Published online Sep 20, 2024. doi: 10.5493/wjem.v14.i3.92589
Revised: June 19, 2024
Accepted: July 10, 2024
Published online: September 20, 2024
Processing time: 210 Days and 17.8 Hours
The possible existence of an acini–islet–acinar (AIA) reflex, involving mutual amylase and insulin interactions, was investigated in the current acute experiment on pigs.
To confirm the existence of an AIA reflex and justify the placement of the exocrine and endocrine pancreatic components within the same organ.
The study was performed on six pigs under general anesthesia. An intravenous glucose tolerance test was performed, with a bolus infusion of 50% glucose to the jugular vein, while amylase (5000 U/kg) or vehicle intrapancreatic infusions were administered via the pancreaticoduodenalis cranialis artery during 30 min with a 1 mL/min flow rate.
The amylase infusion to pancreatic arterial circulation inhibited and delayed the insulin release peak which is usually associated with the highest value of blood glucose and is typically observed at 15 min after glucose infusion, for > 1 h. The intrapancreatic infusion of the vehicle (saline) did not have any effect on the time frame of insulin release. Infusion of 1% bovine serum albumin changed the insulin release curve dramatically and prolonged the high range of insulin secretion, far beyond the glucose peak.
Intrapancreatic arterial infusion of amylase interrupted the integrated glucose–insulin interactions. This confirms an AIA reflex and justifies placement of the exocrine and endocrine pancreatic components within the same organ.
Core tip: The acini–islet–acinar axis involves the inhibition of insulin production by amylase and the stimulation of amylase release by insulin, which is of particular importance with regards to glucose regulation. There is increasing evidence that amylase levels correspond to overall metabolic health, with low amylase levels being significantly associated with the development of metabolic syndrome, diabetes, and obesity. Disruption of the integrated glucose–insulin interactions by intrapancreatic arterial amylase infusion in the present study confirms the existence of an AIA reflex and justifies placement of the exocrine and endocrine pancreatic components within the same organ.
- Citation: Pierzynowska K, Wychowański P, Zaworski K, Woliński J, Donaldson J, Szkopek D, Roszkowicz-Ostrowska K, Kondej A, Pierzynowski SG. Amylase intrapancreatic infusion delays insulin release during an intravenous glucose tolerance test, proof of acini–islet–acinar interactions. World J Exp Med 2024; 14(3): 92589
- URL: https://www.wjgnet.com/2220-315x/full/v14/i3/92589.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i3.92589
The postulated acini–islet–acinar (AIA) reflex, which integrates and justifies the existence of the exocrine and endocrine pancreas within the same organ, requires the presence of amylase in the interstitial fluid surrounding the pancreatic islets and acinar cells[1].
Another contemporary question in terms of gastroenterology is how the pancreatic enzymes reach the bloodstream and whether such a transition is a sign of health or disease. For example, pancreatitis development was believed to be associated with high serum amylase levels at the beginning of the century[2]. Nowadays, this hypothesis has been abandoned and high serum amylase levels are recognized to be noninformative for the diagnosis of pancreatitis. There is a growing number of publications to date that show a correlation between serum amylase levels and metabolic health, with low amylase levels being significantly associated with the development of metabolic syndrome, diabetes and obesity[3,4].
Leakage of the pancreatic enzymes from the acinar cells to the interstitial fluid is the commonly accepted hypothesis regarding the source of pancreatic enzymes in the bloodstream. The approximate concentration of pancreatic enzymes in the interstitial fluid that would ensure their existing levels in the peripheral blood (often measured for diagnostic purposes), needs to be several fold or even several hundred fold higher than their concentration observed in the blood.
Amylase, infused via the pancreaticoduodenalis cranialis artery into the pancreas should penetrate the endothelium in the pancreatic vascular system, and its concentration in the infusate should be high, to influence amylase levels in the interstitial space. Since amylase and other enzymes are smaller than the estimated size of the endothelial holes of 70 kD, which block larger macromolecules (e.g., albumins) from migration to the intestinal fluid, exogenous amylase can enter the pancreatic interstitial fluid.
The aim of the present study was to prove that exogenously increased levels of amylase in the pancreatic interstitial fluid would inhibit insulin release.
The acute experiment was performed on six, Swedish Landrace × Yorkshire × Hampshire pigs of both genders (40–50 kg), that were fasted overnight. The pigs were premedicated intramuscularly, for sedation, with a mixture of: medetomidine 1 mg/m2, midazolam 5 mg/m2, ketamine 8 mg/kg and methadone 0.2 mg/kg. An intravenous cannula was inserted into the marginal ear vein. Drugs to induce anesthesia were administered through the cannula: propofol 2 mg/kg and lidocaine 1 mg/kg. Preoxygenation of 3 L/min with a mask was started during induction of anesthesia. The incision site was epidurally anesthetized with 1% lidocaine. Anesthesia was maintained with isoflurane a concentration of 1%–1.5% and oxygen flow at 1.5–2.0 L/min. An analgesic precaution of intramuscular metamizole at 50 mg/kg and intravenous bolus of fentanyl at 2 μg/kg was administered, followed by a constant-rate infusion of fentanyl in 0.9% NaCl throughout the operation, at a dose of 1.5 μg/kg/h. Monitoring of vital functions [blood oxygen saturation (spO2), end tidal carbon dioxide (EtCO2), pulse rate (PR), noninvasive blood pressure (NIBP): systolic blood pressure (SBP), diastolic blood pre
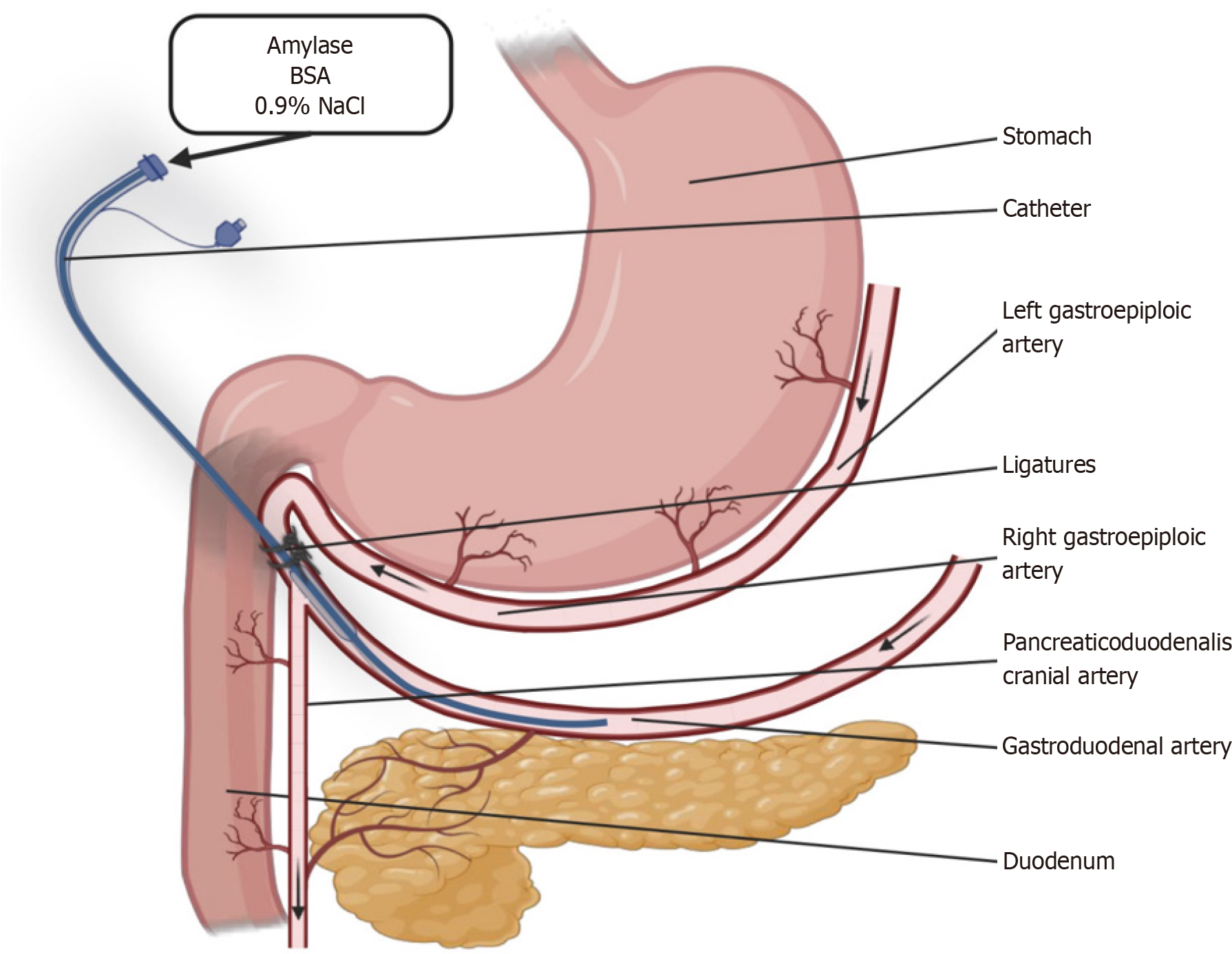
After the surgical level of anesthesia was reached, jugular vein catheters were surgically implanted in the pigs. Following the jugular vein catheter surgery, the abdominal cavity of each pig was opened, along the linea alba, directly after the sternum, with a 15-cm long incision. After localization of the gastroduodenalis artery, the bifurcation of the pancreaticoduodenalis cranialis artery was located (Figure 1). From this point onwards the gastroduodenalis artery becomes the right gastric epiploic artery. The latter was then gently dissected, 2–3 cm cranially to the pancreaticoduodenalis cranialis artery. Two situation silk 3-0 sutures were placed under the right gastric epiploic artery in the place of its dissection and an incision was made with microtip scissors.
A silastic catheter, with an outer diameter of 0.02 mm, was inserted through the incision in the epiploic artery, towards the pancreaticoduodenalis cranialis artery. The tip of the catheter was situated 2–3 cm behind the pancreaticodoudenalis cranialis artery bifurcation from the gastroduodenalis artery and the right epiploic artery was sutured shut, under and over the inserted catheter. Blood flow to the pancreas and duodenum was ensured via the gastroduodenalis artery, while blood flow to the right epiploic artery was supplied via the left epiploic artery. All infusions to the catheter with the tip beyond the pancreaticodoudenalis cranialis artery were directed to the pancreas and duodenum. Approximately 70% of the blood supply to the pancreas originates from the pancreaticoduodenalis cranialis artery in pigs. Localization of the catheter tips and confirmation of the infusion to the pancreas was confirmed using Evans blue, postmortem.
After insertion of the catheters into the jugular vein and gastroduodenalis artery, the pigs were left to stabilize for 30 min. Anesthesia was induced and maintained almost identical for all pigs for the duration of the experiment, with necessary adjustments to ensure animal wellbeing. After the 30-min stabilization period, a baseline blood sample was obtained and the respective infusions begun. A bolus injection of 50% glucose (1 g/kg) to the jugular vein catheter, lasting for 2 min, with a parallel 30-min infusion of (with an infusion rate of 1 mL/min into the pancreatic arterial circulation) either: (1) α-amylase (5000 U/kg) in saline – pigs 1 and 4; (2) 1%, bovine serum albumin (BSA) in saline – pigs 2 and 3; or (3) saline (0.9 % NaCl) – pigs 5 and 6. After the 2-h experiment, the pigs were killed by overdose of pentobarbital, infused via the jugular vein catheter.
Blood samples obtained during the experiment were collected via the jugular vein catheter at 1 min prior to glucose infusion and at 15, 30, 60, 90 and 120 min after the glucose infusion and transferred to BD Vacutainer® glass K3EDTAtubes (BD Diagnostics, Franklin Lakes, NJ, USA).
The blood samples were immediately placed on ice before they were centrifuged at 3000 × g for 15 min at 4°C, and plasma was separated and stored at -80°C until further analysis. Blood glucose concentrations were measured directly following blood sampling using a glucometer and test strips (Accu-Chek Aviva, Roche Diagnostics, Germany). Plasma insulin concentrations were measured using a porcine insulin ELISA kit (cat# 10-1200-01; Mercodia, Uppsala, Sweden).
Data are expressed as individual values. Area under the curve (AUC) values are expressed as mean ± SD. AUC cal
Before the main experiment was performed, several pilot studies were done to investigate the impact of the anesthesia and manipulation with arterial catheter implantation on the insulin response during the intravenous glucose tolerance test (IVGTT).
All parameters that were monitored while the pigs were under anesthesia, such as spO2 (97%–100%), EtCO2 (35–45 mmHg), PR (70–100 bpm), SBP (120–140 mmHg), DBP (60–80 mmHg), MAP (60–100 mmHg), and temperature (37.5–38.5°C) were maintained within the physiological range during the entire experimental period and anesthesia. Based on the above-mentioned parameters on the cardiac monitor, the anesthesiologist regulated the depth of anesthesia by altering the concentration of isoflurane and oxygen flow in the respiratory circuit or administered propofol through a venflon in the marginal ear vein.
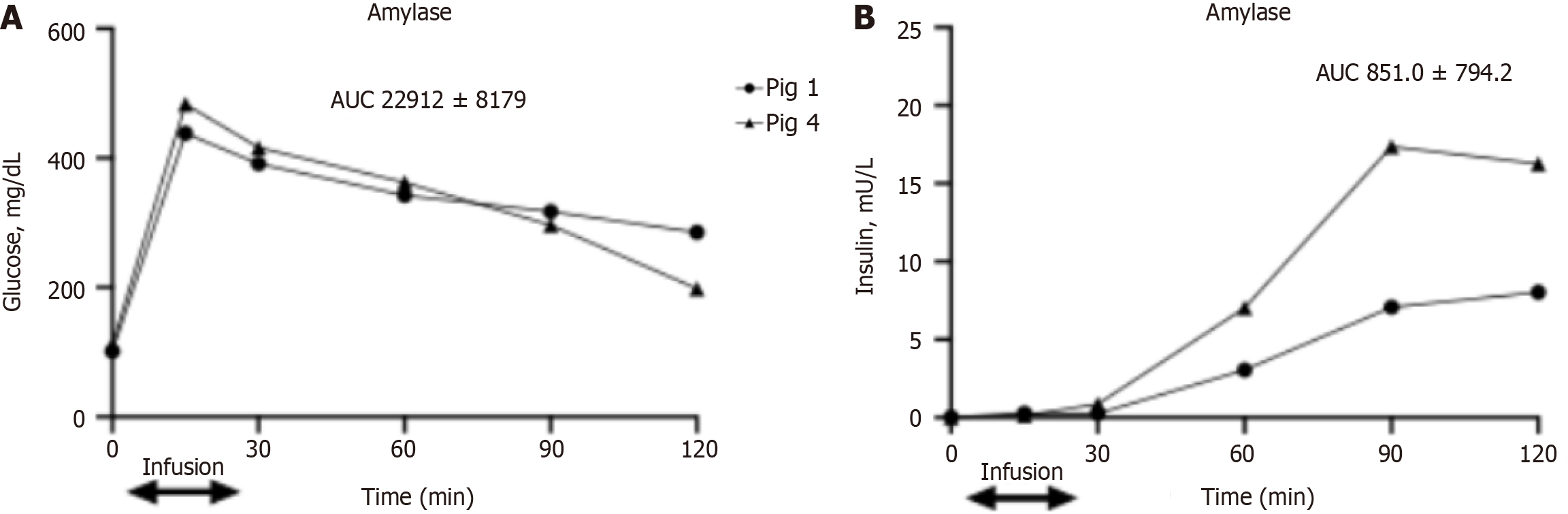
In pigs infused with amylase to the intrapancreatic arterial circulation (pigs 1 and 4), glucose levels during the IVGTT reached a peak, increasing from 80–100 mg/dL to 450–500 mg/dL, at 15 min after glucose injection (Figure 2A).
Surprisingly, peak blood insulin levels did not match peak glucose levels and were observed as late as 90 min after glucose injection and persisted until the last measured point at 2 h following glucose injection. Peak insulin levels varied between pigs, ranging from 5 to 23 mU/L, while basal levels were around 1 mU/L (Figure 2B), and were still on the same level as that measured at the 15-min time point, when the highest blood glucose values were observed.
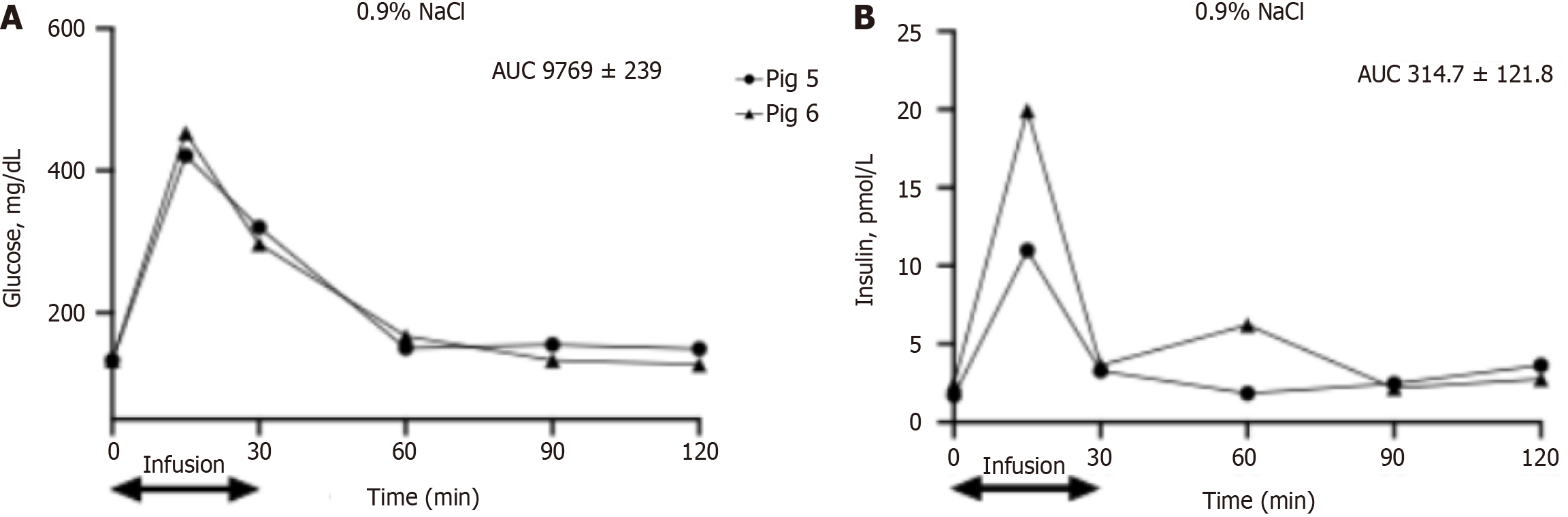
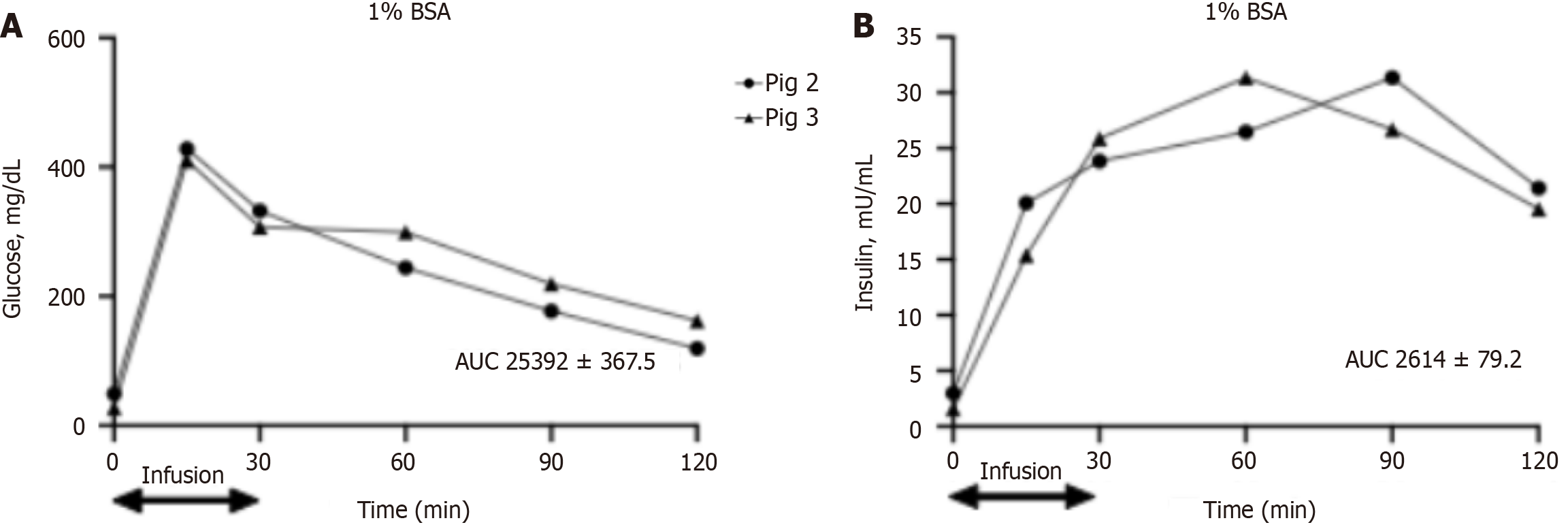
In pigs that obtained an intrapancreatic infusion of 0.9% NaCl and 1% BSA, blood glucose levels were similar to those observed in pigs treated with amylase and reached a peak at 15 min following glucose infusion, from 60–100 to 450–470 mg/dL (Figures 3A and 4A, respectively).
Insulin levels peaked at 15 min after glucose infusion from 2 to 10–20 mU/L in pigs that obtained an intrapancreatic infusion of 0.9% NaCl as a vehicle (pigs 5 and 6). Insulin levels in these pigs returned to basal values at 30 min after glucose infusion. In pigs treated with 1% BSA (pigs 2 and 3), insulin levels increased at 15 min after glucose infusion, to 15–29 mU/L, and then reached a plateau of 22–30 mU/L and remained stable during all measurements at 30, 60, 90 and 120 min following glucose infusion (Figures 3B and 4B).
During the pilot experiments, the effects of the anesthesia on the insulin response during an IVGTT was evaluated, since previous studies showed that isoflurane anesthesia reduces the reactivity of insulin to glucose during infusion[5]. The results of the pilot studies, as well as the latter experimental results from a previous study confirm that isoflurane could reduce the insulin response to an IVGTT and limit glucose metabolism, as compared to the results obtained from conscious pigs[6]. However, in our experiment on conscious, healthy pigs, we did not observe a shift in the insulin response or a prolonged insulin response during the IVGTT. In contrast, a slight delay and long-lasting insulin response to the IVGTT was observed in exocrine-pancreas-insufficient pigs in a previous study[7]. Following the pancreatic duct ligation (PDL) surgery, the amylase concentration in the pancreatic interstitial fluid is increased. In that way our infusion of amylase to the intrapancreatic arterial circulation can mimic PDL-provoked changes in amylase concentration in the interstitial fluid. A previous study by our laboratory showed a shift in the insulin response during an intraduodenal glucose tolerance test in low dose streptozotocin treated pigs which develop type 2 diabetes, following parallel amylase infusion[6].
The current pancreatic perfusion experiment was performed to prove the existence of an AIA axis, in that the infusion of exogenous amylase, via the arterial circulation to the pancreas, could to some extent mimic the appearance of host amylase in the interstitial fluid, as well as its influence on the insulin-producing cells in the pancreatic islets of Lan
Both α-amylase, with a molecular weight of 58.4 kDa, as well as BSA, with molecular weight of 66 kDa, can partially (not easily) penetrate the interstitial fluid when applied arterially, since it is postulated that the endothelium of blood vessels is permeable to molecules < 70 kDa. Thus, it was assumed that amylase as the test molecule and BSA, which was used as a positive control, as well as 0.9% NaCl which was used as a vehicle, could reach the interstitial fluid and finally, the beta cells in the pancreatic islets. Considering the postulation by Lifson et al[10], concerning the existence of blood vessel circuits in the pancreas, similar to the renal and liver rete mirabille arterio arteriosum and vene venosum, one could suspect that infused molecules would reach both the islets of Langerhans and the acinar cell structures. The specificity of the pancreatic islets blood flow[11] additionally ensures that amylase infused to the pancreatic arterial circulation can reach the endocrine pancreas.
Amylase infusion in the current study delayed the glucose-stimulated insulin release for > 1 h during the IVGTT. There was no enhancing of insulin release even despite the parallel loading of glucose, with blood glucose levels reaching an extraordinarily high peak (400 mg/dL) within 15 min of loading. Importantly the inhibitory effect of amylase on insulin release was still observed for a further 30 min after the amylase infusion was stopped. Neither the NaCl infusion nor the 1% BSA infusion inhibited the immediate insulin release observed in response to the glucose loading.
In contrast, BSA infusion to the pancreatic artery strongly enhanced insulin secretion for the entire experimental period of 2 h. This observation needs to be further investigated in future studies to highlight the effects of plasma proteins on insulin release and resistance[12].
In summary, the inhibition of insulin secretion by intrapancreatic amylase confirms the existence of an AIA reflex. The results of the present study should however be considered in the context of a serious limitation in that acute conditions can affect the sensitivity of insulin release during an IVGTT[13]. Thus, similarly designed, chronic experiments are going to be carried out in the near future.
| 1. | Pierzynowski SG, Gregory PC, Filip R, Woliński J, Pierzynowska KG. Glucose homeostasis dependency on acini-islet-acinar (AIA) axis communication: a new possible pathophysiological hypothesis regarding diabetes mellitus. Nutr Diabetes. 2018;8:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Matull WR, Pereira SP, O'Donohue JW. Biochemical markers of acute pancreatitis. J Clin Pathol. 2006;59:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Nakajima K. Low serum amylase and obesity, diabetes and metabolic syndrome: A novel interpretation. World J Diabetes. 2016;7:112-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Zhan F, Chen J, Yan H, Wang S, Zhao M, Zhang S, Lan X, Maekawa M. Association of Serum Amylase Activity and the Copy Number Variation of AMY1/2A/2B with Metabolic Syndrome in Chinese Adults. Diabetes Metab Syndr Obes. 2021;14:4705-4714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 5. | Tanaka K, Kawano T, Tomino T, Kawano H, Okada T, Oshita S, Takahashi A, Nakaya Y. Mechanisms of impaired glucose tolerance and insulin secretion during isoflurane anesthesia. Anesthesiology. 2009;111:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Pierzynowska K, Oredsson S, Pierzynowski S. Amylase-Dependent Regulation of Glucose Metabolism and Insulin/Glucagon Secretion in the Streptozotocin-Induced Diabetic Pig Model and in a Rat Pancreatic Beta-Cell Line, BRIN-BD11. J Diabetes Res. 2020;2020:2148740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Lozinska L, Weström B, Prykhodko O, Lindqvist A, Wierup N, Ahrén B, Szwiec K, Pierzynowski SG. Decreased insulin secretion and glucose clearance in exocrine pancreas-insufficient pigs. Exp Physiol. 2016;101:100-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Pierzynowski SG, Mårtensson H, Weström BR, Ahrén B, Uvnäs-moberg K, Karlsson BW. Cholecystokinin (ck 33) stimulates pancreatic secretion via a local intestinal mechanism in pigs. Biomed Res. 1993;14:217-221. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Kiela P, Zabielski R, Podgurniak P, Midura M, Barej W, Gregory P, Pierzynowski SG. Cholecystokinin-8 and vasoactive intestinal polypeptide stimulate exocrine pancreatic secretion via duodenally mediated mechanisms in the conscious pig. Exp Physiol. 1996;81:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Lifson N, Kramlinger KG, Mayrand RR, Lender EJ. Blood flow to the rabbit pancreas with special reference to the islets of Langerhans. Gastroenterology. 1980;79:466-473. [PubMed] |
| 11. | Jansson L, Barbu A, Bodin B, Drott CJ, Espes D, Gao X, Grapensparr L, Källskog Ö, Lau J, Liljebäck H, Palm F, Quach M, Sandberg M, Strömberg V, Ullsten S, Carlsson PO. Pancreatic islet blood flow and its measurement. Ups J Med Sci. 2016;121:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Bae JC, Seo SH, Hur KY, Kim JH, Lee MS, Lee MK, Lee WY, Rhee EJ, Oh KW. Association between Serum Albumin, Insulin Resistance, and Incident Diabetes in Nondiabetic Subjects. Endocrinol Metab (Seoul). 2013;28:26-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Ishihara H, Kallus FT, Giesecke AH Jr. Intravenous glucose tolerance test during anaesthesia in dogs: insulin response and glucose clearance. Can Anaesth Soc J. 1981;28:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |