eye drops for ocular surface disorders. Curr Opin Allergy Clin Immunol. 2021;21:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 99. | Anitua E, de la Fuente M, Muruzabal F, Riestra A, Merayo-Lloves J, Orive G. Plasma rich in growth factors (PRGF) eye drops stimulates scarless regeneration compared to autologous serum in the ocular surface stromal fibroblasts. Exp Eye Res. 2015;135:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 100. | Kojima T, Higuchi A, Goto E, Matsumoto Y, Dogru M, Tsubota K. Autologous serum eye drops for the treatment of dry eye diseases. Cornea. 2008;27 Suppl 1:S25-S30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 101. | Alvarado-Villacorta R, García-Carmona KP, Martínez-Pardo ME, Vázquez-Maya L. Allogeneic Limbal Epithelial Transplantation Modified With Solid Platelet-Rich Plasma for Bilateral Limbal Stem Cell Deficiency. Cornea. 2020;39:1311-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Akagun N, Ozer PA, Gazyagci S. Rapid healing of a persistent corneal epithelial defect (PCED) with autologous serum treatment. Niger J Clin Pract. 2020;23:123-125. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 103. | Márquez-de-Aracena R, Montero-de-Espinosa I, Muñoz M, Pereira G. [Subconjunctival application of plasma platelet concentrate in the treatment of ocular burns. Preliminary results]. Arch Soc Esp Oftalmol. 2007;82:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 104. | Wilczyński M. [The use of autologous serum in the treatment of the ocular surface diseases]. Klin Oczna. 2009;111:363-368. [PubMed] |
| 105. | Soni NG, Jeng BH. Blood-derived topical therapy for ocular surface diseases. Br J Ophthalmol. 2016;100:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 106. | Hassan A, Telandro A, Barguigua A, Baba M, Körber N. Evaluation of the Use of Highly Concentrated Autologous Platelet-Rich Plasma and Platelet-Rich Fibrin Membrane to Improve the Outcome in the Management of Severe Dry Eye Disease, Corneal Neurotrophic Ulcer and Corneal Burn. Cureus. 2024;16:e51794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 107. | Rodríguez Calvo-de-Mora M, Domínguez-Ruiz C, Barrero-Sojo F, Rodríguez-Moreno G, Antúnez Rodríguez C, Ponce Verdugo L, Hernández Lamas MDC, Hernández-Guijarro L, Villalvilla Castillo J, Fernández-Baca Casares I, Prat Arrojo I, Borroni D, Alba-Linero C, Zamorano-Martín F, Moreno-Guerrero A, Rocha-de-Lossada C. Autologous versus allogeneic versus umbilical cord sera for the treatment of severe dry eye disease: a double-blind randomized clinical trial. Acta Ophthalmol. 2022;100:e396-e408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 108. | Franchini M, Cruciani M, Mengoli C, Marano G, Capuzzo E, Pati I, Masiello F, Veropalumbo E, Pupella S, Vaglio S, Liumbruno GM. Serum eye drops for the treatment of ocular surface diseases: a systematic review and meta-analysis. Blood Transfus. 2019;17:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 109. | Azari AA, Rapuano CJ. Autologous serum eye drops for the treatment of ocular surface disease. Eye Contact Lens. 2015;41:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 110. | Alio JL, Arnalich-Montiel F, Rodriguez AE. The role of "eye platelet rich plasma" (E-PRP) for wound healing in ophthalmology. Curr Pharm Biotechnol. 2012;13:1257-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 111. | Nugent RB, Lee GA. Ophthalmic use of blood-derived products. Surv Ophthalmol. 2015;60:406-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 112. | Hsiung C, Liu YT, Su CY, Hsiung CC, Hung KH, Yeh LK. Production of Modified Autologous Conditioned Serum and Ex Vivo Assessment of Its Healing Potential in Murine Corneal Epithelium. J Vis Exp. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 113. | Gabriel C, Marks DC, Henschler R, Schallmoser K, Burnouf T, Koh MBC. Eye drops of human origin-Current status and future needs: Report on the workshop organized by the ISBT Working Party for Cellular Therapies. Vox Sang. 2023;118:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 114. | Maclennan S, Hartwig D, Geerling G. [Experiences with a centralised national service for autologous serum eyedrops in England]. Ophthalmologe. 2008;105:639-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 115. | Dietrich T, Weisbach V, Seitz B, Jacobi C, Kruse FE, Eckstein R, Cursiefen C. [Manufacture of autologous serum eye drops for out-patient therapy : cooperation between ophthalmic clinic and transfusion medicine department]. Ophthalmologe. 2008;105:1036-1038, 1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 116. | Yang Y, Zhu X, Yang J, Shi A, Jiang M, Peng Y, Li M, Wang Y, Yuan H. An Environmental Control Experiment for Contamination of the Production and Storage of 20% Autologous Serum Eye Drops. Curr Eye Res. 2020;45:1364-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 117. | Gus PI, Marinho D, Zelanis S, Belló-Klein A, Locatelli C, Nicola F, Kunzler AL, Fernandes TR, Carraro CC, Barbosa L. A Case-Control Study on the Oxidative Balance of 50% Autologous Serum Eye Drops. Oxid Med Cell Longev. 2016;2016:9780193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 118. | Dalmon CA, Chandra NS, Jeng BH. Use of autologous serum eyedrops for the treatment of ocular surface disease: first US experience in a large population as an insurance-covered benefit. Arch Ophthalmol. 2012;130:1612-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Fischer KR, Opitz A, Böeck M, Geerling G. Stability of serum eye drops after storage of 6 months. Cornea. 2012;31:1313-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 120. | Koffler BH. Autologous serum therapy of the ocular surface with novel delivery by platelet concentrate gel. Ocul Surf. 2006;4:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 121. | Tahmaz V, Gehlsen U, Sauerbier L, Holtick U, Engel L, Radojska S, Petrescu-Jipa VM, Scheid C, Hallek M, Gathof B, Cursiefen C, Steven P. Treatment of severe chronic ocular graft-versus-host disease using 100% autologous serum eye drops from a sealed manufacturing system: a retrospective cohort study. Br J Ophthalmol. 2017;101:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 122. | Mahelková G, Veselá V, Seidler Štangová P, Židlická A, Dotřelová D, Fales I, Skalická P, Jirsová K. [Tear Osmolarity in Patients with Severe Dry Eye Syndrome Before and After Autologous Serum Treatment: a Comparison with Tear Osmolarity in Healthy Volunteers]. Cesk Slov Oftalmol. 2015;71:184-188. [PubMed] |
| 123. | Campbell JDM, Ahmad S, Agrawal A, Bienek C, Atkinson A, Mcgowan NWA, Kaye S, Mantry S, Ramaesh K, Glover A, Pelly J, MacRury C, MacDonald M, Hargreaves E, Barry J, Drain J, Cuthbertson B, Nerurkar L, Downing I, Fraser AR, Turner ML, Dhillon B. Allogeneic Ex Vivo Expanded Corneal Epithelial Stem Cell Transplantation: A Randomized Controlled Clinical Trial. Stem Cells Transl Med. 2019;8:323-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 124. | Holan V, Trosan P, Cejka C, Javorkova E, Zajicova A, Hermankova B, Chudickova M, Cejkova J. A Comparative Study of the Therapeutic Potential of Mesenchymal Stem Cells and Limbal Epithelial Stem Cells for Ocular Surface Reconstruction. Stem Cells Transl Med. 2015;4:1052-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 125. | Nakamura T, Inatomi T, Cooper LJ, Rigby H, Fullwood NJ, Kinoshita S. Phenotypic investigation of human eyes with transplanted autologous cultivated oral mucosal epithelial sheets for severe ocular surface diseases. Ophthalmology. 2007;114:1080-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 126. | Lomas RJ, Chandrasekar A, Macdonald-Wallis C, Kaye S, Rauz S, Figueiredo FC. Patient-reported outcome measures for a large cohort of serum eye drops recipients in the UK. Eye (Lond). 2021;35:3425-3432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 127. | van der Meer PF, Verbakel SK, Honohan Á, Lorinser J, Thurlings RM, Jacobs JFM, de Korte D, Eggink CA. Allogeneic and autologous serum eye drops: a pilot double-blind randomized crossover trial. Acta Ophthalmol. 2021;99:837-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 128. | Kreimei M, Sorkin N, Boutin T, Slomovic AR, Rootman DS, Chan CC. Patient-reported outcomes of autologous serum tears for the treatment of dry eye disease in a large cohort. Ocul Surf. 2019;17:743-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 129. | Hung Y, Elder MJ, Rawstron JA, Badami KG. A retrospective crossover study of autologous and allogeneic serum eye drops for the management of ocular surface disease. Transfus Med. 2019;29:69-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 130. | Giannaccare G, Carnevali A, Senni C, Logozzo L, Scorcia V. Umbilical Cord Blood and Serum for the Treatment of Ocular Diseases: A Comprehensive Review. Ophthalmol Ther. 2020;9:235-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 131. | Harritshøj LH, Nielsen C, Ullum H, Hansen MB, Julian HO. Ready-made allogeneic ABO-specific serum eye drops: production from regular male blood donors, clinical routine, safety and efficacy. Acta Ophthalmol. 2014;92:783-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 132. | Rybickova I, Vesela V, Fales I, Skalicka P, Jirsova K. Apoptosis of conjunctival epithelial cells before and after the application of autologous serum eye drops in severe dry eye disease. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 133. | Liu J, Liu ZG, Shao Y, Chen JY, Li W, Lin H. [The clinical efficiency of calf blood extract gel on moderate to severe dry eye induced by chronic graft versus host diseases after bone marrow transplantation]. Zhonghua Yan Ke Za Zhi. 2013;49:32-36. [PubMed] |
| 134. | Pinna A, Nuvoli E, Blasetti F, Posadinu MA, Boscia F. Plasmapheresis, Intravenous Immunoglobulins, and Autologous Serum Eyedrops in the Acute Eye Complications of Toxic Epidermal Necrolysis. Eur J Ophthalmol. 2017;27:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 135. | Wei RH, Thomas PB, Samant DM, Schechter JE, Mircheff AK, Trousdale MD. Autoimmune dacryoadenitis and sialadenitis induced in rabbits by intravenous injection of autologous lymphocytes activated ex vivo against lacrimal antigens. Cornea. 2012;31:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 136. | Thomas PB, Samant DM, Selvam S, Wei RH, Wang Y, Stevenson D, Schechter JE, Apparailly F, Mircheff AK, Trousdale MD. Adeno-associated virus-mediated IL-10 gene transfer suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis. Invest Ophthalmol Vis Sci. 2010;51:5137-5144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 137. | Thomas PB, Zhu Z, Selvam S, Samant DM, Stevenson D, Mircheff AK, Schechter JE, Song SW, Trousdale MD. Autoimmune dacryoadenitis and keratoconjunctivitis induced in rabbits by subcutaneous injection of autologous lymphocytes activated ex vivo against lacrimal antigens. J Autoimmun. 2008;31:116-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 138. | Zhu Z, Stevenson D, Schechter JE, Mircheff AK, Crow RW, Atkinson R, Ritter T, Bose S, Trousdale MD. Tumor necrosis factor inhibitor gene expression suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 139. | Singh S, Sharma S, Basu S. Rabbit models of dry eye disease: Current understanding and unmet needs for translational research. Exp Eye Res. 2021;206:108538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 140. | Lin ZR, Wu HP, Xie ZW, Luo SR, Fang X, Yan L, Liu ZS, Dong N, Shang XM. [Efficacy of deproteinized calf blood extract eye drops on early recovery after pterygium surgery]. Zhonghua Yan Ke Za Zhi. 2019;55:134-140. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 141. | Yeh SI, Chu TW, Cheng HC, Wu CH, Tsao YP. The Use of Autologous Serum to Reverse Severe Contact Lens-induced Limbal Stem Cell Deficiency. Cornea. 2020;39:736-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 142. | Di Girolamo N, Bosch M, Zamora K, Coroneo MT, Wakefield D, Watson SL. A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation. 2009;87:1571-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 143. | Marchand M, Harissi-Dagher M, Germain M, Thompson P, Robert MC. Serum drops for ocular surface disease: national survey of Canadian cornea specialists. Can J Ophthalmol. 2018;53:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 144. | Creuzot-Garcher C, Lafontaine PO, Brignole F, Pisella PJ, d'Athis P, Bron A, Lapierre V, Baudouin C. [Treating severe dry eye syndromes with autologous serum]. J Fr Ophtalmol. 2004;27:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 145. | Badami KG, McKellar M. Reactions to serum eye drops-New Zealand experience and review of the literature. Transfus Med. 2024;34:61-65. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 146. | Trindade M, Rodrigues M, Pozzebon ME, Aranha FJP, Colella MP, Fernandes A, Fornazari DO, de Almeida Borges D, Vigorito AC, Alves M. A plethora of ocular surface manifestations in a multidisciplinary ocular graft-versus-host disease unit. Sci Rep. 2022;12:15926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 147. | Geerling G, Unterlauft JD, Kasper K, Schrader S, Opitz A, Hartwig D. [Autologous serum and alternative blood products for the treatment of ocular surface disorders]. Ophthalmologe. 2008;105:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 148. | Thanathanee O, Phanphruk W, Anutarapongpan O, Romphruk A, Suwan-Apichon O. Contamination risk of 100% autologous serum eye drops in management of ocular surface diseases. Cornea. 2013;32:1116-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 149. | Partal A, Scott E. Low-cost protocol for the production of autologous serum eye drops by blood collection and processing centres for the treatment of ocular surface diseases. Transfus Med. 2011;21:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 150. | Li J, Zhang X, Zheng Q, Zhu Y, Wang H, Ma H, Jhanji V, Chen W. Comparative Evaluation of Silicone Hydrogel Contact Lenses and Autologous Serum for Management of Sjögren Syndrome-Associated Dry Eye. Cornea. 2015;34:1072-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 151. | Ang LP, Nakamura T, Inatomi T, Sotozono C, Koizumi N, Yokoi N, Kinoshita S. Autologous serum-derived cultivated oral epithelial transplants for severe ocular surface disease. Arch Ophthalmol. 2006;124:1543-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 152. | Kumari N, Kusumesh R, Kumari R, Sinha BP, Singh V. Comparative evaluation of effectiveness of twenty versus fifty percent autologous serum eye drops in treatment of dry eye. Indian J Ophthalmol. 2023;71:1603-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 153. | Jover Botella A, Márquez Peiró JF, Márques K, Monts Cambero N, Selva Otaolaurruchi J. Effectiveness of 100% autologous serum drops in ocular surface disorders. Farm Hosp. 2011;35:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 154. | Barbaro V, Nasti AA, Raffa P, Migliorati A, Nespeca P, Ferrari S, Palumbo E, Bertolin M, Breda C, Miceli F, Russo A, Caenazzo L, Ponzin D, Palù G, Parolin C, Di Iorio E. Personalized Stem Cell Therapy to Correct Corneal Defects Due to a Unique Homozygous-Heterozygous Mosaicism of Ectrodactyly-Ectodermal Dysplasia-Clefting Syndrome. Stem Cells Transl Med. 2016;5:1098-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 155. | Wang DK, Zhang SE, Su YX, Zheng GS, Yang WF, Liao GQ. Microvascular Submandibular Gland Transplantation for Severe Keratoconjunctivitis Sicca: A Single-Institution Experience of 61 Grafts. J Oral Maxillofac Surg. 2018;76:2443-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 156. | Yu GY, Wu LL, Cai ZG, Lv L, Cong X. [A 20-year study on microvascular autologous transplantation of submandibular gland for treatment of severe dry eye]. Beijing Da Xue Xue Bao Yi Xue Ban. 2018;50:1-4. [PubMed] |
| 157. | Ono K, Yokoo S, Mimura T, Usui T, Miyata K, Araie M, Yamagami S, Amano S. Autologous transplantation of conjunctival epithelial cells cultured on amniotic membrane in a rabbit model. Mol Vis. 2007;13:1138-1143. [PubMed] |
| 158. | Aggarwal S, Colon C, Kheirkhah A, Hamrah P. Efficacy of autologous serum tears for treatment of neuropathic corneal pain. Ocul Surf. 2019;17:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 159. | Jirsova K, Brejchova K, Krabcova I, Filipec M, Al Fakih A, Palos M, Vesela V. The application of autologous serum eye drops in severe dry eye patients; subjective and objective parameters before and after treatment. Curr Eye Res. 2014;39:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 160. | Chiang CC, Lin JM, Chen WL, Tsai YY. Allogeneic serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Cornea. 2007;26:861-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 161. | Fasolo A, Pedrotti E, Passilongo M, Marchini G, Monterosso C, Zampini R, Bohm E, Birattari F, Franch A, Barbaro V, Bertolin M, Breda C, Di Iorio E, Ferrari B, Ferrari S, Meneguzzi M, Ponzin D. Safety outcomes and long-term effectiveness of ex vivo autologous cultured limbal epithelial transplantation for limbal stem cell deficiency. Br J Ophthalmol. 2017;101:640-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 162. | Selver OB, Durak I, Gürdal M, Baysal K, Ates H, Ozbek Z, Wang Z, Wu A, Wolosin JM. Corneal recovery in a rabbit limbal stem cell deficiency model by autologous grafts of tertiary outgrowths from cultivated limbal biopsy explants. Mol Vis. 2016;22:138-149. [PubMed] |
| 163. | Luengo Gimeno F, Lavigne V, Gatto S, Croxatto JO, Correa L, Gallo JE. One-year follow-up of epithelial corneal cell sheet allografts mounted on platelet poor plasma in rabbits. Mol Vis. 2009;15:2771-2779. [PubMed] |
| 164. | Alio JL, Rodriguez AE, WróbelDudzińska D. Eye platelet-rich plasma in the treatment of ocular surface disorders. Curr Opin Ophthalmol. 2015;26:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 165. | Varghese VM, Prasad T, Kumary TV. Optimization of culture conditions for an efficient xeno-feeder free limbal cell culture system towards ocular surface regeneration. Microsc Res Tech. 2010;73:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 166. | Kang MJ, Lee JH, Hwang J, Chung SH. Efficacy and safety of platelet-rich plasma and autologous-serum eye drops for dry eye in primary Sjögren's syndrome: a randomized trial. Sci Rep. 2023;13:19279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 167. | Kim KM, Shin YT, Kim HK. Effect of autologous platelet-rich plasma on persistent corneal epithelial defect after infectious keratitis. Jpn J Ophthalmol. 2012;56:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 168. | Periman LM, Perez VL, Saban DR, Lin MC, Neri P. The Immunological Basis of Dry Eye Disease and Current Topical Treatment Options. J Ocul Pharmacol Ther. 2020;36:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 169. | Avila MY. Restoration of human lacrimal function following platelet-rich plasma injection. Cornea. 2014;33:18-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 170. | Wiącek MP, Gosławski W, Grabowicz A, Sobuś A, Kawa MP, Baumert B, Paczkowska E, Milczarek S, Osękowska B, Safranow K, Zawiślak A, Lubiński W, Machaliński B, Machalińska A. Long-Term Effects of Adjuvant Intravitreal Treatment with Autologous Bone Marrow-Derived Lineage-Negative Cells in Retinitis Pigmentosa. Stem Cells Int. 2021;2021:6631921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 171. | Lee GA, Chen SX. Autologous serum in the management of recalcitrant dry eye syndrome. Clin Exp Ophthalmol. 2008;36:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 172. | Pezzotta S, Del Fante C, Scudeller L, Rossi GC, Perotti C, Bianchi PE, Antoniazzi E. Long-term safety and efficacy of autologous platelet lysate drops for treatment of ocular GvHD. Bone Marrow Transplant. 2017;52:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |